1. Introduction
The demographic aging associated with an improvement in well-being has led to an increase in the population over 65 years of age, carrying in parallel the increase in chronic pathologies associated with age. However, in the case of neurodegenerative diseases such as Alzheimer’s or Parkinson’s, we do not have sufficient knowledge about the initial causes for their approach and consequently for effective treatment, beyond the treatment of the associated clinical symptoms.
Neurodegenerative diseases currently represent a silent pandemic and a real challenge for the scientific community since, while we know different modifiable factors associated with their appearance, we also know other non-modifiable factors such as sex or age. During aging, the brain undergoes alterations at the level of energy metabolism, mainly related to glucose intake or insulin signalling, altering balances between routes. However, this circumstance is not comparable to that which could occur in the case of AD, where the described glucose hypometabolism occurs before clinical symptoms begin to manifest. In both circumstances, sexual differences have been described.
Within the older population, differences regarding sex in the prevalence of Alzheimer’s disease (sporadic or non-genetic cause) and Parkinson’s are evident, the first being mainly associated with the female sex, especially after menopause and in the case of Parkinson’s disease it is related to the male sex. In fact, both in Alzheimer’s disease (AD) and in depression, women show a higher prevalence of the disease and a greater deterioration of memory or more severe cognitive symptoms in both disorders (Gutierrez-Lobos et al., 2002; Irvine et al., 2012; McPherson et al., 1999). While, in the case of males with Parkinson’s disease, they present greater cognitive alterations than women with this same disease(Nicoletti et al., 2017; Szewczyk-Krolikowski et al., 2014).
Therefore, sex hormones could have an important role in the modelling of the structure and plasticity of the hippocampus, a brain structure linked to cognition, which can contribute to greater vulnerability within each sex by type of disease. This evidence makes us think that evolutionary, anatomophysiological and metabolic differences have been determined primarily by the hormonal profile of the female and male sex, as well as by the synthesis of endogenous cerebral oestradiol, especially in the context of cognition and neuroplasticity, which could shed light on the differences in the mentioned neurodegenerative pathologies.
2. Evolutionary Characteristics in the Human Brain
The brain, apart from being an organ governing the most fine and precise bodily functions, is also the central component of human identity, so understanding this organ is essential to understand why we are what we are. The human lineage appears to have arisen from changes in neuronal connectivity, where slight changes in the connectome could lead to deep and specific functional changes. The connectome is made up of countless types of neuronal cells and their specific synaptic connections that make up the central components of circuits and neural networks (van den Heuvel et al., 2016). The latest advances in imaging technology show the immense complexity of the human connectome, leading us to understand that our understanding of its organization and function at the level of long-range projections, local synaptic circuits, and intracellular signalling is still incomplete. In fact, the latest estimates suggest that there may be between several hundred trillion and more than a quadrillion synapses in the human CNS (Silbereis et al., 2016), with an average of 164 trillion synapses in the neocortex of young adult men (Tang et al., 2001). On the other hand, the white matter of young adults contains between 149,000 and 176,000 km of myelinated axons (Marner et al., 2003), which can give rise to hundreds of thousands of distinct long-range projection systems (Irimia et al., 2012). Within its enormous complexity, the topology and stability/reliability of the connectome are fundamental for the establishment of the patterns of dynamic activity that underlie the cognition and specific behaviour of each species (Markov et al., 2013; Mesulam, 2000; van den Heuvel et al., 2016). In fact, the peculiarities of the human connectome appear to be unique in the pattern of neocortical myelination, which may have implications for the speed of conduction along the axons (Glasser et al., 2014; Miller et al., 2012; Olmos-Serrano et al., 2016). If we compare the human brain with that of other primates such as the macaque, it has a larger and less myelinated total axon surface, which mainly represents the association areas (Glasser et al., 2014) which points and reinforces the idea that humans have reorganized the corticopetal, intracortical and long-range corticofugal projection systems, especially those associated with the prefrontal and temporal association cortices, which are regions involved in higher-order cognitive functions. Therefore, both local circuits and long-range networks have undergone structural, molecular, and functional reorganization during human evolution, and these characteristics may have evolved independently of the described increase in brain volume (Sousa et al., 2017).
In this sense, and from an evolutionary point of view, the hippocampus would soon become a critical brain structure for decision-making, determining the importance of incoming sensory signals. Once this critical decision-making capacity is established, the hippocampus would be in charge of decision-making. That is, if the hippocampus indicates that a neuronal input is important, it is likely that the information will be stored in memory, consolidating long-term verbal or symbolic memories.
The hippocampus, and its adjacent structures of the temporal and parietal lobes, collectively called the hippocampal formation, has numerous connections, indirect with different regions of the cerebral cortex, as well as with the basal structures of the limbic system: the amygdala, the hypothalamus, the septum and the mammillary bodies. Therefore, almost any type of sensory experience causes the activation of at least one part of the hippocampus, which in turn distributes many output signals to the anterior thalamus, the hypothalamus and other parts of the limbic system, especially through the fornix, an important communication pathway. Thus, the hippocampus is a channel through which incoming sensory signals can initiate behavioral reactions for different purposes. In fact, the stimulation of different areas of the hippocampus can cause almost any of the different patterns of behaviour, such as pleasure, anger, passivity or excess sexual desire (Lisman et al., 2017; Takehara-Nishiuchi et al., 2013).
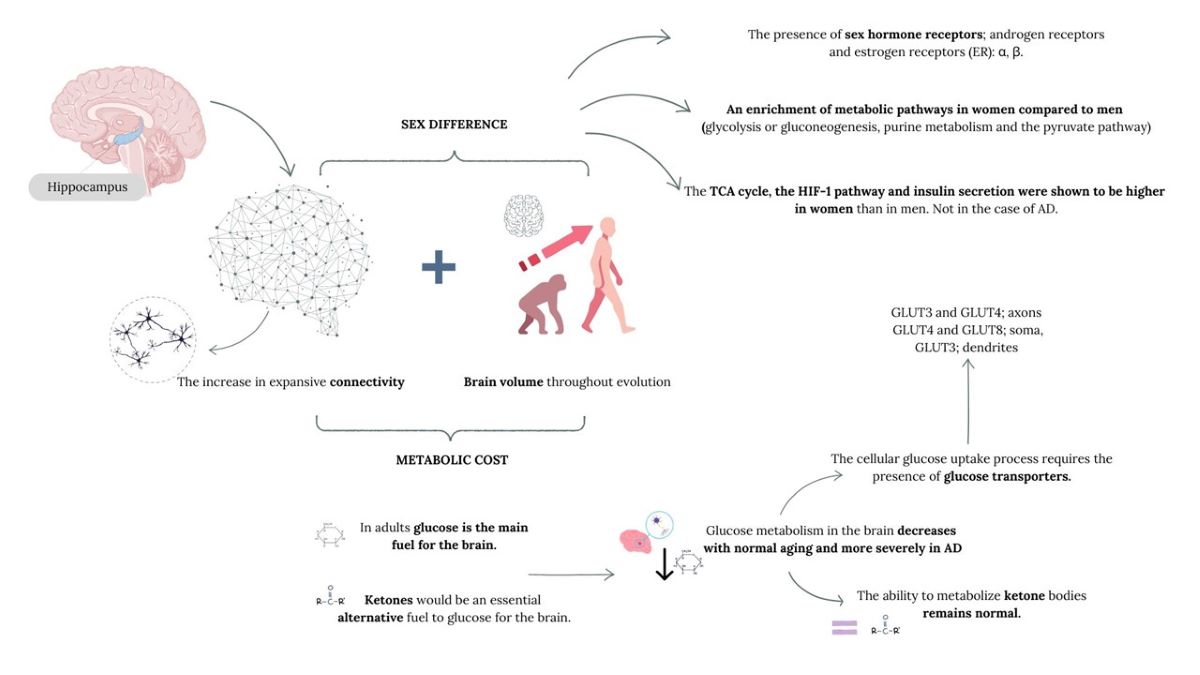
However, the increase in expansive connectivity and brain volume throughout evolution carries a concomitant problem, the metabolic cost. That is, generating and maintaining an increasingly complex connectome has a substantial metabolic cost, since the human brain uses 18% of the body’s oxygen at rest, but it represents only 2.5% of the total body weight of a human being (Kety and Schmidt, 1948). This circumstance required that during human evolution molecular adaptations were produced to maintain high levels of neuronal activity, along with changes in energy allocation and diet (Aiello and Wheeler, 1995; Khaitovich et al., 2008; Pontzer et al., 2016; Wrangham et al., 1999).
3. Energy Metabolism in the Human Brain.
Energy metabolism is crucial for the evolution of the human brain. An adult human brain already requires nearly 20% of energy expenditure, this figure rises in the case of newborns, who require >50% of energy consumption, for an approximate weight of 11% of body weight (Belanger et al., 2011; Kennedy and Sokoloff, 1957). Therefore, the management and use of energy is a very important point in relation to brain size (Laughlin and Sejnowski, 2003).
Taking the evolution of lipid metabolism as an example, metabolic evolution has occurred through several mechanisms, which have led to the metabolic state of current higher organisms, including humans. Firstly, specific steps of energy metabolism were concentrated in specialized tissues, such as the liver and adipose tissue. Secondly, hormonal and neurological control of cellular functions would develop, which would change gene expression and concentrations of signalling metabolites. A third mechanism is gene duplication, where a copy of a gene would be sufficient to carry out the original function, and a final mechanism, multifunctionality, that is, the use of an existing protein to perform a new role while still performing its original function (Wang et al., 2014).
However, in the brain there is no net intake of fatty acids (Pardridge, 1991), which is paradoxical given the high energy needs of the brain. It is clear that circulating fatty acids do not enter the brain and do not directly “feed” cerebral energy metabolism, but they do perform two important indirect functions in brain energy; covering a substantial fraction of the energy needs of organs other than the brain, thus saving glucose for use by the brain, and as a source of ketone bodies, which do enter the brain and provide an important source of energy during fasting (Blomqvist et al., 1995; Hasselbalch et al., 1995; Owen et al., 1967). In fact, body fat at birth provides insurance for the brain between intakes, as fuel and nutrient. In this way, in non-human primates and premature babies, who do not have body fat or have very little, this possibility is compromised (McCance and Widdowson, 1974). Before birth, the deposition of fat in the human foetus represents 90% of its weight gain (Battaglia and Meschia, 1978; Bell et al., 1985) and therefore is a protective mechanism of the high energy needs of the human brain after birth, having a large reserve of fat available to produce ketones. For this reason, in infants, blood ketones are always slightly elevated, regardless of feeding status, a circumstance that does not occur in fed adults. In fact, in human foetuses at mid-gestation, ketones are not only an alternative fuel, but they appear to be an essential fuel, as they provide up to 30% of the energy the brain needs at that age (Adam et al., 1975).
