 Graphical abstract
Graphical abstract. Simvastatin favored Lipid Metabolism in B16.F10 Melanoma Cells via modulation of the constitutive level of HIF-1. Abbreviations: Akt: Protein kinase B, FAO: Fatty Acid Oxidation, FAS: Fatty Acid Synthesis, HIF-1: Hypoxia-Inducible Factor 1, HMG-CoA: 3-Hydroxy-3-Methylglutaryl-Coenzyme A, HMC-CR: HMG-CoA Reductase, OXPHOS: Oxidative Phosphorylation, PPP: Pentose Phosphate Pathway, PUFAs: Polyunsaturated Fatty Acids, SIM: Simvastatin, TCA Cycle: Tricarboxylic Acid Cycle. Created in BioRender. Gabriela, G. (2024)
BioRender.com/j03y911
1. Introduction
Statins have antitumor effects when administrated in doses 100-500 times higher than those needed to lower cholesterol biosynthesis (Coimbra et al, 2010; Alupei et al, 2014; Licarete et al, 2017; Porfire et al, 2014; Alupei et al, 2015). Thus, our data proved that simvastatin (SIM), a lipophilic statin strongly inhibited B16.F10 murine melanoma cell proliferation in vivo and in vitro via strong suppression of the expression of subunit α of hypoxia inducible factor 1 (HIF-1 α) (Alupei et al, 2014; Licarete et al, 2017; Alupei et al, 2015) under hypoxic (Licarete et al, 2017) as well as normoxic conditions (Alupei et al, 2014). Notably, hypoxia-stabilized expression of HIF-1 α is essential for regulation of multiple cancer-related metabolic pathways such as activation of metabolic genes involved in increasing glycolysis, fatty acid synthesis (FAS), pentose phosphate pathway (PPP), and nucleotide biogenesis (Kumar et al, 2013; DeBerardinis and Chandel, 2016; Munir et al, 2019; Shiratori et al, 2019). It is known that one of the hallmarks of cancer cells remains the HIF-1-regulated metabolism reprogramming which supports cell growth, survival, metastasis, and also resistance to therapies (Warburg, 1956; Warburg 1925; Phan et al, 2014; Cazzaniga et al, 2015; Pavlova and Thompson, 2016). Thus, HIF-1 suppresses pyruvate dehydrogenase (PDH), via activation of PDH kinase-1 (PDK-1) expression, resulting in inhibition of the mitochondrial metabolism (tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS)), which ensures cancer cell survival under hypoxia (Kumar et al, 2013; DeBerardinis and Chandel, 2016; Munir et al, 2019; Shiratori et al, 2019). Besides the role of HIF-1 to ensure tumor survival and progression under hypoxic stress conditions, normoxic expression of HIF-1α in melanoma tumors is associated with increased aggressiveness of these cancer cells (Mills et al, 2009; Kuphal et al, 2010; Alupei et al, 2014). In tight connection with our earlier observation about the suppresive effect of SIM on normoxic HIF-1α expression (Alupei et al, 2014), we aimed to explore if by interfering with HIF-1α key metabolic regulator in normoxic conditions, the cell's metabolism was influenced. RNA-seq revealed expression changes induced by SIM in genes involved in energy and biosynthesis metabolic pathways. To validate these data, we evaluated the expression and catalytic activities of several proteins such as fatty acid synthase (FASN), stearylCoA desaturase 1 (SCD1) and phosphofructokinase 1 (PFK1). Our results indicated that low-dose SIM treatment (at IC50 value) influenced B16.F10 melanoma cells metabolism to adapt and become resistant to the treatment. The metabolism was redirected towards the synthesis of fatty acids (FAs), mainly favoring unsaturated fatty acids (UFAs) abundance. This metabolic shift could potentially result in elevated prostaglandin synthesis, bioactive lipids with various signaling roles in the progression of cancer (Wang and Dubois, 2010; Wang et al, 2015; Wang et al, 2022; Ferreira et al, 2018; Yoshida et al, 1998; Gomes et al, 2018).
2. Materials and Methods
Materials and Methods should be described with sufficient details to allow others to replicate and build on published results. Please note that publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Interventionary studies involving animals or humans, and other studies require ethical approval must list the authority that provided approval and the corresponding ethical approval code.
2.1.Cell line and Culture Conditions
B16.F10 murine melanoma cells (ATCC; CRL-6457) were cultured in DMEM (Lonza Group, Basel, Switzerland CH) as a monolayer at 37°C in 5% CO2 and 20% O2 atmosphere, as previously described (Alupei et al, 2014; Patras et al, 2021).
2.2. SIM Solutions
SIM was purchased from Sigma-Aldrich Chemie GmbH, Germany. SIM IC50 (3.5 µM), as determined in our previous study (Alupei et al, 2014) was prepared from the stock solution of 24 mM SIM into the culture medium shortly before use. Cells were incubated with 3.5 µM SIM for 24h.
2.3. RNA-Seq
B16.F10 cells were seeded as 1x106 cells/flask and treated with 3.5 µM SIM for 24h at 37°C. Then, the RNA isolation was performed using peqGOLD Total RNA isolation kit (PeqLab, Erlangen, DE), including the DNase digestion treatment of the lysates (peqGOLD DNase I Digest Kit, PeqLab, Erlangen, DE), to avoid DNA contamination, according to the manufacturer’s instructions (Hemmrich et al, 2010). The RNA was eluted with 50 μl of RNase-free water. The quantification of total RNA was performed by Invitrogen Qubit 4 Fluorometer (Thermo Scientific, MA, USA) with Broad Range (BR) Assay kit (Thermo Scientific, MA, USA), while RNA quality was determined using NanoDrop 1000 (Thermo Scientific, Ma, USA) according to the manufacturer's protocol (Zhang et al, 2020). To remove rRNA from the total RNA Ribo-Zero rRNA removal beads (Illumina, Inc.) were used. Further, to generate strand-specific cDNA libraries, TruSeq Stranded Total RNA (Illumina, Inc.) was used followed by quality control procedure (Qubit 2.0 Fluorometer, Thermo Scientific, USA; Agilent 2100 bioanalyzer, Argilent Technologies, USA). The qualified libraries were then subjected to sequencing by high-throughput Illumina NovaSeq 6000 sequencing platform, PE 150 (Illumina, Inc.). Three biological replicates were used for each experimental group. Library construction and sequencing were performed by Novogene Corporation (UK).
2.4. RNA-seq Data Analysis
The clean obtained sequencing data were mapped to the mouse reference transcriptome by HISAT2 software (v2.0.5) (
Kim et al, 2015). For the estimation of transcript abundance of coding genes as Fragments Per Kilobase of transcript per Million mapped reads (FPKM), StringTie-eB software (v1.3.1)
(Pertea et al, 2015) was used, then the differential expressed transcripts were identified by Ballgown package 2.22.0
(Robinson et al, 2010; Pertea et al, 2016). To measure the level of expression of each gene in SIM-treated group in comparison with its expression in control group, fold regulation (FR) values were calculated. Benjamini-Hochberg method was applied to adjust p-values for multiple testing (
Benjamini and Yekutieli, 1995; Benjamini and Yekutieli, 2001). Genes with a FR cutoff of ± 1.5, a
p-value less than 0.05 and a false discovery rate (FDR) of 20% were considered differentially expressed (
Barbariga et al, 2019; Floares et al, 2011). Furthermore, pathway annotation of differential genes was performed in KEGG database (
https://www.genome.jp/kegg/pathway.html), testing the statistical enrichment of mRNAs with KOBAS software (v.3.0). Hierarchical clustering of expression Z-scores of differentially expressed genes (DEG) considered of interest was done in R, using heatmap.2 function. Z-scores were calculated for each row (each DEG) as follows, z=(x-μ)/σ, where x is the count value of the gene in a replicate, μ the mean of all count values of this gene in all replicates, and σ the corresponding standard deviation (SD) (
Weinguny et al, 2020).
2.5. Quantitative Real-Time PCR (RT-qPCR)
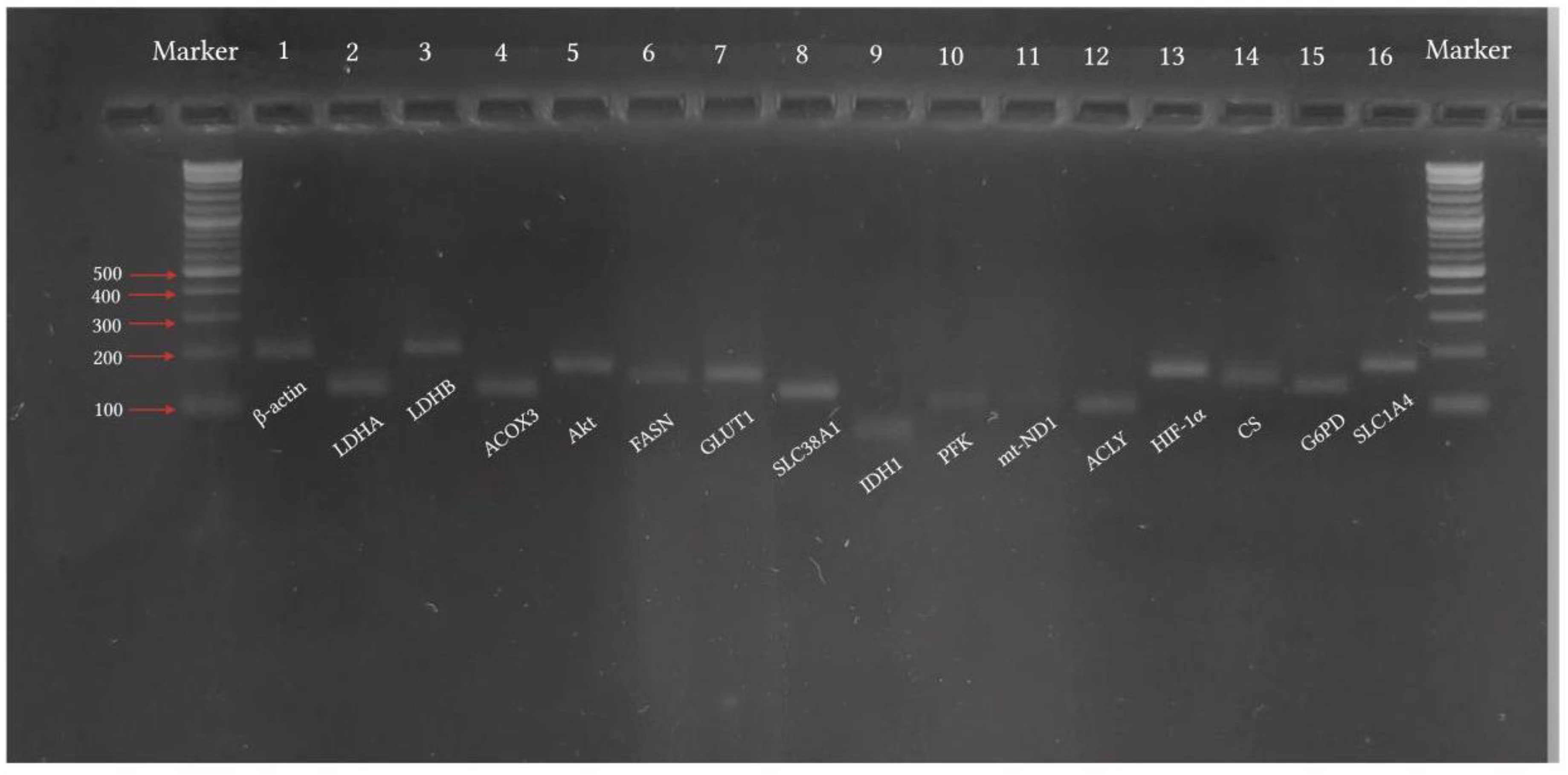
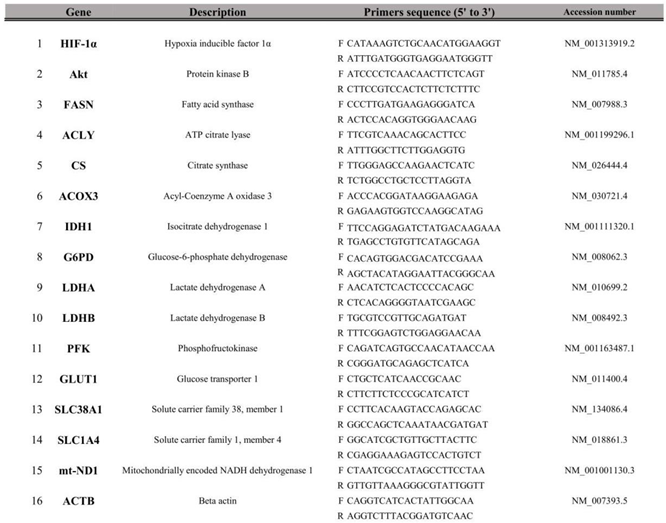
RT-qPCR analysis was used to validate RNA-seq data. Total RNA (1 µg) for each group was reverse-transcribed into cDNA, using iScriptTM Reverse transcription Supermix (#1708841, Bio-Rad Laboratories (Pacific) Pty Ltd, Gladesville, Australia) in a total volume of 20 µl. The complete reaction mix was incubated at 25°C for 5 min for priming, followed by reverse transcription at 46°C for 20 min and finally at 95°C for 1 min for reverse transcriptase inactivation. Real-time quantitative PCR was performed using SsoFastTM EvaGreenR Supermix (#172-5200, Bio-Rad Laboratories (Pacific) Pty Ltd, Gladesville, Australia). cDNA (1 µl) was amplified with 500 mM specific primers. The primers sequences are mentioned in Table 1 (purchased from Eurogentec, Seraing, Belgium). The following setup was used for the amplification: enzyme activation at 95°C for 30 sec, 40 cycles of denaturation at 95°C for 5 min, and annealing at 55°C for 5 sec, followed by 65°C-95°C for 5 sec (in 0.5°C inc). PCR and subsequent 1% agarose gel electrophoresis were used for validation of primer specificity (Figure 1).All reactions were performed in CFX96TM Real-Time System (C1000 Touch Thermo Cycler, Bio-Rad Laboratories (Pacific) Pty Ltd, Gladesville, Australia) according to manufacturer’s instructions. Relative mRNA expression was quantified using the ΔΔCt method (Ct is threshold cycle) and normalized to β-actin mRNA as an internal control (Livak and Schmittgen, 2001).
Figure 1.
Validation of primer specificity by agarose electrophoresis of PCR products. Abbreviations: ACLY: ATP citrate lyase; ACOX3: acyl-CoA oxidase 3; b-actin: beta-actin; CS: citrate synthase; FASN: fatty acid synthase; GLUT1: glucose transporter type 1; G6PD: glucose-6-phosphate dehydrogenase; HIF-1α: hypoxia-inducible factor 1 alpha; IDH1: isocitrate dehydrogenase 1; LDHA: lactate dehydrogenase A; LDHB: lactate dehydrogenase B; AkT: protein kinase B; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; PFK: phosphofructokinase; SCD1: stearoyl-CoA desaturase 1; SLC1A4: solute carrier family 1 member 4; SLC38A1: solute carrier family 38 member 1.
Figure 1.
Validation of primer specificity by agarose electrophoresis of PCR products. Abbreviations: ACLY: ATP citrate lyase; ACOX3: acyl-CoA oxidase 3; b-actin: beta-actin; CS: citrate synthase; FASN: fatty acid synthase; GLUT1: glucose transporter type 1; G6PD: glucose-6-phosphate dehydrogenase; HIF-1α: hypoxia-inducible factor 1 alpha; IDH1: isocitrate dehydrogenase 1; LDHA: lactate dehydrogenase A; LDHB: lactate dehydrogenase B; AkT: protein kinase B; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; PFK: phosphofructokinase; SCD1: stearoyl-CoA desaturase 1; SLC1A4: solute carrier family 1 member 4; SLC38A1: solute carrier family 38 member 1.
Figure 2.
Relative expression of genes involved in metabolic pathways assesed by RT-qPCR. The figure presents the relative expression of 15 reference genes involved in metabolic pathways in B16.F10 melanoma cells treated with SIM, compared to the gene expression in the untreated group. β-actin served as a housekeeping gene in the qPCR analysis. The data are expressed as mean ± SD. At least three replicates were used for the quantification of each gene. Statistical significance was asssesed using Student’s T-test. ns: not significant; * p < 0.05; ** p < 0.01. Abbreviations: ACLY: ATP citrate lyase; ACOX3: acyl-CoA oxidase 3; b-actin: beta-actin; CS: citrate synthase; FASN: fatty acid synthase; GLUT1: glucose transporter type 1; G6PD: glucose-6-phosphate dehydrogenase; HIF-1α: hypoxia-inducible factor 1 alpha; IDH1: isocitrate dehydrogenase 1; LDHA: lactate dehydrogenase A; LDHB: lactate dehydrogenase B; AkT: protein kinase B; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; PFK: phosphofructokinase; SCD1: stearoyl-CoA desaturase 1; SLC1A4: solute carrier family 1 member 4; SLC38A1: solute carrier family 38 member 1.
Figure 2.
Relative expression of genes involved in metabolic pathways assesed by RT-qPCR. The figure presents the relative expression of 15 reference genes involved in metabolic pathways in B16.F10 melanoma cells treated with SIM, compared to the gene expression in the untreated group. β-actin served as a housekeeping gene in the qPCR analysis. The data are expressed as mean ± SD. At least three replicates were used for the quantification of each gene. Statistical significance was asssesed using Student’s T-test. ns: not significant; * p < 0.05; ** p < 0.01. Abbreviations: ACLY: ATP citrate lyase; ACOX3: acyl-CoA oxidase 3; b-actin: beta-actin; CS: citrate synthase; FASN: fatty acid synthase; GLUT1: glucose transporter type 1; G6PD: glucose-6-phosphate dehydrogenase; HIF-1α: hypoxia-inducible factor 1 alpha; IDH1: isocitrate dehydrogenase 1; LDHA: lactate dehydrogenase A; LDHB: lactate dehydrogenase B; AkT: protein kinase B; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; PFK: phosphofructokinase; SCD1: stearoyl-CoA desaturase 1; SLC1A4: solute carrier family 1 member 4; SLC38A1: solute carrier family 38 member 1.
