Submitted:
19 September 2024
Posted:
20 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Role of Oxidative Stress in Neurodegenerative Diseases
2.1. Alzheimer’s Disease (AD)
2.2. Parkinson’s Disease (PD)
2.3. Huntington's Disease (HD)
2.4. Amyotrophic Lateral Sclerosis (ALS)
3. Antioxidant Effects of Aromatic Plant Extracts in Neurodegenerative Diseases
3.1. Polyphenol Antioxidants
| Compounds | Types of study | Cell line(s)/animal model(s) | Type of Disease | Mechanism of action/metabolic effects | References |
|---|---|---|---|---|---|
| Curcumin | In vitro | Aβ-induced SH-SY5Y cells | AD | Restore mitochondrial membrane potential; Reduce the expression of mitochondrial apoptotic proteins such as cytochrome c, caspase-3 and Bax; Restore normalized activity and expression of SOD1, SOD2 and catalase; Reduce the expression of total GSK-3β and phospho-Ser9-GSK-3β |
[89] |
| In vivo | Rotenone-induced swiss albino mice | PD | Reduce alpha-synaptic nucleoprotein aggregation; Elevate levels of DA and NA in brain tissue; Reduce MDA levels and increasing SOD, GSH activity |
[87] | |
| In vivo | 6-OHDA-induced SD rat | PD | Elevate dopamine levels; Increase SOD and GSH activity |
[109] | |
| In vitro | Aβ1-42 induction in primary cortical neurons of Wistar albino rats | AD | Decrease MDA level, inhibit lipid peroxidation; Elevate GSH, CAT levels and increase SOD activity; Increase neurotrophic factor levels |
[110] | |
| In vivo | 6-OHDA-induced SD rats | PD | Reduce MDA content, inhibit lipid peroxidation; Elevate SOD and GPx levels and increases Ach activity. |
[111] | |
| In vivo+ In vitro | 6-OHDA-induced SD rats+ SD rat primary neurons | PD | Increase SOD and GPx levels and decreased MDA levels; Increase Wnt3a, β-catenin protein and mRNA expression and c-myc and cyclinD1 mRNA expression |
[88] | |
| In vitro | AβO-induced SH-SY5Y cells | AD | Reduce ROS generation and attenuates oxidative stress Inhibit Tau hyperphosphorylation; |
[112] | |
| Chlorogenic Acid | In vivo | MPTP-induced Swiss albino mice | PD | Increase mtGSH Mn-SOD levels inhibited the activation of pro-apoptotic proteins (including Bax and caspase-3) while increasing the expression of anti-apoptotic proteins (e.g. Bcl-2) | [94] |
| In vitro | Aβ-induced primary hippocampal neurons of SD rats | AD | Elevate SOD and GPx levels | [113] | |
| In vivo | APP/PS1 transgenic mice | AD | Reduce the expression of IL-1β, IL-6 and TNF-α; Reduce the levels of MDA and H2O2, elevate the levels of SOD, CAT and GPx, and inhibit ROS generation; Reduce Aβ deposition and attenuate neuronal damage; Activate the SIRT1/PGC-1α signaling pathway |
[95] | |
| In vivo+ In vitro | Rotenone-induced C57BL/6 mice+ GLUTag cells | PD | Reduce MDA content in the striatum and cortex, elevate GSH levels, and attenuate oxidative damage; Restore the expression of colonic GPR-40 and GPR-43; Up-regulate the expression of GLP-1 receptor in colon, striatum and cortex; Reduce the accumulation of α-synuclein; Reduce dopaminergic neuron loss |
[114] | |
| Ellagic Acid | In vitro | Aβ25-35-induced PC12 cells | AD | Inhibit ROS production; Reduce calcium production. |
[115] |
| In vivo | AlCl3-induced Wistar rat | AD | Elevate SOD and GSH levels; Restore the normal structure of neurons; Down-regulate APP and caspase expression |
[116] | |
| In vivo | QA-induced Wistar rat | HD | Attenuate AchE activity; Increase the level of CAT in the cortex, restore the level of SOD in the cerebral cortex and the level of GSH in the striatum; Decrease the levels of IL-6, TNF-α; Decrease the level of caspase-3 |
[104] | |
| Honokiol | In vivo+ In vitro | hSOD1-G93A transgenic mice + NSC-34 cells transfected with SOD1 G93A | ALS | Activate NRF2-ARE pathway, increases GSH, CAT, GSR activities, and decreases MDA content; Restore mitochondrial function and morphology |
[108] |
| In vivo+ In vitro | Aβ1-42-induced C57BL/6 mice + AβO-induced primary hippocampal neurons of SD rats | AD | Decrease the production of Bax and caspase-9, increases the expression of Bcl2, and reduce neuronal apoptosis; Inhibit ROS generation, attenuate oxidative stress |
[117] |
3.2. Terpenoid Antioxidants
| Compounds | Types of study | Cell line(s)/animal model(s) | Type of Disease | Mechanism of action/metabolic effects | References |
|---|---|---|---|---|---|
| Linalool | In vivo | Aβ-induced C57BL/6 mice | AD | Elevate SOD and GPx activities; Decrease malondialdehyde levels; Decrease the expression of caspase-9 and caspase-3 |
[144] |
| In vivo | AD Drosophila model + Aβ-induced SD rats | AD | Reduce the level of ROS in the optic disc of Drosophila larvae; Reduce the level of lipid peroxidation product 4-HNE in brain tissue of AD rats |
[126] | |
| In vivo | AlCl3-induced C57BL/6 mouse | AD | Reduce MDA content and inhibits lipid peroxidation; Restore normal levels of SOD and GPx; Activate the Nrf2/HO-1 signaling pathway to reduce oxidative stress; Upregulate CaMKII protein level, improving synaptic plasticity; Increase the level of BDNF |
[145] | |
| In vivo+ In vitro | MPP+-induced SHSY5Y cells + MPTP-induced C57BL/6 mice | PD | Increase protein expression of nuclear Nrf2 and cytoplasmic HO-1 and down-regulatedgp91phox expression in SHSY5Y cells; Inhibite ROS generation and attenuate oxidative stress; |
[127] | |
| Safranal | In vitro | rotenone-induced dopaminergic neurons | PD | Inhibition of ROS generation in dopaminergic neurons by Nrf2-induced downstream antioxidant enzyme genes including GST, GCLs, NQO1 and HO-1-inducible | [146] |
| In vivo | Aβ1-40-induced Wistar rat | AD | Reduce MDA, ROS, protein carbonyl, IL-1β, IL-6, TNF α, NF-kB, and apoptotic biomarkers including cystatinase 3 and acetylcholinesterase activities in hippocampus, and improve SOD activity and mitochondrial membrane potential levels | [133] | |
