Submitted:
20 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- ●Peer-reviewed;
- ●Availability of the full-text publication;
- ●Availability in English.
3. Results
3.1. Gut–Brain Axis
3.2. Role of the Environment
3.3. Stem Cells
3.4. Still-Scattered Genetic Puzzle
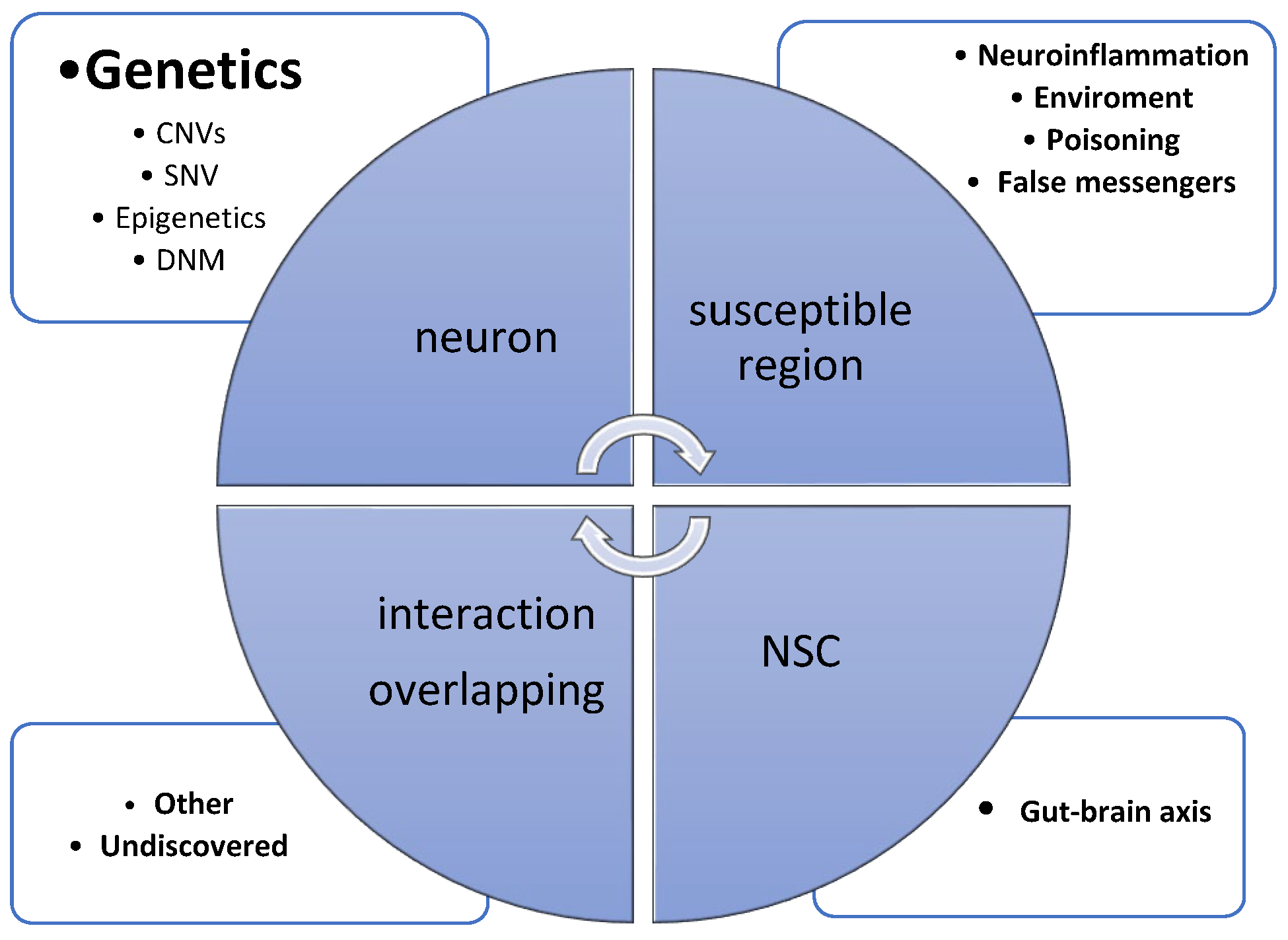
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kanner,L.J. Autistic disturbances of affective contact. Nerv. Child 1943,2,217-250.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Christensen DL, Maenner MJ, Bilder D, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 4 Years - Early Autism and Developmental Disabilities Monitoring Network, Seven Sites, United States, 2010, 2012, and 2014. MMWR Surveill Summ. 2019;68(2):1- 19. Published 2019 Apr 12. doi:10.15585/mmwr.ss6802a1. [CrossRef]
- Croen LA, Grether JK, Hoogstrate J, Selvin S. The changing prevalence of autism in California. J Autism Dev Disord 2002; 32:207–15. 10.1023/A:1015453830880.
