Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Kale ‘Oldenbor F1’ and ‘Redbor F1’ Cultivation and Biofortification with 8-Hydroxy-7-Iodo-5-Quinolinesulfonic Acid
2.2. Analysis in Plant Material
2.3. Animal Study
2.4. Iodine Content in Urine, Faeces and Selected Tissues
2.5. Analysis of Serum
2.6. Statistical Analysis
3. Results
3.1. Iodine Content and Basic Chemical Composition in Plant Material
3.2. Body Weight Gain, Weight of Selected Organs
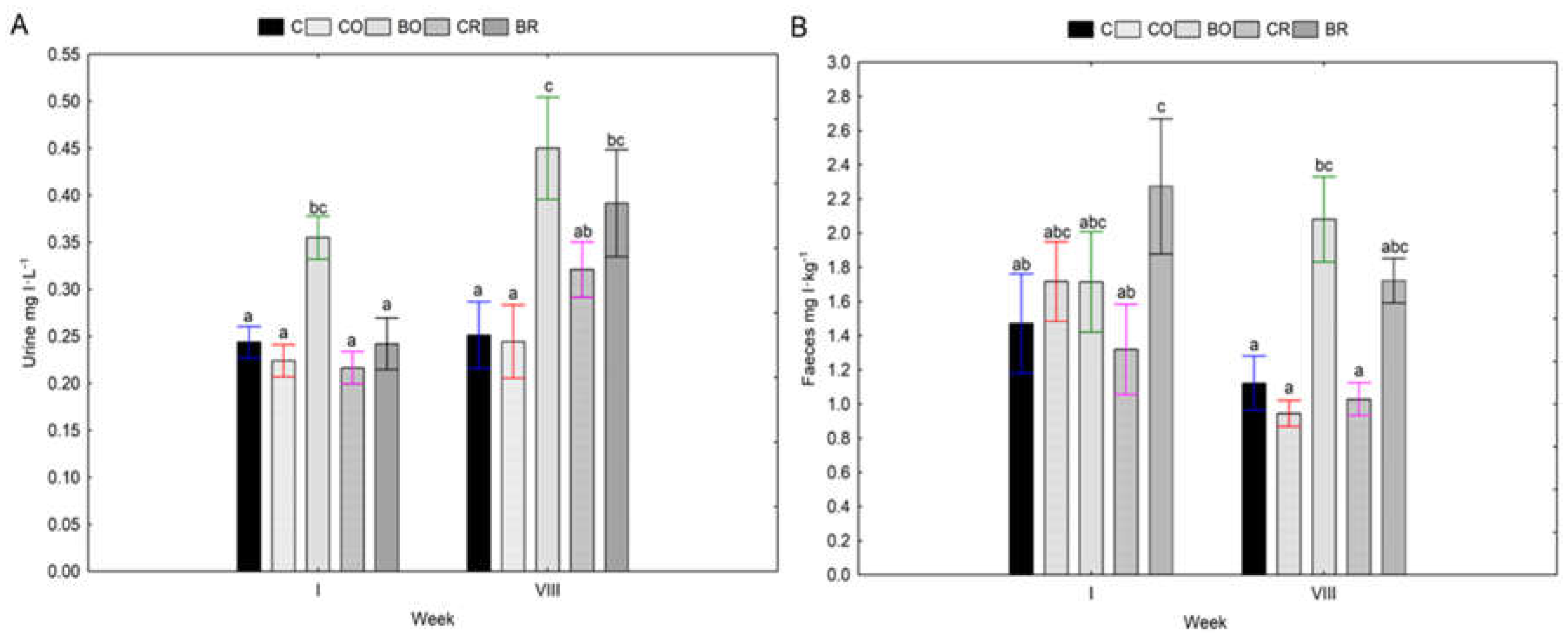
3.3. Iodine Excretion in Urine, Faeces and Selected Organs
3.4. Selected Biochemical Parameters
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Grasberger, H.; Refetoff, S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Current Opinion in Pediatrics, 2011, 23(4): 421-428. [CrossRef]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet, 2008, 4:372(9645):1251-1262.
