1. Introduction
The depletion of fossil fuels and the negative environmental impact of their extraction and combustion [
1] have prompted scientists to explore the development of alternative renewable energy sources, such as biomass energy [
2,
3,
4]. Bio-oil obtained by biomass through rapid pyrolysis are an alternative to fossil fuels [
5]. Bio-oil is a complex mixture that may contain water and fine solid particles. The molecular weight of the bio-oil can range from 370 to 1000 g/mol and is highly dependent on the biomass source [
6]. In general, it contains various chemicals such as organic acids, alcohols, aldehydes, ketones, phenols, esters, ethers, furans and etc. The biggest disadvantage of bio-oil obtained by rapid pyrolysis is that the high content of water and oxygen compounds results in poor quality, high viscosity, unstable storage, low calorific value, and high acidity.
Different materials have been proposed as catalysts for the pyrolysis process. Acid catalysts such as microporous zeolite, mesoporous aluminosilicate, and metal-modified zeolite have been extensively studied. Acid catalysts can promote dehydration, decarbonization, cracking and aromatization reactions. Moreover, aromatization at the zeolite acid site leads to the formation of valuable monocyclic aromatic hydrocarbons and BTX. ZSM-5 catalyst can obtain higher polycyclic aromatic hydrocarbons (PAHs) and lower monocyclic aromatic hydrocarbons (MAHs). Adding transition metal to ZSM-5 catalyst can decrease the yield of PAHs and increase the yield of MAHs [7, 8].
2. Experimental
2.1. Raw Materials
The coconut coat is dried and set aside. The dried coconut coat is cut into pieces and crushed with a grinder until the particle size is less than 250 μm. Then the coconut coat powder is screened with a 60-mesh sieve. The zeolite of USY was used as catalyst.
Microwave Pyrolysis of Coconut Clothes
The coconut coated powder and zeolite of USY catalyst are mixed evenly in a certain proportion and put into the material boat. Then, the material boat is placed in the middle of CY-PY1100C-M microwave pyrolysis furnace (Hunan Changyi Microwave Technology Co., LTD.) for catalytic pyrolysis. The pyrolysis furnace is purged with nitrogen (200 mL/min) for 30min, and then the nitrogen flow is reduced to the set speed. The outlet of the pyrolysis furnace is successively connected with the condensing tube and the washing cylinder equipped with water. After the parameter is set, the experiment was started. During the experiment, the nitrogen gas will drive out the pyrolysis steam and diffuse into the round bottom flask with the condensate tube and the washing cylinder with water.
2.2. Single Factor Experiment Design
Reaction temperature, reaction time, heating rate, nitrogen flow rate, catalyst to coconut mass ratio, 5 single factors were set. The influence of different factors on the yield of oil, water, gas and solid of reaction products and the influence of different factors on the composition of product oil were investigated. After pyrolysis, collect the condensed liquid and separate the oil and water with dichloromethane and weigh it separately; the solid yield is determined by the solid residue in the material boat; the gas yield is obtained by the difference method; the calculation formula is as follows:
YS=m1/m0 (1)
YL=m2/m0 (2)
YG=100%-YS-YL (3)
YW=m3/m0 (4)
YO=YL-YW (5)
YS, YL, YG, YW and YO respectively represent the solid yield, liquid yield, gas yield, aquatic yield, and bio-oil yield; m0 indicates the biomass mass, m1 represents the mass of solid, m2 indicate the liquid mass after the reaction, and m3 represents the mass of water obtained by dichloromethane extraction after the reaction.
2.3. Characterization of Zeolite of USY Catalyst and Analysis of Catalytic Pyrolysis Products
The zeolite of USY catalyst was characterized for XRD (5-90, step length 0.01, scan rate 10/min), SEM, and BET. Then the biochar were tested by BET and SEM. For the bio-oil using gas chromatography (GC-MS) -analyzer, the chromatographic conditions of GC-MS analysis are up to 50℃ for 5min, 10℃/min to 300℃, for 20min; HP-5 column; inlet temperature of 250℃; shunt ratio of 20:1; the carrier gas is helium and the flow rate is 1 mL/min.
3. Results and Discussion
3.1. The Test Results of the Catalyst
3.1.1. The XRD Representation Results of the Zeolite of USY
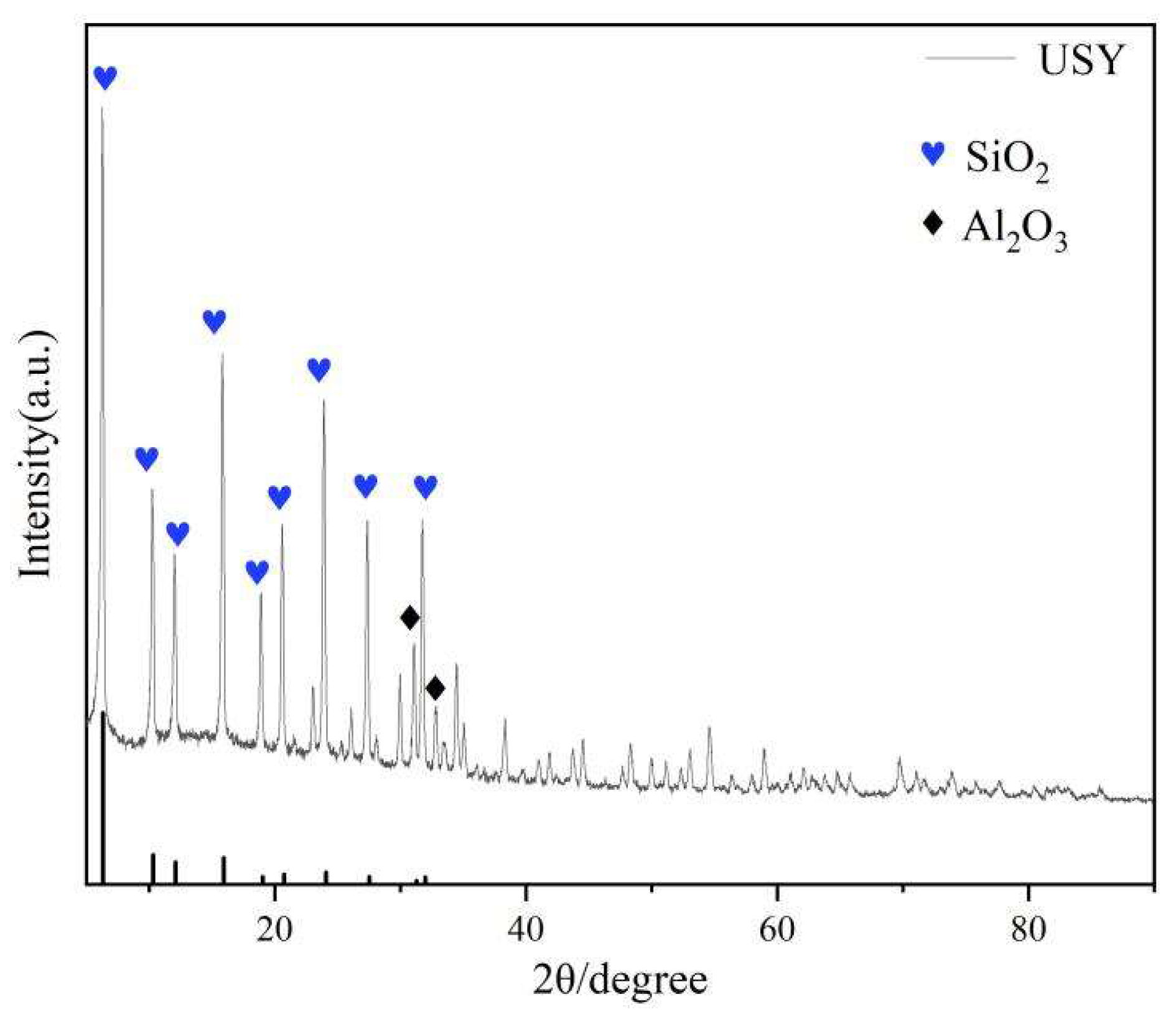
The characterization results by XRD showed that the characteristic peaks of catalyst zeolite of USY clearly combined with the standard spectrum PDF # 45-0112 formed the characteristic peaks of SiO2 at 6.31°, 10.31°, 12.97°, 15.93°, 19.03°, 20.74°, 24.07°, 27.53° and Al2O3 at 31.04° 32.76°.