However, in adults, glucose is the main fuel for the brain. The brain can oxidize ketones, but it does not oxidize the fatty acids from which they come. If food is restricted, the body’s glucose reserves (glycogen) last less than 24 h. and without ketones, brain function would be quickly compromised or it would be necessary to degrade muscle protein to release amino acids that can be converted into glucose. Therefore, ketones would be an essential alternative fuel to glucose for the brain (Cunnane and Crawford, 2003). In fact, Cunnane and his colleagues have recently demonstrated that, while glucose metabolism in the brain decreases with normal aging and more severely in AD, the ability to metabolize ketone bodies, the other source of brain energy (during starvation or the ketogenic diet), remains normal in older people and patients with AD as their use depends on the plasma concentrations of the main ketones; acetoacetate and 3OH-hydroxybutyrate (Croteau et al., 2018; Cunnane et al., 2016), according to data from studies of people following prolonged fasting regimens or a diet rich in ketone precursors, medium chain triglycerides (MCT). In fact, some analyses, but of small sample size, in patients with AD or mild cognitive impairment show that metabolic interventions can improve cognitive processing (Brandt et al., 2019; Ota et al., 2019). These therapeutic strategies were recently termed “neuroketotherapeutics” and have shown some efficacy in preventing cognitive decline in people with AD (Taylor et al., 2019) .
Therefore, the cerebral metabolic rate of glucose is low during foetal development, increases linearly after birth, and reaches its maximum around 3 years of age, remains high during the first decade of life and then gradually decreases during the second decade of life until it reaches the rate of glucose utilization observed in the early years of adulthood (Chugani, 2021). However, glucose needs to cross the BBB before entering the extracellular cerebral space. The glucose transporter, GLUT1 is the fundamental vehicle that facilitates the entry of glucose into the brain, (Pearson et al., 2013), which is regulated by 17 β oestradiol (Shi and Simpkins, 1997).
4. Sexual Differences in Brain Evolution. Metabolic Differences
The brain of mammals is a sexually dimorphic organ. Throughout the evolutionary process, the human brain has undergone enlargement, which on average, is greater in the case of men, with a total brain volume larger than women. This difference was also established in relation to certain brain regions, however, they were discarded when adjusted to the intracranial or total brain volume as a correction factor (Kaczkurkin et al., 2019; Ritchie et al., 2018), approaching or equalling the volumes of specific structures in both sexes (van den Heuvel et al., 2016). Studies in humans do show sex differences in relation to functional brain connectivity (Ingalhalikar et al., 2014; Liu et al., 1999; Matsuzaki et al., 2010), as well as in relation to energy metabolism. Women have more interhemispheric connections compared to men, while men have strong intrahemispheric connections compared to women and metabolic differences intrinsic to male and female physiology have also been described, therefore considering it fundamental to value biological sex in brain metabolism as well as in associated pathologies, such as Alzheimer’s disease, characterized by a higher prevalence in the female sex and cerebral glycosidic hypometabolism and a significant affectation of cognitive functions,. In this context, it is important to point out the hippocampus(Babcock et al., 2021). In structure where there are sex differences in relation to its volume both at a regional level and in connectivity(Persson et al., 2014; Yagi and Galea, 2019). Although variations of it have not been described throughout development, we must take into account that there are variables, which perhaps are not always documented, that throughout life can affect its volume such as early adversity (Colle et al., 2017), the phase of the menstrual cycle (Lisofsky et al., 2015), the state of parity (Hoekzema et al., 2017), hormonal therapy (Wnuk et al., 2012), the menopausal state (Goto et al., 2011), the genotype (Everaerd et al., 2012) and testosterone levels in men (Lord et al., 2008) and therefore these factors must also be contemplated to correctly understand the sexual differences of the hippocampus.
The enormous plasticity of the hippocampus is mainly due to the presence of adult neurogenesis in the dentate gyrus (Christie and Cameron, 2006; Eriksson et al., 1998; Neves et al., 2008), fluctuations in the density of the dendritic column/synapses, dendritic arborization (McEwen, 2018) and electrophysiological plasticity (Artola et al., 2006; Whitlock et al., 2006). This plasticity is modified in a sex-dependent manner, either basally or by exposure to certain factors such as stress.
Hippocampal differences in relation to sex are linked to the presence of sex hormone receptors such as androgen receptors (AR) and oestrogen receptors (ER): α, β, the G protein-coupled protein receptor (GPER), as well as a high concentration of glucocorticoid and mineralocorticoid receptors compared to other regions of the brain, which makes the hippocampus more vulnerable to their ligands (McEwen and Milner, 2017; McEwen et al., 1968; Moguilewsky and Raynaud, 1980; Wang et al., 2013).
From a metabolic point of view, in the hippocampus, recent transcriptional studies (Maffioli et al., 2022) have described an enrichment of metabolic pathways in women compared to men such as glycolysis or gluconeogenesis, purine metabolism and the pyruvate pathway, while the metabolism of amino acids such as Ala/Asp/Glu, Arg biosynthesis and glutaminergic synapses are decreased in healthy women compared to men. In addition, the TCA cycle, the hypoxia-inducible factor-1 (HIF-1) pathway and insulin secretion were shown to be higher (more active) in women than in men. However, this relationship is nullified in the case of AD in women.
An increase in proteins involved in the transport and uptake of insulin-like growth factor (IGF) has also been described in healthy women compared to men, but in the case of Alzheimer’s disease, there is a decrease in proteins involved in the response to insulin stimulus and in the regulation of insulin secretion by GLP1 (glucagon-like peptide-1), some of which are involved in GABAergic synapses and circadian synchronization. In addition, proteomic data show a higher expression of insulin-like growth factor binding protein 7 (IGFBP7) in women with AD. The secretion of this protein is positively regulated in response to oxidative stress and is related to insulin resistance and the alteration of insulin signalling in AD, being a critical regulator of memory consolidation (Agbemenyah et al., 2014). However, in the case of men, a significant difference has been observed in the expression of mitochondrial pyruvate transporter 1 (MPC1), in AD compared to healthy men. MPC1 appears to play a central role in glucose-stimulated insulin secretion, systemic glucose homeostasis in β cells and in the state of insulin resistance (McCommis et al., 2016). Therefore, these data would point to different pathophysiological mechanisms active in men and women in AD.
Glucose Metabolism and Transporters in the Hippocampus
In terms of total glucose consumption in the brain, the extent of glucose requirements for neuronal metabolism is still not clear, not only due to the consumption by other cells but also because glucose can be metabolized mainly by astrocytes into lactate, which can be exported and eventually used as fuel by neurons. However, neurons uptake and metabolize glucose through glycolysis as the main fuel for their normal function, with the proper maintenance of glucose metabolism being crucial to "sustain" learning and memory tasks, as its decrease leads to cognitive deficits (Gold et al., 2013; McEwen and Reagan, 2004). This cellular glucose uptake process requires the presence of glucose transporters, as glucose is a polar molecule, insoluble in the plasma membrane, thus essential for glucose flux.
In human hippocampal neurons, both glucose transporters 3 and 4 have been described in axons, GLUT4 and GLUT8 in soma, and GLUT3 in dendrites (Yonamine et al., 2023a; Yonamine et al., 2023b). GLUT3 is a low-capacity glucose transporter expressed in the plasma membrane of neuropil segments, ensuring basal support of glucose to neuronal activity. As for GLUT8, it has been described in hippocampal neurons, astrocytes, and microglia in murine models but intracellularly (Lizak et al., 2019; McEwen and Reagan, 2004; Mueckler and Thorens, 2013; Zhao and Keating, 2007), initially not playing a significant role in cerebral glucose consumption, as there is no evidence of intracellular free glucose concentration, especially in neurons expressing GLUT4. GLUT8 is capable of translocating in response to insulin in the hippocampus, although despite responding to insulin in blastocysts, it has not been confirmed in neurons. Furthermore, GLUT8 shows a very low Km for glucose transport, compromising a potential significant role in hippocampal glucose metabolism.
In the early 1990s, the presence of GLUT4 was described in the medulla oblongata and hypothalamus, later in the cerebellum, and finally in the dentate gyrus of the hippocampus. It was subsequently reported that the GLUT4 protein translocate to the plasma membrane in response to insulin, both in rat hippocampal neurons and in the human neuronal cell line SH-SY5Y, like adipose and muscle tissues. Additionally, similar to skeletal muscle cells where GLUT4 also translocate due to muscle contractions, in primary rat hippocampal neurons, action potential firing also triggers the insertion of GLUT4 into the plasma membrane, especially in axonal nerve terminals. These data attribute to hippocampal GLUT4 protein the same functional role it plays in insulin-sensitive classic tissues, positioning this transporter as a key player in glucose utilization in the hippocampus. Thus, two transporters are fundamental in glucose transport and therefore for the maintenance of hippocampal cognitive functions; GLUT1, which is responsible for glucose transport across the BBB, supplying glucose to the brain interstice, and GLUT4, which regulates glucose influx into neurons. The latter is crucial in insulin-stimulated peripheral glucose uptake, playing a fundamental role in the metabolic homeostasis of hippocampal neurons in physiological and pathological conditions resulting from neuronal homeostasis disruption (Yonamine et al., 2023a).
5. Cerebral Energy Metabolism and Steroid Hormone Receptors
It is a fact that oestrogens are the master regulator that acts through a network of receptors ensuring an effective cerebral response by regulating energy metabolism through the coordination of transcriptional and signalling pathways (Brinton et al., 2015). Oestrogens, mainly 17 β oestradiol (E2), exert important modulatory functions in the CNS through genomic and non-genomic mechanisms. The sources of E2 for the brain include circulating E2 or testosterone secreted from peripheral tissues and that quickly accesses the CNS and E2 synthesized in neurons and glial cells. The effects of E2 are mediated by 2 types of oestrogen receptors (ER): ERα and ERβ. These receptors act as transcription factors that mediate the genomic effects of E2 and as membrane-associated proteins that trigger rapid non-genomic effects. Some of these rapid effects are also mediated by guanine protein-coupled receptors, including G protein-coupled ER-1 (GPER1, also known as GPR30) (Cersosimo and Benarroch, 2015).
