1. Introduction
Agribusiness plays a crucial role in Brazi
l’s economy, accounting for 23% of the countr
y’s gross domestic product (GDP) and contributing to nearly one-third of the national GDP. In 2022 alone, agribusiness generated 47% of the country’s total exports. In the state of Pará, agribusiness stands out, representing an average of 21% of the GDP in local municipalities. Pará holds the second-largest cattle production in the nation, with a livestock population of approximately 26 million, of which around 750,000 are buffaloes. The majority of this buffalo population is concentrated in the Marajó Archipelago, where buffaloes play a significant economic, sanitary, and cultural role. Particularly, buffaloes are central to the region’s dairy and cheese-making industries, which are vital sources of livelihood for the local population [
1,
2].
Marajó Island was the pioneer in buffalo farming in Brazil, having introduced these animals from India. Since then, they have become an integral part of the island’s tourism and food production industries [
3]. Buffaloes on Marajó Island have continuous interaction with humans, and any decline in their health or productivity leads to severe economic repercussions for the producers. Additionally, zoonotic diseases from buffaloes can pose public health risks. Among the various diseases affecting buffaloes, trypanosomiasis, caused by
Trypanosoma vivax, is of particular concern due to its impact on milk and meat production, as well as on the animal
s’ ability to perform labor-intensive tasks. This hemoparasitic infection causes anemia, jaundice, lethargy, and decreased milk production, among other symptoms, leading to diminished productivity and economic losses for producers [
4].
Originally from sub-Saharan Africa,
T. vivax has spread across Africa, Asia, Central America, and South America, threatening livestock industries in these regions. In Brazil, the parasite has been reported in all five major regions, becoming a significant agricultural concern in various states and biomes [
5]. The parasite has developed resistance to commonly used treatments like isometamidium hydrochloride [
6]. Additionally, research has shown that recombinant ApoL1 protein, although promising in other regions, is unsuitable for treatment in cattle and buffalo due to its renal toxicity [
7]. The limited availability of effective treatment options, such as diminazene aceturate, adds to the challenge, as this drug, while effective in equines [
8], can cause toxicity in certain species like camelids [
9]. On the prevention front, advances have been made, such as the development of a vaccine targeting the IFX glycoprotein of
T. vivax, but its availability is still limited [
10].
This study focuses on diagnosing natural infections of T. vivax in buffaloes from Soure, located in Marajó Island, Pará, aiming to contribute valuable data on the epidemiology and management of this disease in one of Brazil’s most important buffalo farming regions.
2. Materials and Methods
2.1. Ethical and Biosafety Considerations
All activities were conducted following appropriate animal handling protocols in accordance with the approval certificate from the Ethics Committee on the Use of Animals at the Federal Rural University of the Amazon (CEUA/UFRA) under number 6531300620 (ID 000203).
2.2. Anamnesis, Clinical Inspection, and Blood Collection
Prior to blood collection, anamnesis and clinical inspection of the animals were performed through visual examination. Ectoparasites, sanitary management, reproductive management, and feeding parameters were recorded, along with observations of sex, age group, and body condition score.
Blood samples were collected from 72 Murrah buffaloes (males and females) aged between 8 and 36 months, with a body condition score of 2 (scale of 1 to 5) and appearing clinically healthy. The samples were taken during the foot-and-mouth disease vaccination campaign (random and convenience sampling). A total of 1 mL of blood was collected, stored in 1.5 mL microtubes containing 50 µL of EDTA, homogenized, and frozen at -20°C. In buffalo calves, blood was collected from the jugular vein using sterile 3 mL syringes and 40x12 gauge needles. In adults, blood was collected from the lateral and dorsal nasal veins using sterile 3 mL syringes and 25x7 gauge needles [
11].
The sampling locations were all in Soure/PA on Marajó Island, and collections took place in July 2024, during a period of dry and mild rainfall at the end of the Amazon summer, allowing access to the sampling sites.