| In vivo | 3-NP-induced Wistar rats | HD | Prevent 3-NP-induced increasing in nitrite and MDA levels, as well as decreasing in SOD, catalase activity, and GSH. | [134] | |
| In vivo | QA-induced Wistar rat | HD | Reduction of QA-induced lipid peroxidation and oxidative DNA damage prevents QA-generated reduction of hippocampal thiol redox and antioxidant status. | [147] | |
| β-Caryophyllene | In vivo | Rotenone- induced Wistar rats | PD | Prevent the loss of dopaminergic neurons and striatal nerve fibers; Reduce MDA level bar and prevent GSH depletion; Restore SOD and CAT activity; Reduce IL-1β, IL-6, and TNF-a levels; Reduce COX-2 and iNOS expression. |
[136] |
| In vitro | MPP+-treated SH-SY5Y cells | PD | Inhibit MPP+-induced lactate dehydrogenase release and ROS production and increase intracellular GSH and GPx activity; Decrease caspase-3 and Bax levels and increase Bcl-2 expression; |
[148] | |
| In vivo | MPTP-induced C57BL/6 mice | PD | Enhancement of NQO1 expression and enzyme activity inhibits oxidative stress-induced cell death in MPTP-exposed dopaminergic neurons | [149] | |
| Limonene | In vivo | Rotenone- induced Wistar rats | PD | Decrease dopaminergic neuron loss; Increase levels of BDNF and decreased accumulation of alpha-synuclein; Decrease MDA levels and increase activity/concentration of SOD, catalase and GSH; Reduce levels of TNF-α, IL-1β and IL-6; Reduce expression of iNOS, COX-2, P-NF-κ B and P-I κ B in the striatum; Reduce ROT-induced phosphorylation of MAPK signaling proteins in the striatum |
[139] |
| 1,8-cineole | In vitro | Aβ1-42-induced C.elegans | AD | Significantly reduce ROS levels; Activate the SKN-1/Nrf-2 pathway and upregulates the expression of SKN-1, GCS-1 and GST-4" |
[137] |
| In vitro | Aβ25-35-induced PC12 cells | AD | CIN significantly reduced ROS levels in AB25-35 cells in a dose-dependent relationship | [150] | |
| Arteannuin | In vivo+ In vitro | MPTP-induced C57BL/6 mice + (MPP+)-induced PC12 cells | PD | Increase the level of Nrf2 DNA binding activity and its regulated proteins HO-1 and NQO1 in PC12 cells and mouse brain tissue Attenuate the cytotoxicity of MPP+ and decreased the level of ROS; Reduce mitochondrial membrane potential and cleaved cysteine-3 activity Reduce dopaminergic neuron loss in mice |
[143] |
3.3. Flavonoid Antioxidants
| Compounds | Types of study | Cell line(s)/animal model(s) | Type of Disease | Mechanism of action/metabolic effects | References |
|---|---|---|---|---|---|
| Quercetin | In vitro | H2O2-induced SH-SY5Y cells | AD | Reduce hydrogen peroxide-induced reactive oxygen species production, apoptosis, β-site amyloid precursor protein cleaving enzyme 1 expression and Aβ accumulation in SH-SY5Y cells | [157] |
| In vivo | Streptozotocin-induced Wistar rat | AD | Increase SOD and CAT activities; Elevate total antioxidant levels in hippocampus; Reduce MDA content and inhibit lipid peroxidation |
[169] | |
| In vivo | AlCl3-induced Wistar rats | AD | Decrease AchE activity Increase the expression level of antioxidant enzymes SOD1, CAT and GPx, decrease the expression level of iNOS, and reduce ROS generation; Increase the expression of anti-apoptotic gene Bcl2 and decrease the expression of pro-apoptotic gene BAX |
[158] | |
| In vivo | 6-OHDA-induced Wistar rat | PD | Reduce AchE activity; Decrease MDA content and inhibit lipid peroxidation; Increase the activity of SOD, GPx, CAT |
[159] | |
| In vitro | H2O2-induced PC12 cells | PD | Reduce ROS generation, lower MDA content and inhibit lipid peroxidation; Enhance the activities of CAT, SOD, and GPx; Increase Bcl2, decreased Bax expression, decrease expression of cleaved caspase-3 and p53, and decrease apoptosis |
[170] | |
| In vivo | 3-NP-induced Wistar rat | HD | Restoration of SOD and CAT activity; Restoration of mitochondrial function. |
[160] | |
| Naringin | In vivo | Aβ1-42 and manganese induction in Wistar rats | AD | Attenuate elevation of MDA and nitrite concentrations and restore CAT and GSH levels Restore mitochondrial enzyme complex (I, II and IV) activity and enhance the protective effect of AChE; Attenuate the elevation of TNF-α; Reduce the precipitation of Aβ |
[171] |
| In vivo | AlCl3-induced albino rat | AD | Reduce cerebellar iNOS expression and phosphorylation of Tau protein Decrease cerebellar iNOS expression and Tau protein phosphorylation |
[162] | |
| In vitro | 6-OHDA-induced Parkinsonian zebrafish + 6-OHDA-induced SH-SY5Y cells | PD | Increase GSH, SOD and CAT levels and attenuates oxidative stress; Decrease ROS production; Increase mitochondrial membrane potential; Downregulate the expression levels of lrrk2, polg and caspase9 genes |
[172] | |
| In vivo | Vanadium-induced Wistar rats | PD | Effective improvement of GPx, CAT | [173] | |
| In vivo | MPTP-induced C57BL/6J mice | PD | Increase the activity of glutathione reductase and catalase, reduce the content of LPO, and reverse the toxic effect of MPTP. | [174] | |
| In vivo | 3-NP-induced Wistar rats | HD | Significantly reduced lipid peroxidation, nitrite concentration, restored superoxide dismutase and catalase activity | [163] | |
| Hesperidin | In vivo | AlCl3-induced Albino Wistar rats | AD | Elevate GSH levels, increase SOD, CAT, GPx activities, and reduce oxidative stress; Decrease Bax levels, increase Bcl2 levels, and decrease cellular autophagy |
[175] |
| In vitro | Rotenone-induced SK-N-SH cells | PD | Increase GSH levels and activities of SOD, CAT, and GPx; Inhibite the generation of ROS; Increase intracellular ATP levels; Restore mitochondrial membrane potential; Increase Bcl-2 expression and decreased Bax expression |
[165] | |