Studies developed by Cooke et al (2017) reviewed the implication of oestrogens in male brain physiology, considering that local aromatization of testosterone to oestradiol is necessary for the development of the male brain being the effect of testosterone indirect on it. In fact, in the absence of testosterone, undifferentiated brains develop as females. It also establishes that the normal differentiation of the male brain is susceptible to be altered by perinatal exposure to endocrine disruptors (e.g., BPA) (Bai et al., 2011; Faber and Hughes, 1991). Thus, cerebral sexual dimorphism is indirectly controlled by testosterone after local conversion to E2, although other genes of the sex chromosomes could also affect this process. In relation to the steroid receptors that are expressed in the male brain, both aromatase and ERα, ERβ and GPER have been described (Kyi-Tha-Thu et al., 2015; Stanic et al., 2014). The locally produced E2 is considered a cerebral neurosteroid (Baulieu, 1998) and its effects on the brain in the case of men are mediated by the mentioned receptors, as well as its neuroprotective function of oestradiol (Arevalo et al., 2015). In the case of women, the linkage of oestrogens in cerebral energy metabolism becomes especially relevant in a vital transition state for women such as menopause. In fact, in women after menopause, those who receive oestrogen replacement therapy have a different pattern of brain metabolism than women who do not receive oestrogen therapy. However, these results should be taken with caution as there is an important relationship with the time along the perimenopause at which the treatment is administered (Henderson, 2014). In this context, the analysis of 18F-FDGPET, have demonstrated indicators of hypometabolism in regions of the brain necessary for learning and memory. In fact, hippocampal hypometabolism, after a 2-year observation period, was evident in women during menopause, both in the parahippocampal gyrus and the temporal lobe area, the medial prefrontal cortex and the posterior cingulate cortex, and oestrogen replacement therapy prevented hypometabolism in each of these brain regions and preserved memory function (Maki and Resnick, 2000; Rasgon et al., 2014a; Rasgon et al., 2005). In this sense, it has been established that the metabolism of glucose in the brain is fundamental for neurological function and that evidence of hypometabolism is evident several decades before the diagnosis of neurodegenerative diseases such as Alzheimer’s disease (Mosconi et al., 2009; Mosconi et al., 2014; Rasgon et al., 2014b). Oestrogen signalling supports and sustains glucose metabolism in the brain by regulating the expression of glucose transporters, resulting in increased glucose uptake, stimulating aerobic glycolysis. Oestrogens regulate energy metabolism in the brain through oestrogen receptors, GPER, ER-α and ER-β, activation of PI3K and Akt and MAPK-ERK signalling pathways. In fact, in brain regions linked to learning and memory, such as the hippocampus, the amygdala, the cingulate cortex and the retrosplenial cortex, oestrogen receptors are present (McEwen et al., 2012). At this point, we must ask ourselves whether the effect of oestrogens on the process of brain aging affects men and women in the same way, since in the case of men the absence of periods of changes like menopause for women, as well as a longer exposure to testosterone in the case of men, would make us presuppose different aging processes, linked to the exposure of sex hormones (
Figure 1).
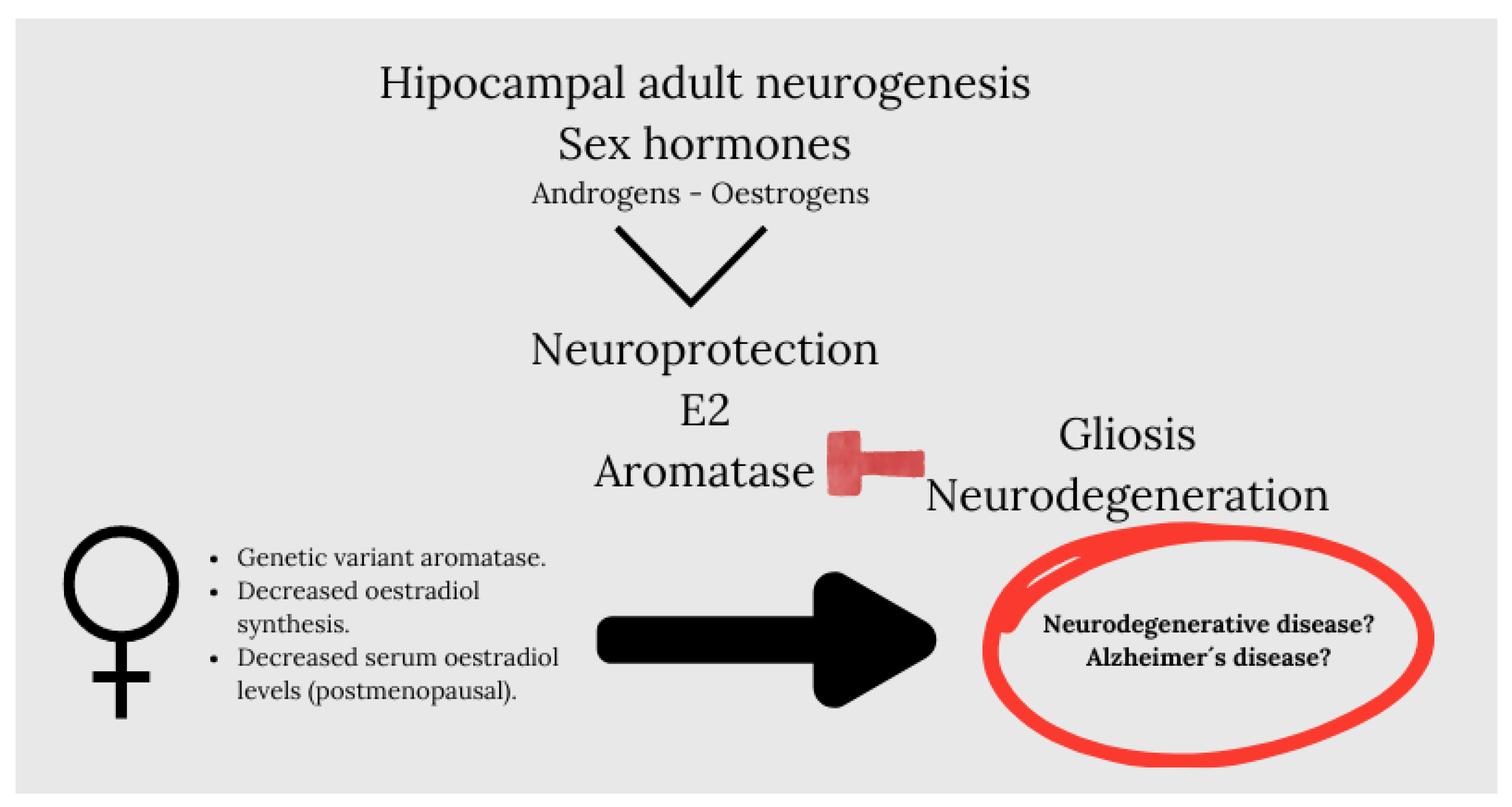
In this context, we must note that sex hormones such as androgens and oestrogens are potent modulators of adult neurogenesis in the hippocampus. Oestrogens modulate neurogenesis in women, but to a lesser extent in men (Barker and Galea, 2008), while androgens modulate neurogenesis in males (Hamson et al., 2013; Swift-Gallant et al., 2018), but it is not known if they modulate neurogenesis in women (Mahmoud et al., 2016). An interesting review developed by Arevalo et al.,(Arevalo et al., 2015) highlights oestradiol as a neuroprotector in male and female brain and established that the synthesis of brain-derived oestradiol regulates synaptic plasticity, the adult neurogenesis, reproductive and aggressive behaviour, pain processing, affect and cognition. It is revealing that they put on stage aromatase, an enzyme that generates oestradiol, which, under pathological conditions, increases in neurons and is induced de novo in astrocytes as an endogenous neuroprotective mechanism. Thus, the inhibition or silencing of cerebral aromatase increases gliosis and neurodegeneration after a brain injury. The importance of aromatase as a neuroprotective molecule in humans is suggested by the existence of genetic variants of the enzyme that confer a higher risk of Alzheimer’s disease (Chace et al., 2012; Iivonen et al., 2004; Medway et al., 2014). These genetic variants of aromatase may cause a decrease in the synthesis of oestradiol in the brain, which, together with a decrease in serum levels of oestradiol in postmenopausal women or serum levels of testosterone in older men, may increase the risk of developing neurodegenerative diseases (
Figure 2).
Interestingly, the expression of aromatase increases in the astrocytes of the human prefrontal cortex in the late stages of Alzheimer’s disease, a phenomenon that has been interpreted as part of a rescue program (Luchetti et al., 2011). In the human brain, aromatase mRNA, aromatase protein, and aromatase activity have been detected in the hippocampus, the amygdala, the preoptic area, the hypothalamus, and the neocortex (Azcoitia et al., 2021; Azcoitia et al., 2011; Stoffel-Wagner et al., 1999). Initially, it was thought that the function of cerebral oestradiol was restricted to the regulation of reproductive behaviour and neuroendocrine processes in the hypothalamus (Roselli, 2007). However, locally produced E2 also protects the brain against a variety of neurological and neurodegenerative disorders, including Alzheimer’s disease (AD) (Melcangi et al., 2011; Saldanha et al., 2009). In fact, studies developed by Prange Kiel, et al., (Prange-Kiel et al., 2016), showed the presence of aromatase in sections of the hippocampus of post-mortem human brain tissue, in all regions of the cornu ammonis (CA1, CA3, and CA4) and in the dentate gyrus (DG). Most of the cells positive for aromatase were neurons and according to the location and size of the neurons, it was concluded that the neurons positive for aromatase in the cornu ammonis and DG were pyramidal cells and granular cells, respectively, with a small percentage of non-neuronal cells (Arevalo et al., 2015). On the other hand, a study carried out by Kakimoto et al. (Kakimoto et al., 2016) conducted with a sample size of 963 cognitively normal humans, addresses the brain changes in both sexes throughout normal aging. They observe a reduction in gray matter volumes predominantly lateral associated with age, as already described earlier (Taki et al., 2011), as well as significant specific differences by sex in the progression and pattern of reduction of various areas. Thus, a rapid decrease was observed from the age of 60 in men, while in women a gradual decrease was observed from the age of 40. This difference was linked to hormonal events such as childbirth or menopause and to social issues in the case of men, such as retirement. As for the changes in brain metabolism related to age, their studies were in agreement with those described by other authors, describing a glucose hypometabolism associated with age in the medial frontal cortex(Yoshizawa et al., 2014), with the peculiarity that they not only observed a change in metabolism but also in volume, since glucose hypometabolism was also associated with brain atrophy associated with age. Studies with PET technique to assess glucose metabolism, showed a decrease in glucose metabolism in men and women in the medial parietal cortex and the lateral frontal cortex. In the case of men, it was associated with a visuospatial sensor(Corbetta et al., 2000), while in the case of women it was associated with an integrative region of speech processing that included Broca’s area and a complex region of speech processing(Grewe et al., 2005). According to these authors, these metabolic differences decreased as age advanced, maintaining differences only in the male parietal prefrontal cortex and female ventrolateral cortex. Already in studies developed in the last century, (Coffey et al., 1998) showed that the morphological characteristics of the brain in humans seem to be sensitive to the effects of age and sex, as we have mentioned earlier, showing, at least according to the data reviewed, that the woman’s brain is more sensitive to these factors, specifically in relation to menopause, where neuronal levels of glucose transporters decrease, which would coincide with the appearance of cerebral hypometabolism (Ding et al., 2013). The brain would adapt to this decrease in the availability of glucose by increasing the dependence on ketone bodies as an alternative fuel to generate acetyl-CoA necessary to enter the TCA cycle and, ultimately, generate ATP through complexes of mitochondrial redox transporters. The depletion of ketone bodies derived from hepatic lipid metabolism, can lead to the metabolism of fatty acids derived from the brain to generate ketone bodies through oxidation processes in glial cells (Harlow et al., 2012). We must bear in mind that as Brinton et al (Brinton et al., 2015) concludes, reproductive senescence, that is, menopause, is driven by the ovaries but also by the brain. In fact, the gonadotropin-releasing hormone (GnRH), stimulates the synthesis of E2 in the ovary, but also activates the synthesis of E2 in the hippocampus (Prange-Kiel et al., 2016), (Prange-Kiel et al., 2008; Prange-Kiel et al., 2013)
6. Alteration of Glucose Metabolism: Insulin
In addition to endogenous brain oestrogens, circulating oestrogens, and aromatase activity, there is another molecule directly involved in cerebral glucose metabolism; insulin.
