Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
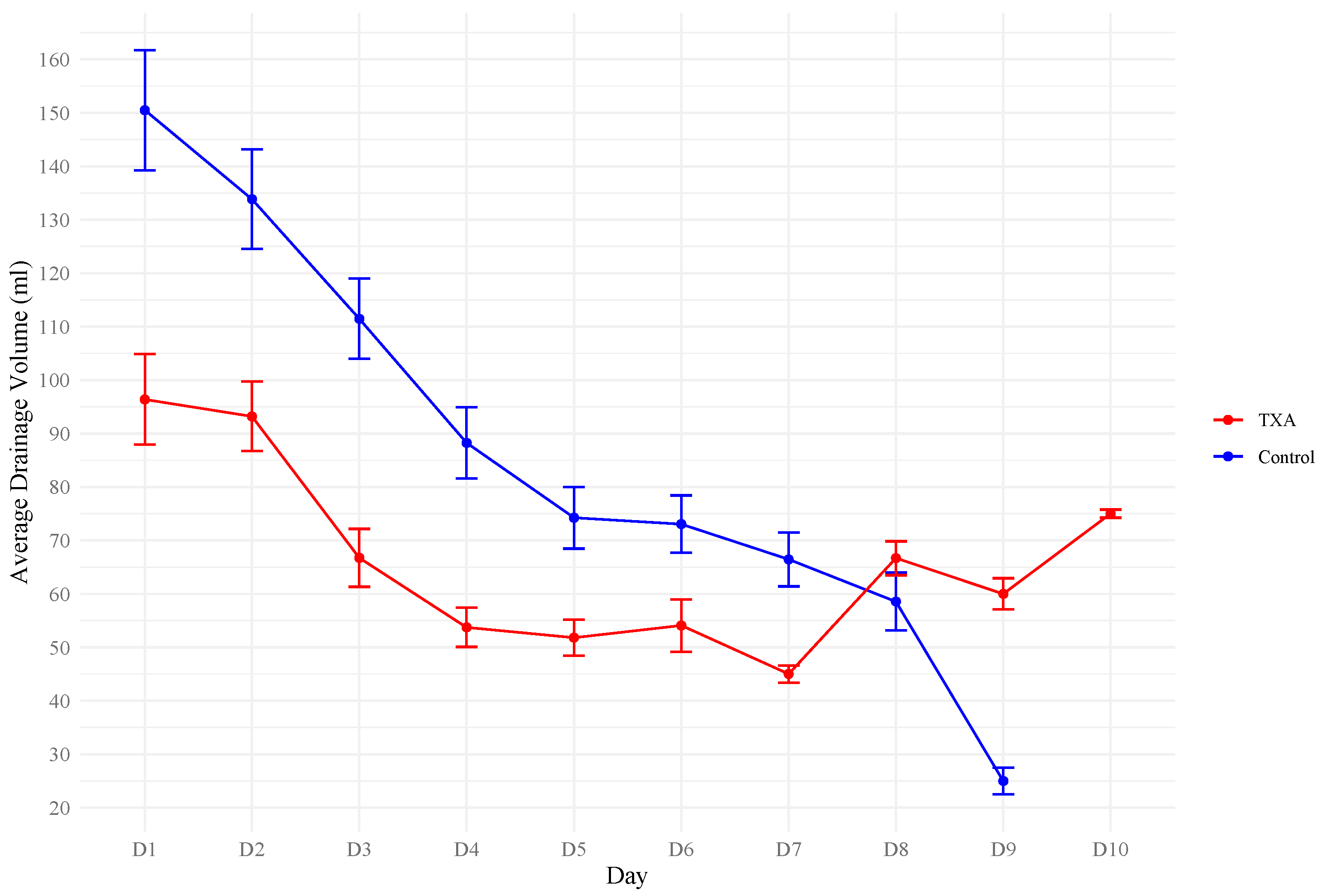
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamoto S, Hijikata-Okunomiya A, Wanaka K, Okada Y, Okamoto U. Enzyme-Controlling Medicines: Introduction. Semin Thromb Hemost. 1997;23(06):493-501. [CrossRef]
- Lee JH. Effect of Topical Tranexamic Acid on Seroma Formation in a Rat Mastectomy Model. Published online 2022.