| Glycyrrhizic acid | In vivo | 3-NP-induced Wistar albino rats | HD | Restore GSH, SOD, and Nrf2 activity, inhibits malondialdehyde activity Decrease TNF-α, IL-1β, and IL-6 levels and reduce inflammatory response, and Increase BDNF content, improve neuronal damage |
[168] |
| In vivo | Rotenone-induced Wistar rats | PD | Increase antioxidant enzyme activity, inhibit glutathione depletion, inhibit lipid peroxidation, and attenuate dopaminergic neuron loss | [46] |
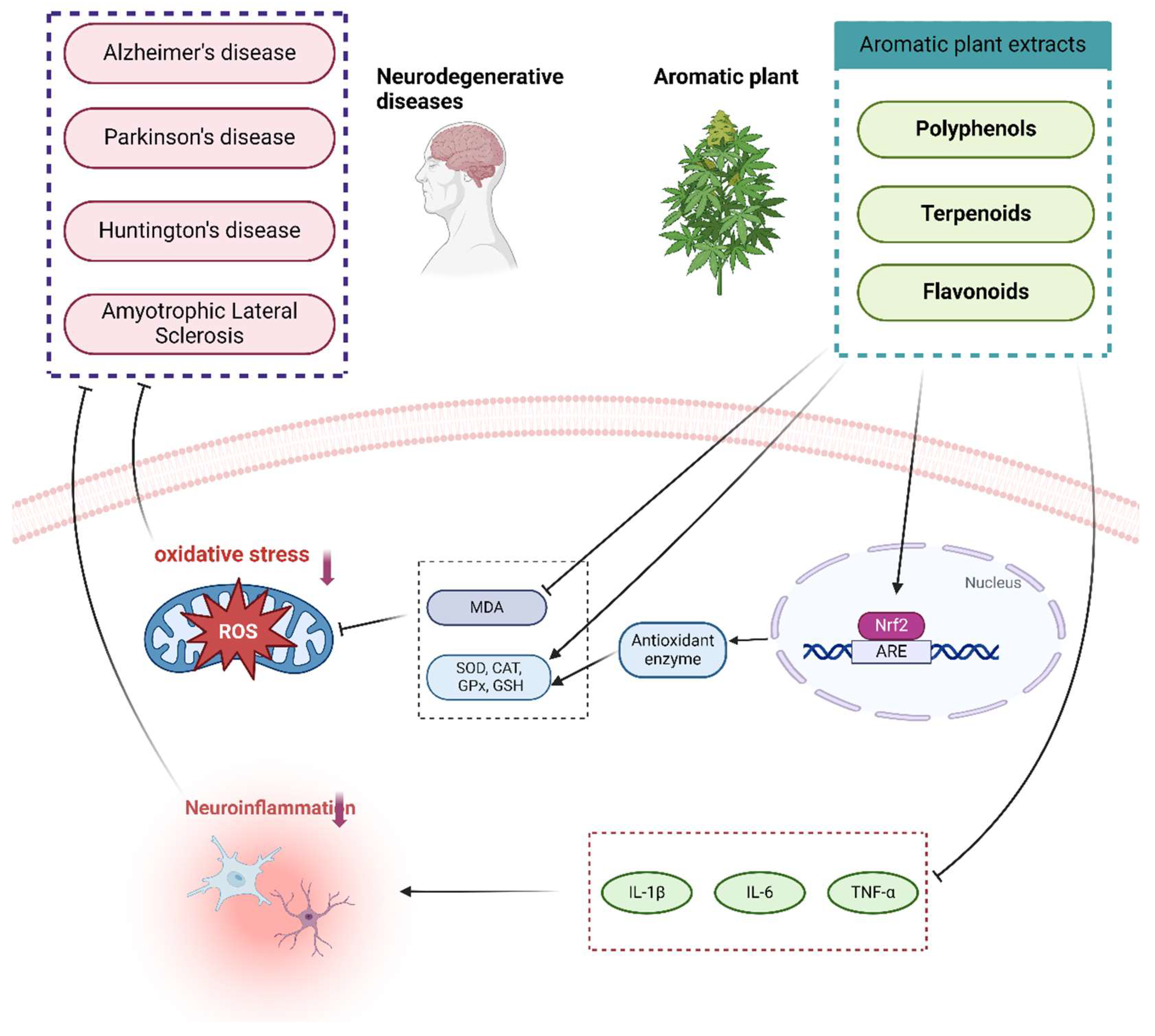
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Du, W.; Zhao, Y.; Lim, K.; Lu, L.; Zhang, C.; Li, L. Mitochondria targeting drugs for neurodegenerative diseases-Design, mechanism and application. Acta Pharm Sin B 2022, 12, 2778-2789. [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci Ther 2017, 23, 5-22. [CrossRef]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int J Mol Sci 2024, 25. [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11. [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 2018, 15, 490-503. [CrossRef]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants (Basel) 2023, 12. [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 2017, 39, 73-82. [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 2015, 97, 55-74. [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013, 2013, 956792. [CrossRef]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease. Prog Neurobiol 2016, 147, 1-19. [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 2010, 4, 118-126. [CrossRef]
- Cadet, J.; Davies, K.J.A. Oxidative DNA damage & repair: An introduction. Free Radic Biol Med 2017, 107, 2-12. [CrossRef]
- Perry, N.; Perry, E. Aromatherapy in the management of psychiatric disorders: clinical and neuropharmacological perspectives. CNS Drugs 2006, 20, 257-280. [CrossRef]
- Proestos, C.; Varzakas, T. Aromatic Plants: Antioxidant Capacity and Polyphenol Characterisation. Foods 2017, 6. [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res 2007, 21, 308-323. [CrossRef]
- Ma, Y.; Li, Y.; Yin, R.; Guo, P.; Lei, N.; Li, G.; Xiong, L.; Xie, Y. Therapeutic potential of aromatic plant extracts in Alzheimer's disease: Comprehensive review of their underlying mechanisms. CNS Neurosci Ther 2023, 29, 2045-2059. [CrossRef]
- Villemure, C.; Bushnell, M.C. Mood influences supraspinal pain processing separately from attention. J Neurosci 2009, 29, 705-715. [CrossRef]
- Haze, S.; Sakai, K.; Gozu, Y. Effects of fragrance inhalation on sympathetic activity in normal adults. Jpn J Pharmacol 2002, 90, 247-253. [CrossRef]
- Sun, Z.T.; Ma, C.; Li, G.J.; Zheng, X.Y.; Hao, Y.T.; Yang, Y.; Wang, X. Application of Antibody Fragments Against Aβ With Emphasis on Combined Application With Nanoparticles in Alzheimer's Disease. Front Pharmacol 2021, 12, 654611. [CrossRef]
- Behl, T.; Kaur, I.; Fratila, O.; Brata, R.; Bungau, S. Exploring the Potential of Therapeutic Agents Targeted towards Mitigating the Events Associated with Amyloid-β Cascade in Alzheimer's Disease. Int J Mol Sci 2020, 21. [CrossRef]
- Coronel, R.; Bernabeu-Zornoza, A.; Palmer, C.; Muñiz-Moreno, M.; Zambrano, A.; Cano, E.; Liste, I. Role of Amyloid Precursor Protein (APP) and Its Derivatives in the Biology and Cell Fate Specification of Neural Stem Cells. Mol Neurobiol 2018, 55, 7107-7117. [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer's Disease. Which Comes First: The Chicken or the Egg? Antioxidants (Basel) 2021, 10. [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid Med Cell Longev 2017, 2017, 2525967. [CrossRef]
- Ferreiro, E.; Oliveira, C.R.; Pereira, C.M.F. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol Dis 2008, 30, 331-342. [CrossRef]
- Hwang, S.; Kim, J.K. Effects of NADPH Oxidase Inhibitors and Mitochondria-Targeted Antioxidants on Amyloid β(1-42)-Induced Neuronal Deaths in Mouse Mixed Cortical Cultures. Chonnam Med J 2018, 54, 159-166. [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Aragno, M.; Borghi, R.; Autelli, R.; Giliberto, L.; Muraca, G.; Danni, O.; Zhu, X.; Smith, M.A.; et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem 2008, 104, 683-695. [CrossRef]
- Ibáñez-Salazar, A.; Bañuelos-Hernández, B.; Rodríguez-Leyva, I.; Chi-Ahumada, E.; Monreal-Escalante, E.; Jiménez-Capdeville, M.E.; Rosales-Mendoza, S. Oxidative Stress Modifies the Levels and Phosphorylation State of Tau Protein in Human Fibroblasts. Front Neurosci 2017, 11, 495. [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer's disease. Ageing Res Rev 2022, 77, 101619. [CrossRef]
- Dias-Santagata, D.; Fulga, T.A.; Duttaroy, A.; Feany, M.B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J Clin Invest 2007, 117, 236-245. [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 2021, 17, 157-172. [CrossRef]
- Faller, P.; Hureau, C. A bioinorganic view of Alzheimer's disease: when misplaced metal ions (re)direct the electrons to the wrong target. Chemistry 2012, 18, 15910-15920. [CrossRef]
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson's disease. Science 2003, 302, 819-822. [CrossRef]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and Pathogenesis of Parkinson's Syndrome. Annu Rev Pathol 2023, 18, 95-121. [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson's disease. Lancet 2024, 403, 293-304. [CrossRef]
- Robea, M.A.; Balmus, I.M.; Ciobica, A.; Strungaru, S.; Plavan, G.; Gorgan, L.D.; Savuca, A.; Nicoara, M. Parkinson's Disease-Induced Zebrafish Models: Focussing on Oxidative Stress Implications and Sleep Processes. Oxid Med Cell Longev 2020, 2020, 1370837. [CrossRef]
- Imbriani, P.; Martella, G.; Bonsi, P.; Pisani, A. Oxidative stress and synaptic dysfunction in rodent models of Parkinson's disease. Neurobiol Dis 2022, 173, 105851. [CrossRef]
- Ammal Kaidery, N.; Ahuja, M.; Thomas, B. Crosstalk between Nrf2 signaling and mitochondrial function in Parkinson's disease. Mol Cell Neurosci 2019, 101, 103413. [CrossRef]