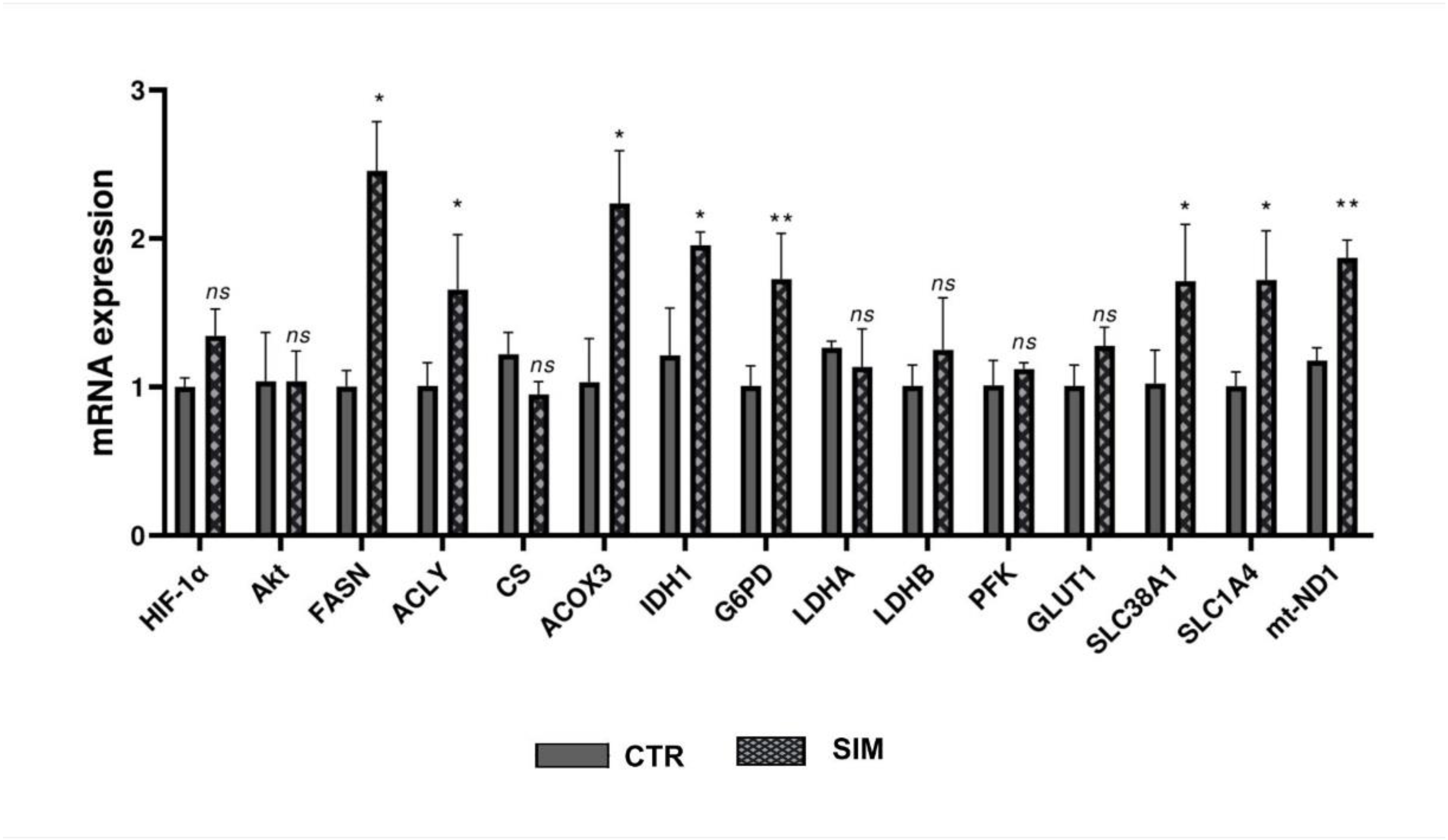
Table 1.
Primers sequences for targeted mouse genes used for RT-qPCR.
Table 1.
Primers sequences for targeted mouse genes used for RT-qPCR.
2.6. Cell Lysates Preparation
Prior to inducing cell lysis, the B16.F10 cell monolayers were washed with PBS and the viable adherent cells were detached with Trypsin 1x (5 min incubation), then centrifuged at 260 x g for 5 minutes. Thereafter, the obtained pellets were resuspended in 250 μl lysis buffer as described previously (Rauca et al, 2018). The homogenates were kept on ice for 30 minutes, followed by centrifugation at 4°C at 12 000 x g for 10 minutes. The supernatants were collected and stored at −80°C before further molecular measurements (Luput et al, 2018). Bradford assay was performed to determine the total protein concentration in the cell lysates (Sigma-Aldrich; Merck KGaA) (Bradford, 1976; Alupei et al, 2014; Rauca et al, 2018).
2.7. Assessment of Lactate Dehydrogenase (LDH) Activity
To assess the LDH activity, the decrease of nicotinamide adenine dinucleotide (NADH) absorbance at 340 nm was monitored in 10 μl of cell lysates. The variation of absorbance is directly proportional to the LDH activity, caused by conversion of NADH to NAD+, while lactate is transformed into pyruvic acid. The determination was performed using Jasco V-530 UV-VIS spectrophotometer, following the protocol described by Huijgen et al, 1997. The values were represented as catalytic activity mU/mg protein. Three independent experiments were performed.
2.8. Determination of PFK1 Activity
To test the activity of the glycolysis rate-limiting enzyme PFK1, Phosphofructokinase Assay Kit (MAK093, Sigma-Aldrich Chemie GmbH, Germany) was used. The principle of the assay is based on the coupled conversion of fructose-6-phosphate to fructose-1,6-diphosphate by the enzyme mix, resulting adenosine diphosphate (ADP) which is further converted to adenosine monophosphate (AMP) and NADH (Alice et al, 2002). Then, NADH reduces a colorless probe, in a way dependent of the PFK activity. The obtained values were represented as miliunits (mU) of PFK1 activity/ml after measuring absorbance at 450 nm. Three independent experiments were performed.
2.9.Glucose 6-Phosphate Dehydrogenase (G6PD) Activity Assay
To evaluate G6PD activity, the first and rate-limiting enzyme of the PPP, 1x106 cells were homogenized in 100 μl ice-cold PBS, for each experimental group. The measurements were performed using G6PD activity assay kit (MAK015, Sigma-Aldrich Chemie GmbH, Germany) according to the supplier’s instructions. Thus, 20 μl of homogenate/sample was used. After the oxidation of glucose-6-phosphate by the enzyme mix, a colorimetric product was generated, and the intensity was monitored at 450 nm by a microplate reader (FLUOstar Omega (BMG, Labtech, Offenburg, Germany) in kinetic mode for 30 min. Catalytic activity in mU/ml had been used to express the results (Hu et al, 2019). Three independent experiments were performed.
2.10. Assessment of Citrate Synthase (CS) Activity
The investigation of activity of CS, the enzyme which catalyzes the synthesis of citrate from oxaloacetate in Krebs cycle, was performed by CS activity kit, (MAK193, Sigma-Aldrich Chemie GmbH, Germany), using 20 μl sample lysate/group. To quantify CS activity, the assay measures the color development of TNB chromophore (2-nitro-5-thiobenzoic acid), produced from Ellman's Reagent, 5,5'-Dithiobis-2-Nitrobenzoic Acid (DTNB) and the CoA-SH generated during the citrate synthesis. The colorimetric product is directly proportional to the amount of active enzyme present in the samples (Srere, 1969; Petrohai et al, 2005). The absorbance measurements at 412 nm were performed using a microplate reader (FLUOstar Omega (BMG, Labtech, Offenburg, Germany) in kinetic mode for 30 min. Each step was done according to the provider’s instructions. The enzyme’s activity was reported as mU/mL. Each sample was tested three times.
2.11. Western Blot Analysis
To assess the effect of the SIM on the expression of several critical protein players involved in the energy metabolism, western blot analysis was performed as described previously (Rauca et al, 2018; Rauca et al, 2021). Thus, to determine HIF-1α levels in cell lysates, 80 μg of total proteins from each lysate were loaded/per lane onto a 7.5% polyacrylamide gel. To reveal the expression of SCD1, 60 μg of total protein were used in each group, respectively 50 μg of total protein for acyl-Coenzyme A oxidase 3 (ACOX3) and mitochondrially encoded NADH dehydrogenase 1 (mt-ND1). To quantify FASN, we used 40 μg of total protein, while for facilitated glucose transporters, solute carrier family 2 member 1 (SLC2a1) / glucose transporter 1 (GLUT1), total protein kinase B (t-Akt) also known as serine/threonine-specific protein kinases and phosphorilated Akt (pAkt) 30 μg of total protein lysates were needed. To detect the levels of these proteins 10% polyacrylamide gel was used. To block membranes, 5% skimmed milk powder dissolved in TBS-T (0.1% Tween) was used for 1 h at room temperature (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Then, the membranes were immunoblotted overnight at 4°C against the primary antibodies as follows, rabbit monoclonal IgG anti-mouse HIF-1α diluted 1:500 (ab179483, Abcam, Cambridge, United Kingdom), rabbit monoclonal IgG anti-mouse SCD1 1:500 (2794, Cell Signaling, United States), rabbit polyclonal IgG anti-mouse ACOX3 1:1000 dilution (PA5-22373, Pierce, Thermo Fisher Scientific, Inc.), rabbit polyclonal IgG anti-mouse mt-ND1 1:1000 (SAB5700797, Sigma-Aldrich Chemie GmbH, Germany), mouse monoclonal IgG anti-mouse FASN 1:1000 (sc-48357, Santa Cruz Biotechnology, Inc., United States), rabbit polyclonal IgG anti-mouse GLUT1 1:1000 (SAB4502803, Sigma-Aldrich Chemie GmbH, Germany), rabbit monoclonal IgG anti-mouse t-Akt 1:1000 (4685, Cell Signaling, United States), rabbit polyclonal IgG anti-mouse p-Akt 1:1000 (sc-7985, Santa Cruz Biotechnology, Inc., United States). As primary antibody to test loading control, β-actin 1:1000 diluted rabbit polyclonal IgG anti-mouse β-actin (A2103, Merck, Darmstadt, GER) was used. As secondary antibodies, a goat anti-mouse horseradish peroxidase (HRP)-conjugated IgG (sc-2005, Santa Cruz Biotechnology, Inc., United States) was utilized for FASN detection, while goat anti-rabbit IgG HRP-conjugated was used to quantify HIF-1α, SCD1, ACOX3, mt-ND1, GLUT1, t-Akt and p-Akt (sc-2004, Santa Cruz Biotechnology, Inc., United States). Each secondary antibody was diluted 1:2500 in TBS-T and the membranes were incubated for 1 h at room temperature. Moreover, membranes were washed in TBS-T three times for 5 minutes before and after the incubation with the secondary antibody (Rauca et al, 2018; Rauca et al, 2021). Data from at least three independent experiments were presented as mean ± SD and each protein’s level of expression was compared to control group expression.
2.12. Statistical Analysis
Mean ± SD was used to report data obtained from different experiments. Unpaired Student’s t-test was used to statistically compare the effects of SIM-treatment on different enzymes activities / levels with those from untreated cell lysates. GraphPad Prism version 7 for Windows (San Diego, CA, USA) was used to conduct all statistical analyses. A p-value lower than 0.05 was defined as significant.
3. Results
SIM hindered key regulatory molecules of cancer cell metabolism reprogramming
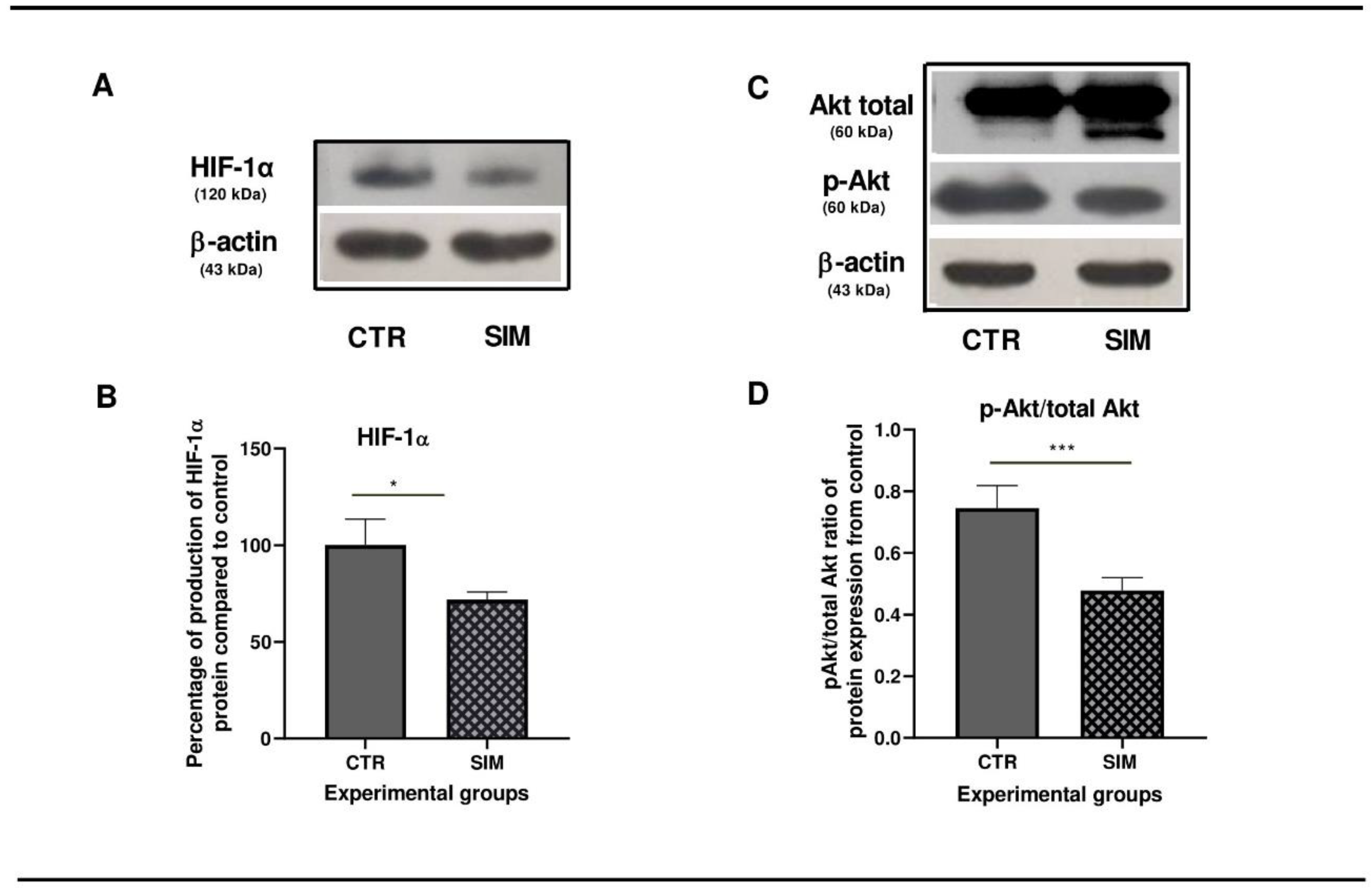
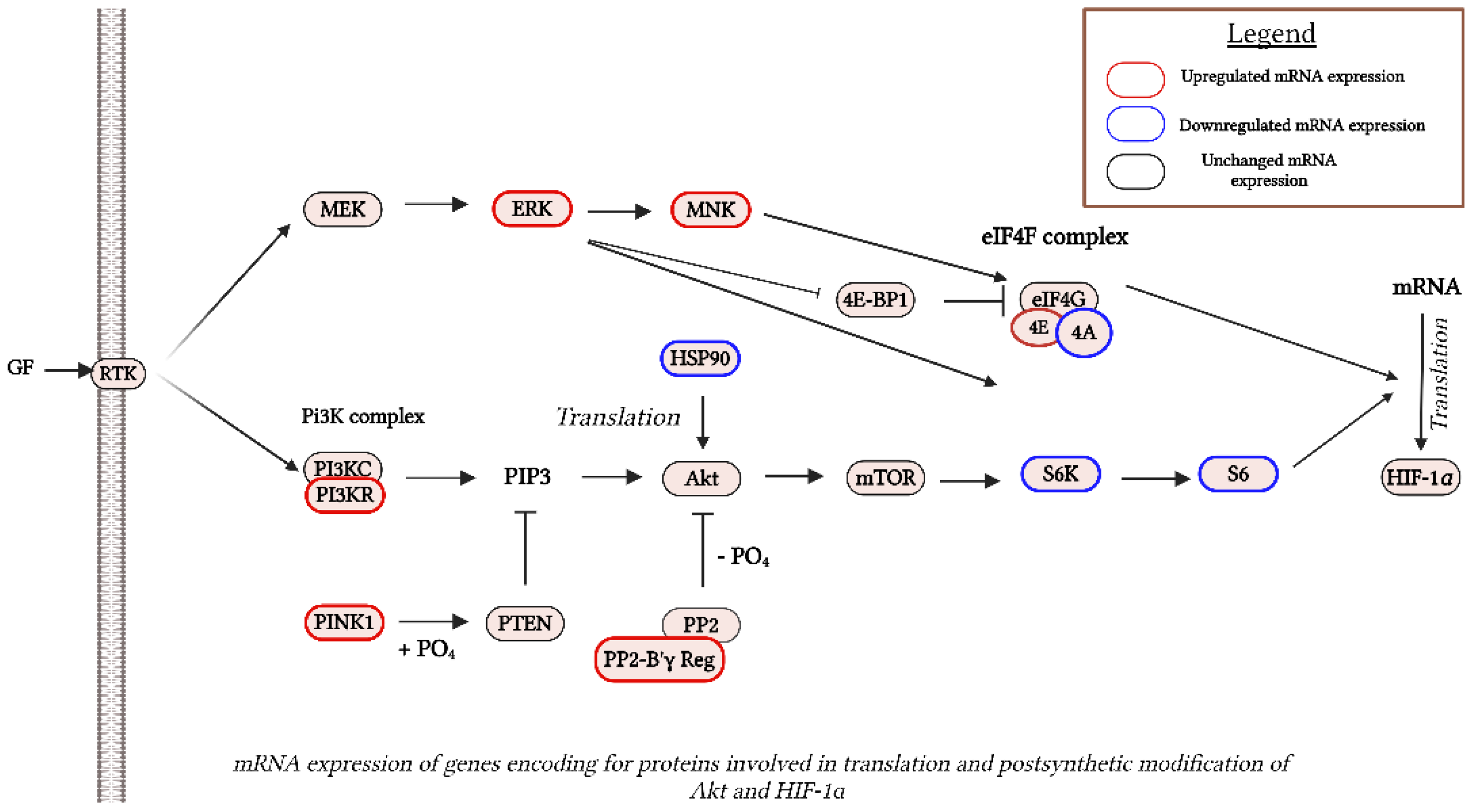
Based on our previous data that showed that 3.5 µM SIM induced inhibition of B16.F10 murine melanoma cell proliferation by 50% under normoxia (Alupei et al, 2014), we assessed the effects of this lipophilic statin on the production of two regulatory proteins for cancer cell aggressiveness and metabolism reprogramming: pAkt (Cheung et al, 2008; Stahl et al, 2004; Davies et al, 2009; Kwong and Davies, 2013; McGrail et al, 2022) and HIF-1 (Lartigau et al, 1997; Kuphal et al, 2010; Mouriaux et al, 2014; Koch et al, 2015; Stine et al, 2015). Therefore, mRNA expression was assessed via RNA-seq analysis, RT-qPCR and protein levels via western blot analysis. Our data suggested no changes in the mRNA levels corresponding to both regulators, in the SIM-treated group compared to the untreated group (Supplementary Table S1. All genes; Figure 1). Nevertheless, SIM exerted suppressive effects on protein production for the subunit ɑ of HIF-1 (HIF-1ɑ) by 30% (p<0.01) (Figure 3 A,B), and significantly reduced the ratio of the active form of Akt/total Akt production by 1.4 fold (p<0.001) (Figure 3 C,D) in comparison with the control protein levels. These data suggested a regulatory mechanism modulated by SIM at the translational level. In tight connection with this finding, significant changes were identified in the mRNA profile for proteins involved in postsynthetic modification of both Akt, such as: heat shock protein 90 (HSP90) (300 times reduction after treatment compared to control, p<0.05), and PTEN induced putative kinase 1 (PINK1) (by almost 2 times, p<0.05) (Taipale and Jarosz, 2010; Junttila and Westermarck, 2008; Benitez, et al, 2017), as well as in HIF-1ɑ translation: eukaryotic translation initiation factor 4A1 (eIF4A1) (FR=-17.75, p<0.05), ribosomal protein S6 kinase polypeptide 2 (Rps6kb2) (FR=-3, p<0.05) and ribosomal protein S6 (RPS6) (FR=-1.6, p<0.05) (Semenza, 2012; Zhang et al, 2014; Lehman et al, 2022) (Table 2; Figure 4).
Table 2.
DEG in Akt and HIF-1 translation.
Table 2.
DEG in Akt and HIF-1 translation.
Figure 3.