2.3. DNA Extraction
DNA extraction was carried out using the Phenol/Chloroform method adapted from Sambrook et al. [
12]. The reagents used were prepared and diluted for research purposes at the Laboratory of Serology and Molecular Biology at the Institute of Animal Health and Production – ISPA/UFRA.
2.4. Polymerase Chain Reaction (PCR)
For the diagnosis of
Trypanosoma vivax, the cathepsin L-like gene was targeted using primers TviCatL1 (
5′GCC ATC GCC AAG TAC CTC GCC GA
3′) and DTO 155 (5’TTA GAA TTC CCA GGA GTT CTT GAT GAT CCA GTA3’), which amplify a 177 bp product at a concentration of 5 pmol, as standardized by Cortez et al. [
13]. The nucleotide mix from Ludwig Biotechnology was diluted to a final concentration of 10 mM.
The reaction was prepared with a final volume of 25 µL, consisting of 13.375 µL Milli-Q® ultrapure water, 5 µL Colorless GoTaq® Flexi Buffer, 0.5 µL dNTP mixture, 1 µL 25 mM MgCl2 solution, 1 µL TviCatL1, 1 µL DTO 155, 1 µL GoTaq® Hot Start Polymerase (5u/µL), and 3 µL of DNA. A known T. vivax-positive buffalo blood sample was used as a positive control. The reactions were performed using a Thermal Cycler 2720.
The thermocycling conditions were as follows: 1 cycle of initial denaturation at 94°C for 2 minutes; 40 cycles of denaturation at 94°C for 30 seconds, annealing at 59.6°C for 30 seconds, and extension at 72°C for 1 minute; followed by 1 cycle of final extension at 72°C for 5 minutes.
2.5. Electrophoresis
After polymerization of a 1.5% agarose gel, 5 µL of each PCR reaction product was mixed with 1.5 µL GelRed (fluorescent dye) and 1.5 µL Blue Juice Gel Loading Buffer (molecular weight agent). The voltage and current of the electrophoresis equipment were set to 120V and 700A, respectively. After 45 minutes of electrophoresis, the results were visualized using a Vilber Lourmat UV transilluminator (312NM, 21x26 cm).
3. Results