Figure 1.
The XRD characterization results of the zeolite of USY.
Figure 1.
The XRD characterization results of the zeolite of USY.
3.1.2. BET Characterization Test Results for the Zeolite of USY
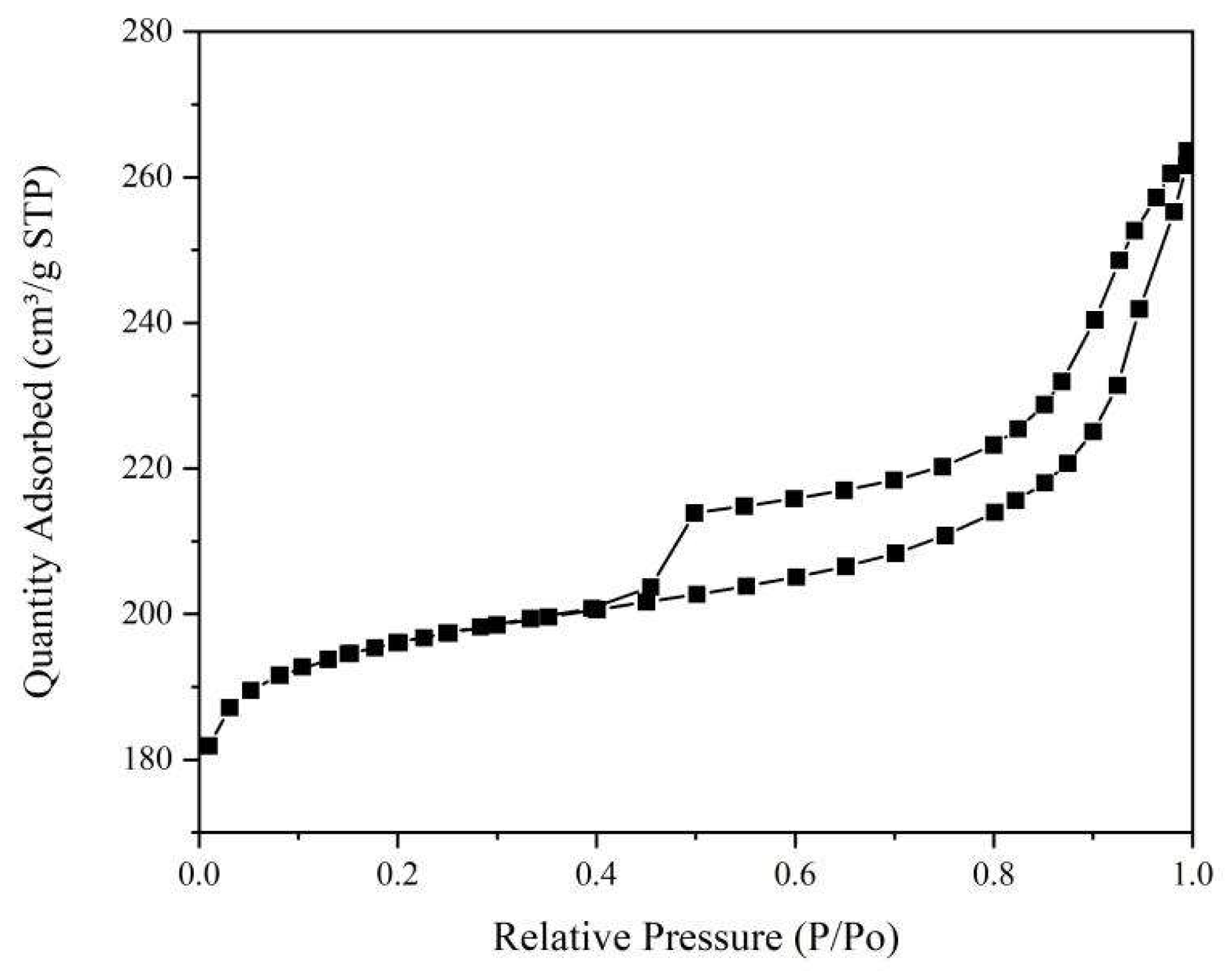
From
Table 1, it can be seen that the specific surface area of the zeolite of USY is 792.2876m
2/g. The hole volume is 0.407810cm
3/g, and the average pore diameter is 6.0828nm. From
Figure 2, it can be seen that the nitrogen absorption-desorption diagram of the zeolite of USY is the IV curve.
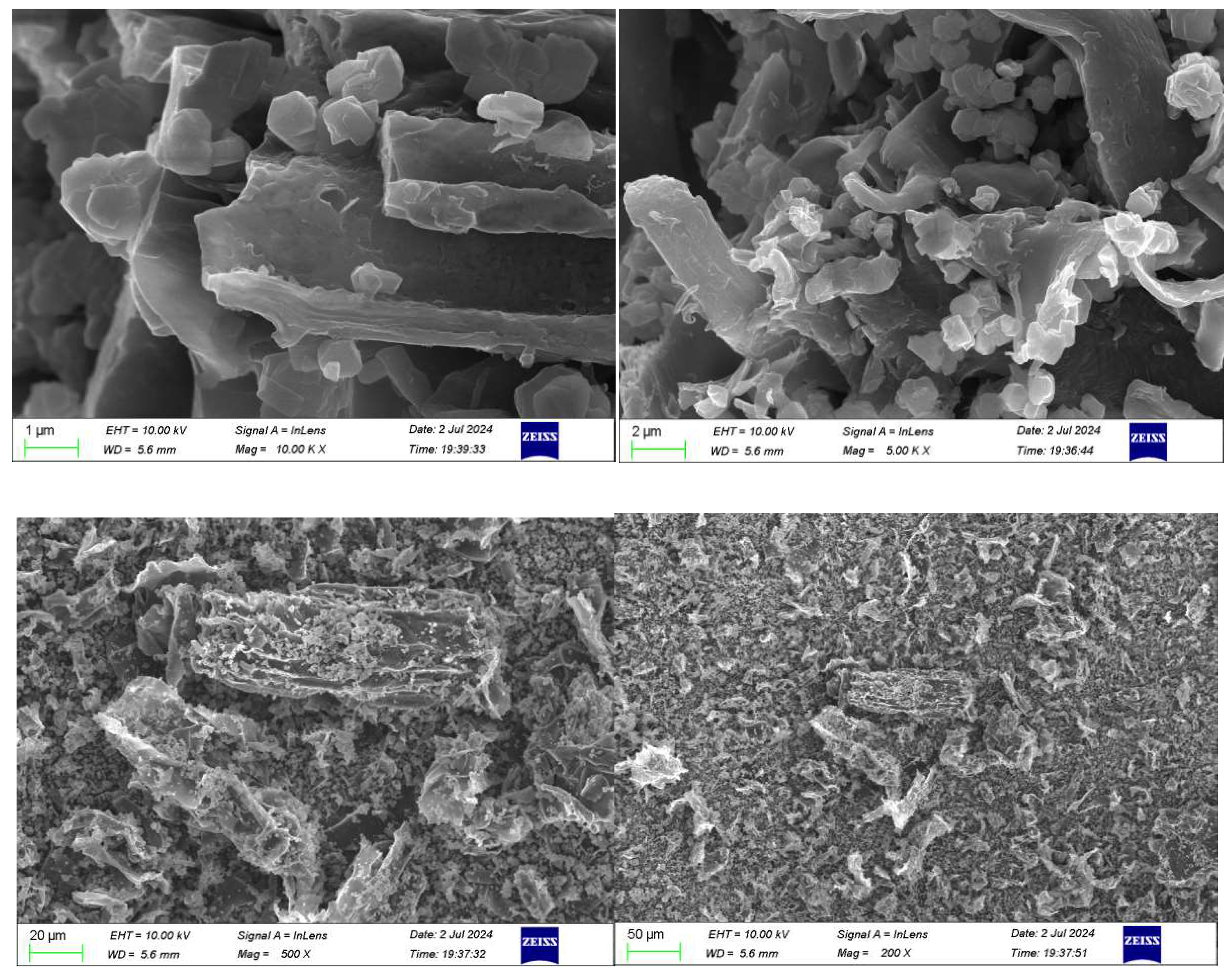
3.1.3. The SEM Characterization Results of the Zeolite of USY
The catalyst zeolite of USY was observed with 1μm-200nm resolution electron microscopy, from
Figure 3, the zeolite of USY has a close-packed hexagonal structure similar to diamond, and the particle surface is relatively smooth.
