1. Introduction
Diatraea saccharalis Fabricius (Lepidoptera: Crambidae) is an agricultural pest that inhabits the Americas, mainly attacking sugarcane (
Saccharum spp.), corn (
Zea mays L.), sorghum (
Sorghum bicolor L. Moench) and rice (
Oryza sativa L.). The damage caused by
D. saccharalis to these crops is economically significant[
1,
2,
3,
4], resulting from drilling and opening of galleries along the stems by the caterpillars, destroying the vascular system[
5]. As the caterpillars penetrate and develop inside the stems, it is possible to observe a mass composed of plant tissue and fecal residues close to the entrance closure[
2].
Visual detection of the symptoms, the infestation and, above all, its potential for damage is quite difficult. In rice,
D. saccharalis has been difficult to control, because when the main symptoms that demonstrate its attack, “dead heart” and “white panicle”, are noticed, the damage inside the stems has already occurred, making it very difficult to exterminate the caterpillars inside. Another important aspect is that the number of attacked stems is always higher than the number of stems showing both symptoms [
2]. For this reason, the recommendation to monitor the insect during the stages of greatest rice susceptibility has been reinforced, around 50 days after seedling emergence, by recording the presence of eggs and adults.
However, using traditional sampling methods, the practice of monitoring this pest becomes highly expensive and challenging for tropical and long-scale agriculture, such as in Brazil. The pest’s rapid and unpredictable population growth requires frequent surveys, for if spaced just a week apart, they can initially show a balanced situation and, later, populations that are found inside the stems at densities that exceeded the action levels. In this case, early detection is crucial to avoid yield loss, as effective treatments in correct time can allow plants to recover.
The development of advanced electronics, Geographic Information Systems and remote sensing have enabled significant advances in the practice of precision agriculture. Irrigation, fertilization, disease and weed detection, and yield mapping are some examples of crop management practices that are being transformed by remote sensing [
6]. Recent technological advances in imaging and sensor networks have demonstrated the technique's potential for application to automated monitoring of arthropod pests, optimizing the use of insecticides and reducing yield losses in large-scale agriculture[
7,
8].
Among the remote sensing techniques, thermography can potentially be used in agriculture, in the areas of plant physiology and plant protection. In recent years, a broad spectrum of applications has been found for thermal infrared imaging in plants, based on its relationship to transpiration and stomatal conductance. Thermal infrared remote sensing detects energy emitted in the thermal infrared band (8 – 14 μm), which is reflected by vegetation over long distances, and can be used to detect changes in plant transpiration and water content caused by biotic and abiotic factors, as well as to identify changes in the temperature of the plant canopy as a result of changes in physiological variables related to stress[
9]. For example, the infrared thermal analysis has been used to i) evaluate plant responses to soil water availability conditions[
10,
11] high-throughput phenotyping and selection of drought tolerant genotypes[
12,
13,
14] estimation of chlorophyll concentration[
15] injury detection and/or presence in cultivated plants or stored grains caused by different biological agents, such as phytopathogens[
16,
17,
18,
19,
20,
21,
22] and pest arthropods[
23,
24,
25].
The objective of the present study was to evaluate and model the responses of two rice cultivars of contrasting susceptibility to D. saccharalis attack using infrared thermal imaging.