- Baker SK, Strickland S. A critical role for plasminogen in inflammation. Journal of Experimental Medicine. 2020;217(4):e20191865. [CrossRef]
- Draxler DF, Medcalf RL. The Fibrinolytic System—More Than Fibrinolysis? Transfusion Medicine Reviews. 2015;29(2):102-109. [CrossRef]
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10). [CrossRef]
- Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. The Lancet. 2017;389(10084):2105-2116. [CrossRef]
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344(may17 1):e3054-e3054. [CrossRef]
- Prudovsky I, Kacer D, Zucco VV, et al. Tranexamic acid: Beyond antifibrinolysis. Transfusion. 2022;62(S1). [CrossRef]
- Walker PF, Foster AD, Rothberg PA, Davis TA, Bradley MJ. Tranexamic acid decreases rodent hemorrhagic shock-induced inflammation with mixed end-organ effects. PLoS One. 2018;13(11):e0208249. [CrossRef]
- Teng Y, Feng C, Liu Y, Jin H, Gao Y, Li T. Anti-inflammatory effect of tranexamic acid against trauma-hemorrhagic shock-induced acute lung injury in rats. Exp Anim. 2018;67(3):313-320. [CrossRef]
- Jimenez JJ, Iribarren JL, Lorente L, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007;11(6):R117. [CrossRef]
- Lei Y, Xie J, Huang Q, Huang W, Pei F. Additional benefits of multiple-dose tranexamic acid to anti-fibrinolysis and anti-inflammation in total knee arthroplasty: a randomized controlled trial. Arch Orthop Trauma Surg. 2020;140(8):1087-1095. [CrossRef]
- Peng Z, Ban K, LeBlanc A, Kozar RA. Intraluminal Tranexamic Acid Inhibits Intestinal Sheddases and Mitigates Gut and Lung Injury and Inflammation in a Rodent Model of Hemorrhagic Shock. J Trauma Acute Care Surg. 2016;81(2):358-365. [CrossRef]
- Reichel CA, Lerchenberger M, Uhl B, et al. Plasmin Inhibitors Prevent Leukocyte Accumulation and Remodeling Events in the Postischemic Microvasculature. PLoS One. 2011;6(2):e17229. [CrossRef]
- Hyman YG, Vischer TL. Protease inhibitors reduce the reverse passive arthus reaction. Agents and Actions. 1978;8(5):532-535. [CrossRef]
- Calpin GG, McAnena PF, Davey MG, et al. The role of tranexamic acid in reducing post-operative bleeding and seroma formation in breast surgery: A meta-analysis. The Surgeon. 2023;21(4):e183-e194. [CrossRef]
- Oertli D, Laffer U, Haberthuer F, Kreuter U, Harder F. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. British Journal of Surgery. 2005;81(6):856-859. [CrossRef]
- Lohani KR, Kumar C, Kataria K, Srivastava A, Ranjan P, Dhar A. Role of tranexamic acid in axillary lymph node dissection in breast cancer patients. Breast J. 2020;26(7):1316-1320. [CrossRef]
- Seth AK, Hirsch EM, Kim JYS, et al. Hematoma After Mastectomy With Immediate Reconstruction An Analysis of Risk Factors in 883 Patients. Annals of Plastic Surgery. 2013;71(1):20-23. [CrossRef]
- Bloom JA, Foroutanjazi S, Erlichman Z, et al. The Use of Hemostatic Agents to Decrease Bleeding Complications in Breast Cancer Surgery. The American Surgeon. 2023;89(3):395-400. [CrossRef]
- Marinescu SA, Bejinariu LG, Marina MC, Giuglea C. Complications related to breast reconstruction after mastectomy using multiple surgical techniques – a national and international comparative analysis.