3.2. Characterization Test Results of the Biochar
3.2.1. BET Characterization of Biochar
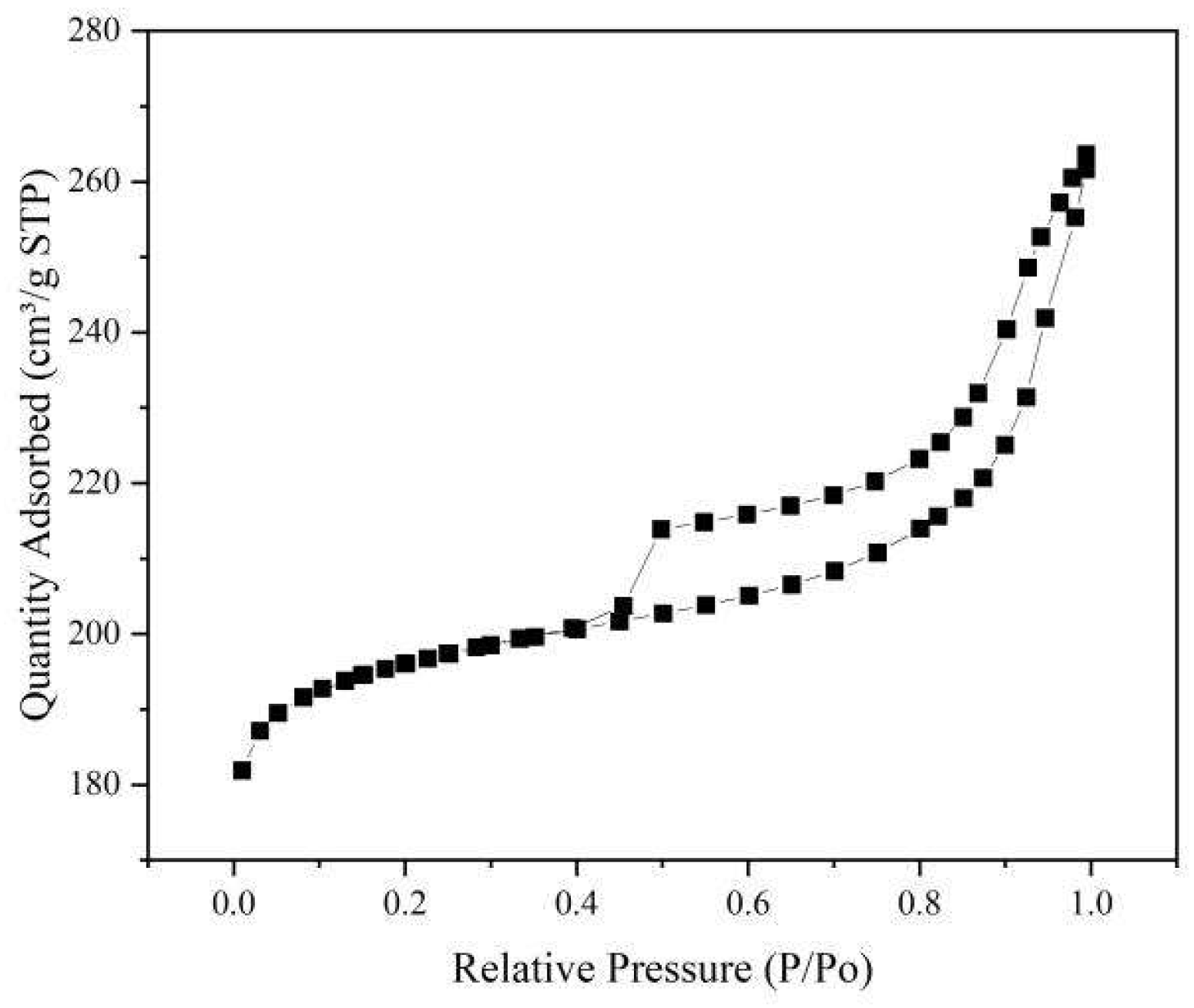
As can be seen from
Table 2, the specific surface area of the catalytic biochar is 290.2271m
2/g and has a large specific surface area, with a pore capacity of 0.168938cm
3/g and a mean pore diameter of 7.8690nm. From
Figure 5, the nitrogen absorption-desorption curve of catalytic pyrolysis biochar is type IV curve.
3.2.2. The Test Results of the SEM Representation of Biochar
According to
Figure 6 SEM, both biochar powders showed a certain flake structure, and the surface structure of biochar mixed with zeolite of USY pyrolysis contained a large number of zeolite of USY solid particles.
4. Analysis of the Catalytic Pyrolysis Process of Coconut Coat
4.1. The Effect of Reaction Temperature
Nitrogen flow rate 110 ml/min, heating rate 20℃/min, reaction time 15min, catalyst: coconut-coated powder =1:1, change the reaction temperature, the results are shown in Table
Table 3 and
Figure 7.