2. Results
2.1. Insect Damage and Plant Variables
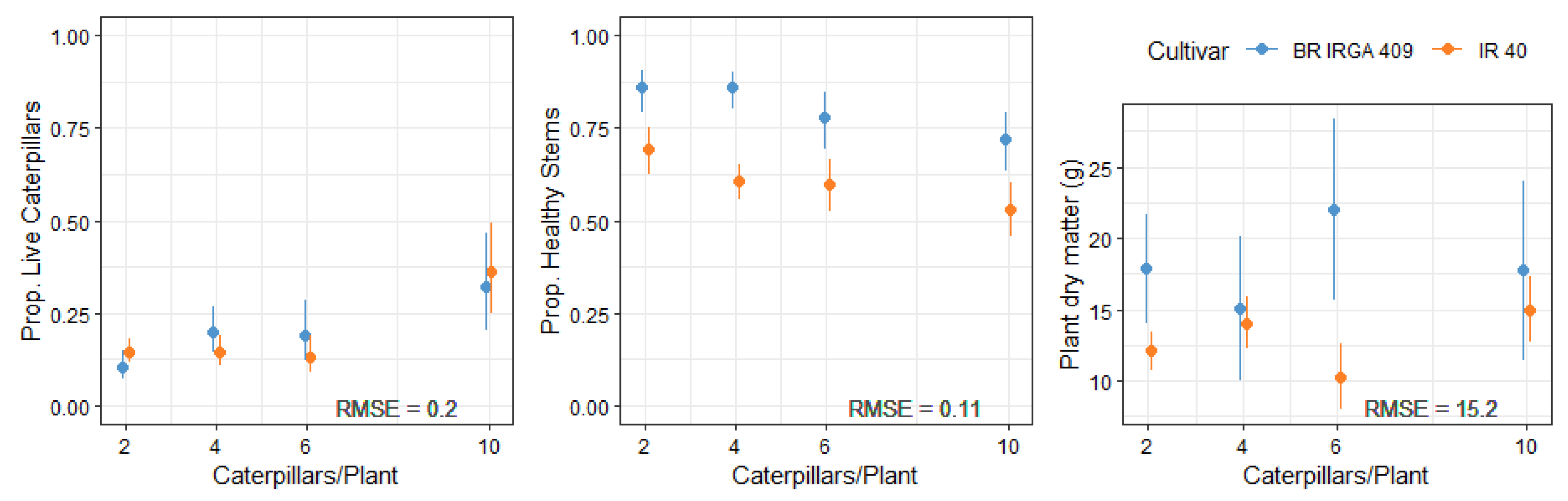
The proportion of live caterpillars increased with infestation, and did not differ from one cultivar to another in any infestation level (
p > 0.05). In both cultivars, less than 40% of caterpillars were found alive in the end of the experiment (
Figure 2).
The proportion of healthy stems presented a linear decrease as a function of the number of caterpillars of
D. saccharalis per plant in both cultivars, which means are significantly (
p < 0.05) different in all infestation levels (
Figure 2). IR 40 presented, in general, more damaged stems than BR IRGA 409. The proportion of healthy stems of IR 40 decreased from 0.69 to 0.53 according to infestation, while BR IRGA 409 decreased from 0.86 to 0.72.
Plant dry matter did not present a clear trend as infestation increased, and, in general, BR IRGA 409 presented higher values.
2.2. Thermometric Measurements
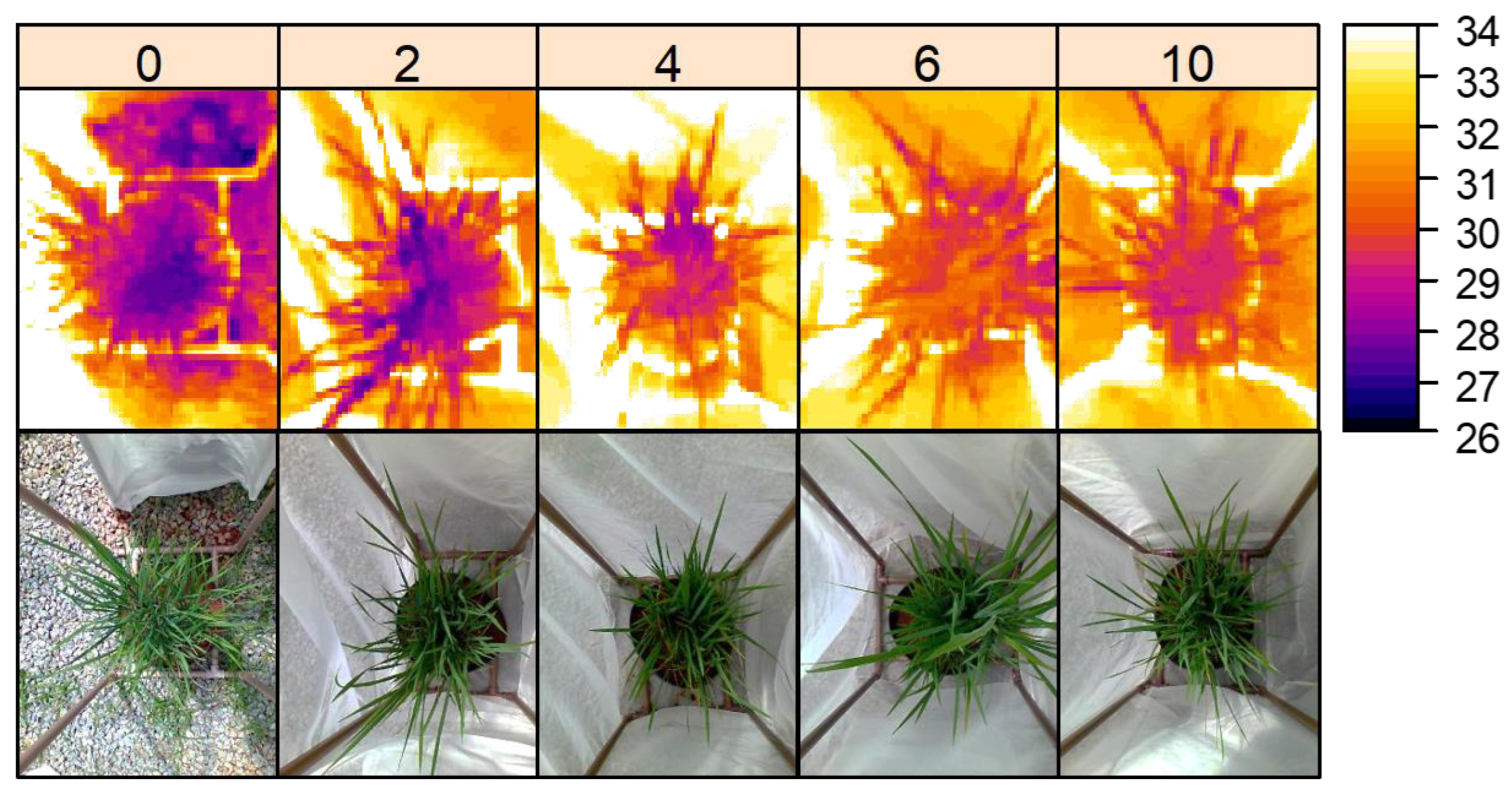
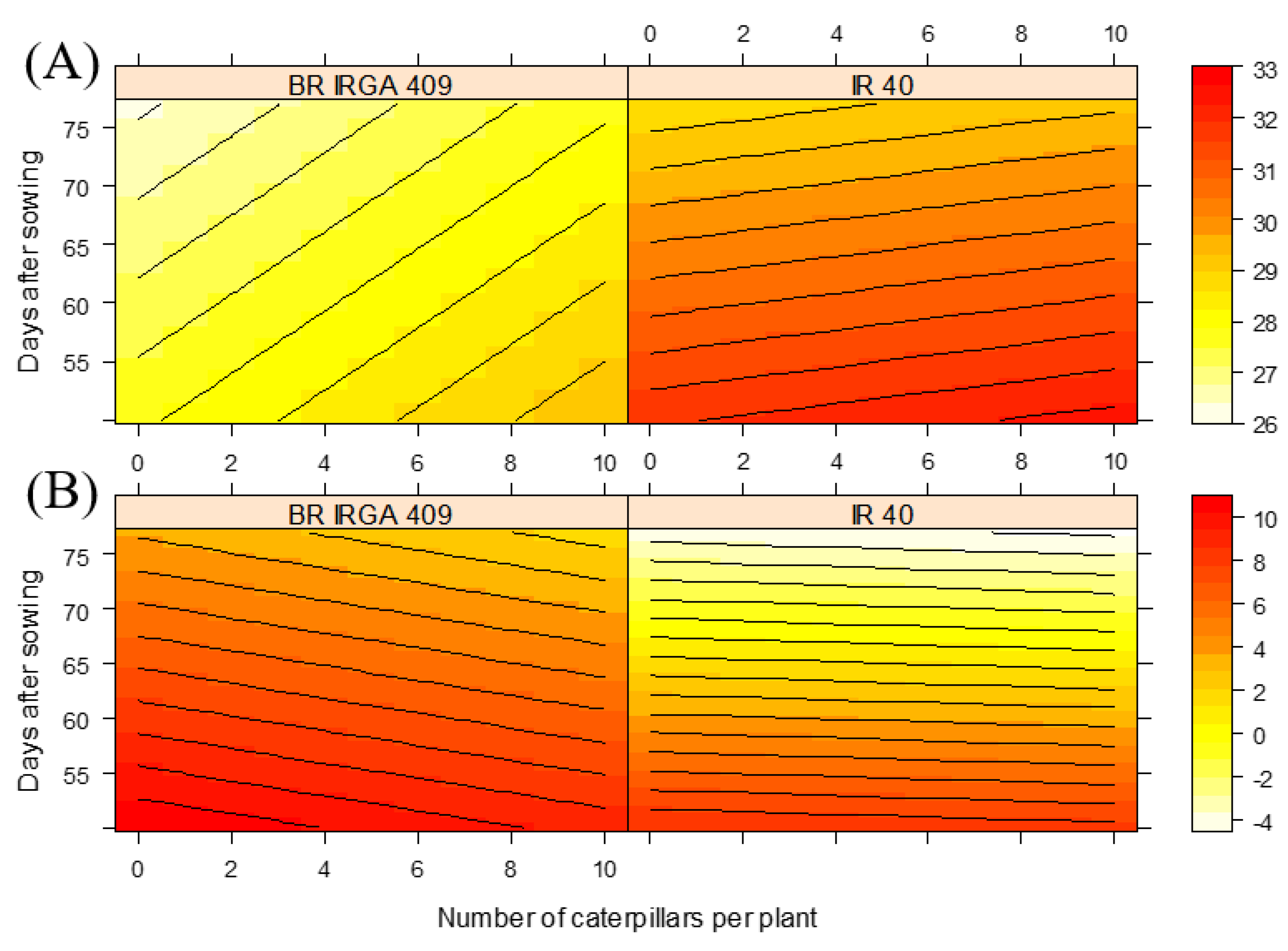
Infestation by