It should be noted that the effects of insulin in the hippocampus may not exactly coincide with those observed in other parts of the body. In fact, some of its effects are mechanically impossible in the hippocampus; for example, GLUT4 is expressed in neurons (DiNuzzo et al., 2011[McEwen, 2004 #120)] and glycogen, on the contrary, is only produced in astrocytes (Heni et al., 2011), so it is unlikely that insulin effects the conversion of glucose to glycogen through GLUT4-mediated glucose uptake in the brain. In general, brain insulin signalling and GLUT4 regulation may differ from those of peripheral tissues. However, it is true that hyperinsulinemia and insulin resistance have significant effects on cognitive impairment and play an important role in AD, being more prevalent in women (Cetinkalp et al., 2014). In fact, hyperglycaemia is a possible risk factor for the development of AD, as well as low insulin response, i.e., insulin resistance.
The possible mediating mechanisms of insulin action come from studies showing a binding relationship between T2DM and the development of AD, and showing that AD patients have a reduction in insulin signalling in the hippocampus along with brain hypometabolism and beta-amyloid (Aβ) accumulation. (Mayeux and Stern, 2012; Pearson-Leary and McNay, 2012) (Luchsinger, 2010a, b; Maffioli et al., 2022)
Under physiological conditions, the activation of insulin signalling requires the binding of insulin to the insulin receptor (IR), which auto-phosphorylates on Tyr residues (e.g., Tyr1158/1162/1163) and promotes the receptor tyrosine kinase-mediated phosphorylation of its substrate (IRS1) on specific Tyr residues (e.g. 632). Once activated, IRS1 works as a scaffold protein, driving the activation of the PI3K/Akt axis, which is critical for linking upstream effectors (IR and IRS1) with downstream proteins mediating insulin neurotrophic outcomes. Activation of the PI3K/Akt axis is regulated by the phosphatase PTEN, which reduces PIP3 levels required for Akt activation as well as for increasing the expression of PKCζ. Akt promotes the phosphorylation of several targets among which are: GSK3β (on Ser9, inhibitory site) and AS160 (on Thr642, activating site). This latter, together with PKCζ, are responsible for the translocation of GLUT4-containing vesicles to the plasma membrane to mediate glucose uptake. Furthermore, Akt stimulates the upregulation of HKII, which is a pivotal enzyme involved in glucose metabolism and thus energy production. During the development of brain insulin resistance, a dysregulation of a number of these proteins was observed. In particular, the brain insulin resistance phenomenon shows key markers such as reduced IR protein levels and/or increased IRS1 inhibitory phosphorylation levels (e.g., Ser636), that are responsible for the uncoupling between IR and IRS1. As a result, despite that insulin binds to IR, reduced activation of its downstream effectors was found (Tramutola et al., 2020).Therefore, the inhibition of IRS1 is responsible for the uncoupling between IR and IRS1.
The scientific literature undoubtedly links the development of cerebral insulin resistance and hippocampal affection with the risk of developing Alzheimer’s disease (Arnold et al., 2018; Hamze et al., 2022; McNay and Recknagel, 2011). In fact, Alzheimer’s disease has already been characterized as type 3 diabetes (de la Monte and Wands, 2008; Michailidis et al., 2022).
Data provided by Tramutola et al. (Tramutola et al., 2020)suggest the existence of defects in the mechanisms responsible for the translocation of GLUT4 to the plasma membrane. Although most glucose uptake in neurons occurs through GLUT3, insulin-regulated GLUT4 is also co-expressed with GLUT3 in brain regions related to cognitive behaviour (Apelt et al., 1999). Insulin activation induces the translocation of GLUT4 to the neuronal cell membrane through an Akt-dependent mechanism (Marko et al., 2020; McEwen and Reagan, 2004) and is believed to improve glucose flow to neurons during periods of high metabolic demand, such as learning (Pearson-Leary and McNay, 2016).
6.1. GLUT4 as an Insulin-Oestradiol Mediator
However, along with GLUT4, the vesicles also contain other proteins, some of which are involved in the regulation of memory processes, such as insulin-like growth factor 2 (IGF2) receptors (IGF2R), insulin-regulated aminopeptidase (IRAP), and the Ras GTPase activator-like protein IQGAP1 (Agis-Balboa et al., 2011; Fernando et al., 2008; Schrick et al., 2007). Therefore, the recruitment of GLUT4 to the cell surface will simultaneously recruit these proteins (and vice versa), so that the increase in GLUT4 in the plasma membrane could be a correlation with the cognitive effects of insulin, associated with the peptides with which it is co-transported. The presence of IRAP in GSVs can promote GLUT4 protein stability and regulate GLUT4 compartmentalization and recycling from endosomes to GSVs after its translocation and subsequent endocytosis (Jordens et al., 2010).
The GLUT4 translocation pathway shows points of connection with the action pathways induced through ER α and β, specifically in relation to glucose metabolism through PI3k and Akt. Thus, the binding of oestradiol to membrane-associated α and β-ER activates numerous signalling cascades, many of which are linked to neuroprotection. It also activates the insulin-like growth factor 1 (IGF1) receptor (IGF1R), which mediates neuroprotective signals through the transcriptional increase of IGF1 and inducing the interaction of the ER receptor with the p85 catalytic subunit of PI3K, composed of this and the p110 unit, allowing the formation of a complex formed by ER, IGF1R and the components of the IGF1R signalling pathway such as insulin receptor substrate IRS1, PI3K, Akt and GSK3. Through this pathway, oestradiol downregulates Tau phosphorylation (Arevalo et al., 2015).
In this context, the study of postmortem brains with AD and mild cognitive impairment showed clear signs of brain insulin resistance, i.e., reduced insulin receptor (IR) and increased serine (inhibitory) phosphorylation of insulin receptor substrate 1 (IRS1), particularly in the hippocampus, cortex and hypothalamus(Biessels and Reagan, 2015; Tramutola et al., 2020).In this sense, although there are few studies related to the relationship between IRS1 and brain oestrogens, it is true that this connection could occur through the IRS1/PI3K and Akt molecules and ERα and β (
Figure 3).
6.2. Role of GLUT4 in Hippocampal Cognitive Processes: Importance of IRAP
Hippocampal cognitive processes and glucose metabolism are intimately linked processes and connected through insulin. Insulin is a key player in the cognitive processes of the hippocampus: specific blockade of endogenous intrahippocampal insulin significantly impairs spatial working memory, while physiological doses administered to the hippocampus improve it. In fact, the alteration of glucose supply to neurons because of dysregulation of the insulin-sensitive glucose transporter GLUT4, authors such as Mc Nay and Pearson propose as a unifying mechanism that explains, at least in part, the comorbidity between type II diabetes and AD (McNay and Recknagel, 2011; McNay et al., 2013).
GLUT4 is a very important piece in neuronal carbohydrate metabolism, in addition to being the predominant glucose transporter in adipose and muscle tissue. In these tissues, insulin regulates glucose uptake by controlling the number of transporters on the cell surface and acts within minutes to mobilize GLUT4 from intracellular reserves. In fact, one of the main current treatments for type II diabetes and/or insulin resistance is metformin, which increases insulin sensitivity of tissues by increasing GLUT4 expression (Herman et al., 2022).
At the beginning of this century, the presence of the GLUT4 transporter in the mouse hippocampus along with the insulin-regulated protein (IRAP) was described (Fernando et al., 2008). The results shown by Chai et al. suggest that, in the hippocampus, where IRAP and GLUT4 colocalize in the same vesicles, there is an activity-dependent glucose uptake mechanism. This system can be modulated by IRAP ligands, such as angiotensin IV, and depends on both IRAP and GLUT4. Therefore, the discovery of IRAP expressed in intracellular vesicles of the neuronal soma, where GLUT4 is also localized, in brain regions involved in cognitive function, as a binding site for memory-facilitating peptides such as Ang IV, would represent the description of a new function for this enzyme in a murine model (Albiston et al., 2008; Fernando et al., 2007).
Thus, it has been shown that glucose flow through GLUT4 is necessary for cognitive improvement by hippocampal AngIV, suggesting that IRAP is not an alternative to GluT4 as the transducer of the cognitive effects of insulin(McNay and Pearson-Leary, 2020).
In this context, the results obtained by Ismail et al. (Ismail et al., 2017) show a molecular link between cholesterol, brain glucose, and the brain renin-angiotensin system, all of which are affected in some neurodegenerative diseases. Specifically, they observed 27-hydroxycholesterol (27OH) was able to curb the ability of AngIV to stimulate glucose uptake and inhibit the catalytic activity of IRAP. Recently, the 27OH has been defined as an endogenous selective estrogen receptor modulator (SERM)(He and Nelson, 2017), with activities in atherosclerosis, osteoporosis, breast and prostate cancers, and neurodegenerative diseases. Although the blood-brain barrier is impermeable to cholesterol and, therefore, a cholesterol-rich diet does not modify intracranial cholesterol concentrations, 27OH is a slightly more polar metabolite than cholesterol and can cross the blood-brain barrier. In this sense, a recent study by Brooks et al. on late-onset AD described the reduction of ERα and an elevation of ERβ in association with a high level of cholesterol/27OH. The same pattern of reduced ERα and elevated ERβ is also found in the hippocampus of AD patients.
7. Conclusions
Brain development characteristics in both sexes lead us to consider pathological differences at the brain level for each sex, along with the described plasticity of hippocampal tissue and the distinct pattern of receptors present in this tissue, making it susceptible to different ligands and defining its response to them. Therefore, we could deduct from the review conducted that a comprehensive understanding of the functional, anatomical, and functional differences in brain tissue, particularly the hippocampus, as well as the energy metabolism where hormones like insulin and oestradiol play a crucial role, along with glucose and its transporters and related molecules such as insulin-regulated aminopeptidase, would provide us with valuable information for comprehending and subsequently studying pathologies resulting ultimately from the aforementioned processes, and associated with sex, such as neurodegenerative pathologies, specifically Alzheimer’s disease.
Author’s Contribution
JMM-M and VC-H; Investigation, Methodology, Writing-Review & Editing, MR-R, MJR-M and MJR-E; Visualization, Writing - Review & Editing, JMM-M and VC-H: Writing-Review & Editing, Resources. MdPC-G, Conceptualization, Visualization, Investigation, Writing-Original Draft, Writing-Review & Editing. All authors read and approved the final document.