- Leem, E.; Kim, S.R. Limited therapeutic potential of astrocyte elevated gene-1 transduction in an animal model of Parkinson's disease. Neural Regen Res 2020, 15, 1850-1851. [CrossRef]
- Leem, E.; Kim, H.J.; Choi, M.; Kim, S.; Oh, Y.S.; Lee, K.J.; Choe, Y.S.; Um, J.Y.; Shin, W.H.; Jeong, J.Y.; et al. Upregulation of neuronal astrocyte elevated gene-1 protects nigral dopaminergic neurons in vivo. Cell Death Dis 2018, 9, 449. [CrossRef]
- Nam, J.H.; Leem, E.; Jeon, M.T.; Jeong, K.H.; Park, J.W.; Jung, U.J.; Kholodilov, N.; Burke, R.E.; Jin, B.K.; Kim, S.R. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: neuroprotective mechanisms of hRheb(S16H) in a model of Parkinson's disease. Mol Neurobiol 2015, 51, 487-499. [CrossRef]
- Araújo, B.; Caridade-Silva, R.; Soares-Guedes, C.; Martins-Macedo, J.; Gomes, E.D.; Monteiro, S.; Teixeira, F.G. Neuroinflammation and Parkinson's Disease-From Neurodegeneration to Therapeutic Opportunities. Cells 2022, 11. [CrossRef]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson's Disease. Cells 2023, 12. [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid Redox Signal 2017, 27, 989-1010. [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson's disease. Neural Regen Res 2021, 16, 1383-1391. [CrossRef]
- Krishnamoorthy, A.; Sevanan, M.; Mani, S.; Balu, M.; Balaji, S.; P, R. Chrysin restores MPTP induced neuroinflammation, oxidative stress and neurotrophic factors in an acute Parkinson's disease mouse model. Neurosci Lett 2019, 709, 134382. [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Haque, M.E. Glycyrrhizic acid Attenuates Neuroinflammation and Oxidative Stress in Rotenone Model of Parkinson's Disease. Neurotox Res 2016, 29, 275-287. [CrossRef]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson's disease. J Neurosci Res 2007, 85, 919-934. [CrossRef]
- Leathem, A.; Ortiz-Cerda, T.; Dennis, J.M.; Witting, P.K. Evidence for Oxidative Pathways in the Pathogenesis of PD: Are Antioxidants Candidate Drugs to Ameliorate Disease Progression? Int J Mol Sci 2022, 23. [CrossRef]
- Al-Zaid, F.S.; Hurley, M.J.; Dexter, D.T.; Gillies, G.E. Neuroprotective role for RORA in Parkinson's disease revealed by analysis of post-mortem brain and a dopaminergic cell line. NPJ Parkinsons Dis 2023, 9, 119. [CrossRef]
- Ayton, S.; Lei, P.; McLean, C.; Bush, A.I.; Finkelstein, D.I. Transferrin protects against Parkinsonian neurotoxicity and is deficient in Parkinson's substantia nigra. Signal Transduct Target Ther 2016, 1, 16015. [CrossRef]
- Sian-Hülsmann, J.; Mandel, S.; Youdim, M.B.; Riederer, P. The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem 2011, 118, 939-957. [CrossRef]
- Wakamatsu, K.; Fujikawa, K.; Zucca, F.A.; Zecca, L.; Ito, S. The structure of neuromelanin as studied by chemical degradative methods. J Neurochem 2003, 86, 1015-1023. [CrossRef]
- Longhena, F.; Faustini, G.; Spillantini, M.G.; Bellucci, A. Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology. Int J Mol Sci 2019, 20. [CrossRef]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson's disease pathogenesis. Biochim Biophys Acta Proteins Proteom 2019, 1867, 890-908. [CrossRef]
- Vonsattel, J.P.G.; Difiglia, M. Huntington Disease. Journal of Neuropathology & Experimental Neurology 1998, 57, 369-384. [CrossRef]
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell 1993, 72, 971-983. [CrossRef]
- Sorolla, M.A.; Reverter-Branchat, G.; Tamarit, J.; Ferrer, I.; Ros, J.; Cabiscol, E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med 2008, 45, 667-678. [CrossRef]
- Stack, E.C.; Matson, W.R.; Ferrante, R.J. Evidence of oxidant damage in Huntington's disease: translational strategies using antioxidants. Ann N Y Acad Sci 2008, 1147, 79-92. [CrossRef]
- Long, J.D.; Matson, W.R.; Juhl, A.R.; Leavitt, B.R.; Paulsen, J.S. 8OHdG as a marker for Huntington disease progression. Neurobiol Dis 2012, 46, 625-634. [CrossRef]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. Int J Neurosci 2020, 130, 1047-1062. [CrossRef]
- Enokido, Y.; Tamura, T.; Ito, H.; Arumughan, A.; Komuro, A.; Shiwaku, H.; Sone, M.; Foulle, R.; Sawada, H.; Ishiguro, H.; et al. Mutant huntingtin impairs Ku70-mediated DNA repair. J Cell Biol 2010, 189, 425-443. [CrossRef]
- Hands, S.; Sajjad, M.U.; Newton, M.J.; Wyttenbach, A. In vitro and in vivo aggregation of a fragment of huntingtin protein directly causes free radical production. J Biol Chem 2011, 286, 44512-44520. [CrossRef]
- Giorgini, F.; Guidetti, P.; Nguyen, Q.; Bennett, S.C.; Muchowski, P.J. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet 2005, 37, 526-531. [CrossRef]
- Chen, C.M.; Wu, Y.R.; Cheng, M.L.; Liu, J.L.; Lee, Y.M.; Lee, P.W.; Soong, B.W.; Chiu, D.T. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington's disease patients. Biochem Biophys Res Commun 2007, 359, 335-340. [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363-1380. [CrossRef]
- Cappello, V.; Vezzoli, E.; Righi, M.; Fossati, M.; Mariotti, R.; Crespi, A.; Patruno, M.; Bentivoglio, M.; Pietrini, G.; Francolini, M. Analysis of neuromuscular junctions and effects of anabolic steroid administration in the SOD1G93A mouse model of ALS. Mol Cell Neurosci 2012, 51, 12-21. [CrossRef]
- Shibata, N.; Nagai, R.; Miyata, S.; Jono, T.; Horiuchi, S.; Hirano, A.; Kato, S.; Sasaki, S.; Asayama, K.; Kobayashi, M. Nonoxidative protein glycation is implicated in familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Acta Neuropathol 2000, 100, 275-284. [CrossRef]
- Ohta, Y.; Nomura, E.; Shang, J.; Feng, T.; Huang, Y.; Liu, X.; Shi, X.; Nakano, Y.; Hishikawa, N.; Sato, K.; et al. Enhanced oxidative stress and the treatment by edaravone in mice model of amyotrophic lateral sclerosis. J Neurosci Res 2019, 97, 607-619. [CrossRef]