- Newschaffer CJ, Falb MD, Gurney JG. National autism prevalence trends from United States special education data. Pediatrics 2005;115: e277–82. 10.1542/peds.2004-1958 s: changes in the California caseload, an update: June 1987– June 2007. Sacramento, CA: California Health and Human Services Agency, Department of Developmental Services; 2007.
- Saito M, Hirota T, Sakamoto Y, Adachi M, Takahashi M, Osato-Kaneda A, Kim YS, Leventhal B, Shui A, Kato S, Nakamura K. Prevalence and cumulative incidence of autism spectrum disorders and the patterns of co-occurring neurodevelopmental disorders in a total population sample of 5-year-old children. Mol Autism. 2020 May 14;11(1):35. doi: 10.1186/s13229-020-00342-5. PubMed PMID: 32410700. [CrossRef] [PubMed]
- DSM-5 American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https://doi.org/10.1176/appi.books.9780890425596. [CrossRef]
- de Leeuw A, Happé F, Hoekstra RA. A Conceptual Framework for Understanding the Cultural and Contextual Factors on Autism Across the Globe. Autism Res. 2020 Feb 21. doi: 10.1002/aur.2276. Review. PubMed PMID:32083402. [CrossRef] [PubMed]
- De Sales-Millán A, Aguirre-Garrido JF, González-Cervantes RM, Velázquez-Aragón JA. Microbiome-Gut-Mucosal-Immune-Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology. Behav Sci (Basel). 2023 Jun 30;13(7):548. doi: 10.3390/bs13070548. [CrossRef]
- Peralta-Marzal LN, Prince N, Bajic D, Roussin L, Naudon L, Rabot S, Garssen J, Kraneveld AD, Perez-Pardo P. The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int J Mol Sci. 2021 Sep 17;22(18):10052. doi: 10.3390/ijms221810052. [CrossRef]
- Fattorusso A, Di Genova L, Dell'Isola GB, Mencaroni E, Esposito S. Autism Spectrum Disorders, and the Gut Microbiota. Nutrients. 2019 Feb 28;11(3). pii: E521. doi:10.3390/nu11030521. Review. PubMed PMID: 30823414; PubMed Central PMCID: PMC6471505. [CrossRef] [PubMed]
- Iglesias-Vázquez L, Van Ginkel Riba G, Arija V, Canals J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients. 2020 Mar 17;12(3). pii: E792. doi: 10.3390/nu12030792. Review. PubMed PMID: 32192218; PubMed Central PMCID: PMC7146354. [CrossRef] [PubMed]
- Srikantha P, Mohajeri MH. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int J Mol Sci. 2019 Apr 29;20(9):2115. doi: 10.3390/ijms20092115. PMID: 31035684; PMCID: PMC6539237. [CrossRef] [PubMed]
- Siracusano M, Arturi L, Riccioni A, Noto A, Mussap M, Mazzone L. Metabolomics: Perspectives on Clinical Employment in Autism Spectrum Disorder. Int J Mol Sci. 2023 Aug 29;24(17):13404. doi: 10.3390/ijms241713404. PMID: 37686207; PMCID: PMC10487559. [CrossRef] [PubMed]
- Davoli-Ferreira M, Thomson CA, McCoy KD. Microbiota and Microglia Interactions in ASD. Front Immunol. 2021 May 25;12:676255. doi: 10.3389/fimmu.2021.676255. eCollection 2021. [CrossRef]
- Ho LKH, Tong VJW, Syn N, Nagarajan N, Tham EH, Tay SK, Shorey S, Tambyah PA, Law ECN. Gut microbiota changes in children with autism spectrum disorder: a systematic review. Gut Pathog. 2020 Feb 3; 12:6. doi: 10.1186/s13099-020-0346-1. eCollection 2020. Review. PubMed PMID: 32025243; PubMed Central PMCID: PMC6996179. [CrossRef] [PubMed]
- Sumathi T, Manivasagam T, Thenmozhi AJ. The Role of Gluten in Autism. Adv Neurobiol. 2020; 24:469-479. doi: 10.1007/978-3-030-30402-7_14. Review. PubMed PMID: 32006368. [CrossRef] [PubMed]
- Croall ID, Hoggard N, Hadjivassiliou M. Gluten and Autism Spectrum Disorder. Nutrients. 2021 Feb 9;13(2):572. doi: 10.3390/nu13020572. [CrossRef]
- McCaulley ME. Autism spectrum disorder and mercury toxicity: use of genomic and epigenetic methods to solve the etiologic puzzle. Acta Neurobiol Exp (Wars). 2019;79(2):113-125. Review. PubMed PMID: 31342948.