- Andersson, M.; Takkouche, B.; Egli, I.; Allen, H.E.; Benoist, B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organization, 2005, 83(7), 518-25. PMCID: PMC2626287.
- O’Donnell, M.; Mente, A.; Alderman, M.H.; Brady, A.J.B.; Diaz, R.; Gupta, R.; et al. Salt and cardiovascular disease: insufficient evidence to recommend low sodium intake. European Heart Journal, 2020, 41: 3363–73. [CrossRef]
- World Health Organization. People in the WHO European Region at greater risk of iodine deficiency due to changing diets. Available online: https://www.who.int/azerbaijan/news/item/28-06-2024-people-in-the-who-european-region-at-greater-risk-of-iodine-deficiency-due-to-changing-diets (accessed on 24 June 2024).
- Meharg, A. Marschner’s Mineral Nutrition of Higher Plants. 3rd edition. Edited by P. Marschner. Amsterdam, Netherlands: Elsevier/Academic Press, 2011, pp. 684. Experimental Agriculture, 2012, 48(2): 305-305. [CrossRef]
- Kiferle, C.; Gonzali, S.; Holwerda, H.T.; Real Ibaceta, R.; Perata, P. Tomato fruits: a good target for iodine biofortification. Frontiers in Plant Science, 2013, 4:205. [CrossRef]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; et al. Iodine accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves grown in floating system technique. Frontiers in Plant Science, 2019, 10:1494. [CrossRef]
- Sabatino, L.; D’Anna, F.; Iapichino, G.; Moncada, A.; D’Anna, E.; De Pasquale, C. Interactive effects of genotype and molybdenum supply on yield and overall fruit quality of tomato. Frontiers in Plant Science, 2019, 9: 1922. [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of selenium enrichment and type of application on yield, functional quality and mineral composition of curly endive grown in a hydroponic system. Agronomy, 2019, 9:207. [CrossRef]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D’Anna, F, et al. Protein hydrolysates and mo-biofortification interactively modulate plant performance and quality of ‘canasta’ lettuce grown in a protected environment. Agronomy, 2021, 11:1023. [CrossRef]
- Sabatino, L.; La Bella, S.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C.; et al. Selenium biofortification and grafting modulate plant performance and functional fures of cherry tomato grown in a soilless system. Scientia Horticulturae, 2021, 285: 110095. [CrossRef]
- Sabatino. L.; Di Gaudio, F.; Consentino, B.B.; Rouphael, Y.; El-Nakhel, C.; La Bella, S.; et al. Iodine biofortification counters micronutrient deficiency and improve functional quality of open field grown curly endive. Horticulturae, 2021, 7(3): 58. [CrossRef]
- La Bella, S.; Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; et al. Impact of ecklonia maxima seaweed extract and mo foliar trments on biofortification, spinach yield, quality and NUE. Plants, 2021, 10(6): 1139. [CrossRef]
- Puccinelli, M.; Landi, M.; Maggini, R.; Pardossi, A.; Incrocci, L. Iodine biofortification of sweet basil and lettuce grown in two hydroponic systems. Scientia Horticulturae, 2021, 276: 109783. [CrossRef]
- Garg, M. et al. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Frontiers in Nutrition, 2018, 5:12. [CrossRef]
- Bouis, H.E. et al. Biofortification - a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Science, 2010, 50:20-32. [CrossRef]
- Gómez-Galera, S.; Rojas, E.; Sudhakar, D. et al. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Research, 2010, 19, 165–180. [CrossRef]
- Basharat, A.; Asghar, A.; Muhammad, T.; Shafaqat, A. Growth, seed yield and quality of mungbean as influenced by foliar application of iron sulphate. Pakistan Journal of Life and Social Sciences, 2014, 12(1): 20-25.