D. Saccharalis caused an increase in leaf temperature, with a practically linear temperature gradient being distributed according to the level of infestation, from 0 to 10 caterpillars per plant (
Figure 1). Furthermore, there was a significant difference (
p < 0.05) in the general averages (the entire experimental period) of leaf surface temperature of rice cultivars, at all levels of infestation submitted (
Figure 3). Although plants not infested with IR 40 had higher leaf temperatures, both cultivars responded to the level of infestation. It was found that, although BR IRGA 409 presented a slightly more sensitive response to the level of infestation, as observed by the orientation of the regression line, IR 40 presented higher average leaf temperatures than BR IRGA 409, which varied from 26 to 28.5 ºC, while IR 40 had a temperature above 30 ºC.
It was observed, in both cultivars, but with more evidence in IR 40, that leaf temperatures were higher in the first 15 days after infestation, that is, between the 50th and 65th day after sowing (
Figure 3). The difference between cultivars in relation to temperature was also evidenced by the calculated difference between air and leaf surface temperatures throughout the infestation period, with IR 40 presenting a leaf surface temperature closest to the air temperature.
3. Discussion
Rapid and accurate quantification of pest attack symptoms, before they cause (early) economic damage, is important from the point of view of IPM and crop protection, especially when it comes to reducing the use of insecticides and consequently greater profitability and environmental security[
26]. Based on the results of this study, which were obtained from experiments conducted over two agricultural years, it was found that thermal infrared images were capable of differentially diagnosing
D. saccharalis injuries in rice plants of the IR 40, with a susceptibility reaction, and BR IRGA 409, with a resistance reaction[
5]. Leaf surface temperature values were significantly higher in the IR 40 cultivar, showing to be related to biotic stress.
