1. Introduction
The rise in the incidence of gestational diabetes mellitus (GDM) and its related complications necessitates the search for potentially modifiable risk factors and new solutions. Pre-pregnancy BMI and weight gain during pregnancy significantly influence newborn weight. McBain R.D. et al. (2016) demonstrated in their study that an increase in BMI by more than 4 kg/m² before the second pregnancy raised the risk of fetal macrosomia (aRR 4.06; 95% CI 2.25-7.34) and GDM (aRR 1.97; 95% CI 1.22–3.19) [
1]. It is known that fetal macrosomia complicates 20% of pregnancies in obese women. After the 20th week of pregnancy, in response to maternal hyperglycemia, hypertrophy of the fetal pancreatic β-cells occurs, leading to hyperinsulinemia and hyperlipidemia. By the time of delivery, there is a significant increase in fetal adipose tissue and the development of fetal macrosomia [
2,
3,
4]. Results of a meta-analysis of 30 studies from 1950-2011 showed that the risk of developing a large fetus doubles with a BMI >30 kg/m² [
5]. Obesity significantly complicates the course of pregnancy and contributes to the development of GDM. Currently, diet therapy is the main therapeutic method for managing patients with GDM.
The changes in circulating lipid profiles in maternal blood depending on adherence to rational nutrition among mothers with GDM and the development of fetal macrosomia is a complex and not fully understood process, involving both behavioral factors (“eating behavior”) and genetic ones. Szabo A.J. (2019) proposed the hypothesis that lipids are not only a source of nutrients but also serve as initiators of adipocyte differentiation in the fetus, explaining the development of fetal macrosomia, excess body weight, and obesity in the future for children born to mothers with metabolic diseases [
6]. It is suggested that transported fatty acids initiate the transformation of mesenchymal stem cells into adipocytes through the activation of transcription factors in the fetus. Accelerated transplacental transfer of fatty acids leads to excessive formation of adipocytes in the fetus, resulting in the development of excess body weight in the fetus. The source of the transferred fatty acids is “free” fatty acids, the hydrolysis products of triglycerides, and components of polyunsaturated fatty acids from the mother’s phospholipids. Glucose remains an important precursor for alpha-glycerophosphate, from which most fatty acids are esterified [
7,
8].
The aim of the study was to examine the features of the plasma lipid profile of pregnant women with GDM during all trimesters of pregnancy depending on adherence to dietary therapy.
2. Results
2.1. Clinical Characteristics
In the first stage, we conducted a comparative analysis of the clinical characteristics of the study groups. The analysis of the results between the groups of women examined is presented in
Table S1. The analysis of the main and control groups did not reveal significant differences in age and anthropometric data of patients before pregnancy. However, within the group analysis showed differences between subgroups with gestational diabetes mellitus (GDM), depending on adherence to the diet prescribed by the doctor. Pregnant women with GDM who did not follow the diet were older (37 (31; 39), p=0.08) and had significantly higher body weight before pregnancy (81 (66; 86), p=0.004). Pre-pregnancy BMI and weight gain by the time of delivery significantly differed between the main and control groups (22.6 (20.1; 26.5), 21.2 (19.5; 22.9, p=0.03; 14 (11; 17), 11 (9; 15), p=0.009). A significant contribution to these differences was made by pregnant women with GDM who did not follow the diet, having the highest BMI (26.4 (24.5; 27.6), p=0.003) and weight gain (13 (10; 16), p=0.006) among all examined patients. Of particular interest is the significantly higher fetal mass according to ultrasound data at 32 weeks of gestation, depending on the mother
s’ adherence to the diet (1899 (1717; 2110), 2132 (1865; 229), p=0.04). Patients in the main group (unlike the control) were significantly more often delivered by cesarean section (18 (60%), 27 (34%), p=0.02). Among them, operative delivery was often planned (13 (43%), 14 (18%), p=0.01), with this indicator being the highest among patients with GDM who did not follow the diet, reaching up to 60%. This is likely due to combined factors such as the presumed large fetal size (>4000g) with unprepared birth pathways and the insistence of the women. The weight of the newborn in this subgroup of patients was the highest (3810 (3317; 4122), p=0.14). Hospital discharge times for mothers significantly differed: patients in the main group stayed longer in the hospital on average (5 (3; 5), 4 (3; 5), p=0.02), regardless of adherence to the diet before childbirth. Outcomes for the newborns (early neonatal complications, Apgar score at 1 and 5 minutes, discharge time from the hospital) did not significantly differ between the main and control groups
2.2. Lipid Profile Analysis
In the study, HPLC-MS analysis of blood samples from patients in the main and control groups was performed at three time points: at 11-13, 24-26, and 30-32 weeks of pregnancy. A total of 164 lipids were identified, belonging to 11 classes: sphingomyelins (SM), phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylserines (PS), phosphatidylinositols (PI), phosphatidylglycerols (PG), phosphatidic acids (PA), diglycerides (DG), triglycerides (TG), and fatty acids (FA).