- Smoleń, S.; Sady, W. Influence of iodine form and application method on the effectiveness of iodine biofortification, nitrogen metabolism as well as the content of mineral nutrients and heavy metals in spinach plants (Spinacia oleracea L.). Scientia Horticulturae, 2012, 143, 176–183. [CrossRef]
- Medrano-Macias, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; & Benavides Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Frontiers in Plant Science, 2016, 7:1146. [CrossRef]
- Golob, A.; Novak, T.; Maršić, K. N.; Šircelj, H.; Stibilj, V.; Jerše, A., & et al. Biofortification with selenium and iodine changes morphological properties of Brassica oleracea L. var. gongylodes) and increases their contents in tubers. Plant Physiology and Biochemistry, 2020, 150, 234–243. [CrossRef]
- Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I.; Leszczyńska, T. Chemical composition of lettuce (Lactuca sativa L.) biofortified with iodine by KIO3 , 5-Iodo-, 3.5-Diiodosalicylic acid in a Hydrophonic Cultivation. Agronomy, 2020, 10 (7), 1022. [CrossRef]
- Smoleń, S.; Kowalska, I.; Skoczylas, Ł.; Tabaszewska, M.; Pitala, J.; Mrożek, J.; Kovacik, P. Effectiveness of enriching lettuce with iodine using 5-iodosalicylic and 3,5-diiodosalicylic acids and the chemical composition of plants depending on the type of soil in a pot experiment. Food Chemistry, 2022, 382, 132347. [CrossRef]
- Halka, M.; Smoleń, S.; Czernicka, M.; Klimek-Chodacka, M.; Pitala, J.; Tutaj, K. Iodine biofortification through expression of HMT, SAMT and S3H genes in Solanum lycopersicum L. Plant physiology and biochemistry, 2019, 144, 35–48. [CrossRef]
- Halka, M.; Smoleń, S.; Ledwożyw-Smoleń, I.; Sady, W. Iodosalicylates and iodobenzoates supplied to tomato plants affect the antioxidative and sugar metabolism differently than potassium iodide. Folia Horticulturae, 2019, 31 (2), 385–400. [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorganic & Medicinal Chemistry, 2021, 32, 115973. [CrossRef]
- Satheesh, N. & Fanta, S. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food & Agriculture, 2020, 6. 1-32. [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: review of the scientific dvidence behind the statement. Critical Reviews in Food Science and Nutrition, 2019, 59 (15), 2411–2422. [CrossRef]
- Total Dietary Fiber—Assay Procedure—Megazyme. Available online: https://www.megazyme.com/documents/Assay (accessed on 1 February 2024).
- AOAC. Official methods of analysis (18th ed.). Gaithersburg: Association of Official Analytical Chemists International, 2006.
- Standard Operation Procedure. Ref. Ares. 2020; p. 1848056-31/03/2020. Available online: https://ec.europa.eu/research/participants/documents/downloadPublicdocumentIds=080166e5cd8d669b&appId=PPGMS (accessed on 1 February 2024).
- Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; Smoleń, S.; Rakoczy, R.; Skoczylas, Ł.; Leszczyńska, T.; Ledwożyw-Smoleń, I. Effect of lettuce biofortified with iodine by soil fertilization on iodine concentration in various tissues and selected biochemical parameters in serum of Wistar rats. Journal of Functional Foods, 2015, 14: 479-486. [CrossRef]
- Smoleń, S.; Kowalska, I.; Kovácik, P.; Sady, W.; Grzanka, M.; Kutman, U.B. Changes in the Chemical Composition of Six Lettuce Cultivars (Lactuca sativa L.) in Response to Biofortification with Iodine and Selenium Combined with Salicylic Acid Application. Agronomy, 2019, 9, 660. [CrossRef]
- PN-EN: 15111:2008. Foodstuffs—Determination of Trace Elements—Determination of Iodine Content by ICP-MS (Inductively Coupled Plasma Mass Spectrometry). Polish Committee for Standardisation: Warsaw, Poland, 2008. Available online: https://sklep.pkn.pl/pn-en-15111-2008p.html (accessed on 22 January 2024).