The injury caused by
D. saccharalis feeding on the inner part of the rice stems affected the physiology of the plants in a similar way to water stress. As demonstrated by Godfrey et al[
27], plants damaged by the formation of galleries by species similar to the stem borer or with water stress generally respond with a reduction in net photosynthetic rate, stomatal conductance and intercellular CO
2 concentration, and with an increase in leaf temperature. Probably, feeding the inner part of the rice stalks reduced the movement of water from the soil to the upper photosynthetic leaves. As a result, the plant proved unable to conduct water through the vascular system obstructed/destroyed by the caterpillars, even if the roots were in soil with adequate moisture reserves. The plant appeared to respond as if it were under water stress, partially closing its stomata, conserving water by reducing its loss through transpiration. Therefore, the fact that the cultivar IR 40 had the highest proportion of damaged stems explains the increase in leaf surface temperature, as well as the differences in this variable compared to BR IRGA 409. Soroker et al[
28], using a thermal monitoring system, detected palm trees infested with larvae of the red palm weevil,
Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), about three weeks before the (visual) appearance of symptoms. According to the authors, the injuries resulting from the attack of the larvae caused water stress, generating higher temperatures in the plant canopy compared to uninfested palm trees. The potential of thermal infrared remote sensing techniques for early detection of pests in crop plants has been confirmed by some other studies[
29,
30,
31]
The relationship between population levels or arthropod damage to cultivated plants and rates of production loss can be directly affected by a resistant cultivar, distinguishing itself in each of the plant resistance categories[
32,
33]. In the present study, although thermal images detected
D. saccharalis infestation in both cultivars, regardless of the level and period of infestation, leaf temperature was significantly higher in cultivar IR 40 than in cultivar BR IRGA 409, which is resistant to attack by
D. saccharalis[
5]. Cultivars that, comparatively, are capable of presenting smaller changes in physiological parameters, such as photosynthetic rate, transpiration and stomatal conductance, when exposed to insect attack, often present greater tolerance to these[
34].This also means that the development and validation of systems and technologies applied to automated monitoring of arthropod pests, such as thermal imaging, must consider the reaction of the cultivar to the pest, in order not to underestimate or overestimate the result.
Our study demonstrated the potential of using infrared thermal imaging in detecting
D. saccharalis infestation and associated symptoms in rice cultivars. This is an initial evaluation to apply a non-invasive detection method of
D. saccharalis in rice before its widespread establishment. Despite the encouraging prospects, given that stem borer sampling is destructive and laborious to carry out in rice fields, it is still necessary to further study this technique to take it to the field, given its high sensitivity to environmental factors, such as temperature, radiation, rain and wind, which can directly influence the estimates[
17]. Furthermore, other aspects that should be considered in future studies involve analyzing the response of plants under multiple stressors[
8], for example, attack by more than one type of pest at the same time, pest attack to plants with and without water/nutritional deficiency, among other combinations.
4. Materials and Methods
4.1. Study Site and Plant Material
Two experiments were carried out in two agricultural years, harvests 2019/2020 and 2021/2022, both at the Instituto Federal Goiano, Campus Urutaí, located in the Brazilian Cerrado, State of Goiás (17º 27' 49 "S; 48º 12' 06" W; average altitude of 807 m). Two commercial rice cultivars, IR 40 and BR IRGA 409, were selected for the experiments. This selection was based on a broad study on evaluating the resistance of rice accessions to
D. saccharalis[
5], in which IR 40 and BR IRGA 409 demonstrated susceptibility and resistance behavior, respectively. The cultivar IR 40 (International Rice Research Institute, Philippines, 1977) has “modern type” plants, a cycle of 119 days, and a productive potential of 5.0 t ha
-1[
35]. BR IRGA 409 (Embrapa/Instituto Rio-grandense do Arroz, Brazil, 1982) is a cultivar that also presents “modern type” plants, cycle of 120 - 130 days, and productive potential of 10.1 t ha
-1.
The seeds were sown in plastic trays with commercial substrate (Maxfertil, Pouso Redondo, SC, Brazil). Twenty days after emergence, three seedlings were transplanted into 5 L plastic pots filled with Red Oxisol, with 42% clay, taken from an experimental area with a known use history, receiving 0.75 kg of limestone per m3 of soil and 200 g of the formulated fertilizer 08-30-10 (NPK) per m3 of soil. The pots were watered daily by the greenhouse's irrigation system, and remained in this environment throughout the experiment period. Cultural treatments were carried out in accordance with the technical research recommendations for rice cultivation, but without the application of insecticides.
4.2. Insects and Experimental Procedures of Infestation
D. saccharalis eggs were obtained from Laboratório Biocana, located in Itumbiara, Goiás, Brazil. Egg masses were kept in plastic cups (300 mL) in a controlled environment [25 ± 2 ºC; 12:12h (Light:Darkness); 70% relative humidity] until the caterpillars hatch.
