Submitted:
18 September 2024
Posted:
20 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. PROOF Subjects Characteristics
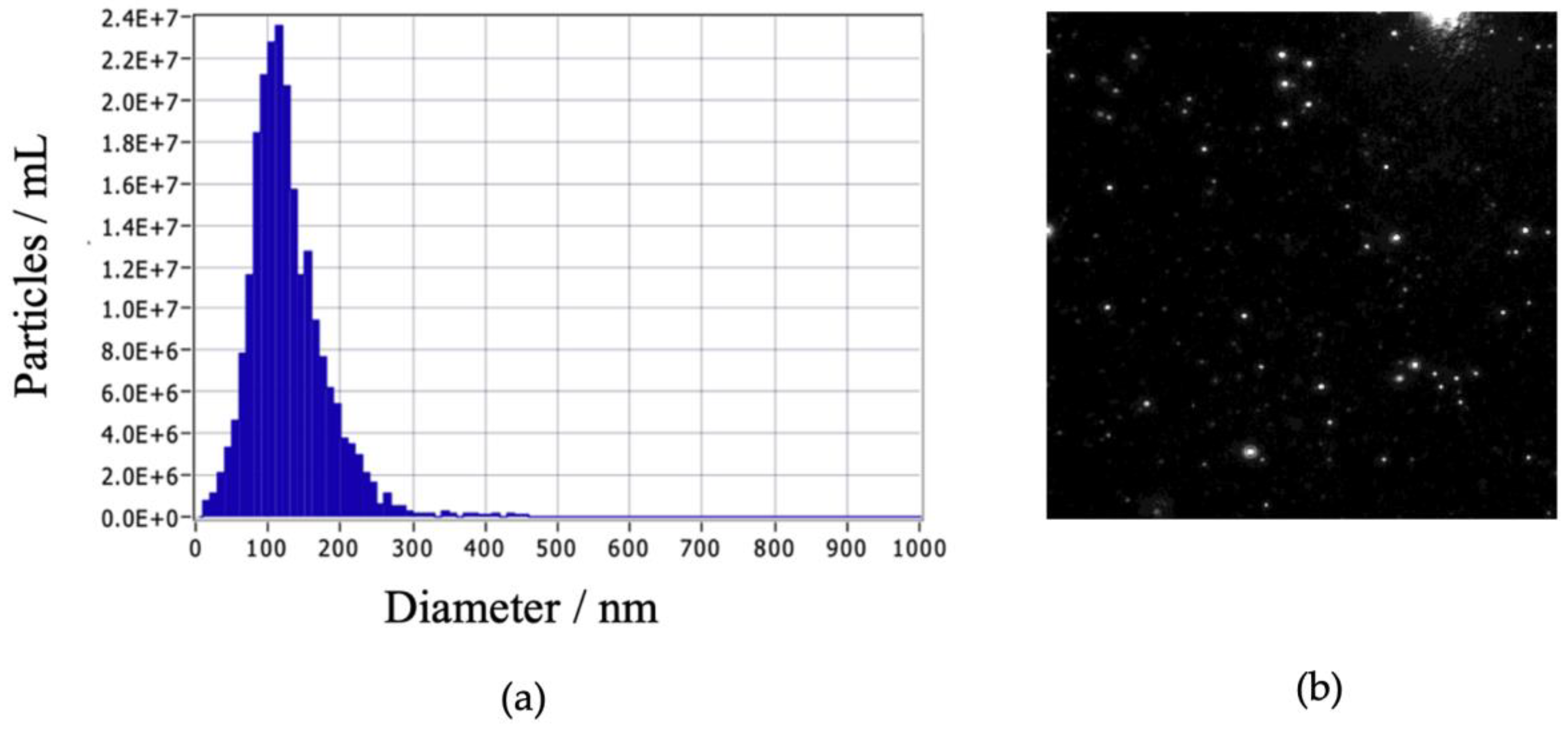
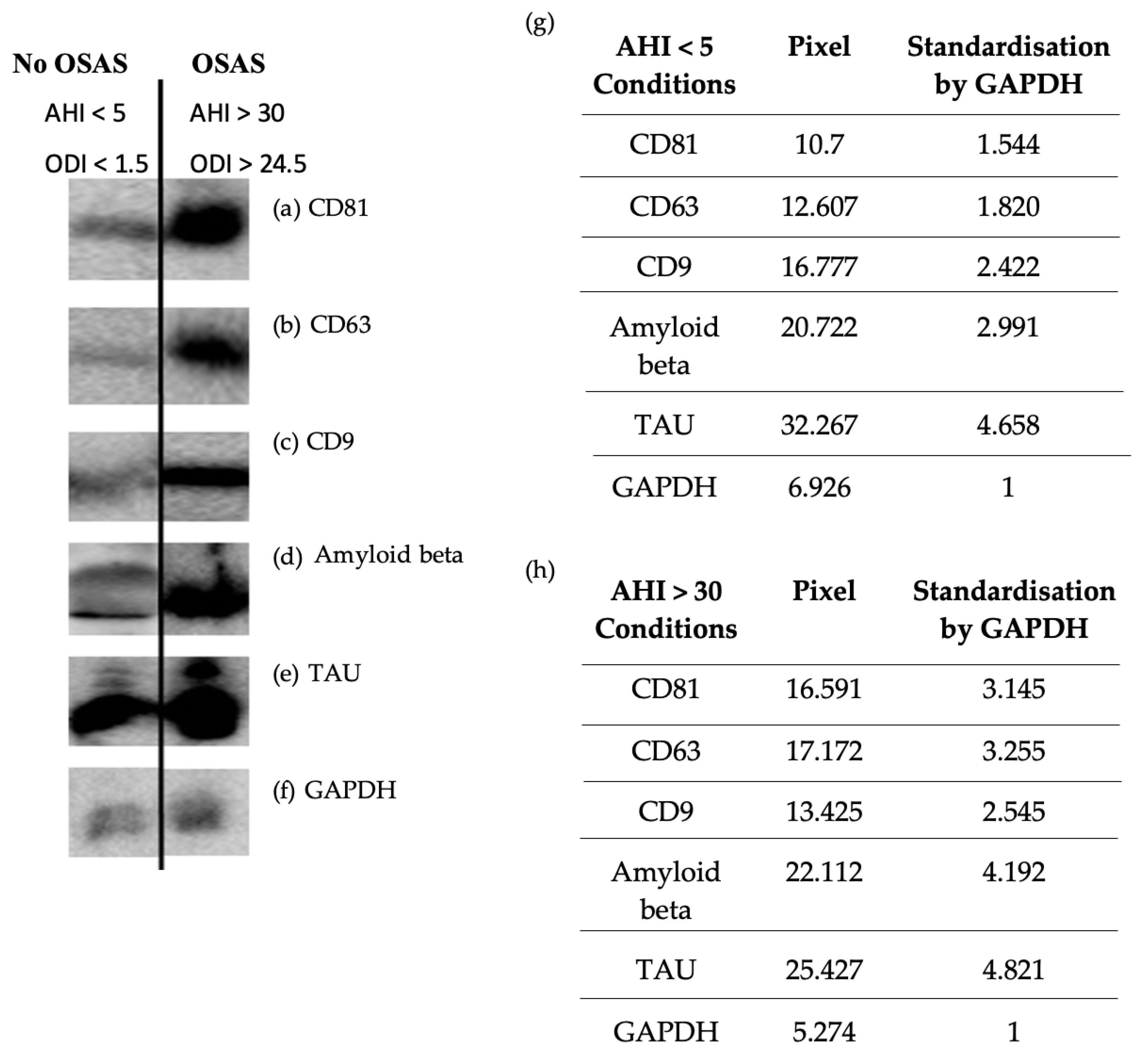
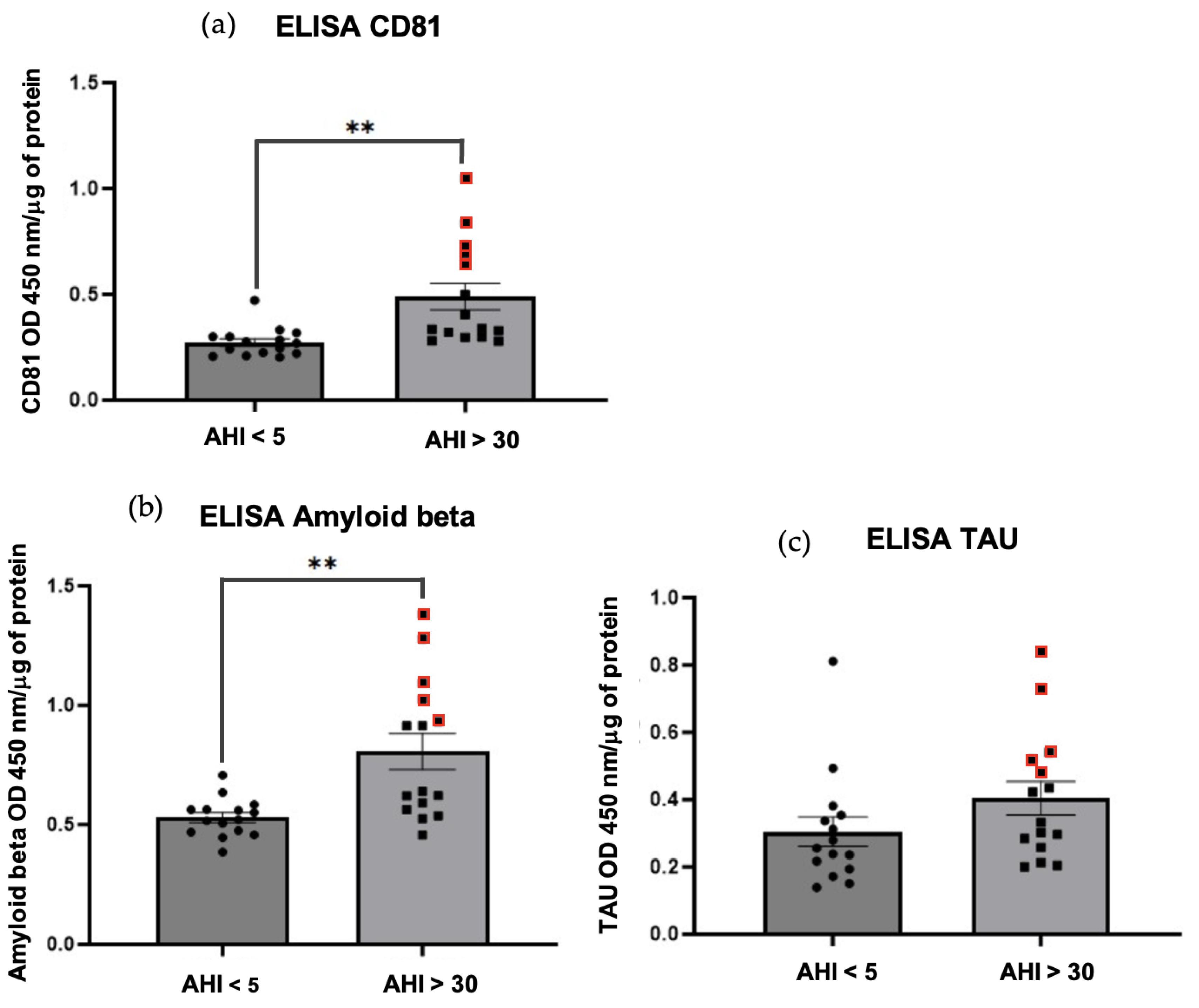
2.2. Characterization of Exosomes - Western Blot and Homemade ELISA