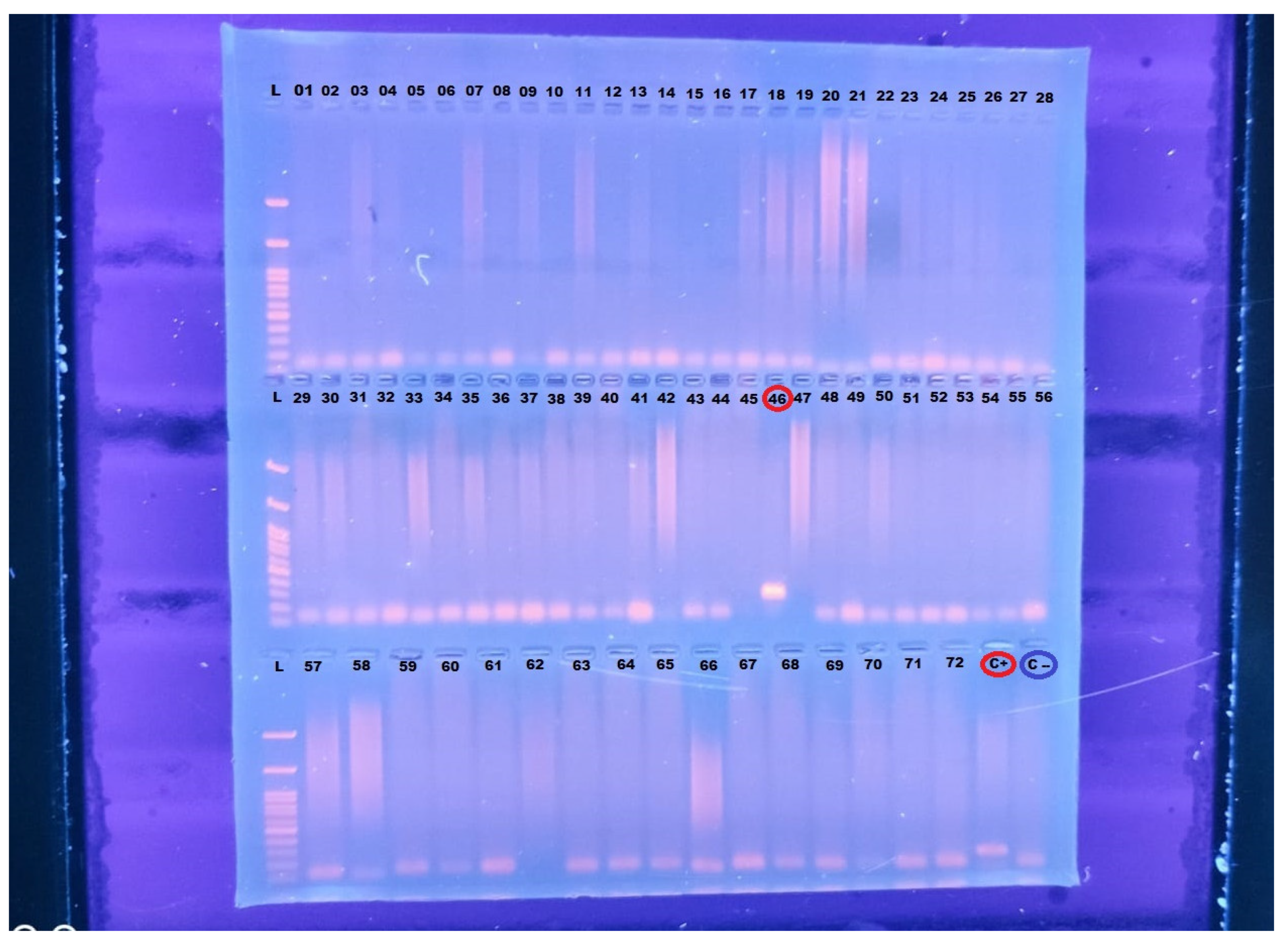
Out of the 72 Murrah buffaloes sampled for the presence of Trypanosoma vivax using conventional PCR, only one sample (Sample 46) tested positive (
Figure 1). The positive sample was obtained from an adult female, aged between 24 and 36 months, with a body condition score of 2 (on a scale of 1 to 5), and despite testing positive for T. vivax, the animal appeared clinically healthy.
The body condition score of most of the sampled buffaloes was consistently low, averaging a score of 2. This suboptimal condition is likely attributed to the poor nutritional quality of the pastures during the dry season in the region. Furthermore, the buffaloes in the Marajó Island area generally suffer from inadequate sanitary, reproductive, and nutritional management, which is reflected in the overall health and productivity of the herd. This deficiency, coupled with the region’s extremely low Human Development Index (HDI), places significant constraints on the productivity of buffalo herds in comparison to other regions of Brazil, despite Marajó Island hosting the largest buffalo population in the country.
The molecular diagnostics confirmed the presence of T. vivax in one animal, and the PCR reaction successfully amplified the 177 base pair products specific to T. vivax cathepsin L-like gene, confirming the infection. The remaining 71 samples were negative for T. vivax under the same testing conditions.
This finding highlights the low prevalence of T. vivax in the region, although the inadequate management conditions, particularly the nutritional deficiencies and lack of health interventions, may exacerbate the susceptibility of the local buffalo population to parasitic infections. Further epidemiological studies are warranted to better understand the transmission dynamics and to assess the overall health impact of T. vivax on buffaloes in this unique ecological setting.