- Adrien C, Katia M, Marie-Lucile B, Alice R, Claire B, Roman R. Prevention of lymphocele or seroma after mastectomy and axillary lymphadenectomy for breast cancer: systematic review and meta-analysis. Sci Rep. 2022;12(1):10016. [CrossRef]
- Barwell J, Campbell L, Watkins RM, Teasdale C. How long should suction drains stay in after breast surgery with axillary dissection? Ann R Coll Surg Engl. 1997;79(6):435-437.
- Srivastava V, Basu S, Shukla VK. Seroma Formation after Breast Cancer Surgery: What We Have Learned in the Last Two Decades. J Breast Cancer. 2012;15(4):373. [CrossRef]
- Kumar S, Lal B, Misra MC. Post-mastectomy seroma: a new look into the aetiology of an old problem. J R Coll Surg Edinb. 1995;40(5):292-294.
- Sampathraju S, Rodrigues G. Seroma Formation after Mastectomy: Pathogenesis and Prevention. Indian J Surg Oncol. 2010;1(4):328-333. [CrossRef]
- Pogson CJ, Adwani A, Ebbs SR. Seroma following breast cancer surgery. European Journal of Surgical Oncology (EJSO). 2003;29(9):711-717. [CrossRef]
- Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20(5):497-555.
- Patey DH, Dyson WH. The Prognosis of Carcinoma of the Breast in Relation to the Type of Operation Performed. Br J Cancer. 1948;2(1):7-13.
- McWHIRTER R. The value of simple mastectomy and radiotherapy in the treatment of cancer of the breast. Br J Radiol. 1948;21(252):599-610. [CrossRef]
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87(6):1048-1053.
- Crowe J Joseph P, Kim JA, Yetman R, Banbury J, Patrick RJ, Baynes D. Nipple-Sparing Mastectomy: Technique and Results of 54 Procedures. Archives of Surgery. 2004;139(2):148-150. [CrossRef]
- Bostwick J. Total mastectomy with breast skin and volume reduction using an inverted T incision. Plastic and reconstructive breast surgery. 1990;2:1048-1054.
- Querci della Rovere G, Nava M, Bonomi R, Catanuto G, Benson JR. Skin-reducing mastectomy with breast reconstruction and sub-pectoral implants. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2008;61(11):1303-1308. [CrossRef]
- Suga H, Shiraishi T, Shibasaki Y, Takushima A, Harii K. Predictive Factors for Drainage Volume after Expander-based Breast Reconstruction. Plastic and Reconstructive Surgery - Global Open. 2016;4(6):e727. [CrossRef]
- Lim YM, Lew DH, Roh TS, Song SY. Analysis of factors that affect drainage volume after expander-based breast reconstruction. Arch Plast Surg. 2020;47(01):33-41. [CrossRef]
- Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK. Seroma Formation Following Breast Cancer Surgery. Breast Journal. 2003;9(5):385-388. [CrossRef]
- Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H, Montazeri A. Seroma formation after surgery for breast cancer. World J Surg Onc. 2004;2(1):44. [CrossRef]
- Petrek JA. Axillary Lymphadenectomy: A Prospective, Randomized Trial of 13 Factors Influencing Drainage, Including Early or Delayed Arm Mobilization. Arch Surg. 1990;125(3):378. [CrossRef]
- Loo W, Chow L. Factors predicting seroma formation after mastectomy for Chinese breast cancer patients. Indian J Cancer. 2007;44(3):99. [CrossRef]
- Kuroi K, Shimozuma K, Taguchi T, et al. Pathophysiology of seroma in breast cancer. Breast Cancer. 2005;12(4):288-293. [CrossRef]
- Khansa I, Hendrick RGJ, Shore A, Meyerson J, Yang M, Boehmler JHI. Breast Reconstruction with Tissue Expanders: Implementation of a Standardized Best-Practices Protocol to Reduce Infection Rates. Plastic and Reconstructive Surgery. 2014;134(1):11. [CrossRef]
- Lardi AM, Dreier K, Junge K, Farhadi J. The use of tranexamic acid in microsurgery—is it safe? Gland Surg. 2018;7(S1):S59-S63. [CrossRef]
- Liechti R, Van De Wall BJM, Hug U, Fritsche E, Franchi A. Tranexamic Acid Use in Breast Surgery: A Systematic Review and Meta-Analysis. Plastic & Reconstructive Surgery. 2023;151(5):949-957. [CrossRef]
- Ausen K, Fossmark R, Spigset O, Pleym H. Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. British Journal of Surgery. 2015;102(11):1348-1353. [CrossRef]
- Eldesouky MS, Ashour HSA, Shahin MA. Effect of topical application of tranexamic acid on reduction of wound drainage and seroma formation after mastectomy.