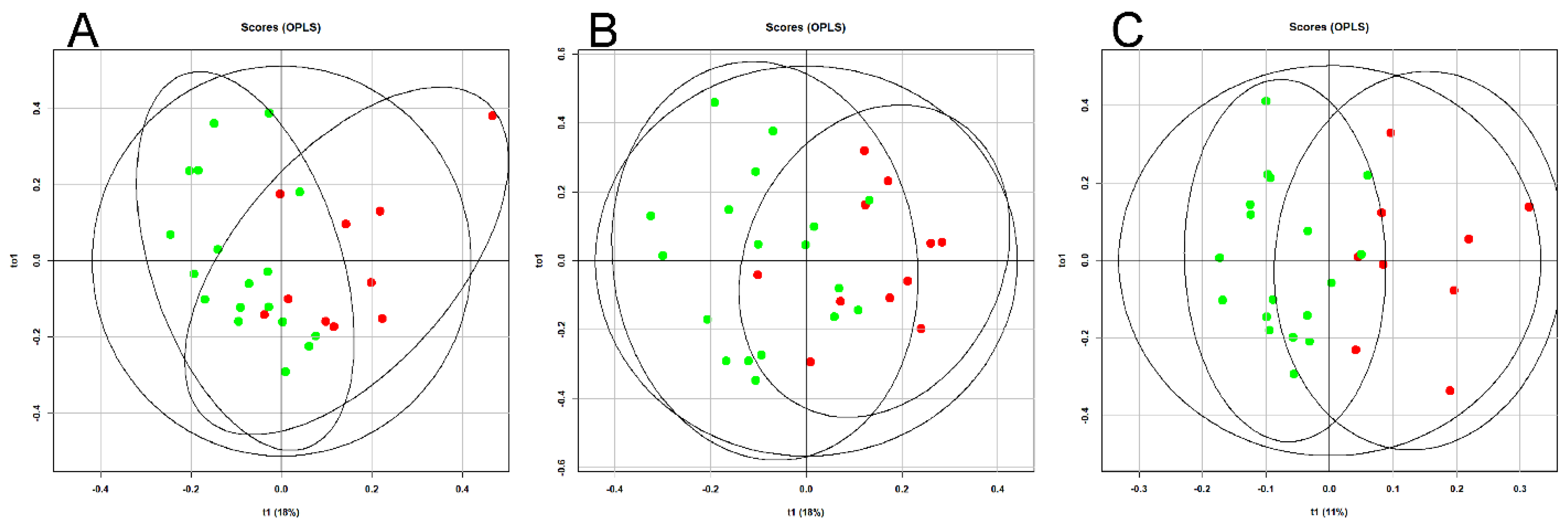
An OPLS analysis of the lipid profile of the blood plasma of patients with GDM was performed over time (11-13, 24-26, and 30-32 weeks of pregnancy), depending on adherence to diet (
Figure 1).
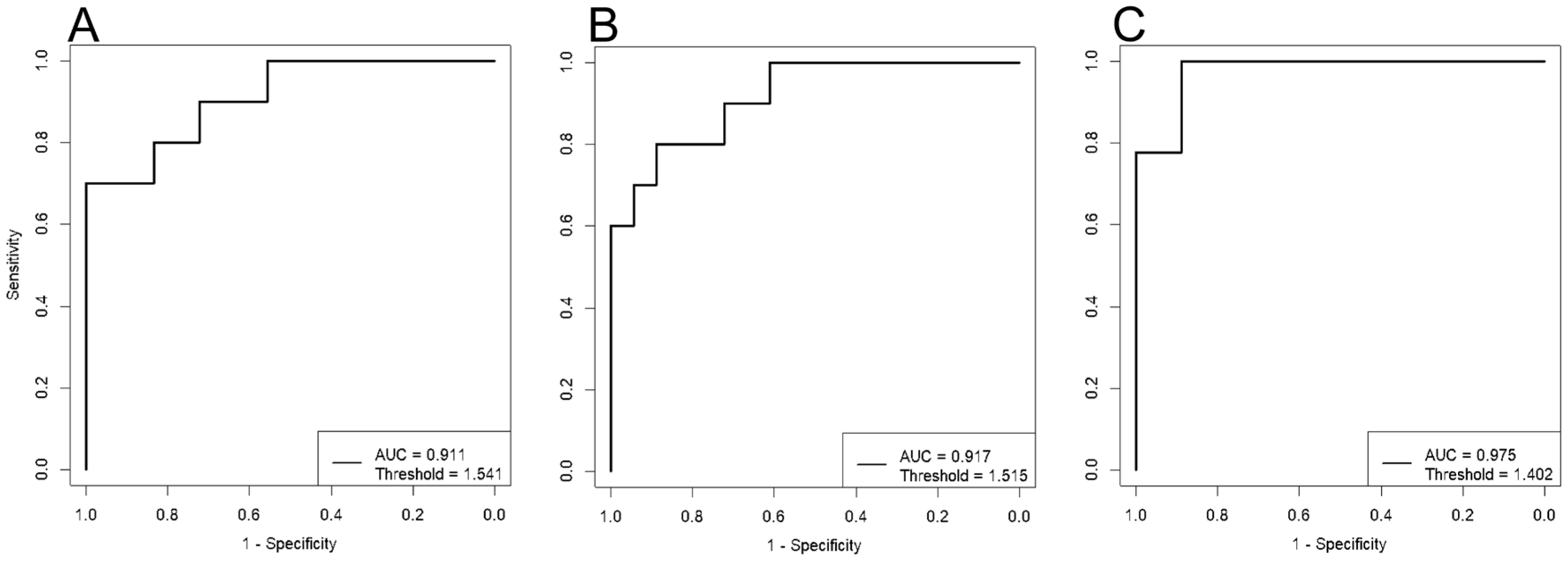
Clustering of the data points indicates a significant impact of dietary therapy on blood lipid composition, starting from the first trimester of pregnancy. ROC analysis demonstrated high effectiveness of the developed models. The lowest AUC value was 0.91 for the “11-13 weeks” model, with sensitivity and specificity of 0.8 and 1, respectively (
Figure 2,
Table 1). The highest AUC value was 0.98 for the “30-32 weeks” model, with sensitivity and specificity of 1 and 0.9, respectively (
Figure 2,
Table 1).