Protein levels of two regulatory molecules after 3.5 µM SIM-administration in B16.F10 cells under normoxia. (A, C) Western blot images of the expression levels of HIF-1α, total Akt and pAkt. β-actin was used as loading control; (B) Percentages of the HIF-1α levels in SIM-treated cells (SIM) in comparison with the production of the same protein in control lysates (CTR); (D) The ratio of expression levels of pAkt protein of total Akt levels in comparison with the production of the same protein in control lysates (CTR). Data were expressed as mean ± SD of at least three independent measurements; * p < 0.05; *** p < 0.001. Abbreviations: β-actin: beta-actin; CTR: control group; HIF-1α: hypoxia-inducible factor 1 alpha; pAkt: phosphorylated Akt; SIM: simvastatin treated group; total Akt: total Akt (protein kinase B).
Figure 3.
Protein levels of two regulatory molecules after 3.5 µM SIM-administration in B16.F10 cells under normoxia. (A, C) Western blot images of the expression levels of HIF-1α, total Akt and pAkt. β-actin was used as loading control; (B) Percentages of the HIF-1α levels in SIM-treated cells (SIM) in comparison with the production of the same protein in control lysates (CTR); (D) The ratio of expression levels of pAkt protein of total Akt levels in comparison with the production of the same protein in control lysates (CTR). Data were expressed as mean ± SD of at least three independent measurements; * p < 0.05; *** p < 0.001. Abbreviations: β-actin: beta-actin; CTR: control group; HIF-1α: hypoxia-inducible factor 1 alpha; pAkt: phosphorylated Akt; SIM: simvastatin treated group; total Akt: total Akt (protein kinase B).
Figure 4.
mRNA levels of genes encoding for proteins involved in translation and postsynthetic modification of Akt and HIF-1 proteins after SIM-administration. Red outlines indicate the up-regulated genes and green outlines indicate the down-regulated genes, revealed by RNA-Seq analysis. KEGG pathway maps served as sources for all reaction stages. Created in BioRender. Gabriela, G. (2024) BioRender.com/l37i799. Abbreviations: 4E-BP1: eukaryotic initiation factor 4E-binding protein 1; Akt: protein kinase B; ERK: extracellular signal-regulated kinase; eIF4F: eukaryotic initiation factor 4F; GF: growth factor; HIF-1α: hypoxia-inducible factor 1 alpha; HSP90: heat shock protein 90; mTOR: mechanistic target of rapamycin; MEK: MAPK/ERK kinase; MNK: MAPK-interacting kinase; PI3KC: phosphoinositide 3-kinase catalytic subunit; PI3KR: phosphoinositide 3-kinase regulatory subunit; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PP2: protein phosphatase 2; PTEN: phosphatase and tensin homolog; PINK1: PTEN-induced kinase 1; PO4: phosphate group; PPR-B ɣ Reg: protein phosphatase 2, regulatory subunit B', gamma; RTK: receptor tyrosine kinase; S6: ribosomal protein S6; S6K: ribosomal protein S6 kinase.
Figure 4.
mRNA levels of genes encoding for proteins involved in translation and postsynthetic modification of Akt and HIF-1 proteins after SIM-administration. Red outlines indicate the up-regulated genes and green outlines indicate the down-regulated genes, revealed by RNA-Seq analysis. KEGG pathway maps served as sources for all reaction stages. Created in BioRender. Gabriela, G. (2024) BioRender.com/l37i799. Abbreviations: 4E-BP1: eukaryotic initiation factor 4E-binding protein 1; Akt: protein kinase B; ERK: extracellular signal-regulated kinase; eIF4F: eukaryotic initiation factor 4F; GF: growth factor; HIF-1α: hypoxia-inducible factor 1 alpha; HSP90: heat shock protein 90; mTOR: mechanistic target of rapamycin; MEK: MAPK/ERK kinase; MNK: MAPK-interacting kinase; PI3KC: phosphoinositide 3-kinase catalytic subunit; PI3KR: phosphoinositide 3-kinase regulatory subunit; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PP2: protein phosphatase 2; PTEN: phosphatase and tensin homolog; PINK1: PTEN-induced kinase 1; PO4: phosphate group; PPR-B ɣ Reg: protein phosphatase 2, regulatory subunit B', gamma; RTK: receptor tyrosine kinase; S6: ribosomal protein S6; S6K: ribosomal protein S6 kinase.
3.1. SIM Treatment Altered Expression of mRNA for SREBP1 and FOXM1 - Two Important Regulators of Cholesterol Biosynthesis and Tumor Development
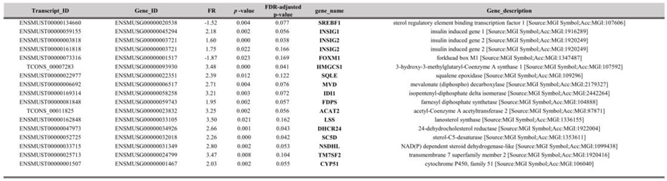
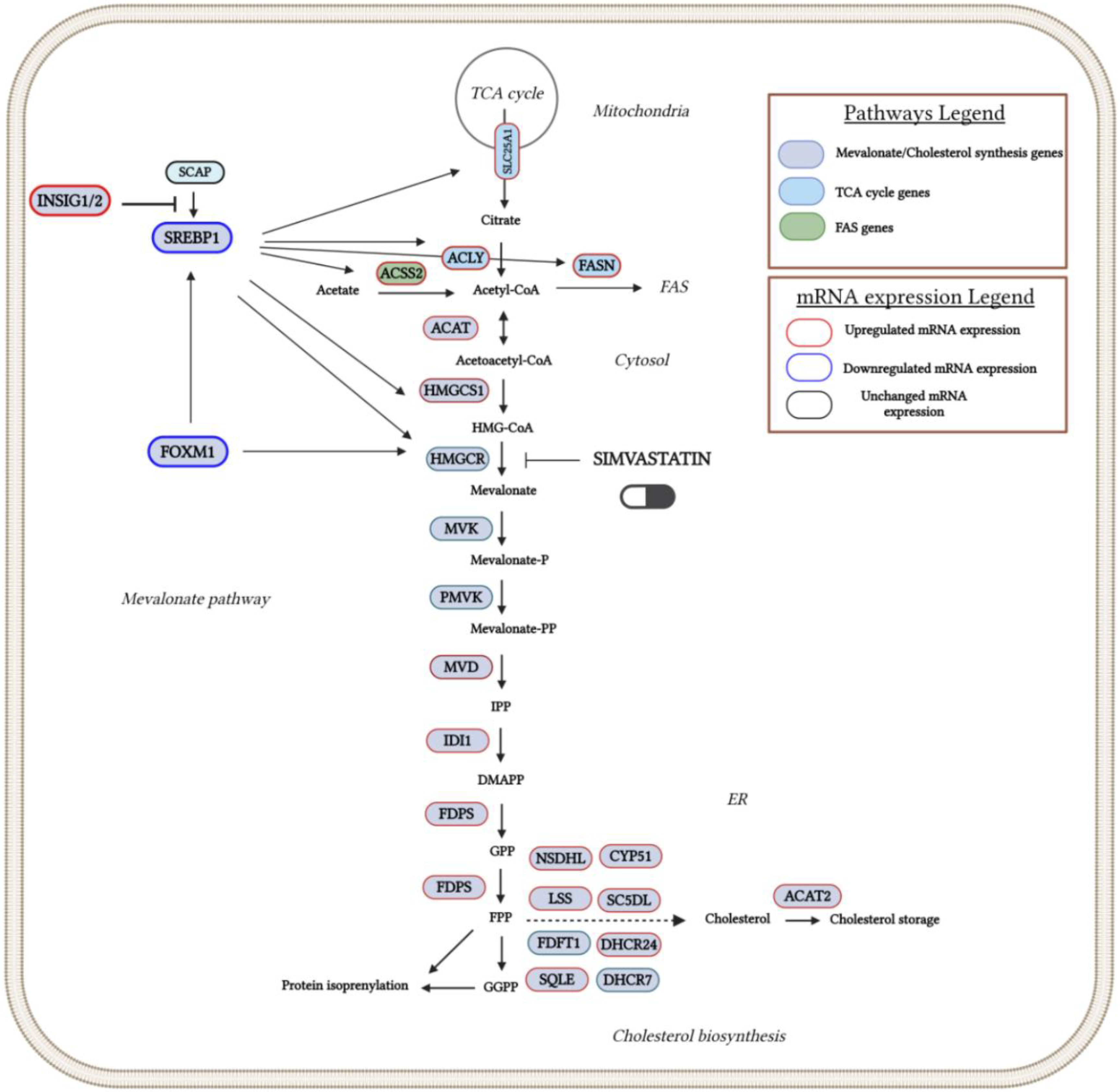
As statins are proven to be competitive inhibitors of cholesterol biosynthesis (Istvan and Deisenhofer, 2001), as well as due to the importance of this pathway for tumor development and progression (Silvente and Poirot, 2014; Kuzu et al, 2016), we explored the effects of SIM on the transcriptomic profile of the cholesterol biosynthesis and regulation. Our data indicated that two positive regulators of cholesterol biosynthesis, such as sterol regulatory element binding proteins (SREBP1) (Weber et al, 2004), as well as of forkhead box M1 (FOXM1) factor associated with mevalonate pathway regulation, tumorigenesis and glucose metabolism (Koo et al, 2012; Zhang et al, 2017; Wang et al, 2016), were significantly reduced by SIM (Table 3; Figure 5). Moreover, insulin-induced gene proteins 1 and 2 (INSIG-1 and INSIG-2), responsible for maintaining SRBEP in inactive form (Yang et al, 2002; Yabe et al, 2002), were both 2-fold overexpressed after the same treatment (Table 3; Figure 5). In addition to these findings, except for the gene encoding for the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-Coenzyme A reductase (HMG-CR) (Supplementary Table S1. All genes), transcripts of genes encoding for most enzymes of the same pathway were upregulated in response to SIM-treatment, as follows: 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 (HMG-CS1, FR=3.48; p<0.001), squalene epoxidase (SQLE, FR=2.38; p<0.05), mevalonate (diphospho) decarboxylase (MVD, FR=2.7; p<0.01), isopentenyl-diphosphate isomerase (IDI1, FR=3.21; p<0.01), farnesyl diphosphate synthase (FDPS, FR=1.95; p<0.01), and acetyl-coenzyme A acetyl-transferase 2 (ACAT2, FR=3.25; p<0.01) (Table 3). These findings suggest that SIM-induced inhibition of key regulatory molecules was counteracted via up-regulation of genes encoding enzymes for isoprenoids production, which are important metabolites for postsynthetic modification of proteins which are involved in signaling pathways related to the melanoma cell proliferation (Bjarnadottir et al, 2015; Kimbung et al, 2016; Lazaro-Mixteco et al, 2022).
Table 3.
DEG in cholesterol metabolism pathway.
Table 3.
DEG in cholesterol metabolism pathway.
Figure 5.
Overview of the cholesterol metabolism pathway and its regulation by SREBP1 and FOXM1 regulatory factors in SIM-treated melanoma cells. Red outlines indicate the up-regulated genes and green outlines indicate the down-regulated genes which were revealed by RNA-Seq analysis. KEGG pathway maps served as sources for all reaction stages. Created in BioRender. Gabriela, G. (2024) BioRender.com/g44x434. Abbreviations: ACAT: acetyl-CoA acetyltransferase; ACAT2: acetyl-CoA acetyltransferase 2; ACLY: ATP citrate lyase; ACSS2: acyl-CoA synthetase short-chain family member 2; CYP51: cytochrome P450 family 51; DHCR24: 24-dehydrocholesterol reductase; DHCR7: 7-dehydrocholesterol reductase; FDPS: farnesyl diphosphate synthase; FAS: fatty acid synthase; FASN: fatty acid synthase; FDFT1: farnesyl-diphosphate farnesyltransferase 1 (squalene synthase); FOXM1: forkhead box M1; HMGCR: 3-hydroxy-3-methylglutaryl-CoA reductase; HMGCS1: 3-hydroxy-3-methylglutaryl-CoA synthase 1; INSIG1/2: insulin induced gene 1/2; IDI1: isopentenyl-diphosphate delta isomerase 1; LSS: lanosterol synthase; MVD: mevalonate diphosphate decarboxylase; MVK: mevalonate kinase; NSDHL: NAD(P) dependent steroid dehydrogenase-like; PMVK: phosphomevalonate kinase; SC5DL: sterol-C5-desaturase-like; SCAP: SREBP cleavage-activating protein; SREBP1: sterol regulatory element-binding protein 1; SQLE: squalene epoxidase.
Figure 5.
Overview of the cholesterol metabolism pathway and its regulation by SREBP1 and FOXM1 regulatory factors in SIM-treated melanoma cells. Red outlines indicate the up-regulated genes and green outlines indicate the down-regulated genes which were revealed by RNA-Seq analysis. KEGG pathway maps served as sources for all reaction stages. Created in BioRender. Gabriela, G. (2024) BioRender.com/g44x434. Abbreviations: ACAT: acetyl-CoA acetyltransferase; ACAT2: acetyl-CoA acetyltransferase 2; ACLY: ATP citrate lyase; ACSS2: acyl-CoA synthetase short-chain family member 2; CYP51: cytochrome P450 family 51; DHCR24: 24-dehydrocholesterol reductase; DHCR7: 7-dehydrocholesterol reductase; FDPS: farnesyl diphosphate synthase; FAS: fatty acid synthase; FASN: fatty acid synthase; FDFT1: farnesyl-diphosphate farnesyltransferase 1 (squalene synthase); FOXM1: forkhead box M1; HMGCR: 3-hydroxy-3-methylglutaryl-CoA reductase; HMGCS1: 3-hydroxy-3-methylglutaryl-CoA synthase 1; INSIG1/2: insulin induced gene 1/2; IDI1: isopentenyl-diphosphate delta isomerase 1; LSS: lanosterol synthase; MVD: mevalonate diphosphate decarboxylase; MVK: mevalonate kinase; NSDHL: NAD(P) dependent steroid dehydrogenase-like; PMVK: phosphomevalonate kinase; SC5DL: sterol-C5-desaturase-like; SCAP: SREBP cleavage-activating protein; SREBP1: sterol regulatory element-binding protein 1; SQLE: squalene epoxidase.
3.2. SIM Effects on Transcriptomic Profile of Metabolism of B16.F10 Melanoma Cells Under Normoxia
As SIM affected the protein synthesis of two metabolic reprogramming regulators HIF-1α and Akt, we investigated by RNA-seq the mRNA levels of genes encoding for proteins involved in several metabolic pathways regulated by these factors. To validate this data, RT-qPCR was used to assess the expression of HIF-1α, Akt and direct/indirect targets in the metabolic pathways analysed (Figure 1). We found full concordance between RT-qPCR data and RNA-Seq results in terms of both the amount and the direction of expression changes. For FASN, isocitrate dehydrogenase 1 (IDH1) and solute carrier family 1 glutamate transporter member 4 (SLC1A4) genes, the expression levels were slightly higher than those obtained by RNA-Seq, while the expression of all the others was comparable between the two techniques.
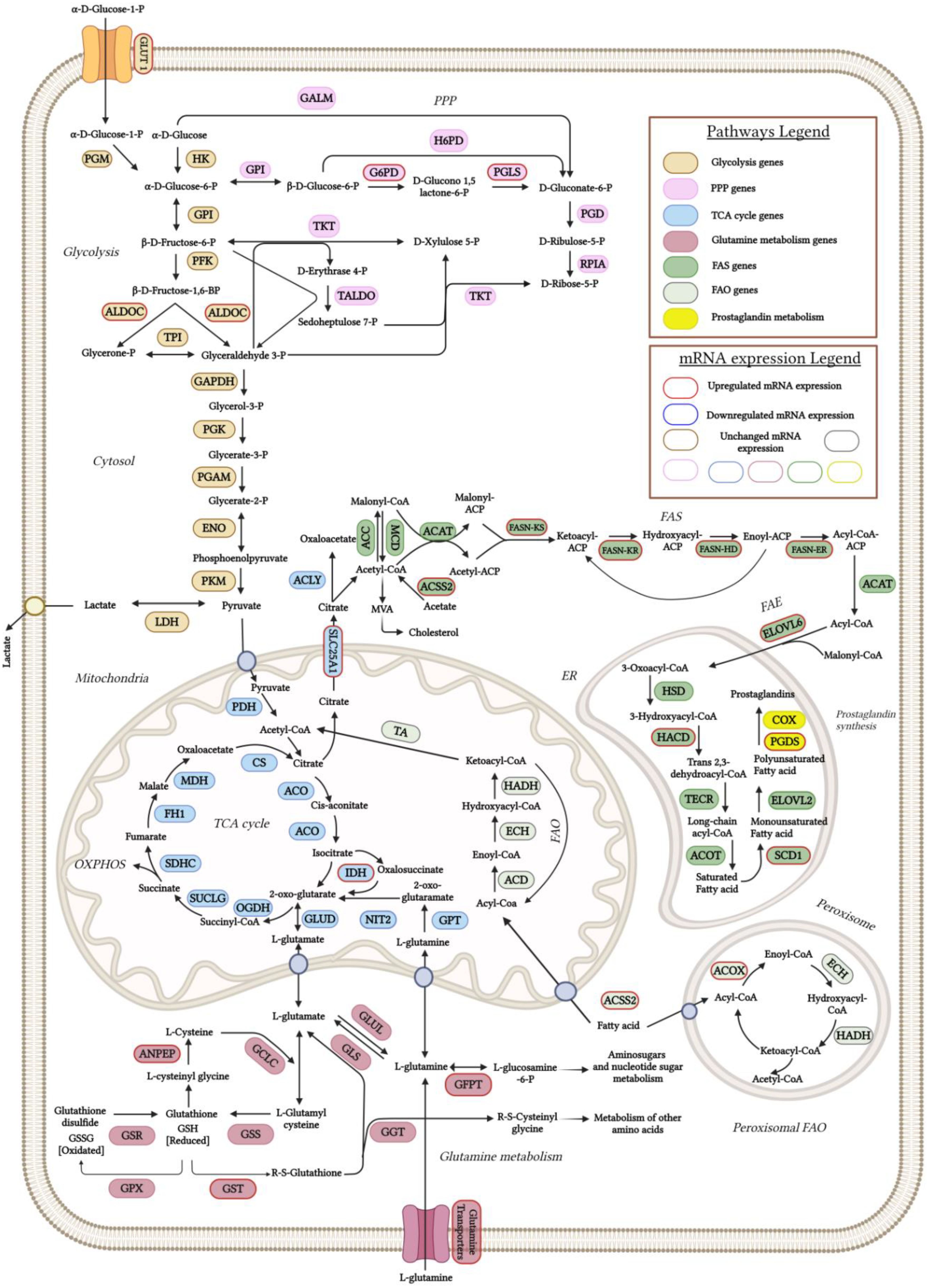
Our data revealed a panel of 1513 out of 15905 genes with at least 1.5 FR in expression after SIM treatment, with 817 transcripts upregulated, and 696 downregulated compared to the control group (
Supplementary Table S1. All genes). Functional enrichment analysis showed that SIM treatment favored the transcription of genes encoding proteins which are involved in glucose, glutamine and fatty acids transport, glycolysis, PPP, TCA cycle, OXPHOS, fatty acids synthesis (FAS), fatty acids elongation (FAE), peroxisomal β-oxidation (FAO) and suppressed transcripts related to ribonucleotide synthesis, OXPHOS and mitochondrial biogenesis as showed by the clustering of DEG in the hierarchical representation (
Figure 6 and
Figure 7; Table 4). Namely, SIM treatment doubled the mRNA levels of genes encoding for glucose metabolic pathways such as glycolysis: aldolase C (Aldoc) (FR=2.06;
p=0.0027), enolase 2 (Eno2) (FR=1.96;
p=0.0248) and facilitated glucose transporter solute carrier family 2 member 6 (SLC2A6 or GLUT6) (FR=2.5-fold increase;
p=0.0108) and PPP: G6PD (FR=1.5;
p=0.0003) and 6-phosphogluconolactonase (PGLS) (FR=1.87;
p=0.0305) compared to the control levels (
Table 4; Figure 6 and 7). Although there were increased mRNA levels of genes encoding for proteins involved in mitochondrial metabolism, such as IDH1 and several electron transport chain (ETC) subunits, one subunit of the ETC complex I, NADH dehydrogenase: Ubiquinone Oxidoreductase Subunit B10 (Ndufb10) was downregulated (FR=-1.53;
p=0.0294) and the mitochondrial biogenesis was affected by SIM treatment (nuclear respiratory factor 1 (NRF1) downregulation;
p<0.05) (
Table 4; Figure 7 and 8). Moreover, our data suggested that SIM administration favored both lipolysis and FAS as increased levels of transcripts were noted for lipolysis enzymes: adipose triglyceride lipase (ATGL) by twofold (FR=1.92;
p<0.05), monoacylglycerol lipase (MAGL) by ninefold (FR=9.18;
p<0.05), phospholipase D1 (PLD1) by fivefold (FR=5.28;
p=0.017), and for FAS and FAE: solute carrier family 25 member 1 (SLC25A1) (FR=1.96;
p=0.001) transporter of mitochondrial citrate into the cytosol (
Hlouschek et al, 2018) and ATP citrate lyase (ACLY), the enzyme responsible for linking of TCA to cytosolic FAS (FR=1.65;
p=0.0037), FASN (FR=1.98;
p=0.002) and elongation of long chain fatty acids family, member 6 (ELOVL6; FR=2.02;
p=0.004) (
Table 4; Figure 6 and 7). Also, SIM favored UFAs abundance by upregulating SCD1 mRNA expression by twofold (FR=1.86;
p=0.005) (
Table 4; Figure 6 and 7). Interestingly, SIM did not significantly influenced mitochondrial FAO (
Supplementary Table S1. All genes), but upregulated mRNA level of ACOX3, the rate-limiting enzyme of the peroxisomal FAO (FR=2.13;
p=0.0065) (
Table 4; Figure 6 and 7). All these data linked with the upregulation of mRNA level for prostaglandin D2 synthase
(PGDS) (FR=2.81;
p=0.001), suggested that SIM modulated an increase in prostaglandin synthesis in melanoma cells (
Table 4; Figure 6 and 7). Our transcriptomics findings indicated that excepting for glutamine related ribonucleotide synthesis, SIM enhanced glutamine utilization, implied by increased expression of glutamine transporters or enzymes such as SLC1A4 (FR=1.55;
p=0.0041), alanyl aminopeptidase (ANPEP) (FR=4.69;
p=0.0214) and glutamine fructose-6-phosphate transaminase 1 (GFPT1) (FR=1.5;
p=0.0043) (
Table 4; Figure 7). Because glutamine metabolism can replenish TCA cycle intermediates, it provides a carbon and nitrogen source for energy, nucleotide and lipid synthesis, promoting cell proliferation (
Kovacević and Morris, 1972; Mohamed et al, 2014; Zacharias et al, 2017; Demas et al, 2019; Gaglio et al, 2011; Kerr et al, 2016). Altogether, our RNA-seq data suggested that SIM treatment enhanced cell metabolism at the mRNA level, mainly by upregulating mRNA expression of genes encoding for: transporters of glucose, glutamine and FAs; glucose metabolism; mitochondrial oxidative metabolism and lipid metabolism in B16.F10 melanoma cells.
