1. Introduction
Multiple myeloma is a hematological malignancy of BM-resident clonal PCs characterized by uncontrolled cell growth and overproduction of nonfunctional Igs. Multiple myeloma belongs to the plasma cell disorders group, including MGUS, smoldering myeloma, solitary bone plasmacytoma, solitary extraosseous plasmacyoma and Amyloidosis [1,2]. The etiology of MM has been associated with exposure to several factors such as chemical substances, toxins, and ionizing radiation; also, with the emergence of recurrent genetic alterations such as acquired mutations in growth promoter or suppressor genes, and genes involved in cell metabolism and development, and with chromosomal translocations and DNA hyperdiploidy [3,4].
Multiple myeloma is the second most frequent hematologic malignancy after non-Hodgkin's lymphoma and mainly affects the population over 65 years of age. For 2020, the worldwide incidence and mortality rates for MM were respectively 1.8 and 1.1 per 100,000 population, according to Globocan; a higher incidence rate was observed in men compared to women [5]. Patients affected by MM present the “CRAB criteria”, which refer to hypercalcemia (C), renal failure (R), anemia (A), and bone disease (B). Additionally, patients experience fatigue or dyspnea related to anemia, persistent bone pain due to the bone compromise, recurrent infections by different agents (virus and bacteria) resulting from the synthesis of non-functional immunoglobulins (Igs), radiculopathies, altered mental status associated with hypercalcemia, and the displacement of the BM normal resident cells by the MPCs [3,6,7].
A defining clinical feature of MM is the development of osteolytic bone disease (OBD), resultant from an altered bone homeostasis. This condition causes high activation and differentiation of osteoclasts and marked inhibition of the development and function of osteoblasts and osteocytes, ensuing a high rate of bone resorption and low osteogenesis. Consequently, osteolytic lesions form, even in patients in complete remission, affecting mainly the vertebrae and proximal long bones. At diagnosis, about 85% of patients with MM show some degree of bone involvement, which may correlate with tumor burden and prognosis of the disease [8]. Another defining clinical feature of MM is the hyperviscosity syndrome, related to the elevated synthesis and secretion of proteins, particularly Igs, by the MPCs. These Igs accumulate in the distal segment of nephrons, where they induce the formation of casts that block the glomerular pathway and trigger renal failure. In addition, the high levels of circulating proteins slow down blood flow, impeding the adequate microcirculation and generating tissue hypoxia. From the clinical point of view, the hyperviscosity syndrome also translates into different neurological alterations such as headache, dizziness, ataxia, and seizures; ophthalmological issues such as visual alterations and retinal exudates; and cardiovascular system/stromal problems that generate heart failure by the expansion of the plasma volume together with the tissular hypoxia [9].
The diagnosis of MM according to the criteria of the is based on a clonal bone marrow PCs count greater than or equal to 10% and the demonstration of an extramedullary plasmacytoma identified by biopsy, among other variables [2,10] (
Table 1). Additionally, the diagnosis of MM includes the detection of genetic alterations such as trisomies, del (17p), and various chromosomal translocations involving chromosome 14, where the gene encoding the Ig heavy chain (Cr 14q32) is located (18,19). Throughout the disease, additional cytogenetic changes that arise secondary to the development of MM, such as loss of p53 (del17p), RAS gene mutations, and MYC gene-associated translocations, can be detected. Such genetic alterations are also crucial for MM staging and the prognosis of the disease [11,12] (
Table 2)
2. Role of MSCs Cells in the Development, Maintenance, and Progression of Multiple Mye-Loma
MSCs are non-hematopoietic multipotent cells derived from the embryonic mesoderm (lateral and paraxial mesoderm) and ectoderm (neural crest) [14]. MSCs can differentiate into cells of mesodermal (osteocytes, adipocytes, and chondrocytes), ectodermal (neurocytes), and endodermal (hepatocytes) lineages. MSCs were first isolated from BM and, so far, have been obtained from adipose tissue, endometrium, amniotic and syno-vial fluids, and umbilical cord, supporting the hypothesis that MSCs can regulate hem-atopoiesis, the immune system and some events associated with tissue regeneration. MSCs are identified in vitro as adherent cells that express surface markers such as CD73, CD90, and CD105 in the absence of hematopoietic antigens (CD14, CD34, CD45, and HLA-DR); they differentiate in vitro into cell populations such as chondrocytes, osteocytes, and adipocytes [15]. This phenotypic characterization of the human bone mar-row MSCs was recently revised and modified by the ISO International Standard 24651 [16]. MSCs respond to signals from the microenvironment through Toll-like receptors (TLRs). Activation through TLR4 promotes a pro-inflammatory phenotype (MSC1) while via TLR3 induces an immunosuppressive phenotype (MSC2); in this way, innate and adaptive immunity are modulated [17]. MSCs can move towards sites of inflammation, tissue damage, and tumor microenvironments, by a homing process like migrating leukocytes to sites of inflammation.