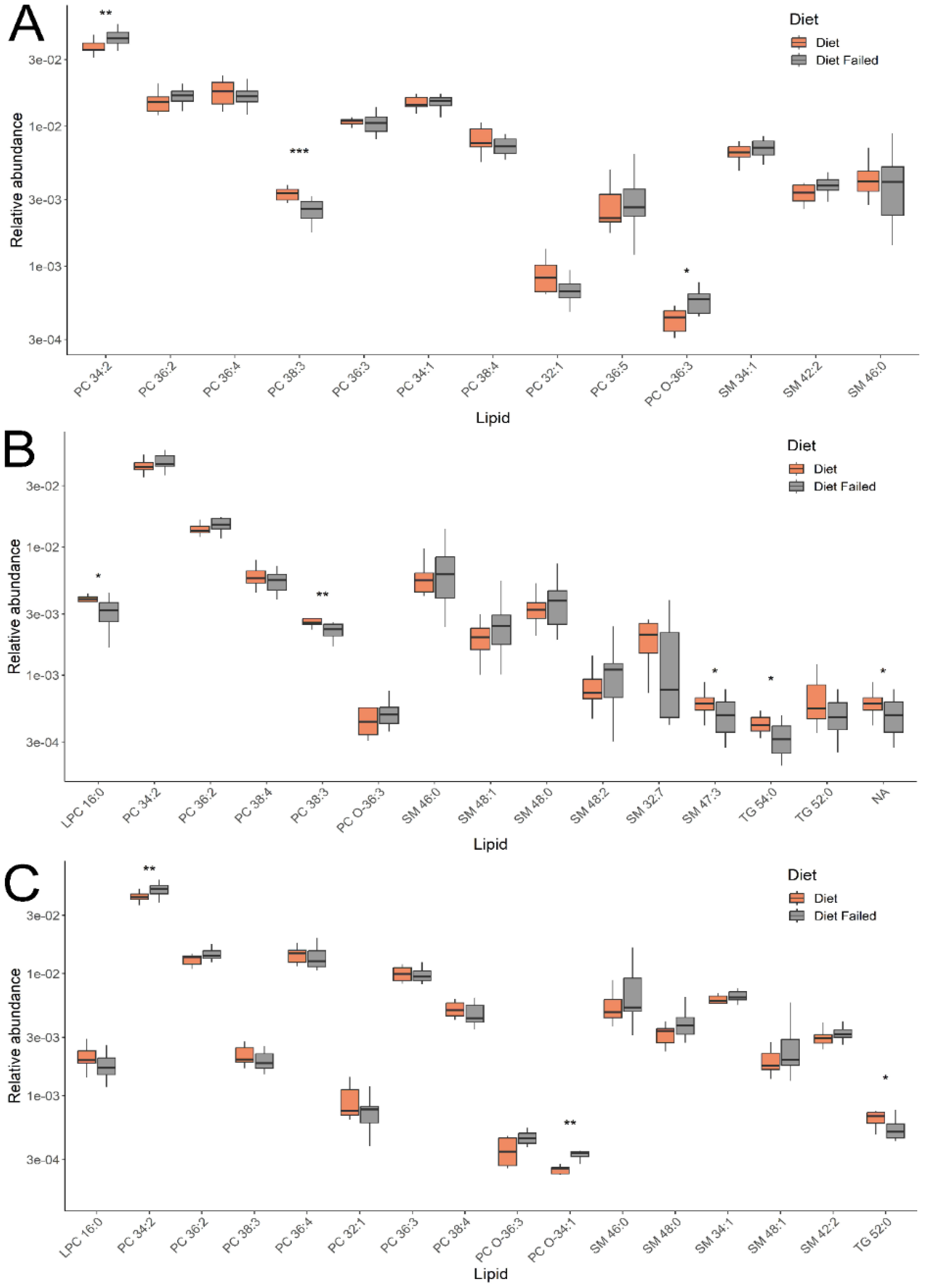
Lipids with the greatest contribution to sample classification in the OPLS-DA models were identified by a VIP value > 1. Their levels for the groups under consideration are presented in
Figure 3. Comparing groups of pregnant women with gestational diabetes, who either adhered to or did not adhere to a diet, identified 4 classes of lipids: phosphatidylcholines (PC), sphingomyelins, lysophosphatidylcholines, and triglycerides. In the first trimester (11-13 weeks), lipids such as phosphatidylcholines and sphingomyelins were detected, and in the second and third trimesters (24-26 and 30-32 weeks), lysophosphatidylcholines and triglycerides were added to these.