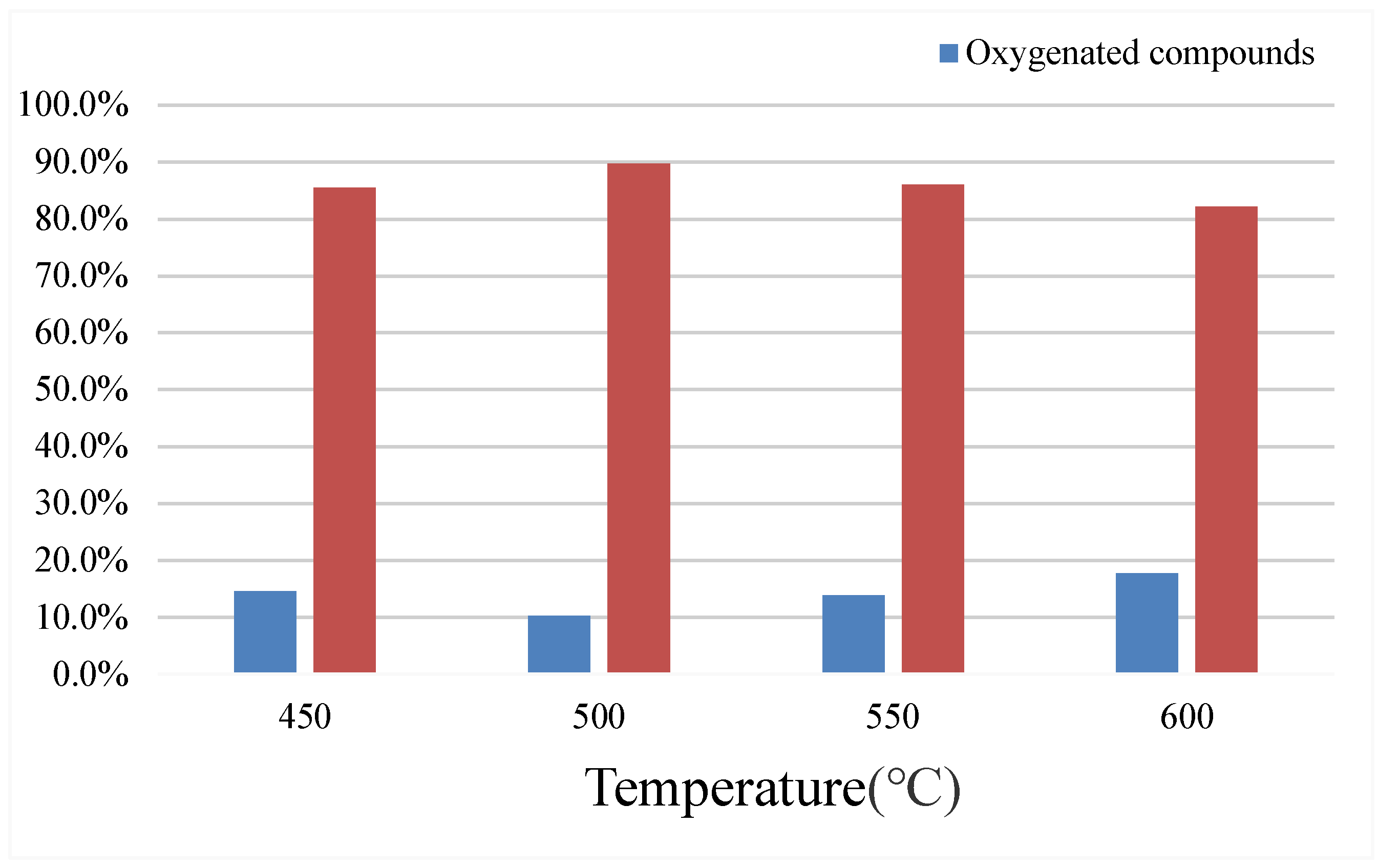
According to
Table 3 and
Figure 7, when only the reaction temperature is changed, with the increase of the reaction temperature, the yield of the bio-oil decreases first and then increases, while the yield of water increases first and then decreases. The solid yield reached a lowest of 26.9% at 550℃ and a highest of 31.48% at 600℃. The gas yield was lowest 28.45% at 500℃ and highest 42.34% at 550℃. The reaction temperature showed the lowest solid yield at 550℃, but the highest gas yield, indicating a deep catalytic pyrolysis of the coconut coat at 550℃. At the reaction temperature of 500℃, although the lowest yield of oil was 4.97%, the lowest amount of oxygen compounds was 10.3%, and the highest content of aromatic compounds was 89.7%. The highest amount of oxygenated compounds at 600℃ was 17.8%, and the lowest aromatic compound content was 82.2%. Therefore, the appropriate temperature range for the catalytic pyrolysis was between 500 and 550℃.
4.2. Heating Rate
The reaction temperature is 500 °C, the nitrogen flow is 110ml/min, the reaction time is 15min, catalyst: coconut powder = 1: 1, change the heating rate, see
Table 4 and
Figure 8.
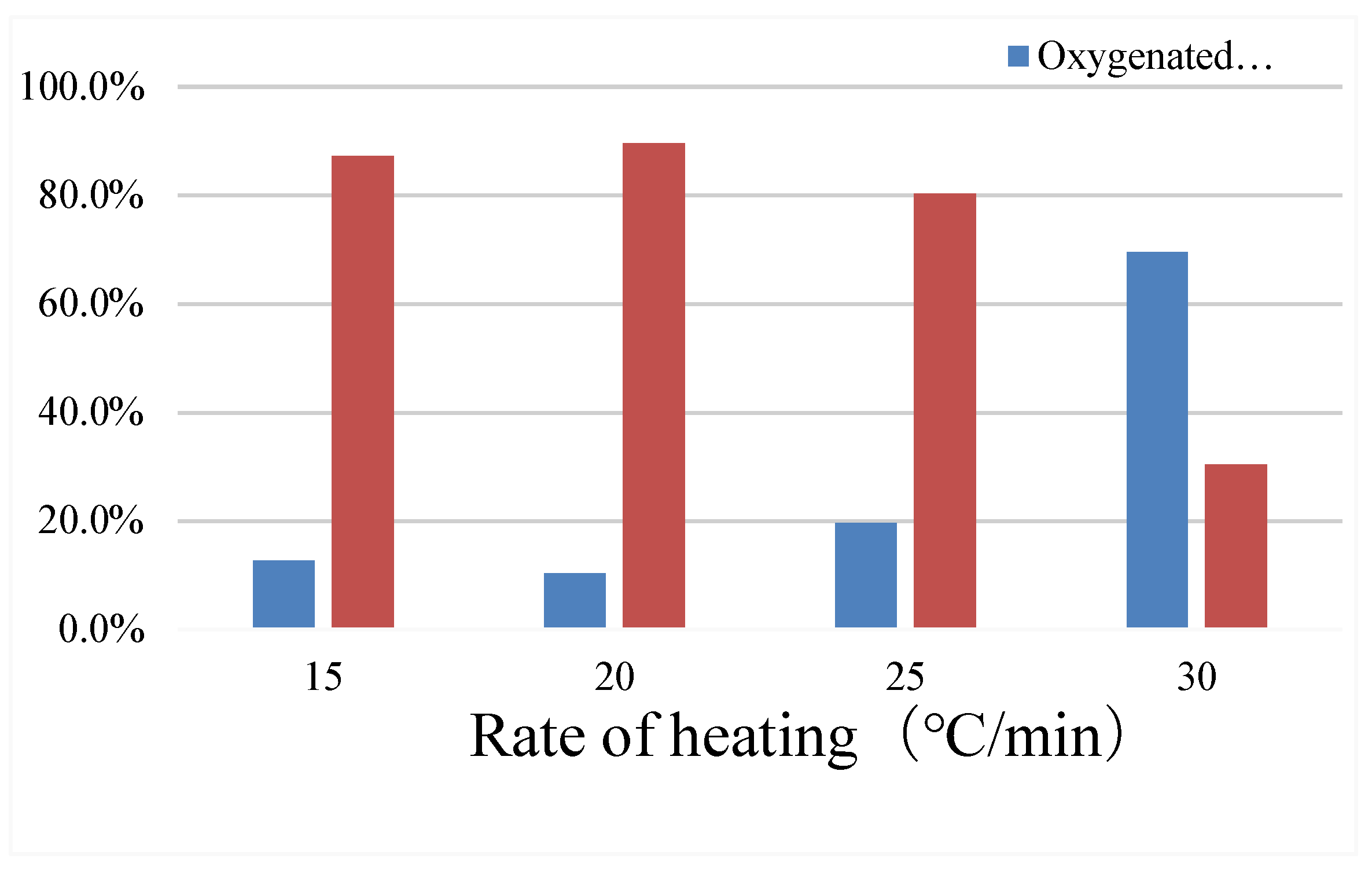
According to
Table 3.2 and
Figure 8, the catalytic cracking oil yield with increasing heating rate reached 11.87% at 25℃/min, and the aquatic rate was 24.68%, while the amount of oxygenated compounds was 19.6%, which was also higher. At 30℃/min, the oil yield reached the lowest 0.09%, the solid yield reached 33.47%, the gas yield reached the largest 41.81%, but the amount of oxygenated compounds in the bio-oil reached the largest 69.6%, and the aromatic compound reached the lowest 30.4%. Too high heating rate will reduce the oil yield and also increase the amount of oxygenated compounds in the bio-oil. The high heating rate reduces the proportion of the pyrolysis oil for further catalytic cracking reaction, that is, the pyrolysis product fails to react adequately with the catalyst during the high temperature pyrolysis process, leading to the high level of the oxygen-containing compound.