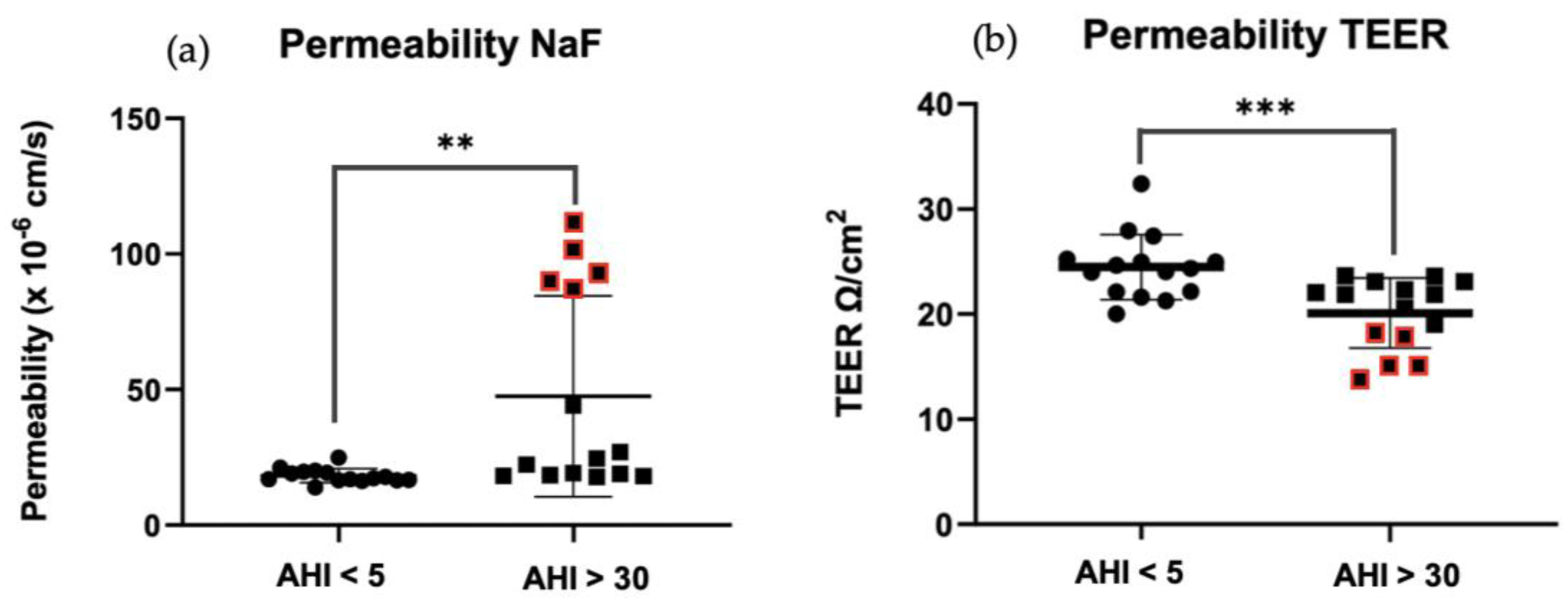
2.3. Impact of Exosomes on the BBB Model - Monolayer of HBEC-5i Endothelial Cells in Conditioned Medium of HA Astrocytes
2.3.1. Permeability Tests - Na-F and TEER
2.4. TJs Expressions: ZO-1, Claudin-5 and Occludin
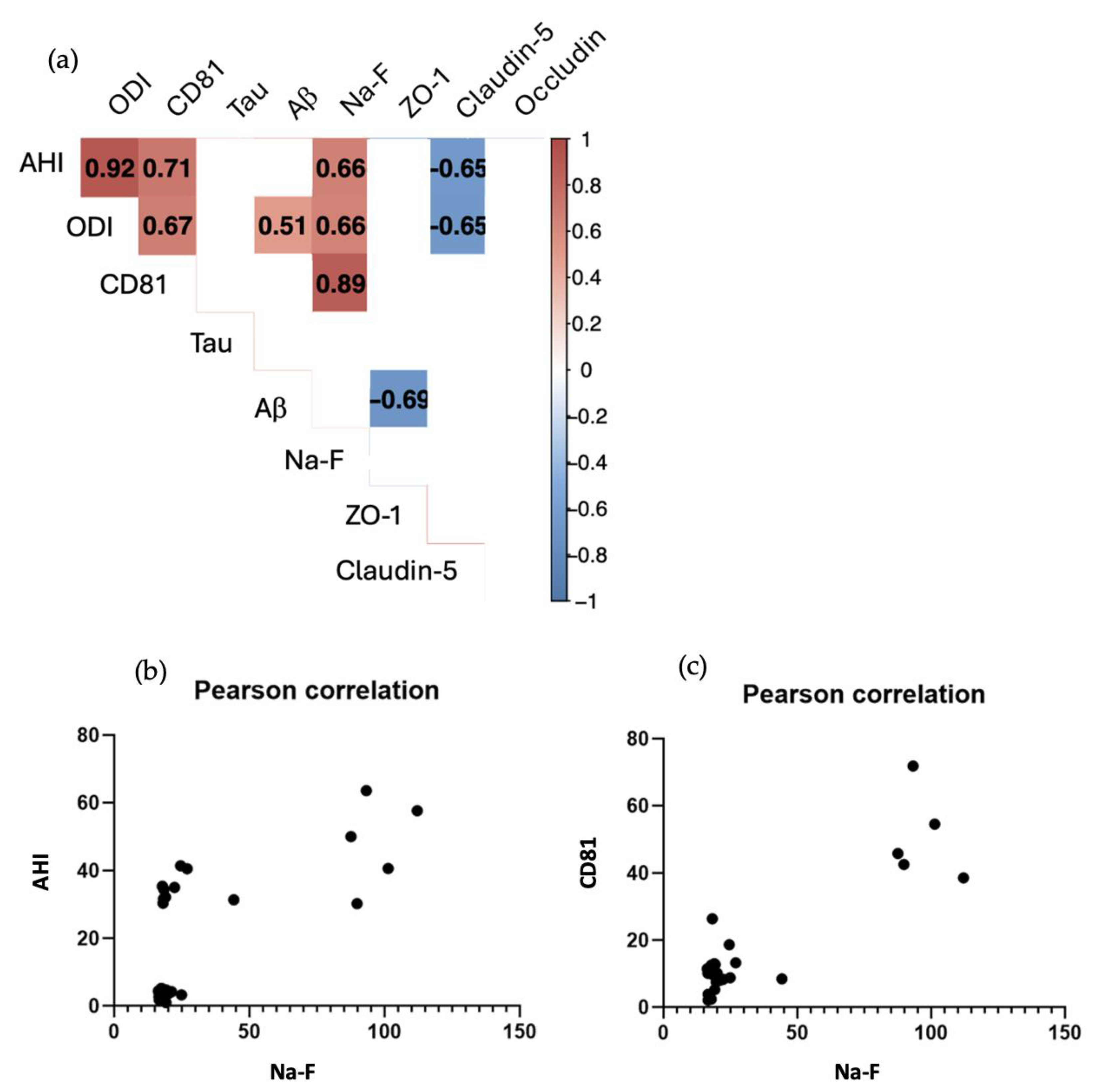
2.5. Correlation Tests
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Biological Samples
4.3. Chemicals and Reagents
4.3.1. Cell Culture
4.3.2. Permeability Measurement
4.3.3. Exosome Isolation & Characterization
4.3.4. ELISA
4.4. Blood Serum Exosome Isolation and Purification
4.5. Protein Measurements
4.6. Exosomes Characterization
4.6.1. Western Blot
4.6.2. Homemade ELISA
4.6.3. Nanoparticle Tracking Analysis (NTA)
4.7. In Vitro BBB Model
4.8. Permeability Measurements – Barrier Properties
4.8.1. Trans Endothelial Electrical Resistance (TEER)
4.8.2. Sodium-Fluorescein
4.9. Protein Expression and Quantification
4.10. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Norman D, Loredo JS. Obstructive Sleep Apnea in Older Adults. Clin Geriatr Med. févr 2008;24(1):151-65. [CrossRef]
- Beaudin AE, Waltz X, Hanly PJ, Poulin MJ. Impact of obstructive sleep apnoea and intermittent hypoxia on cardiovascular and cerebrovascular regulation: Obstructive sleep apnoea, intermittent hypoxia and vascular regulation. Exp Physiol. 1 juill 2017;102(7):743-63.
