Submitted:
19 September 2024
Posted:
19 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Diffusion Based on Thermodynamic Solution Theory
2.1. Thermodynamic Solution Theory
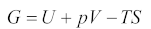
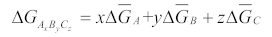
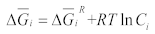
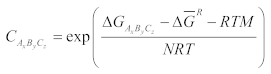
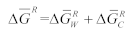
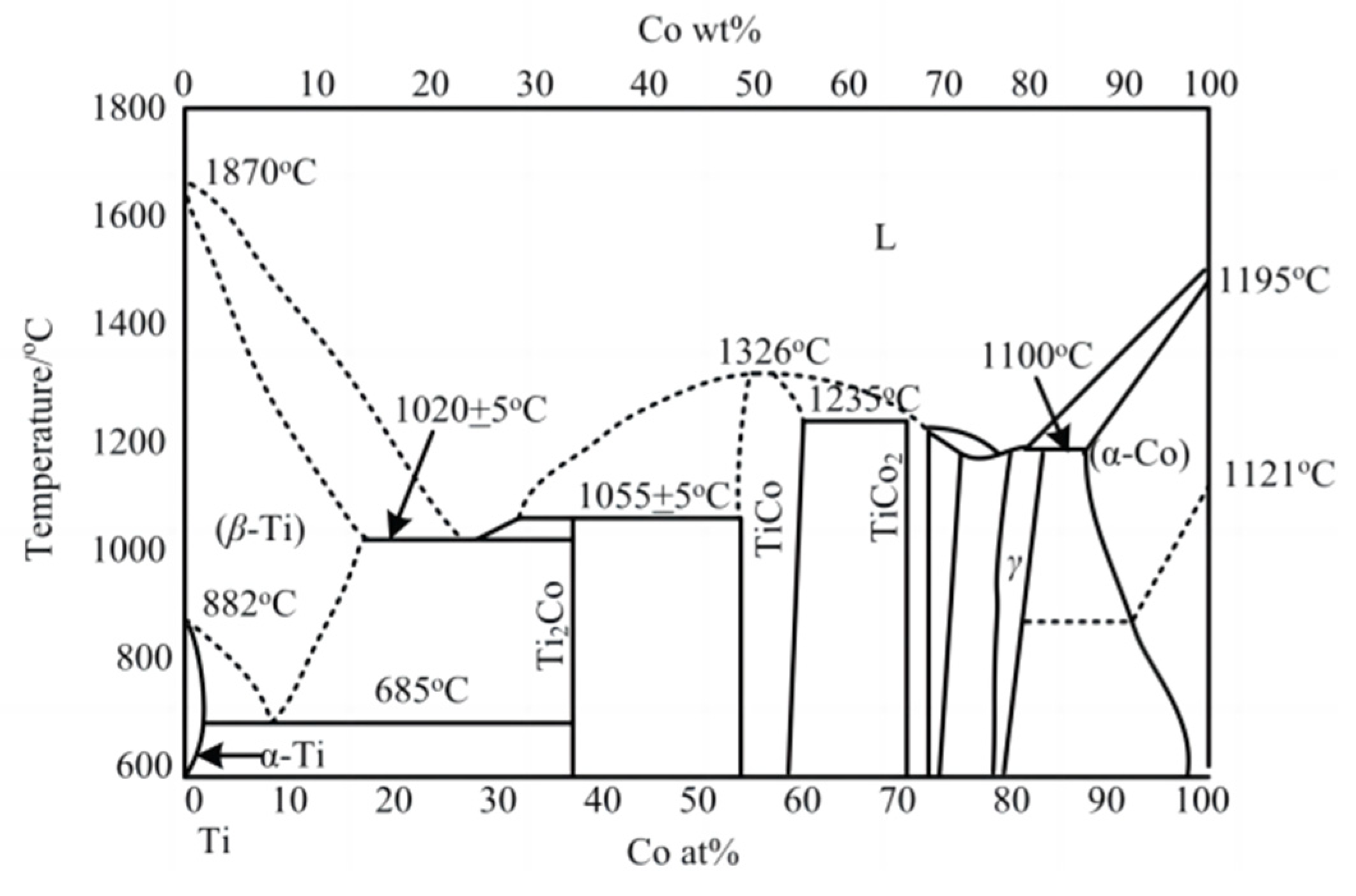
2.2. Solubility Calculation of Base Materials in Titanium
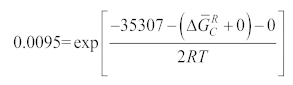
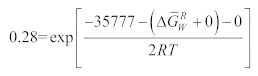
2.3. Solubility Calculation of Coating Materials in Titanium
3. Experimental Introduction
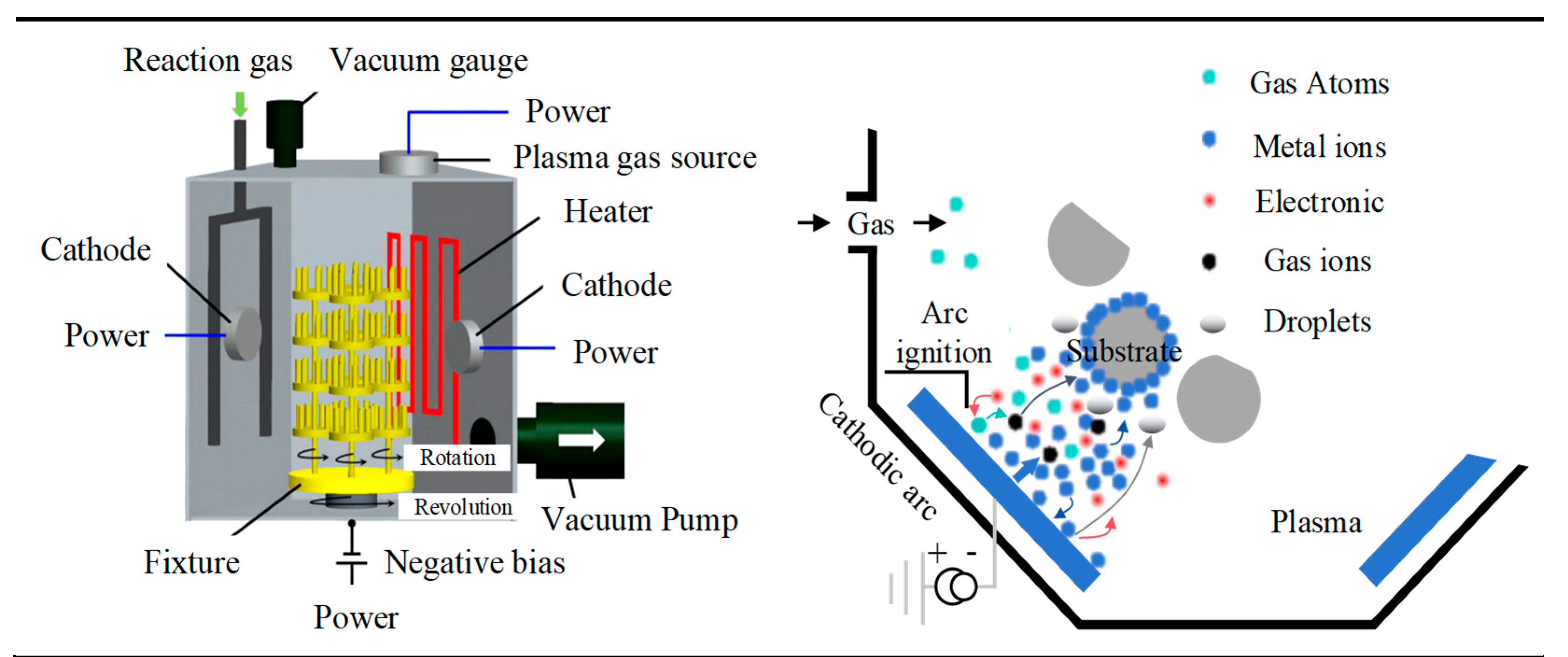
3.1. Coating Experiment
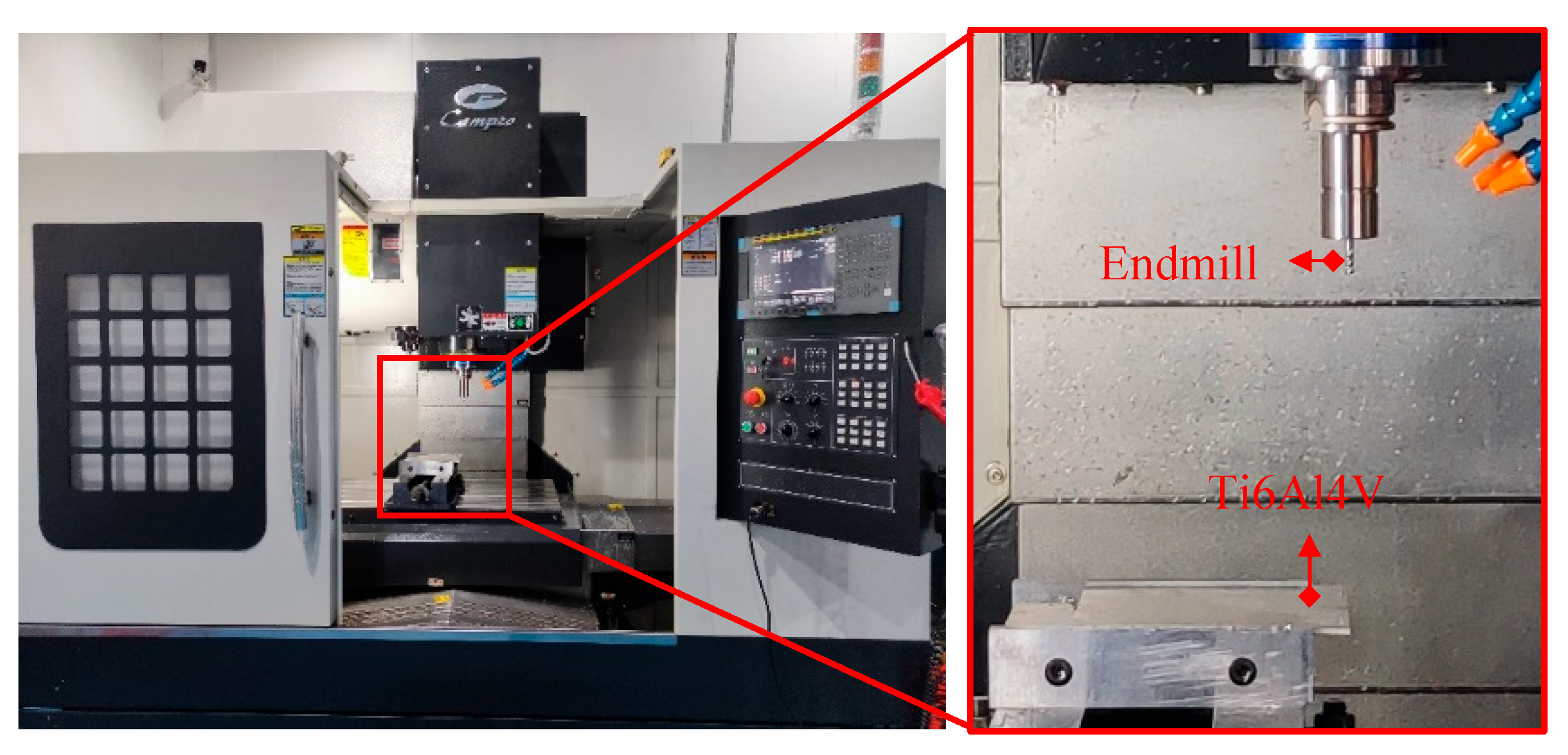
3.2. Milling Experiment
4. Results and Discussion