4.3. Nitrogen Flow
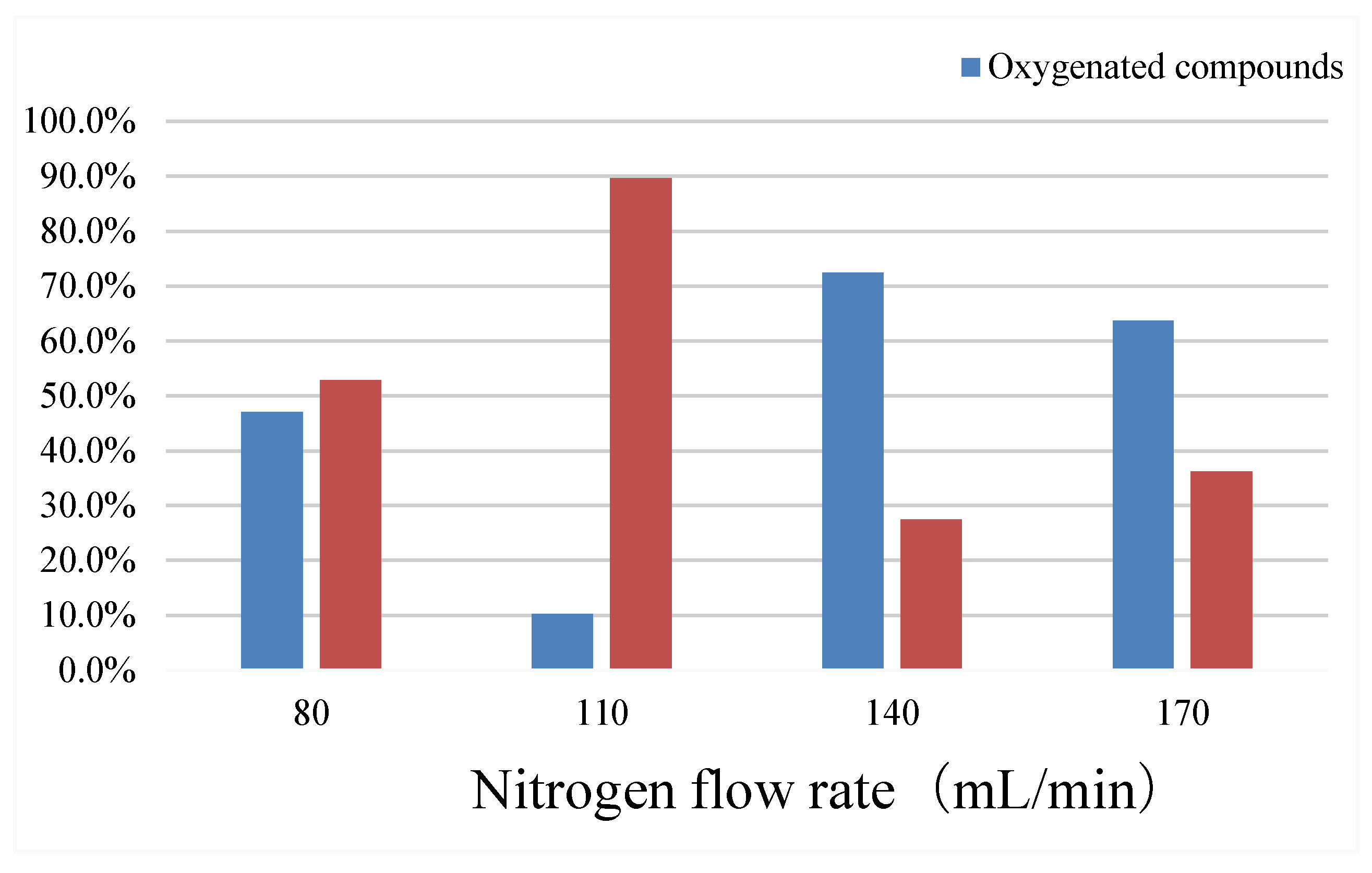
The reaction temperature is 500 °C, the heating rate is 20 °C/min, the reaction time is 15min, catalyst: coconut powder = 1: 1, change the results of nitrogen flow, show in
Table 5 and
Figure 9.
With the increase of nitrogen flow, oil production is the first to fall and then rise, aquatic product rate is the first rise and then fall, the amount of oxygen compounds is the first fall and then rise, and the content of aromatic compounds rises first and then fall. At 110 mL/min, the oil yield was the lowest and the highest aromatic compounds. Therefore, although too high and too low nitrogen flow is conducive to the increase of oil yield, it is not conducive to the catalytic cracking of thermal cracking oil to reduce the amount of oxygen compounds and improve the content of aromatic compounds.
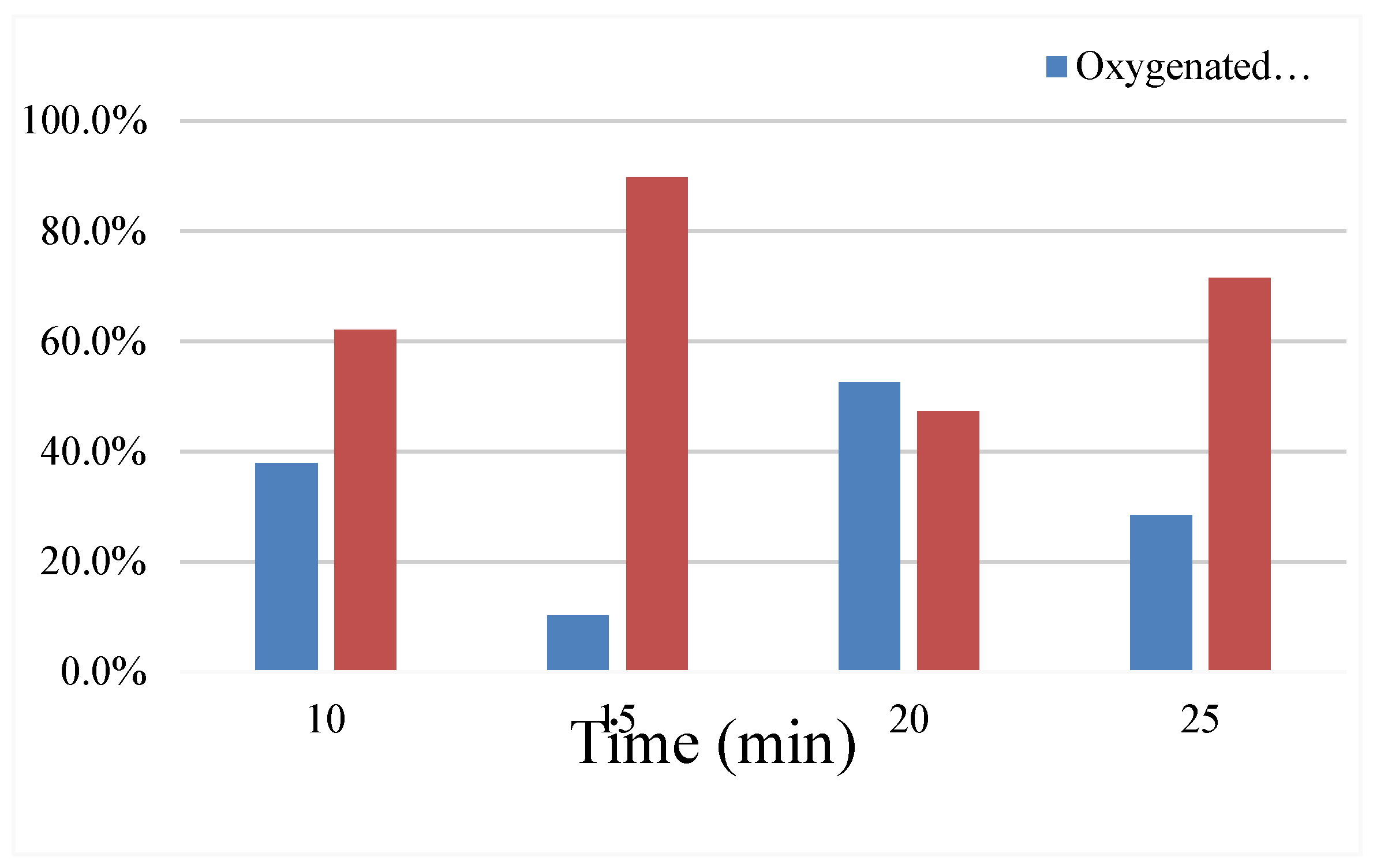
4.4. Reaction Time
The reaction temperature is 500 °C, the temperature heating rate is 20 °C/min, the nitrogen flow rate is 110ml/min, catalyst: coconut powder = 1: 1, change the reaction time results, show in
Table 6 and
Figure 10.