4. Discussion
The detection of
Trypanosoma vivax in buffaloes in the Marajó Island, Amazon Biome, raises significant concerns regarding the epidemiology and transmission of trypanosomiasis in this region. Despite the pathogenic potential of
T. vivax, which can lead to acute disease in cattle [
14], no clinical signs were observed in the buffaloes sampled in this study, including the one positive animal. This aligns with the general understanding that buffaloes tend to be more resistant to diseases compared to cattle, often acting as asymptomatic reservoirs of pathogens like
T. vivax [
15,
16]. This silent carrier state poses a risk to susceptible herds, as the infected buffaloes can serve as a source of infection without showing obvious symptoms.
In South America,
T. vivax transmission is believed to occur mainly through mechanical vectors such as biting flies, given the absence of the biological vector, the tsetse fly (
Glossina spp.) [
14]. Studies, including this one, suggest that mechanical transmission by local fly species like horn flies, stable flies, and horseflies is likely the main route. However, other potential vectors, such as buffalo lice, have been detected with
T. vivax DNA, although their role in transmission remains uncertain [
17]. Blood contact through biting flies likely facilitated the transmission in this case.
The low detection rate of
T. vivax (1.39% of 72 samples) in this study may reflect the chronic stage of infection in the animals, where parasitemia tends to be low and intermittent. Similar findings have been reported in other studies where low parasite loads during the chronic phase can lead to difficulty in detection, especially using PCR-based diagnostics [
17]. The use of alternative diagnostic techniques, such as fluorescent fragment length barcoding (FFLB), has been suggested as more sensitive than PCR for detecting
T. vivax [
18]. Additionally, the choice of diagnostic methods, including serology, plays a crucial role in minimizing false negatives, as seen in studies using recombinant proteins for more accurate detection in cattle [
19].
The positive buffalo identified in this study likely acted as an asymptomatic carrier, given the absence of clinical signs. This asymptomatic carrier state is concerning, as it allows the animal to silently spread the parasite within the herd [
19,
20]. Furthermore, the animal’s nutritional status may have contributed to its immune system’s ability to control the infection, making it less likely to show symptoms [
20]. The timing of the sample collection during the dry season, when pastures were scarce, may have further influenced the immune response and parasite load.
Environmental factors, such as the dry season, likely played a role in the low prevalence of
T. vivax observed in this study. Previous studies have shown an association between the rainy season and the proliferation of biting flies, which in turn increases the prevalence of
T. vivax in livestock [
21]. The low fly density during the dry season may have reduced the transmission rates, contributing to the low number of positive cases in this study. Further research conducted during different seasons could provide more insights into the seasonal dynamics of
T. vivax transmission in this region.
In conclusion, this is the first reported case of T. vivax in buffaloes from Marajó Island, diagnosed through PCR. Although only one animal tested positive, this study highlights the importance of buffaloes as potential reservoirs of T. vivax in the Amazon Biome. Future research should focus on expanding the use of more sensitive diagnostic methods, investigating other potential vectors, and examining the role of buffaloes in the epidemiology of T. vivax in South America. Additionally, further studies on the nutritional status and immune response of buffaloes could provide valuable information on their resistance to trypanosomiasis.
5. Conclusion
This study provides valuable insights into the molecular diagnostic evidence of Trypanosoma vivax in buffaloes from Soure, Marajó Island, within the Amazon Biome. By utilizing molecular techniques, we have identified and confirmed the presence of T. vivax in buffaloes, adding to the existing serological and molecular data from other regions in Northern Brazil. This research not only fills a crucial gap in the understanding of trypanosomiasis in the Amazon but also underscores the need for ongoing surveillance and research in this under-studied area.