- Reeves, P. G. Components of the AIN-93 diets as improvements in the AIN-76A diet. Journal of Nutrition, 1997, 127, 838–841.
- Friedewald, W. T.; Fredrick, D. S.; & Levy, R. I. Estimation of concentration of low-density lipoprotein cholesterol in plasma, without use of preparative ultracentrifuge. Clinical Chemistry, 1972, 18(6), 499–502.
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 1979, 95(2): 351-8. [CrossRef]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Hayirlioglu-Ayaz, S. Nutrient contents of kale (Brassica oleraceae L. var. acephala DC). Food Chemistry, 2006, 96(4), 572–579. [CrossRef]
- Dyląg, A.; Smoleń, S.; Wisła-Świder, A.; Kowalska, I.; Sularz, O.; Krzemińska, J.; Pitala, J.; & Koronowicz, A. Evaluation of the chemical composition and nutritional value of lettuce (Lactuca sativa L.) biofortified in hydroponics with iodine in the form of iodoquinolines. Frontiers in plant science, 2023, 14, 1288773. [CrossRef]
- Krzemińska, J.; Smoleń, S.; Kowalska, I.; Pitala, J.; Sularz, O.; Koronowicz, A. Effect of Biofortification with Iodine by 8-Hydroxy-7-iodo-5-quinolinesulfonic Acid and 5-Chloro-7-iodo-8-quinolinol on the Chemical Composition and Antioxidant Properties of Potato Tubers (Solanum tuberosum L.) in a Pot Experiment. Applied Science, 2023, 13, 4659. [CrossRef]
- Krawczyk, K.; Smoleń, S.; Wisła-Świder, A.; Kowalska, I.; Kiełbasa, D.; Pitala, J.; Krzemińska, J.; Waśniowska, J.; Koronowicz, A. Kale (Brassica oleracea L. var. sabellica) biofortified with iodoquinolines: Effectiveness of enriching with iodine and influence on chemical composition. Scientia Horticulturae, 2024, 323, 112519. [CrossRef]
- Prade, T.; Muneer, F.; Berndtsson, E.; Nynäs, A.L.S.; Svensson. S.E.; Newson, W.R.; Johansson, E. Protein fractionation of broccoli (Brassica oleracea, var. Italica) and kale (Brassica oleracea, var. Sabellica) residual leaves — A pre-feasibility assessment and evaluation of fraction phenol and fibre content. Food and Bioproducts Processing, 2021, 130, 229-243. [CrossRef]
- Pitura, K.; Jarosz, Z. Chemical composition and biological value of kale depending on the varied mineral fertilization. Agronomy Science, 2020, 78 (4), 97-107. https://orcid.org/0000-0002-1561-4457.
- Korus, A. Kale: a Valuable Brassica Vegetable. Part II. The nutritional Value of kale. Fermentation and Fruit and Vegetable Industry, 2015, 2. [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Metabolic Effects of Dietary Fiber and Prevention of Diabetes. The Journal of Nutrition, 2008, 138(3): 439–442. [CrossRef]
- Piątkowska, E.; Kopeć, A.; Bieżanowska-Kopeć, R.; Pysz, M.; Kapusta-Duch, J.; Koronowicz, A., et al. The Impact of Carrot Enriched in Iodine through Soil Fertilization on Iodine Concentration and Selected Biochemical Parameters in Wistar Rats. PLoS ONE, 2016, 11(4): e0152680. [CrossRef]
- Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Smoleń, S.; Skoczylas, Ł.; Leszczyńska, T.; Sady, W. The Iodine Content in Urine, Faeces and Selected Organs of Rats Fed Lettuce Biofortified with Iodine Through Foliar Application. Biological Trace Element Research, 2016, 174, 347–355. [CrossRef]
- Sherer, T.T.; Thrall, K.D.; Bull, R.J. Comparison of toxicity induced by iodine and iodide in male and female rats. Journal of Toxicology and Environmental Health, 1991, 32 (1), 89–101. [CrossRef]
- Kanno, J.; Matsuoka, C.; Furuta, K. et al. Tumor Promoting Effect of Goitrogens on the Rat Thyroid. Toxicologic Pathology, 1990, 18(2): 239-246. [CrossRef]
- WHO/UNICEF. Iodine deficiency in Europe: a continuing public health problem. Geneva, Switzerland, 2007. ISBN ISBN: 9789241593960.