Fifty days after sowing, plants were infested with first-instar caterpillars of D. saccharalis. To do so, Eppendorf-type tubes containing caterpillars were distributed over the plant sheaths in each pot, which were covered with a voile fabric cage (0.5 × 0.5 × 1.0 m) throughout the experiment, to avoid insect escape and natural enemies. For both cultivars, the following infestation levels were used: 0, 2, 4, 6 and 10 caterpillars/plant. Each infestation level was replicated on three plants. A completely randomized experimental design was adopted, consisting of five treatments (infestation levels) and three replications, totaling 15 cages or plots for each cultivar (IR 40 and BR IRGA 409).
4.3. Data Collection and Analysis
At three-day intervals after manual infestation, digital images of the experimental units were captured using the FLIR® C2 IR camera (FLIR Systems, Wilsonville, OR, USA), with a 41° × 31° field of view, 60×80 pixel of optic resolution, spectral range of 7.5 - 14 μm, radiometric resolution of 0.1 ºC, absolute precision of 2 ºC and emissivity adjustment ε = 0.98. The images were taken together with a thermo-hygrometer to obtain the atmospheric temperature and relative humidity, which were recorded between 10 am and 2 pm, at a standard height of 1 m above ground level (top of the cages). Leaf surface temperature was obtained from three random points on the plant (
Figure 1).
After 30 days of infestation, at the end of the experiment, the plants were cut off at the base of the stems and taken to the laboratory to evaluate the number of live and dead caterpillars (NLC, NDC), number of damaged and healthy stems (NDS, NHS), and aerial part dry mass (DM, g) in an oven (105 ± 2 ºC for 24 h).
A generalized linear mixed model was fitted to data obtained from leaf temperature as a function of infestation level, throughout the infestation period (repeated measures), using the F-test to detect significant differences (
p < 0.05) between Cultivars. Generalized Additive Models for Location, Scale and Shape – GAMLSS [
36] were fitted to data of the variables related to resistance: plant dry matter (normal distribution) and the proportions of NLC and NHS (beta distribution). Akaike’s information criterion (AIC) and the root-mean-squared-error (RMSE) were used to choose and evaluate the goodness-of-fit of models. The analyses were performed with the R software (
www.r-project.org).
Data Availability Statement
References
- Bortoli, S. A. D., Dória, H. O. S., Albergaria, N. M. M. S., & Botti, M. V. (2005). Biological aspects and damage of Diatraea saccharalis (Lepidoptera: Pyralidae) in sorghum, under different doses of nitrogen and potassium. Ciência e Agrotecnologia, 29(2), 267-273. [CrossRef]
- Ferreira, E., Barrigossi, J. A. F., de Castro, E. D. M., & dos Santos, A. B. (2004). Yield losses by stem borer (Diatraea saccharalis Fab. 1794) (Lepidoptera: Pyralidae) in upland rice gonotypes. Pesquisa Agropecuária Tropical, 34(2), 99-103.
- Vilela, M., Santos, A. J. N., Simeone, M. L. U. F., da Costa Parrella, R. A., Silva, D. D., Parreira, D. F., Okumura, F., Schaffert, R. E., & Mendes, S. M. (2017). Influence of Diatraea saccharalis (Lepidoptera: Crambidae) infestation on sweet sorghum productivity and juice quality. African Journal of Agricultural Research, 12(39), 2877-2885. [CrossRef]
- Sandhu, H. S., & Cherry, R. H. (2017). Sugarcane borer Diatraea saccharalis (F.) (Lepidoptera: Crambidae), injury and survival in energy cane versus sugarcane. Sugar Tech, 20 (5), 558-565. [CrossRef]
- Correa, F., Silva, C. L. T., Pelosi, A. P., Almeida, A. C. S., Heinrichs, E. A., Barrigossi, J. A. F., & Jesus, F. G. (2017). Resistance in 27 rice cultivars to sugarcane borer (Lepidoptera: Crambidae). Journal of Economic Entomology, 111(1), 422-427. [CrossRef]
- Nansen, C., & Elliot, N. (2016). Remote sensing and reflectance profiling in Entomology. Annual Review of Entomology, 61, 139-158. [CrossRef]
- Backoulou, G. F., Elliott, N.C., Giles, K., Phoofolo, M., & Catana, V. (2011). Development of a method using multispectral imagery and spatial pattern metrics to quantify stress to wheat fields caused by Diuraphis noxia. Computers and Electronics in Agriculture, 75(1), 64-70. [CrossRef]
- Iost Filho, F. H., Pazini, J. de B., Medeiros, A. D., Rosalen, D. L., & Yamamoto, P. T. (2022). Assessment of injury by four major pests in soybean plants using hyperspectral proximal imaging. Agronomy, 12(7), e1516. [CrossRef]
- Zheng, Q., Huang, W., Xia, Q., Dong, Y., Ye, H., Jiang, H., Chen, S., Huang, S. Remote sensing monitoring of rice diseases and pests from different data sources: A review. Agronomy, v. 13, n. 7, e1851, 2023.