- Pessah IN, Lein PJ, Seegal RF, Sagiv SK. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol. 2019 Sep;138(3):363-387. doi: 10.1007/s00401-019-01978-1. Epub 2019 Apr 11. Review. PubMed PMID: 30976975; PubMed Central PMCID: PMC6708608. [CrossRef] [PubMed]
- Tizabi Y, Bennani S, El Kouhen N, Getachew B, Aschner M. Interaction of Heavy Metal Lead with Gut Microbiota: Implications for Autism Spectrum Disorder.Biomolecules. 2023 Oct 19;13(10):1549. doi: 10.3390/biom13101549. PMID:37892231; PMCID: PMC10605213. [CrossRef] [PubMed]
- Torres G, Mourad M, Iqbal S, Moses-Fynn E, Pandita A, Siddhartha SS, Sood RA, Srinivasan K, Subbaiah RT, Tiwari A, Leheste JR. Conceptualizing Epigenetics and the Environmental Landscape of Autism Spectrum Disorders. Genes (Basel). 2023 Aug 30;14(9):1734. doi: 10.3390/genes14091734. PMID: 37761876; PMCID: PMC10531442. [CrossRef] [PubMed]
- Yui K, Imataka G, Yoshihara S Lipid-Based Molecules on Signaling Pathways in Autism Spectrum Disorder. Int J Mol Sci. 2022 Aug 29;23(17):9803. doi: 10.3390/ijms23179803. [CrossRef]
- Lagod PP, Naser SA. The Role of Short-Chain Fatty Acids and Altered Microbiota Composition in Autism Spectrum Disorder: A Comprehensive Literature Review. Int J Mol Sci. 2023 Dec 13;24(24):17432. doi: 10.3390/ijms242417432. [CrossRef]
- Lampiasi N, Bonaventura R, Deidda I, Zito F, Russo R. Inflammation and the Potential Implication of Macrophage-Microglia Polarization in Human ASD: An Overview. Int J Mol Sci. 2023 Jan 31;24(3):2703. doi: 10.3390/ijms24032703. [CrossRef]
- Fan G, Ma J, Ma R, Suo M, Chen Y, Zhang S, Zeng Y, Chen Y. Microglia Modulate Neurodevelopment in Autism Spectrum Disorder and Schizophrenia. Int J Mol Sci.2023 Dec 9;24(24):17297. doi: 10.3390/ijms242417297. PMID: 38139124; PMCID: PMC10743577. [CrossRef] [PubMed]
- Hughes HK, R J Moreno, Ashwood P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav Immun. 2023 Feb;108:245-254. doi: 10.1016/j.bbi.2022.12.001. Epub 2022 Dec 6. [CrossRef]
- Whiteley P, Marlow B, Kapoor RR, Blagojevic-Stokic N, Sala R). Autoimmune Encephalitis and Autism Spectrum Disorder. Front Psychiatry. 2021 Dec 17;12:775017. doi: 10.3389/fpsyt.2021.775017. eCollection 2021. [CrossRef]
- Arenella M, Matuleviciute R, Tamouza R, Leboyer M, McAlonan G, Bralten J, Murphy D. Immunogenetics of autism spectrum disorder: A systematic literature review. Brain Behav Immun. 2023 Nov;114:488-499. doi: 10.1016/j.bbi.2023.09.010. Epub 2023 Sep 16. PMID: 37717669. [CrossRef] [PubMed]
- Malaguarnera M, Cauli O. Effects of L-Carnitine in Patients with Autism Spectrum Disorders: Review of Clinical Studies. Molecules. 2019 Nov 22;24(23). pii: E4262. doi: 10.3390/molecules24234262. Review. PubMed PMID: 31766743; PubMed Central PMCID: PMC6930613. [CrossRef] [PubMed]
- Bankaitis VA, Xie Z. The neural stem cell/carnitine malnutrition hypothesis: new prospects for effective reduction of autism risk? J Biol Chem. 2019 Dec 13;294(50):19424-19435. doi: 10.1074/jbc.AW119.008137. Epub 2019 Nov 7. Review. PubMed PMID: 31699893; PubMed Central PMCID: PMC6916470. [CrossRef] [PubMed]