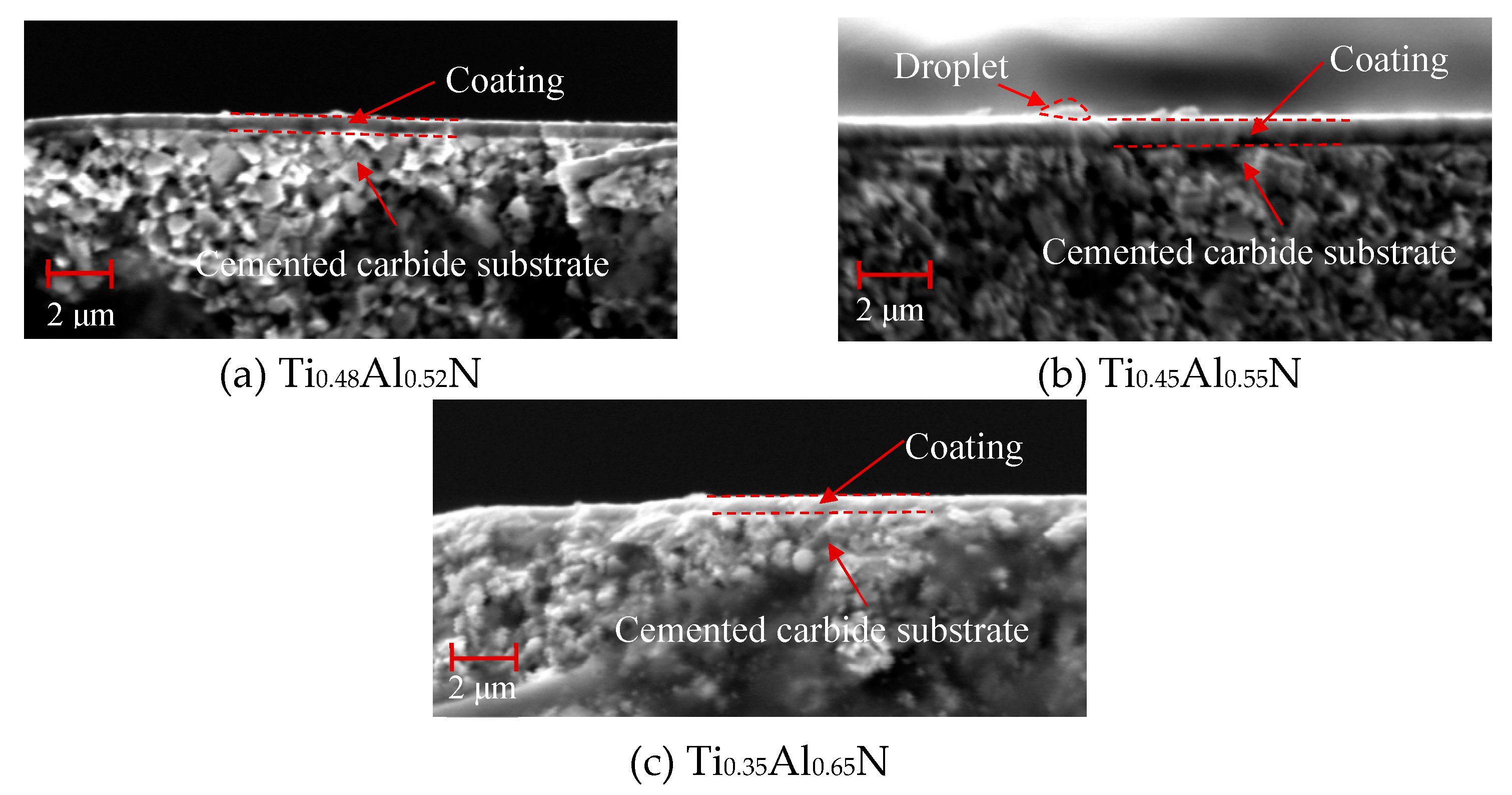
4.1. Base Structure and Morphology
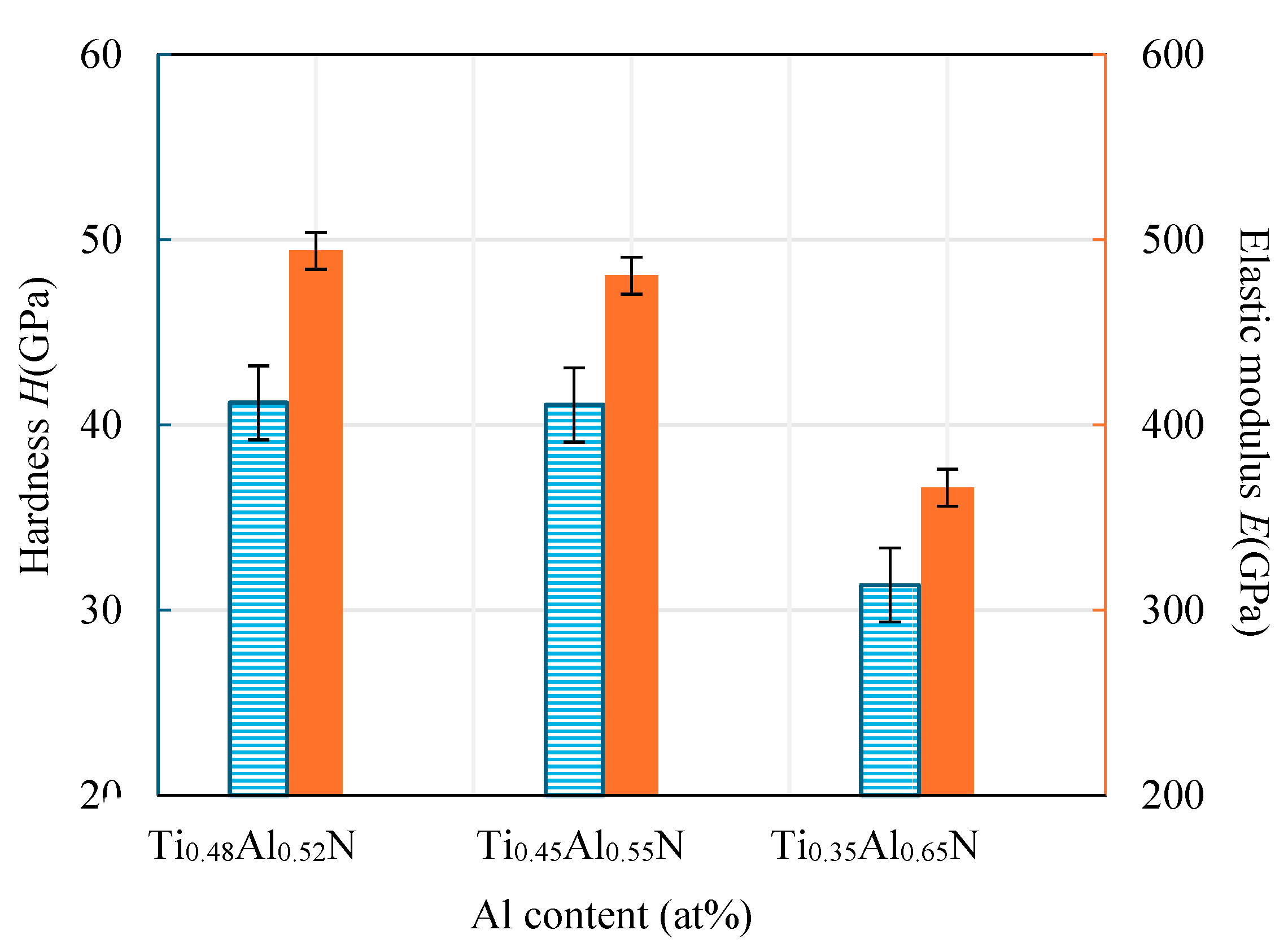
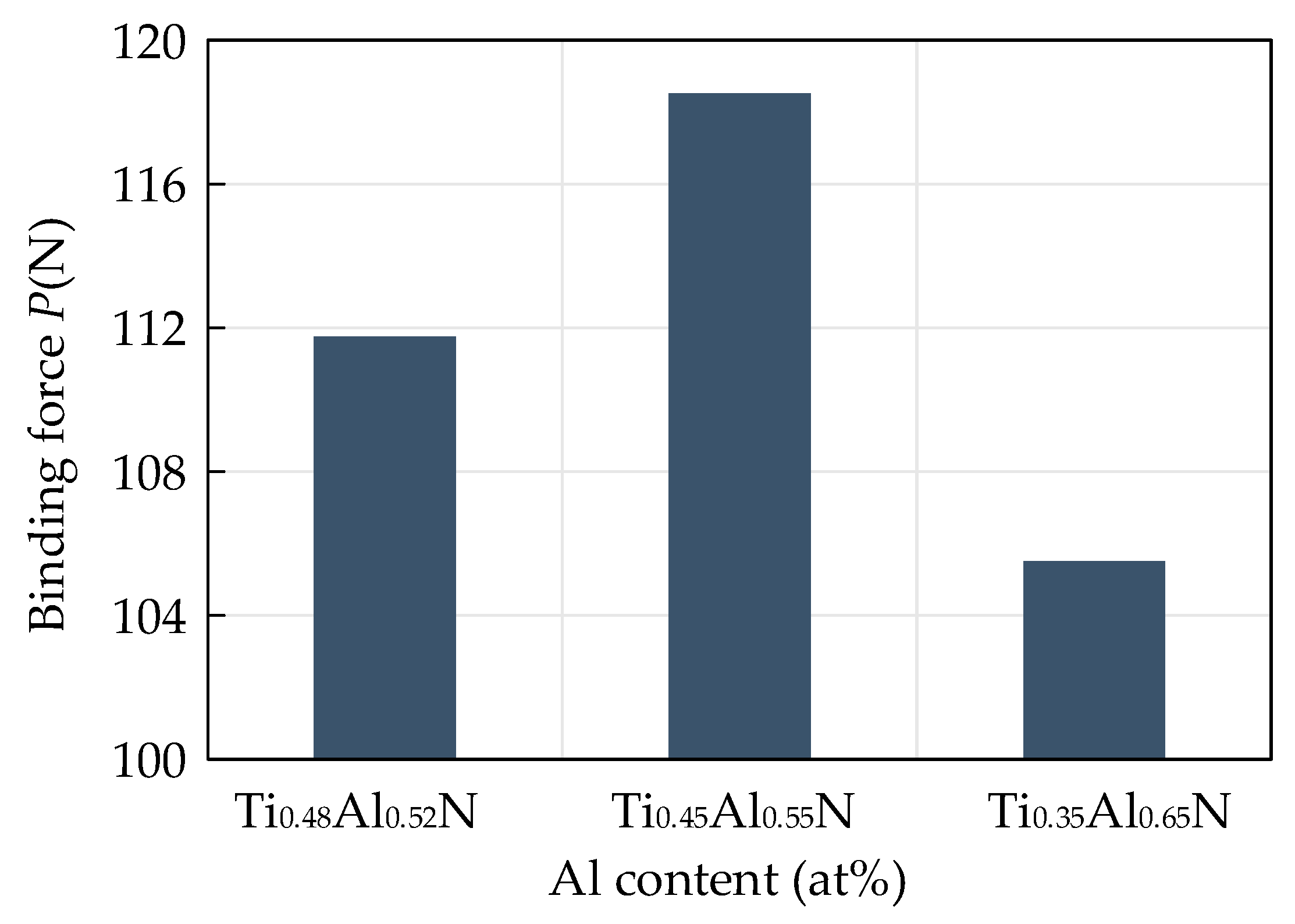
4.2. Mechanical Properties of Base Layer
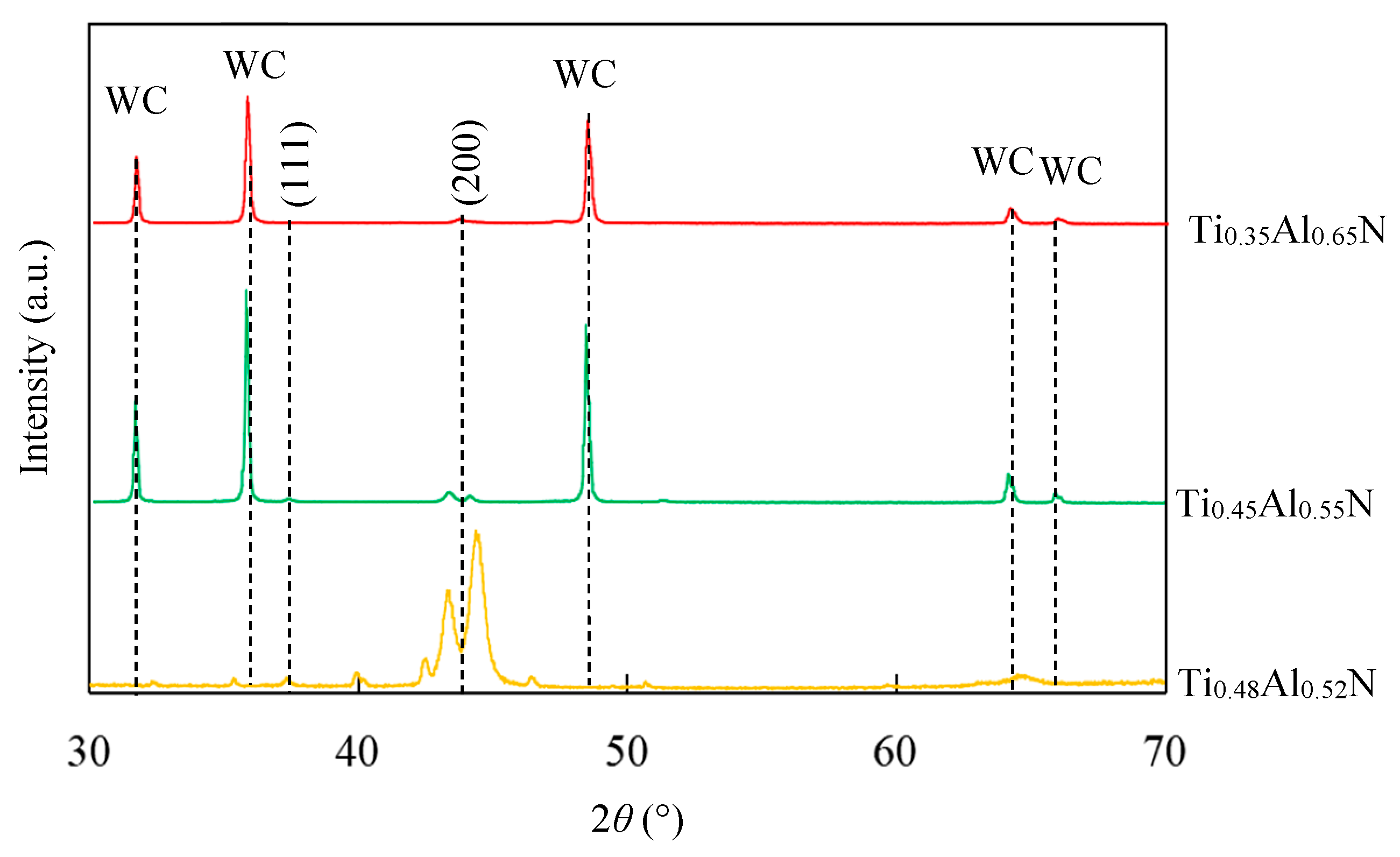
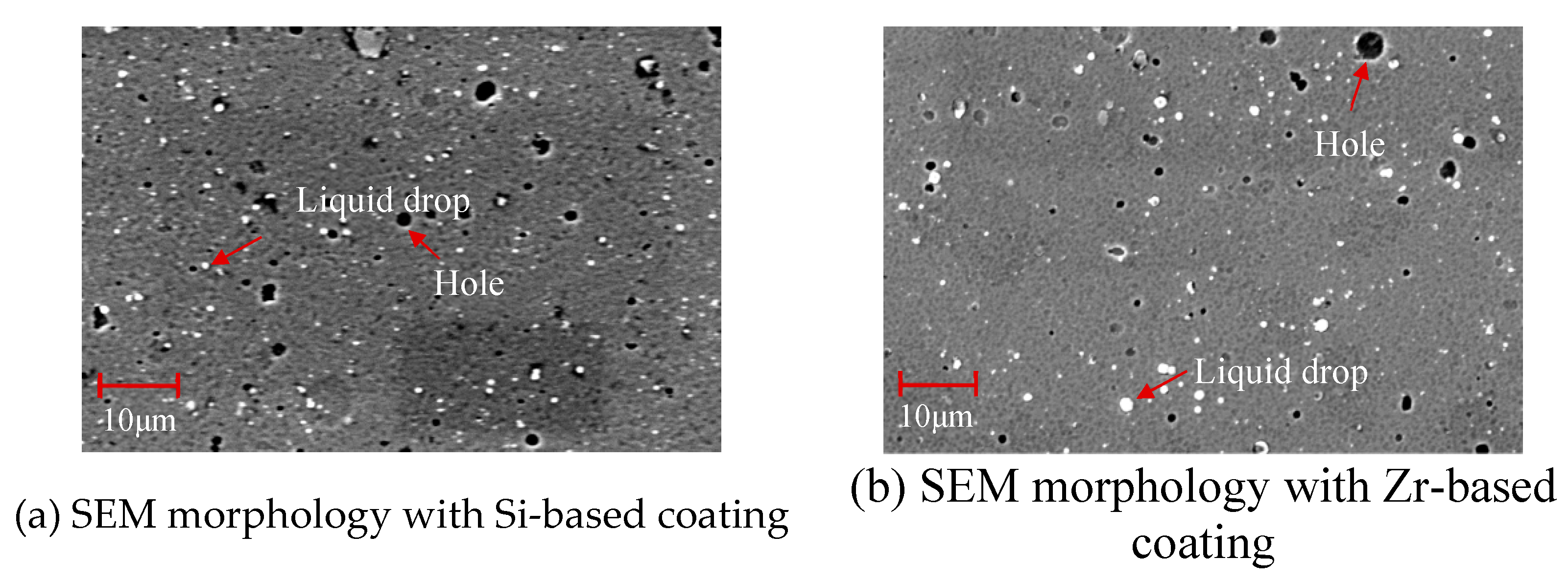
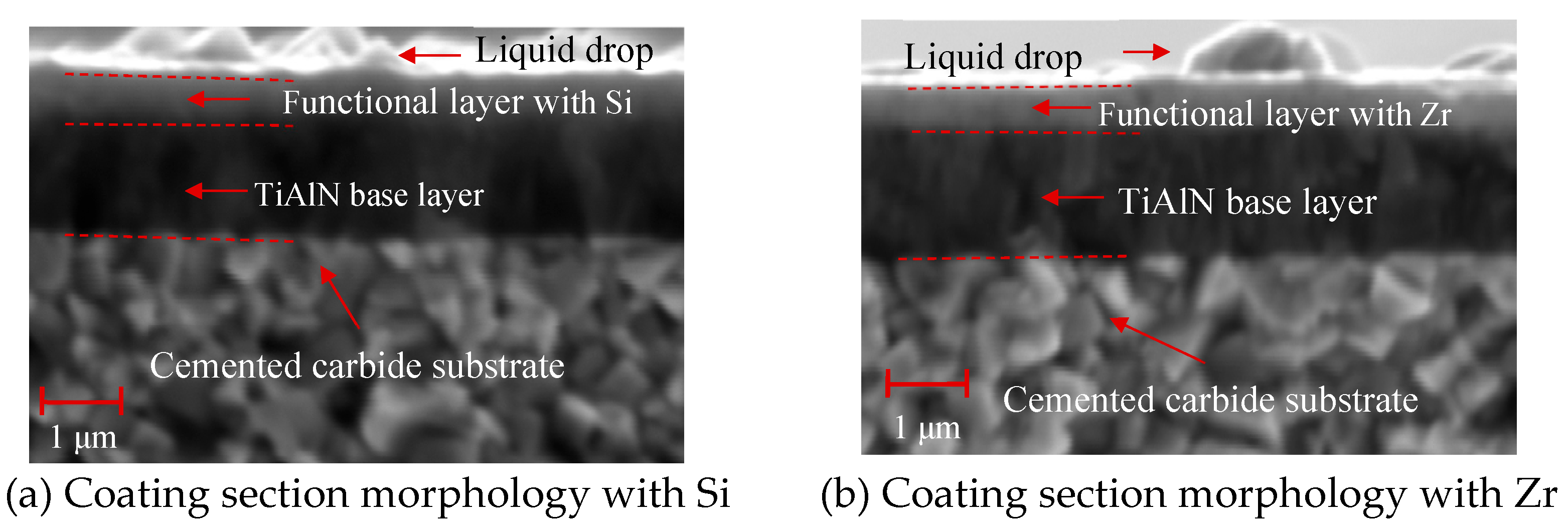
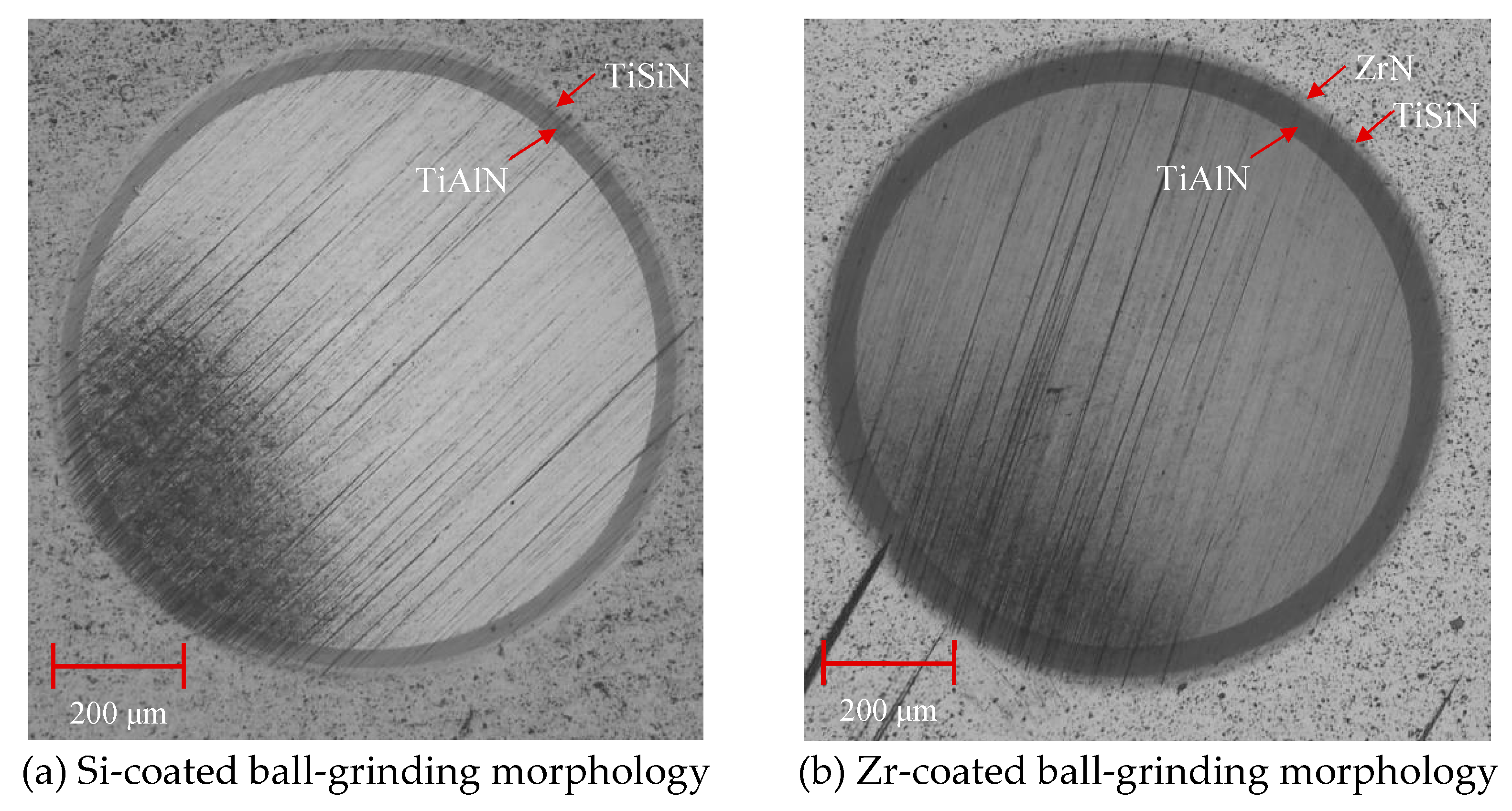
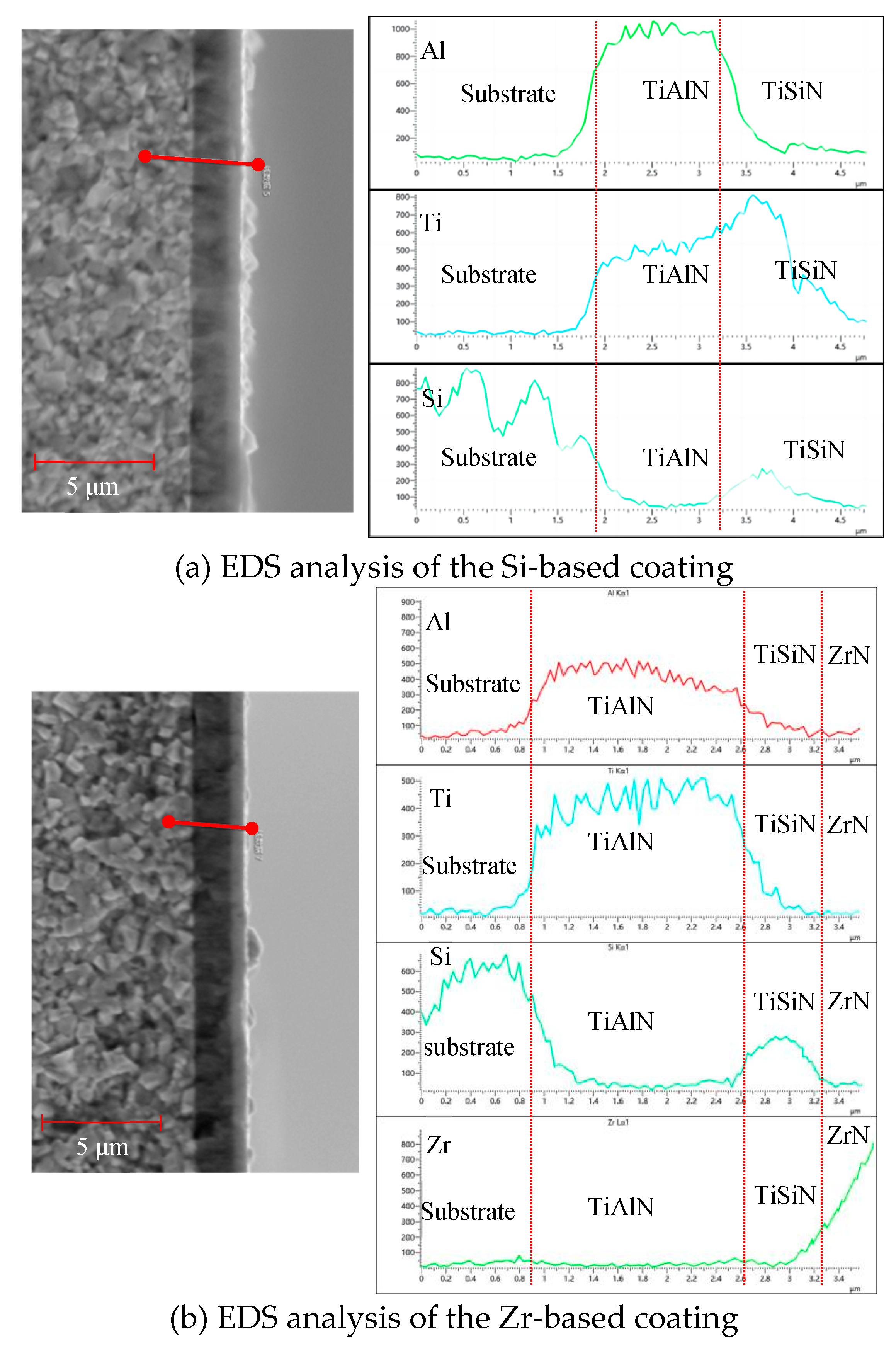