Table 1.
Diagnosis of MM according to the criteria of the International Myeloma Working Group.
Table 1.
Diagnosis of MM according to the criteria of the International Myeloma Working Group.
| Criteria |
Definition / Comment |
| Clonal bone marrow PCs ≥ 10%, or biopsy-proven bony or extramedullary plasmacytoma |
The percentage of PCs in the BM will ideally be calculated in a core biopsy specimen. Plasma cells’ clonality is established by demonstrating Kappa/Lambda light chain restriction by flow cytometry, immunohistochemistry, or immunofluorescence. |
| One or more defining events of Multiple Myeloma (MM) |
Myeloma-defining events: Evidence of end-organ damage caused by the plasma cell proliferative disorder, specifically:
- ○
Hypercalcemia: serum calcium > 0.25 mmol/L (> 1 mg/dL) higher than the upper limit of normal or > 2.75 mmol/L (> 11 mg/dL). - ○
Renal insufficiency: creatinine clearance < 40 mL/min or serum creatinine > 177mol/L (> 2 mg/dL). - ○
Anemia: hemoglobin value of > 2 g/dL below the lowest limit of normal, or a hemoglobin value < 10g/dL - ○
Bone lesions: one or more osteolytic lesion on skeletal radiography, CT, or PET/CT.
Biomarkers of malignancy
- ○
60% or greater clonal plasma cells on bone marrow examination - ○
Serum involved / uninvolved free light chain ratio of 100 or greater, provided the absolute level of the involved light chain is at least 100 mg/L - ○
More than one focal lesion on MRI that is at least 5 mm or greater in size (17,18).
|
Table 2.
Genetic Alterations Associated with Groups Risk of MM.
Table 2.
Genetic Alterations Associated with Groups Risk of MM.
| Risk |
Genetic Alteration |
Gen / Chromosome involved |
Patients with MM (%) |
| Standard Risk |
Trisomies |
Chromosomes 3, 5, 7, 9, 11, 15, and 19 |
42% |
| t(11;14) |
CCND1 |
14 – 15% |
| t(6;14) |
CCND3 |
5% |
| High Risk |
t(4;14)* |
FGFR3 and MMSET
|
12 – 15% |
| t(14;16) |
c-MAF |
3 – 4% |
| t(14;20) |
MAFB |
< 1.5% |
| Trisomy 21 |
Chromosome 21 |
– |
| Gain (1q21) |
CKS1B |
17 – 33% |
| del(17p)** |
TP53 |
6.6 – 11% |
Homing begins with the recruitment and activation of MSCs by complement proteins (C1q, C3α and C5α) and inflammatory cytokines that induce the expression of adhesion molecules such as VLA-4; this molecule binds to VCAM-1 present on endothelial cells, promoting the sequestration of MSCs in the vas-cular endothelium. Subsequently, growth factors released by the affected tissue, such as SDF-1, HGF, and MIP-1, bind to their receptors on MSCs, triggering the intracellular ac-tivation of various signaling cascades [18].
