1. Introduction
Surgical site infections (SSIs) are among the most common healthcare-associated infections (HAIs), accounting for approximately 31% of all HAIs globally [
1]. They represent a significant economic and social burden for patient care and healthcare systems [
2]. Numerous studies reported variable SSI rates, ranging from 2% to over 20% depending on the surgical procedure and healthcare setting [
2]. The prevalence of SSIs varies according to the times of surgeries, patient populations, and healthcare facilities. Several studies have documented fluctuating SSIs rates worldwide [
3]. In Italy, the SNICh study group reported data on non-prosthetic surgery from the Italian SSI surveillance program from 2009 to 2011 [
4]. The analysis revealed that SSIs were observed in 1,628 cases (2,6%) with 60% of SSIs being diagnosed through 30-day post-discharge surveillance [
4]. Another study found a higher prevalence of SSIs up to 11% [
5]; in this study risk factors associated with a higher incidence of SSI were found to be elderly age (>55 years), diabetes mellitus (especially hyperglycemia in the perioperative period), immunocompromised patients (mainly HIV and immunosuppressive therapy patients), surgeon skill (higher in senior professors compared with junior residents), nature of the cases, (emergency surgeries), placement of drains, wound class (highest in dirty wounds), type of closure (multilayer closure), prolonged duration of hospital stay, longer duration of surgery (>2 hours), type of surgery (highest in cholecystectomy). The highest rates of causative organisms for SSIs found were
Staphylococcus aureus,
Escherichia coli and
Klebsiella ssp [
6]. So, the occurrence of SSIs is influenced by many factors, including patient characteristics, surgical techniques, and microbial pathogens. Patient-related factors include advanced age, obesity, smoking, immunosuppression, and comorbidities such as diabetes mellitus [
7,
8,
9]. Procedure-related factors include the complexity of the intervention, duration, perioperative blood loss, and the use of devices [
10]. 70% of HAIS were, in fact, related to an invasive device [
11] Healthcare system-related risk factors encompass inadequate sterilization techniques, inappropriate antimicrobial prophylaxis, and poor compliance with infection prevention and poor adherence to infection prevention protocols [
12].
The pathogens responsible for SSIs primarily originated from the patient’s endogenous skin microbiota or exogenous sources [
13]. Gram-positive bacteria, particularly
Staphylococcus aureus and coagulase-negative
Staphylococcus, were the most common pathogens including methicillin-sensitive
Staphylococcus (MSSA) and methicillin-resistant staphylococci (MRSA) [
14]. Their ability to form biofilms and antibiotic resistance contribute to their persistence and virulence at surgical wound sites, posing additional challenges for treating and preventing these infections [
15]. Gram-negative bacteria, such as
Escherichia coli, were also frequently involved and commonly observed in abdominal or urinary tract HAIs [
16]. Several studies highlight the role of
E. coli as a primary pathogen in these infections, which are of concern due to their potential association with antimicrobial resistance and extended-spectrum beta-lactamase (ESBL) production [
13]. Even more emerging infections are those derived from
Enterococcus spp., particularly
Enterococcus faecalis and
Enterococcus faecium. Their intrinsic resistance to various antibiotics, including vancomycin, posed challenges to treatment and prevention strategies [
14,
15].
In this scenario, the prevention of SSIs requires a multifaceted approach. Key measures include optimizing the patient before surgery, adhering to sterile techniques during the procedure, appropriate antimicrobial prophylaxis, meticulous hand hygiene, aseptic wound care, and enhanced surveillance [
12]. Additionally, implementing interventions, such as those recommended by the Healthcare Infection Control Practices Advisory Committee (HICPAC), has significantly reduced SSI rates [
16]. In the last century, HICPAC improved a bundle for the prevention of HAIs based on the use of aseptic procedures, disinfection of the skin and correct use of antibiotics for SSIs. Moreover, in our country, the Gelli-Bianco law introduced the national health guidelines system including the prophylaxis antibiotic therapy pre- and post-surgery [
17].
Based on these premises, our study aims to evaluate adherence to and appropriateness of perioperative-antibiotic prophylaxis use in a province of southern Italy and to assess potential risk factors that may negatively affect the progression of SSIs.
2. Results
We collected a total of 117 patients in the study of which 70,9% (n=83) were from Thoracic Surgery, and 34 were from Vascular one (29,05%), with a mean age of 63 (± 12.36 SD). All the examined patients were immunocompetent and data about socio-demographic value were represented in
Table 1. All patients contacted chose to apply for this study.
Table 1.
Distribution of the study sample according to socio-demographic data and risk factors.
Table 1.