- Bruijn, L.I.; Houseweart, M.K.; Kato, S.; Anderson, K.L.; Anderson, S.D.; Ohama, E.; Reaume, A.G.; Scott, R.W.; Cleveland, D.W. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 1998, 281, 1851-1854. [CrossRef]
- Barber, S.C.; Shaw, P.J. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med 2010, 48, 629-641. [CrossRef]
- Xiao, Y.; Karam, C.; Yi, J.; Zhang, L.; Li, X.; Yoon, D.; Wang, H.; Dhakal, K.; Ramlow, P.; Yu, T.; et al. ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol Res 2018, 138, 25-36. [CrossRef]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: a review. Cell Death Dis 2018, 9, 348. [CrossRef]
- Carrí, M.T.; Ferri, A.; Cozzolino, M.; Calabrese, L.; Rotilio, G. Neurodegeneration in amyotrophic lateral sclerosis: the role of oxidative stress and altered homeostasis of metals. Brain Res Bull 2003, 61, 365-374. [CrossRef]
- Tokuda, E.; Watanabe, S.; Okawa, E.; Ono, S. Regulation of Intracellular Copper by Induction of Endogenous Metallothioneins Improves the Disease Course in a Mouse Model of Amyotrophic Lateral Sclerosis. Neurotherapeutics 2015, 12, 461-476. [CrossRef]
- Tokuda, E.; Okawa, E.; Watanabe, S.; Ono, S. Overexpression of metallothionein-I, a copper-regulating protein, attenuates intracellular copper dyshomeostasis and extends lifespan in a mouse model of amyotrophic lateral sclerosis caused by mutant superoxide dismutase-1. Hum Mol Genet 2014, 23, 1271-1285. [CrossRef]
- Qin, X.; Wu, P.; Wen, T.; Jia, R.; Zhang, R.; Jin, J.; Hu, F.; Chen, Q.Y.; Dang, J. Comparative assessment of blood Metal/metalloid levels, clinical heterogeneity, and disease severity in amyotrophic lateral sclerosis patients. Neurotoxicology 2022, 89, 12-19. [CrossRef]
- Aydemir, D.; Surucu, S.; Basak, A.N.; Ulusu, N.N. Evaluation of the Hematological and Serum Biochemistry Parameters in the Pre-Symptomatic and Symptomatic Stages of ALS Disease to Support Early Diagnosis and Prognosis. Cells 2022, 11. [CrossRef]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: focus on neuronal regeneration. Crit Rev Food Sci Nutr 2022, 62, 3421-3436. [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in Health and Diseases: Alzheimer's Disease and Curcumin Analogues, Derivatives, and Hybrids. Int J Mol Sci 2020, 21. [CrossRef]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J Cell Physiol 2018, 233, 830-848. [CrossRef]
- Liao, F.; Liu, L.; Luo, E.; Hu, J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch Oral Biol 2018, 92, 32-37. [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24. [CrossRef]
- Lou, S.; Gong, D.; Yang, M.; Qiu, Q.; Luo, J.; Chen, T. Curcumin Improves Neurogenesis in Alzheimer's Disease Mice via the Upregulation of Wnt/β-Catenin and BDNF. Int J Mol Sci 2024, 25. [CrossRef]
- Gomez-Sequeda, N.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Combination of Tramiprosate, Curcumin, and SP600125 Reduces the Neuropathological Phenotype in Familial Alzheimer Disease PSEN1 I416T Cholinergic-like Neurons. Int J Mol Sci 2024, 25. [CrossRef]
- Tripathi, S.; Bhawana. Epigenetic Orchestration of Neurodegenerative Disorders: A Possible Target for Curcumin as a Therapeutic. Neurochem Res 2024. [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 2007, 595, 105-125. [CrossRef]
- Motawi, T.K.; Sadik, N.A.H.; Hamed, M.A.; Ali, S.A.; Khalil, W.K.B.; Ahmed, Y.R. Potential therapeutic effects of antagonizing adenosine A(2A) receptor, curcumin and niacin in rotenone-induced Parkinson's disease mice model. Mol Cell Biochem 2020, 465, 89-102. [CrossRef]
- Wang, Y.L.; Ju, B.; Zhang, Y.Z.; Yin, H.L.; Liu, Y.J.; Wang, S.S.; Zeng, Z.L.; Yang, X.P.; Wang, H.T.; Li, J.F. Protective Effect of Curcumin Against Oxidative Stress-Induced Injury in Rats with Parkinson's Disease Through the Wnt/ β-Catenin Signaling Pathway. Cell Physiol Biochem 2017, 43, 2226-2241. [CrossRef]
- Huang, H.C.; Xu, K.; Jiang, Z.F. Curcumin-mediated neuroprotection against amyloid-β-induced mitochondrial dysfunction involves the inhibition of GSK-3β. J Alzheimers Dis 2012, 32, 981-996. [CrossRef]
- Tošović, J.; Marković, S.; Dimitrić Marković, J.M.; Mojović, M.; Milenković, D. Antioxidative mechanisms in chlorogenic acid. Food Chem 2017, 237, 390-398. [CrossRef]
- Ye, J.; Gao, Y.; Ji, M.; Yang, Y.; Wang, Z.; Wang, B.; Jin, J.; Li, L.; Wang, H.; Xu, X.; et al. Oral SMEDDS promotes lymphatic transport and mesenteric lymph nodes target of chlorogenic acid for effective T-cell antitumor immunity. J Immunother Cancer 2021, 9. [CrossRef]
- Wang, X.; Fan, X.; Yuan, S.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food Funct 2017, 8, 2924-2934. [CrossRef]
- Hussein, R.M.; Sawy, D.M.; Kandeil, M.A.; Farghaly, H.S. Chlorogenic acid, quercetin, coenzyme Q10 and silymarin modulate Keap1-Nrf2/heme oxygenase-1 signaling in thioacetamide-induced acute liver toxicity. Life Sci 2021, 277, 119460. [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of DA Neurons in a Parkinsonian Mouse Model. Oxid Med Cell Longev 2020, 2020, 6571484. [CrossRef]
- Shi, D.; Hao, Z.; Qi, W.; Jiang, F.; Liu, K.; Shi, X. Aerobic exercise combined with chlorogenic acid exerts neuroprotective effects and reverses cognitive decline in Alzheimer's disease model mice (APP/PS1) via the SIRT1/ /PGC-1α/PPARγ signaling pathway. Front Aging Neurosci 2023, 15, 1269952. [CrossRef]
- Anees Ahmed, K.; Ubaid ur, R.; Moazzam Rafiq, K.; Amna, S.; Tariq, M.; Muneeb, K. Essential oil eugenol: sources, extraction techniques and nutraceutical perspectives. RSC Advances 2017. [CrossRef]
- Taleuzzaman, M.; Jain, P.; Verma, R.; Iqbal, Z.; Mirza, M.A. Eugenol as a Potential Drug Candidate: A Review. Curr Top Med Chem 2021, 21, 1804-1815. [CrossRef]