- Ramírez, A. J. F., Coelho, R.D., Pizani, M. A. M., & Silva, C. J. da (2015). Determination of crop water stress index for tomato cherry (Lycopersicum Solanum var. cerasiforme.) using a thermal camera [in Portuguese]. Revista Brasileira de Agricultura Irrigada, 9(4), 218-224. [CrossRef]
- Thapa, S., Stewart, B. A., Xue, Q., Rhoades, M. B., Angira, B., & Reznik, J. (2018). Canopy temperature, yield, and harvest index of corn as affected by planting geometry in a semi-arid environment. Field Crops Research, 227(1), 110-118. [CrossRef]
- Gonzalez-Dugo, V., Hernandez, P., Solis, I., & Zarco-Tejada, P. (2015). Using high-resolution hyperspectral and thermal airborne imagery to assess physiological condition in the context of wheat phenotyping. Remote Sensing, 7(10), 13586-13605. [CrossRef]
- Rischbeck, P., Cardellach, P., Mistele, B., & Schmidhalter, U. (2017). Thermal phenotyping of stomatal sensitivity in spring barley. Journal of Agronomy and Crop Science, 203(6), 483-493. [CrossRef]
- Biju, S., Fuentes, S., & Gupta, D. (2018). The use of infrared thermal imaging as a non-destructive screening tool for identifying drought-tolerant lentil genotypes. Plant Physiology and Biochemistry, 127, 11-24. [CrossRef]
- Elarab, M., Ticlavilca, A. M., Torres-Rua, A. F., Maslova, I., & McKee, M. (2015). Estimating chlorophyll with thermal and broadband multispectral high resolution imagery from an unmanned aerial system using relevance vector machines for precision agriculture. International Journal of Applied Earth Observation and Geoinformation, 43, 32-42. [CrossRef]
- Berdugo, C. A., Zito, R., Paulus, S., & Mahlein, A. K. (2014). Fusion of sensor data for the detection and differentiation of plant diseases in cucumber. Plant Pathology, 63(6), 1344-1356. [CrossRef]
- Mahlein, A-K. (2016). Plant disease detection by imaging sensors - Parallels and specific demands for precision agriculture and plant phenotyping. Plant Disease, 100(2), 241-251. [CrossRef]
- Ortiz-Bustos, C. M., María L. Pérez-Bueno, M. L., Barón, M., & Molinero-Ruiz, L. (2017). Use of blue-green fluorescence and thermal imaging in the early detection of sunflower infection by the root parasitic weed Orobanche cumana Wallr. Frontiers in Plant Science, 8, e833. [CrossRef]
- Hatton et al., 2018.