Table 4.
DEG in metabolism.
Table 4.
DEG in metabolism.
Figure 6.
Schematic overview of the effects of SIM on the expression of genes encoding for proteins involved in metabolic pathways in B16.F10 murine melanoma cells. Red outlines indicate the up-regulated genes and green outlines indicate the down-regulated genes which were revealed by RNA-Seq analysis. KEGG pathway maps served as sources for all reaction stages. Created in BioRender. Gabriela, G. (2024) BioRender.com/h93z324. Abbreviations: ACAT: acetyl-CoA acetyltransferase; ACLY: ATP citrate lyase; ACOX: acyl-CoA oxidase; ACSS2: acyl-CoA synthetase short-chain family member 2; ACC: acetyl-CoA carboxylase; ACOT: acyl-CoA thioesterase; ANPEP: alanyl aminopeptidase; CS: citrate synthase; COX: cytochrome c oxidase; ELOVL2: elongation of very long chain fatty acids protein 2; ELOVL6: elongation of very long chain fatty acids protein 6; ENO: enolase; FAD: flavin adenine dinucleotide; FASN-KS: fatty acid synthase keto synthase; FASN-KR: fatty acid synthase keto reductase; FASN-HD: fatty acid synthase hydroxydecarboxylase; FASN-ET: fatty acid synthase enoyl reductase; FAO: fatty acid oxidation; FAS: fatty acid synthase; FH1: fumarate hydratase 1; GALM: galactose-1-phosphate mutase; GCLC: gamma-glutamylcysteine ligase catalytic subunit; GFPT: glutamine-fructose-6-phosphate transaminase; GCLC: gamma-glutamylcysteine synthetase; GLUL: glutamine synthetase; GLUD: glutamate dehydrogenase; G6PD: glucose-6-phosphate dehydrogenase; GPT: glutamate-pyruvate transaminase; GPI: glucose-6-phosphate isomerase; GSR: glutathione reductase; GSS: glutathione synthetase; GST: glutathione S-transferase; HACD: 3-hydroxyacyl-CoA dehydratase; HADH: hydroxyacid dehydrogenase; H6PD: hexose-6-phosphate dehydrogenase; HK: hexokinase; HSD: hydroxysteroid dehydrogenase; IDH: isocitrate dehydrogenase; MCD: malonyl-CoA decarboxylase; MDH: malate dehydrogenase; NIT2: nitrite reductase; OGDH: 2-oxoglutarate dehydrogenase; PDH: pyruvate dehydrogenase; PFK: phosphofructokinase; PGD: 6-phosphogluconate dehydrogenase; PGDS: prostaglandin D2 synthase; PGK: phosphoglycerate kinase; PGK: phosphoglycerate kinase; PGLS: 6-phosphogluconolactonase; PGM: phosphoglucomutase; PGAM: phosphoglycerate mutase; PKM: pyruvate kinase M; PPP: pentose phosphate pathway; RPIA: ribose-5-phosphate isomerase A; SCD1: stearoyl-CoA desaturase 1; SCO: sterol-14α-demethylase; SDHC: succinate dehydrogenase cytochrome b subunit; SLC25A1: solute carrier family 25 member 1; SUCLG: succinate-CoA ligase gamma subunit; TAC: tricarboxylic acid cycle; TA: transaldolase; TALDO: transaldolase; TKT: transketolase; TGAPDH: 3-phosphoglycerate dehydrogenase; TNK: tyrosine kinase; TECR: trans-2-enoyl-CoA reductase.
Figure 6.
Schematic overview of the effects of SIM on the expression of genes encoding for proteins involved in metabolic pathways in B16.F10 murine melanoma cells. Red outlines indicate the up-regulated genes and green outlines indicate the down-regulated genes which were revealed by RNA-Seq analysis. KEGG pathway maps served as sources for all reaction stages. Created in BioRender. Gabriela, G. (2024) BioRender.com/h93z324. Abbreviations: ACAT: acetyl-CoA acetyltransferase; ACLY: ATP citrate lyase; ACOX: acyl-CoA oxidase; ACSS2: acyl-CoA synthetase short-chain family member 2; ACC: acetyl-CoA carboxylase; ACOT: acyl-CoA thioesterase; ANPEP: alanyl aminopeptidase; CS: citrate synthase; COX: cytochrome c oxidase; ELOVL2: elongation of very long chain fatty acids protein 2; ELOVL6: elongation of very long chain fatty acids protein 6; ENO: enolase; FAD: flavin adenine dinucleotide; FASN-KS: fatty acid synthase keto synthase; FASN-KR: fatty acid synthase keto reductase; FASN-HD: fatty acid synthase hydroxydecarboxylase; FASN-ET: fatty acid synthase enoyl reductase; FAO: fatty acid oxidation; FAS: fatty acid synthase; FH1: fumarate hydratase 1; GALM: galactose-1-phosphate mutase; GCLC: gamma-glutamylcysteine ligase catalytic subunit; GFPT: glutamine-fructose-6-phosphate transaminase; GCLC: gamma-glutamylcysteine synthetase; GLUL: glutamine synthetase; GLUD: glutamate dehydrogenase; G6PD: glucose-6-phosphate dehydrogenase; GPT: glutamate-pyruvate transaminase; GPI: glucose-6-phosphate isomerase; GSR: glutathione reductase; GSS: glutathione synthetase; GST: glutathione S-transferase; HACD: 3-hydroxyacyl-CoA dehydratase; HADH: hydroxyacid dehydrogenase; H6PD: hexose-6-phosphate dehydrogenase; HK: hexokinase; HSD: hydroxysteroid dehydrogenase; IDH: isocitrate dehydrogenase; MCD: malonyl-CoA decarboxylase; MDH: malate dehydrogenase; NIT2: nitrite reductase; OGDH: 2-oxoglutarate dehydrogenase; PDH: pyruvate dehydrogenase; PFK: phosphofructokinase; PGD: 6-phosphogluconate dehydrogenase; PGDS: prostaglandin D2 synthase; PGK: phosphoglycerate kinase; PGK: phosphoglycerate kinase; PGLS: 6-phosphogluconolactonase; PGM: phosphoglucomutase; PGAM: phosphoglycerate mutase; PKM: pyruvate kinase M; PPP: pentose phosphate pathway; RPIA: ribose-5-phosphate isomerase A; SCD1: stearoyl-CoA desaturase 1; SCO: sterol-14α-demethylase; SDHC: succinate dehydrogenase cytochrome b subunit; SLC25A1: solute carrier family 25 member 1; SUCLG: succinate-CoA ligase gamma subunit; TAC: tricarboxylic acid cycle; TA: transaldolase; TALDO: transaldolase; TKT: transketolase; TGAPDH: 3-phosphoglycerate dehydrogenase; TNK: tyrosine kinase; TECR: trans-2-enoyl-CoA reductase.
3.3. Investigation of the SIM Effects the Metabolism of B16.F10 Melanoma Cells at Protein Level
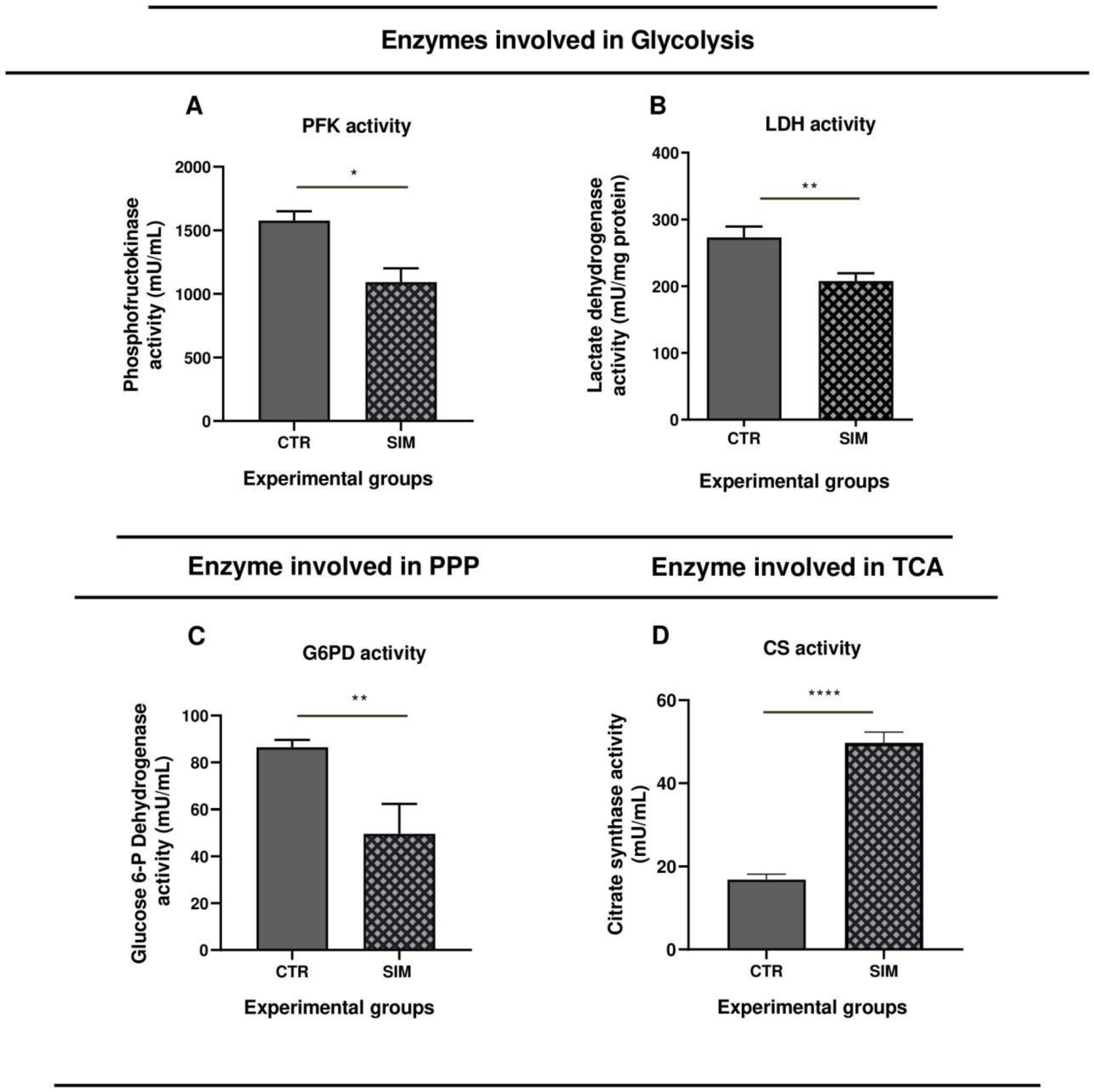
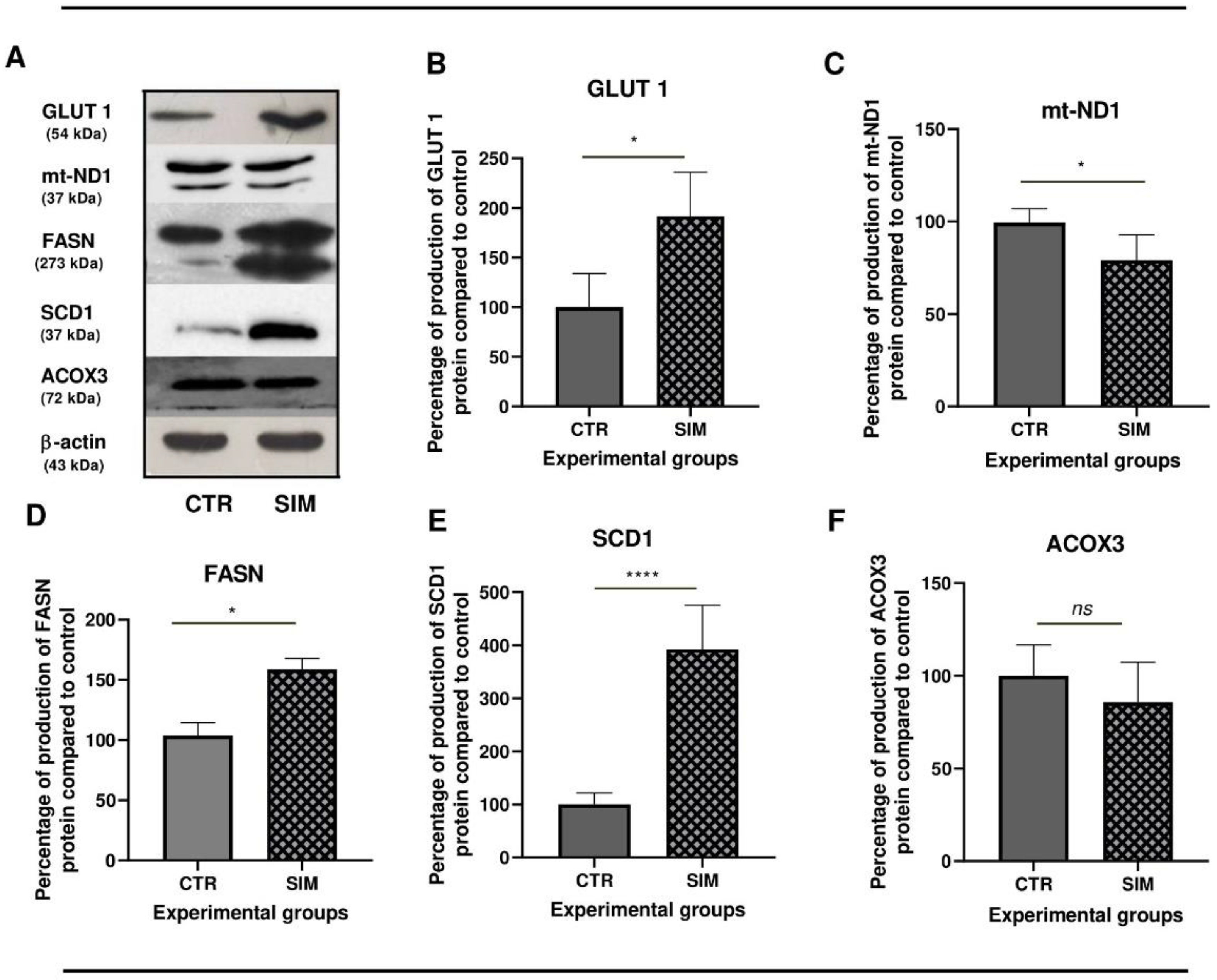
To compare transcriptomics data results with the effects of SIM at protein level, we checked several proteins with a key role in cancer cell metabolism for their catalytic activity, through enzymatic assays (Figure 9) and protein production (Figure 10) via western blot analysis. Firstly, to assess if SIM treatment had the ability to modulate the glucose metabolism, we investigated whether the uptake of glucose through one isoform of the family of facilitated glucose transporters, GLUT1 was affected by treatment. While SIM did not affect GLUT1 mRNA expression (p>0.05) (Supplementary Table S1. All genes), it doubled the protein levels (Figure 10A,B), suggesting an enhanced uptake of glucose in SIM-treated cells. Although our transcriptomics data suggested that SIM upregulated expression of genes involved in glycolysis (Table 4), the catalytic activities of key enzymes were reduced by the treatment by almost 30% (p<0.0001) for one of the rate-limiting enzymes of the pathway - PFK1 (Figure 9A), and by 25% for LDH (Figure 9B), indirectly suggesting reduced lactate production in SIM-treated group. Also, our transcriptomics data indicated upregulation of transcripts of genes in the PPP; however, the catalytic activity of G6PD, the rate-limiting enzyme, was decreased by 30% (p<0.01) (Figure 9C) indicating an overall reduced pathway activity. Hence, this alteration likely led to a decrease in the nicotinamide adenine dinucleotide phosphate (NADPH) synthesis, affecting both redox homeostasis and nucleotide synthesis in which PPP intermediates take part in. Despite transcriptomics information revealing no changes in expression of genes in TCA cycles, except IDH1 (Supplementary Table S1. All genes), there was a substantial threefold increase (p<0.0001) in the catalytic activity of CS (Figure 9D), the first rate-limiting enzyme, indicating a mild re-triggering of TCA cycle activity due to SIM administration compared to control. Although our transcriptomics data suggested that SIM upregulated expression of genes involved in OXPHOS and peroxisomal FAO, the protein expression of mt-ND1 was slightly reduced in SIM-treated cells compared to untreated cells (Table 4; Figure 10A,C). Using transcriptomics data we found that at mRNA level, both saturated FAS main enzyme FASN, and SCD1 the enzyme responsible for converting saturated FAs (SFAs) to UFAs exhibited significant increase in expression in SIM group compared to the control (Table 4). The expression signature was also seen at the protein level, with a significant twofold increase for FASN (Table 4; Figure 10 A,D), and threefold increase for SCD1 (Figure 10A,E) compared to control. The ACOX3 protein expression, enzyme crucial for peroxisomal FAO, was similar in SIM compared to the control (Table 4; Figure 10A,F). The observed increase in lipid metabolism and the preference for UFAs production in treated cells suggested an expected raise in the availability of monounsaturated fatty acids (MUFAs), providing protective effects against lipid peroxidation and endoplasmic reticulum (ER) stress (Gonzalez et al, 2018) and of polyunsaturated fatty acids (PUFAs), potentially leading to enhanced prostaglandins synthesis with significant implications for cellular processes (Wang et al, 2015; Ferreira et al, 2017).