- Pontes, N.H.L.; Reis, T.; Vasconcelos, C.F.M.; Aragão, P.; Souza, R.B.; Catunda Junior, F.E.A.; Aguiar, L.M.V.; Cunha, R. Impact of eugenol on in vivo model of 6-hydroxydopamine-induced oxidative stress. Free Radic Res 2021, 55, 556-568. [CrossRef]
- Goyal, A.; Solanki, A.; Verma, A. Preclinical Evidence-based Review on Therapeutic Potential of Eugenol for the Treatment of Brain Disorders. Curr Mol Med 2023, 23, 390-400. [CrossRef]
- Liu, Z.; Niu, W.; Yang, X.; Wang, Y. Effects of combined acupuncture and eugenol on learning-memory ability and antioxidation system of hippocampus in Alzheimer disease rats via olfactory system stimulation. J Tradit Chin Med 2013, 33, 399-402. [CrossRef]
- Salem, A.M.; Mohammaden, T.F.; Ali, M.A.M.; Mohamed, E.A.; Hasan, H.F. Ellagic and ferulic acids alleviate gamma radiation and aluminium chloride-induced oxidative damage. Life Sci 2016, 160, 2-11. [CrossRef]
- Sanadgol, N.; Golab, F.; Tashakkor, Z.; Taki, N.; Moradi Kouchi, S.; Mostafaie, A.; Mehdizadeh, M.; Abdollahi, M.; Taghizadeh, G.; Sharifzadeh, M. Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis. Pharm Biol 2017, 55, 1679-1687. [CrossRef]
- Lin, W.; Liu, G.; Kang, X.; Guo, P.; Shang, Y.; Du, R.; Wang, X.; Chen, L.; Yue, R.; Kong, F.; et al. Ellagic acid inhibits high glucose-induced injury in rat mesangial cells via the PI3K/Akt/FOXO3a signaling pathway. Exp Ther Med 2021, 22, 1017. [CrossRef]
- Bains, M.; Kaur, J.; Akhtar, A.; Kuhad, A.; Sah, S.P. Anti-inflammatory effects of ellagic acid and vanillic acid against quinolinic acid-induced rat model of Huntington's disease by targeting IKK-NF-κB pathway. Eur J Pharmacol 2022, 934, 175316. [CrossRef]
- Guo, S.; Xu, J.J.; Wei, N.; Han, J.Y.; Xue, R.; Xu, P.S.; Gao, C.Y. Honokiol Attenuates the Memory Impairments, Oxidative Stress, Neuroinflammation, and GSK-3β Activation in Vascular Dementia Rats. J Alzheimers Dis 2019, 71, 97-108. [CrossRef]
- Li, C.G.; Ni, C.L.; Yang, M.; Tang, Y.Z.; Li, Z.; Zhu, Y.J.; Jiang, Z.H.; Sun, B.; Li, C.J. Honokiol protects pancreatic β cell against high glucose and intermittent hypoxia-induced injury by activating Nrf2/ARE pathway in vitro and in vivo. Biomed Pharmacother 2018, 97, 1229-1237. [CrossRef]
- Xia, S.; Lin, H.; Liu, H.; Lu, Z.; Wang, H.; Fan, S.; Li, N. Honokiol Attenuates Sepsis-Associated Acute Kidney Injury via the Inhibition of Oxidative Stress and Inflammation. Inflammation 2019, 42, 826-834. [CrossRef]
- Zhou, Y.; Tang, J.; Lan, J.; Zhang, Y.; Wang, H.; Chen, Q.; Kang, Y.; Sun, Y.; Feng, X.; Wu, L.; et al. Honokiol alleviated neurodegeneration by reducing oxidative stress and improving mitochondrial function in mutant SOD1 cellular and mouse models of amyotrophic lateral sclerosis. Acta Pharm Sin B 2023, 13, 577-597. [CrossRef]
- Lv, H.; Liu, J.; Wang, L.; Zhang, H.; Yu, S.; Li, Z.; Jiang, F.; Niu, Y.; Yuan, J.; Cui, X.; et al. Ameliorating effects of combined curcumin and desferrioxamine on 6-OHDA-induced rat mode of Parkinson's disease. Cell Biochem Biophys 2014, 70, 1433-1438. [CrossRef]
- Alamro, A.A.; Alsulami, E.A.; Almutlaq, M.; Alghamedi, A.; Alokail, M.; Haq, S.H. Therapeutic Potential of Vitamin D and Curcumin in an In Vitro Model of Alzheimer Disease. J Cent Nerv Syst Dis 2020, 12, 1179573520924311. [CrossRef]
- Song, S.; Nie, Q.; Li, Z.; Du, G. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol Res Pract 2016, 212, 247-251. [CrossRef]
- Yu, H.; Yamashita, T.; Hu, X.; Bian, Z.; Hu, X.; Feng, T.; Tadokoro, K.; Morihara, R.; Abe, K. Protective and anti-oxidative effects of curcumin and resveratrol on Aβ-oligomer-induced damage in the SH-SY5Y cell line. J Neurol Sci 2022, 441, 120356. [CrossRef]
- Shi, M.; Sun, F.; Wang, Y.; Kang, J.; Zhang, S.; Li, H. CGA restrains the apoptosis of Aβ(25-35)-induced hippocampal neurons. Int J Neurosci 2020, 130, 700-707. [CrossRef]
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; Kale, R.; Sayyed, A.A.; Behera, S.K.; Khairnar, A. Chlorogenic Acid: a Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson's Disease by GLP-1 Secretion. Mol Neurobiol 2022, 59, 6834-6856. [CrossRef]
- Shen, Y.C.; Juan, C.W.; Lin, C.S.; Chen, C.C.; Chang, C.L. NEUROPROTECTIVE EFFECT OF TERMINALIA CHEBULA EXTRACTS AND ELLAGIC ACID IN PC12 CELLS. Afr J Tradit Complement Altern Med 2017, 14, 22-30. [CrossRef]
- Ramadan, W.S.; Alkarim, S. Ellagic Acid Modulates the Amyloid Precursor Protein Gene via Superoxide Dismutase Regulation in the Entorhinal Cortex in an Experimental Alzheimer's Model. Cells 2021, 10. [CrossRef]
- Wang, M.; Li, Y.; Ni, C.; Song, G. Honokiol Attenuates Oligomeric Amyloid β1-42-Induced Alzheimer's Disease in Mice Through Attenuating Mitochondrial Apoptosis and Inhibiting the Nuclear Factor Kappa-B Signaling Pathway. Cell Physiol Biochem 2017, 43, 69-81. [CrossRef]
- Chakraborty, K.; Krishnan, S.; Joy, M. Antioxidative oxygenated terpenoids with bioactivities against pro-inflammatory inducible enzymes from Indian squid, Uroteuthis (Photololigo) duvaucelii. Nat Prod Res 2021, 35, 909-920. [CrossRef]
- Tousif, M.I.; Nazir, M.; Riaz, N.; Saleem, M.; Tauseef, S.; Azam, S.M.; Arfan Yawer, M.; Zengin, G. Terpenoids as Human Neutrophil Elastase (HNE) Inhibitors: A Comprehensive Review of Natural Anti-inflammatory Isoprenoids. Chembiochem 2023, 24, e202300346. [CrossRef]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg Chem 2022, 124, 105817. [CrossRef]
- Abreu, L.S.; do Nascimento, Y.M.; Costa, R.D.S.; Guedes, M.L.S.; Souza, B.; Pena, L.J.; Costa, V.C.O.; Scotti, M.T.; Braz-Filho, R.; Barbosa-Filho, J.M.; et al. Tri- and Diterpenoids from Stillingia loranthacea as Inhibitors of Zika Virus Replication. J Nat Prod 2019, 82, 2721-2730. [CrossRef]
- Mony, T.J.; Elahi, F.; Choi, J.W.; Park, S.J. Neuropharmacological Effects of Terpenoids on Preclinical Animal Models of Psychiatric Disorders: A Review. Antioxidants (Basel) 2022, 11. [CrossRef]