Acknowledgments
Declared none.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adam, P.A., Raiha, N., Rahiala, E.L., Kekomaki, M., 1975. Oxidation of glucose and D-B-OH-butyrate by the early human fetal brain. Acta paediatrica Scandinavica 64, 17-24. [CrossRef]
- Agbemenyah, H.Y.; Agis-Balboa, R.C.; Burkhardt, S.; Delalle, I.; Fischer, A. Insulin growth factor binding protein 7 is a novel target to treat dementia. Neurobiol. Dis. 2014, 62, 135–143. [CrossRef]
- Agis-Balboa, R.C.; Arcos-Diaz, D.; Wittnam, J.; Govindarajan, N.; Blom, K.; Burkhardt, S.; Haladyniak, U.; Agbemenyah, H.Y.; Zovoilis, A.; Salinas-Riester, G.; et al. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011, 30, 4071–4083. [CrossRef]
- Aiello, L.C.; Wheeler, P. The Expensive-Tissue Hypothesis: The Brain and the Digestive System in Human and Primate Evolution. Curr. Anthr. 1995, 36, 199–221. [CrossRef]
- Albiston, A.L.; Morton, C.J.; Ng, H.L.; Pham, V.; Yeatman, H.R.; Ye, S.; Fernando, R.N.; De Bundel, D.; Ascher, D.B.; Mendelsohn, F.A.O.; et al. Identification and characterization of a new cognitive enhancer based on inhibition of insulin-regulated aminopeptidase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 4209–4217. [CrossRef]
- Apelt, J.; Mehlhorn, G.; Schliebs, R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J. Neurosci. Res. 1999, 57, 693–705. [CrossRef]
- Arevalo, M.-A.; Azcoitia, I.; Garcia-Segura, L.M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2014, 16, 17–29. [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [CrossRef]
- Artola, A.; Von Frijtag, J.C.; Fermont, P.C.J.; Gispen, W.H.; Schrama, L.H.; Kamal, A.; Spruijt, B.M. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur. J. Neurosci. 2006, 23, 261–272. [CrossRef]
- Azcoitia, I., Mendez, P., Garcia-Segura, L.M., 2021. Aromatase in the Human Brain. Androgens: clinical research and therapeutics 2, 189-202. [CrossRef]
- Azcoitia, I.; Yague, J.; Garcia-Segura, L. Estradiol synthesis within the human brain. Neuroscience 2011, 191, 139–147. [CrossRef]
- Babcock, K.R., Page, J.S., Fallon, J.R., Webb, A.E., 2021. Adult Hippocampal Neurogenesis in Aging and Alzheimer’s Disease. Stem cell reports 16, 681-693. [CrossRef]
- Bai, Y.; Chang, F.; Zhou, R.; Jin, P.-P.; Matsumoto, H.; Sokabe, M.; Chen, L. Increase of Anteroventral Periventricular Kisspeptin Neurons and Generation of E2-Induced LH-Surge System in Male Rats Exposed Perinatally to Environmental Dose of Bisphenol-A. Endocrinology 2011, 152, 1562–1571. [CrossRef]
- Barker, J.; Galea, L. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience 2008, 152, 888–902. [CrossRef]
- Battaglia, F.C.; Meschia, G. Principal substrates of fetal metabolism.. Physiol. Rev. 1978, 58, 499–527. [CrossRef]
- Baulieu, E. Neurosteroids: A novel function of the brain. Psychoneuroendocrinology 1998, 23, 963–987. [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [CrossRef]
- Bell, A.W.; Battaglia, F.C.; Makowski, E.L.; Meschia, G. Relationship between Metabolic Rate and Body Size in Fetal Life. Neonatology 1985, 47, 120–123. [CrossRef]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [CrossRef]
- Blomqvist, G.; Thorell, J.O.; Ingvar, M.; Grill, V.; Widen, L.; Stone-Elander, S. Use of R-beta-[1-11C]hydroxybutyrate in PET studies of regional cerebral uptake of ketone bodies in humans. Am. J. Physiol. Metab. 1995, 269, E948–E959. [CrossRef]
- Brandt, J.; Buchholz, A.; Henry-Barron, B.; Vizthum, D.; Avramopoulos, D.; Cervenka, M.C. Preliminary Report on the Feasibility and Efficacy of the Modified Atkins Diet for Treatment of Mild Cognitive Impairment and Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 969–981. [CrossRef]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 2015, 11, 393–405. [CrossRef]
- Cersosimo, M.G., Benarroch, E.E., 2015. Estrogen actions in the nervous system: Complexity and clinical implications. Neurology 85, 263-273. [CrossRef]
- Cetinkalp, S.; Simsir, I.Y.; Ertek, S. Insulin Resistance in Brain and Possible Therapeutic Approaches. Curr. Vasc. Pharmacol. 2014, 12, 553–564. [CrossRef]
- Coffey, C.E., Lucke, J.F., Saxton, J.A., Ratcliff, G., Unitas, L.J., Billig, B., Bryan, R.N., 1998. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Archives of neurology 55, 169-179. [CrossRef]
- Colle, R.; Segawa, T.; Chupin, M.; Dong, M.N.T.K.T.; Hardy, P.; Falissard, B.; Colliot, O.; Ducreux, D.; Corruble, E. Early life adversity is associated with a smaller hippocampus in male but not female depressed in-patients: a case–control study. BMC Psychiatry 2017, 17, 71. [CrossRef]
- Corbetta, M.; Kincade, J.M.; Ollinger, J.M.; McAvoy, M.P.; Shulman, G.L. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 2000, 3, 292–297. [CrossRef]
- Croteau, E.; Castellano, C.-A.; Richard, M.A.; Fortier, M.; Nugent, S.; Lepage, M.; Duchesne, S.; Whittingstall, K.; Turcotte, .E.; Bocti, C.; et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 551–561. [CrossRef]
- Cunnane, S.C., Courchesne-Loyer, A., St-Pierre, V., Vandenberghe, C., Pierotti, T., Fortier, M., Croteau, E., Castellano, C.A., 2016. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Annals of the New York Academy of Sciences 1367, 12-20. [CrossRef]
- Cunnane, S.C.; Crawford, M.A. Survival of the fattest: fat babies were the key to evolution of the large human brain. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2003, 136, 17–26. [CrossRef]
- Chace, C., Pang, D., Weng, C., Temkin, A., Lax, S., Silverman, W., Zigman, W., Ferin, M., Lee, J.H., Tycko, B., Schupf, N., 2012. Variants in CYP17 and CYP19 cytochrome P450 genes are associated with onset of Alzheimer’s disease in women with down syndrome. Journal of Alzheimer’s disease : JAD 28, 601-612. [CrossRef]
- Christie, B.R.; Cameron, H.A. Neurogenesis in the adult hippocampus. Hippocampus 2006, 16, 199–207. [CrossRef]
- Chugani, H.T., 2021. Hypermetabolism on Pediatric PET Scans of Brain Glucose Metabolism: What Does It Signify? Journal of nuclear medicine: official publication, Society of Nuclear Medicine 62, 1301-1306. [CrossRef]
- de la Monte, S.M., Wands, J.R., 2008. Alzheimer’s disease is type 3 diabetes-evidence reviewed. Journal of diabetes science and technology 2, 1101-1113. [CrossRef]
- Ding, F., Yao, J., Rettberg, J.R., Chen, S., Brinton, R.D., 2013. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PloS one 8, e79977. [CrossRef]
- DiNuzzo, M., Maraviglia, B., Giove, F., 2011. Why does the brain (not) have glycogen? BioEssays : news and reviews in molecular, cellular and developmental biology 33, 319-326. [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [CrossRef]
- Everaerd, D.; Gerritsen, L.; Rijpkema, M.; Frodl, T.; van Oostrom, I.; Franke, B.; Fernández, G.; Tendolkar, I. Sex Modulates the Interactive Effect of the Serotonin Transporter Gene Polymorphism and Childhood Adversity on Hippocampal Volume. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 2012, 37, 1848–1855. [CrossRef]
- Faber, K.A.; Hughes, C.L. The Effect of Neonatal Exposure to Diethylstilbestrol, Genistein, and Zearalenone on Pituitary Responsiveness and Sexually Dimorphic Nucleus Volume in the Castrated Adult Rat1. Biol. Reprod. 1991, 45, 649–653. [CrossRef]
- Fernando, R.N., Albiston, A.L., Chai, S.Y., 2008. The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus--potential role in modulation of glucose uptake in neurones? The European journal of neuroscience 28, 588-598. [CrossRef]
- Fernando, R.N.; Luff, S.E.; Albiston, A.L.; Chai, S.Y. Sub-cellular localization of insulin-regulated membrane aminopeptidase, IRAP to vesicles in neurons. J. Neurochem. 2007. [CrossRef]
- Glasser, M.F.; Goyal, M.S.; Preuss, T.M.; Raichle, M.E.; Van Essen, D.C. Trends and properties of human cerebral cortex: Correlations with cortical myelin content. NeuroImage 2014, 93, 165–175. [CrossRef]
- Gold, P.E., Newman, L.A., Scavuzzo, C.J., Korol, D.L., 2013. Modulation of multiple memory systems: from neurotransmitters to metabolic substrates. Hippocampus 23, 1053-1065. [CrossRef]
- Goto, M.; Abe, O.; Miyati, T.; Inano, S.; Hayashi, N.; Aoki, S.; Mori, H.; Kabasawa, H.; Ino, K.; Yano, K.; et al. 3 Tesla MRI detects accelerated hippocampal volume reduction in postmenopausal women. J. Magn. Reson. Imaging 2010, 33, 48–53. [CrossRef]
- Grewe, T., Bornkessel, I., Zysset, S., Wiese, R., von Cramon, D.Y., Schlesewsky, M., 2005. The emergence of the unmarked: a new perspective on the language-specific function of Broca’s area. Human brain mapping 26, 178-190. [CrossRef]
- Gutiérrez-Lobos, K.; Scherer, M.; Anderer, P.; Katschnig, H. The influence of age on the female/male ratio of treated incidence rates in depression. BMC Psychiatry 2002, 2, 3–3. [CrossRef]
- Hamson, D.K.; Wainwright, S.R.; Taylor, J.R.; Jones, B.A.; Watson, N.V.; Galea, L.A.M. Androgens Increase Survival of Adult-Born Neurons in the Dentate Gyrus by an Androgen Receptor-Dependent Mechanism in Male Rats. Endocrinology 2013, 154, 3294–3304. [CrossRef]
- Hamzé, R.; Delangre, E.; Tolu, S.; Moreau, M.; Janel, N.; Bailbé, D.; Movassat, J. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Shared Molecular Mechanisms and Potential Common Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 15287. [CrossRef]