- Sforza E, Roche F. Sleep Apnea Syndrome and Cognition. Front Neurol [Internet]. 2012 [cité 13 juin 2023];3. Disponible sur: http://journal.frontiersin.org/article/10.3389/fneur.2012.00087/abstract. 10.3389/fneur.2012.00087.
- Launois SH, Pépin JL, Lévy P. Sleep apnea in the elderly: A specific entity? Sleep Med Rev. avr 2007;11(2):87-97.
- Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: A meta-review: Cognitive function in OSA: a meta-review. Respirology. janv 2013;18(1):61-70.
- Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, et al. Cognitive Function and Sleep Related Breathing Disorders in a Healthy Elderly Population: the Synapse Study. Sleep. avr 2010;33(4):515-21. [CrossRef]
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 15 oct 2012;08(05):597-619.
- Guerra S, Carsin AE, Keidel D, Sunyer J, Leynaert B, Janson C, et al. Health-related quality of life and risk factors associated with spirometric restriction. Eur Respir J. mai 2017;49(5):1602096. [CrossRef]
- Sforza E, Roche F, Chapelle C, Pichot V. Internight Variability of Apnea-Hypopnea Index in Obstructive Sleep Apnea Using Ambulatory Polysomnography. Front Physiol. 9 juill 2019;10:849. [CrossRef]
- Sun H, Gao Y, Li M, Zhang S, Shen T, Yuan X, et al. Altered amyloid-β and tau proteins in neural-derived plasma exosomes in obstructive sleep apnea. Sleep Med. juin 2022;94:76-83. [CrossRef]
- Kerner NA, Roose SP. Obstructive Sleep Apnea is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am J Geriatr Psychiatry. juin 2016;24(6):496-508. [CrossRef]
- Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: Addressing the blood–brain barrier. Sleep Med Rev. févr 2014;18(1):35-48. [CrossRef]
- Puech C, Hodin S, Forest V, He Z, Mismetti P, Delavenne X, et al. Assessment of HBEC-5i endothelial cell line cultivated in astrocyte conditioned medium as a human blood-brain barrier model for ABC drug transport studies. Int J Pharm. nov 2018;551(1-2):281-9. [CrossRef]
- Daneman R, Prat A. The Blood–Brain Barrier. Cold Spring Harb Perspect Biol. janv 2015;7(1):a020412.
- Khalyfa A, Kheirandish-Gozal L, Gozal D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir Physiol Neurobiol. oct 2018;256:143-56. [CrossRef]
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. juin 2015;11(6):600. [CrossRef]
- Pan W, Kastin AJ. Can sleep apnea cause Alzheimer’s disease? Neurosci Biobehav Rev. nov 2014;47:656-69.
- Baril AA, Carrier J, Lafrenière A, Warby S, Poirier J, Osorio RS, et al. Biomarkers of dementia in obstructive sleep apnea. Sleep Med Rev. déc 2018;42:139-48. [CrossRef]
- Ayloo S, Gu C. Transcytosis at the blood–brain barrier. Curr Opin Neurobiol. août 2019;57:32-8.
- Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. Elucidation of Exosome Migration Across the Blood–Brain Barrier Model In Vitro. Cell Mol Bioeng. déc 2016;9(4):509-29. [CrossRef]
- Wood MJ, O’Loughlin AJ, Lakhal S. Exosomes and the blood–brain barrier: implications for neurological diseases. Ther Deliv. sept 2011;2(9):1095-9. [CrossRef]
- Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 23 avr 2021;19(1):47. [CrossRef]
- Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. sept 2020;Volume 15:6917-34. [CrossRef]
- Cao Y, Xu W, Liu Q. Alterations of the blood-brain barrier during aging. J Cereb Blood Flow Metab. 21 mars 2024;0271678X241240843. [CrossRef]
- Knox EG, Aburto MR, Clarke G, Cryan JF, O’Driscoll CM. The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry. juin 2022;27(6):2659-73. [CrossRef]
- Voirin AC, Celle S, Perek N, Roche F. Sera of elderly obstructive sleep apnea patients alter blood–brain barrier integrity in vitro: a pilot study. Sci Rep. 9 juill 2020;10(1):11309. [CrossRef]
- Intermittent Hypoxia and Its Impact on Nrf2/HIF-1α Expression and ABC Transporters: An in Vitro Human Blood-Brain Barrier Model Study. Cell Physiol Biochem. 16 déc 2020;54(6):1231-48.
- Sapin E, Peyron C, Roche F, Gay N, Carcenac C, Savasta M, et al. Chronic Intermittent Hypoxia Induces Chronic Low-Grade Neuroinflammation in the Dorsal Hippocampus of Mice. Sleep. 1 oct 2015;38(10):1537-46. [CrossRef]
- Alkhalifa AE, Al-Ghraiybah NF, Odum J, Shunnarah JG, Austin N, Kaddoumi A. Blood–Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int J Mol Sci. 14 nov 2023;24(22):16288.
- Michalicova A, Majerova P, Kovac A. Tau Protein and Its Role in Blood–Brain Barrier Dysfunction. Front Mol Neurosci. 30 sept 2020;13:570045. [CrossRef]
- Ahmad AA, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and Pericytes Differentially Modulate Blood—Brain Barrier Characteristics during Development and Hypoxic Insult. J Cereb Blood Flow Metab. févr 2011;31(2):693-705. [CrossRef]
- Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood–brain barrier. Brain Res. août 2004;1018(1):1-9. [CrossRef]
- Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, et al. Deficiency of Zonula Occludens-1 Causes Embryonic Lethal Phenotype Associated with Defected Yolk Sac Angiogenesis and Apoptosis of Embryonic Cells. Mostov K, éditeur. Mol Biol Cell. juin 2008;19(6):2465-75. [CrossRef]
- Zhou J, Camacho M, Tang X, Kushida CA. A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness. Sleep Med. juill 2016;23:99-108. [CrossRef]
- Salminen A, Kauppinen A, Kaarniranta K. Hypoxia/ischemia activate processing of Amyloid Precursor Protein: impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J Neurochem. févr 2017;140(4):536-49. [CrossRef]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci. 25 juill 2006;103(30):11172-7.
- Brunello CA, Merezhko M, Uronen RL, Huttunen HJ. Mechanisms of secretion and spreading of pathological tau protein. Cell Mol Life Sci. mai 2020;77(9):1721-44. [CrossRef]
- Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. août 2019;15(8):1071-80.
- Teunissen CE, Verberk IMW, Thijssen EH, Vermunt L, Hansson O, Zetterberg H, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. janv 2022;21(1):66-77.