When the reaction time reached 20min, the oil yield and solid yield reached the highest, 14.61% and 34.21%, respectively, and the lowest gas yield was 20.97%. At the reaction time of 25min, the aquatic product rate reached the lowest 26.79%, and the gas yield reached the highest 32.83%. However, the amount of oxygenated compounds at 10min, 20min and 25min were 37.9%, 52.7% and 28.4%, respectively, and the amount of oxygenated compounds was much higher than 10.3% for 15min. It shows that the degree of thermal cracking of coconut clothes also increases with the increase of reaction time. The catalytic cracking of coconut pyrolysis oil has the best effect in the reaction is 15min. The catalytic cracking of the pyrolysis oil to produce aromatic compounds, and the oxygen in oxygenated compounds is mainly removed in the form of water.
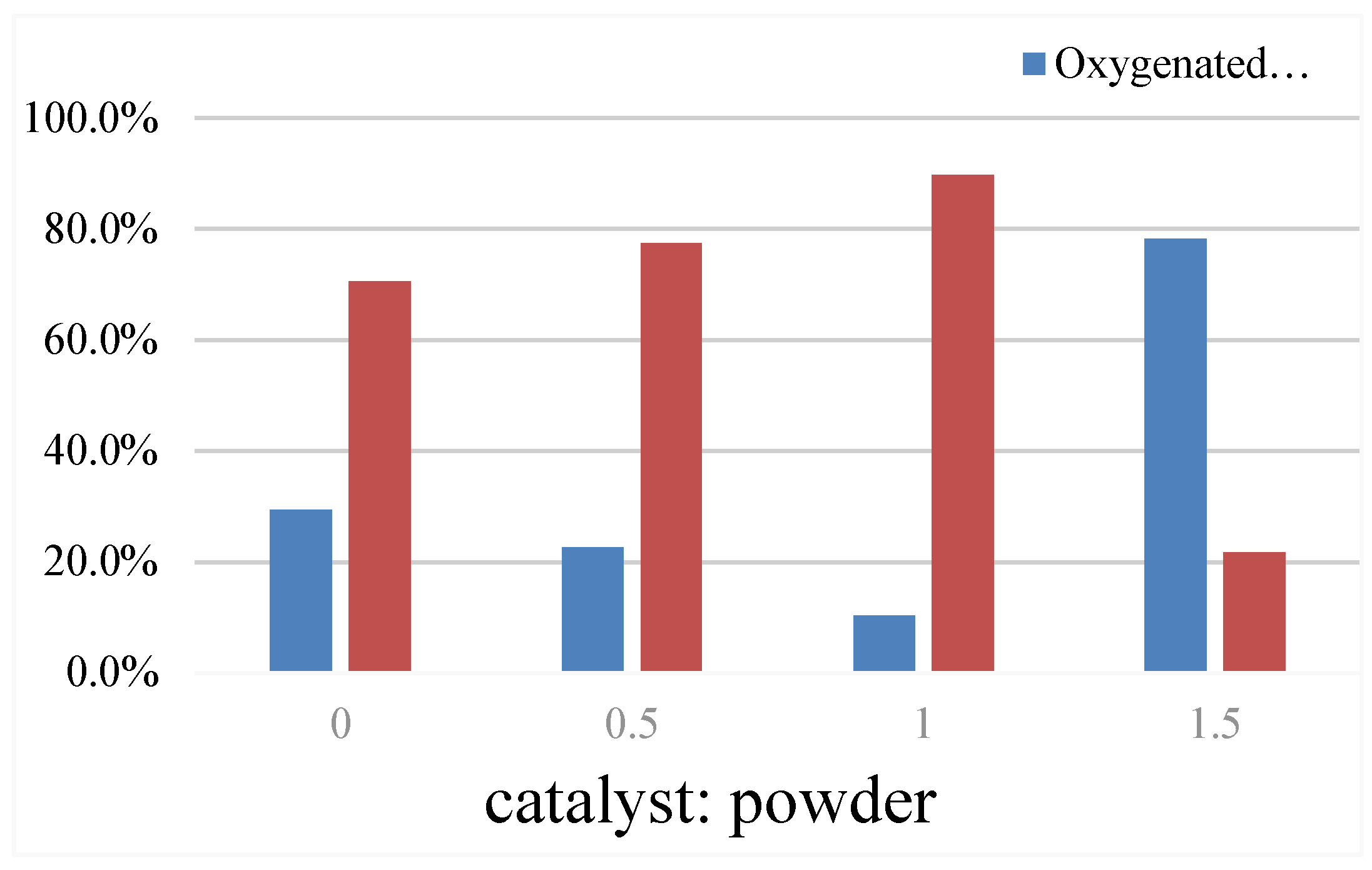
4.5. Mass Ratio of the Catalyst to the Coconut-Coated Powder
The reaction temperature is 500 °C, the reaction time is 15min, the heating rate is 20 °C/min, and the nitrogen flow rate is 110ml/min. The quality ratio of catalyst and coconut powder is shown in
Table 7 and
Figure 11.
With the increase of the catalyst quality ratio, the oil yield showed a trend of increasing after decreasing, and the maximum oil yield reached 10.58% at 0g of the catalyst. The aquatic rate showed a trend of gradual increase, also at the catalyst 0g, the aquatic rate reached the lowest 16.97%. The solid yield reached a maximum of 37.27% at mass ratio of 0.5 and the gas yield of 36.69% at mass ratio 0. The appropriate amount of catalyst can cause the catalytic cracking of the coconut pyrolysis oil, which reduces the oxygenated compounds in the pyrolysis oil, increases the aromatic compounds, and the yield of water, indicating that the oxygenated compounds in the cracking oil produce most water through catalytic cracking, so as to remove oxygen. Although the largest oil yield is obtained without the catalyst, the oxygen content reaches 29.4%. With the addition of the catalyst, the amount of oxygenated compounds gradually decreases, but too much catalyst will increase the amount of oxygenated compounds to the highest 78.3%. Therefore, the high amount of catalyst is not conducive to the catalytic cracking reaction.
4.6. Analysis of the Catalytic Pyrolysis Mechanism
For the catalytic pyrolysis of coconut coat, the reaction temperature was 500℃, and the other process conditions were the same. The results of the cracking oil components obtained from the comparative experiment of coconut coat pyrolysis are shown in
Table 8.
As can be seen from
Table 8, the distribution of coconut oil components is more than catalytic pyrolysis. The pyrolysis components of coconut are mainly phenol, phenanthrene and other polycyclic aromatic hydrocarbons, containing 26.36%, 23.24% and 47.29%, respectively. The catalytic pyrolysis oil components include phenanthrene, metaxylene, 4-hydroxy-4-methyl-2-pentenone, other polycyclic aromatic hydrocarbons and ethylbenzene, containing 41.87%, 14.02%, 10.32%, 31.45% and 2.34%, respectively. The analysis of the components of the experimental cracking oil showed that the high temperature catalytic reaction of phenol, 2-methyl phenol, 2-methyl naphthalene and naphthalene through zeolite of USY catalyst mainly produced phenanthrene and pyrene, followed by interxylene. Therefore, the zeolite of USY catalyst performs the catalytic pyrolysis and condensation reaction of phenolics and naphthalene compounds at high temperature, and the catalytic pyrolysis product is mainly metaxylene
The nitrogen flow rate was 110ml/min, the heating rate was 20°C/min, the reaction time was 15min, the catalyst: coconut-coated powder = 1:1, and the analysis results of the catalytic cracking oil components with changing the reaction temperature were shown in
Table 9.