- Zarco-Tejada, P. J., Camino, C., Beck, P. S. A., Calderon, R., Hornero, A., Hernández-Clemente, R., Kattenborn, T., Montes-Borrego, M., Susca, L., Morelli, M., Gonzalez-Dugo, V., North, P. R. J., Landa, B. B., Boscia, D., Saponari, M., & Navas-Cortes, J. A. (2018). Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nature Plants, 4(7), 432-439. [CrossRef]
- Zhu, W., Chen, H., Ciechanowska, I., & Spaner, D. (2018). Application of infrared thermal imaging for the rapid diagnosis of crop disease. IFAC-PapersOnLine, 51(17), 424-430. [CrossRef]
- Hasan, A., Mutaqin, K. H., Taufik, M., & Hidayat, S. H. (2022). The potential of a low-cost thermal camera for early detection of temperature changes in virus-infected chili plants. Journal of ICT Research & Applications, 17(1), 17-28, 2022. [CrossRef]
- Chelladurai, V., Kaliramesh, S., & Jayas, D. S. (2012). Detection of Callosobruchus maculatus (F.) infestation in mung bean (Vigna radiata) using thermal imaging technique. In: NABEC-CSBE/SCGAB 2012 Joint Meeting and Technical Conference, Orillia, Canada. Canadian Society for Bioengineering. Proceedings, 15-18.
- Golomb, O., Alchanatis, V., Cohen, Y., Levin, N., Cohen, Y., & Soroker, V. (2015). Detection of red palm weevil infected trees using thermal imaging. In: Stafford, J. V. (Ed.). Precision Agriculture '15 (pp. 643-650). Wageningen, Netherlands: Wageningen Academic.
- Yones, M. S., Arafat, S., Abou, H. A. F., Abd Elrahman, H. A., & Dah, H. F. (2012). Determination of the best timing for control application against cotton leaf worm using remote sensing and geographical information techniques. The Egyptian Journal of Remote Sensing and Space Sciences, 15(2), 151-160. [CrossRef]
- Prabhakar, M., Prasad, Y. G., & Rao, M. N. (2011). Remote sensing of biotic stress in crop plants and its applications for pest management. In: Venkateswarlu, B., Shanker, A. K., Shanker, C., Maheswari, M. (Ed.s). Crop stress and its management: Perspectives and strategies (pp. 517-545). Dordrecht, Netherlands: Springer.
- Godfrey, L. D., Holtzer, T. O., & Norman, J. M. (1991). Effects of European corn borer (Lepidoptera: Pyralidae) tunneling and drought stress on field corn gas exchange parameters. Journal of Economic Entomology, 84(4), 1370-1380. [CrossRef]
- Soroker, V., Suma, P., Pergola, A., Cohen, Y., Alchanatis, V., Golomb, O., Goldshtein, E., Hetzroni, A., Galazan, L., Kontodimas, D., Pontikakos, C., Zorovic, M., & Brandstetter, M. (2013). Early detection and monitoring of red palm weevil: Approaches and challenges (pp. 16-18). In: Palm pest Mediterranean conference, 2013, Nice: France. Proceedings. Association Française de Protection des Plantes.
- Chaerle L, Van Der Straeten D. Imaging techniques and the early detection of plant stress. Trends Plant Sci. 2000;5(11):495-501. [CrossRef]
- Takács, S., Bottomley, H., Andreller, I., Zaradnik, T., Schwarz, J., Bennett, R., Strong, W., & Gerhard, G. (2009). Infrared radiation from hot cones on cool conifers attracts seed-feeding insects. Proceedings: Biological Sciences, 276(1657), 649-655. [CrossRef]
- Hoffmann, N., Schröder, T., Schlüter, F., & Meinlschmidt, P. (2013). Potential of infrared thermography to detect insect stages and defects in young trees. Journal für Kulturpflanzen, 65(9), 337-346. [CrossRef]
- Painter, R. H. (1951). Insect resistance in crop plants. New York: The Macmillan Co.
- Eigenbrode, S. D., & Trumble, J.T. (1994). Host plant resistance to insects in integrated pest management in vegetable crops. Journal of Agricultural Entomology, 11(3), 201-224.
- Kerchev, P. I., Fenton, B., Foyer, C. H., & Hancock, R. D. (2012). Plant responses to insect herbivory: interactions between photosynthesis, reactive oxygen species and hormonal signalling pathway. Plant, Cell and Environment, 35(2), 441-453. [CrossRef]
- Khush, G. S., & Virk, P.S. (2005). IR varieties and their impact. Los Baños: International Rice Research Institute.
- Rigby, R. A., & Stasinopoulos, D. M. (2005). Generalized additive models for location, scale and shape (with discussion). Journal of the Royal Statistical Society Series C: Applied Statistics, 54(3), 507-554. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).