Interest is sparked by the different levels of changes in individual lipids within classes, which persist dynamically from the first to the third trimester, depending on adherence to a diet in gestational diabetes (GD). For instance, the level of phosphatidylcholine PC 34:2 (across all three trimesters) and sphingomyelins SM 46:0, 48:1, 48:0, 48:2 (in the second and third trimesters) increased among women not following the diet, while PC 38:3 (across all three trimesters) and SM 32:7, 47:3 (in the second trimester) decreased. The levels of triglycerides TG 54:0 (in the second and third trimesters) and 52:0 (in the second trimester) and lysophosphatidylcholine LPC 16:0 (in the second and third trimesters) among women not adhering to the GD diet remained lowered. These changes were significant for a number of lipids presented in
Figure 3. Thus, the analysis of the plasma lipid composition in women allowed us to differentiate patients with GD who adhered to and neglected the diet throughout pregnancy (in all three trimesters).
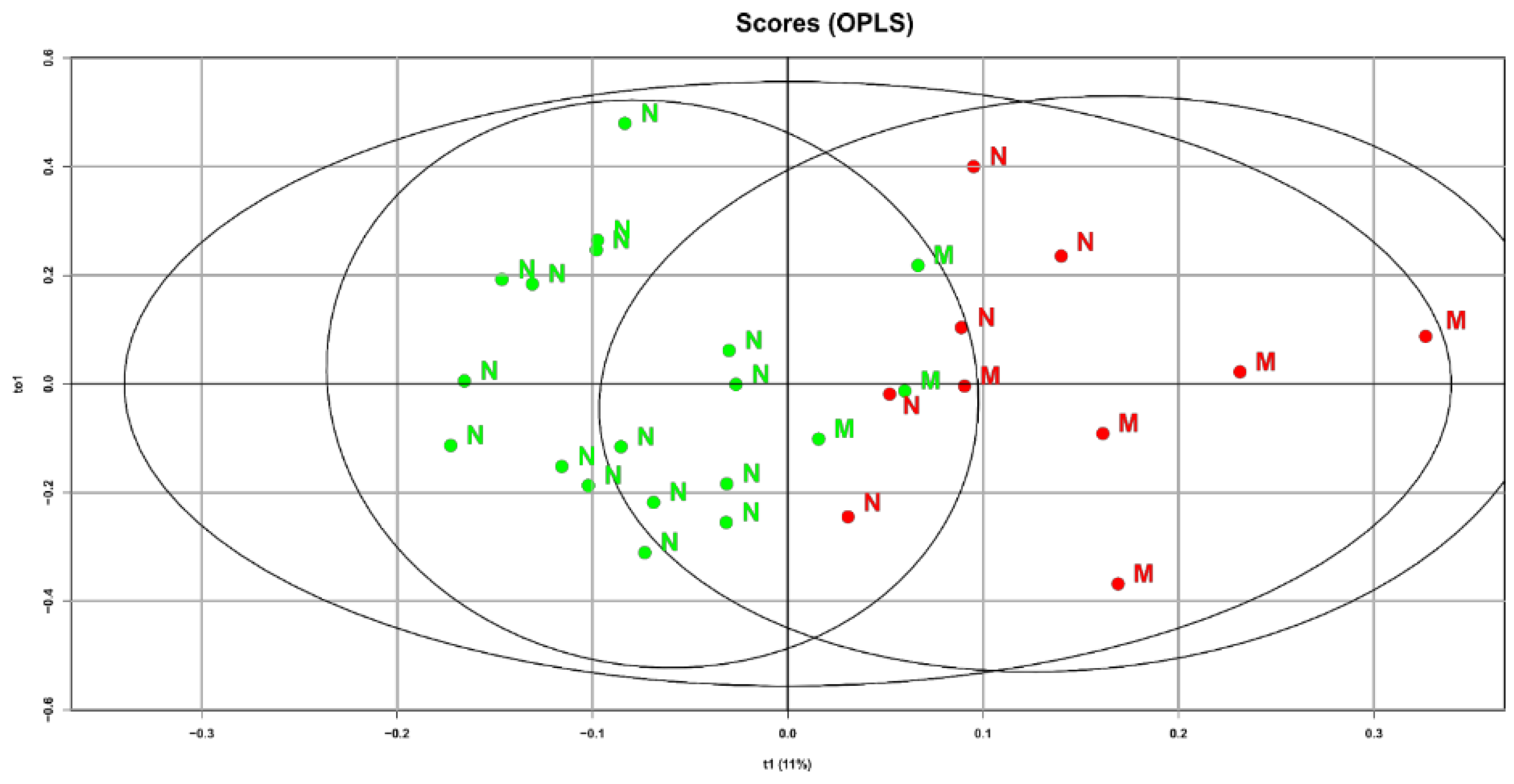
The characteristics of the lipid spectrum may be associated not only with maternal factors but also with fetal ones. Therefore, to the already obtained OPLS analysis data, we additionally included a fetal factor, namely birth weight. In choosing this clinical indicator, we relied on obtained data indicating a high percentage of macrosomia (reaching up to 50%) among women with gestational diabetes who did not follow a diet. A special interest is the identified connection between diet and the maternal blood lipid composition at 32 weeks of pregnancy and the newborn’s weight (
Figure 4).