- Kirchgessner, M.; He, J.; Windisch, W. Homeostatic adjustments of iodine metabolism and tissue iodine to widely varying iodine supply in 125I labeled rats. Journal of Animal Physiology and Animal Nutrition, 1999, 82(5): 238–250. [CrossRef]
- Winger, R.J.; König, J.; House, D.A. Technological issues related to food enrichment with iodine. Trendy Food Sci Tech, 2008, 19:94–101.
- Cai, J.; Fang, Y.; Jing, D.; Xu, S.; Ming, J.; Gau, B.; Shen, H.; Zhang, R.; Ji, Q. Reference intervals of thyroid hormones in a previously iodine-deficient but presently more than adequate area of Western China: a population-based survey. Endocrine Journal, 2016, 63 (4), 381-388. [CrossRef]
- Konturek, S. Human physiology. Gastrointestinal tract and endocrine glands (Vol. 5). Kraków, Poland: Scientific Publishing House: DWN (in Polish), 1994.
- Schneider, M. J.; Fiering, S. N.; Thai, B.; Wu, S. Y.; St Germain, E.; Parlow, A. F.; St Germain, D. L.; & Galton, V. A. Targeted disruption of the type 1 selenodeiodinase Gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology, 2006, 147(1), 580–589. [CrossRef]
- Bianco, A. C.; Kim, B. W. Deiodinases: Implications of the local control of thyroid hormone action. The Journal of Clinical Investigation, 2014, 116,(10), 2571–2579. [CrossRef]
- George, C.; Leslie, S.W.; Minter, D.A. Hyperuricemia. In: Stat Pearls, Treasure Island (FL): Stat Pearls Publishing, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459218/ (accessed on 25 May 2024).
- Pal, S.; Khossousi, A.; Binns, C.; Dhaliwal, S.; Ellis, V. The effect of a fibre supplement compared to a healthy diet on body composition, lipids, glucose, insulin and other metabolic syndrome risk factors in overweight and obese individuals. British Journal of Nutrition, 2011, 105(1): 90-100. [CrossRef]
- Pippi, B.; Reginatto, P.; Monte, M.; Rosa, G.; Zafaneli Bergamo, V.; Flores Dalla Lana, D.; Lettieri Teixeira, M.; Lopardi Franco, L.; Alves Ricardo, J.; Andrade Saulo, F.; Meneghello Fuentefria, A. Evaluation of 8-Hydroxyquinoline Derivatives as Hits for Antifungal Drug Design. Medical Mycology, 2011, 55(7): 763–773. [CrossRef]
- Tkaczewska, J.; Jamróz, E.; Piątkowska, E.; Borczak, B.; Kapusta-Duch, J.; Morawska, M. Furcellaran-Coated Microcapsules as Carriers of Cyprinus carpio Skin-Derived Antioxidant Hydrolysate: An In Vitro and In Vivo Study. Nutrients, 2019, 11, 2502. [CrossRef]