- Letizia, C.S.; Cocchiara, J.; Lalko, J.; Api, A.M. Fragrance material review on linalool. Food Chem Toxicol 2003, 41, 943-964. [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf B Biointerfaces 2018, 171, 566-578. [CrossRef]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed Pharmacother 2019, 118, 109295. [CrossRef]
- Yuan, C.; Shin, M.; Park, Y.; Choi, B.; Jang, S.; Lim, C.; Yun, H.S.; Lee, I.S.; Won, S.Y.; Cho, K.S. Linalool Alleviates Aβ42-Induced Neurodegeneration via Suppressing ROS Production and Inflammation in Fly and Rat Models of Alzheimer's Disease. Oxid Med Cell Longev 2021, 2021, 8887716. [CrossRef]
- Chang, W.H.; Hsu, H.T.; Lin, C.C.; An, L.M.; Lee, C.H.; Ko, H.H.; Lin, C.L.; Lo, Y.C. Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson's Disease. Int J Mol Sci 2024, 25. [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: effect on oxidative stress and mechanisms of action. Crit Rev Food Sci Nutr 2022, 62, 3232-3249. [CrossRef]
- Alayunt Ö, N.; Aksoy, L.; Karafakioğlu, Y.S.; Sevimli, S. Assessment of Anti-inflammatory and Antioxidant Properties of Safranal on CCI4-Induced Oxidative Stress and Inflammation in Rats. An Acad Bras Cienc 2019, 91, e20181235. [CrossRef]
- Xue, Y.; Jin, W.; Xue, Y.; Zhang, Y.; Wang, H.; Zhang, Y.; Guan, S.; Chu, X.; Zhang, J. Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca(2+) homeostasis. J Pharmacol Sci 2020, 143, 156-164. [CrossRef]
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases (Review). Exp Ther Med 2023, 25, 235. [CrossRef]
- Fazeli, E.; Ghalibaf, M.H.E.; Forouzanfar, F. Neuroprotective Potency of Safranal Against Neurological Disorders. Curr Mol Med 2023, 23, 952-959. [CrossRef]
- Baluchnejadmojarad, T.; Mohamadi-Zarch, S.M.; Roghani, M. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid β-induced rat model of Alzheimer's disease: underlying mechanisms. Metab Brain Dis 2019, 34, 1747-1759. [CrossRef]
- Fotoohi, A.; Moloudi, M.R.; Hosseini, S.; Hassanzadeh, K.; Feligioni, M.; Izadpanah, E. A Novel Pharmacological Protective Role for Safranal in an Animal Model of Huntington's Disease. Neurochem Res 2021, 46, 1372-1379. [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by β-Caryophyllene: A Focus on the Nervous System. Antioxidants (Basel) 2021, 10. [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem 2016, 418, 59-70. [CrossRef]
- Tan, X.; Xu, R.; Li, A.P.; Li, D.; Wang, Y.; Zhao, Q.; Long, L.P.; Fan, Y.Z.; Zhao, C.X.; Liu, Y.; et al. Antioxidant and anti-Alzheimer's disease activities of 1,8-cineole and its cyclodextrin inclusion complex. Biomed Pharmacother 2024, 175, 116784. [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J Food Biochem 2021, 45, e13566. [CrossRef]
- Eddin, L.B.; Azimullah, S.; Jha, N.K.; Nagoor Meeran, M.F.; Beiram, R.; Ojha, S. Limonene, a Monoterpene, Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Modulating Neuroinflammation, Hippo Signaling and Apoptosis in Rats. Int J Mol Sci 2023, 24. [CrossRef]
- Collins, J.M.; King, A.E.; Woodhouse, A.; Kirkcaldie, M.T.; Vickers, J.C. The effect of focal brain injury on beta-amyloid plaque deposition, inflammation and synapses in the APP/PS1 mouse model of Alzheimer's disease. Exp Neurol 2015, 267, 219-229. [CrossRef]
- Kiss, E.; Kins, S.; Zöller, Y.; Schilling, S.; Gorgas, K.; Groß, D.; Schlicksupp, A.; Rosner, R.; Kirsch, J.; Kuhse, J. Artesunate restores the levels of inhibitory synapse proteins and reduces amyloid-β and C-terminal fragments (CTFs) of the amyloid precursor protein in an AD-mouse model. Mol Cell Neurosci 2021, 113, 103624. [CrossRef]
- Xia, L.; Pang, Y.; Li, J.; Wu, B.; Du, Y.; Chen, Y.; Luo, M.; Wang, Y.; Dong, Z. Dihydroartemisinin Induces O-GlcNAcylation and Improves Cognitive Function in a Mouse Model of Tauopathy. J Alzheimers Dis 2021, 84, 239-248. [CrossRef]
- Lim, H.S.; Park, G. Artemisinin protects dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in a mouse model of Parkinson's disease. Biomed Pharmacother 2024, 170, 115972. [CrossRef]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci 2017, 174, 21-27. [CrossRef]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. The Protective Effect of Lavender Essential Oil and Its Main Component Linalool against the Cognitive Deficits Induced by D-Galactose and Aluminum Trichloride in Mice. Evid Based Complement Alternat Med 2017, 2017, 7426538. [CrossRef]
- Pan, P.K.; Qiao, L.Y.; Wen, X.N. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson's disease through regulating Keap1/Nrf2 signaling pathway. Cell Mol Biol (Noisy-le-grand) 2016, 62, 11-17.
- Sadeghnia, H.R.; Kamkar, M.; Assadpour, E.; Boroushaki, M.T.; Ghorbani, A. Protective Effect of Safranal, a Constituent of Crocus sativus, on Quinolinic Acid-induced Oxidative Damage in Rat Hippocampus. Iran J Basic Med Sci 2013, 16, 73-82.
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) ameliorates MPP+ induced cytotoxicity. Biomed Pharmacother 2018, 103, 1086-1091. [CrossRef]
- Flores-Soto, M.E.; Corona-Angeles, J.A.; Tejeda-Martinez, A.R.; Flores-Guzman, P.A.; Luna-Mujica, I.; Chaparro-Huerta, V.; Viveros-Paredes, J.M. β-Caryophyllene exerts protective antioxidant effects through the activation of NQO1 in the MPTP model of Parkinson's disease. Neurosci Lett 2021, 742, 135534. [CrossRef]
- Khan, A.; Vaibhav, K.; Javed, H.; Tabassum, R.; Ahmed, M.E.; Khan, M.M.; Khan, M.B.; Shrivastava, P.; Islam, F.; Siddiqui, M.S.; et al. 1,8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: relevance to Alzheimer's disease. Neurochem Res 2014, 39, 344-352. [CrossRef]
- Jeong, S.H.; Kim, H.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Won, C.K.; Park, K.I.; Kim, E.; Heo, J.D.; Kim, H.W.; et al. Flavones: The Apoptosis in Prostate Cancer of Three Flavones Selected as Therapeutic Candidate Models. Int J Mol Sci 2023, 24. [CrossRef]