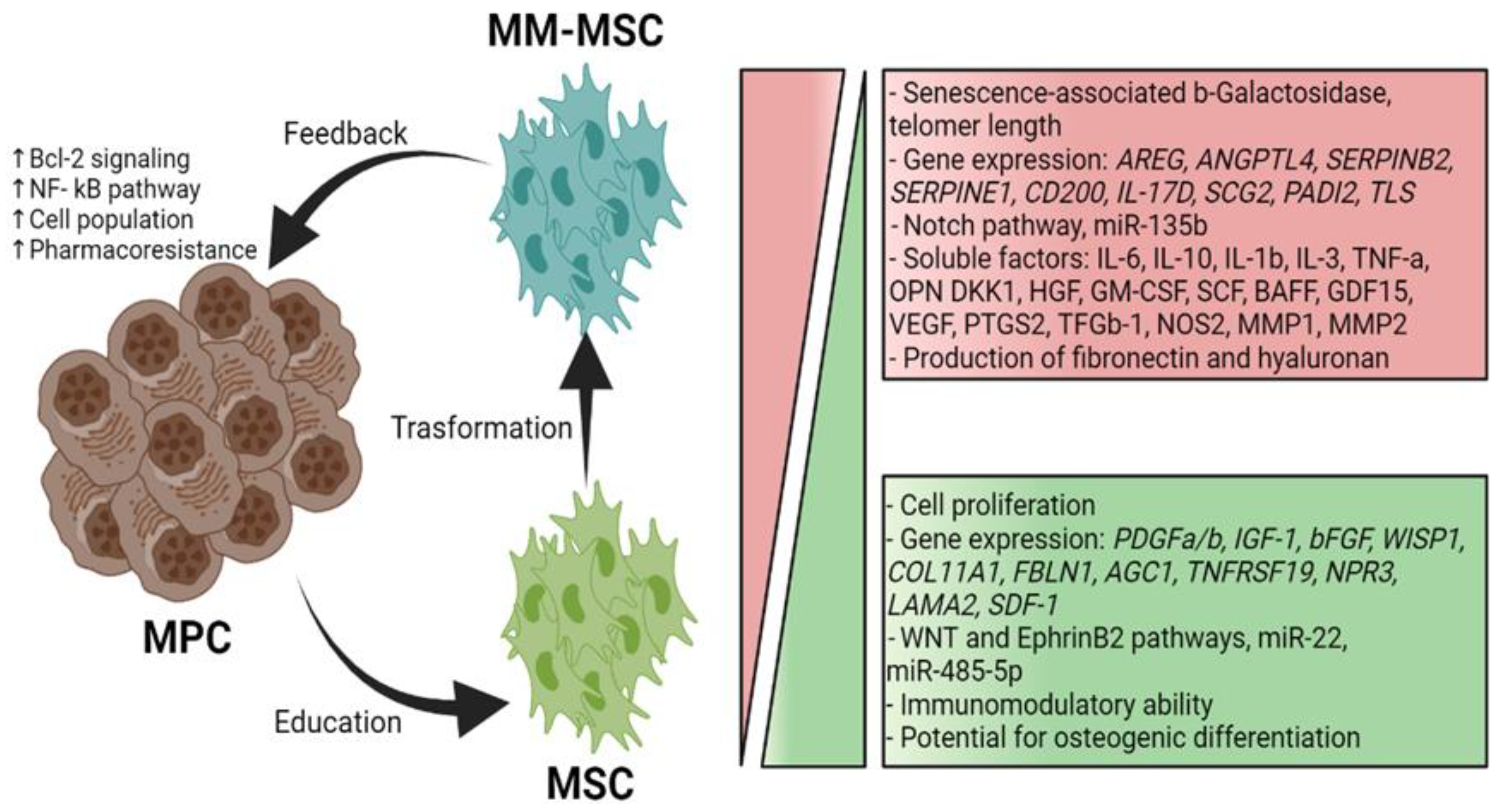
MSCs residing in the MM microenvironment with MPCs or MM-associated MSCs (MM-MSCs) exhibit specific characteristics that contribute to the growth, survival, and chemoresistance of MPCs. A bidirectional communication exists between the tumor cells and MSCs through soluble factors and adhesion molecules [19]. Several studies have shown that approximately 145 genes are expressed to a greater extent in MM-MSCs than in normal MSCs; these genes induce increased synthesis of IL-1β, GDF-15, IL-6 and DKK1, promoting MM development [20]. The MM-MSCs also overexpress adhesion molecules such as ICAM-1 and VCAM-1 that facilitate their interaction with the MPCs and exhibit increased expression of fibronectin and type IV collagen that, upon interacting with MPCs, trigger the activation of the NF-κB pathway. This pathway confers pharmacoresistance to the tumor cells and positively regulates the transcription of genes involved in cell proliferation, angiogenesis, metastasis, inflammation, and suppression of apoptosis (
Figure 1) [21]. Moreover, the MSCs participate in the MM microenvironment by secreting soluble factors such as IL-1β, IL-3, IL-6, IL-10, DKK1, VEGF, BAFF, GM-CSF, TNF-α, HGF, and SCF; these factors influence tumor survival, growth, and immunomodulation, and contribute to the development of the pathological lesions characteristic of MM [19].
The high levels of IL-6 secreted by the MM-MSCs are consistent with studies showing this phenomenon is induced by the MPCs themselves. In addition, MM-MSCs can secrete MIP-1α, which significantly inhibits osteoblast functionality and promotes tumor survival; these events contribute to bone disease progression and MM development. Interestingly, MPCs express higher levels of CXCR4 in the presence of SDF-1-secreting MM-MSCs; thus, these cells attract MPCs and promote their adhesion to the BM [19,22]. MM-MSCs also secrete exosomes with a high content of miR-146a, a miRNA associated with increased activity of the NF-κB, NOTCH, JAK/STAT, and MAPK pathways in MPCs, as well as the secretion of high levels of IL-6, IL-8, IL-1β, TNF-α, CXCL1, IP-10, and CCL5. These factors enhance distant migration of tumor cells [23] (
Figure 1).