- Lee KM, Hawi ZH, Parkington HC, Parish CL, Kumar PV, Polo JM, Bellgrove MA, Tong J. The application of human pluripotent stem cells to model the neuronal and glial components of neurodevelopmental disorders. Mol Psychiatry. 2020 Feb;25(2):368-378. doi: 10.1038/s41380-019-0495-0. Epub 2019 Aug 27. Review. PubMed PMID: 31455859. [CrossRef] [PubMed]
- Vicari S, Napoli E, Cordeddu V, Menghini D, Alesi V, Loddo S, Novelli A, Tartaglia M. Copy number variants in autism spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jun 8; 92:421-427. doi: 10.1016/j.pnpbp.2019.02.012. Epub 2019 Feb 20. Review. PubMed PMID: 30797015. [CrossRef] [PubMed]
- Bourgeron, T., 2016. Current knowledge on the genetics of autism and propositions for future research. C. R. Biol. 339 (7–8), 300–307. https://doi.org/10.1016/j.crvi.2016.05.004. [CrossRef]
- Rylaarsdam L, Guemez-Gamboa A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front Cell Neurosci. 2019 Aug 20; 13:385. doi: 10.3389/fncel.2019.00385. eCollection 2019. Review. PubMed PMID: 31481879; PubMed Central PMCID: PMC6710438. [CrossRef] [PubMed]
- Genovese A, Butler MG. Clinical Assessment, Genetics, and Treatment Approaches in Autism Spectrum Disorder (ASD). Int J Mol Sci. 2020 Jul 2;21(13):4726. doi: 10.3390/ijms21134726. [CrossRef]
- Yoon SH, Choi J, Lee WJ, Do JT. Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. J Clin Med. 2020 Mar 31;9(4). pii: E966. doi: 10.3390/jcm9040966. Review. PubMed PMID: 32244359. [CrossRef] [PubMed]
- Al-Dewik N, Alsharshani M. New Horizons for Molecular Genetics Diagnostic and Research in Autism Spectrum Disorder. Adv Neurobiol. 2020; 24:43-81. doi: 10.1007/978-3-030-30402-7_2. Review. PubMed PMID: 32006356. [CrossRef] [PubMed]
- Nakanishi M, Anderson MP, Takumi T. Recent genetic and functional insights in autism spectrum disorder. Curr Opin Neurol. 2019 Aug;32(4):627-634. doi: 10.1097/WCO.0000000000000718. Review. PubMed PMID: 31135459; PubMed Central PMCID: PMC6959126. [CrossRef] [PubMed]
- De Rubeis, S., He, X., Goldberg, A. P., Poultney, C. S., Samocha, K., Cicek, A. E., et al. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215. doi:10.1038/nature13772. [CrossRef]
- Durand, C. M., Perroy, J., Loll, F., Perrais, D., Fagni, L., Bourgeron, T., et al. (2012). SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an action-dependent mechanism. Mol. Psychiatry 17, 71–84. doi:10.1038/mp.2011.57. [CrossRef]
- Giovedí, S., Corradi, A., Fassio, A., and Benfenati, F. (2014). Involvement of Synaptic Genes in the Pathogenesis of Autism Spectrum Disorders: The Case of Synapsins. Front. Pediatr.2. doi:10.3389/fped.2014.00094. [CrossRef]
- Jamain, S., Quach, H., Betancur, C., Råstam, M., Colineaux, C., Gillberg, I. C., et al. (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet.34, 27–29. doi:10.1038/ng1136. [CrossRef]
- Schmunk, G., and Gargus, J. J. (2013). Channelopathy pathogenesis in autism spectrum disorders. Front. Genet.4. doi:10.3389/fgene.2013.00222. [CrossRef]
- Stessman, H. A. F., Xiong, B., Coe, B. P., Wang, T., Hoekzema, K., Fenckova, M., et al. (2017). Targeted sequencing identifies 91 neurodevelopmental disorder risk genes with autism and 1094 developmental disability biases. Nat. Genet. 49, 515–526. doi:10.1038/ng.3792. [CrossRef]