- Safran T, Vorstenbosch J, Viezel-Mathieu A, Davison P, Dionisopoulos T. Topical Tranexamic Acid in Breast Reconstruction: A Double-Blind Randomized Controlled Trial. Plastic & Reconstructive Surgery. 2023;152(4):699-706. [CrossRef]
- Weissler JM, Banuelos J, Jacobson SR, et al. Intravenous Tranexamic Acid in Implant-Based Breast Reconstruction Safely Reduces Hematoma without Thromboembolic Events. Plastic & Reconstructive Surgery. 2020;146(2):238-245. [CrossRef]
- Gogna S, Goyal P. Prospective randomized study on effect of tranexamic acid on wound drainage following modified radical mastectomy for cancer breast. Int J Cur Res. 2015;7:16192-16194.
- Ausen K, Hagen AI, Østbyhaug HS, et al. Topical moistening of mastectomy wounds with diluted tranexamic acid to reduce bleeding: randomized clinical trial. BJS Open. 2020;4(2):216-224. [CrossRef]
- Wolter A, Scholz T, Pluto N, Diedrichson J, Arens-Landwehr A, Liebau J. Subcutaneous mastectomy in female-to-male transsexuals: Optimizing perioperative and operative management in 8 years clinical experience. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2018;71(3):344-352. [CrossRef]
- Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38(5):375-381. [CrossRef]
- Murray JD, Elwood ET, Jones GE, Barrick R, Feng J. Decreasing expander breast infection: A new drain care protocol. Can J Plast Surg. 2009;17(1):17-21.
- Hanna KR, Tilt A, Holland M, et al. Reducing Infectious Complications in Implant Based Breast Reconstruction: Impact of Early Expansion and Prolonged Drain Use. Ann Plast Surg. 2016;76 Suppl 4:S312-315. [CrossRef]
- Lembo F, Cecchino LR, Parisi D, Portincasa A. Reduction of seroma and improvement of quality of life after early drain removal in immediate breast reconstruction with tissue expander. Preliminary report from a randomized controlled study. J Plast Reconstr Aesthet Surg. 2021;74(10):2565-2572. [CrossRef]
- Bayasgalan M, Munhoz AM, Shellock FG. Breast Tissue Expander With Radiofrequency Identification Port: Assessment of MRI Issues. American Journal of Roentgenology. 2020;215(1):159-164. [CrossRef]
- Matsubayashi F, Takahashi T, Miyauchi H, Ito Y, Harada A, Yoshioka Y. Modeling of a tissue expander with a radiofrequency identification port in postmastectomy radiation therapy planning. J Radiat Res. 2024;65(3):360-368. [CrossRef]
- Stillaert FBJL, Lannau B, Landuyt KV, Blondeel PN. The Prepectoral, Hybrid Breast Reconstruction: The Synergy of Lipofilling and Breast Implants. Plastic and Reconstructive Surgery Global Open. 2020;8(7). [CrossRef]