Samples from GDM patients who did not follow the diet and gave birth to children with fetal macrosomia are shifted to the right area of the graph. This indicates that the direction of changes in the lipid profile during the development of macrosomia and diet therapy are diametrically opposite. Based on the obtained data, it can be assumed that after validating the models, they could be used to refine predictions of macrosomia development based on the plasma lipid profile at various stages of pregnancy
3. Discussion
To date, it is known that the primary therapeutic method for correcting hyperglycemia is diet therapy, excluding easily digestible carbohydrates. In our study, 30 women with gestational diabetes (the main group) were on diet therapy. Of all the patients on diet therapy, 10 women admitted to not following the diet regimen. An intra-group analysis of patients with gestational diabetes, depending on adherence to the diet prescribed by the doctor, revealed a number of differences between the two subgroups. Patients not adhering to the diet were older, had significantly higher body weight and BMI before pregnancy, which may indirectly indicate established poor eating habits and psychological difficulties in this group with independently normalizing their diet according to the doctor’s recommendations. Lu Liu et al., in their work, demonstrated that a pre-pregnancy BMI reduction of 10–15% for women with obesity and 5% for overweight women was associated with a significant decrease in adverse perinatal complications (gestational diabetes, hypertensive disorders, macrosomia) [
9]. Patients in the main group (unlike the control) were significantly more often delivered by cesarean section. The frequency of operative delivery among patients with gestational diabetes significantly exceeded that of the control group, reaching 60% among women who did not follow the diet. Also, in this subgroup of women, larger babies were more frequently born. As a result, of the 20 patients who followed the diet, only 3 (15.0%) developed fetal macrosomia, whereas in women neglecting the diet, large babies were born in 50.0% of cases.
When comparing patients with gestational diabetes mellitus (GDM) who adhered to and did not adhere to a diet, four classes of lipids related to phosphatidylcholines (PC), sphingomyelins, lysophosphatidylcholines, and triglycerides were identified. At 11-13 weeks, phosphatidylcholines and sphingomyelins were detected, and at 24-26 and 30-32 weeks of pregnancy, the list was supplemented with lysophosphatidylcholines and triglycerides. Phosphatidylcholines and sphingomyelins are major components of cell membranes, involved in signal transduction processes, including the activation of insulin-related pathways [
10,
11]. Changes in the composition of fatty acids and phospholipids in cells can reduce tissue sensitivity to insulin, which is a key factor in the development of GDM. Phosphatidylcholines can interact with other lipid classes, such as triglycerides and free fatty acids, which can also affect metabolism and the energy status of cells involved in insulin secretion. Changes in lipid composition, including types like phosphatidylcholines and sphingomyelins, may be associated with various metabolic disorders, such as insulin resistance and obesity.
Changes in blood lipid composition due to not following a diet in GDM were recorded starting from the first trimester of pregnancy. A distinctive feature of the plasma lipid profile in these women was the multidirectional level of changes in specific lipids within classes, persisting dynamically from the first to the third trimester. At three points during the study, covering all three trimesters of pregnancy, we recorded an increase in the level of phosphatidylcholine PC 34:2 and a decrease in PC 38:3 among women not adhering to a diet. A similar pattern was observed for the sphingomyelin group, where SM 46:0, 48:1, 48:0, 48:2 increased among women not following a diet in the second and third trimesters, while SM 32:7, 47:3 decreased. The level of triglycerides (TG 54:0 and 52:0) and lysophosphatidylcholine (LPC 16:0) among women not adhering to a diet during GDM remained low.
In 2021, Christopher Papandreou et al. published a study focusing on the relationship between changes in circulating metabolites during weight loss in the context of dietary therapy in patients with obesity. The cohort study included 162 participants who achieved a weight loss of ≥8% during an initial 8-week low-calorie diet, followed by a 12-week observation period. Targeted metabolite profiling (123 metabolites) was performed using three different platforms: proton nuclear magnetic resonance, liquid chromatography-mass spectrometry, and gas chromatography-mass spectrometry. Changes in levels of several types of lipids and citric acid were significantly associated with weight loss, total body fat amount, and abdominal fat distribution. The authors found a decrease in concentrations of lysophosphatidylcholines LPC 14:0, LPC 20:3, phosphatidylcholine PC 32:2, PC 38:3, sphingomyelin SM 32:2, and an increase in citric acid concentration during the dynamic 12-week observation post-weight loss. The authors conclude that there is an existing connection between weight loss and changes in types of lipids and citric acid. These changes likely reflect lipid metabolism in the body and are an important factor in controlling weight gain and the development of obesity [
12]. An earlier study from 2007 also showed an association of PC 34:4 with obesity. The authors demonstrated that a higher plasma level of PC 34:4 was associated with an increased risk of obesity in adults. The absence of dependence on hereditary factors was also demonstrated by conducting a study on a group of monozygotic twins [
13]. However, it should be considered that factors like progressive pregnancy, fetal growth, and the presence of already diagnosed gestational diabetes (GDM) can significantly contribute to the maternal blood lipid profile and explain conflicting data on the decrease of several metabolites (PC 38:3, SM 32:7, SM 47:3, TG 54:0, and 52:0, LPC 16:0) over time, against the backdrop of diet cessation in our cohort of pregnant women with GDM. A study from 2020 on lipidomic profiling of 114 plasma samples from overweight or obese pregnant women at three points: before pregnancy, at 15 weeks, and at 35 weeks, revealed changes in 17 lipids, mainly PC and SM, and their connection to GDM [
14]. Furthermore, published data (2020) track a large cohort of women with GDM post-delivery (2 years of observation) to identify metabolic changes and the development of type 2 diabetes. In the longitudinal study, metabolic changes from the initial inclusion point to 2 years of observation were analyzed as a trajectory of type 2 diabetes progression. When creating a predictive model, the authors identified a distinct metabolic signature in the early postpartum period, which predicted the development of type 2 diabetes (AUC 0.883 (95% CI 0.820-0.945, p < 0.001)). The most striking finding at the baseline level was the overall increase in amino acids (AA), as well as diacylglycerophospholipids and a decrease in sphingolipids and acylalkylglycerophospholipids among women with an onset of type 2 diabetes. Dynamic observation of women with type 2 diabetes showed the preservation or increase of AA regulation and a decrease in sphingolipid and acylalkylglycerophospholipid regulation. The authors found a metabolic signature predicting the transition from GDM to type 2 diabetes in the early postpartum period. They concluded that metabolic dysregulation is present several years before the onset of type 2 diabetes and can be detected in the early postpartum period, among women with GDM [
15]. Similar data were shown in an experimental study by Moritz Liebmann et al., conducted on two mouse models, with and without pre-existing glucose tolerance impairment at the time of pregnancy onset. Mice with initial glucose intolerance exhibited pronounced hyperlipidemia during pregnancy with elevated levels of free fatty acids, triglycerides, and an increased atherogenic index, while hepatic sphingomyelin concentrations decreased in the face of increased plasma sphingosine-1-phosphate (S1P) concentrations. These mice showed impairments in hepatic weight regulation and changes in the metabolism of free fatty acids, accompanied by impaired translocation of fatty acid translocase into the hepatocellular plasma membrane [
16]. Thus, elevated concentrations of free fatty acids against a decrease in certain sphingolipid fractions may be a predictor of a more severe course of GDM and high risks for the development of type 2 diabetes.