- Jiao J, Zhang M, Yang P, Huang Y, Hu X, Cai J, Yang C, Si-Tu M, Zhang H, Fu L, Guo K, Huang Y. [Analysis of common genetic variants associated with neuro synapse development among 60 family trios affected with sporadic autism spectrum disorders]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2020 Jan 10;37(1):1-4. doi: 10.3760/cma.j.issn.1003-9406.2020.01.001. Chinese. PubMed PMID: 31922584. [CrossRef] [PubMed]
- Short, P. J., McRae, J. F., Gallone, G., Sifrim, A., Won, H., Geschwind, D. H., et al. (2018). De novo mutations in regulatory elements in neurodevelopmental disorders. Nature 555, 611–616. 1072 doi:10.1/nature25983. [CrossRef]
- Turner, T. N., Coe, B. P., Dickel, D. E., Hoekzema, K., Nelson, B. J., Zody, M. C., et al. (2017). Genomic patterns of de novo mutation in simplex autism. Cell171, 710- 722.e12. doi: 10.1016/j.cell.2017.08.047. [CrossRef]
- Turner, T. N., Hormozdiari, F., Duyzend, M. H., McClymont, S. A., Hook, P. W., Iossifov, I., et al. (2016). Genome Sequencing of Autism-Affected Families Reveals Disruption of Putative Noncoding Regulatory DNA. Am. J. Hum. Genet. 98,58–74. doi: 10.1016/j.ajhg.2015.11.023. [CrossRef]
- Carney, R. M., Wolpert, C. M., Ravan, S. A., Shahbazian, M., Ashley-Koch, A., Cuccaro, M. L., et al. (2003). Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol.28, 205–211.C.
- Stessman, H. A. F., Xiong, B., Coe, B. P., Wang, T., Hoekzema, K., Fenckova, M., et al. (2017). Targeted sequencing identifies 91 neurodevelopmental disorder risk genes with autism and developmental disability biases. Nat. Genet. 49, 515–526. doi:10.1038/ng.3792. [CrossRef]
- Tran, S. S., Jun, H.-I., Bahn, J. H., Azghadi, A., Ramaswami, G., Van Nostrand, E. L., et al. (2019). Widespread RNA editing dysregulation in brains from autistic individuals. Nat. Neurosci. 22, 113325–36. doi:10. /s41593-018-0287-x. 38. [CrossRef]
- D’Gama, A. M., and Walsh, C. A. (2018). Somatic mosaicism and neurodevelopmental disease. Nat. Neurosci.21, 1504. doi:10.1038/s41593-018-0257- 3. [CrossRef]
- Poduri, A., Evrony, G. D., Cai, X., and Walsh, C. A. (2013). Somatic Mutation, Genomic Variation, and Neurological Disease.Science341, 1237758. doi:10.1126/science.1237758. [CrossRef]
- Ronemus, M., Iossifov, I., Levy, D., and Wigler, M. (2014). The role of de novomutations in the genetics of autism spectrum disorders. Nat. Rev. Genet.15, 133– 141. doi:10.1038/nrg3585. [CrossRef]
- Krupp, D. R., Barnard, R. A., Duffourd, Y., Evans, S. A., Mulqueen, R. M., Bernier, R., et al. (2017). Exonic Mosaic Mutations Contribute Risk for Autism Spectrum Disorder. Am. J. Hum. Genet.844101, 369–390. doi: 10.1016/j.ajhg.2017.07.016. [CrossRef]
- Lim, E. T., Uddin, M., De Rubeis, S., Chan, Y., Kamumbu, A. S., Zhang, X., et al. (2017). Rates, distribution, and implications of postzygotic mosaic mutations in autism spectrum disorder. Nat. 878Neurosci.20, 1217–1224. doi:10.1038/nn.4598. [CrossRef]
- Rasia-Filho, A. A., Londero, R. G., and Achaval, M. (2000). Functional activities of the amygdala: an overview.J. Psychiatry Neurosci.25, 14–23.