- Chiu WK, Fracol M, Feld LN, Qiu CS, Kim JYS. Judging an Expander by Its Cover: A Propensity-Matched Analysis of the Impact of Tissue Expander Surface Texture on First-Stage Breast Reconstruction Outcomes. Plast Reconstr Surg. 2021;147(1):1e-6e. [CrossRef]
- Lee KT, Park HY, Jeon BJ, Mun GH, Bang SI, Pyon JK. Does the Textured-Type Tissue Expander Affect the Outcomes of Two-Stage Prosthetic Breast Reconstruction? A Propensity Score Matching Analysis between Macrotextured and Microtextured Expanders. Plast Reconstr Surg. 2021;147(3):545-555. [CrossRef]
- Suh YC, Kim JK, Kim NR, et al. A comparative study of pre- or subpectoral expander position with the fenestrated Acellular dermal matrix anterior coverage, on drainage volume and Seroma Formation after Non-Nipple-Sparing Mastectomy. J Plast Reconstr Aesthet Surg. 2021;74(9):2237-2243. [CrossRef]
- Ozturk CN, Ozturk C, Magner WJ, Ali A, Diehl J, Sigurdson SL. Seroma After Breast Reconstruction With Tissue Expanders: Outcomes and Management. Ann Plast Surg. 2023;91(3):331-336. [CrossRef]
| Variable | TXA N = 83 | Controls N = 72 | P-Value |
|---|---|---|---|
| Age a | 50 (46, 58) | 48 (42, 62) | 0.722 d |
| BMI a | 22.0 (20.8, 26.0) | 22.3 (20.3, 25.3) | 0.380 d |
| DM b | 1 (1.4) | 0 (0) | 0.281 c |
| Hypertension b | 10 (12.1) | 6 (8.3) | 0.448 c |
| Heart disease b | 7 (8.4) | 5 (6.9) | 0.729 c |
| Kidney disease b | 4 (4.8) | 0 (0) | 0.059 c |
| Active smoking b | 27 (32.5) | 17 (23.6) | 0.219 c |
| Chronic NSAID b | 2 (2.8) | 1 (1.2) | 0.478 c |
| Antiaggregation b | 1 (1.2) | 4 (5.6) | 0.126 c |
| Anticoagulation b | 2 (2.4) | 1 (1.4) | 0.645 c |
| Previous thromboembolic event b | 0 (0) | 4 (5.6) | 0.030 c |
| Previous radiotherapy b | 7 (8.4) | 0 (0) | 0.012 c |
| Neoadjuvant chemotherapy b | 21 (25.3) | 11 (15.3) | 0.124 c |
| Thrombocytes a | 253 (220, 312) | 269 (219, 331) | 0.236 d |
| Quick a | 103 (96, 113) | 98.5 (90, 106) | 0.012 d |
| INR a | 1 (0.9, 1) | 1 (1, 1) | 0.002 d |
| Variable | TXA N = 83 | Controls N = 72 | P-Value |
|---|---|---|---|
| Breast | |||
| Rightb | 41 (49.4) | 30 (41.7) | 0.335 c |
| Leftb | 42 (50.6) | 42 (58.3) | 0.335 c |
| Resection weight (g) a | 332 (228, 550) | 316.5 (218.5, 485.5) | 0.588 d |
| Type of mastectomy | <0.001 c | ||
| Skin-sparingb | 14 (16.9) | 23 (31.9) | |
| Nipple-sparingb | 36 (43.4) | 45 (62.5) | |
| Skin-reducingb | 33 (39.8) | 4 (5.6) | |
| Expander placement | <0.001 c | ||
| Prepectoralb | 71 (85.5) | 29 (40.3) | |
| Subpectoralb | 12 (14.5) | 43 (59.7) | |
| Expander | <0.001 c | ||