4.3. Structure and Morphology of Functional Layer
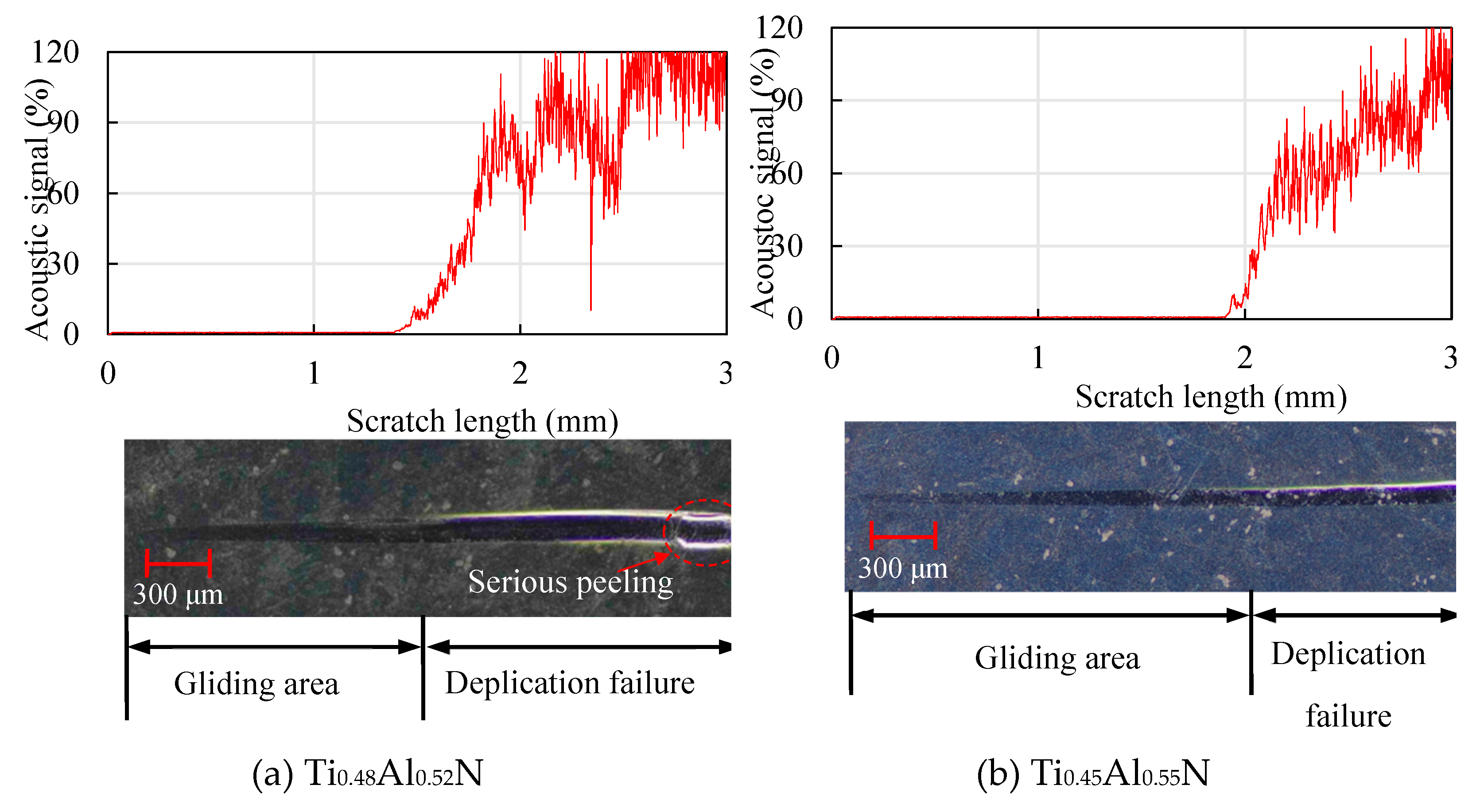
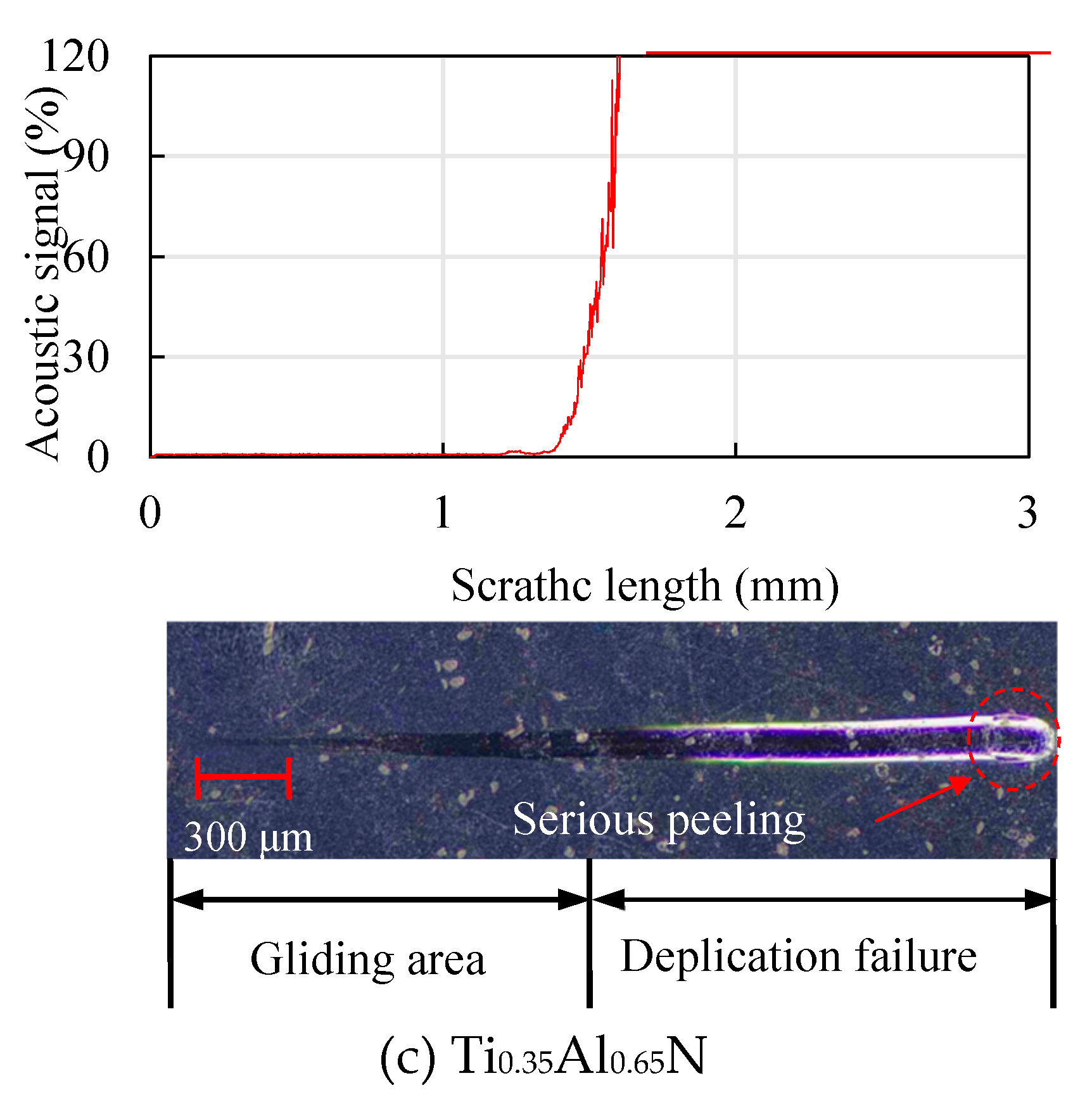
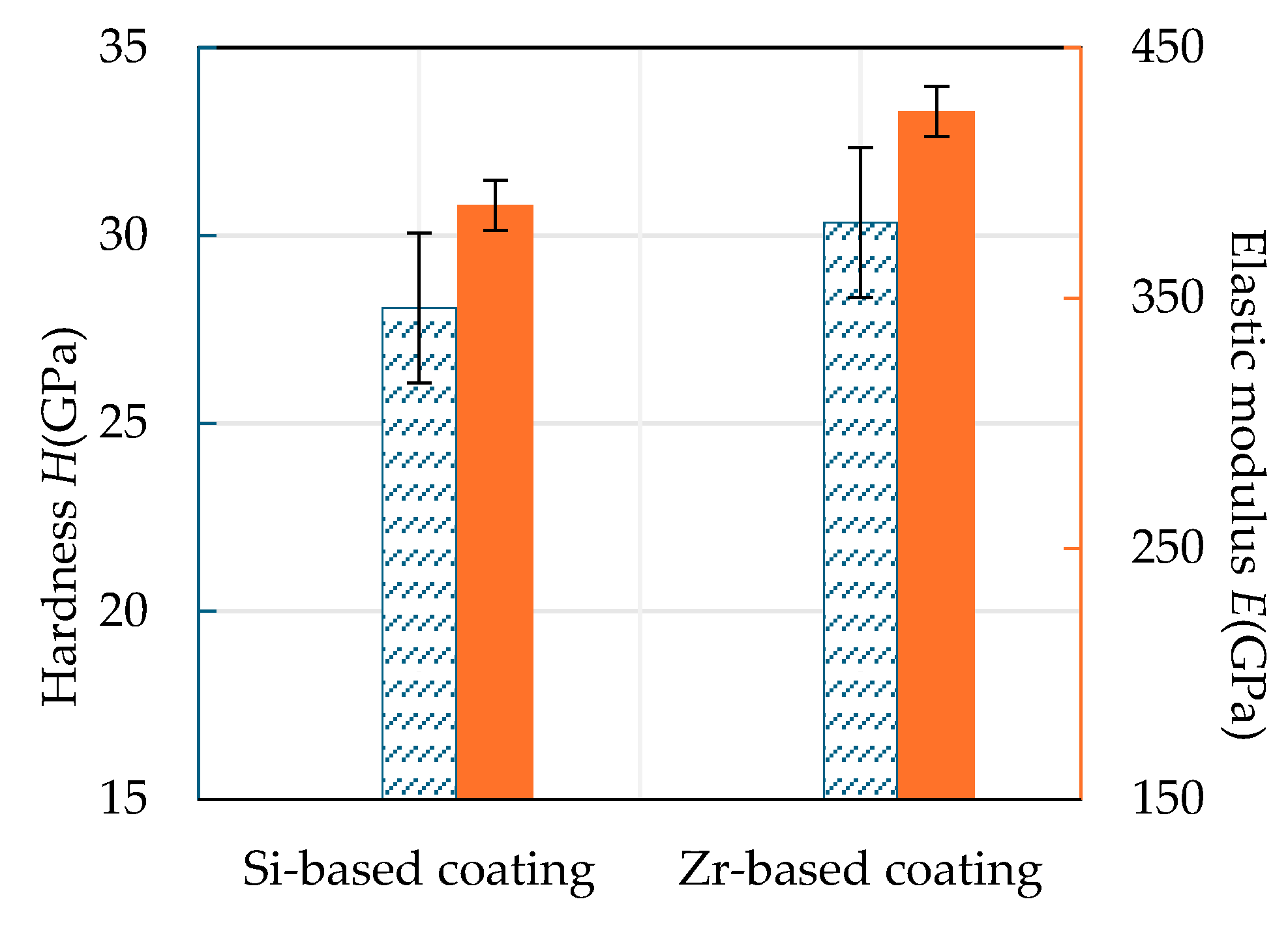
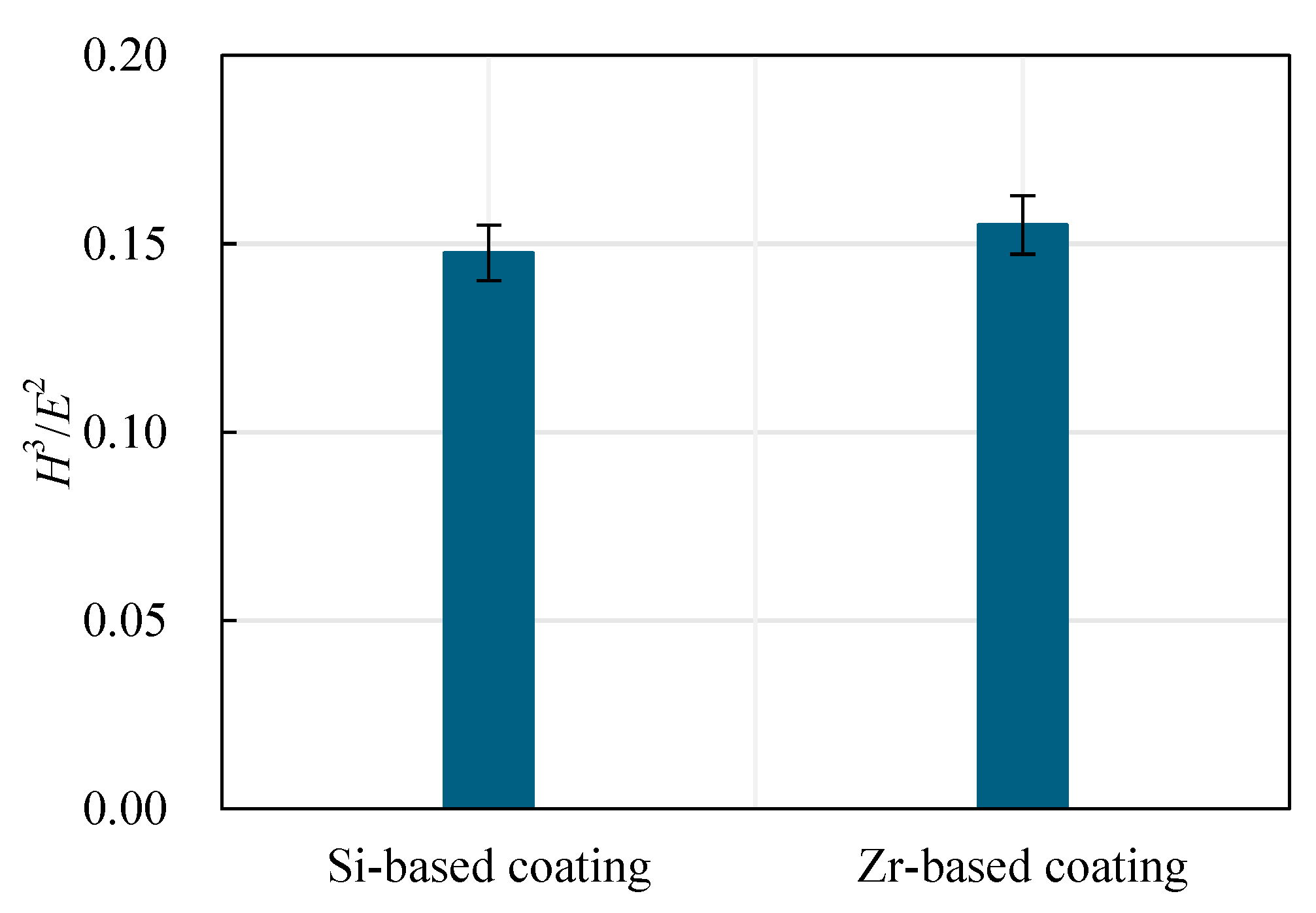
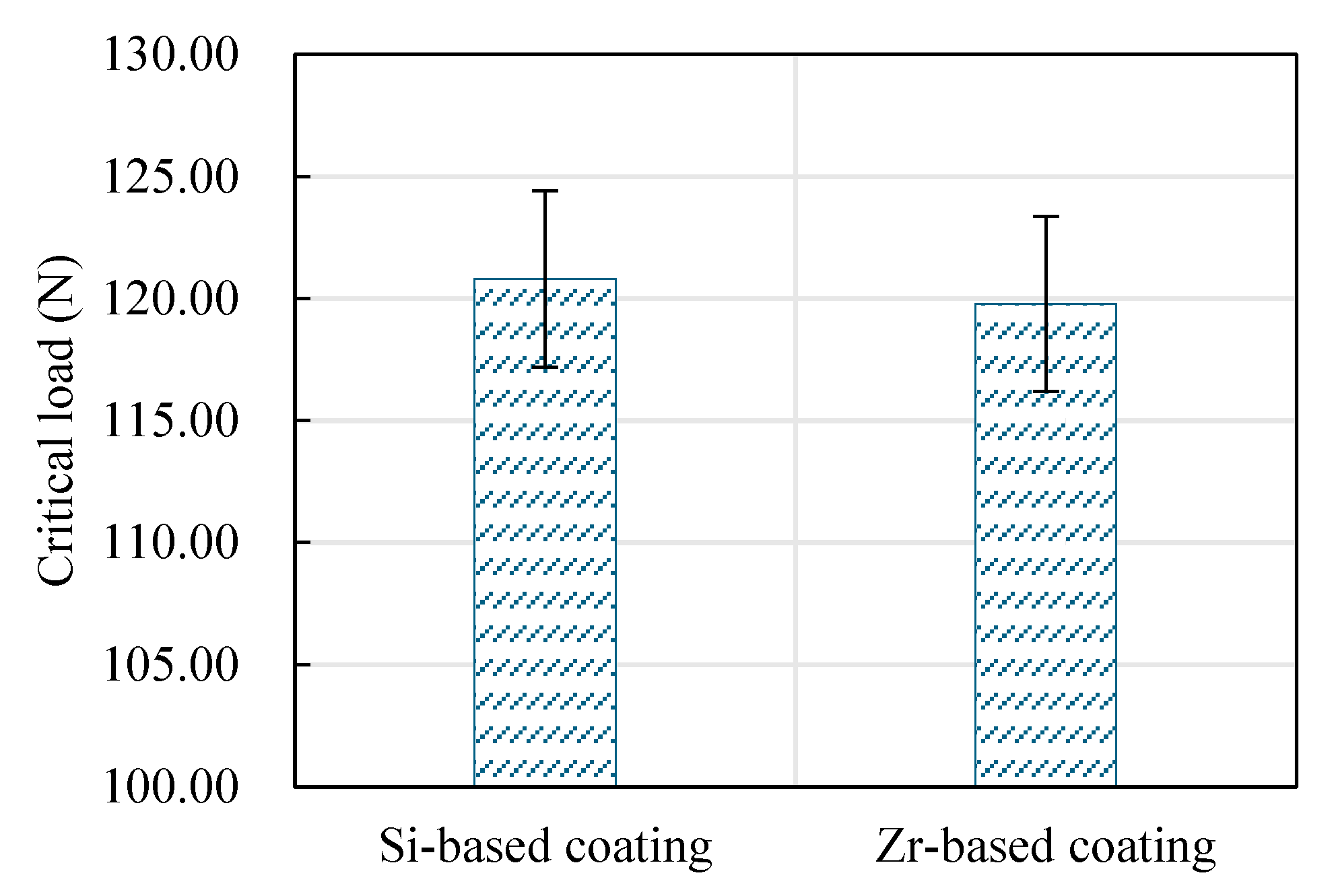
4.4. Analysis of Mechanical Properties of Functional Layer
4.5. Mechanical Property Analysis of Functional Layer