Figure 7.
Supervised hierarchical clustering analysis of DEG in the control versus SIM-treated group in B16.F10 melanoma cells under normoxic conditions. Heatmap Clustering of 31 genes was generated using normalized FPKM values. Each column represents a biological replicate/group (CTR_R1, CTR_R2, CTR_R3 replicates of the gene expression in untreated cells and SIM_R1, SIM_R2, SIM_R3 replicates of the gene expression in SIM-treated cells), and each row represents a specific transcript. The level of each gene's expression in a single sample is displayed in accordance with the color scale, red color represents relative increase in transcript abundance (positive Z-scores; 0 < Z-score ≤ 1.5), blue color represents relative decrease (negative Z-scores; -1.50 ≤ Z-score < 0), while white indicates median gene expression (Z-score close to zero). Names of the corresponding metabolic pathway for each gene were listed on the right of the heatmap. Genes with FR ± 1.5, p < 0.05, FDR 20% were considered differentially expressed. Abbreviations: ACLY: ATP citrate lyase; ACSS2: acyl-CoA synthetase short-chain family member 2; ACOX3: acyl-CoA oxidase 3; ANPEP: alanyl aminopeptidase; ATGL: adipose triglyceride lipase; COX6B1: cytochrome c oxidase subunit 6B1; ELOVL6: elongation of very long chain fatty acids protein 6; ELOVL7: elongation of very long chain fatty acids protein 7; ENO2: enolase 2; FASN: fatty acid synthase; GFPT1: glutamine-fructose-6-phosphate transaminase 1; GSTT3: glutathione S-transferase theta 3; HACD2: 3-hydroxyacyl-CoA dehydratase 2; IDH1: isocitrate dehydrogenase 1; MAGL: monoacylglycerol lipase; mt-ATP8: mitochondrial ATP synthase subunit 8; mt-CO2: mitochondrial cytochrome c oxidase subunit 2; mt-CYTB: mitochondrial cytochrome b; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; mt-ND2: mitochondrial NADH dehydrogenase subunit 2; mt-ND4: mitochondrial NADH dehydrogenase subunit 4; mt-ND4L: mitochondrial NADH dehydrogenase subunit 4L; NRF1: nuclear respiratory factor 1; PGLS: 6-phosphogluconolactonase; PGDS: prostaglandin D2 synthase; PLD1: phospholipase D1; RRM1: ribonucleotide reductase subunit M1; RRM2: ribonucleotide reductase subunit M2; SCD1: stearoyl-CoA desaturase 1; SLC2A6: solute carrier family 2 member 6; SLC25A1: solute carrier family 25 member 1; SLC27A4: solute carrier family 27 member 4; SLC38A1: solute carrier family 38 member 1; SLC38A2: solute carrier family 38 member 2; SLC1A4: solute carrier family 1 member 4; SLC38A1: solute carrier family 38 member 1.
Figure 7.
Supervised hierarchical clustering analysis of DEG in the control versus SIM-treated group in B16.F10 melanoma cells under normoxic conditions. Heatmap Clustering of 31 genes was generated using normalized FPKM values. Each column represents a biological replicate/group (CTR_R1, CTR_R2, CTR_R3 replicates of the gene expression in untreated cells and SIM_R1, SIM_R2, SIM_R3 replicates of the gene expression in SIM-treated cells), and each row represents a specific transcript. The level of each gene's expression in a single sample is displayed in accordance with the color scale, red color represents relative increase in transcript abundance (positive Z-scores; 0 < Z-score ≤ 1.5), blue color represents relative decrease (negative Z-scores; -1.50 ≤ Z-score < 0), while white indicates median gene expression (Z-score close to zero). Names of the corresponding metabolic pathway for each gene were listed on the right of the heatmap. Genes with FR ± 1.5, p < 0.05, FDR 20% were considered differentially expressed. Abbreviations: ACLY: ATP citrate lyase; ACSS2: acyl-CoA synthetase short-chain family member 2; ACOX3: acyl-CoA oxidase 3; ANPEP: alanyl aminopeptidase; ATGL: adipose triglyceride lipase; COX6B1: cytochrome c oxidase subunit 6B1; ELOVL6: elongation of very long chain fatty acids protein 6; ELOVL7: elongation of very long chain fatty acids protein 7; ENO2: enolase 2; FASN: fatty acid synthase; GFPT1: glutamine-fructose-6-phosphate transaminase 1; GSTT3: glutathione S-transferase theta 3; HACD2: 3-hydroxyacyl-CoA dehydratase 2; IDH1: isocitrate dehydrogenase 1; MAGL: monoacylglycerol lipase; mt-ATP8: mitochondrial ATP synthase subunit 8; mt-CO2: mitochondrial cytochrome c oxidase subunit 2; mt-CYTB: mitochondrial cytochrome b; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; mt-ND2: mitochondrial NADH dehydrogenase subunit 2; mt-ND4: mitochondrial NADH dehydrogenase subunit 4; mt-ND4L: mitochondrial NADH dehydrogenase subunit 4L; NRF1: nuclear respiratory factor 1; PGLS: 6-phosphogluconolactonase; PGDS: prostaglandin D2 synthase; PLD1: phospholipase D1; RRM1: ribonucleotide reductase subunit M1; RRM2: ribonucleotide reductase subunit M2; SCD1: stearoyl-CoA desaturase 1; SLC2A6: solute carrier family 2 member 6; SLC25A1: solute carrier family 25 member 1; SLC27A4: solute carrier family 27 member 4; SLC38A1: solute carrier family 38 member 1; SLC38A2: solute carrier family 38 member 2; SLC1A4: solute carrier family 1 member 4; SLC38A1: solute carrier family 38 member 1.
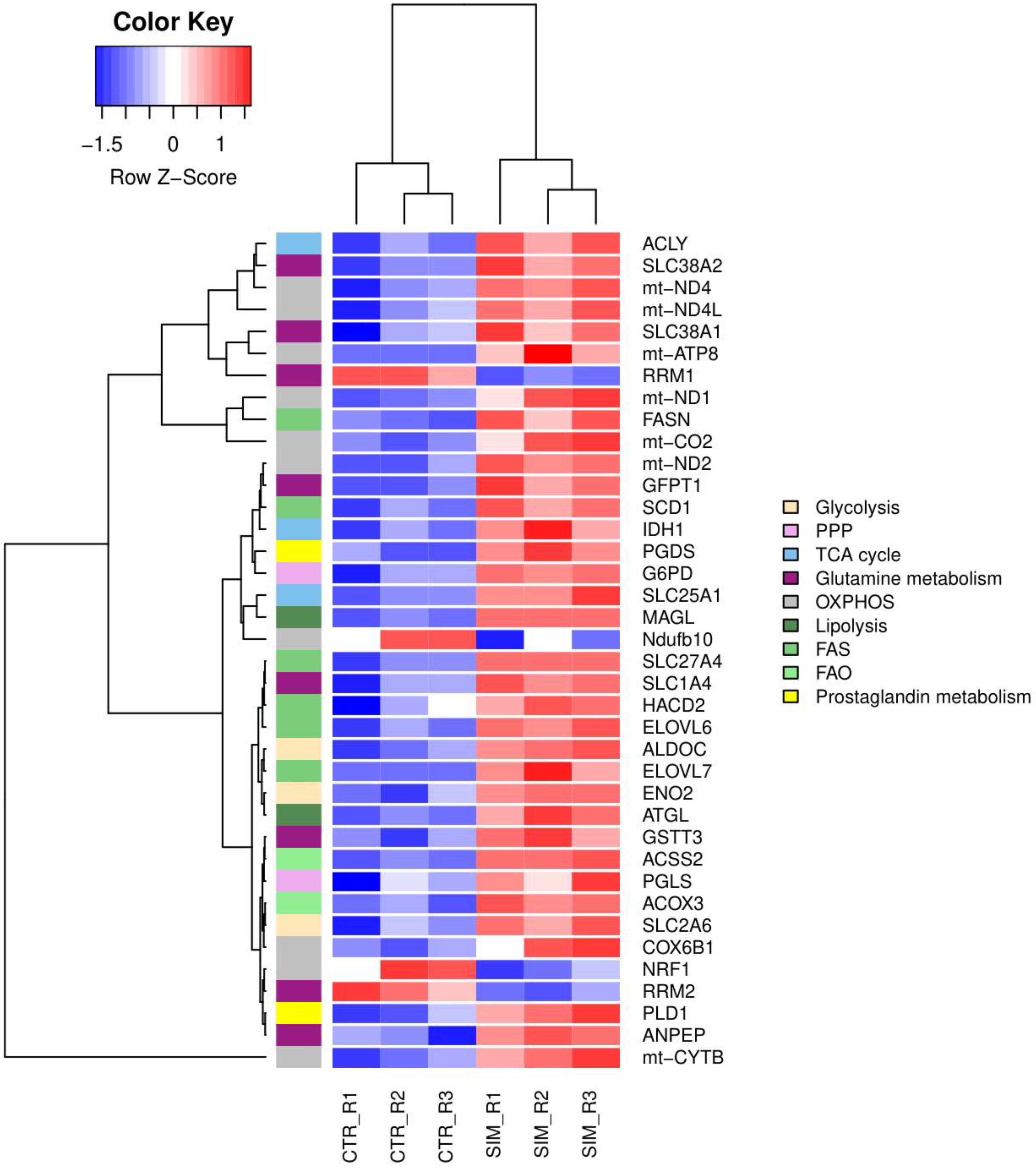
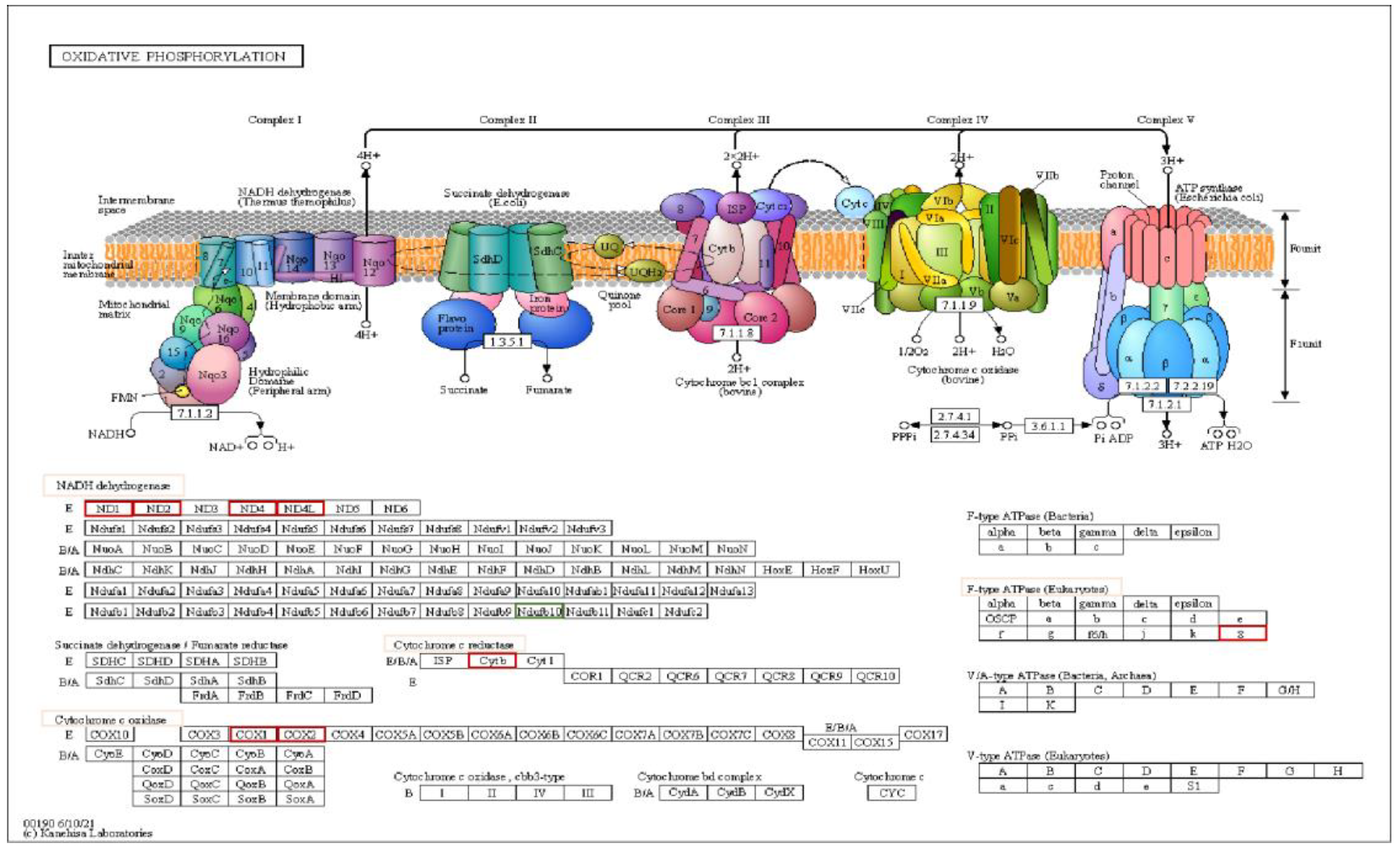
Figure 8.
Graphical visualization via KEGG pathways of significant DEG in SIM-treated cells compared to control cells involved in OXPHOS. Highlighted by red squares are genes that were up-regulated, while green indicate down-regulated genes in every complex of the respiratory chain system. Pink squares show the gene complexes whose expression had been influenced by SIM. Genes with FR ± 1.5, p<0.05 and FDR 20% were considered DEG. Abbreviations: COX6B1: cytochrome c oxidase subunit 6B1; DEG: differentially expressed genes; FDR: false discovery rate; FR: fold regulation; KEGG: Kyoto Encyclopedia of Genes and Genomes; mt-ATP8: mitochondrial ATP synthase subunit 8; mt-CO2: mitochondrial cytochrome c oxidase subunit 2; mt-CYTB: mitochondrial cytochrome b; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; mt-ND2: mitochondrial NADH dehydrogenase subunit 2; mt-ND4: mitochondrial NADH dehydrogenase subunit 4; mt-ND4L: mitochondrial NADH dehydrogenase subunit 4L; Ndufb10: NADH dehydrogenase:Ubiquinone Oxidoreductase Subunit B10; OXPHOS: oxidative phosphorilation.
Figure 8.
Graphical visualization via KEGG pathways of significant DEG in SIM-treated cells compared to control cells involved in OXPHOS. Highlighted by red squares are genes that were up-regulated, while green indicate down-regulated genes in every complex of the respiratory chain system. Pink squares show the gene complexes whose expression had been influenced by SIM. Genes with FR ± 1.5, p<0.05 and FDR 20% were considered DEG. Abbreviations: COX6B1: cytochrome c oxidase subunit 6B1; DEG: differentially expressed genes; FDR: false discovery rate; FR: fold regulation; KEGG: Kyoto Encyclopedia of Genes and Genomes; mt-ATP8: mitochondrial ATP synthase subunit 8; mt-CO2: mitochondrial cytochrome c oxidase subunit 2; mt-CYTB: mitochondrial cytochrome b; mt-ND1: mitochondrial NADH dehydrogenase subunit 1; mt-ND2: mitochondrial NADH dehydrogenase subunit 2; mt-ND4: mitochondrial NADH dehydrogenase subunit 4; mt-ND4L: mitochondrial NADH dehydrogenase subunit 4L; Ndufb10: NADH dehydrogenase:Ubiquinone Oxidoreductase Subunit B10; OXPHOS: oxidative phosphorilation.
4. Discussion
The present work provides a follow-up of our previous findings that inhibition of the constitutive expression of HIF-1 in B16.F10 murine melanoma cells is one of the main actions behind the cytotoxicity caused by SIM on these cancer cells (
Alupei et al, 2014; Licarete et al, 2017). As HIF-1 is an essential regulator of cancer cell metabolism under hypoxia, this study aimed to investigate whether SIM-induced reduction of the normoxic levels of HIF-1 might also affect melanoma cell metabolism (
DeBerardinis and Chandel, 2016; Munir et al, 2019; Shiratori et al, 2019). Since the translation of subunit α of HIF-1 (HIF-1α) is tightly linked to Akt/mTOR signaling (
Harada et al, 2009) and Akt is also a well-known central regulator of cancer metabolic reprogramming (
Semenza et al, 1996; Kuphal et al, 2010; Kwong and Davies, 2013; Koch et al, 2015;
Choi et al, 2016; Li et al, 2008; Xiao et al, 2017; Tian et al, 2023;
Harada et al, 2009;
Majumder et al, 2004), we also explored SIM effects on Akt mRNA and protein production in melanoma cells. Our data proved that pAkt/total Akt is also reduced after SIM-administration compared to control (
Figure 3), these findings being consistent with previous reports (
Ghosh-Choudhury et al, 2010; Hwang et al, 2011; Park et al, 2013). As SIM inhibited these two connected regulatory proteins, we further analyzed whether the energy and biosynthesis metabolic pathways of melanoma cells (
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10) were affected. In tight relation with the inhibitory effect of SIM on rate-limiting enzyme of cholesterol biosynthesis pathway (
Endo, 2008; Taylor et al, 2013; Beckwitt et al, 2018), the expression of the genes associated with isoprenoids production was assessed. Our transcriptomic results might suggest that inhibitory effects exerted by SIM on melanoma cell cholesterol metabolism were counteracted via upregulation of mRNA expression of genes encoding enzymes for isoprenoids production which finally will enhance cancer cell survival rates
(Bjarnadottir et al, 2015; Kimbung et al, 2016; Lazaro-Mixteco et al, 2022). With regard to glucose metabolism, although no changes at mRNA levels for PFK1 and LDH (
Supplementary Table S1) were noted in SIM-treated cells, catalytic activities of PFK1 and LDH were decreased while protein level of GLUT1 was elevated
(Figure 9 A,B; Figure 10 A,B), probably signaled through HIF-1-independent mechanisms (
Vander Heiden et al, 2001; Elstrom et al, 2004; Düvel et al, 2010; Jacobs et al, 2008; Yun et al 2009). Moreover, despite increased G6PD and PGD mRNA expression (
Table 4; Figures 6, 7) after SIM administration, catalytic activity of G6PD was suppressed (
Figure 9C), indicating a decrease in the glucose use via PPP. These data might suggest that in SIM-treated cells, low rates of glycolysis and PPP might be compensated via an enhanced influx of glucose via GLUT1 and reduced lactate production (
Le et al, 2010; Haugrud et al, 2014; Circu et al, 2017; Li and Cui, 2023) which in turn, will provide enough pyruvate to mitochondria ensuring metabolites for TCA cycle activity. Restoring activity of the TCA cycle was also supported by the data regarding the substantial increase in the catalytic activity of CS (
Figure 9D) as well as mRNA levels for IDH1 and glutamine metabolism (
Figures 2, 6, 7, Table 4, and
Supplementary Table S1) (
Chen et al, 2019; Ding et al, 2021; Aurora et al, 2022; Song et al, 2017). Nevertheless, the reduction of mt-ND1 protein levels (
Figure 10A,C) as well as overexpression of mRNA for SLC25A1 suggested that biosynthetic role of TCA was prioritized over its energy role in SIM-treated cells compared to untreated cells (
Table 4; Figures 6, 7) (
Simonnet et al, 2002; DeBerardinis et al, 2008; Lin et al, 2012; Chen et al, 2014; Mycielska et al, 2006; Cai et al, 2020; Isidoro et al, 2004; Chae et al, 2016). In addition to the efflux of citrate via SLC25A1 from mitochondrion to cytosol, SIM treatment favored the transcription of lipolysis enzymes (such as, MAGL and ATGL) (
Table 4 and
Figures 6, 7) which linked to cancer cell aggressiveness (
Hu et al, 2014; Nomura et al, 2010; Zhu et al, 2016), via accumulation of available FAs to support the synthesis of lipid-derived signaling molecules (
Wymann and Schneiter, 2008). Previous studies proved that in hypoxia, Akt promotes FAs and lipid synthesis by favoring FASN and ACLY availability (by transcription activation and protein phosphorylation) (
Berwick et al, 2002; Bauer et al, 2005). In normoxia, FASN expression was favored both at mRNA and protein level in SIM treated group compared to the control (
Table 4, Figure 10 A,D), suggesting high rates of FAS which give anti-apoptotic advantages contributing to chemoresistance (
Yang et al, 2011; Papaevangelou et al, 2018). However, we did not identified a negative-feedback of FASN over pAkt, or
vice versa in normoxic conditions, compared to the published data from hypoxic conditions (
Wang et al, 2013) (
Figure 3 A,C; Figure 10 A,D). Also, the elevated ACLY mRNA expression in SIM-treated cells supported our hypothesis about the role of TCA cycle to produce citrate for
de novo FAS (
Table 4, Figure 6,7). For protective effects against lipid peroxidation and ER-related stress, SIM-treated cells boosted both SCD1 mRNA and protein level to reduce SFAs level and increase UFAs level (
Table 4; Figures 6,7
and 10 A,E) (
Gonzalez et al, 2018; Piccolis et al, 2019; Ackerman et al, 2018) and upregulated ELOVL genes to favor FAE (
Table 4, Figure 6 and
Figure 7) (
Su et al, 2018; Naganuma et al, 2011). No significant increase in mitochondrial or peroxisomal FAO was seen in the SIM-treated group (
Figure 10 A,F), despite the reduced HIF-1 and pAkt protein levels, which prevent lipid catabolism in hypoxia (
Szutowicz et al, 1974; Flavin et al, 2010; Fazolini et al, 2015; Zhang, 2015; Huang et al, 2018). This suggested cancer cells might need less energy to survive than previously assumed (
Bartman et al, 2023). Our findings point to upregulation of prostaglandin synthesis post-treatment, considering the favored lipid metabolism that leads to high PUFAs availability (
Koundouros and Poulogiannis, 2020) and the increased PGD2 and PLD1 mRNA levels (
Table 4; Figure 7). The dual pro/anti-inflammatory role of PGDS remains debatable (
Breyer et al, 2001; Rajakariar et al, 2007; Fukuoka et al, 2015; Dash et al, 2022; Jiang et al, 2020), yet PGDS could serve as a significant diagnostic marker and treatment target (
Jiang et al, 2020).