Author Contributions
Conceptualization, F.M.S.M. and A.d.R.C.; methodology, F.M.S.M., K.C.G.d.P.A. and A.d.R.C.; formal analysis, F.M.S.M. and K.C.G.d.P.A.; investigation, F.M.S.M., K.C.G.d.P.A. and A.d.R.C.; data curation, F.M.S.M. and F.M.S.; writing—original draft preparation, F.M.S.M., G.G.C. and A.d.R.C.; writing—review and editing, F.M.S. The author has read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Profa. Dra. Andrea Maria Góes Negrão and Msc Elem Cristina Macedo Barra do ISPA (Instituto da Produção e Saúde Animal) da UFRA (Universidade Federal Rural da Amazônia); CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPESPA (Fundação Amazônia de Amparo a Estudos e Pesquisas do Estado do Pará); CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior finance code: 001, and and PROPESP-UFPA (Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Federal do Pará) for paying the publication fee for this article via the Programa Institucional de Apoio à Pesquisa (PAPQ/2023-2024).
Conflicts of Interest
The author declares no conflicts of interest.
References
- Barbosa, J.D.; Possidonio, B.I.d.O.; dos Santos, J.B.; Oliveira, H.G.d.S.; Sousa, A.I.d.J.; Barbosa, C.C.; Beuttemmuller, E.A.; Silveira, N.d.S.e.S.; Brito, M.F.; Salvarani, F.M. Leucoderma in Buffaloes (Bubalus bubalis) in the Amazon Biome. Animals 2023, 13, 1665. [CrossRef]
- Nascimento, A.J.d.S.N., Nardi Junior, G.d., Oliveira, P.A.d.., Barbosa-Fantin, B.R., Dantas, A. Bubalinocultura no Brasil: principais raças, características e importância ao agronegócio. Peer Review. 2023, 5, 19–30. [CrossRef]
- Barbosa, J.D.; Martins, F.M.S.; Vieira, E.V.; Silva, R.P.d.L.; Bomjardim, H.d.A.; Silva, M.X.; Salvarani, F.M. Anti-Leptospira Antibodies in Buffaloes on Marajó Island. Ruminants 2023, 3, 182-188. [CrossRef]
- Castelli, G.S.N.; Silva, R.E.; Costa, A.P.; Marcili, A. Trypanosoma vivax: uma breve revisão. Braz. J. Develop. 2021, 7, 109155-109171. [CrossRef]
- Algehani, A.M.G.; Jaber, F.A.; Khan, A.; Alsulami, M.N. Review on trypanosomiasis and their prevalence in some country on the Red Sea. Braz. J. Biol. 2023, 83, e251671. [CrossRef]
- Castilho neto, K.J.G.A.; Garcia, A.B.C.F.; Fidelis júnior, O.L.; Nagata, W.B.; André, M.R.; Teixeira, M.M.G.; Machado, R.Z.; Cadioli, F.A. Follow-up of dairy cattle naturally infected by Trypanosoma vivax after treatment with isometamidium chloride. Braz J Vet Parasitol. 2021, 30, e020220. [CrossRef]
- Jaramillo, I.L.; Ruiz, J.D.; Agudelo, P.M. Cytotoxic Effect of ApoL1r in Bovine Epithelial Kidney Cells as an Alternative Treatment for Trypanosoma spp.in Cattle. Arch. Nephrol. Urol. 2023, 6, 85-89. [CrossRef]
- Silva, A.S.; Zanette, R.A.; Otto, M.A.; Gressler, L.T.; Pereira, P.L.; Monteiro, S.G. Aceturato de diminazeno no tratamento de eqüinos infectados naturalmente por Trypanosoma evansi no município de Cruz Alta-RS, Brasil. Vet. Zootec. 2009, 16, 74-79. https://rvz.emnuvens.com.br/rvz/article/view/1302/824 .