A particular interest is drawn to the significantly greater fetal mass according to ultrasound data at 32 weeks of gestation, when mothers with gestational diabetes mellitus (GDM) do not follow a diet (1899(1717; 2110), 2132(1865;229), p=0.04). It is likely that by 32 weeks of pregnancy, this separation becomes irreversible for the subsequent newborn’s weight (ultrasound fetal data at 32 weeks and results of OPLS analysis of the plasma lipid profile). According to a study by Robert J D’Arcy et al., accelerated fetal growth begins long before the diagnosis of GDM. The authors conclude on the necessity of finding early methods to identify pregnancies at high risk for fetal macrosomia due to GDM, as current methods (such as measuring glycated hemoglobin levels in the first trimester) are ineffective for these purposes [
17]. This confirms the effectiveness of diet therapy in patients with GDM and adherence to proper nutrition principles during the planning of pregnancy to prevent fetal macrosomia. It can be assumed that the development of fetal macrosomia in women with GDM significantly depends on adherence to diet therapy: the higher the patient compliance, the more often babies with normal fetal weight were born (85% vs 50%). The samples of patients with fetal macrosomia are shifted to the right area of the graph, closer to the samples of patients who did not follow diet therapy. This means that the direction of changes in the lipid spectrum during the development of macrosomia and diet therapy are diametrically opposite. This confirms on a molecular level that diet therapy allows normalization not only of carbohydrate but also lipid metabolism in the mother and fetus.
4. Materials and Methods
4.1. Study Desing
To study the clinical and anamnestic characteristics, obstetric and perinatal outcomes of pregnancies complicated by gestational diabetes mellitus (diet therapy), an observational case-control study was conducted at the National Medical Research Center for Obstetrics, Gynecology, and Perinatology Named after Academician V.I. Kulakov of the Ministry of Healthcare of the Russian Federation. Samples were collected throughout 2023.
The study included 110 women who were observed during pregnancy and childbirth at the National Medical Research Center for Obstetrics Gynecology and Perinatology Named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. Blood samples were taken from the cubital vein at 11-13, 24-26, and 30-32 weeks of pregnancy.
Inclusion criteria for the main group (n=30): Caucasian race, singleton pregnancy, newborn weight from 2501 g to 4999 g, presence of gestational diabetes, and consent of the patient to participate in the study. Inclusion criteria for the control group (n=80): absence of gestational diabetes, singleton pregnancy, newborn weight from 2501 g to 3999 g, and consent of the patient to participate in the study. Exclusion criteria included type 1 and 2 diabetes mellitus, any somatic pathology in the decompensation stage, oncological diseases, autoimmune diseases, asthma in the stage of medicinal compensation, and multiple pregnancies. A semi-quantitative assessment of plasma lipid levels was performed using mass spectrometry. For a deeper data analysis, the main group was stratified based on adherence or refusal of the diet prescribed by an endocrinologist into two subgroups, consisting of 20 and 10 women, respectively. The frequencies of excessive, insufficient, and recommended total weight gain during pregnancy among the women in the groups and subgroups were calculated using the criteria of the Institute of Medicine of the USA (2009).
Several stages were provided for in the study to address the tasks set. The second stage included conducting a longitudinal study (study of time points) among the group of women with gestational diabetes, where blood was collected at three points (at 11-13, 24-26, and 30-32 weeks of pregnancy) and lipidomic analysis of plasma was performed using HPLC-MS/MS, along with statistical analysis of the data obtained depending on the adherence of women with gestational diabetes to the diet prescribed by their doctor.
Ultrasound examinations were performed on all pregnant women at scheduled times (11-14 weeks, 18-21 weeks, and 30-32 weeks of pregnancy). Diagnostics of carbohydrate metabolism disorders were carried out in the first and third trimesters of pregnancy by fasting venous blood glucose levels, and in the second trimester by the results of an oral glucose tolerance test.