3. MSCs and Their Role on the Development of Osteolytic Bone Disease and Distant Migration of Malignant Plasma Cells in Multiple Myeloma
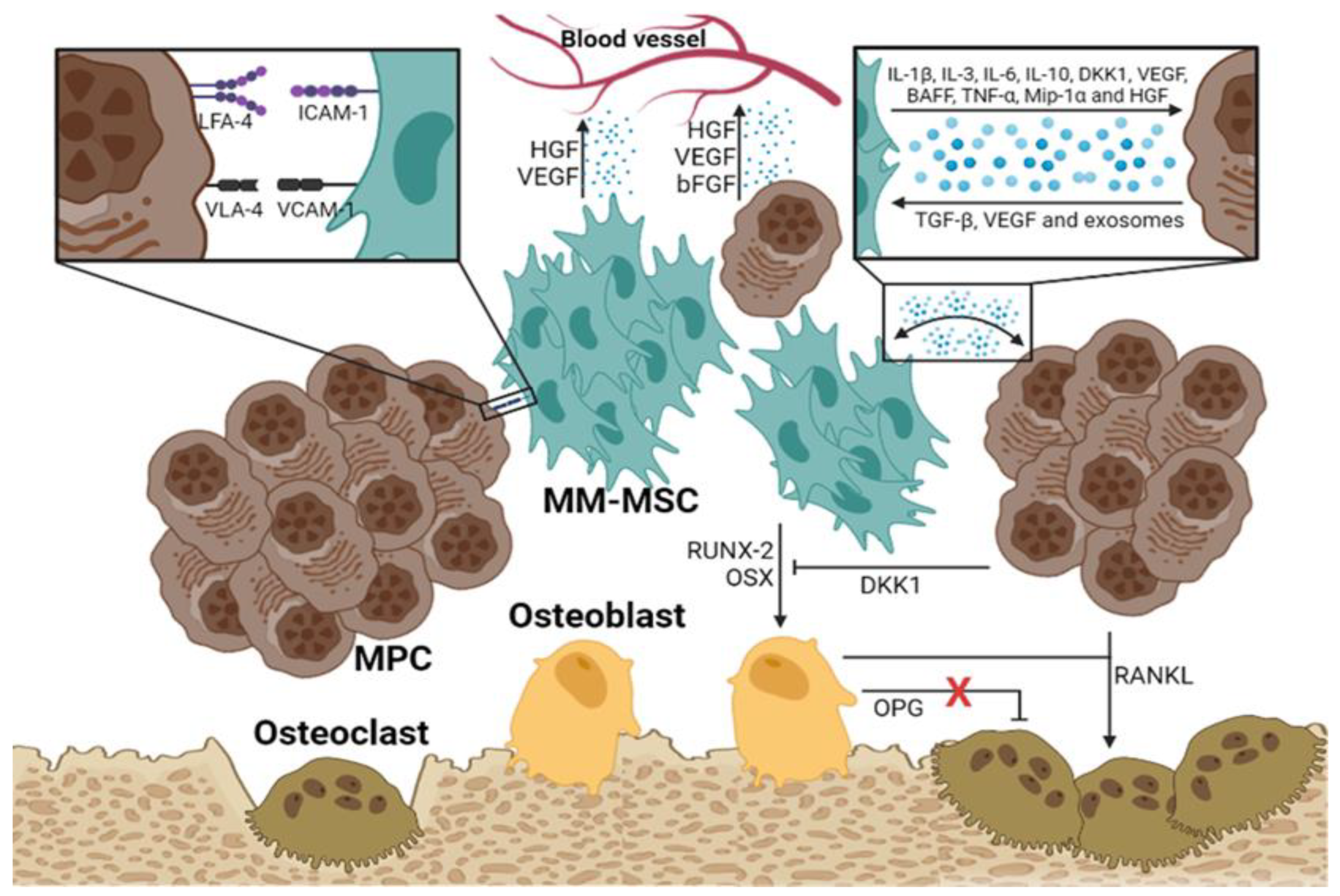
Several mechanisms have been described linking MM-MSCs to the development of osteolytic bone disease in patients with MM. Several studies indicate that the Notch signaling pathway is very active in MM-MSCs, where it downregulates the expression of RUNX2, a critical factor for their osteogenic differentiation [25,26]. In addition, MM-MSCs exhibit an abnormal expression of some molecules, such as miR-135b, which correlates inversely with bone alkaline phosphatase activity [27]. PPARγ2 mediates another mechanism associated with the development of osteolytic lesions; this transcription factor is involved in the regulation of fatty acid storage, glucose metabolism, and activation of adipogenesis and is essential in the suppression of osteoblastogenesis, highly active in MM-MSCs [28]. Finally, HGF secreted by the MM-MSCs and osteoclasts is another relevant factor in the progression of the osteolytic bone disease. HGF interacts with its receptor on both osteoblasts and osteoclasts. In osteoblasts, HGF prevents the transcription of RUNX-2 and OSX, disrupting their differentiation; however, it also induces the secretion of IL-11, which promotes osteoclast activation (
Figure 2).