Distribution of the study sample according to socio-demographic data and risk factors.
| |
N |
% |
| Mean age± SD |
63± 12.36 |
| Body Mass Index |
|
|
<17
18-24
25-29
>30 |
4
35
44
15 |
3%
29,9%
37,6%
12,8% |
Sex
Male
Female |
85
32 |
72,6%
27,3% |
Smoke
Actual smoker
Not smoker
Past Smoker |
39
39
27 |
33,3%
33,3%
23,07% |
| Diabetes |
26 |
22,2% |
| Immunosuppressive therapy |
11 |
9,4% |
Table 2.
Distribution of the study sample according to clinical data.
Table 2.
Distribution of the study sample according to clinical data.
| |
N |
% |
Type of operation (NAS-NCR)
Clean
Clean Contaminated
Contaminated |
18 15.3%
97 82.9%
2 17.09% |
| Use of blood transfusion or derivatives |
5 |
4.27% |
| Implants |
8 |
6.8% |
| Videoendoscopy |
53 |
45.2% |
ASA Score
1
2
>3 |
4
31
82 |
3.4%
26.49%
70.08% |
Duration of surgery (min)
<15
15-44
45-60
>60 |
4
49
16
40 |
3.4%
4.8%
13.67%
34.2% |
We found that 11.7% (n=10) of the sample had a long hospitalization length (>15 days) for the Vascular Surgery ward was 9.44± 10.24 SD days and for Thoracic Surgery was 8.89±9.92 SD. Analyzing the individual risk factors of the population, approximately 72.54% (n=85) of the sample had a BMI ≥25, of which 25.74% (n=30) were smokers and about 20% (n=24) were diabetics.
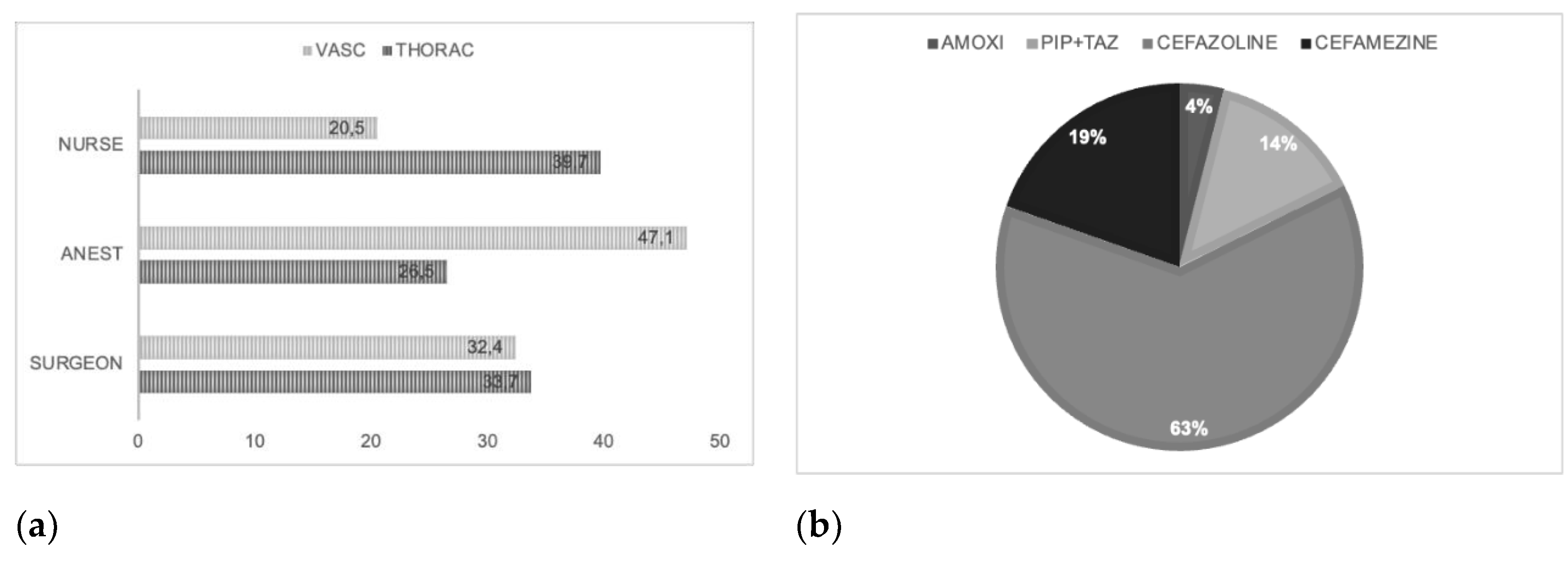
In most cases, the healthcare liability of administration antibiotics is of the anesthetist (see
Figure 1a) and the main class of antibiotics administered is cefazolin in line with National Guideline System (SNLG) indications (see
Figure 1b) [
18].
In most cases the administration of antibiotics is mainly after surgical procedures such as indicated by Table 3.
Table 3.