- Harlow, S.D., Gass, M., Hall, J.E., Lobo, R., Maki, P., Rebar, R.W., Sherman, S., Sluss, P.M., de Villiers, T.J., Group, S.C., 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19, 387-395. [CrossRef]
- Hasselbalch, S.G.; Knudsen, G.M.; Jakobsen, J.; Hageman, L.P.; Holm, S.; Paulson, O.B. Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am. J. Physiol. Metab. 1995, 268, E1161–E1166. [CrossRef]
- He, S.; Nelson, E.R. 27-Hydroxycholesterol, an endogenous selective estrogen receptor modulator. Maturitas 2017, 104, 29–35. [CrossRef]
- Henderson, V.W., 2014. Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause. The Journal of steroid biochemistry and molecular biology 142, 99-106. [CrossRef]
- Heni, M.; Hennige, A.M.; Peter, A.; Siegel-Axel, D.; Ordelheide, A.-M.; Krebs, N.; Machicao, F.; Fritsche, A.; Häring, H.-U.; Staiger, H. Insulin Promotes Glycogen Storage and Cell Proliferation in Primary Human Astrocytes. PLOS ONE 2011, 6, e21594. [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [CrossRef]
- Hoekzema, E.; Barba-Müller, E.; Pozzobon, C.; Picado, M.; Lucco, F.; García-García, D.; Soliva, J.C.; Tobeña, A.; Desco, M.; A Crone, E.; et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 2016, 20, 287–296. [CrossRef]
- Iivonen, S.; Corder, E.; Lehtovirta, M.; Helisalmi, S.; Mannermaa, A.; Vepsäläinen, S.; Hänninen, T.; Soininen, H.; Hiltunen, M. Polymorphisms in the CYP19 gene confer increased risk for Alzheimer disease. Neurology 2004, 62, 1170–1176. [CrossRef]
- Ingalhalikar, M., Smith, A., Parker, D., Satterthwaite, T.D., Elliott, M.A., Ruparel, K., Hakonarson, H., Gur, R.E., Gur, R.C., Verma, R., 2014. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences of the United States of America 111, 823-828. [CrossRef]
- Irimia, A.; Chambers, M.C.; Torgerson, C.M.; Van Horn, J.D. Circular representation of human cortical networks for subject and population-level connectomic visualization. NeuroImage 2012, 60, 1340–1351. [CrossRef]
- Irvine, K., Laws, K.R., Gale, T.M., Kondel, T.K., 2012. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. Journal of clinical and experimental neuropsychology 34, 989-998. [CrossRef]
- Ismail, M.-A.-M.; Mateos, L.; Maioli, S.; Merino-Serrais, P.; Ali, Z.; Lodeiro, M.; Westman, E.; Leitersdorf, E.; Gulyás, B.; Olof-Wahlund, L.; et al. 27-Hydroxycholesterol impairs neuronal glucose uptake through an IRAP/GLUT4 system dysregulation. J. Exp. Med. 2017, 214, 699–717. [CrossRef]
- Jordens, I.; Molle, D.; Xiong, W.; Keller, S.R.; McGraw, T.E. Insulin-regulated Aminopeptidase Is a Key Regulator of GLUT4 Trafficking by Controlling the Sorting of GLUT4 from Endosomes to Specialized Insulin-regulated Vesicles. Mol. Biol. Cell 2010, 21, 2034–2044. [CrossRef]
- Kaczkurkin, A.N., Raznahan, A., Satterthwaite, T.D., 2019. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 44, 71-85. [CrossRef]
- Kakimoto, A.; Ito, S.; Okada, H.; Nishizawa, S.; Minoshima, S.; Ouchi, Y. Age-Related Sex-Specific Changes in Brain Metabolism and Morphology. J. Nucl. Med. 2015, 57, 221–225. [CrossRef]
- Kennedy, C.; Sokoloff, L. An Adaptation of the Nitrous Oxide Method to the Study of the Cerebral Circulation in Children; Normal Values for Cerebral Blood Flow and Cerebral Metabolic Rate in Childhood1. J. Clin. Investig. 1957, 36, 1130–1137. [CrossRef]
- Kety, S.S.; Schmidt, C.F. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values 1. J. Clin. Investig. 1948, 27, 476–483. [CrossRef]
- Khaitovich, P.; E Lockstone, H.; Wayland, M.T.; Tsang, T.M.; Jayatilaka, S.D.; Guo, A.J.; Zhou, J.; Somel, M.; Harris, L.W.; Holmes, E.; et al. Metabolic changes in schizophrenia and human brain evolution. Genome Biol. 2008, 9, R124–R124. [CrossRef]
- Kyi-Tha-Thu, C.; Okoshi, K.; Ito, H.; Matsuda, K.-I.; Kawata, M.; Tsukahara, S. Sex differences in cells expressing green fluorescent protein under the control of the estrogen receptor-α promoter in the hypothalamus of mice. Neurosci. Res. 2015, 101, 44–52. [CrossRef]
- Laughlin, S.B.; Sejnowski, T.J. Communication in Neuronal Networks. Science 2003, 301, 1870–1874. [CrossRef]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017, 20, 1434–1447. [CrossRef]
- Lisofsky, N.; Mårtensson, J.; Eckert, A.; Lindenberger, U.; Gallinat, J.; Kühn, S. Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage 2015, 118, 154–162. [CrossRef]
- Liu, X.; Kim, C.S.; Kurbanov, F.T.; Honzatko, R.B.; Fromm, H.J. Dual Mechanisms for Glucose 6-Phosphate Inhibition of Human Brain Hexokinase. J. Biol. Chem. 1999, 274, 31155–31159. [CrossRef]
- Lizák, B.; Szarka, A.; Kim, Y.; Choi, K.-S.; Németh, C.E.; Marcolongo, P.; Benedetti, A.; Bánhegyi, G.; Margittai, . Glucose Transport and Transporters in the Endomembranes. Int. J. Mol. Sci. 2019, 20, 5898. [CrossRef]
- Lord, C.; Buss, C.; Lupien, S.J.; Pruessner, J.C. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiol. Aging 2006, 29, 95–101. [CrossRef]
- Luchetti, S., Bossers, K., Van de Bilt, S., Agrapart, V., Morales, R.R., Frajese, G.V., Swaab, D.F., 2011. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer’s disease. Neurobiology of aging 32, 1964-1976. [CrossRef]
- Luchsinger, J.A., 2010a. Diabetes, related conditions, and dementia. Journal of the neurological sciences 299, 35-38. [CrossRef]
- Luchsinger, J.A., 2010b. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? Journal of Alzheimer’s disease : JAD 20, 723-736. [CrossRef]
- Maffioli, E.; Murtas, G.; Rabattoni, V.; Badone, B.; Tripodi, F.; Iannuzzi, F.; Licastro, D.; Nonnis, S.; Rinaldi, A.M.; Motta, Z.; et al. Insulin and serine metabolism as sex-specific hallmarks of Alzheimer’s disease in the human hippocampus. Cell Rep. 2022, 40, 111271. [CrossRef]
- Mahmoud, R.; Wainwright, S.R.; Galea, L.A. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front. Neuroendocr. 2016, 41, 129–152. [CrossRef]
- Maki, P.M.; Resnick, S.M. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol. Aging 2000, 21, 373–383. [CrossRef]
- Marko, D.M.; Foran, G.; Vlavcheski, F.; Baron, D.C.; Hayward, G.C.; Baranowski, B.J.; Necakov, A.; Tsiani, E.; MacPherson, R.E.K. Interleukin-6 Treatment Results in GLUT4 Translocation and AMPK Phosphorylation in Neuronal SH-SY5Y Cells. Cells 2020, 9, 1114. [CrossRef]
- Markov, N.T.; Ercsey-Ravasz, M.; Van Essen, D.C.; Knoblauch, K.; Toroczkai, Z.; Kennedy, H. Cortical High-Density Counterstream Architectures. Science 2013, 342, 578–+. [CrossRef]
- Marner, L.; Nyengaard, J.R.; Tang, Y.; Pakkenberg, B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 2003, 462, 144–152. [CrossRef]
- Matsuzaki, T., Sasaki, K., Tanizaki, Y., Hata, J., Fujimi, K., Matsui, Y., Sekita, A., Suzuki, S.O., Kanba, S., Kiyohara, Y., Iwaki, T., 2010. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 75, 764-770. [CrossRef]
- Mayeux, R., Stern, Y., 2012. Epidemiology of Alzheimer disease. Cold Spring Harbor perspectives in medicine 2.
- McCance, R.A., Widdowson, E.M., 1974. The determinants of growth and form. Proceedings of the Royal Society of London. Series B, Biological sciences 185, 1-17.
- McCommis, K.S.; Hodges, W.T.; Bricker, D.K.; Wisidagama, D.R.; Compan, V.; Remedi, M.S.; Thummel, C.S.; Finck, B.N. An ancestral role for the mitochondrial pyruvate carrier in glucose-stimulated insulin secretion. Mol. Metab. 2016, 5, 602–614. [CrossRef]
- McEwen, B.S. Redefining neuroendocrinology: Epigenetics of brain-body communication over the life course. Front. Neuroendocr. 2018, 49, 8–30. [CrossRef]
- McEwen, B.S.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms.. Behav. Neurosci. 2012, 126, 4–16. [CrossRef]
- McEwen, B.S.; Milner, T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2016, 95, 24–39. [CrossRef]
- McEwen, B.S.; Reagan, L.P. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur. J. Pharmacol. 2004, 490, 13–24. [CrossRef]
- Mcewen, B.S.; Weiss, J.M.; Schwartz, L.S. Selective Retention of Corticosterone by Limbic Structures in Rat Brain. Nature 1968, 220, 911–912. [CrossRef]
- McNay, E.C.; Pearson-Leary, J. GluT4: A central player in hippocampal memory and brain insulin resistance. Exp. Neurol. 2019, 323, 113076–113076. [CrossRef]
- McNay, E.C.; Recknagel, A.K. Brain insulin signaling: A key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol. Learn. Mem. 2011, 96, 432–442. [CrossRef]
- McNay, E.C., Sandusky, L.A., Pearson-Leary, J., 2013. Hippocampal insulin microinjection and in vivo microdialysis during spatial memory testing. Journal of visualized experiments : JoVE, e4451. [CrossRef]
- McPherson, S., Back, C., Buckwalter, J.G., Cummings, J.L., 1999. Gender-related cognitive deficits in Alzheimer’s disease. Int Psychogeriatr 11, 117-122. [CrossRef]
- Medway, C.; Combarros, O.; Cortina-Borja, M.; Butler, H.T.; A Ibrahim-Verbaas, C.; Bruijn, R.F.A.G.d.; Koudstaal, P.J.; van Duijn, C.M.; Ikram, M.A.; Mateo, I.; et al. The sex-specific associations of the aromatase gene with Alzheimer’s disease and its interaction with IL10 in the Epistasis Project. Eur. J. Hum. Genet. 2013, 22, 216–220. [CrossRef]
- Melcangi, R.; Panzica, G.; Garcia-Segura, L. Neuroactive steroids: focus on human brain. Neuroscience 2011, 191, 1–5. [CrossRef]
- Mesulam, M., 2000. Brain, mind, and the evolution of connectivity. Brain and cognition 42, 4-6.