As MPCs migrate distantly, they remodel the new microenvironment and convert it into a space favorable for their proliferation and survival. It has been proposed that MM-MSCs actively participate in the distant migration of MPCs by regulating GAP junctions which are made up of molecules such as Cx43 [29]. The MM-MSCs express a higher level of Cx43 than normal MSCs; this feature enhances the migration of MPCs in vitro. Another mechanism involved in the distant migration of neoplastic cells is the secretion of matrix metalloproteinase-9 (MMP-9) by MM-MSCs, which facilitates the mobilization of MPCs into the extracellular matrix. Rethnam M et al. demonstrated that normal BM-MSCs secrete elevated levels of TIMP-1 (MMP-9 inhibitor); however, in the presence of MPCs, the TIMP-1 levels decrease, and the production of MMP-9 by MSCs is favored [30].
4. Biological Features of RPs and Their Role in the Bone Marrow Microenvironment in MM
Platelets recently released from BM megakaryocytes have an elevated mRNA content and are termed young, immature or reticulated platelets (RPs). These platelets are the youngest, most reactive, and largest circulating population, and have an average lifespan of 24 to 36 hours, which allows them to be identified as markers of early megakaryopoiesis. RPs undergo progressive RNA degradation resulting in a decreased mean platelet volume until they finally transform into mature platelets [31]. Studies of platelet metabolism have shown that RPs have higher thrombotic activity than mature platelets, owing to an elevated intracellular content of ADP, PF4, ATP, AMP, TxB2, and glycogen, and increased uptake and release of serotonin. Compared to mature platelets, RPs express higher levels of membrane glycoproteins and integrins (GPIb, GPIIb/IIIa, GPIV, and VLA-6) and adhesion molecules (P-selectin) that improve their adhesion to components of their environment [32]. In this regard, Lador A et al. observed a higher expression of platelet activation markers (P-selectin and annexin-V) in RPs than in mature platelets. Additionally, methionine and leucine incorporation studies have shown that RPs translate their RNA into proteins [33].
The tumor microenvironment promotes different types of interactions between platelets and other cell populations through homotypic and heterotypic adhesions. Platelet-tumor cell aggregates (heterotypic adhesions) are mediated by integrin-like adhesion molecules that bind to several proteins (fibrinogen, fibrin, fibronectin, vitronectin, thrombospondin), selectins (e.g., P-selectin), Ig superfamily proteins (e.g., PECAM-1), and leucine-rich glycoproteins (e.g., GPIb/V/IX) [34]. In the particular case of MM, heterotypic adhesion between platelets and MPCs is mediated by P-selectin, PECAM-1, and CD162; MPCs had previously been shown to express CD38, a ligand for PECAM-1 present on the cell surface of platelets. These heterotypic junctions also induce the release of mediators (ATP, histamine, serotonin) that regulate blood vessel permeability, as well as eicosanoid metabolites (TxA2 and 12-HETE) which promote endothelial cell retraction to expose the basement membrane, facilitating tumor cell extravasation. Additionally, platelets can release the MMPs 1, 2, and 9 that participate in the degradation of the extracellular matrix and facilitate tumor invasion [34,35].
Schumacher D et al. demonstrated that upon activation by tumor cells, platelets activate, secrete their granules, and release ATP, which acts on P2Y2 receptors of endothelial cells, easing the access of tumor cells to the underlying basement membrane and enhancing their distant migration [36]. Additionally, Ward Y et al. demonstrated that platelet activation triggers granule secretion through CD97; this molecule also participates in the proximal CD97-LPAR heterodimer signaling cascade mediated by LPA, that increases tumor invasiveness [37].
In patients with MM, there is a positive correlation between the Ig levels and the percentage of RPs; this correlation is associated with increased platelet destruction that promotes thrombopoiesis and results in an elevated circulating platelet count. Notably, despite the lack of scientific evidence on the specific role of RPs in the pathophysiology of MM, the available information suggests the existence of chronic platelet activation and platelet dysfunction resulting from "platelet exhaustion" [38]. Thus, mature platelets become cells of minor importance in the pathophysiology of MM. In contrast, RPs, which have not been chronically activated by the tumor cells and do not exhibit the "platelet exhaustion" phenotype, could participate more actively in the pathogenesis of this hematologic malignancy. Malignant plasma cells could exacerbate the activation of RPs through molecules such as ADP, TxA2, HMGB1, and thrombin, consequently increasing their surface expression of GPs and P-selectin; these molecules would bind to MPCs favoring their survival in the bloodstream. Moreover, the interaction of RPs with MPCs may trigger the degranulation of the former and the secretion of large amounts of ATP, which favors the migration of tumor cells by the association between platelets and endothelial cells, as previously mentioned in the study of Schumacher D et al [36].
In contrast to mature platelets, RPs have a high RNA content; this feature has been associated with increased platelet reactivity, adhesion, aggregation, and secretion of granules. Gidlöf O et al. demonstrated in vitro that the platelet miRNA content decreases after activation but rises significantly in culture supernatants; from there, endothelial cells can take it up. Thus, platelets with high RNA content can regulate gene expression of other cell populations through vertical transfer, an extracellular vesicle-dependent mechanism [39].