Administration of antibiotics first, during and past surgical procedure versus qualitative characteristics.
Table 3.
Administration of antibiotics first, during and past surgical procedure versus qualitative characteristics.
| Administration of antibiotic |
First of surgical procedure |
During surgical procedure |
After surgical procedure |
p value |
| Total |
40.2 (n= 47) |
2.6 (n= 3) |
43.6 (n= 51) |
|
Gender
Males
Females |
68.1 (n= 32)
31.9 (n= 15) |
66.7 (n= 2)
33.3 (n= 1) |
68.6 (n= 35)
31.4 (n= 16) |
p=0.662
p=0.851
p=0.391 |
Diabetes
Yes
Not |
78.7 (n= 37)
21.3 (n= 10) |
66.7 (n= 2)
33.3 (n= 1) |
82.4 (n= 42)
17.6 (n= 9) |
p=0.740
p=0.326
p=0.295 |
Use of corticosteroids
Yes
Not |
87.2(n= 41)
12.8 (n= 6) |
100 (n= 3)
0 (n= 0) |
80.4 (n= 41)
19.6 (n= 10) |
p=0.510
p=0.356
p=0.001 |
ICD9 CM code
Fiberoptic endoscopy
Drainage
Angioplasty
Lung resections
Biopsies
|
21.3 (n= 10)
27.7 (n= 13)
36.2 (n= 17)
10.6 (n= 5)
4.3 (n= 2)
|
33.3 (n= 1)
33.3 (n= 1)
33.3 (n= 1)
0 (n= 0)
0 (n= 0)
|
21.6 (n= 11)
33.3 (n= 17)
27.5 (n= 14)
9.8 (n= 5)
7.8 (n= 4) |
p= 0.049
p=0.733
p=0.076 |
Type of surgical procedure
In emergency
Election
|
34 (n= 16)
63.8 (n= 30) |
33.3 (n= 1)
66.7 (n= 2) |
19.6 (n= 10)
78.4 (n= 40) |
p=0.001
p=0.005
p=0.346 |
Classification of surgical procedure
Clean
Contaminated clean
Contaminated |
12.8 (n= 6)
87.2 (n= 41)
0 (n= 0) |
0 (n= 0)
100 (n= 3)
0 (n= 0) |
9.8 (n= 5)
88.2 (n= 45)
2 (n= 1) |
p=0.280
p=0.418
p=0.337 |
Prosthesis implant
Yes
Not |
17(n= 8)
2.1 (n= 1) |
100 (n= 3)
0 (n= 0) |
92.2 (n= 47)
3.9 (n= 2) |
p=0.001
p=0.001
p=0.364 |
Blood transfusion
Yes
Not |
87.2 (n= 41)
8.5 (n= 4) |
100 (n= 3)
0 (n= 0) |
88.2 (n= 45)
7.8 (n= 4) |
p=0.001
p=0.001
p=0.060 |
Time of antibiotic administration
Within 60 minutes
Two hours first |
55.3 (n= 26)
17 (n= 8) |
33.3(n= 1)
0 (n= 0) |
78.4 (n= 40)
19.6 (n= 10) |
p=0.001
p=0.007
p=0.051 |
At the same time, in 6.8% of cases, we found the onset of an infection first to recovery of the patient (community infection).
We can state, regarding the comparison of qualitative characteristics and timing of antibiotic prophylaxis, the following observations: in 6.8% of the sample, HAIs were detected, and in half of the sample, the administration of an antibiotic, particularly beta-lactamase, was noted after surgery (approximately 43.6% of procedures).
Concerning gender, a higher prevalence of postoperative antibiotic administration was found in males, although no statistically significant difference was observed in antibiotic administration across the three phases studied (p > 0.05).
Among diabetics, approximately 82.4% (n=42) received postoperative antibiotics, but no statistically significant difference were highlighted in our study (p > 0.05).
The association between preoperative systemic corticosteroid use and postoperative antibiotic administration was statistically significant (p = 0.001), with about 80.4% (n=41) of corticosteroid users and 19.6% (n=10) non-corticosteroid users receiving postoperative antibiotic therapy.
Additionally, when comparing the type of surgical intervention, coded using ICD-9-CM, significance was found for preoperative administration (p = 0.049), particularly for video endoscopy (21.3%), drainage (27.7%), angioplasty (36.2%), pulmonary resection (10.6%), and biopsies (4.2%).
Regarding the type of procedure, whether emergency or elective, there was a significant difference in preoperative and intraoperative antibiotic administration (p = 0.005), particularly in association with preoperative administration (p = 0.001).