| Allergan (Natrelle 133)b | 0 (0) | 65 (90.3) | |
| Mentor (CPX4)b | 18 (21.7) | 5 (6.9) | |
| Motiva (Flora)b | 65 (78.3) | 2 (2.8) | |
| Sentinel lymph node biopsy b | 49 (59.0) | 49 (68.1) | 0.245 c |
| Axillar lymph node dissection ad continuitatemb | 6 (7.2) | 6 (8.3) | 0.797 c |
| Axillar access b | 39 (47.0) | 41 (56.9) | 0.216 c |
| Use of synthetic mesh b | 10 (12.1) | 14 (19.4) | 0.204 c |
| Use of biological mesh (acellular dermal matrix) b | 11 (13.3) | 0 (0) | 0.001 c |
| Variable | TXA N = 83 | Controls N = 72 | P-Value |
|---|---|---|---|
| Hematoma b | 5 (6.0) | 4 (5.6) | 0.901 c |
| Seroma b | 5 (6.0) | 3 (4.2) | 0.602 c |
| Duration of drain placement (days) a | 3.3 (1.8) | 4.6 (1.6) | <0.001 d |
| Length of hospital stay (days) a | 5.3 (1.8) | 6.2 (1.5) | 0.001 d |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Estimate (SE) | P-Value | 95% CI | Estimate (SE) | P-Value | 95% CI |
| Age (per year) | 0.1 (0.2) | 0.520 | -0.2 - 0.5 | 0.1 (0.1) | 0.401 | -0.2 - 0.4 |
| BMI (per kg/m2) | 0.9 (0.5) | 0.098 | -0.2 - 2.0 | 0.3 (0.6) | 0.607 | -0.9 - 1.5 |
| Current smoking | -7.1 (4.6) | 0.118 | -16.1 - 1.8 | -4.4 (3.7) | 0.234 | -11.5 - 2.8 |
| Type of mastectomy (Skin-sparing as reference) | ||||||
| Nipple-sparing | -9.0 (5.0) | 0.069 | -18.8 - 0.7 | -3.6 (4.1) | 0.387 | -11.7 - 4.5 |
| Skin-reducing | -17.0 (5.8) | 0.004 | -28.4 - (-5.5) | -10.8 (5.1) | 0.034 | -20.8 - (-0.8) |
| Biologic mesh (acellular dermal matrix) | -18.6 (7.9) | 0.018 | -34.1 - (-3.2) | -2.7 (6.6) | 0.690 | -15.7 - 10.4 |
| Type of expander (Mentor (CPX4) as reference) | ||||||
| Allergan (Natrelle 133) | 1.9 (5.4) | 0.729 | -8.8 - 12.5 | -18.4 (8.1) | 0.023 | -34.3 - (-2.5) |
| Motiva (Flora) | -25.0 (5.4) | <0.001 | -35.6 - (-14.4) | -23.5 (5.0) | <0.001 | -33.3 - (-13.8) |
| Axillar lymph node dissection ad continuitatem | 20.3 (7.7) | 0.009 | 5.2 - 35.4 | 18.3 (5.8) | 0.002 | 6.9 - 29.7 |
| Resection weight (per gram) | 0.03 (0.01) | 0.002 | 0.01 - 0.05 | 0.04 (0.01) | 0.001 | 0.01 - 0.06 |
| Hematoma | 20.2 (8.7) | 0.020 | 3.2 - 37.3 | 23.7 (6.7) | <0.001 | 10.6 - 36.8 |
| Tranexamic acid | -23.3 (3.7) | <0.001 | -30.6 - (-16.1) | -19.0 (8.1) | 0.020 | -35.0 - (-3.0) |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Estimate (SE) | P-Value | 95% CI | Estimate (SE) | P-Value | 95% CI | |
| Age (per year) | -0.004 (0.01) | 0.759 | -0.03 - 0.02 | -0.01 (0.01) | 0.468 | -0.03 - 0.02 | |
| BMI (per kg/m2) | 0.1 (0.04) | 0.033 | 0.007 - 0.2 | 0.0 (0.1) | 0.995 | -0.1 - 0.1 | |
| Current smoking | -0.7 (0.3) | 0.050 | -1.3 - (-0.001) | -0.5 (0.3) | 0.148 | -1.1 - 0.2 | |