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [CrossRef]
- Miller, D.J., Duka, T., Stimpson, C.D., Schapiro, S.J., Baze, W.B., McArthur, M.J., Fobbs, A.J., Sousa, A.M., Sestan, N., Wildman, D.E., Lipovich, L., Kuzawa, C.W., Hof, P.R., Sherwood, C.C., 2012. Prolonged myelination in human neocortical evolution. Proceedings of the National Academy of Sciences of the United States of America 109, 16480-16485. [CrossRef]
- Moguilewsky, M.; Raynaud, J. Evidence for a specific mineralocorticoid receptor in rat pituitary and brain. J. Steroid Biochem. 1980, 12, 309–314. [CrossRef]
- Mosconi, L.; Mistur, R.; Switalski, R.; Brys, M.; Glodzik, L.; Rich, K.; Pirraglia, E.; Tsui, W.; De Santi, S.; de Leon, M.J. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology 2009, 72, 513–520. [CrossRef]
- Mosconi, L.; Murray, J.; Tsui, W.H.; Li, Y.; Spector, N.; Goldowsky, A.; Williams, S.; Osorio, R.; McHugh, P.; Glodzik, L.; et al. Brain imaging of cognitively normal individuals with 2 parents affected by late-onset AD. Neurology 2014, 82, 752–760. [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [CrossRef]
- Neves, G.; Cooke, S.F.; Bliss, T.V.P. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat. Rev. Neurosci. 2008, 9, 65–75; Erratum in 2008, 13, 878. [CrossRef]
- Nicoletti, A., Vasta, R., Mostile, G., Nicoletti, G., Arabia, G., Iliceto, G., Lamberti, P., Marconi, R., Morgante, L., Barone, P., Quattrone, A., Zappia, M., 2017. Gender effect on non-motor symptoms in Parkinson’s disease: are men more at risk? Parkinsonism Relat Disord 35, 69-74. [CrossRef]
- Olmos-Serrano, J.L.; Kang, H.J.; Tyler, W.A.; Silbereis, J.C.; Cheng, F.; Zhu, Y.; Pletikos, M.; Jankovic-Rapan, L.; Cramer, N.P.; Galdzicki, Z.; et al. Down Syndrome Developmental Brain Transcriptome Reveals Defective Oligodendrocyte Differentiation and Myelination. Neuron 2016, 89, 1208–1222. [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2018, 690, 232–236. [CrossRef]
- Owen, O.E., Morgan, A.P., Kemp, H.G., Sullivan, J.M., Herrera, M.G., Cahill, G.F., Jr., 1967. Brain metabolism during fasting. The Journal of clinical investigation 46, 1589-1595. [CrossRef]
- Pardridge, W.M., 1991. Blood-brain barrier transport of glucose, free fatty acids, and ketone bodies. Advances in experimental medicine and biology 291, 43-53. [CrossRef]
- Pearson-Leary, J., McNay, E.C., 2012. Intrahippocampal administration of amyloid-beta(1-42) oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. Journal of Alzheimer’s disease : JAD 30, 413-422. [CrossRef]
- Pearson-Leary, J.; McNay, E.C. Novel Roles for the Insulin-Regulated Glucose Transporter-4 in Hippocampally Dependent Memory. J. Neurosci. 2016, 36, 11851–11864. [CrossRef]
- Pearson, T.S.; Akman, C.; Hinton, V.J.; Engelstad, K.; De Vivo, D.C. Phenotypic Spectrum of Glucose Transporter Type 1 Deficiency Syndrome (Glut1 DS). Curr. Neurol. Neurosci. Rep. 2013, 13, 342. [CrossRef]
- Persson, J.; Spreng, R.N.; Turner, G.; Herlitz, A.; Morell, A.; Stening, E.; Wahlund, L.-O.; Wikström, J.; Söderlund, H. Sex differences in volume and structural covariance of the anterior and posterior hippocampus. NeuroImage 2014, 99, 215–225. [CrossRef]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr. Biol. 2016, 26, 410–417. [CrossRef]
- Prange-Kiel, J.; Dudzinski, D.A.; Pröls, F.; Glatzel, M.; Matschke, J.; Rune, G.M. Aromatase Expression in the Hippocampus of AD Patients and 5xFAD Mice. Neural Plast. 2016, 2016, 1–11. [CrossRef]
- Prange-Kiel, J.; Jarry, H.; Schoen, M.; Kohlmann, P.; Lohse, C.; Zhou, L.; Rune, G.M. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J. Cell Biol. 2008, 180, 417–426. [CrossRef]
- Prange-Kiel, J., Schmutterer, T., Fester, L., Zhou, L., Imholz, P., Brandt, N., Vierk, R., Jarry, H., Rune, G.M., 2013. Endocrine regulation of estrogen synthesis in the hippocampus? Progress in histochemistry and cytochemistry 48, 49-64. [CrossRef]
- Rasgon, N.L.; Geist, C.L.; Kenna, H.A.; Wroolie, T.E.; Williams, K.E.; Silverman, D.H.S. Prospective Randomized Trial to Assess Effects of Continuing Hormone Therapy on Cerebral Function in Postmenopausal Women at Risk for Dementia. PLOS ONE 2014, 9, e89095. [CrossRef]
- Rasgon, N.L.; Kenna, H.A.; Wroolie, T.E.; Williams, K.E.; DeMuth, B.N.; Silverman, D.H. Insulin resistance and medial prefrontal gyrus metabolism in women receiving hormone therapy. Psychiatry Res. 2014, 223, 28–36. [CrossRef]
- Rasgon, N.L., Silverman, D., Siddarth, P., Miller, K., Ercoli, L.M., Elman, S., Lavretsky, H., Huang, S.C., Phelps, M.E., Small, G.W., 2005. Estrogen use and brain metabolic change in postmenopausal women. Neurobiology of aging 26, 229-235. [CrossRef]
- Ritchie, S.J.; Cox, S.R.; Shen, X.; Lombardo, M.V.; Reus, L.M.; Alloza, C.; A Harris, M.; Alderson, H.L.; Hunter, S.; Neilson, E.; et al. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 2018, 28, 2959–2975. [CrossRef]
- Roselli, C.F., 2007. Brain aromatase: roles in reproduction and neuroprotection. The Journal of steroid biochemistry and molecular biology 106, 143-150. [CrossRef]
- Saldanha, C.J., Duncan, K.A., Walters, B.J., 2009. Neuroprotective actions of brain aromatase. Frontiers in neuroendocrinology 30, 106-118. [CrossRef]
- Schrick, C., Fischer, A., Srivastava, D.P., Tronson, N.C., Penzes, P., Radulovic, J., 2007. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron 55, 786-798. [CrossRef]
- Shi, J.; Simpkins, J.W.; Obrenovitch, T.P.; Krause, D.N.; Duckles, S.P.; Pelligrino, D.A.; Mann, G.E.; Yudilevich, D.L.; Sobrevia, L. 17 beta-Estradiol modulation of glucose transporter 1 expression in blood-brain barrier. Am. J. Physiol. Metab. 1997, 272, E1016–E1022. [CrossRef]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89, 248–268. [CrossRef]
- Sousa, A.M.; Meyer, K.A.; Santpere, G.; Gulden, F.O.; Sestan, N. Evolution of the Human Nervous System Function, Structure, and Development. Cell 2017, 170, 226–247. [CrossRef]
- Stanić, D.; Dubois, S.; Chua, H.K.; Tonge, B.; Rinehart, N.; Horne, M.K.; Boon, W.C. Characterization of Aromatase Expression in the Adult Male and Female Mouse Brain. I. Coexistence with Oestrogen Receptors α and β, and Androgen Receptors. PLOS ONE 2014, 9, e90451. [CrossRef]
- Stoffel-Wagner, B.; Watzka, M.; Schramm, J.; Bidlingmaier, F.; Klingmüller, D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J. Steroid Biochem. Mol. Biol. 1999, 70, 237–241. [CrossRef]
- Swift-Gallant, A.; Duarte-Guterman, P.; Hamson, D.K.; Ibrahim, M.; Monks, D.A.; Galea, L.A.M. Neural androgen receptors affect the number of surviving new neurones in the adult dentate gyrus of male mice. J. Neuroendocr. 2018, 30, e12578. [CrossRef]
- Szewczyk-Krolikowski, K., Tomlinson, P., Nithi, K., Wade-Martins, R., Talbot, K., Ben-Shlomo, Y., Hu, M.T., 2014. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord 20, 99-105. [CrossRef]
- Takehara-Nishiuchi, K.; Insel, N.; Hoang, L.T.; Wagner, Z.; Olson, K.; Chawla, M.K.; Burke, S.N.; Barnes, C.A. Activation Patterns in Superficial Layers of Neocortex Change Between Experiences Independent of Behavior, Environment, or the Hippocampus. Cereb. Cortex 2012, 23, 2225–2234. [CrossRef]
- Taki, Y.; Thyreau, B.; Kinomura, S.; Sato, K.; Goto, R.; Kawashima, R.; Fukuda, H. Correlations among Brain Gray Matter Volumes, Age, Gender, and Hemisphere in Healthy Individuals. PLOS ONE 2011, 6, e22734. [CrossRef]
- Tang, Y.; Nyengaard, J.R.; De Groot, D.M. Total regional and global number of synapses in the human brain neocortex. Synapse 2001, 41, 258–273. [CrossRef]
- Taylor, M.K., Swerdlow, R.H., Sullivan, D.K., 2019. Dietary Neuroketotherapeutics for Alzheimer’s Disease: An Evidence Update and the Potential Role for Diet Quality. Nutrients 11. [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Di Domenico, F.; Head, E.; Butterfield, D.A.; Perluigi, M.; Barone, E. Brain insulin resistance triggers early onset Alzheimer disease in Down syndrome. Neurobiol. Dis. 2020, 137, 104772. [CrossRef]
- van den Heuvel, M.P., Bullmore, E.T., Sporns, O., 2016. Comparative Connectomics. Trends in cognitive sciences 20, 345-361. [CrossRef]
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673. [CrossRef]
- Wang, S.P.; Yang, H.; Wu, J.W.; Gauthier, N.; Fukao, T.; Mitchell, G.A. Metabolism as a tool for understanding human brain evolution: Lipid energy metabolism as an example. J. Hum. Evol. 2014, 77, 41–49. [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning Induces Long-Term Potentiation in the Hippocampus. Science 2006, 313, 1093–1097. [CrossRef]
- Wnuk, A.; Korol, D.L.; Erickson, K.I. Estrogens, hormone therapy, and hippocampal volume in postmenopausal women. Maturitas 2012, 73, 186–190. [CrossRef]
- Wrangham; Jones; Laden; Pilbeam; Brittain, C. The Raw and the Stolen: Cooking and the Ecology of Human Origins. Curr. Anthr. 1999, 40, 567–594. [CrossRef]
- Yagi, S., Galea, L.A.M., 2019. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44, 200-213. [CrossRef]
- Yonamine, C.Y., Michalani, M.L.E., Moreira, R.J., Machado, U.F., 2023a. Glucose Transport and Utilization in the Hippocampus: From Neurophysiology to Diabetes-Related Development of Dementia. International journal of molecular sciences 24. [CrossRef]
- Yonamine, C.Y.; Passarelli, M.; Suemoto, C.K.; Pasqualucci, C.A.; Jacob-Filho, W.; Alves, V.A.F.; Marie, S.K.N.; Correa-Giannella, M.L.; Britto, L.R.; Machado, U.F. Postmortem Brains from Subjects with Diabetes Mellitus Display Reduced GLUT4 Expression and Soma Area in Hippocampal Neurons: Potential Involvement of Inflammation. Cells 2023, 12, 1250. [CrossRef]
- Yoshizawa, H.; Gazes, Y.; Stern, Y.; Miyata, Y.; Uchiyama, S. Characterizing the normative profile of 18F-FDG PET brain imaging: Sex difference, aging effect, and cognitive reserve. Psychiatry Res. 2014, 221, 78–85. [CrossRef]
- Zhao, F.-Q.; Keating, A.F. Functional Properties and Genomics of Glucose Transporters. Curr. Genom. 2007, 8, 113–128. [CrossRef]
Figure 1.