- Dou, Y., Yang, X., Li, Z., Wang, S., Zhang, Z., Ye, A. Y., et al. (2017). Postzygotic single-nucleotide mosaicisms contribute to the etiology of autism spectrum disorder and autistic traits and the origin of mutations. Hum. Mutat.38, 1002–1013. doi:10.1002/humu.23255. [CrossRef]
- Freed, D., and Pevsner, J. (2016). The Contribution of Mosaic Variants to Autism Spectrum Disorder. PLoS Genet.12, e1006245. doi: 10.1371/journal.pgen.1006245. [CrossRef]
- Golzio, C., Willer, J., Talkowski, M. E., Oh, E. C., Taniguchi, Y., Jacquemont, S., et al. (2012). KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 485, 363–367. doi:10.1038/nature11091.726. [CrossRef]
- Escamilla, C. O., Filonova, I., Walker, A. K., Xuan, Z. X., Holehonnur, R., Espinosa, F., et al. (2017). Kctd13 deletion reduces synaptic transmission via increased RhoA. Nature 551, 227–231. doi: 10.1038/nature24470.680Este. [CrossRef]
- Iyer, J., Singh, M. D., Jensen, M., Patel, P., Pizzo, L., Huber, E., et al. (2018). Pervasive genetic interactions modulate neurodevelopmental defects of the autism associated 16p11.2 deletion in Drosophila melanogaster. Nature Communications 9, 2548. doi:10.1038/ s41467-018-04882-6.782. [CrossRef]
- Schroer, R. J., Phelan, M. C., Michaelis, R. C., Crawford, E. C., Skinner, S. A., Cuccaro, M., et al. (1998). Autism and maternally derived aberrations of chromosome 15q. Am. J. Med. Genet.76, 1055327–336.
- Glessner, J. T., Wang, K., Cai, G., Korvatska, O., Kim, C. E., Wood, S., et al. (2009). Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459, 569–573. doi:10.1038/nature07953. [CrossRef]
- Marshall, C. R., and Scherer, S. W. (2012). Detection and characterizationof copy number variation in autism spectrum disorder. Methods Mol. Biol.Clifton NJ838, 115–135. doi:10.1007/978-1-61779-507-7_5. [CrossRef]
- Pinto, D., Pagnamenta, A. T., Klei, L., Anney, R., Merico, D., Regan, R., et al. (2010). Functional impact of global rare copy number variation in autism spectrum disorders. Nature466, 368–372. doi:10.1038/nature09146. [CrossRef]
- Stefansson, H., Meyer-Lindenberg, A., Steinberg, S., Magnusdottir, B., Morgen, K., Arnarsdottir, S., et al. (2014). CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature1085505, 361–366. doi:10. /nature12818. [CrossRef]
- Girirajan, S., Rosenfeld, J. A., Cooper, G. M., Antonacci, F., Siswara, P., Itsara, A., et al. (2010). A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat. Genet.42, 203–209. doi:10.1038/ng.534. [CrossRef]
- Casanova, E. L., Sharp, J. L., Chakraborty, H., Sumi, N. S., and Casanova, M. F. (2016). Genes with high penetrance for syndromic and non-syndromic autism typically function within the nucleus and regulate gene expression. Mol. Autism 7. doi:10.1186/s13229-016-0082-z. [CrossRef]
- Wong, C. C. Y., Meaburn, E. L., Ronald, A., Price, T. S., Jeffries, A. R., Schalkwyk, L. C., et al. (2014). Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol. Psychiatry 19, 495–503. doi:10.1038/mp.2013.41. [CrossRef]
- Kubota, T., and Mochizuki, K. (2016). Epigenetic Effect of Environmental Factors on Autism Spectrum Disorders. Int. J. Environ. Res. Public. Health 13. doi:10.3390/ijerph13050504. [CrossRef]
- Nagarajan, R. P., Hogart, A. R., Gwye, Y., Martin, M. R., and LaSalle, J. M. (2006). Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics 1, e1-11.