Altogether, our study indicated that under normoxia, SIM inhibition of HIF-1 and Akt levels induced different effects on B16.F10 murine melanoma cell metabolism than those described previously under hypoxia (Phan et al, 2014). Our data suggested that SIM treatment orientated lipid metabolism to support isoprenoid and prostaglandin synthesis which might favor melanoma cell survival and aggressiveness. To counteract SIM induced metabolic plasticity, combination therapies should be explored (Yakes et al, 2011; Telang et al, 2014; Jones et al, 2019), potentially using 15-prostaglandin dehydrogenase (15-PGDH) to favor prostaglandin turnover (Backlund et al, 2008) and L-glutamine analogue L-γ-glutamyl-p-nitroanilide (GPNA) to inhibit glutamine transporters (Hassanein et al, 2013; Choi and Park, 2018).
Figure 9.
Effects of SIM on the catalytic activities of specific enzymes in B16.F10 melanoma cells. Graphical representations of catalytic activities of different enzymes in lysates of B16.F10 cells treated with 3.5 μM SIM for 24h in normoxia compared to control: (A) PFK activity expressed as mU/ml; (B) LDH activity expressed as mU/mg protein; (C) G6PD activity expressed as mU/ml; (D) CS activity represented as mU/ml. All data were expressed mean ± SD of at least three independent experiments. T-test was used for statistical analysis of the data; *, p < 0.05; **, p < 0.01; ****, p < 0.0001. Abbreviations: CS: citrate synthase; CTR: control group; G6PD: glucose-6-phosphate dehydrogenase; LDH: lactate dehydrogenase; PFK: phosphofructokinase; SIM: simvastatin treated group. .
Figure 9.
Effects of SIM on the catalytic activities of specific enzymes in B16.F10 melanoma cells. Graphical representations of catalytic activities of different enzymes in lysates of B16.F10 cells treated with 3.5 μM SIM for 24h in normoxia compared to control: (A) PFK activity expressed as mU/ml; (B) LDH activity expressed as mU/mg protein; (C) G6PD activity expressed as mU/ml; (D) CS activity represented as mU/ml. All data were expressed mean ± SD of at least three independent experiments. T-test was used for statistical analysis of the data; *, p < 0.05; **, p < 0.01; ****, p < 0.0001. Abbreviations: CS: citrate synthase; CTR: control group; G6PD: glucose-6-phosphate dehydrogenase; LDH: lactate dehydrogenase; PFK: phosphofructokinase; SIM: simvastatin treated group. .
Figure 10.
Western blot analysis of the effects of SIM on specific protein levels in B16.F10 melanoma cells. Western blot images (A) and their corresponding graphs showing the percentage of protein levels after administration of 3.5 μM SIM for 24h, in normoxia, as compared to control levels of the same proteins; (B) GLUT1 protein expression; (C) mt-ND1 protein expression; (D) FASN protein expression; (E) SCD1 protein expression; (F) ACOX3 and β-actin was used as loading control. All data were expressed mean ± SD of at least three independent experiments. T-test was used for statistical analysis of the data; *, p < 0.05; **, p < 0.01; ns: not significant. Abbreviations: ACOX3: acyl-CoA oxidase 3; β-actin: beta-actin; CTR: control group; FASN: fatty acid synthase; GLUT1: glucose transporter 1; mt-ND1: mitochondrially encoded NADH dehydrogenase 1; SCD1: stearoyl-CoA desaturase 1; SIM: simvastatin treated group.
Figure 10.
Western blot analysis of the effects of SIM on specific protein levels in B16.F10 melanoma cells. Western blot images (A) and their corresponding graphs showing the percentage of protein levels after administration of 3.5 μM SIM for 24h, in normoxia, as compared to control levels of the same proteins; (B) GLUT1 protein expression; (C) mt-ND1 protein expression; (D) FASN protein expression; (E) SCD1 protein expression; (F) ACOX3 and β-actin was used as loading control. All data were expressed mean ± SD of at least three independent experiments. T-test was used for statistical analysis of the data; *, p < 0.05; **, p < 0.01; ns: not significant. Abbreviations: ACOX3: acyl-CoA oxidase 3; β-actin: beta-actin; CTR: control group; FASN: fatty acid synthase; GLUT1: glucose transporter 1; mt-ND1: mitochondrially encoded NADH dehydrogenase 1; SCD1: stearoyl-CoA desaturase 1; SIM: simvastatin treated group.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Funding
This work was supported by L'Oréal - UNESCO “For Women in Science" [Fellowship Programme no. 914, 2020]; the UEFISCDI grant [PN-III-P2-2_1-PED-2021-0411, No. 659PED, 2022] granted to dr. Alina Sesarman.
Declaration of generative AI in scientific writing
Authors declare the use of generative AI (ChatGPT 3.5) in the writing process to improve the readability and language of the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the manuscript.
Abbreviations
15-prostaglandin dehydrogenase (1.15-PGDH), acetyl-Coenzyme A acetyl-transferase 2 (ACAT2), acyl-Coenzyme A oxidase 3 (ACOX3), protein kinase B (also known as serine/threonine-specific protein kinases) (Akt), aldolase C (ALDOC), adenosine monophosphate (AMP), alanyl aminopeptidase (ANPEP), adenosine diphosphate (ADP), adipose triglyceride lipase (ATGL), ATP citrate lyase (ACLY), citrate synthase (CS), differentially expressed genes (DEGs), 5,5'-Dithiobis-2-Nitrobenzoic Acid (DTNB), eukaryotic translation initiation factor 4A1 (eIF4A1), enolase 2 (Eno2), endoplasmic reticulum (ER), electron transport chain (ETC), elongation of long chain fatty acids family (ELOVL), fatty acids elongation (FAE), β-oxidation (FAO), fatty acids synthesis (FAS), fatty acid synthase (FASN), fatty acids (FAs), farnesyl diphosphate synthase (FDPS), forkhead box M1 (FOXM1), glucose 6-Phosphate Dehydrogenase (G6PD), glutamine fructose-6-phosphate transaminase 1 (GFPT1), glucose transporter 1 (GLUT1), L-γ-glutamyl-p-nitroanilide (GPNA), glutathione S-transferase theta 3 (GST theta 3), 3-hydroxy-acyl-CoA dehydrogenase (HACD2), hydroxy-3-methylglutaryl-Coenzyme A reductase (HMG-CR), 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 (HMG-CS1), heat shock protein 90 (HSP90), isopentenyl-diphosphate isomerase (IDI1), isocitrate dehydrogenase 1 (IDH1), insulin-induced gene protein 1 (INSIG-1), insulin-induced gene protein 2 (INSIG-2), Lactate Dehydrogenase (LDH), monoacylglycerol lipase (MAGL), monounsaturated fatty acids (MUFAs), mitochondrially encoded NADH dehydrogenase 1 (mt-ND1), mevalonate (diphospho) decarboxylase (MVD), nicotinamide adenine dinucleotide (NADH), nicotinamide adenine dinucleotide phosphate (NADPH), NADH Oxidoreductase Subunit B10 (Ndufb10), nuclear respiratory factor 1 (NRF1), oxidative phosphorylation (OXPHOS), pyruvate dehydrogenase (PDH), PDH kinase-1 (PDK-1), phosphofructokinase 1 (PFK1), 6-phosphogluconolactonase (PGLS), PTEN induced putative kinase 1 (PINK1), prostaglandin D2 synthase (PGDS), protein kinase B (PKB), phospholipase D1 (PLD1), pentose phosphate pathway (PPP), polyunsaturated fatty acids (PUFAs), ribosomal protein S6 (RPS6), ribosomal protein S6 kinase polypeptide 2 (Rps6kb2), ribonucleotide reductase 1 (RRM1), ribonucleotide reductase 2 (RRM2), simvastatin (SIM), stearyl Co-A desaturase 1 (SCD1), saturated fatty acids (SFA), solute carrier family 1 glutamate transporter, member 4 (SLC1A4), solute carrier family 2, member 1 (GLUT1) (SLC2A1), facilitated glucose transporter solute carrier family 2, member 6 (GLUT6) (SLC2A6), solute carrier family 25 member 1 (SLC25A1), solute carrier transporter member 4 (SLC27A4), solute carrier family 38, member 1 (SLC38A1), squalene epoxidase (SQLE), sterol regulatory element binding proteins (SREBP1), tricarboxylic acid cycle (TCA), unsaturated fatty acids (UFAs).
References
- Ackerman, D., Tumanov, S., Qiu, B., Michalopoulou, E., Spata, M., Azzam, A., Xie, H., Simon, M. C., & Kamphorst, J. J. (2018). Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell reports, 24(10), 2596–2605.e5. https://doi.org/10.1016/j.celrep.2018.08.015.
- Alice, A. F., Pérez-Martínez, G., & Sánchez-Rivas, C. (2002). Existence of a true phosphofructokinase in Bacillus sphaericus: cloning and sequencing of the pfk gene. Applied and environmental microbiology, 68(12), 6410–6415. https://doi.org/10.1128/AEM.68.12.6410-6415.2002.
- Alupei, M. C., Licarete, E., Cristian, F. B., & Banciu, M. (2014). Cytotoxicity of lipophilic statins depends on their combined actions on HIF-1α expression and redox status in B16.F10 melanoma cells. Anti-cancer drugs, 25(4), 393–405. https://doi.org/10.1097/CAD.0000000000000065.
- Alupei, M. C., Licarete, E., Patras, L., & Banciu, M. (2015). Liposomal simvastatin inhibits tumor growth via targeting tumor-associated macrophages-mediated oxidative stress. Cancer letters, 356(2 Pt B), 946–952. https://doi.org/10.1016/j.canlet.2014.11.010.
- Aurora, A. B., Khivansara, V., Leach, A., Gill, J. G., Martin-Sandoval, M., Yang, C., Kasitinon, S. Y., Bezwada, D., Tasdogan, A., Gu, W., Mathews, T. P., Zhao, Z., DeBerardinis, R. J., & Morrison, S. J. (2022). Loss of glucose 6-phosphate dehydrogenase function increases oxidative stress and glutaminolysis in metastasizing melanoma cells. Proceedings of the National Academy of Sciences of the United States of America, 119(6), e2120617119. https://doi.org/10.1073/pnas.2120617119.
- Backlund, M. G., Mann, J. R., Holla, V. R., Shi, Q., Daikoku, T., Dey, S. K., & DuBois, R. N. (2008). Repression of 15-hydroxyprostaglandin dehydrogenase involves histone deacetylase 2 and snail in colorectal cancer. Cancer research, 68(22), 9331–9337. https://doi.org/10.1158/0008-5472.CAN-08-2893.
- Barbariga, M., Vallone, F., Mosca, E., Bignami, F., Magagnotti, C., Fonteyne, P., Chiappori, F., Milanesi, L., Rama, P., Andolfo, A., & Ferrari, G. (2019). The role of extracellular matrix in mouse and human corneal neovascularization. Scientific reports, 9(1), 14272. https://doi.org/10.1038/s41598-019-50718-8.
- Bartman, C. R., Weilandt, D. R., Shen, Y., Lee, W. D., Han, Y., TeSlaa, T., Jankowski, C. S. R., Samarah, L., Park, N. R., da Silva-Diz, V., Aleksandrova, M., Gultekin, Y., Marishta, A., Wang, L., Yang, L., Roichman, A., Bhatt, V., Lan, T., Hu, Z., Xing, X., … Rabinowitz, J. D. (2023). Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature, 614(7947), 349–357. https://doi.org/10.1038/s41586-022-05661-6.
- Bauer, D. E., Hatzivassiliou, G., Zhao, F., Andreadis, C., & Thompson, C. B. (2005). ATP citrate lyase is an important component of cell growth and transformation. Oncogene, 24(41), 6314–6322. https://doi.org/10.1038/sj.onc.1208773.
- Beckwitt, C. H., Brufsky, A., Oltvai, Z. N., & Wells, A. (2018). Statin drugs to reduce breast cancer recurrence and mortality. Breast cancer research : BCR, 20(1), 144. https://doi.org/10.1186/s13058-018-1066-z.
- Benjamini Y, Yekutieli D. (2001). The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 29(4):1165–88. DOI: 10.1214/aos/1013699998.
- Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N., & Golani, I. (2001). Controlling the false discovery rate in behavior genetics research. Behavioural brain research, 125(1-2), 279–284. https://doi.org/10.1016/s0166-4328(01)00297-2.
- Berwick, D. C., Hers, I., Heesom, K. J., Moule, S. K., & Tavare, J. M. (2002). The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. The Journal of biological chemistry, 277(37), 33895–33900. https://doi.org/10.1074/jbc.M204681200.
- Bjarnadottir, O., Kimbung, S., Johansson, I., Veerla, S., Jönsson, M., Bendahl, P. O., Grabau, D., Hedenfalk, I., & Borgquist, S. (2015). Global Transcriptional Changes Following Statin Treatment in Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 21(15), 3402–3411. https://doi.org/10.1158/1078-0432.CCR-14-1403.
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry, 72, 248–254. https://doi.org/10.1006/abio.1976.9999.
- Breyer, R. M., Bagdassarian, C. K., Myers, S. A., & Breyer, M. D. (2001). Prostanoid receptors: subtypes and signaling. Annual review of pharmacology and toxicology, 41, 661–690. https://doi.org/10.1146/annurev.pharmtox.41.1.661.
- Cai, Z., Deng, Y., Ye, J., Zhuo, Y., Liu, Z., Liang, Y., Zhang, H., Zhu, X., Luo, Y., Feng, Y., Liu, R., Chen, G., Wu, Y., Han, Z., Liang, Y., Jiang, F., & Zhong, W. (2020). Aberrant Expression of Citrate Synthase is Linked to Disease Progression and Clinical Outcome in Prostate Cancer. Cancer management and research, 12, 6149–6163. https://doi.org/10.2147/CMAR.S255817.
- Cazzaniga, M., & Bonanni, B. (2015). Relationship Between Metabolic Reprogramming and Mitochondrial Activity in Cancer Cells. Understanding The Anticancer Effect of Metformin and Its Clinical Implications. Anticancer research, 35(11), 5789–5796.
- Chae, Y. C., Vaira, V., Caino, M. C., Tang, H. Y., Seo, J. H., Kossenkov, A. V., Ottobrini, L., Martelli, C., Lucignani, G., Bertolini, I., Locatelli, M., Bryant, K. G., Ghosh, J. C., Lisanti, S., Ku, B., Bosari, S., Languino, L. R., Speicher, D. W., & Altieri, D. C. (2016). Mitochondrial Akt Regulation of Hypoxic Tumor Reprogramming. Cancer cell, 30(2), 257–272. https://doi.org/10.1016/j.ccell.2016.07.004.
- Chen, L., Liu, T., Zhou, J., Wang, Y., Wang, X., Di, W., & Zhang, S. (2014). Citrate synthase expression affects tumor phenotype and drug resistance in human ovarian carcinoma. PloS one, 9(12), e115708. https://doi.org/10.1371/journal.pone.0115708.
- Chen, L., Zhang, Z., Hoshino, A., Zheng, H. D., Morley, M., Arany, Z., & Rabinowitz, J. D. (2019). NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nature metabolism, 1, 404–415. https://doi.org/10.1038/s42255-019-0043-x.
- Cheung, M., Sharma, A., Madhunapantula, S. V., & Robertson, G. P. (2008). Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer research, 68(9), 3429–3439. https://doi.org/10.1158/0008-5472.CAN-07-5867.
- Choi, S. W., Lee, K. S., Lee, J. H., Kang, H. J., Lee, M. J., Kim, H. Y., Park, K. I., Kim, S. L., Shin, H. K., & Seo, W. D. (2016). Suppression of Akt-HIF-1α signaling axis by diacetyl atractylodiol inhibits hypoxia-induced angiogenesis. BMB reports, 49(9), 508–513. https://doi.org/10.5483/bmbrep.2016.49.9.069.
- Choi, Y. K., & Park, K. G. (2018). Targeting Glutamine Metabolism for Cancer Treatment. Biomolecules & therapeutics, 26(1), 19–28. https://doi.org/10.4062/biomolther.2017.178.
- Circu, M. L., Maloney, R. E., & Aw, T. Y. (2017). Low glucose stress decreases cellular NADH and mitochondrial ATP in colonic epithelial cancer cells: Influence of mitochondrial substrates. Chemico-biological interactions, 264, 16–24. https://doi.org/10.1016/j.cbi.2017.01.001.
- Coimbra, M., Banciu, M., Fens, M. H., de Smet, L., Cabaj, M., Metselaar, J. M., Storm, G., & Schiffelers, R. M. (2010). Liposomal pravastatin inhibits tumor growth by targeting cancer-related inflammation. Journal of controlled release : official journal of the Controlled Release Society, 148(3), 303–310. https://doi.org/10.1016/j.jconrel.2010.09.011.