4.2. HPLC-MS/MS Analysis of Plasma Lipid Extracts
Lipid extracts were obtained using a modified Folch method. To 40 μL of blood plasma, 480 μL of a chloroform-methanol mixture (2:1, v/v) was added. The mixture was incubated for 10 minutes, filtered using filter paper, and 150 μL of an aqueous NaCl solution (1 mol/L) was added to the resulting solution. The mixture was centrifuged at 3000 rpm for 5 minutes at room temperature. The organic bottom layer containing lipids was collected and dried under a nitrogen stream, then re-dissolved in an acetonitrile-2-propanol mixture (1:1, v/v) for subsequent mass spectrometric analysis.
The molecular composition of the blood plasma samples was determined using flow injection analysis (FIA) electrospray ionization mass spectrometry with a Maxis Impact qTOF mass spectrometer (Bruker Daltonics, Bremen, Germany) [
18,
19]. Mass spectra were obtained in positive ion mode within the m/z range of 400-1000 with the following settings: capillary voltage of 4.5 kV, nebulizer gas pressure of 0.7 bar, drying gas flow rate of 6 L/min, drying gas temperature of 200 °C. A constant flow of eluents A/B at a 1/1 ratio was supplied at a rate of 20 μL/min by a Dionex UltiMate 3000 binary pump and 5 μL of sample was injected by a Dionex UltiMate 3000 autosampler (ThermoScientific, Bremen, Germany). Acetonitrile/water (60:40, v/v) with 0.1% formic acid and 10 mM ammonium formate was used as eluent A, and acetonitrile/2-propanol/water (90:8:2, v/v/v) with 0.1% formic acid and 10 mM ammonium formate as eluent B. Tandem mass spectrometry (MS/MS) was used only for specific ions to refine their identification. Data-dependent analysis was used for ion identification.
After the MS analysis, 200 mass spectra obtained during sample elution were averaged, normalized by total ion current (TIC), and transformed into an abundance-m/z table for further processing.
4.3. Statistical Analysis
Statistical data processing was performed in RStudio (1.383 GNU) using custom scripts in the R language (4.1.1). Median values (Me) and quartiles (Q1, Q3) were used to describe the quantitative data. Qualitative data were presented as absolute values (%). Comparative analysis for qualitative data was performed using Fisher’s exact test and the χ2-test. Comparative analysis of quantitative data was carried out using the Mann-Whitney test for pairwise comparison of groups. The significance threshold was determined to be 0.05.
Mass spectrometry data analysis was conducted using multivariate analysis OPLS-DA (Orthogonal Projections to Latent Structures Discriminant Analysis), which allows for building statistical models using multivariate data to differentiate samples [
20]. The influence of individual variables (lipids) on the created model was calculated using the Variable Influence on Projection (VIP) parameter. Lipids were identified by exact mass using the Lipid Maps database and characteristic tandem mass spectra
[21,22].
To determine the prognostic significance of features, ROC analysis was performed and ROC curves were constructed with the calculation of the Area Under the Curve (AUC).
5. Conclusions
The data obtained enhance our understanding of the pathogenetic mechanisms involved in the development of gestational diabetes mellitus (GDM) and fetal macrosomia. Proper nutrition during pregnancy seems to play a crucial role in managing not only carbohydrate metabolism but also lipid metabolism for both the mother and the fetus. Early identification in the first trimester of patients who do not adhere to an appropriate nutritional regimen—coupled with consultations with dietitians and clinical psychologists—can effectively motivate these women to adjust their diets before irreversible changes in fetal weight occur. This proactive approach can help reduce the incidence of large-for-gestational-age newborns and those with macrosomia, decrease the frequency of cesarean sections, improve long-term health outcomes for women with GDM and their children, and promote economic efficiency.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1. Summary of clinical characteristics of the group. *—statistically significant differences and statistically significant p-values. Clinical parameters and p-values with statistical significance are highlighted in bold.
Author Contributions
Data curation, V.C., A.T., and V.F..; Formal analysis, N.F. and V.C. Funding acquisition, V.C., V.F. and G.S.; Investigation, N.F., V.C., and A.T..; Methodology, N.F., V.C., and V.F.; Project administration, G.S., V. F.; Resources, V.F., V.C. and G.S.; Software, A.T. and V.C.; Supervision, N.S., V.F. and G.S.; Validation, N.F. and N.S..; Visualization, V.F. and N.F; Writing—original draft, N.F., V.F., and N.S.; Writing—review and editing, V.C., V.F. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation (RSF) grant № 24-64-00006.