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Foods for Human Nutrition, 2008, 63, 163-169. [CrossRef]
| Ingredient (g·kg-1) | C | CO | BO | CR | BR |
|---|---|---|---|---|---|
| Corn starch | 532.49 | 524.72 | 525.00 | 526.04 | 526.62 |
| Saccharose | 100 | 100 | 100 | 100 | 100 |
| Casein | 200 | 200 | 200 | 200 | 200 |
| Soybean oil | 70 | 70 | 70 | 70 | 70 |
| Fibre | 50 | 47.02 | 47.19 | 46.47 | 47 |
| Vitamin mix a | 10 | 10 | 10 | 10 | 10 |
| Mineral mix a | 35 | 35 | 35 b | 35 | 35 b |
| Choline | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| TBHQ c | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| Biofortified kale d | - | - | 10.3 | - | 8.87 |
| Control kale d | - | 10.75 | - | 9.98 | - |
| Control raw kale 'Oldenbor F1’ | Raw biofortified with 8-OH-7-I-5QSA kale 'Oldenbor F1’ | Control raw kale‘Redbor F1’ | Raw biofortified with 8-OH-7-I-5QSA kale ‘Redbor F1’ | |
|---|---|---|---|---|
| Iodine [mg·kg d.m.-1] | 0.18 ± 0.02 a | 2.10 ± 0.04 b | 0.20 ± 0.01 a | 2.43 ± 0.08 c |
| Basic chemical composition | ||||
| Protein | 32.98 ± 0.06 b | 30.74 ± 1.06 b | 26.03 ± 2.31 a | 25.71 ± 0.88 a |
| Crude fat | 5.46 ± 0.13 b | 5.46 ± 0.26 b | 3.24 ± 0.13 a | 3.16 ± 0.15 a |
| Digestible carbohydrates | 17.39 ± 0.77 a | 20.39 ± 0.16 c | 15.53 ± 0.43 b | 18.35 ± 0.86 a |
| Dietary fiber | 29.06 ± 0.82 a | 28.45 ± 0.11 a | 36.39 ± 0.33 c | 34.71 ± 0.81 b |
| Ash | 20.06 ± 0.23 b | 19.21 ± 0.19 a | 21.33 ± 0.09 d | 20.76 ± 0.03 c |
| Type of diet | C | CO | BO | CR | BR |
|---|---|---|---|---|---|
| Body gain (g) | 302.38 ± 27.43 b | 280.88 ± 26.62 ab | 277.50 ± 17.65 a | 273.38 ± 15.32 a | 293.25 ± 20.10 ab |
| FER* | 0.202 ± 0.02 b | 0.187 ± 0.02 ab | 0.185 ± 0.01 a | 0.182 ± 0.01 a | 0.195 ± 0.01 ab |
| Liver (g) | 16.14 ± 2.17 c | 14.14 ± 1.52 b | 13.04 ± 1.35 ab | 12.32 ± 0.98 a | 13.54 ± 1.80 ab |
| Kidney** (g) | 2.68 ± 0.14 a | 2.47 ± 0.14 ab | 2.55 ± 0.24 ab | 2.29 ± 0.48 b | 2.65 ± 0.24 a |
| Heart (g) | 1.27 ± 0.09 b | 1.18 ± 0.08 ab | 1.22 ± 0.08 ab | 1.14 ± 0.07 a | 1.22 ± 0.08 ab |
| Thyroid gland (g) | 0.23 ± 0.06 a | 0.26 ± 0.03 ab | 0.24 ± 0.04 ab | 0.27 ± 0.03 ab | 0.28 ± 0.03 b |