About 70% (n=82) of the interventions in the studio were classified as clean-contaminated. The time of administration was adherent to the guidelines (47% of the administration antibiotics occurred after 60 min from the intervention; of these 62.7% had such an administration intravenous). The comparison between ASA score and preoperative administration was statistically significant (p<0.001) as well as class ASA score vs second dose of antibiotic administration before surgery (p<0.01); also significant was the association between duration of surgery intervention, use of systemic corticosteroids and postoperative administration of antibiotics (p<0.001). No statistically significant correlation was found between the administration of a second dose of antibiotic during surgery and the risk factors examined except for the type of intervention (urgent or in the election; p<0.01), start time of administration (within 60 minutes; two hours before; greater than two hours; p<0,05). The administration of a second dose of antibiotic was not related to the investigated risk factors and administration of a dose of antibiotic after surgery was related to lower albumin values and longer albumin duration. The administration of additional doses of antibiotics after the end of the surgery has generally not proven effective in further reducing the frequency of SSIs.
Another factor considered was the percentage of serum albumin as a predictor of poor surgical outcomes, with a higher likelihood of SSIs.
3. Discussion
SSIs contribute to considerable morbidity and mortality, leading to increased hospital length of stay, readmissions, and healthcare costs [
2]. In our study, we found that in all cases the administration of antibiotics fit the local guidelines. SSIs are common postoperative complications, occurring in 2-5% of all surgical procedures [
15].
Gender disparities in the beginning of SSIs are rarely studied in research. The risk of surgical site infections (SSIs) in abdominal surgery was shown to be lower in women than in men while in cardiac surgery, women had a greater incidence of surgical site infections (5.50 vs. 3.02; p < 0.001), but there was no gender-specific differences in orthopedic or vascular surgery. [
19,
20]
Although the relationship between diabetes and higher incidence of surgical site infections (SSI) as well as the possible connection between hyperglycemia and SSI has been extensively documented in the media in recent years. [
21,
22]. No statistical difference was detected.
In our study we found a statistical correlation between diabetes and use of PAP; in the scientific literature we found several studies that confirm this and, in particular, recent meta-analysis revealed a strong correlation between diabetes and SSI that held true after adjusting for BMI and across a variety of surgical procedures. Even though we were able to confirm a link between pre- and post-operative hyperglycemia and SSI, meta-analyses of trials that adjusted for hyperglycemia still showed that diabetes history was a substantial risk factor [
23].
Numerous inflammatory and autoimmune diseases are treated with chronic steroid therapy, yet side effects are well-known. Regarding the part that continuous steroid therapy plays in predisposing patients to perioperative problems, there is still a gap in the literature. Chronic preoperative steroid medication has been shown by Ranson et al. to be an independent risk factor for perioperative complications, including as wound dehiscence, urinary tract infection, pulmonary embolism, readmission, and nonhome release. Patients who are excessively obese are even more at risk [
24]
ICD-9-CM diagnosis codes for surgical site infections (SSI) have not been extensively studied [
25]. However, in our study when comparing the type of surgical intervention, coded using ICD-9-CM, significance was found for preoperative administration (p = 0.049), particularly for video endoscopy (21.3%), drainage (27.7%), angioplasty (36.2%), pulmonary resection (10.6%), and biopsies (4.2%). Similar results to what was reported by Onyekwelu et al, where the association between Surgical Wound Classification (SWC) and SSI development is not statistically significant (p>0.005) [
26]. This may indicate that the model has predictive value for future SSIs, which was not its initial intended application.
Moreover, the rate of SSI was significantly associated with ASA classification. Similar results have been reported by other studies [
27] such as indicated by the literature, where some authors reported that ASA class 1 decreased the risk for SSIs by 0.3 times as compared to ASA 3 [
28]
Additionally, in patients undergoing prolonged antibiotic treatments, it is certainly less likely to isolate bacteria from the wound swab [
29]. In the literature same association was evaluated in patients undergoing cardiac surgery showing that a longer duration of prophylaxis doesn’t alter the percentage of SSIs even in the long-term [
30].
A recent observational study conducted on patients undergoing cemented hip arthroplasty shows a lower number of reoperations for removal or replacement of the prosthesis when prophylaxis is continued for 24 hours, and an antibiotic is added to the cement [
31]. Three recent studies, one conducted on patients undergoing appendectomy (for non-perforated appendicitis), one on patients undergoing surgery for gastric carcinoma, and a third on patients undergoing gynecological surgery, confirm that the administration of a single perioperative dose of antibiotic has the same effect in preventing surgical site infections as repeated doses. [
32] No evidence continuing antibiotic prophylaxis in the presence of a drain reduces postoperative infectious complications.
Regarding the type of procedure, whether emergency or elective, there was a significant difference in preoperative and intraoperative antibiotic administration (p = 0.005), particularly in association with preoperative administration (p = 0.001); the finding that administration of antibiotics could impact the incidence of SSI is well known. [
33]
In our study about 70% (n=82) of the interventions were classified as clean-contaminated. The time of administration was adherent to the guidelines (47% of the administration antibiotics occurred after 60 min from the intervention; of these 62.7% had such an administration intravenous) confirming literature results [
34].