- Carvalho DZ, St. Louis EK, Schwarz CG, Lowe VJ, Boeve BF, Przybelski SA, et al. Witnessed apneas are associated with elevated tau-PET levels in cognitively unimpaired elderly. Neurology [Internet]. 28 avr 2020 [cité 7 avr 2024];94(17). Disponible sur: https://www.neurology.org/doi/10.1212/WNL.0000000000009315. [CrossRef]
- Andrade AG, Bubu OM, Varga AW, Osorio RS. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. Perry G, Avila J, Moreira PI, Sorensen AA, Tabaton M, éditeurs. J Alzheimers Dis. 12 juin 2018;64(s1):S255-70.
- Ju YES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. févr 2014;10(2):115-9. [CrossRef]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 18 oct 2013;342(6156):373-7.
- Macey PM, Prasad JP, Ogren JA, Moiyadi AS, Aysola RS, Kumar R, et al. Sex-specific hippocampus volume changes in obstructive sleep apnea. NeuroImage Clin. 2018;20:305-17. [CrossRef]
- Berthiaume AA, Schmid F, Stamenkovic S, Coelho-Santos V, Nielson CD, Weber B, et al. Pericyte remodeling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nat Commun. 7 oct 2022;13(1):5912. [CrossRef]
- Barthélémy JC, Pichot V, Dauphinot V, Celle S, Laurent B, Garcin A, et al. Autonomic Nervous System Activity and Decline as Prognostic Indicators of Cardiovascular and Cerebrovascular Events: The ‘PROOF’ Study. Neuroepidemiology. 2007;29(1-2):18-28. [CrossRef]

| Variables | Whole population | OSAS | No OSAS | p |
|---|---|---|---|---|
| Age (y) | 75.8 ± 0.9 | 75.7 ± 0.9 | 75.9 ± 0.9 | 0.621 |
| Sex (F/M) | 19/11 | 6/9 | 13/2 | 0.008 |
| AHI (h-1) | 22.5 ± 21.0 | 41.2 ± 12.6 | 3.8 ± 1.5 | < 0.001 |
| ODI (h-1) | 14.7 ± 15.1 | 28.1 ± 9.3 | 1.4 ± 0.6 | < 0.001 |
| SaO2 min (%) | 88.0 ± 5.2 | 84.3 ± 4.6 | 91.7 ± 2.1 | < 0.001 |
| SaO2moy (%) | 94.4 ± 1.7 | 93.4 ± 2.0 | 95.3 ± 0.7 | < 0.01 |
| %time SaO2 < 90 | 4.8 ± 11.5 | 9.6 ± 15.0 | 0 | < 0.05 |
| Total cholesterol (g.L-1) | 2.3 ± 0.4 | 2.1 ± 0.4 | 2.4 ± .4 | 0.057 |
| Antibody | Name | Anti | Reference | Supplier |
|---|---|---|---|---|
| Primary | CD81 (B-11) |
Anti-mouse | SC-166029 | Santa Cruz Biotechnology |
| Primary | CD63 (MX-49.129.5) |
Anti-mouse | SC-5275 | Santa Cruz Biotechnology |
| Primary | CD9 (ALB 6) |
Anti-mouse | SC-59140 | Santa Cruz Biotechnology |
| Primary | TAU (Tau-13) |
Anti-mouse | SC-21796 | Santa Cruz Biotechnology |
| Primary | Amyloid beta (B-4), |
Anti-mouse | SC-28365 | Santa Cruz Biotechnology |
| Primary | GAPDH (0411) |
Anti-mouse | SC-47724 | Santa Cruz Biotechnology |
| Primary | ZO-1 | Anti-rabbit | 40-2200 | Thermo Fisher |
| Primary | Claudin-5 | Anti-mouse | SC-374221 | Santa Cruz Biotechnology |
| Primary | Occludin | Anti-rabbit | 711500 | Life tech |
| Secondary | m-IgGκBP-HRP | Anti-mouse | SC-516102 | Santa Cruz Biotechnology |
| Secondary | Goat anti-Rabbit IgG, HRP conjugate | Anti-rabbit | 12-348 | Millipore |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