5. MSCs and RPs as Prognostic Value Variables in MM
MSCs have been observed to interact with cancer cells at various stages of cancer progression, influencing their invasive behavior and promoting metastasis formation. In this regard, four main mechanisms have been described [40–42]: (i) Induction of a pro-metastatic state: MSCs have the ability to modify the tumor microenvironment and direct tumor cells towards an invasive pro-metastatic state. It has been observed that MSCs promote the migration, invasion and extravasation of tumor cells, thus facilitating their spread to other organs, possibly through the promotion of epithelial-mesenchymal transition (EMT) or through the induction of phenotypic changes in tumor cells that are associated with modifications in the profile of adhesion molecules, among others. (ii) Interaction with the tumor stroma: MSCs can migrate toward the stroma of primary tumors and also toward metastatic sites, promoting the generation of cancer-associated fibroblasts (CAFs), which have been shown to actively participate in metastatic processes. (iii) Influence on the formation of metastatic niches: It has been suggested that MSCs may participate in the creation and maintenance of metastatic niches, which are favorable microenvironments for the survival and growth of metastatic tumor cells. These niches provide conditions conducive to the colonization of cancer cells at distant sites. Kaplan et al, have shown that bone marrow stromal cells expressing VEGFR1+ migrants form pre-metastatic complexes before the arrival of tumor cells. (iv) Interaction with cells of the immune system: MSCs have the ability to modulate the immune response, which may influence the ability of the immune system to detect and eliminate metastatic tumor cells. In addition, MSCs can suppress the antitumor immune response, facilitating the immune evasion of cancer cells and their ability to form metastases.
Although the association between MSCs and multiple myeloma has been demonstrated [43], the evaluation of MSCs as a possible variable of prognostic value for metastasis is not used in clinical practice. Interestingly, Van der Velden et al have shown that in different types of cancer such as sarcoma, colon and prostate cancer, the number of endogenous mesenchymal stem cells in peripheral blood is increased; which demonstrates that the evaluation of these cells in cancer could have an important clinical impact [44]. Particularly in MM, it would be very useful to evaluate MSCs in peripheral blood and/or bone marrow, possibly due to their association with metastasis, but also due to MM-MSC dysfunction and its association with the appearance of lytic lesions and disease progression.
Regarding the role of platelets as a variable of prognostic value in MM, in clinical practice only their quantification is considered useful [45,46]; however, other aspects such as determination of reticulated platelets or their activation are not currently evaluated. Previous studies have shown that platelets may participate in MM pathophysiology through four mechanisms [33,36–38]: i) Markers of Active Thrombopoiesis: Reticulated platelets are younger and more active than mature platelets, reflecting accelerated thrombopoiesis. In MM, where hematopoiesis is often disrupted, elevated levels of reticulated platelets may indicate active platelet production in response to thrombocytopenia or platelet destruction caused by the disease. ii) Role in the Tumor Microenvironment: Reticulated platelets have been implicated in promoting angiogenesis and tumor growth, contributing to the microenvironment that supports MM progression. These platelets may help facilitate tumor cell extravasation and modify the bone microenvironment, which is significantly affected in MM. iii) Indicator of Platelet Activation: Since reticulated platelets are more reactive, elevated levels of these cells may correlate with heightened platelet activation, which has been observed in MM patients. Platelet activation is linked to a higher risk of thrombotic complications, adding to the disease burden in MM. iv) Prognostic Implications: Increased levels of reticulated platelets in MM may reflect more active disease and a worse prognosis. Monitoring reticulated platelet counts could provide a useful tool for tracking disease progression and treatment response in clinical practice.
6. Conclusions
In conclusion, both mesenchymal stem cells (MSCs) and reticulated platelets (RPs) play crucial roles in the development, maintenance, and progression of multiple myeloma (MM). MSCs create a supportive microenvironment for myeloma cells by secreting cytokines, chemokines, and growth factors that promote cell survival, proliferation, and drug resistance. Moreover, the genetic abnormalities and phenotypic characteristics of MSCs in MM patients further contribute to the aggressiveness of the disease. RPs, as young, highly reactive platelets, are implicated in enhancing angiogenesis, tumor growth, and metastasis. Despite these findings, MSCs and RPs are not yet integrated into routine clinical practice for prognostic evaluation, presenting an opportunity for future advancements in MM treatment and patient management.
Furthermore, the role of RPs as markers of thrombopoietic activity and potential indicators of disease severity and progression in MM patients deserves greater attention. Their involvement in promoting tumor cell migration and facilitating interactions within the bone marrow microenvironment highlights their potential as valuable prognostic markers. This study suggests that assessing MSCs and RPs in clinical practice could provide a more comprehensive understanding of MM biology and enhance strategies for disease monitoring and therapeutic intervention, ultimately improving outcomes for MM patients.