Moreover, in our study, we found that the percentage of serum albumin was a predictor of negative surgical outcomes with an increased probability of SSI as indicated by He et al [
35].
Patients who develop SSIs often require additional surgical interventions, experience impaired wound healing, and suffer from long-term disabilities [
29]. The costs incurred due to SSIs are staggering and, several studies [
36,
37,
38] prevention strategies [
39] Direct medical costs associated with SSIs include prolonged hospital stays, additional surgical procedures, and expensive antibiotic regimens [
40]. Furthermore, indirect costs such as lost productivity, rehabilitation, and long-term care exacerbate the economic impact [
41].
Multiple preventive measures have proven effective in reducing SSIs and associated costs (e.g. hand hygiene, preoperative antimicrobial prophylaxis, careful surgical site preparation, and patient and healthcare personnel education) [
42,
43,
44]. Enhanced surveillance and mandatory reporting systems are essential for monitoring and mitigating SSIs [
45]. Additionally, various cost-containment strategies, such as bundled payment systems and value-based care models, can help optimize resource allocation and reduce the economic burden. The escalating costs of managing SSIs highlight the urgency to implement robust preventive strategies and are thus crucial to reduce both individual and societal healthcare burdens.
4. Materials and Methods
The study was conducted from 2022 to 2023, at the University Hospital “G. Martino” in Messina. Data on the evolution of surgical site infections in patients undergoing Thoracic and Vascular surgery were collected through an ad hoc questionnaire.
The questionnaire was divided into the following sections: a) socio-personal data; b) risk factors (Smoking, diabetes, steroids, etc.); c) clinical data (serum albumin, ongoing clinical infection, drug allergy); d) type of surgery (ICD9 CM code, urgent or elective surgery, video endoscopy surgery, classification of surgery, duration, ASA Physical Status Classification System score of American Anesthesiology Society (ASA score); implantation, type of anesthesia, blood transfusion and blood product transfusions, antibiotics administration before surgery); e) evaluation of adherence to preoperative antibiotic use guidelines; f) data on the healthcare workers (HCWs), responsible for administration/non-administration, type of antibiotic, place of administration, start time, route of administration before, during, and after the surgical procedure.
5. Conclusions
Antibiotic resistance has become a global public health concern, leading to increased morbidity, mortality, and healthcare costs. Monitoring and understanding the spread of antibiotic-resistant bacteria is crucial for effective control and prevention measures [
46]. Since its initial edition, the National Plan to Combat Antimicrobial Resistance (PNCAR) saw the consolidation of ICA surveillance, which included site infections. Surveillance of infections related to the assistance also in support of PNCAR”, coordinated by the Higher Institute of Health. [
47] Surveillance systems play a vital role in the early detection, evaluation, and response to antibiotic resistance by tracking the prevalence of specific antibiotic-resistant strains, such as extended-spectrum β-lactamase (ESBL)-producing
Escherichia coli [
48]. Surgical site infections remain a significant challenge in healthcare settings. Understanding the epidemiology of SSIs, including risk factors, common antibiotic-resistant pathogens, prevention strategies, and their impact on patient outcomes, is essential for implementing effective prevention interventions. By implementing evidence-based guidelines, healthcare professionals’ education and correct antibiotic use can reduce the burden of SSIs by improving patient care. Further research is warranted to explore novel preventive measures and therapeutic interventions to reduce the burden of SSIs. Antimicrobial resistance has become a global health crisis, threatening the effectiveness of antibiotics and rendering once-treatable infections increasingly difficult to manage [
49]. In response to this challenge, the World Health Organization (WHO) introduced the AWaRe classification system as a tool to promote responsible antibiotic prescribing practices [
50]. The Access, Watch, and Reserve (AWaRe) classification system is an evidence-based approach that aims to guide healthcare professionals in the appropriate use of antibiotics.
Antimicrobial stewardship programs can significantly contribute to the overall reduction in antimicrobial resistance [
51]. By improving prescribing practices and promoting appropriate antibiotic use, these programs aim to prolong the effectiveness of existing antimicrobial agents. The evidence presented in this review indicates that effective implementation of stewardship interventions can help optimize antibiotic use, reduce patient harm, and preserve the efficacy of antibiotics for future generations. The findings from recent studies reinforce the need for ongoing research, improved surveillance systems, and continued efforts to implement and evaluate antimicrobial stewardship initiatives to combat the global threat of antimicrobial resistance [
52].