| Visceral fat (g) | 4.39 ± 0.80 a | 3.88 ± 0.68 a | 4.18 ± 0.77 a | 3.52 ± 1.06 a | 3.77 ± 0.58 a |
| Type of diet | C | CO | BO | CR | BR |
|---|---|---|---|---|---|
| Kidney [mg I·kg d.m.-1] |
0.14 ± 0.01 b | 0.13 ± 0.02 ab | 0.17 ± 0.03 c | 0.11 ± 0.02 a | 0.18 ± 0.04 c |
| Liver [mg I·kg d.m.-1] |
0.12 ± 0.02 ab | 0.11 ± 0.02 a | 0.13 ± 0.01 b | 0.11 ± 0.01 a | 0.12 ± 0.01 ab |
| Type of diet | C | CO | BO | CR | BR |
|---|---|---|---|---|---|
| Liver panel | |||||
| AST [U·L-1] | 36.11 ± 9.11 a | 33.53 ± 12.39 a | 30.54 ± 10.49 ab | 31.11 ± 5.03 ab | 19.79 ± 8.35 b |
| ALT [U·L-1] | 26.55 ± 7.84 a | 25.09 ± 6.81 a | 23.74 ± 6.72 a | 15.85 ± 4.84 b | 13.93 ± 4.8 b |
| Bilirubin | |||||
| Total bilirubin [µmol·L-1] | 10.38 ± 6.08 b | 3.73 ± 1.84 a | 3.15 ± 3.17 a | 5.34 ± 3.47 a | 4.28 ± 2.79 a |
| Direct bilirubin [µmol·L-1] | 7.59 ± 3.85 b | 3.35 ± 2.40 a | 2.13 ± 1.27 a | 4.53 ± 4.56 ab | 4.27 ± 1.87 a |
| Uric acid | |||||
| Uric acid [µmol·L-1] | 166.04 ± 62.01 a | 132.35 ± 69.70 a | 116.11 ± 67.30 a | 112.50 ± 48.74 a | 108.29 ± 19.76 a |
| Lipid profile | |||||
| TC [mmol·L-1] | 3.01 ± 0.53 a | 2.94 ± 0.22 a | 2.77 ± 0.30 a | 2.64 ± 0.34 a | 2.55 ± 0.65 a |
| HDL [mmol·L-1] | 1.89 ± 0.18 b | 1.71 ± 0.22 ab | 1.76 ± 0.34 ab | 1.54 ± 0.27 a | 1.64 ± 0.17 ab |
| LDL + VLDL [mmol·L-1] | 1.12 ± 0.37 a | 1.23 ± 0.31 a | 1.01 ± 0.42 a | 1.10 ± 0.20 a | 0.91 ± 0.55 a |
| TG [mmol·L-1] | 1.45 ± 0.62 b | 1.35 ± 0.36 ab | 0.99 ± 0.16 a | 1.15 ± 0.44 ab | 1.05 ± 0.22 ab |
| Antioxidant activity | |||||
| Glutathione reductase [U·L-1] | 375.72 ± 205.75 a | 348.31 ± 174.47 a | 444.23 ± 83.52 a | 378.96 ± 148.61 a | 438.01 ± 68.74 a |
| TAS [mmol·L-1] | 0.89 ± 0.20 ab | 0.87 ± 0.08 a | 1.04 ± 0.08 c | 0.99 ± 0.10 abc | 1.02 ± 0.10 bc |
| TBARs [nmol MDA·mL-1] | 554.52 ±27.36 c | 535.31 ± 88.82 bc | 513.88 ± 49.46 abc | 474.93 ± 63.19 ab | 459.47 ± 66.32 a |
| Hormones | |||||
| TSH [ng·mL-1] | 2.18 ± 0.21 b | 2.02 ± 0.13 a | 1.99 ± 0.08 a | 2.10 ± 0.17 ab | 2.07 ± 0.11 ab |
| T3[pg·mL-1] | 3.81 ± 0.19 ab | 3.92 ± 0.15 b | 3.73 ± 0.06 a | 3.68 ± 0.09 a | 3.84 ± 0.07 ab |
| T4 [ng·mL-1] | 2.75 ± 0.59 a | 2.87 ± 0.44 a | 2.51 ± 0.53 a | 2.59 ± 1.10 a | 2.24 ± 0.84 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