- Csanadi, A., Kayser, C., Donauer, M., Gumpp, V., Aumann, K., Rawluk, J., Prasse, A., zur Hausen, A., Wiesemann, S., Werner, M., & Kayser, G. (2015). Prognostic Value of Malic Enzyme and ATP-Citrate Lyase in Non-Small Cell Lung Cancer of the Young and the Elderly. PloS one, 10(5), e0126357. https://doi.org/10.1371/journal.pone.0126357.
- Dash, P., Ghatak, S., Topi, G., Satapathy, S. R., Ek, F., Hellman, K., Olsson, R., Mehdawi, L. M., & Sjölander, A. (2022). High PGD2 receptor 2 levels are associated with poor prognosis in colorectal cancer patients and induce VEGF expression in colon cancer cells and migration in a zebrafish xenograft model. British journal of cancer, 126(4), 586–597. https://doi.org/10.1038/s41416-021-01595-4.
- Davies, M. A., Stemke-Hale, K., Lin, E., Tellez, C., Deng, W., Gopal, Y. N., Woodman, S. E., Calderone, T. C., Ju, Z., Lazar, A. J., Prieto, V. G., Aldape, K., Mills, G. B., & Gershenwald, J. E. (2009). Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research, 15(24), 7538–7546. https://doi.org/10.1158/1078-0432.CCR-09-1985.
- DeBerardinis, R. J., & Chandel, N. S. (2016). Fundamentals of cancer metabolism. Science advances, 2(5), e1600200. https://doi.org/10.1126/sciadv.1600200.
- DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G., & Thompson, C. B. (2008). The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell metabolism, 7(1), 11–20. https://doi.org/10.1016/j.cmet.2007.10.002.
- Demas, D. M., Demo, S., Fallah, Y., Clarke, R., Nephew, K. P., Althouse, S., Sandusky, G., He, W., & Shajahan-Haq, A. N. (2019). Glutamine Metabolism Drives Growth in Advanced Hormone Receptor Positive Bre ast Cancer. Frontiers in oncology, 9, 686. https://doi.org/10.3389/fonc.2019.00686.
- Ding, H., Chen, Z., Wu, K., Huang, S. M., Wu, W. L., LeBoeuf, S. E., Pillai, R. G., Rabinowitz, J. D., & Papagiannakopoulos, T. (2021). Activation of the NRF2 antioxidant program sensitizes tumors to G6PD inhibition. Science advances, 7(47), eabk1023. https://doi.org/10.1126/sciadv.abk1023.
- Düvel, K., Yecies, J. L., Menon, S., Raman, P., Lipovsky, A. I., Souza, A. L., Triantafellow, E., Ma, Q., Gorski, R., Cleaver, S., Vander Heiden, M. G., MacKeigan, J. P., Finan, P. M., Clish, C. B., Murphy, L. O., & Manning, B. D. (2010). Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular cell, 39(2), 171–183. https://doi.org/10.1016/j.molcel.2010.06.022.
- Elstrom, R. L., Bauer, D. E., Buzzai, M., Karnauskas, R., Harris, M. H., Plas, D. R., Zhuang, H., Cinalli, R. M., Alavi, A., Rudin, C. M., & Thompson, C. B. (2004). Akt stimulates aerobic glycolysis in cancer cells. Cancer research, 64(11), 3892–3899. https://doi.org/10.1158/0008-5472.CAN-03-2904.
- Endo A. (2008). A gift from nature: the birth of the statins. Nature medicine, 14(10), 1050–1052. https://doi.org/10.1038/nm1008-1050.
- Fazolini, N. P., Cruz, A. L., Werneck, M. B., Viola, J. P., Maya-Monteiro, C. M., & Bozza, P. T. (2015). Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell cycle (Georgetown, Tex.), 14(16), 2667–2676. https://doi.org/10.1080/15384101.2015.1041684.
- Ferreira, M. T., Gomes, R. N., Panagopoulos, A. T., de Almeida, F. G., Veiga, J. C. E., & Colquhoun, A. (2018). Opposing roles of PGD2 in GBM. Prostaglandins & other lipid mediators, 134, 66–76. https://doi.org/10.1016/j.prostaglandins.2017.10.002.
- Ferreira, M. T., Gomes, R. N., Panagopoulos, A. T., de Almeida, F. G., Veiga, J. C. E., & Colquhoun, A. (2018). Opposing roles of PGD2 in GBM. Prostaglandins & other lipid mediators, 134, 66–76. https://doi.org/10.1016/j.prostaglandins.2017.10.002.
- Flavin, R., Peluso, S., Nguyen, P. L., & Loda, M. (2010). Fatty acid synthase as a potential therapeutic target in cancer. Future oncology (London, England), 6(4), 551–562. https://doi.org/10.2217/fon.10.11.
- Floares A. G., Floares C., Vermesan O., Popa T., Williams M., Ajibode S.,et al. (2011). Intelligent Clinical Decision Support Systems for Non-invasive Bladder Cancer Diagnosis. Lecture Notes in Computer Science, 253–262. doi:10.1007/978-3-642-21946-7_20.
- Fukuoka, T., Yashiro, M., Kinoshita, H., Morisaki, T., Hasegawa, T., Hirakawa, T., Aomatsu, N., Takeda, H., Maruyama, T., & Hirakawa, K. (2015). Prostaglandin D synthase is a potential novel therapeutic agent for the treatment of gastric carcinomas expressing PPARγ. International journal of cancer, 137(5), 1235–1244. https://doi.org/10.1002/ijc.29392.
- Gaglio, D., Metallo, C. M., Gameiro, P. A., Hiller, K., Danna, L. S., Balestrieri, C., Alberghina, L., Stephanopoulos, G., & Chiaradonna, F. (2011). Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Molecular systems biology, 7, 523. https://doi.org/10.1038/msb.2011.56.
- Ghosh-Choudhury, N., Mandal, C. C., Ghosh-Choudhury, N., & Ghosh Choudhury, G. (2010). Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cellular signalling, 22(5), 749–758. https://doi.org/10.1016/j.cellsig.2009.12.010.
- Gomes, R. N., Felipe da Costa, S., & Colquhoun, A. (2018). Eicosanoids and cancer. Clinics (Sao Paulo, Brazil), 73(suppl 1), e530s. https://doi.org/10.6061/clinics/2018/e530s.
- Gonzalez, P. S., O'Prey, J., Cardaci, S., Barthet, V. J. A., Sakamaki, J. I., Beaumatin, F., Roseweir, A., Gay, D. M., Mackay, G., Malviya, G., Kania, E., Ritchie, S., Baudot, A. D., Zunino, B., Mrowinska, A., Nixon, C., Ennis, D., Hoyle, A., Millan, D., McNeish, I. A., … Ryan, K. M. (2018). Mannose impairs tumour growth and enhances chemotherapy. Nature, 563(7733), 719–723. https://doi.org/10.1038/s41586-018-0729-3.
- Harada, H., Itasaka, S., Kizaka-Kondoh, S., Shibuya, K., Morinibu, A., Shinomiya, K., & Hiraoka, M. (2009). The Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. The Journal of biological chemistry, 284(8), 5332–5342. https://doi.org/10.1074/jbc.M806653200.
- Hassanein, M., Hoeksema, M. D., Shiota, M., Qian, J., Harris, B. K., Chen, H., Clark, J. E., Alborn, W. E., Eisenberg, R., & Massion, P. P. (2013). SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clinical cancer research : an official journal of the American Association for Cancer Research, 19(3), 560–570. https://doi.org/10.1158/1078-0432.CCR-12-2334.
- Haugrud, A. B., Zhuang, Y., Coppock, J. D., & Miskimins, W. K. (2014). Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast cancer research and treatment, 147(3), 539–550. https://doi.org/10.1007/s10549-014-3128-y.
- Hemmrich, K., Denecke, B., Paul, N. E., Hoffmeister, D., & Pallua, N. (2010). RNA Isolation from Adipose Tissue: An Optimized Procedure for High RNA Yield and Integrity. Laboratory Medicine, 41(2), 104–106. https://doi.org/10.1309/LMFSBPUOA19MH5BV.
- Hlouschek, J., Hansel, C., Jendrossek, V., & Matschke, J. (2018). The Mitochondrial Citrate Carrier (SLC25A1) Sustains Redox Homeostasis and Mitochondrial Metabolism Supporting Radioresistance of Cancer Cells With Tolerance to Cycling Severe Hypoxia. Frontiers in oncology, 8, 170. https://doi.org/10.3389/fonc.2018.00170.
- Hu, W. R., Lian, Y. F., Peng, L. X., Lei, J. J., Deng, C. C., Xu, M., Feng, Q. S., Chen, L. Z., Bei, J. X., & Zeng, Y. X. (2014). Monoacylglycerol lipase promotes metastases in nasopharyngeal carcinoma. International journal of clinical and experimental pathology, 7(7), 3704–3713.
- Huang, X., Liu, G., Guo, J., & Su, Z. (2018). The PI3K/AKT pathway in obesity and type 2 diabetes. International journal of biological sciences, 14(11), 1483–1496. https://doi.org/10.7150/ijbs.27173.
- Huijgen, H. J., Sanders, G. T., Koster, R. W., Vreeken, J., & Bossuyt, P. M. (1997). The clinical value of lactate dehydrogenase in serum: a quantitative review. European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies, 35(8), 569–579.
- Hwang, K. E., Na, K. S., Park, D. S., Choi, K. H., Kim, B. R., Shim, H., Jeong, E. T., & Kim, H. R. (2011). Apoptotic induction by simvastatin in human lung cancer A549 cells via Akt signaling dependent down-regulation of survivin. Investigational new drugs, 29(5), 945–952. https://doi.org/10.1007/s10637-010-9450-2.
- Isidoro, A., Martínez, M., Fernández, P. L., Ortega, A. D., Santamaría, G., Chamorro, M., Reed, J. C., & Cuezva, J. M. (2004). Alteration of the bioenergetic phenotype of mitochondria is a hallmark of breast, gastric, lung and oesophageal cancer. The Biochemical journal, 378(Pt 1), 17–20. https://doi.org/10.1042/BJ20031541.
- Istvan, E. S., & Deisenhofer, J. (2001). Structural mechanism for statin inhibition of HMG-CoA reductase. Science (New York, N.Y.), 292(5519), 1160–1164. https://doi.org/10.1126/science.1059344.
- Jiang, J., Pan, W., Xu, Y., Ni, C., Xue, D., Chen, Z., Chen, W., & Huang, J. (2020). Tumour-Infiltrating Immune Cell-Based Subtyping and Signature Gene Analysis in Breast Cancer Based on Gene Expression Profiles. Journal of Cancer, 11(6), 1568–1583. https://doi.org/10.7150/jca.37637.
- Jones, A. T., Narov, K., Yang, J., Sampson, J. R., & Shen, M. H. (2019). Efficacy of Dual Inhibition of Glycolysis and Glutaminolysis for Therapy of Renal Lesions in Tsc2+/- Mice. Neoplasia (New York, N.Y.), 21(2), 230–238. https://doi.org/10.1016/j.neo.2018.12.003.
- Kalyanaraman, B., Cheng, G., Hardy, M., Ouari, O., Lopez, M., Joseph, J., Zielonka, J., & Dwinell, M. B. (2018). A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox biology, 14, 316–327. https://doi.org/10.1016/j.redox.2017.09.020.
- Kerr, E. M., Gaude, E., Turrell, F. K., Frezza, C., & Martins, C. P. (2016). Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature, 531(7592), 110–113. https://doi.org/10.1038/nature16967.
- Kim, D., Langmead, B., & Salzberg, S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nature methods, 12(4), 357–360. https://doi.org/10.1038/nmeth.3317.
- Kimbung, S., Lettiero, B., Feldt, M., Bosch, A., & Borgquist, S. (2016). High expression of cholesterol biosynthesis genes is associated with resistance to statin treatment and inferior survival in breast cancer. Oncotarget, 7(37), 59640–59651. https://doi.org/10.18632/oncotarget.10746.
- Koch, A., Lang, S. A., Wild, P. J., Gantner, S., Mahli, A., Spanier, G., Berneburg, M., Müller, M., Bosserhoff, A. K., & Hellerbrand, C. (2015). Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget, 6(32), 32748–32760. https://doi.org/10.18632/oncotarget.4977-.
- Koo, C. Y., Muir, K. W., & Lam, E. W. (2012). FOXM1: From cancer initiation to progression and treatment. Biochimica et biophysica acta, 1819(1), 28–37. https://doi.org/10.1016/j.bbagrm.2011.09.004.
- Koundouros, N., & Poulogiannis, G. (2020). Reprogramming of fatty acid metabolism in cancer. British journal of cancer, 122(1), 4–22. https://doi.org/10.1038/s41416-019-0650-z.
- Kovacević, Z., & Morris, H. P. (1972). The role of glutamine in the oxidative metabolism of malignant cells. Cancer research, 32(2), 326–333.
- Kumar, K., Wigfield, S., Gee, H. E., Devlin, C. M., Singleton, D., Li, J. L., Buffa, F., Huffman, M., Sinn, A. L., Silver, J., Turley, H., Leek, R., Harris, A. L., & Ivan, M. (2013). Dichloroacetate reverses the hypoxic adaptation to bevacizumab and enhances its antitumor effects in mouse xenografts. Journal of molecular medicine (Berlin, Germany), 91(6), 749–758. https://doi.org/10.1007/s00109-013-0996-2.
- Kuphal, S., Winklmeier, A., Warnecke, C., & Bosserhoff, A. K. (2010). Constitutive HIF-1 activity in malignant melanoma. European journal of cancer (Oxford, England : 1990), 46(6), 1159–1169. https://doi.org/10.1016/j.ejca.2010.01.031.
- Kuzu, O. F., Noory, M. A., & Robertson, G. P. (2016). The Role of Cholesterol in Cancer. Cancer research, 76(8), 2063–2070. https://doi.org/10.1158/0008-5472.CAN-15-2613.
- Kwong, L. N., & Davies, M. A. (2013). Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research, 19(19), 5310–5319. https://doi.org/10.1158/1078-0432.CCR-13-0142.
- Lartigau, E., Randrianarivelo, H., Avril, M. F., Margulis, A., Spatz, A., Eschwège, F., & Guichard, M. (1997). Intratumoral oxygen tension in metastatic melanoma. Melanoma research, 7(5), 400–406. https://doi.org/10.1097/00008390-199710000-00006.
- Lázaro-Mixteco, P. E., González-Coronel, J. M., Hernández-Padilla, L., Martínez-Alcantar, L., Martínez-Carranza, E., López-Bucio, J. S., Guevara-García, Á. A., & Campos-García, J. (2022). Transcriptomics Reveals the Mevalonate and Cholesterol Pathways Blocking as Part of the Bacterial Cyclodipeptides Cytotoxic Effects in HeLa Cells of Human Cervix Adenocarcinoma. Frontiers in oncology, 12, 790537. https://doi.org/10.3389/fonc.2022.790537.
- Le, A., Cooper, C. R., Gouw, A. M., Dinavahi, R., Maitra, A., Deck, L. M., Royer, R. E., Vander Jagt, D. L., Semenza, G. L., & Dang, C. V. (2010). Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proceedings of the National Academy of Sciences of the United States of America, 107(5), 2037–2042. https://doi.org/10.1073/pnas.0914433107.
- Lehman, S. L., Wechsler, T., Schwartz, K., Brown, L. E., Porco, J. A., Devine, W. G., Pelletier, J., Shankavaram, U. T., Camphausen, K., & Tofilon, P. J. (2022). Inhibition of the Translation Initiation Factor eIF4A Enhances Tumor Cell Radiosensitivity. Molecular cancer therapeutics, 21(9), 1406–1414. https://doi.org/10.1158/1535-7163.MCT-22-0037.
- Li, L., Qu, Y., Mao, M., Xiong, Y., & Mu, D. (2008). The involvement of phosphoinositid 3-kinase/Akt pathway in the activation of hypoxia-inducible factor-1alpha in the developing rat brain after hypoxia-ischemia. Brain research, 1197, 152–158. https://doi.org/10.1016/j.brainres.2007.12.059.
- Li, Z., & Cui, J. (2023). Targeting the lactic acid metabolic pathway for antitumor therapy. Molecular therapy oncolytics, 31, 100740. https://doi.org/10.1016/j.omto.2023.100740.
- Licarete, E., Sesarman, A., Rauca, V. F., Luput, L., Patras, L., & Banciu, M. (2017). HIF-1α acts as a molecular target for simvastatin cytotoxicity in B16.F10 melanoma cells cultured under chemically induced hypoxia. Oncology letters, 13(5), 3942–3950. https://doi.org/10.3892/ol.2017.5928.
- Lin, C. C., Cheng, T. L., Tsai, W. H., Tsai, H. J., Hu, K. H., Chang, H. C., Yeh, C. W., Chen, Y. C., Liao, C. C., & Chang, W. T. (2012). Loss of the respiratory enzyme citrate synthase directly links the Warburg effect to tumor malignancy. Scientific reports, 2, 785. https://doi.org/10.1038/srep00785.
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.), 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262.
- Luput, L., Licarete, E., Drotar, D. M., Nagy, A. L., Sesarman, A., Patras, L., Rauca, V. F., Porfire, A., Muntean, D., Achim, M., Tomuta, I., Vlase, L., Catoi, C., Dragos, N., & Banciu, M. (2018). In Vivo Double Targeting of C26 Colon Carcinoma Cells and Microenvironmental Protumor Processes Using Liposomal Simvastatin. Journal of Cancer, 9(2), 440–449. https://doi.org/10.7150/jca.21560.
- Majumder, P. K., Febbo, P. G., Bikoff, R., Berger, R., Xue, Q., McMahon, L. M., Manola, J., Brugarolas, J., McDonnell, T. J., Golub, T. R., Loda, M., Lane, H. A., & Sellers, W. R. (2004). mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nature medicine, 10(6), 594–601. https://doi.org/10.1038/nm1052.
- McGrail, K., Granado-Martínez, P., Esteve-Puig, R., García-Ortega, S., Ding, Y., Sánchez-Redondo, S., Ferrer, B., Hernandez-Losa, J., Canals, F., Manzano, A., Navarro-Sabaté, A., Bartrons, R., Yanes, O., Pérez-Alea, M., Muñoz-Couselo, E., Garcia-Patos, V., & Recio, J. A. (2022). BRAF activation by metabolic stress promotes glycolysis sensitizing NRASQ61-mutated melanomas to targeted therapy. Nature communications, 13(1), 7113. https://doi.org/10.1038/s41467-022-34907-0.
- Mills, C. N., Joshi, S. S., & Niles, R. M. (2009). Expression and function of hypoxia inducible factor-1 alpha in human melanoma under non-hypoxic conditions. Molecular cancer, 8, 104. https://doi.org/10.1186/1476-4598-8-104.
- Mohamed, A., Deng, X., Khuri, F. R., & Owonikoko, T. K. (2014). Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clinical lung cancer, 15(1), 7–15. https://doi.org/10.1016/j.cllc.2013.09.001.
- Mouriaux, F., Sanschagrin, F., Diorio, C., Landreville, S., Comoz, F., Petit, E., Bernaudin, M., Rousseau, A. P., Bergeron, D., & Morcos, M. (2014). Increased HIF-1α expression correlates with cell proliferation and vascular markers CD31 and VEGF-A in uveal melanoma. Investigative ophthalmology & visual science, 55(3), 1277–1283. https://doi.org/10.1167/iovs.13-13345.
- Munir, R., Lisec, J., Swinnen, J. V., & Zaidi, N. (2019). Lipid metabolism in cancer cells under metabolic stress. British journal of cancer, 120(12), 1090–1098. https://doi.org/10.1038/s41416-019-0451-4.