As can be seen from
Table 9, the temperature had a obvious effect on the components of catalytic pyrolysis oil. With the increase of pyrolysis temperature, the content of naphthalene in catalytic pyrolysis oil increased significantly from 0% to 42.27%, while the content of pyrene gradually decreased. When the temperature reaches 600℃, the amount of tricyclic and tetracyclic aromatic hydrocarbons in ananthrene and pyrene decreases to zero, indicating that tricyclic and tetracrocyclic aromatic hydrocarbons such as ananthrene and pyrene are condensed into solid biochar at high temperature.
5. Conclusions
By analyzing the catalyst characterization, the zeolite of USY contains both SiO2 and Al2O3 components. The specific surface area was up to 792.2876m2/g, the pore capacity was 0.407810cm3/g, and the mean pore diameter was 6.0828nm. The specific surface area of biochar after pyrolysis of coconut-coated powder with catalyst zeolite of USY was up to 290.2271m2/g. The exploration of the influence of different experimental parameters on the catalytic pyrolysis reaction was conducted through single-factor experiments. Although the high and low reaction temperature, nitrogen flow, heating rate and reaction time improved the yield of the bio-oil, the amount of oxygen compounds in the bio-oil was relatively high. The reduction of catalyst quantity is beneficial to improve the oil yield but not conducive to increase the content of aromatic compounds. By observing the optimal reaction temperature of 500℃, nitrogen flow rate of 110 mL/min, heating rate of 20℃/min, reaction time of 15min, and the mass ratio of catalyst to coconut coated powder is 1, the oil yield is 4.97%, aquatic product is 35.80%, solid yield 30.78%, gas yield 28.45%, the amount of oxygen compound is 10.3%, and the aromatic content is 89.7%. The analysis of the catalytic pyrolysis mechanism of coconut coat shows that the catalyst catalyzes the oxygen compounds in the cracking oil at high temperature to produce water and aromatic compounds, thus removing oxygen.
Acknowlegments
This work were supported by the Projects of Technical Innovation of Hainan Scientific Research Institutes (KYYSGY2023-002, SQKY2022-0039).
References
- ISAHAK W N R W, HISHAM M W M, YARMO M A, et al. A review on bio-oil production from biomass by using pyrolysis method. Renewable and Sustainable Energy Reviews, 2012, 16 (8): 5910–5923. [CrossRef]
- XIAO R, YANG W. Influence of temperature on organic structure of biomass pyrolysis products. Renewable Energy, 2013, 50: 136–141. [CrossRef]
- GUEDES R E, LUNA A S, TORRES A R. Operating parameters for bio-oil production in biomass pyrolysis: A review. Journal of Analyticaland Applied Pyrolysis, 2018, 129: 134–149. [CrossRef]
- GUPTA G K, MONDAL M K. Bio-energy generation from sagwan sawdust via pyrolysis: Product distributions, characterizations and optimization using response surface methodology. Energy, 2019, 170: 423–437. [CrossRef]
- Li Y, Li B, Zhang X, Chen L, Zhang Q, Wang T, Ma L (2016) Continuous pyrolysis and catalytic upgrading of corncob hydrolysis residue in the combined system of auger reactor and downstream fixed-bed reactor. Energy Convers Manag 122:1–9. [CrossRef]
- Wang S et al. (2017) CHAPTER 6 characterization and separation of bio-oil, in fast pyrolysis of biomass: advances in science and technology. The Royal Society of Chemistry, p 96–116.
- Sun L, Zhang X, Chen L, Zhao B, Yang S, Xie X (2016) Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J Anal Appl Pyrolysis 121:342–346. [CrossRef]
- Stefanidis SD, Karakoulia SA, Kalogiannis KG, Iliopoulou EF, Delimitis A, Yiannoulakis H, Zampetakis T, Lappas AA, Triantafyllidis KS (2016) Natural magnesium oxide (MgO) Biomass Conv. Bioref.catalysts: a cost-effective sustainable alternative to acid zeolites for the in situ upgrading of biomass fast pyrolysis oil. Appl Catal B Environ 196:155–173. [CrossRef]
Figure 2.
Nitrogen absorption-desorption diagram of the zeolite of USY.
Figure 2.
Nitrogen absorption-desorption diagram of the zeolite of USY.
Figure 3.
SEM characterization of zeolite of USY.
Figure 3.
SEM characterization of zeolite of USY.
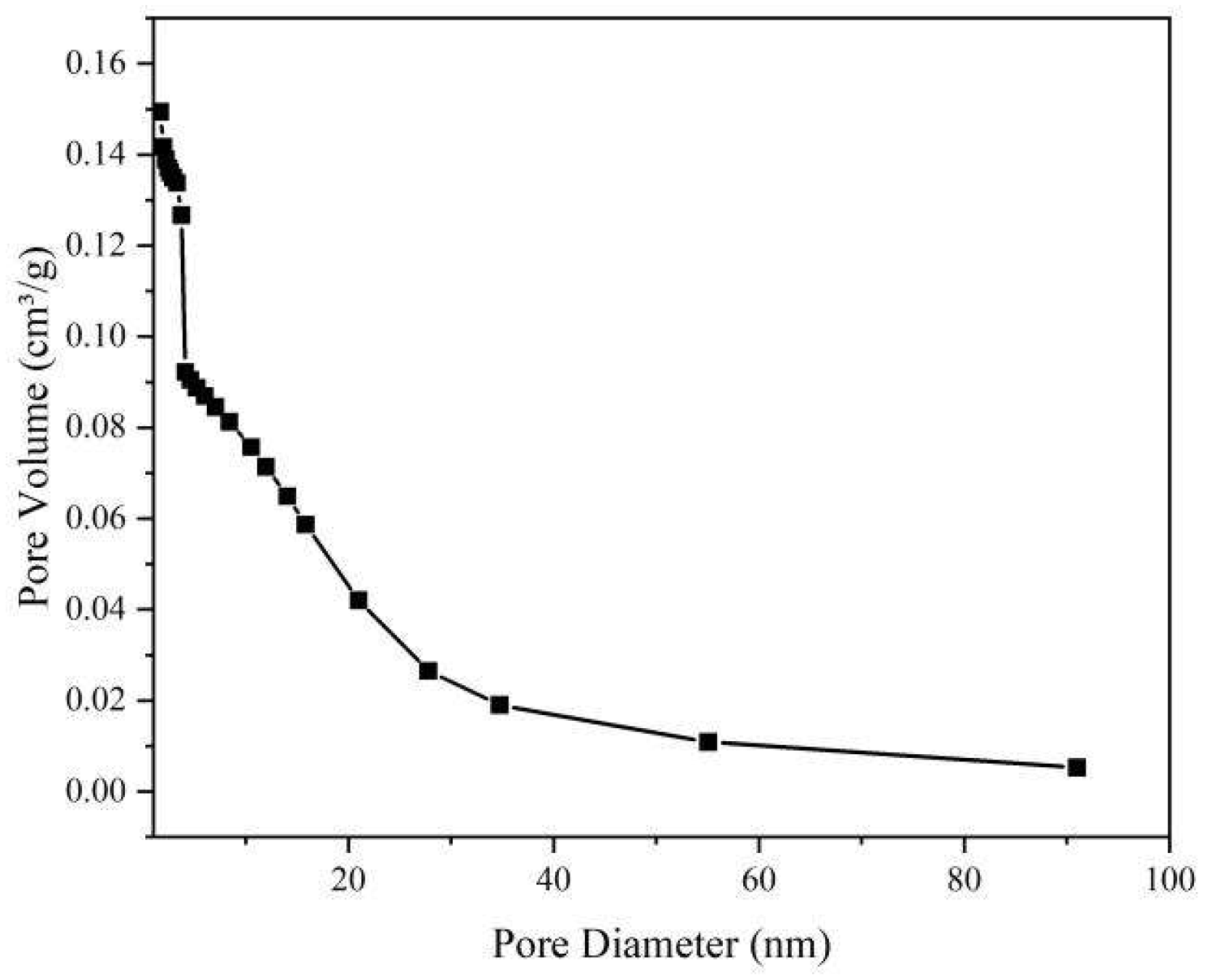
Figure 4.