References
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO Recommendations on Intraoperative and Postoperative Measures for Surgical Site Infection Prevention: An Evidence-Based Global Perspective. Lancet Infect Dis 2016, 16, e288–e303. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Podgorny, K.; Berríos-Torres, S.I.; Bratzler, D.W.; Dellinger, E.P.; Greene, L.; Nyquist, A.-C.; Saiman, L.; Yokoe, D.S.; Maragakis, L.L.; et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update. Infect Control Hosp Epidemiol 2014, 35, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Young, P.Y.; Khadaroo, R.G. Surgical Site Infections. Surg Clin North Am 2014, 94, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Marchi, M.; Pan, A.; Gagliotti, C.; Morsillo, F.; Parenti, M.; Resi, D.; Moro, M.L. ; Sorveglianza Nazionale Infezioni in Chirurgia (SNICh) Study Group The Italian National Surgical Site Infection Surveillance Programme and Its Positive Impact, 2009 to 2011. Euro Surveill 2014, 19, 20815. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Griffiths, B. Cohort Study to Characterise Surgical Site Infections after Open Surgery in the UK’s National Health Service. BMJ Open 2023, 13, e076735. [Google Scholar] [CrossRef]
- Akhter, M.S.J.; Verma, R.; Madhukar, K.P.; Vaishampayan, A.R.; Unadkat, P.C. Incidence of Surgical Site Infection in Postoperative Patients at a Tertiary Care Centre in India. J Wound Care 2016, 25, 210–217. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017, 152, 784. [Google Scholar] [CrossRef]
-
World Health Organization Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, 2018.
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics (Basel) 2022, 11, 69. [Google Scholar] [CrossRef]
- Giufrè, M.; Mazzolini, E.; Cerquetti, M.; Brusaferro, S.; Accogli, M.; Agnoletti, F.; Agodi, A.; Alborali, G.L.; Arghittu, M.; Auxilia, F.; et al. Extended-Spectrum β-Lactamase-Producing Escherichia Coli from Extraintestinal Infections in Humans and from Food-Producing Animals in Italy: A “One Health” Study. Int J Antimicrob Agents 2021, 58, 106433. [Google Scholar] [CrossRef]
- Bartolek Hamp, D.; Cavrić, G.; Prkačin, I.; Houra, K.; Houra, K.; Perović, D.; Ljubičić, T.; Elezović, A. DEVICE-ASSOCIATED HEALTHCARE INFECTION and SEPSIS in INTENSIVE CARE UNIT. Acta Med Croatica 2015, 69, 203–209. [Google Scholar]
- Ling, M.L.; Apisarnthanarak, A.; Abbas, A.; Morikane, K.; Lee, K.Y.; Warrier, A.; Yamada, K. APSIC Guidelines for the Prevention of Surgical Site Infections. Antimicrob Resist Infect Control 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.-Y.; Gan, H.-Q.; Zhou, J.-F.; Gong, Y.-J.; Li, L.-Y.; Zhang, X.-Q.; Meng, Y.; Chen, J.-R.; Liu, W.-J.; Ye, L.; et al. Incidence of and Risk Factors for Surgical Site Infection after Colorectal Surgery: A Multiple-Center Prospective Study of 3,663 Consecutive Patients in China. Int J Infect Dis 2020, 96, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Tholany, J.; Kobayashi, T.; Marra, A.R.; Schweizer, M.L.; Samuelson, R.J.; Suzuki, H. Impact of Infectious Diseases Consultation on the Outcome of Patients with Enterococcal Bacteremia: A Systematic Literature Review and Meta-Analysis. Open Forum Infect Dis 2022, 9, ofac200. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.D.; Cho, K.S.; Moon, Y.J.; Chung, D.Y.; Kang, D.H.; Lee, J.Y. Antibiotic Prophylaxis for Percutaneous Nephrolithotomy: An Updated Systematic Review and Meta-Analysis. PLOS ONE 2022, 17, e0267233. [Google Scholar] [CrossRef]
- O’Hara, L.M.; Thom, K.A.; Preas, M.A. Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Guideline for the Prevention of Surgical Site Infection (2017): A Summary, Review, and Strategies for Implementation. Am J Infect Control 2018, 46, 602–609. [Google Scholar] [CrossRef]
- Albano GD, Rifiorito A, Malta G, Sorrentino ES, Falco V, Firenze A, Argo A, Zerbo S. The Impact on Healthcare Workers of Italian Law n. 24/2017 “Gelli-Bianco” on Patient Safety and Medical Liability: A National Survey. Int J Environ Res Public Health. 2022, 19(14):8448. [CrossRef] [PubMed]
- National Health Institute. Surveillance of Surgical Site Infections. Available online: https://www.epicentro.iss.it/sorveglianza-ica/sorveglianza-infezioni-sito-chirurgico (accessed on 30 August 2024).