- Mycielska, M. E., Broke-Smith, T. P., Palmer, C. P., Beckerman, R., Nastos, T., Erguler, K., & Djamgoz, M. B. (2006). Citrate enhances in vitro metastatic behaviours of PC-3M human prostate cancer cells: status of endogenous citrate and dependence on aconitase and fatty acid synthase. The international journal of biochemistry & cell biology, 38(10), 1766–1777. https://doi.org/10.1016/j.biocel.2006.04.008.
- Naganuma, T., Sato, Y., Sassa, T., Ohno, Y., & Kihara, A. (2011). Biochemical characterization of the very long-chain fatty acid elongase ELOVL7. FEBS letters, 585(20), 3337–3341. https://doi.org/10.1016/j.febslet.2011.09.024.
- Nomura, D. K., Long, J. Z., Niessen, S., Hoover, H. S., Ng, S. W., & Cravatt, B. F. (2010). Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell, 140(1), 49–61. https://doi.org/10.1016/j.cell.2009.11.027.
- Papaevangelou, E., Almeida, G. S., Box, C., deSouza, N. M., & Chung, Y. L. (2018). The effect of FASN inhibition on the growth and metabolism of a cisplatin-resistant ovarian carcinoma model. International journal of cancer, 143(4), 992–1002. https://doi.org/10.1002/ijc.31392.
- Park, Y. H., Jung, H. H., Ahn, J. S., & Im, Y. H. (2013). Statin induces inhibition of triple negative breast cancer (TNBC) cells via PI3K pathway. Biochemical and biophysical research communications, 439(2), 275–279. https://doi.org/10.1016/j.bbrc.2013.08.043.
- Patras, L., Ionescu, A. E., Munteanu, C., Hajdu, R., Kosa, A., Porfire, A., Licarete, E., Rauca, V. F., Sesarman, A., Luput, L., Bulzu, P., Chiroi, P., Tranca, R. A., Meszaros, M. S., Negrea, G., Barbu-Tudoran, L., Potara, M., Szedlacsek, S., & Banciu, M. (2022). Trojan horse treatment based on PEG-coated extracellular vesicles to deliver doxorubicin to melanoma in vitro and in vivo. Cancer biology & therapy, 23(1), 1–16. https://doi.org/10.1080/15384047.2021.2003656.
- Pavlova, N. N., & Thompson, C. B. (2016). The Emerging Hallmarks of Cancer Metabolism. Cell metabolism, 23(1), 27–47. https://doi.org/10.1016/j.cmet.2015.12.006.
- Pertea, M., Kim, D., Pertea, G. M., Leek, J. T., & Salzberg, S. L. (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nature protocols, 11(9), 1650–1667. https://doi.org/10.1038/nprot.2016.095.
- Pertea, M., Pertea, G. M., Antonescu, C. M., Chang, T. C., Mendell, J. T., & Salzberg, S. L. (2015). StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature biotechnology, 33(3), 290–295. https://doi.org/10.1038/nbt.3122.
- Petrohai, A., Nagy, G., Bosze, S., Hudecz, F., Zsiros, E., Paragh, G., Nyárády, Z., Németh, P., & Berki, T. (2005). Detection of citrate synthase-reacting autoantibodies after heart transplantation: an epitope mapping study. Transplant international: official journal of the European Society for Organ Transplantation, 17(12), 834–840. https://doi.org/10.1007/s00147-004-0794-4.
- Phan, L. M., Yeung, S. C., & Lee, M. H. (2014). Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer biology & medicine, 11(1), 1–19. https://doi.org/10.7497/j.issn.2095-3941.2014.01.001.
- Piccolis, M., Bond, L. M., Kampmann, M., Pulimeno, P., Chitraju, C., Jayson, C. B. K., Vaites, L. P., Boland, S., Lai, Z. W., Gabriel, K. R., Elliott, S. D., Paulo, J. A., Harper, J. W., Weissman, J. S., Walther, T. C., & Farese, R. V., Jr (2019). Probing the Global Cellular Responses to Lipotoxicity Caused by Saturated Fatty Acids. Molecular cell, 74(1), 32–44.e8. https://doi.org/10.1016/j.molcel.2019.01.036.
- Porfire, A., Tomuta, I., Muntean, D., Luca, L., Licarete, E., Alupei, M. C., Achim, M., Vlase, L., & Banciu, M. (2015). Optimizing long-circulating liposomes for delivery of simvastatin to C26 colon carcinoma cells. Journal of liposome research, 25(4), 261–269. https://doi.org/10.3109/08982104.2014.987787.
- Qian, X., Hu, J., Zhao, J., & Chen, H. (2015). ATP citrate lyase expression is associated with advanced stage and prognosis in gastric adenocarcinoma. International journal of clinical and experimental medicine, 8(5), 7855–7860.
- Rajakariar, R., Hilliard, M., Lawrence, T., Trivedi, S., Colville-Nash, P., Bellingan, G., Fitzgerald, D., Yaqoob, M. M., & Gilroy, D. W. (2007). Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proceedings of the National Academy of Sciences of the United States of America, 104(52), 20979–20984. https://doi.org/10.1073/pnas.0707394104.
- Rauca, V. F., Licarete, E., Luput, L., Sesarman, A., Patras, L., Bulzu, P., Rakosy-Tican, E., & Banciu, M. (2018). Combination therapy of simvastatin and 5, 6-dimethylxanthenone-4-acetic acid synergistically suppresses the aggressiveness of B16.F10 melanoma cells. PloS one, 13(8), e0202827. https://doi.org/10.1371/journal.pone.0202827.
- Rauca, V. F., Patras, L., Luput, L., Licarete, E., Toma, V. A., Porfire, A., Mot, A. C., Rakosy-Tican, E., Sesarman, A., & Banciu, M. (2021). Remodeling tumor microenvironment by liposomal codelivery of DMXAA and simvastatin inhibits malignant melanoma progression. Scientific reports, 11(1), 22102. https://doi.org/10.1038/s41598-021-01284-5.
- Robinson, M. D., McCarthy, D. J., & Smyth, G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England), 26(1), 139–140. https://doi.org/10.1093/bioinformatics/btp616.
- Semenza G. L. (1998). Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Current opinion in genetics & development, 8(5), 588–594. https://doi.org/10.1016/s0959-437x(98)80016-6.
- Semenza G. L. (2012). Hypoxia-inducible factors in physiology and medicine. Cell, 148(3), 399–408. https://doi.org/10.1016/j.cell.2012.01.021.
- Shiratori, R., Furuichi, K., Yamaguchi, M., Miyazaki, N., Aoki, H., Chibana, H., Ito, K., & Aoki, S. (2019). Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Scientific reports, 9(1), 18699. https://doi.org/10.1038/s41598-019-55296-3.
- Silvente-Poirot, S., & Poirot, M. (2014). Cancer. Cholesterol and cancer, in the balance. Science (New York, N.Y.), 343(6178), 1445–1446. https://doi.org/10.1126/science.1252787.
- Simonnet, H., Alazard, N., Pfeiffer, K., Gallou, C., Béroud, C., Demont, J., Bouvier, R., Schägger, H., & Godinot, C. (2002). Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis, 23(5), 759–768. https://doi.org/10.1093/carcin/23.5.759.
- Song, Z., Wei, B., Lu, C., Li, P., & Chen, L. (2017). Glutaminase sustains cell survival via the regulation of glycolysis and glutaminolysis in colorectal cancer. Oncology letters, 14(3), 3117–3123. https://doi.org/10.3892/ol.2017.6538.
- Srere PA. (1969). Citrate synthase: EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating). Methods Enzymology.13:3-11. doi: 10.1016/0076-6879(69)13005-0.
- Stahl, J. M., Sharma, A., Cheung, M., Zimmerman, M., Cheng, J. Q., Bosenberg, M. W., Kester, M., Sandirasegarane, L., & Robertson, G. P. (2004). Deregulated Akt3 activity promotes development of malignant melanoma. Cancer research, 64(19), 7002–7010. https://doi.org/10.1158/0008-5472.CAN-04-1399.
- Stine, Z. E., Walton, Z. E., Altman, B. J., Hsieh, A. L., & Dang, C. V. (2015). MYC, Metabolism, and Cancer. Cancer discovery, 5(10), 1024–1039. https://doi.org/10.1158/2159-8290.CD-15-0507.
- Su, Y. C., Feng, Y. H., Wu, H. T., Huang, Y. S., Tung, C. L., Wu, P., Chang, C. J., Shiau, A. L., & Wu, C. L. (2018). Elovl6 is a negative clinical predictor for liver cancer and knockdown of Elovl6 reduces murine liver cancer progression. Scientific reports, 8(1), 6586. https://doi.org/10.1038/s41598-018-24633-3.
- Szutowicz, A., Kwiatkowski, J., & Angielski, S. (1979). Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. British journal of cancer, 39(6), 681–687. https://doi.org/10.1038/bjc.1979.120.
- Taylor, F., Huffman, M. D., Macedo, A. F., Moore, T. H., Burke, M., Davey Smith, G., Ward, K., & Ebrahim, S. (2013). Statins for the primary prevention of cardiovascular disease. The Cochrane database of systematic reviews, 2013(1), CD004816. https://doi.org/10.1002/14651858.CD004816.pub5.
- Telang, S., O’Neal, J., Tapolsky, G., Clem, B., Kerr, A., Imbert-Ferndandez, Y., & Chesney, J. (2014). Discovery of a PFKFB3 inhibitor for phase I trial testing that synergizes with the B-Raf inhibitor vemurafenib. Cancer & Metabolism, 2(Suppl 1), P14. https://doi.org/10.1186/2049-3002-2-S1-P14.
- Tian, Y., Zhao, L., Gui, Z., Liu, S., Liu, C., Yu, T., & Zhang, L. (2023). PI3K/AKT signaling activates HIF1α to modulate the biological effects of invasive breast cancer with microcalcification. NPJ breast cancer, 9(1), 93. https://doi.org/10.1038/s41523-023-00598-z.
- Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, N.Y.), 324(5930), 1029–1033. https://doi.org/10.1126/science.1160809.
- Vander Heiden, M. G., Plas, D. R., Rathmell, J. C., Fox, C. J., Harris, M. H., & Thompson, C. B. (2001). Growth factors can influence cell growth and survival through effects on glucose metabolism. Molecular and cellular biology, 21(17), 5899–5912. https://doi.org/10.1128/MCB.21.17.5899-5912.2001.
- Wang, D., & Dubois, R. N. (2010). Eicosanoids and cancer. Nature reviews. Cancer, 10(3), 181–193. https://doi.org/10.1038/nrc2809.
- Wang, D., Fu, L., Sun, H., Guo, L., & DuBois, R. N. (2015). Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology, 149(7), 1884–1895.e4. https://doi.org/10.1053/j.gastro.2015.07.064.
- Wang, D., Wan, B., Zhang, X., Sun, P., Lu, S., Liu, C., & Zhu, L. (2022). Nuclear respiratory factor 1 promotes the growth of liver hepatocellular carcinoma cells via E2F1 transcriptional activation. BMC gastroenterology, 22(1), 198. https://doi.org/10.1186/s12876-022-02260-7.
- Wang, H., Luo, Q. F., Peng, A. F., Long, X. H., Wang, T. F., Liu, Z. L., Zhang, G. M., Zhou, R. P., Gao, S., Zhou, Y., & Chen, W. Z. (2014). Positive feedback regulation between Akt phosphorylation and fatty acid synthase expression in osteosarcoma. International journal of molecular medicine, 33(3), 633–639. https://doi.org/10.3892/ijmm.2013.1602.
- Wang, Y., Yun, Y., Wu, B., Wen, L., Wen, M., Yang, H., Zhao, L., Liu, W., Huang, S., Wen, N., & Li, Y. (2016). FOXM1 promotes reprogramming of glucose metabolism in epithelial ovarian cancer cells via activation of GLUT1 and HK2 transcription. Oncotarget, 7(30), 47985–47997. https://doi.org/10.18632/oncotarget.10103.
- Warburg, O., (1925). The metabolism of carcinoma cells. Journal Cancer Research; 9:148–63. doi: 10.1158/jcr.1925.148.
- WARBURG, O., (1956). On the origin of cancer cells. Science (New York, N.Y.), 123(3191), 309–314. https://doi.org/10.1126/science.123.3191.309.
- Weber, L. W., Boll, M., & Stampfl, A. (2004). Maintaining cholesterol homeostasis: sterol regulatory element-binding proteins. World journal of gastroenterology, 10(21), 3081–3087. https://doi.org/10.3748/wjg.v10.i21.3081.
- Weinguny, M., Klanert, G., Eisenhut, P., Jonsson, A., Ivansson, D., Lövgren, A., & Borth, N. (2020). Directed evolution approach to enhance efficiency and speed of outgrowth during single cell subcloning of Chinese Hamster Ovary cells. Computational and structural biotechnology journal, 18, 1320–1329. https://doi.org/10.1016/j.csbj.2020.05.020.
- Wymann, M. P., & Schneiter, R. (2008). Lipid signalling in disease. Nature reviews. Molecular cell biology, 9(2), 162–176. https://doi.org/10.1038/nrm2335.
- Xiao, Y., Peng, H., Hong, C., Chen, Z., Deng, X., Wang, A., Yang, F., Yang, L., Chen, C., & Qin, X. (2017). PDGF Promotes the Warburg Effect in Pulmonary Arterial Smooth Muscle Cells via Activation of the PI3K/AKT/mTOR/HIF-1α Signaling Pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 42(4), 1603–1613. https://doi.org/10.1159/000479401.
- Yabe, D., Brown, M. S., & Goldstein, J. L. (2002). Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proceedings of the National Academy of Sciences of the United States of America, 99(20), 12753–12758. https://doi.org/10.1073/pnas.162488899.
- Yakes, F. M., Chen, J., Tan, J., Yamaguchi, K., Shi, Y., Yu, P., Qian, F., Chu, F., Bentzien, F., Cancilla, B., Orf, J., You, A., Laird, A. D., Engst, S., Lee, L., Lesch, J., Chou, Y. C., & Joly, A. H. (2011). Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Molecular cancer therapeutics, 10(12), 2298–2308. https://doi.org/10.1158/1535-7163.MCT-11-0264.
- Yang, T., Espenshade, P. J., Wright, M. E., Yabe, D., Gong, Y., Aebersold, R., Goldstein, J. L., & Brown, M. S. (2002). Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell, 110(4), 489–500. https://doi.org/10.1016/s0092-8674(02)00872-3.
- Yang, Y., Liu, H., Li, Z., Zhao, Z., Yip-Schneider, M., Fan, Q., Schmidt, C. M., Chiorean, E. G., Xie, J., Cheng, L., Chen, J. H., & Zhang, J. T. (2011). Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. International journal of biochemistry and molecular biology, 2(1), 89–98.
- Yoshida, T., Ohki, S., Kanazawa, M., Mizunuma, H., Kikuchi, Y., Satoh, H., Andoh, Y., Tsuchiya, A., & Abe, R. (1998). Inhibitory effects of prostaglandin D2 against the proliferation of human colon cancer cell lines and hepatic metastasis from colorectal cancer. Surgery today, 28(7), 740–745. https://doi.org/10.1007/BF02484622.
- Zacharias, N. M., McCullough, C., Shanmugavelandy, S., Lee, J., Lee, Y., Dutta, P., McHenry, J., Nguyen, L., Norton, W., Jones, L. W., & Bhattacharya, P. K. (2017). Metabolic Differences in Glutamine Utilization Lead to Metabolic Vulnerabilities in Prostate Cancer. Scientific reports, 7(1), 16159. https://doi.org/10.1038/s41598-017-16327-z.
- Zhang H. (2015). HIF-1 suppresses lipid catabolism to promote cancer progression. Molecular & cellular oncology, 2(4), e980184. https://doi.org/10.4161/23723556.2014.980184.
- Zhang, D., Liu, J., Mi, X., Liang, Y., Li, J., & Huang, C. (2014). The N-terminal region of p27 inhibits HIF-1α protein translation in ribosomal protein S6-dependent manner by regulating PHLPP-Ras-ERK-p90RSK axis. Cell death & disease, 5(11), e1535. https://doi.org/10.1038/cddis.2014.496.
- Zhang, J. X., Lau, E., Paik, D. T., Zhuge, Y., & Wu, J. C. (2020). High-throughput Preparation of DNA, RNA, and Protein from Cryopreserved Human iPSCs for Multi-omics Analysis. Current protocols in stem cell biology, 54(1), e114. https://doi.org/10.1002/cpsc.114.
- Zhang, J., Niu, Y., & Huang, C. (2017). Role of FoxM1 in the Progression and Epithelial to Mesenchymal Transition of Gastrointestinal Cancer. Recent patents on anti-cancer drug discovery, 12(3), 247–259. https://doi.org/10.2174/1574892812666170424144352.
- Zhu, W., Zhao, Y., Zhou, J., Wang, X., Pan, Q., Zhang, N., Wang, L., Wang, M., Zhan, D., Liu, Z., He, X., Ma, D., Liu, S., & Wang, L. (2016). Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial-mesenchymal transition. Journal of hematology & oncology, 9(1), 127. https://doi.org/10.1186/s13045-016-0361-3.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
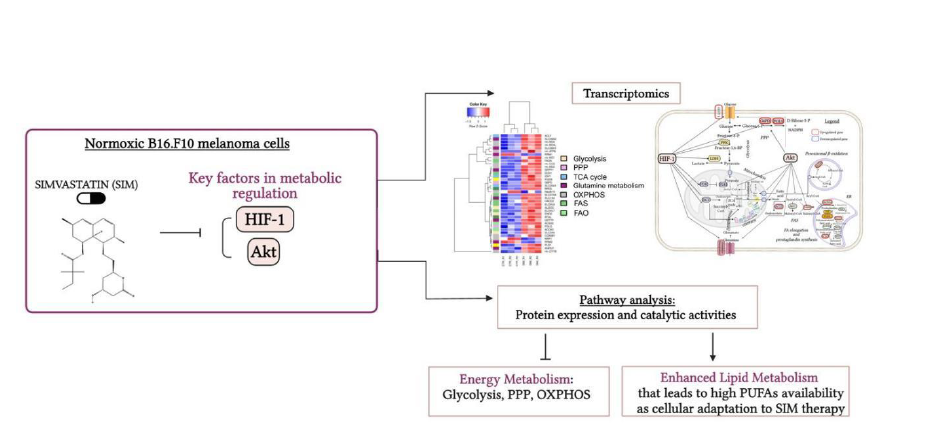
 Graphical abstract. Simvastatin favored Lipid Metabolism in B16.F10 Melanoma Cells via modulation of the constitutive level of HIF-1. Abbreviations: Akt: Protein kinase B, FAO: Fatty Acid Oxidation, FAS: Fatty Acid Synthesis, HIF-1: Hypoxia-Inducible Factor 1, HMG-CoA: 3-Hydroxy-3-Methylglutaryl-Coenzyme A, HMC-CR: HMG-CoA Reductase, OXPHOS: Oxidative Phosphorylation, PPP: Pentose Phosphate Pathway, PUFAs: Polyunsaturated Fatty Acids, SIM: Simvastatin, TCA Cycle: Tricarboxylic Acid Cycle. Created in BioRender. Gabriela, G. (2024) BioRender.com/j03y911
Graphical abstract. Simvastatin favored Lipid Metabolism in B16.F10 Melanoma Cells via modulation of the constitutive level of HIF-1. Abbreviations: Akt: Protein kinase B, FAO: Fatty Acid Oxidation, FAS: Fatty Acid Synthesis, HIF-1: Hypoxia-Inducible Factor 1, HMG-CoA: 3-Hydroxy-3-Methylglutaryl-Coenzyme A, HMC-CR: HMG-CoA Reductase, OXPHOS: Oxidative Phosphorylation, PPP: Pentose Phosphate Pathway, PUFAs: Polyunsaturated Fatty Acids, SIM: Simvastatin, TCA Cycle: Tricarboxylic Acid Cycle. Created in BioRender. Gabriela, G. (2024) BioRender.com/j03y911