- Calderaro, A.; Patanè, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Laganà, G. The Neuroprotective Potentiality of Flavonoids on Alzheimer's Disease. Int J Mol Sci 2022, 23. [CrossRef]
- Basli, A.; Soulet, S.; Chaher, N.; Mérillon, J.M.; Chibane, M.; Monti, J.P.; Richard, T. Wine polyphenols: potential agents in neuroprotection. Oxid Med Cell Longev 2012, 2012, 805762. [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27. [CrossRef]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol Behav 2017, 171, 69-78. [CrossRef]
- Ishisaka, A.; Ichikawa, S.; Sakakibara, H.; Piskula, M.K.; Nakamura, T.; Kato, Y.; Ito, M.; Miyamoto, K.; Tsuji, A.; Kawai, Y.; et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med 2011, 51, 1329-1336. [CrossRef]
- Ho, C.L.; Kao, N.J.; Lin, C.I.; Cross, T.L.; Lin, S.H. Quercetin Increases Mitochondrial Biogenesis and Reduces Free Radicals in Neuronal SH-SY5Y Cells. Nutrients 2022, 14. [CrossRef]
- Amanzadeh Jajin, E.; Esmaeili, A.; Rahgozar, S.; Noorbakhshnia, M. Quercetin-Conjugated Superparamagnetic Iron Oxide Nanoparticles Protect AlCl(3)-Induced Neurotoxicity in a Rat Model of Alzheimer's Disease via Antioxidant Genes, APP Gene, and miRNA-101. Front Neurosci 2020, 14, 598617. [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson's disease induced by 6-hydroxydopamine. Evid Based Complement Alternat Med 2012, 2012, 823206. [CrossRef]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington's disease. Biochim Biophys Acta 2013, 1832, 421-430. [CrossRef]
- Viswanatha, G.L.; Shylaja, H.; Moolemath, Y. The beneficial role of Naringin- a citrus bioflavonoid, against oxidative stress-induced neurobehavioral disorders and cognitive dysfunction in rodents: A systematic review and meta-analysis. Biomed Pharmacother 2017, 94, 909-929. [CrossRef]
- Hassan, H.M.; Elnagar, M.R.; Abdelrazik, E.; Mahdi, M.R.; Hamza, E.; Elattar, E.M.; ElNashar, E.M.; Alghamdi, M.A.; Al-Qahtani, Z.; Al-Khater, K.M.; et al. Neuroprotective effect of naringin against cerebellar changes in Alzheimer's disease through modulation of autophagy, oxidative stress and tau expression: An experimental study. Front Neuroanat 2022, 16, 1012422. [CrossRef]
- Kumar, P.; Kumar, A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington's like symptoms in rats: possible role of nitric oxide. Behav Brain Res 2010, 206, 38-46. [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res 2015, 29, 323-331. [CrossRef]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxid Med Cell Longev 2013, 2013, 102741. [CrossRef]
- Bakr, A.F.; Shao, P.; Farag, M.A. Recent advances in glycyrrhizin metabolism, health benefits, clinical effects and drug delivery systems for efficacy improvement; a comprehensive review. Phytomedicine 2022, 99, 153999. [CrossRef]
- Ban, J.Y.; Park, H.K.; Kim, S.K. Effect of Glycyrrhizic Acid on Scopolamine-Induced Cognitive Impairment in Mice. Int Neurourol J 2020, 24, S48-55. [CrossRef]
- Gendy, A.M.; El-Sadek, H.M.; Amin, M.M.; Ahmed, K.A.; El-Sayed, M.K.; El-Haddad, A.E.; Soubh, A. Glycyrrhizin prevents 3-nitropropionic acid-induced neurotoxicity by downregulating HMGB1/TLR4/NF-κB p65 signaling, and attenuating oxidative stress, inflammation, and apoptosis in rats. Life Sci 2023, 314, 121317. [CrossRef]
- Molaei, A.; Hatami, H.; Dehghan, G.; Sadeghian, R.; Khajehnasiri, N. Synergistic effects of quercetin and regular exercise on the recovery of spatial memory and reduction of parameters of oxidative stress in animal model of Alzheimer's disease. Excli j 2020, 19, 596-612. [CrossRef]
- Bao, D.; Wang, J.; Pang, X.; Liu, H. Protective Effect of Quercetin against Oxidative Stress-Induced Cytotoxicity in Rat Pheochromocytoma (PC-12) Cells. Molecules 2017, 22. [CrossRef]
- Kaur, G.; Prakash, A. Involvement of the nitric oxide signaling in modulation of naringin against intranasal manganese and intracerbroventricular β-amyloid induced neurotoxicity in rats. J Nutr Biochem 2020, 76, 108255. [CrossRef]
- Kesh, S.; Kannan, R.R.; Balakrishnan, A. Naringenin alleviates 6-hydroxydopamine induced Parkinsonism in SHSY5Y cells and zebrafish model. Comp Biochem Physiol C Toxicol Pharmacol 2021, 239, 108893. [CrossRef]
- Adekeye, A.O.; Fafure, A.A.; Ogunsemowo, A.E.; Enye, L.A.; Saka, O.S.; Ogedengbe, O.O. Naringin ameliorates motor dysfunction and exerts neuroprotective role against vanadium-induced neurotoxicity. AIMS Neurosci 2022, 9, 536-550. [CrossRef]
- Sugumar, M.; Sevanan, M.; Sekar, S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int J Neurosci 2019, 129, 534-539. [CrossRef]
- Justin Thenmozhi, A.; William Raja, T.R.; Manivasagam, T.; Janakiraman, U.; Essa, M.M. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer's disease. Nutr Neurosci 2017, 20, 360-368. [CrossRef]
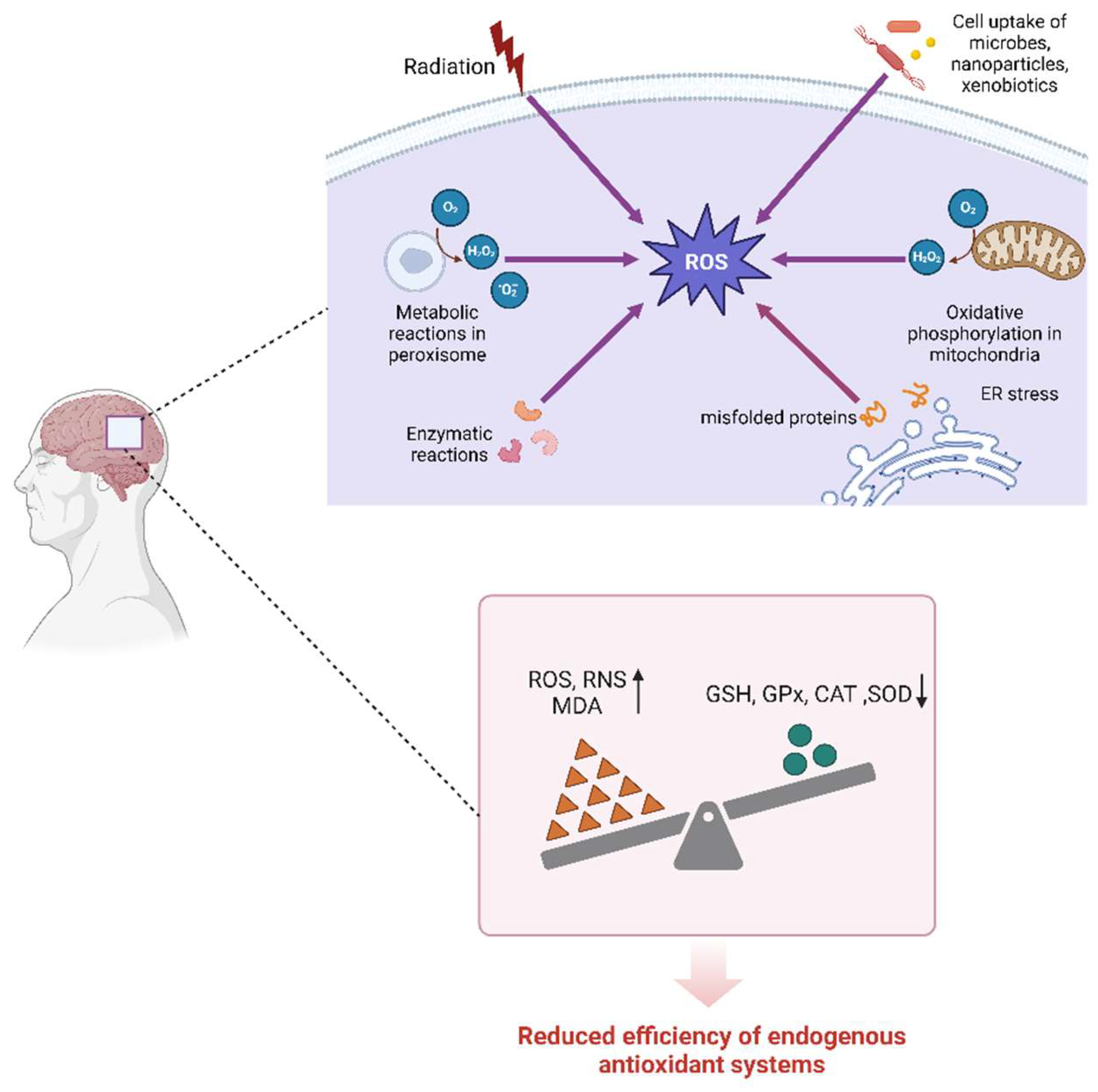
- Hassan, W.; Noreen, H.; Rehman, S.; Kamal, M.A.; da Rocha, J.B.T. Association of Oxidative Stress with Neurological Disorders. Curr Neuropharmacol 2022, 20, 1046-1072. [CrossRef]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974-25990. [CrossRef]
- Delgado, A.; Gonçalves, S.; Romano, A. Mediterranean Diet: The Role of Phenolic Compounds from Aromatic Plant Foods. Foods 2023, 12. [CrossRef]
- Vlčko, T.; Rathod, N.B.; Kulawik, P.; Ozogul, Y.; Ozogul, F. The impact of aromatic plant-derived bioactive compounds on seafood quality and safety. Adv Food Nutr Res 2022, 102, 275-339. [CrossRef]
- Korczowska-Łącka, I.; Słowikowski, B.; Piekut, T.; Hurła, M.; Banaszek, N.; Szymanowicz, O.; Jagodziński, P.P.; Kozubski, W.; Permoda-Pachuta, A.; Dorszewska, J. Disorders of Endogenous and Exogenous Antioxidants in Neurological Diseases. Antioxidants (Basel) 2023, 12. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