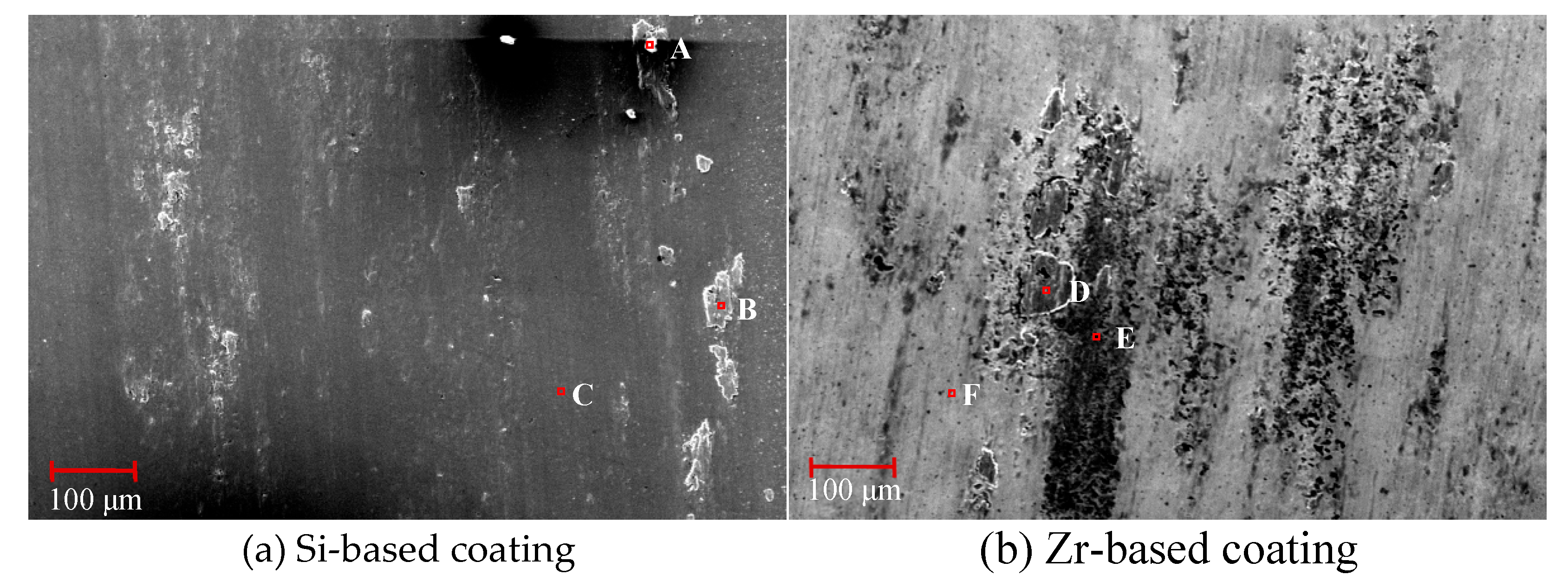
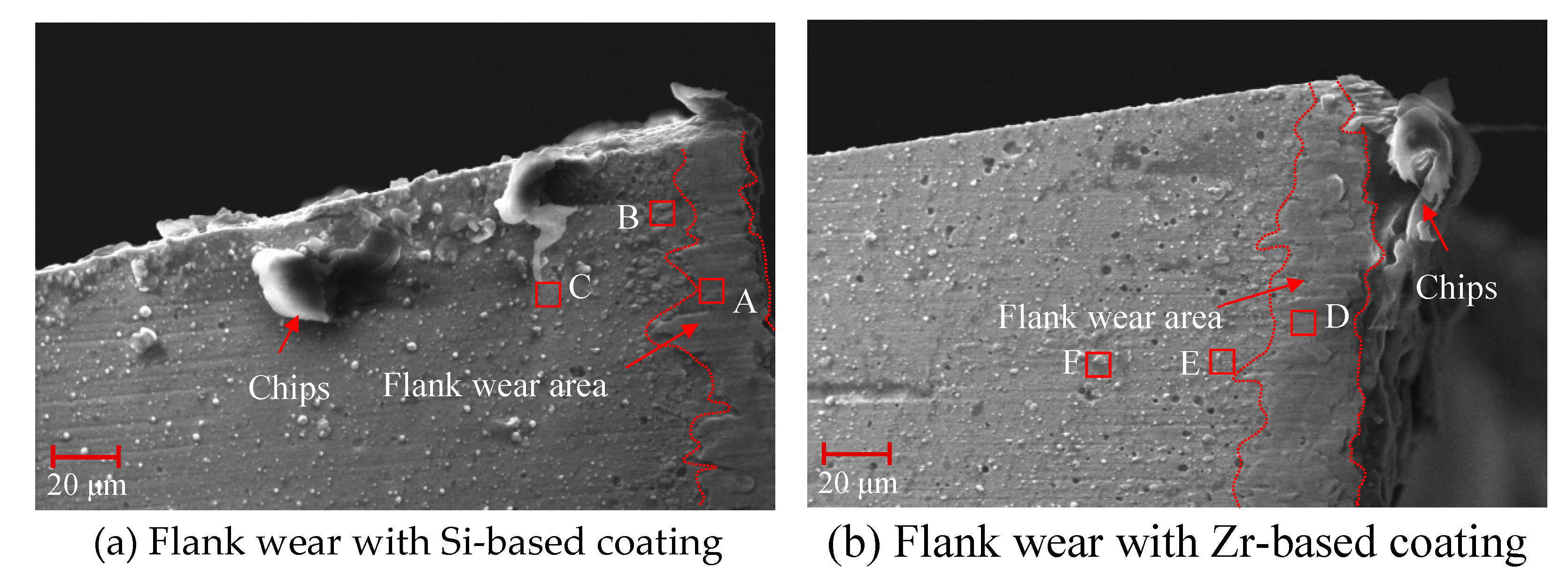
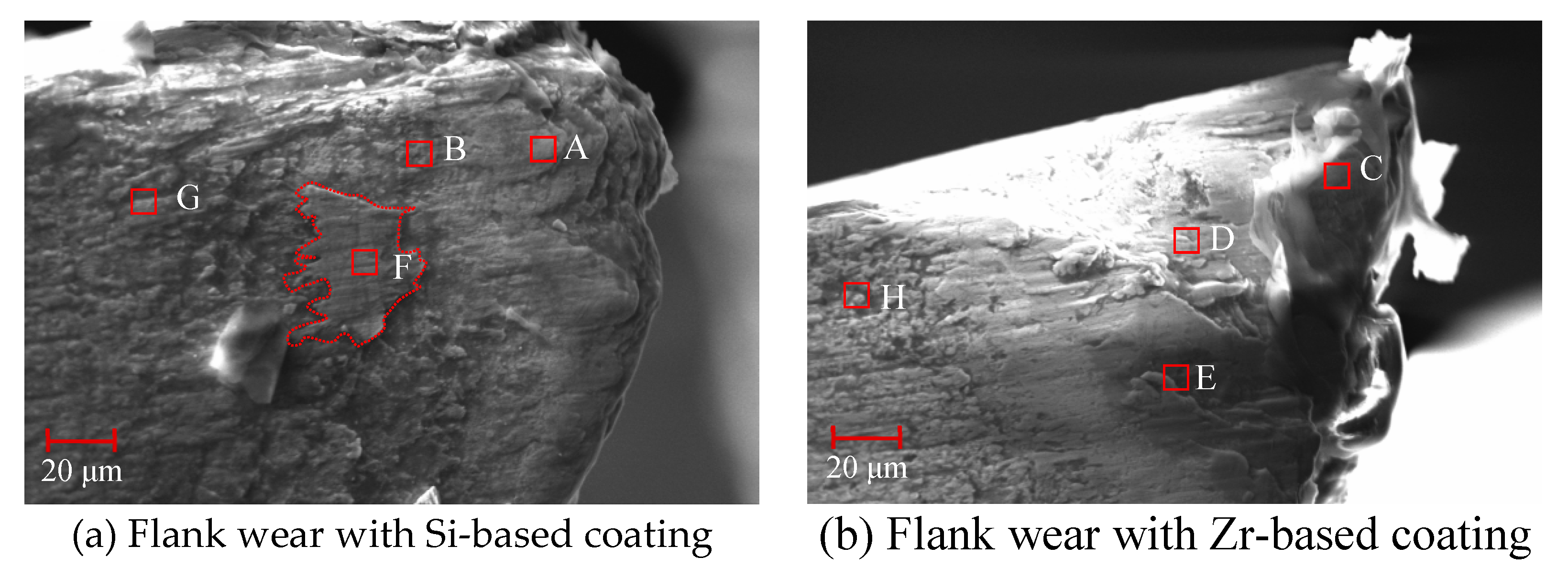
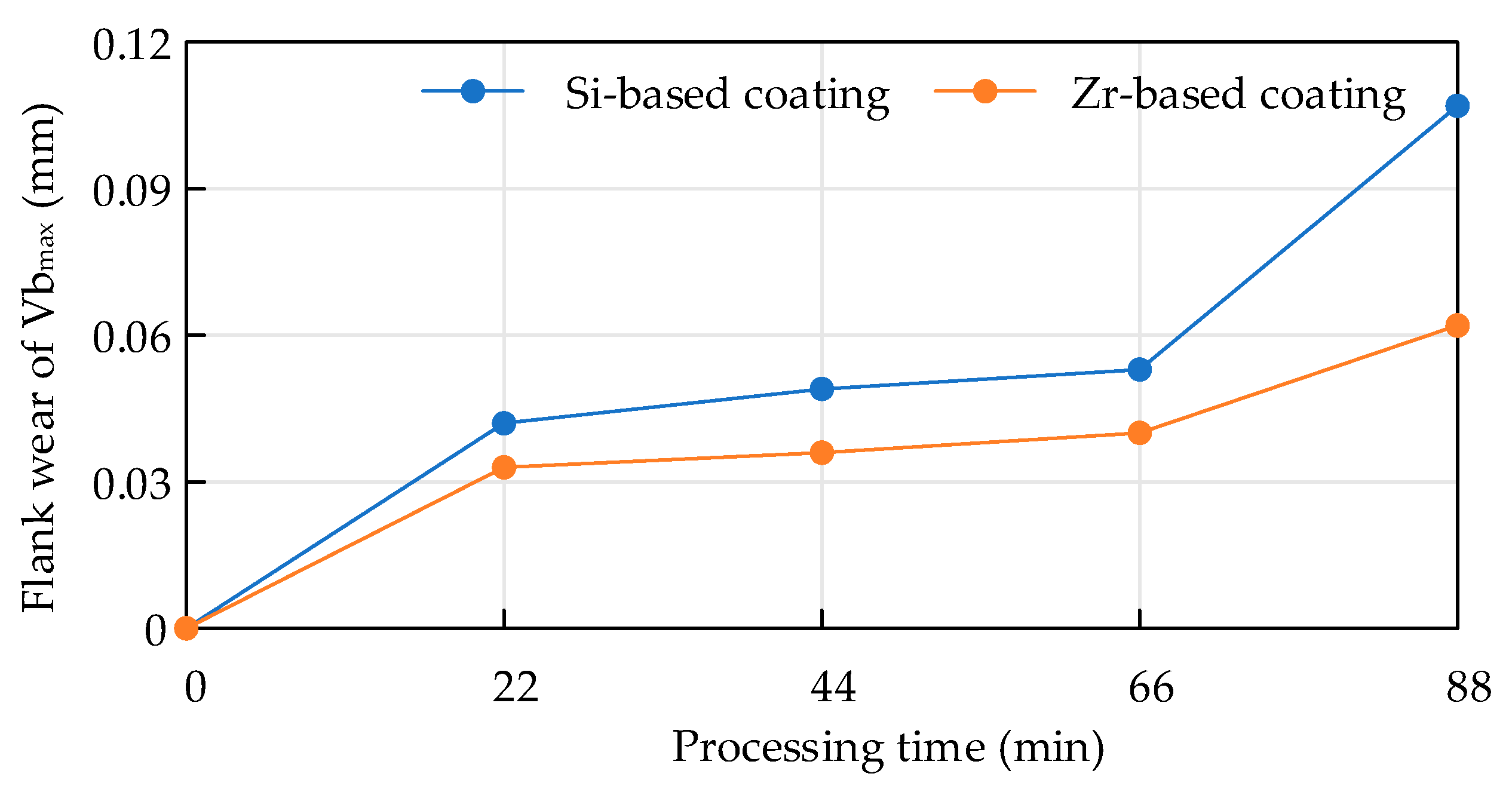
4.6. Analysis of Flank Wear in Milling Ti6Al4V with Different Coatings
5. Conclusion
- During the Ti6Al4V milling process, dissolution and diffusion phenomena easily occur between the tool substrate and the processed material under high temperature conditions, which will reduce the wear and damage resistance of the tool. Based on the thermodynamic solution theory, the dissolution and diffusion of Si and Zr element in Ti at different temperatures are analyzed. The solubility of Si element and Zr element in Ti was found to be much lower than the solubility of W element and Co element, which can effectively prevent the occurrence of element diffusion.