Institutional Review Board Statement
The study was approved by the Ethical Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov (protocol No. 9, dated November 22, 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McBain, R.D.; Dekker, G.A.; Clifton, V.L.; Mol, B.W.; Grzeskowiak, L.E. Impact of inter-pregnancy BMI change on perinatal outcomes: a retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Mouzon, S.H. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic ? Am. J. Obstet. Gynecol. 2011, 204, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.L.; Lind, O.; Abelsen, T.; Olesen, J.; Jørgensen, M.E. Gestational diabetes and macrosomia among Greenlanders. Time to change diagnostic strategy? Int. J. Circumpolar Health 2018, 77, 1528126. [Google Scholar] [CrossRef]
- Olmos, P.; Martelo, G.; Reimer, V.; Rigotti, A.; Busso, D.; Belmar, C.; González, R.; Goldenberg, D.; Samith, B.; Santos, J.L.; et al. La hipótesis de Pedersen no es suficiente: Otros nutrientes además de la glucosa explicarían la macrosomía fetal en pacientes diabéticas gestacionales con sobrepeso y buen control glicémico. Rev. Med. Chil. 2013, 141, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; Wallace, M.; Brennan, L.; McAuliffe, F.M. Early pregnancy maternal urinary metabolomic profile and later insulin resistance and fetal adiposity. J. Matern. Neonatal Med. 2015, 28, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.J. Transferred maternal fatty acids stimulate fetal adipogenesis and lead to neonatal and adult obesity. Med. Hypotheses 2019, 122, 82–88. [Google Scholar] [CrossRef]
- Liu, K.; Ye, K.; Han, Y.; Sheng, J.; Jin, Z.; Bo, Q.; Hu, C.; Hu, C.; Li, L. Maternal and cord blood fatty acid patterns with excessive gestational weight gain and neonatal macrosomia. Asia Pac. J. Clin. Nutr. 2017, 26, 291–297. [Google Scholar] [CrossRef]
- Ciborowski, M.; Zbucka-Kretowska, M.; Bomba-Opon, D.; Wielgos, M.; Brawura-Biskupski-Samaha, R.; Pierzynski, P.; Szmitkowski, M.; Wolczynski, S.; Lipinska, D.; Citko, A.; et al. Potential first trimester metabolomic biomarkers of abnormal birth weight in healthy pregnancies. Prenat. Diagn. 2014, 34, 870–877. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Zhang, Y.; Niu, J.; Li, Z.; Tang, R. Effect of pregravid obesity on perinatal outcomes in singleton pregnancies following in vitro fertilization and the weight-loss goals to reduce the risks of poor pregnancy outcomes: A retrospective cohort study. PLoS One 2020, 15, e0227766. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef]
- Dai, J.; Boghossian, N.S.; Sarzynski, M.A.; Luo, F.; Sun, X.; Li, J.; Fiehn, O.; Liu, J.; Chen, L. Metabolome-Wide Associations of Gestational Weight Gain in Pregnant Women with Overweight and Obesity. Metabolites 2022, 12, 960. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; García-Gavilán, J.; Camacho-Barcia, L.; Toft Hansen, T.; Harrold, J.A.; Sjödin, A.; Halford, J.C.G.; Bulló, M. Changes in Circulating Metabolites During Weight Loss are Associated with Adiposity Improvement, and Body Weight and Adiposity Regain During Weight Loss Maintenance: The SATIN Study. Mol. Nutr. Food Res. 2021, 65, 2001154. [Google Scholar] [CrossRef]
- Pietiläinen, K.H.; Sysi-Aho, M.; Rissanen, A.; Seppänen-Laakso, T.; Yki-Järvinen, H.; Kaprio, J.; Orešič, M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects—A monozygotic twin study. PLoS One 2007, 2, e218. [Google Scholar] [CrossRef]
- Lau, C.H.E.; Taylor-Bateman, V.; Vorkas, P.A.; Graça, G.; Vu, T.H.T.; Hou, L.; Chekmeneva, E.; Ebbels, T.M.D.; Chan, Q.; Van Horn, L.; et al. Metabolic signatures of gestational weight gain and postpartum weight loss in a lifestyle intervention study of overweight and obese women. Metabolites 2020, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Liu, Y.; Ronnett, G.V.; Wu, A.; Cox, B.J.; Dai, F.F.; Rost, H.L.; Gunderson, E.P.; Wheeler, M.B. Amino acid and lipid metabolism in postgestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med. 2020, 17, e1003112. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, M.; Grupe, K.; Asuaje Pfeifer, M.; Rustenbeck, I.; Scherneck, S. Differences in lipid metabolism in acquired versus preexisting glucose intolerance during gestation: role of free fatty acids and sphingosine-1-phosphate. Lipids Health Dis. 2022, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, R.J.; Cooke, I.E.; McKinley, M.; McCance, D.R.; Graham, U.M. First-trimester glycaemic markers as predictors of gestational diabetes and its associated adverse outcomes: A prospective cohort study. Diabet. Med. 2023, 40, e15019. [Google Scholar] [CrossRef] [PubMed]
- Tonoyan, N.M.; Tokareva, A.O.; Chagovets, V.V.; Starodubtseva, N.L.; Kozachenko, I.F.; Adamyan, L. V. Possibilities for predicting recurrent uterine myoma by plasma lipidomic analysis. Obstet. Gynegology 2019, 136–151. [Google Scholar] [CrossRef]
- Odinokova, V.A.; Chagovets, V.V.; Shmakov, R.G.; Starodubtseva, N.L.; Salimova, D.F.; Kononikhin, A.S.; Frankevich, V.E. Features of serum lipidome in pregnant women with fetal macrosomia and a concurrence of macrosomia with gestational diabetes mellitus. Obstet. Gynegology 2019, 44–51. [Google Scholar] [CrossRef]
- Tokareva, A.O.; Chagovets, V.V.; Starodubtseva, N.L.; Nazarova, N.M.; Nekrasova, M.E.; Kononikhin, A.S.; Frankevich, V.E.; Nikolaev, E.N.; Sukhikh, G.T. Feature selection for OPLS discriminant analysis of cancer tissue lipidomics data. J. Mass Spectrom. 2020, 55, e4457. [Google Scholar] [CrossRef]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, 527–532. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).