Author Contributions
Conceptualization, S.M.Q.G. and V.M.R.-P.; investigation, C.A.M.A. and J.L.V.P.; writing—original draft preparation, S.M.Q.G. and V.M.R.-P.; writing—review and editing V.M.R.-P.; supervision, S.M.Q.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol 2016;43:676–81. [CrossRef]
- Fend F, Dogan A, Cook JR. Plasma cell neoplasms and related entities—evolution in diagnosis and classification. Virchows Arch 2023;482:163–77. [CrossRef]
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol 2020;95:548–67. [CrossRef]
- Sadaf H, Hong H, Maqbool M, Emhoff K, Lin J, Yan S, et al. Multiple myeloma etiology and treatment. J Transl Genet Genom 2022;6:63–83. [CrossRef]
- Deo SVS, Sharma J, Kumar S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann Surg Oncol 2022;29:6497–500. [CrossRef]
- Bianchi G, Anderson KC. Understanding biology to tackle the disease: Multiple myeloma from bench to bedside, and back. CA: A Cancer J Clin 2014;64:422–44. [CrossRef]
- Singh B, Gogia P, Kaur P, Guragai N, Maroules M. Hypercalcaemia, Renal Dysfunction, Anaemia, Bone Disease (CRAB Criteria): A Case of Lymphoma. Eur J Case Rep Intern Med 2020;7:002140. [CrossRef]
- Marino S, Roodman GD. Multiple Myeloma and Bone: The Fatal Interaction. Cold Spring Harb Perspect Med 2018;8:a031286. [CrossRef]
- Brigle K, Rogers B. Pathobiology and Diagnosis of Multiple Myeloma. Semin Oncol Nurs 2017;33:225–36. [CrossRef]
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48. [CrossRef]
- Malard F, Neri P, Bahlis NJ, Terpos E, Moukalled N, Hungria VTM, et al. Multiple myeloma. Nat Rev Dis Prim 2024;10:45. [CrossRef]
- Atencia-Flórez C, Quintero-Valencia C, Mondragón-Arismendy M, Cardona-Arias A, Regino-Agamez C, Vélez-Urrego J. Clinical, Laboratory, Cytometry and Cytogenetic Characteristics of a Cohort of Patients Diagnosed with Multiple Myeloma for the First Time in a Third-Level Hospital in Medellín, Colombia, Survival after 8 Years of Follow-Up. Int J Hematol-Oncol Stem Cell Res 2021;17:28–38. [CrossRef]
- Ramo A, Kuriakose P. Cytogenetic Changes in Multiple Myeloma Timeline: A Tertiary Center’s Experience. Blood 2020;136:40–1. [CrossRef]
- Sheng, G. The developmental basis of mesenchymal stem/stromal cells (MSCs). BMC Dev Biol 2015;15:44. [CrossRef]
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 2015;35:e00191. [CrossRef]
- Viswanathan S, Blanc KL, Ciccocioppo R, Dagher G, Filiano AJ, Galipeau J, et al. An International Society for Cell and Gene Therapy Mesenchymal Stromal Cells (MSC) Committee perspectives on International Standards Organization/Technical Committee 276 Biobanking Standards for bone marrow-MSCs and umbilical cord tissue–derived MSCs for research purposes. Cytotherapy 2023;25:803–7. [CrossRef]
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010;5:e10088. [CrossRef]
- Lou S, Duan Y, Nie H, Cui X, Du J, Yao Y. Mesenchymal stem cells: Biological characteristics and application in disease therapy. Biochimie 2021;185:9–21. [CrossRef]
- Reagan MR, Ghobrial IM. Multiple Myeloma Mesenchymal Stem Cells: Characterization, Origin, and Tumor-Promoting Effects. Clin Cancer Res 2012;18:342–9. [CrossRef]
- Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007;21:1079–88. [CrossRef]
- Yang H, Zheng Y, Zhang Y, Cao Z, Jiang Y. Mesenchymal stem cells derived from multiple myeloma patients protect against chemotherapy through autophagy-dependent activation of NF-κB signaling. Leuk Res 2017;60:82–8. [CrossRef]
- Lu Y, Zheng C, Zhang W, Liu X, Zhou Z, Wang Z, et al. Characterization of the biological and transcriptomic landscapes of bone marrow-derived mesenchymal stem cells in patients with multiple myeloma. Cancer Cell Int 2024;24:116. [CrossRef]
- Gu J, Wang M, Wang X, Li J, Liu H, Lin Z, et al. Exosomal miR-483-5p in Bone Marrow Mesenchymal Stem Cells Promotes Malignant Progression of Multiple Myeloma by Targeting TIMP2. Front Cell Dev Biol 2022;10:862524. [CrossRef]
- Xu S, Veirman KD, Becker AD, Vanderkerken K, Riet IV. Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target? Leukemia 2018;32:1500–14. [CrossRef]