- Seixas, J. N.; Orlando, D. R.; Wouters, F.; Wouters, A. T. B.; Varaschin. M. S.; Raymundo, D. L. Aspectos patológicos da intoxicação por aceturato de diminazeno em camelídeos sul-americanos. Pesq. Vet. Bras. 2017, 37, 1509-151. [CrossRef]
- Autheman, D.; Crosnier, C.; Clare, S.; Goulding, D.A.; Brandt, C.; Harcourt, K. Tolley, C.; Galaway, F.; Khushu, M.; Ong, H.; Romero-Ramirez, A.; Duffy, C.W.; Jackson, A.P.; Wright, G.J. An invariant Trypanosoma vivax vaccine antigen induces protective immunity. Nature 2021, 595, 96-100. [CrossRef]
- Fontes, D. G.; Monteiro, M.V.B.; Jorge, E.M., Oliveira, C.M.C.; Ritter, R.A.; Barbosa Neto, J. D.; Silva Filho, E. d.; Monteiro, F.O.B. Perfil hematológico e bioquímico de búfalos (Bubalus bubalis) na Amazônia Oriental. Pesq. Vet. Bras. 2014, 34, 57–63. [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring, 1989, 345p.
- Cortez, A.P.; Rodrigues, A.C.; Garcia, H.A.; Neves, L.; Batista, J.S.; Bengaly, Z.; Paiva, F.; Teixeira, M.M.G. Cathepsin L-like genes of Trypanosoma vivax from Africa and South America - characterisation, relationships and diagnostic implications. Mol. Cell. Probes. 2009, 23, 44-51. [CrossRef]
- Shaw, J. J.; Lainson, R. Trypanosoma vivax in Brazil. Ann Trop Med Parasitol. 1972, 66, 25-32. [CrossRef]
- Gomes, R. A; Machado, R.A.; Starke-Buzetti, W.A.; Bonesso, M.A. Resposta imune-humoral de búfalos (Bubalus bubalis) contra Anaplasma marginale (THEILER, 1910). Rev. Bras. Parasitol. Vet. 2008, 17, 73-80. [CrossRef]
- Florentin, A.S.; Perez, H.A.G.; Rodrigues, C.M.F.; Dubois, E.F.; Monzón, C.M.; Teixeira, M.M.G. Molecular epidemiological insights into Trypanosoma vivax in Argentina: From the endemic Gran Chaco to outbreaks in the Pampas. Transbound Emerg Dis. 2021, 00, 1–11. [CrossRef]
- Dyonisio, G.H.S.; Batista, H.R.; Silva, R.E.; Azevedo, R.C.F.; Costa, J.O.J.; Manhães, I.B.O.; Tonhosolo, R.; Genari, S.M.; Minervino, A.H.H.; Marcili, A. Molecular Diagnosis and Prevalence of Trypanosoma vivax (Trypanosomatida: Trypanosomatidae) in Buffaloes and Ectoparasites in the Brazilian Amazon Region. J. Med. Entom. 2020, 58, 403-407. [CrossRef]
- Pérez, H.A.G.; Rodrigues, C.M.F.; Pivat, I.H.V.; Fuzato, A.C.R.; Camargo, E.P.; Minervino, A.H.H.; Teixeira, M.M.G. High Trypanosoma vivax infection rates in water buffalo and cattle in the Brazilian Lower Amazon. Parasit. Interl. 2020, 79, 102162. [CrossRef]
- Bontempi, I.A.; Arias, D.G.; Castro, G.V.; Peverengo, L.M.; Díaz, G.F.; Allassia, M.; Greif, G.; Marcipar, I. Improved serodiagnosis of Trypanosoma vivax infections in cattle reveals high infection rates in the livestock regions of Argentina. PLoS Negl. Trop. Dis. 2024, 18, e0012020. [CrossRef]
- Souza, E.M. d.; Starke-Buzetti, W.A.; Ferreira, F.P.; Neves, M.F.; Machado, R.Z. Humoral immune response of water buffalo monitored with three different antigens of Toxocara vitulorum. Vet. Parasit. 2004, 122, 67–78. [CrossRef]
- Cadioli, F.A.; Barnabé, P.A.; Machado, R.Z.; Teixeira, M.C.A.; André, M.R.; Sampaio, P.H.; Fidélis Júnior, O.L. Teixeira, M.M.G.; Marquez, L.C. First report of Trypanosoma vivax outbreak in dairy cattle in São Paulo state, Brazil. Rev. Bras. Parasitol. Vet. 2012, 21, 118-124. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).