The sources of E2 for the brain include circulating E2 or testosterone secreted from peripheral tissues and that quickly accesses the CNS and E2 synthesized in neurons and glial cells. The effects of E2 are mediated by 2 types of oestrogen receptors (ER): ERα and ERβ (Cersosimo and Benarroch, 2015). Oestrogen signalling supports and sustains glucose metabolism in the brain by regulating the expression of glucose transporters, resulting in increased glucose uptake, stimulating aerobic glycolysis. Oestrogens regulate energy metabolism in the brain through oestrogen receptors, GPER, ER-α and ER-β, activation of PI3K and Akt and MAPK-ERK signalling pathways. In fact, in brain regions linked to learning and memory, such as the hippocampus, the amygdala, the cingulate cortex and the retrosplenial cortex, oestrogen receptors are present (McEwen et al., 2012). In this context, we need to consider whether the process of brain ageing affects men and women equally, taking into account the discrepancy in oestrogen and testosterone exposure in both sexes, for example in the case of the menopausal process in the case of women or the longer exposure to testosterone in the case of men.
Figure 1.
The sources of E2 for the brain include circulating E2 or testosterone secreted from peripheral tissues and that quickly accesses the CNS and E2 synthesized in neurons and glial cells. The effects of E2 are mediated by 2 types of oestrogen receptors (ER): ERα and ERβ (Cersosimo and Benarroch, 2015). Oestrogen signalling supports and sustains glucose metabolism in the brain by regulating the expression of glucose transporters, resulting in increased glucose uptake, stimulating aerobic glycolysis. Oestrogens regulate energy metabolism in the brain through oestrogen receptors, GPER, ER-α and ER-β, activation of PI3K and Akt and MAPK-ERK signalling pathways. In fact, in brain regions linked to learning and memory, such as the hippocampus, the amygdala, the cingulate cortex and the retrosplenial cortex, oestrogen receptors are present (McEwen et al., 2012). In this context, we need to consider whether the process of brain ageing affects men and women equally, taking into account the discrepancy in oestrogen and testosterone exposure in both sexes, for example in the case of the menopausal process in the case of women or the longer exposure to testosterone in the case of men.

Figure 2.
Sex hormones such as androgens and oestrogens are potent modulators of adult neurogenesis in the hippocampus. Arevalo et al., highlights oestradiol as a neuroprotector in male and female brain. Also, aromatase, an enzyme that generates oestradiol, under pathological conditions, increases in neurons and is induced de novo in astrocytes as an endogenous neuroprotective mechanism. Thus, the inhibition or silencing of cerebral aromatase increases gliosis and neurodegeneration. Genetic variants of aromatase may cause a decrease in the synthesis of oestradiol in the brain that together with a decrease in serum levels of oestradiol in postmenopausal women, or serum levels of testosterone in older men, may increase the risk of developing neurodegenerative diseases.
Figure 2.
Sex hormones such as androgens and oestrogens are potent modulators of adult neurogenesis in the hippocampus. Arevalo et al., highlights oestradiol as a neuroprotector in male and female brain. Also, aromatase, an enzyme that generates oestradiol, under pathological conditions, increases in neurons and is induced de novo in astrocytes as an endogenous neuroprotective mechanism. Thus, the inhibition or silencing of cerebral aromatase increases gliosis and neurodegeneration. Genetic variants of aromatase may cause a decrease in the synthesis of oestradiol in the brain that together with a decrease in serum levels of oestradiol in postmenopausal women, or serum levels of testosterone in older men, may increase the risk of developing neurodegenerative diseases.
Figure 3.
Under physiological conditions, the activation of insulin signalling requires the binding of insulin to the insulin receptor (IR), which auto-phosphorylates on Tyr residues (e.g., Tyr1158/1162/1163) and promotes the receptor tyrosine kinase-mediated phosphorylation of its substrate (IRS1) on specific Tyr residues (e.g. 632). Once activated, IRS1 works as a scaffold protein, driving the activation of the PI3K/Akt axis, which is critical for linking upstream effectors (IR and IRS1). Activation of the PI3K/Akt axis is regulated by the phosphatase PTEN, which reduces PIP3 levels required for Akt activation as well as for increasing the expression of PKCζ. Akt promotes the phosphorylation of several targets among which are: GSK3β (on Ser9, inhibitory site) and AS160 (on Thr642, activating site). This latter, together with PKCζ, are responsible for the translocation of GLUT4-containing vesicles to the plasma membrane to mediate glucose uptake. along with GLUT4, the vesicles also contain other proteins, some of which are involved in the regulation of memory processes, such as insulin-like growth factor 2 (IGF2) receptors (IGF2R), insulin-regulated aminopeptidase (IRAP), and the Ras GTPase activator-like protein IQGAP1 (Agis-Balboa et al., 2011; Fernando et al., 2008; Schrick et al., 2007). Therefore, the recruitment of GLUT4 to the cell surface will simultaneously recruit these proteins (and vice versa), so that the increase in GLUT4 in the plasma membrane could be a correlation with the cognitive effects of insulin, associated with the peptides with which it is co-transported. The GLUT4 translocation pathway shows points of connection with the action pathways induced through ER α and β, specifically in relation to glucose metabolism through PI3k and Akt the binding of oestradiol to membrane-associated α and β-ER activates numerous signalling cascades, many of which are linked to neuroprotection. It also activates the insulin-like growth factor 1 (IGF1) receptor (IGF1R), which mediates neuroprotective signals through the transcriptional increase of IGF1 ( Arévalo et al., 2015).
Figure 3.
Under physiological conditions, the activation of insulin signalling requires the binding of insulin to the insulin receptor (IR), which auto-phosphorylates on Tyr residues (e.g., Tyr1158/1162/1163) and promotes the receptor tyrosine kinase-mediated phosphorylation of its substrate (IRS1) on specific Tyr residues (e.g. 632). Once activated, IRS1 works as a scaffold protein, driving the activation of the PI3K/Akt axis, which is critical for linking upstream effectors (IR and IRS1). Activation of the PI3K/Akt axis is regulated by the phosphatase PTEN, which reduces PIP3 levels required for Akt activation as well as for increasing the expression of PKCζ. Akt promotes the phosphorylation of several targets among which are: GSK3β (on Ser9, inhibitory site) and AS160 (on Thr642, activating site). This latter, together with PKCζ, are responsible for the translocation of GLUT4-containing vesicles to the plasma membrane to mediate glucose uptake. along with GLUT4, the vesicles also contain other proteins, some of which are involved in the regulation of memory processes, such as insulin-like growth factor 2 (IGF2) receptors (IGF2R), insulin-regulated aminopeptidase (IRAP), and the Ras GTPase activator-like protein IQGAP1 (Agis-Balboa et al., 2011; Fernando et al., 2008; Schrick et al., 2007). Therefore, the recruitment of GLUT4 to the cell surface will simultaneously recruit these proteins (and vice versa), so that the increase in GLUT4 in the plasma membrane could be a correlation with the cognitive effects of insulin, associated with the peptides with which it is co-transported. The GLUT4 translocation pathway shows points of connection with the action pathways induced through ER α and β, specifically in relation to glucose metabolism through PI3k and Akt the binding of oestradiol to membrane-associated α and β-ER activates numerous signalling cascades, many of which are linked to neuroprotection. It also activates the insulin-like growth factor 1 (IGF1) receptor (IGF1R), which mediates neuroprotective signals through the transcriptional increase of IGF1 ( Arévalo et al., 2015).
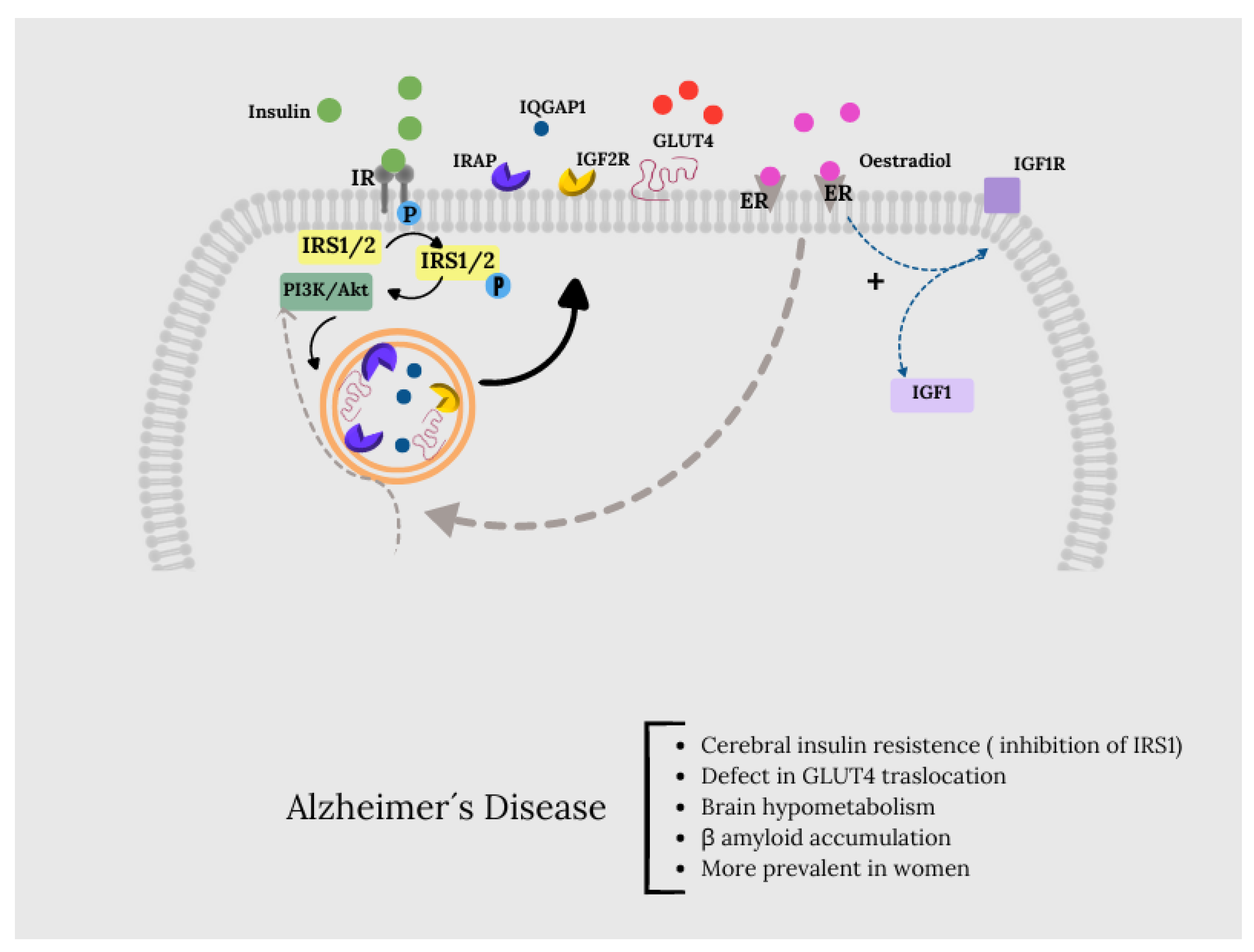
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).