- Xu S, Santini GC, Veirman KD, Broek IV, Leleu X, Becker AD, et al. Upregulation of miR-135b Is Involved in the Impaired Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients. PLoS ONE 2013;8:e79752. [CrossRef]
- Li B, Xu H, Han H, Song S, Zhang X, Ouyang L, et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene 2018;37:5508–19. [CrossRef]
- Garcia-Gomez A, Sanchez-Guijo F, Cañizo MCD, Miguel JFS, Garayoa M. Multiple myeloma mesenchymal stromal cells: Contribution to myeloma bone disease and therapeutics. World J Stem Cells 2014;6:322. [CrossRef]
- Liu Z, Liu H, He J, Lin P, Tong Q, Yang J. Myeloma cells shift osteoblastogenesis to adipogenesis by inhibiting the ubiquitin ligase MURF1 in mesenchymal stem cells. Sci Signal 2020;13. [CrossRef]
- Zhang Y, Wang Z, Zhang L, Zhou D, Sun Y, Wang P, et al. Impact of connexin 43 coupling on survival and migration of multiple myeloma cells. Arch Méd Sci : AMS 2017;13:1335–46. [CrossRef]
- Rethnam M, Tan DQ, Suda T. Myeloma cells self-promote migration by regulating TAB1-driven TIMP-1 expression in mesenchymal stem cells. Biochem Biophys Res Commun 2021;534:843–8. [CrossRef]
- Meijden PEJ van der, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol 2019;16:166–79. [CrossRef]
- Hannawi B, Hannawi Y, Kleiman N. Reticulated Platelets: Changing Focus from Basics to Outcomes. Thromb Haemost 2018;118:1517–27. [CrossRef]
- Lador A, Leshem-Lev D, Spectre G, Abelow A, Kornowski R, Lev EI. Characterization of surface antigens of reticulated immature platelets. J Thromb Thrombolysis 2017;44:291–7. [CrossRef]
- Li, N. Platelets in cancer metastasis: To help the “villain” to do evil. Int J Cancer 2016;138:2078–87. [CrossRef]
- Liao K, Zhang X, Liu J, Teng F, He Y, Cheng J, et al. The role of platelets in the regulation of tumor growth and metastasis: the mechanisms and targeted therapy. MedComm 2023;4:e350. [CrossRef]
- Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-Derived Nucleotides Promote Tumor-Cell Transendothelial Migration and Metastasis via P2Y2 Receptor. Cancer Cell 2013;24:130–7. [CrossRef]
- Ward Y, Lake R, Faraji F, Sperger J, Martin P, Gilliard C, et al. Platelets Promote Metastasis via Binding Tumor CD97 Leading to Bidirectional Signaling that Coordinates Transendothelial Migration. Cell Rep 2018;23:808–22. [CrossRef]
- O’Sullivan LR, Meade-Murphy G, Gilligan OM, Mykytiv V, Young PW, Cahill MR. Platelet hyperactivation in multiple myeloma is also evident in patients with premalignant monoclonal gammopathy of undetermined significance. Br J Haematol 2021;192:322–32. [CrossRef]
- Gidlöf O, Brug M van der, Öhman J, Gilje P, Olde B, Wahlestedt C, et al. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 2013;121:3908–17. [CrossRef]
- Tang J, Chen Y, Wang C, Xia Y, Yu T, Tang M, et al. The role of mesenchymal stem cells in cancer and prospects for their use in cancer therapeutics. MedComm 2024;5. [CrossRef]
- Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer 2017;16:31. [CrossRef]
- Slama Y, Ah-Pine F, Khettab M, Arcambal A, Begue M, Dutheil F, et al. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. Int J Mol Sci 2023;24:13511. [CrossRef]
- García-Sánchez D, González-González A, Alfonso-Fernández A, Dujo-Gutiérrez MD, Pérez-Campo FM. Communication between bone marrow mesenchymal stem cells and multiple myeloma cells: Impact on disease progression. World J Stem Cells 2023;15:421–37. [CrossRef]
- Velden DL van der, Houthuijzen JM, Roodhart JML, Werkhoven E van, Voest EE. Detection of endogenously circulating mesenchymal stem cells in human cancer patients. Int J Cancer 2018;143:2516–24. [CrossRef]
- Charalampous C, Goel U, Kapoor P, Binder M, Buadi F, Dingli D, et al. Association of thrombocytopenia with disease burden, high-risk cytogenetics, and survival in newly diagnosed multiple myeloma patients treated with novel therapies. Clin Lymphoma Myeloma Leuk 2024. [CrossRef]
- Mai EK, Hielscher T, Bertsch U, Salwender HJ, Zweegman S, Raab MS, et al. Predictors of early morbidity and mortality in newly diagnosed multiple myeloma: data from five randomized, controlled, phase III trials in 3700 patients. Leukemia 2024;38:640–7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).