- Langelotz C, Mueller-Rau C, Terziyski S, Rau B, Krannich A, Gastmeier P, Geffers C. Gender-Specific Differences in Surgical Site Infections: An Analysis of 438,050 Surgical Procedures from the German National Nosocomial Infections Surveillance System. Viszeralmedizin. 2014, 30:114-7. [CrossRef] [PubMed]
- Aghdassi SJS, Schröder C, Gastmeier P. Gender-related risk factors for surgical site infections. Results from 10 years of surveillance in Germany. Antimicrob Resist Infect Control. 2019 3;8:95. [CrossRef] [PubMed]
- Gachabayov M, Senagore AJ, Abbas SK, Yelika SB, You K, Bergamaschi R. Perioperative hyperglycemia: an unmet need within a surgical site infection bundle. Tech Coloproctol. 2018, 22, 201-207. Epub 2018 Mar 6. 22. [CrossRef] [PubMed]
- Pennington Z, Lubelski D, Westbroek EM, Ahmed AK, Passias PG, Sciubba DM. Persistent Postoperative Hyperglycemia as a Risk Factor for Operative Treatment of Deep Wound Infection After Spine Surgery. Neurosurgery 2020, 87, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, Bertran E, Jaber L. Diabetes and Risk of Surgical Site Infection: A Systematic Review and Meta-analysis. Infect Control Hosp Epidemiol. 2016, 7, 88-99. Epub 2015 Oct 27. [CrossRef] [PubMed]
- Ranson WA, White SJW, Cheung ZB, Mikhail C, Ye I, Kim JS, Cho SK. The Effects of Chronic Preoperative Steroid Therapy on Perioperative Complications Following Elective Posterior Lumbar Fusion. Global Spine J. Epub 2018 May 10. 2018, 8, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Olsen MA, Ball KE, Nickel KB, Wallace AE, Fraser VJ. Validation of ICD-9-CM Diagnosis Codes for Surgical Site Infection and Noninfectious Wound Complications After Mastectomy. Infect Control Hosp Epidemiol. 2017, 38, 334-339. [CrossRef] [PubMed]
- Onyekwelu I, Yakkanti R, Protzer L, Pinkston CM, Tucker C, Seligson D. Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient. J Am Acad Orthop Surg Glob Res Rev. 2017, 3:e022. [CrossRef] [PubMed]
- Johnson, A.P. Surveillance of Antibiotic Resistance. Philos Trans R Soc Lond B Biol Sci 2015, 370, 20140080. [Google Scholar] [CrossRef]
- Papadopoulos A, Machairas N, Tsourouflis G, Chouliaras C, Manioti E, Broutas D, Kykalos S, Daikos GL, Samarkos M, Vagianos C. Risk Factors for Surgical Site Infections in Patients Undergoing Emergency Surgery: A Single-centre Experience. In Vivo. 2021, 35, 3569-3574. [CrossRef] [PubMed]
- Pinchera, B.; Buonomo, A.R.; Schiano Moriello, N.; Scotto, R.; Villari, R.; Gentile, I. Update on the Management of Surgical Site Infections. Antibiotics (Basel) 2022, 11, 1608. [Google Scholar] [CrossRef]
- Sartelli, M.; Boermeester, M.A.; Cainzos, M.; Coccolini, F.; de Jonge, S.W.; Rasa, K.; Dellinger, E.P.; McNamara, D.A.; Fry, D.E.; Cui, Y.; et al. Six Long-Standing Questions about Antibiotic Prophylaxis in Surgery. Antibiotics (Basel) 2023, 12, 908. [Google Scholar] [CrossRef]
- Taheriazam, A.; Saeidinia, A. Two-Stage Revision of Infected Hip Prosthesis after Post-Operative Antibiotic Therapy: An Observational Study. Medicine (Baltimore) 2023, 102, e32878. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Paajanen, H.; Rautio, T.; Nordström, P.; Aarnio, M.; Rantanen, T.; Tuominen, R.; Hurme, S.; Virtanen, J.; Mecklin, J.-P.; et al. Antibiotic Therapy vs Appendectomy for Treatment of Uncomplicated Acute Appendicitis. JAMA 2015, 313, 2340. [Google Scholar] [CrossRef] [PubMed]
- Crader MF, Varacallo M. Preoperative Antibiotic Prophylaxis. [Updated 2023 Aug 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442032/ (accessed on 30 August 2024).