| Type of mastectomy (Skin-sparing as reference) | |||||||
| Nipple-sparing | -0.7 (0.4) | 0.062 | -1.5 - 0.04 | -0.3 (0.4) | 0.360 | -1.0 - 0.4 | |
| Skin-reducing | -0.9 (0.4) | 0.042 | -1.8 - (-0.04) | -0.5 (0.4) | 0.236 | -1.4 - 0.3 | |
| Biologic mesh (acellular dermal matrix) | -1.3 (0.6) | 0.021 | -2.5 - (-0.2) | -0.5 (0.6) | 0.399 | -1.6 - 0.6 | |
| Type of expander (Mentor (CPX4) as reference) | |||||||
| Allergan (Natrelle 133) | 0.1 (0.5) | 0.753 | -0.7 - 1.0 | -0.7 (0.7) | 0.313 | -2.0 - 0.7 | |
| Motiva (Flora) | -1.3 (0.5) | 0.004 | -2.2 - (-0.4) | -1.3 (0.5) | 0.004 | -2.2 - (-0.4) | |
| Axillar lymph node dissection ad continuitatem | 1.2 (0.6) | 0.036 | 0.08 - 2.3 | 1.0 (0.5) | 0.055 | -0.02 - 2.0 | |
| Resection weight (per gram) | 0.003 (0.001) | <0.001 | 0.002 - 0.004 | 0.003 (0.001) | 0.001 | 0.001 - 0.005 | |
| Hematoma | 1.1 (0.7) | 0.105 | -0.2 - 2.4 | 1.2 (0.6) | 0.049 | 0.005 - 2.3 | |
| Tranexamic acid | -1.3 (0.3) | <0.001 | -1.9 - (-0.7) | -0.7 (0.7) | 0.341 | -2.0 - 0.7 | |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Estimate (SE) | P-Value | 95% CI | Estimate (SE) | P-Value | 95% CI | |
| Age (per year) | 0.005 (0.01) | 0.720 | -0.02 - 0.03 | -0.003 (0.01) | 0.819 | -0.03 - 0.02 | |
| BMI (per kg/m2) | 0.1 (0.03) | <0.001 | 0.1 - 0.2 | 0.095 (0.047) | 0.043 | 0.003 - 0.2 | |
| Current smoking | -0.5 (0.3) | 0.110 | -1.1 - 0.1 | -0.286 (0.290) | 0.326 | -0.9 - 0.3 | |
| Type of mastectomy (Skin-sparing as reference) | |||||||
| Nipple-sparing | -0.7 (0.3) | 0.045 | -1.3 - (-0.02) | -0.267 (0.330) | 0.419 | -0.9 - 0.4 | |
| Skin-reducing | -0.6 (0.4) | 0.134 | -1.4 - 0.2 | -0.513 (0.405) | 0.207 | -1.3 - 0.3 | |
| Biologic mesh (acellular dermal matrix) | -0.8 (0.5) | 0.117 | -1.9 - 0.2 | 0.097 (0.533) | 0.855 | -1.0 - 1.2 | |
| Type of expander (Mentor (CPX4) as reference) | |||||||
| Allergan (Natrelle 133) | -0.005 (0.4) | 0.991 | -0.8 - 0.8 | -0.782 (0.649) | 0.230 | -2.1 - 0.5 | |
| Motiva (Flora) | -1.1 (0.4) | 0.005 | -1.9 - (-0.3) | -1.236 (0.391) | 0.002 | -2.0 - (-0.5) | |
| Axillar lymph node dissection ad continuitatem | 0.4 (0.5) | 0.409 | -0.6 - 1.4 | 0.248 (0.461) | 0.591 | -0.7 - 1.2 | |
| Resection weight (per gram) | 0.003 (0.001) | <0.001 | 0.002 - 0.004 | 0.002 (0.001) | 0.025 | 0.0 - 0.004 | |
| Hematoma | 0.6 (0.6) | 0.338 | -0.6 - 1.7 | 0.965 (0.535) | 0.073 | -0.1 - 2.0 | |
| Tranexamic acid | -0.9 (0.3) | 0.001 | -1.4 - (-0.4) | -0.611 (0.650) | 0.349 | -1.9 - 0.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