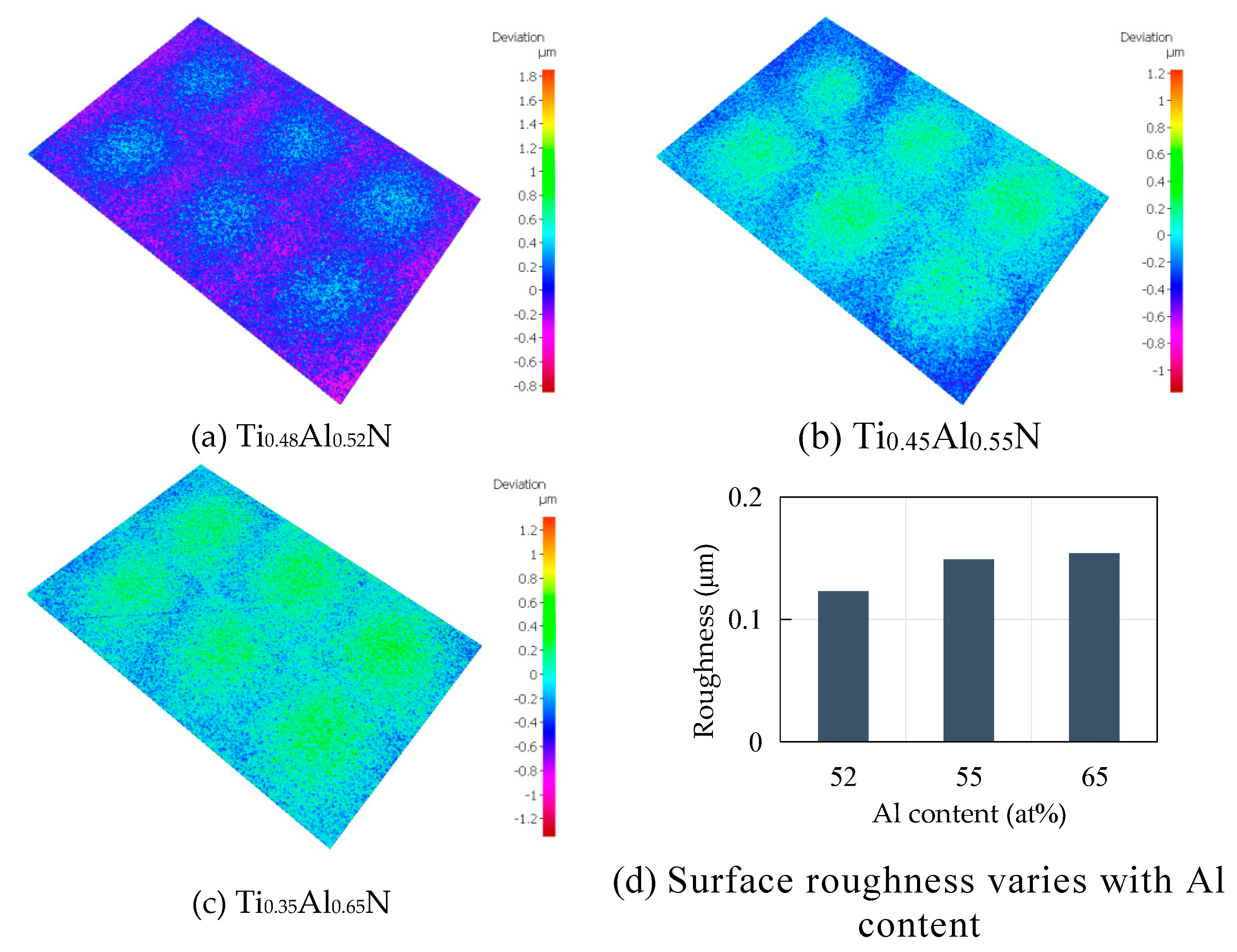
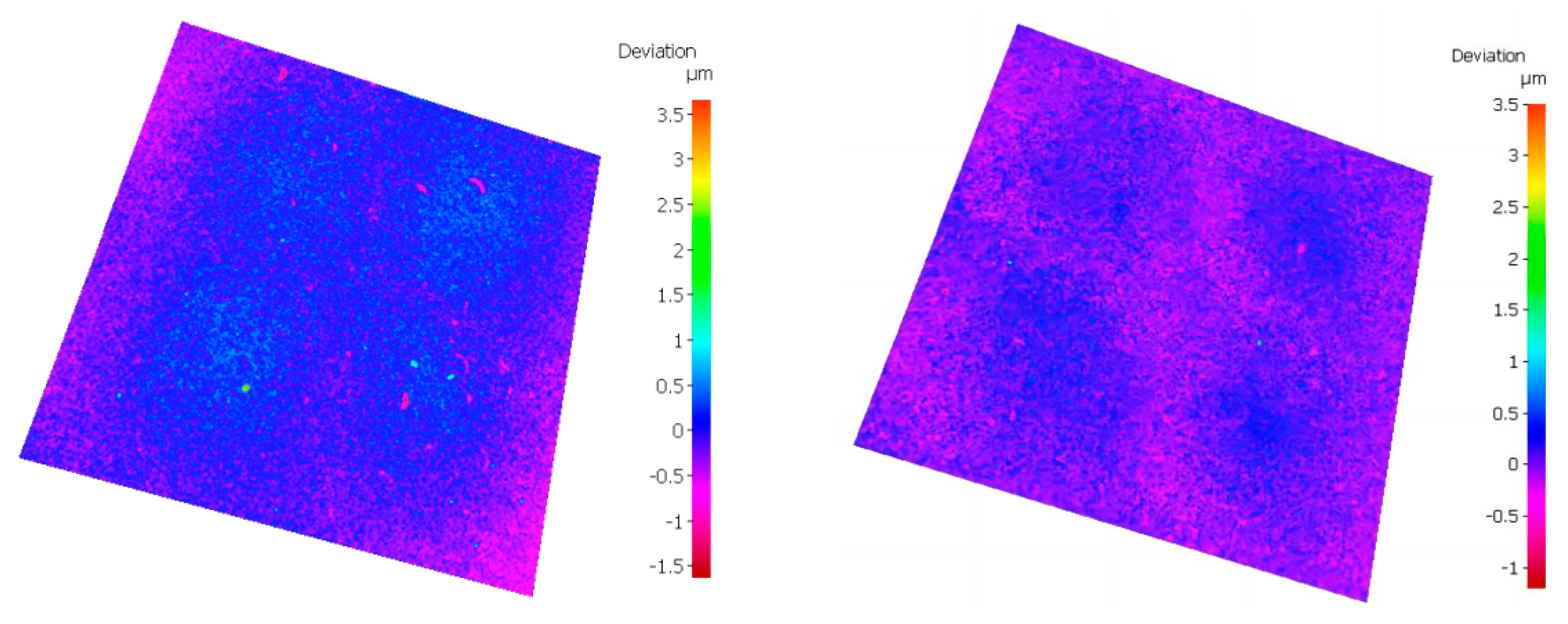
- Conduct research on base coatings with different Ti: Al ratios to obtain the proportion of Re-containing cemented carbide base coating elements. The surface roughness of the base layer increases with the increase of Al element content, and the hardness and elastic modulus increase with the increase of Al element. The Ti: Al ratio decreases with the increase of content. When the Ti: Al ratio is 50:50, there is a higher bonding force between the substrate and the base layer.
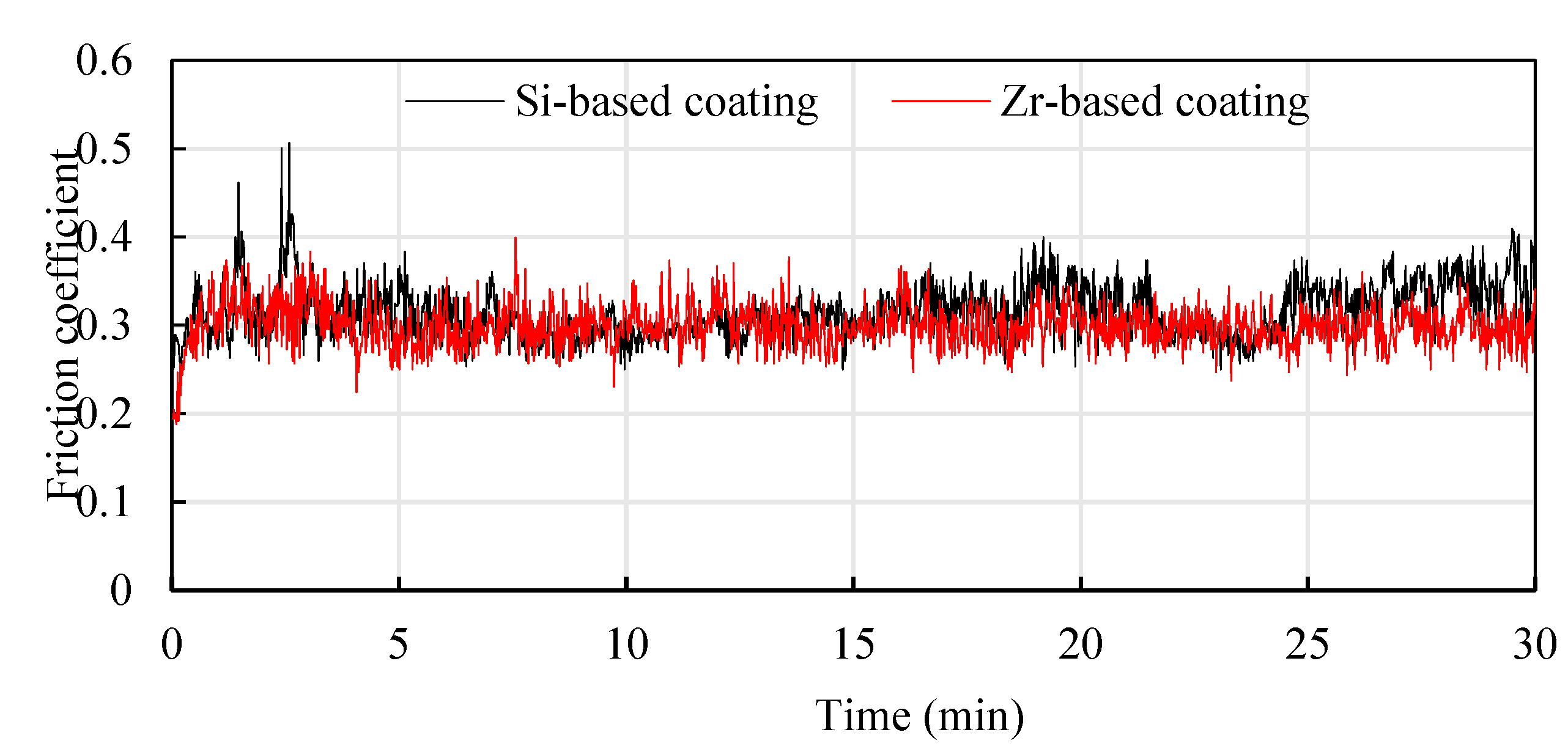
- Analyzing the performance of the functional layers TiSiN and TiSiN/ZrN, it was found that the addition of Zr element reduced the surface roughness of the coating. Compared with the TiSiN coating, the addition of Zr element to TiSiN/ZrN reduced the surface roughness of the coating and increased the density of the coating, the hardness and elastic modulus of the coating were improved. Friction and wear experiments were conducted with Ti6Al4V and it was found that the Zr-based coating obtained a lower friction coefficient.
- The tool was prepared for Ti6Al4V milling experiments and the initial and mid-term tool wear were analyzed. It was found that the addition of Zr element effectively suppressed the occurrence of bonding wear and effectively extended the cutting life of the tool.
References
- JAWAID A, SHARIF S, KOKSAL S. Evaluation of wear mechanisms of coated carbide tools when face milling titanium alloy[J]. Journal of Materials Processing Technology, 2000, 99(1-3): 266-274.
- C. Chim, X.Z. Ding, X.T. Zeng, S. Zhang, Oxidation resistance of TiN, CrN, TiAlN and CrAlN coatings deposited by lateral rotating cathode arc, Thin Solid Films 517 (2009) 4845–4849.
- H. Liu, J.X. Deng, H.B. Cui, Y.Y. Chen, J. Zhao, Friction and wear properties of TiN, TiAlN, AlTiN and CrAlN PVD nitride coatings, Int. J. Refract. Met. Hard 31 (2012) 82–88.
- Chen L, Paulitsch J, Du Y, et al. Thermal stability and oxidation resistance of Ti–Al–N coatings[J]. Surface and Coatings Technology, 2012, 206(11-12): 2954-2960.