Porore distribution of catalytic pyrolysis.
Figure 4.
Porore distribution of catalytic pyrolysis.
Figure 5.
Nitrogen absorption-desorption diagram of catalytic pyrolysis biochar.
Figure 5.
Nitrogen absorption-desorption diagram of catalytic pyrolysis biochar.
Figure 6.
The results of the biochemical carbon SEM (including the catalyst zeolite of USY).
Figure 6.
The results of the biochemical carbon SEM (including the catalyst zeolite of USY).
Figure 7.
Composition of the bio-oil at different temperatures.
Figure 7.
Composition of the bio-oil at different temperatures.
Figure 8.
The composition of bio-oil at different heating rate.
Figure 8.
The composition of bio-oil at different heating rate.
Figure 9.
Composition of the bio-oil at different nitrogen flow rates.
Figure 9.
Composition of the bio-oil at different nitrogen flow rates.
Figure 10.
The composition of bio-oil in different reactions time.
Figure 10.
The composition of bio-oil in different reactions time.
Figure 11.
Composition of the bio-oil under the different quality ratio.
Figure 11.
Composition of the bio-oil under the different quality ratio.
Table 1.
The zeolite of USY specific surface area, pore diameter and hole volume.
Table 1.
The zeolite of USY specific surface area, pore diameter and hole volume.
| Catalyst |
SBET (m2/g) |
VBJH (cm3/g) |
DBJH (nm) |
| USY |
792.2876 |
0.407810 |
6.0828 |
Table 2.
Biochar specific surface area and pore diameter, hole volume.
Table 2.
Biochar specific surface area and pore diameter, hole volume.
| Catalyst |
SBET (m2/g) |
VBJH (cm3/g) |
DBJH (nm) |
| Biochar |
290.2271 |
0.168938 |
7.8690 |
Table 3.
Yield and composition of the products at different temperatures.
Table 3.
Yield and composition of the products at different temperatures.
| Number |
Reaction Temperature (℃) |
Oil (%) |
Water (%) |
Solids (%) |
Gas (%) |
Oxygenated Compounds (%) |
Aromatic Compounds (%) |
| 1 |
450 |
7.94 |
24.03 |
29.37 |
38.67 |
14.6 |
85.5 |
| 2 |
500 |
4.97 |
35.80 |
30.78 |
28.45 |
10.3 |
89.7 |
| 3 |
550 |
5.35 |
25.41 |
26.90 |
42.34 |
13.9 |
86.1 |
| 4 |
600 |
9.27 |
24.69 |
31.48 |
34.56 |
17.8 |
82.2 |
Table 4.
Yield and composition of products at different heating rates.
Table 4.
Yield and composition of products at different heating rates.
| Number |
Heating Rate (℃/min) |
Oil (%) |
Water (%) |
Solids (%) |
Gas (%) |
Oxygenated Compounds (%) |
Aromatic Compounds (%) |
| 1 |
15 |
9.07 |
26.70 |
24.68 |
39.55 |
12.6 |
87.4 |
| 2 |
20 |
4.97 |
35.80 |
30.78 |
28.45 |
10.3 |
89.7 |
| 3 |
25 |
11.87 |
24.68 |
32.48 |
30.97 |
19.6 |
80.4 |
| 4 |
30 |
0.09 |
24.63 |
33.47 |
41.81 |
69.6 |
30.4 |
Table 5.
Yield and composition of products under different nitrogen flow rates.
Table 5.
Yield and composition of products under different nitrogen flow rates.
| Number |
Nitrogen Flow Rate (mL/min) |
Oil (%) |
Water (%) |
Solids (%) |
Gas (%) |
Oxygenated Compounds (%) |
Aromatic Compounds (%) |
| 1 |
80 |
12.86 |
25.10 |
32.34 |
29.70 |
47.1 |
52.9 |
| 2 |
110 |
4.97 |
35.80 |
30.78 |
28.45 |
10.3 |
89.7 |
| 3 |
140 |
12.36 |
24.59 |
32.93 |
30.11 |
72.5 |
27.5 |
| 4 |
170 |
11.33 |
26.49 |
33.06 |
29.12 |
63.7 |
36.3 |
Table 6.
The yield and composition of products at different reaction times.
Table 6.
The yield and composition of products at different reaction times.
| Number |
Reaction Time (min) |
Oil (%) |
water (%) |
Solids (%) |
Gas (%) |
Oxygenated Compounds (%) |
Aromatic Compounds (%) |
| 1 |
10 |
8.22 |
32.92 |
32.46 |
26.41 |
37.9 |
62.1 |
| 2 |
15 |
4.97 |
35.80 |
30.78 |
28.45 |
10.3 |
89.7 |
| 3 |
20 |
14.61 |
30.22 |
34.21 |
20.97 |
52.7 |
47.3 |
| 4 |
25 |
10.47 |
26.79 |
30.01 |
32.73 |
28.4 |
71.6 |
Table 7.
Yield and composition of products under different quality ratio.
Table 7.
Yield and composition of products under different quality ratio.
| Number |
Catalyst: Powder |
Oil (%) |
Water (%) |
Solids (%) |
Gas (%) |
Oxygenated Compounds (%) |
Aromatic Compounds (%) |
| 1 |
0 |
10.58 |
16.97 |
35.75 |
36.69 |
29.4 |
70.6 |
| 2 |
0.50 |
10.49 |
20.65 |
37.27 |
31.59 |
22.7 |
77.3 |
| 3 |
1.00 |
4.97 |
35.80 |
30.78 |
28.45 |
10.3 |
89.7 |
| 4 |
1.5 |
5.89 |
38.33 |
26.79 |
28.99 |
78.3 |
21.7 |
Table 8.
Analysis of the comparative experimental pyrolysis oil components.
Table 8.
Analysis of the comparative experimental pyrolysis oil components.
| Component |
Pyrolysis |
Catalytic Pyrolysis |
| 4-Hydroxy-4-methyl-2-pentanone |
0.00 |
10.32 |
| ethylbenzene |
0.33 |
2.34 |
| m-xylene |
1.82 |
14.02 |
| paraxylene |
0.37 |
0.00 |
| phenol |
26.36 |
0.00 |
| 2-Methamphetamine |
0.59 |
0.00 |
| naphthalene |
12.25 |
7.16 |
| 2-Methylnaphthalene |
1.25 |
0.00 |
| biphenyl |
2.48 |
0.00 |
| acenaphthene |
10.6 |
10.32 |
| diphenylene-oxide |
2.7 |
0.00 |
| fluorene |
3.48 |
0.00 |
| Phenanthrene |
23.24 |
41.87 |
| fluoranthene |
6.75 |
0.00 |
| pyrene |
1.1 |
13.97 |
| 1-Phenylnaphthalene |
1.56 |
0.00 |
| 4H-Cyclopenta[def]phenanthrene |
5.12 |
0.00 |
Table 9.
Analysis of the catalytic cracking oil components at different temperatures.
Table 9.
Analysis of the catalytic cracking oil components at different temperatures.
| Component |
450℃ |
500℃ |
550℃ |
600℃ |
| The 4-hydroxy-4-methyl-2-pentanone |
14.55 |
10.32 |
0.00 |
17.8 |
| diacetone alcohol |
0.00 |
0.00 |
13.92 |
0.00 |
| ethylbenzene |
4.55 |
2.34 |
2.64 |
5.8 |
| m-xylene |
20.34 |
14.02 |
13.74 |
34.13 |
| p-xylene |
6.56 |
0.00 |
0.00 |
0.00 |
| naphthalene |
0.00 |
7.16 |
17.27 |
42.27 |
| acenaphthene |
15.49 |
10.32 |
16.36 |
0.00 |
| phenanthrene |
24.29 |
41.87 |
21.38 |
0.00 |
| fluoranthene |
0.00 |
0.00 |
4.15 |
0.00 |
| pyrene |
14.22 |
13.97 |
10.53 |
0.00 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).