- Misha G, Chelkeba L, Melaku T. Incidence, risk factors and outcomes of surgical site infections among patients admitted to Jimma Medical Center, South West Ethiopia: Prospective cohort study. Ann Med Surg (Lond). 2021 Mar, 65, 102247. [CrossRef] [PubMed]
- He, Z.; Zhou, K.; Tang, K.; Quan, Z.; Liu, S.; Su, B. Perioperative Hypoalbuminemia Is a Risk Factor for Wound Complications Following Posterior Lumbar Interbody Fusion. Journal of Orthopaedic Surgery and Research 2020, 15, 538. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Collinsworth, A.; Flutura, Hasa; Griffin, L. Incidence and Impact of Surgical Site Infections on Length of Stay and Cost of Care for Patients Undergoing Open Procedures. Surg Open Sci 2022, 11, 1–18. [Google Scholar] [CrossRef]
- Atesok, K.; Papavassiliou, E.; Heffernan, M.J.; Tunmire, D.; Sitnikov, I.; Tanaka, N.; Rajaram, S.; Pittman, J.; Gokaslan, Z.L.; Vaccaro, A.; et al. Current Strategies in Prevention of Postoperative Infections in Spine Surgery. Global Spine J 2020, 10, 183–194. [Google Scholar] [CrossRef]
- Piednoir, E.; Robert-Yap, J.; Baillet, P.; Lermite, E.; Christou, N. The Socioeconomic Impact of Surgical Site Infections. Front Public Health 2021, 9, 712461. [Google Scholar] [CrossRef]
- Iskandar, K.; Sartelli, M.; Tabbal, M.; Ansaloni, L.; Baiocchi, G.L.; Catena, F.; Coccolini, F.; Haque, M.; Labricciosa, F.M.; Moghabghab, A.; et al. Highlighting the Gaps in Quantifying the Economic Burden of Surgical Site Infections Associated with Antimicrobial-Resistant Bacteria. World J Emerg Surg 2019, 14, 50. [Google Scholar] [CrossRef]
- Perencevich, E.N.; Sands, K.E.; Cosgrove, S.E.; Guadagnoli, E.; Meara, E.; Platt, R. Health and Economic Impact of Surgical Site Infections Diagnosed after Hospital Discharge. Emerg Infect Dis 2003, 9, 196–203. [Google Scholar] [CrossRef]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of Surgical Site Infection on Healthcare Costs and Patient Outcomes: A Systematic Review in Six European Countries. J Hosp Infect 2017, 96, 1–15. [Google Scholar] [CrossRef]
- Kang, M.; Andrew, M.E.; Farishta, A.; Oltmann, S.C.; Sreeramoju, P.V. Best Practices and a Business Case for Surgical Site Infection Prevention. AORN J 2023, 117, 277–290. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect Dis 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Squeri, R.; Genovese, C.; Palamara, M. a. R.; Trimarchi, G.; La Fauci, V. “Clean Care Is Safer Care”: Correct Handwashing in the Prevention of Healthcare Associated Infections. Ann Ig 2016, 28, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.; Dalmasso, P.; Politano, G.; Furmenti, M.F.; Quattrocolo, F.; Zotti, C.M. Surgical Site Infections in Italy, 2009–2015: Incidence, Trends, and Impact of Surveillance Duration on Infection Risk. Surg Infect (Larchmt) 2019, 20, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.; Caricato, P.; Mele, F.; Leonardelli, M.; Duma, S.; Gorini, E.; Stellacci, A.; Bavaro, D.F.; Diella, L.; Saracino, A.; et al. Analysis of Italian Requests for Compensation in Cases of Responsibility for Healthcare-Related Infections: A Retrospective Study. Front Public Health 2023, 10, 1078719. [Google Scholar] [CrossRef]
- EpiCentro. Piano Nazionale Di Contrasto All’Antibiotico-Resistenza (PNCAR) 2022-2025. Available online: https://www.epicentro.iss.it/antibiotico-resistenza/pncar-2022 (accessed on 12 August 2024).
- Silvestri, M.; Dobrinja, C.; Scomersi, S.; Giudici, F.; Turoldo, A.; Princic, E.; Luzzati, R.; de Manzini, N.; Bortul, M. Modifiable and Non-Modifiable Risk Factors for Surgical Site Infection after Colorectal Surgery: A Single-Center Experience. Surg Today 2017, 48, 338–345. [Google Scholar] [CrossRef]
- Smith, D.; Dushoff, J.; Perencevich, E.N.; Harris, A.; Levin, S.A. Persistent Colonization and the Spread of Antibiotic Resistance in Nosocomial Pathogens: Resistance Is a Regional Problem. Proc Natl Acad Sci U S A 2004, 101, 3709–3714. [Google Scholar] [CrossRef]
- World Healt Organization. AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use, 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 12 August 2024).
- Johnson, A.P.; Woodford, N. Global Spread of Antibiotic Resistance: The Example of New Delhi Metallo-β-Lactamase (NDM)-Mediated Carbapenem Resistance. J Med Microbiol 2013, 62, 499–513. [Google Scholar] [CrossRef]
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) Point Prevalence Survey: Developing Hospital-Quality Indicators of Antibiotic Prescribing for Children. J Antimicrob Chemother 2016, 71, 1106–1117. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).