- AN Qinglong, CHEN Jie, TAO Zhengrui, et al. Experimental investigation on tool wear characteristics of PVD and CVD coatings during face milling of Ti-6242S and Ti-555 titanium alloys[J]. International Journal of Refractory Metals and Hard Materials, 2020, 86: 105091.
- KURAM, E. The effect of monolayer TiCN-, AlTiN-, TiAlN-and two layers TiCN+ TiN-and AlTiN+ TiN-coated cutting tools on tool wear, cutting force, surface roughness and chip morphology during high-speed milling of Ti6Al4V titanium alloy[J]. Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture, 2018, 232(7): 1273-1286.
- Yi J Y, Chen K H, Xu Y C, et al. Performance of AlTiBN and AlTiTaN coatings during milling of titanium[J]. Surface Engineering, 2019, 35(6): 501-506.
- NIU Qiulin, CHEN Ming, MING Weiwei, et al. Evaluation of the performance of coated carbide tools in face milling TC6 alloy under dry condition[J]. The International Journal of Advanced Manufacturing Technology, 2013, 64(5): 623-631.
- NIU Qiulin, AN Qinglong, CHEN Ming, et al. Wear mechanisms and performance of coated inserts during face milling of TC11 and TC17 alloys[J]. Machining Science and Technology, 2013, 17(3): 483-495.
- Biksa A, Yamamoto K, Dosbaeva G, et al. Wear behavior of adaptive nano-multilayered AlTiN/MexN PVD coatings during machining of aerospace alloys[J]. Tribology International, 2010, 43(8): 1491-1499.
- SRINIVASAN B, RAO M S R, RAO B C. On the development of a dual-layered diamond-coated tool for the effective machining of titanium Ti-6Al-4V alloy[J]. Journal of Physics D: Applied Physics, 2016, 50(1): 015302.
- THEPSONTHI T, OZEL T. Experimental and finite element simulation based investigations on micro-milling Ti-6Al-4V titanium alloy: Effects of cBN coating on tool wear[J]. Journal of Materials Processing Technology, 2013, 213(4): 532-542.
- CALISKAN H, KUCUKKOSE M. The effect of aCN/TiAlN coating on tool wear, cutting force, surface finish and chip morphology in face milling of Ti6Al4V superalloy[J]. International Journal of Refractory Metals and Hard Materials, 2015, 50: 304-312.
- VOLOSOVA M A, FYODOROV S V, OPLESHIN S, et al. Wear resistance and titanium adhesion of cathodic arc deposited multi-component coatings for carbide end mills at the trochoidal milling of titanium alloy[J]. Technologies, 2020, 8(3): 38.
- Zhang, Z.; Wang, Q. J.; Mo, W. Metallography and Heat Treatment of Titanium[M]. Metallurgical industry press, 2009.
- Fan, Y. H.; Hao, Z. P.; Zheng. M. L.; Sun, F. L.; Niu, S. L. Tool diffusion wear mechanism in high efficiency machining Ti6Al4V[C]//Key Engineering Materials. Trans Tech Publications Ltd, 2014, 579: 3-7.
- Wang, W. G.; Zhang, H. J.; Wang, Q. Z.; Ma, Z. Y.; Chen, L. Q. Effects of Carbide Inhibitor on Microstructures and Mechanical Properties of Ultrafine Grained Carbide Cement WC-2.5TiC-10Co[J]. Chinese Journal of Material Research, 2015, 29, 881-888. [CrossRef]
- Zhou, X. K.; Wang, K.; Li, C. S.; Wang, Q.; Wu, S.; Liu, J. X. Effect of ultrafine gradient cemented carbides substrate on the performance of coating tools for titanium alloy high speed cutting[J]. International Journal of Refractory Metals and Hard Materials, 2019, 84, 105024. [CrossRef]
- Liang Y J, Che Y C. Inorganic substances thermodynamic data handbook[J]. 1994.
- Bhanumurthy K, Laik A, Kale G B. Novel method of Evaluation of Diffusion coefficients in Ti-Zr system[C]//Defect and Diffusion Forum. Trans Tech Publications Ltd, 2008, 279: 53-62.
- Xuming Zha. Mechanical and cutting properties of bilayer and nano-multilayer[D]. Huaqiao University, 2020.


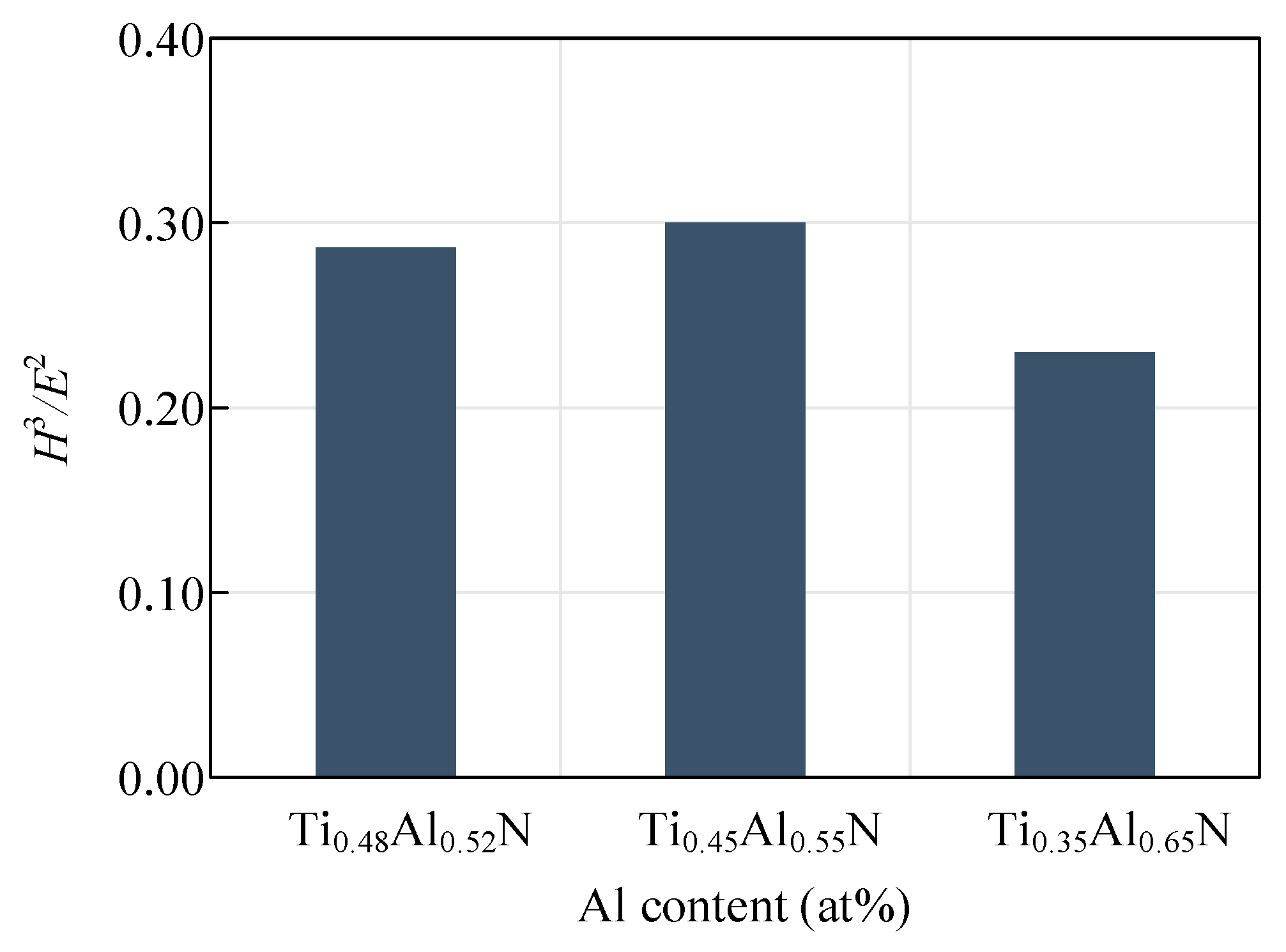


| T/K | 400 | 600 | 800 | 920 | 1000 | 1200 | 1400 |
|---|---|---|---|---|---|---|---|
| ΔGWC/J | -37700 | -36886 | -36287 | -35891 | -35777 | -35307 | -34853 |
| T/K | 400 | 600 | 800 | 920 | 1000 | 1200 |
|---|---|---|---|---|---|---|
| CWC/% | 0.92×10-3 | 0.02 | 0.18 | 0.44 | 0.68 | 1.6 |
| T/K | 800 | 900 | 1000 | 1200 |
|---|---|---|---|---|
| CCo/% | 3 | 4 | 4.5 | 8 |
| Reactivity | T/K | 953 | 1053 |
|---|---|---|---|
| Ti+3/5Si→1/5Ti5Si3 Ti+4/5Si→1/5Ti5Si4 Ti+Si→TiSi Ti+2Si→TiSi2 |
 |
-117100 -130600 -137900 -171700 |
-115500 -130600 -135200 -170600 |
| Diameter (mm) | Number of blades (-) | Helix angle (°) | Rake angle (°) | Relief angle (°) | Blade width (mm) | Coating materials |
|---|---|---|---|---|---|---|
| 6 | 4 | 38–40 | 5 | 10 | 0.45 | TiAlN/TiSiN TiAlN/TiSiN/ZrN |
| Processed material | Milling form | n (r/min) | f (mm/r) | ap (mm) | ae (mm) |
|---|---|---|---|---|---|
| Ti6Al4V | Side milling | 3713 | 0.088 | 6 | 0.3 |
| Position | Ti | Al | Si | Zr | O | N | C |
|---|---|---|---|---|---|---|---|
| A | 74.78 | 4.40 | 1.43 | 0.00 | 9.83 | 1.24 | 8.31 |
| B | 55.93 | 6.22 | 0.00 | 0.00 | 24.41 | 5.47 | 7.96 |
| C | 31.84 | 5.71 | 4.59 | 0.00 | 17.82 | 35.59 | 4.44 |
| D | 57.02 | 5.04 | 0.00 | 0.24 | 25.29 | 4.98 | 7.03 |
| E | 29.47 | 17.29 | 0.82 | 0.12 | 21.11 | 23.09 | 8.10 |
| F | 5.11 | 1.69 | 0.00 | 38.82 | 13.68 | 19.68 | 21.02 |
| Position | Ti | Al | Si | Zr | O | N | C | V |
|---|---|---|---|---|---|---|---|---|
| A | 24.31 | 0.3 | 2.26 | - | 16.10 | 23.24 | 33.58 | 0.20 |
| B | 27.29 | 0.37 | 2.87 | - | - | 47.46 | 22.01 | - |
| C | 25.82 | 0.50 | 3.17 | - | - | 48.19 | 22.32 | - |
| D | 28.93 | 8.18 | 0.49 | 12.08 | - | 26.70 | 24.11 | - |
| E | 8.44 | 2.60 | - | 10.75 | 7.09 | 21.86 | 49.27 | - |
| F | 6.32 | 2.32 | - | 10.20 | 11.54 | 14.97 | 54.65 | - |
| Poision | Ti | Al | Si | Zr | O | N | C | V | W | Co |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 70.20 | 2.79 | - | - | 8.15 | - | 15.56 | 3.29 | ||
| B | 20.21 | 4.52 | - | - | 12.4 | - | 45.84 | 0.75 | 10.35 | 5.26 |
| C | 48.39 | 1.51 | - | - | - | - | 46.47 | 2.62 | 0.25 | 0.76 |
| D | 79.19 | 3.56 | 0.24 | - | - | - | 13.36 | 3.62 | 0.05 | - |
| E | 46.84 | 0.95 | 2.51 | - | 36.64 | - | 8.25 | 2.14 | - | - |
| F | 71.83 | 6.13 | - | - | - | - | 19.02 | 3.02 | - | - |
| G | 34.60 | 1.43 | 2.24 | - | 25.24 | 7.39 | 27.69 | - | - | - |
| H | 15.72 | 3.51 | - | 10.65 | 20.66 | 3.46 | 45.24 | - | 0.13 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).