- Nagarajan, R. P., Patzel, K. A., Martin, M., Yasui, D. H., Swanberg, S. E., Hertz Picciotto, I., et al. (2008). MECP2 promoter methylation and X chromosome inactivation in autism. Autism Res. 1, 169–178. doi:10.1002/aur.24. [CrossRef]
- Xu, X., Li, C., Gao, X., Xia, K., Guo, H., Li, Y., et al. (2018). Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res. 28, 48–68. doi:10.1038/cr.2017.132. [CrossRef]
- Lee, S. Y., Ramirez, J., Franco, M., Lectez, B., Gonzalez, M., Barrio, R., et al. (2014). Ube3a, the E3 ubiquitin ligase causing Angelman syndrome and linked to autism, regulates protein homeostasis through the proteasomal shuttle Rpn10. Cell. Mol. Life Sci. 71, 2747–2758. doi:10.1007/s00018-867 013-1526-7. [CrossRef]
- Puram, S. V., Kim, A. H., Park, H.-Y., Anckar, J., and Bonni, A. (2013). The ubiquitin receptor S5a/Rpn10 links centrosomal proteasomes with dendrite development in the mammalian brain. Cell Rep. 4, 19–30. doi: 10.1016/j.celrep.2013.06.006. [CrossRef]
- Yi, J. J., Paranjape, S. R., Walker, M. P., Choudhury, R., Wolter, J. M., Fragola, G., et al. (2017). The autism-linked UBE3A T485A mutant E3 ubiquitin ligase activates the Wnt/β-catenin pathway by inhibiting the proteasome. J. Biol. Chem. 292, 12503– 12515. doi:10.1074/jbc.M117.788448. [CrossRef]
- Ladd-Acosta, C., Hansen, K. D., Briem, E., Fallin, M. D., Kaufmann, W. E., and Feinberg, A. P. (2014). Common DNA methylation alterations in multiple brain regions in autism. Mol. Psychiatry 19, 862–871. doi:10.1038/mp.2013.114. [CrossRef]
- Tremblay MW, Jiang YH. DNA Methylation and Susceptibility to Autism Spectrum Disorder. Annu Rev Med. 2019 Jan 27; 70:151-166. doi: 10.1146/annurev-med 120417-091431. Review. PubMed PMID: 30691368; PubMed Central PMCID: PMC6597259. [CrossRef] [PubMed]
- Mor, M., Nardone, S., Sams, D. S., and Elliott, E. (2015). Hypomethylation of miR 142 promoter and upregulation of microRNAs that target the oxytocin receptor gene in the autism prefrontal cortex. Mol. Autism 6. doi:10.1186/s13229-015-0040-1. [CrossRef]
- Nóbrega IS, Teles E Silva AL, Yokota-Moreno BY, Sertié AL. The Importance of Large-Scale Genomic Studies to Unravel Genetic Risk Factors for Autism. Int J Mol Sci. 2024 May 27;25(11):5816. doi: 10.3390/ijms25115816. PMID: 38892002; PMCID: PMC11172008. [CrossRef] [PubMed]
| Gene | Variations | Locus | Function | Clinical consequences of alteration |
|---|---|---|---|---|
| SYN1, SYN2 | Deletion, duplication, SNV | chrX:47,571,901-47,619,857 and chr3:12,004,388-12,192,032 | Synapse formation | X-linked delayed intellectual development, X-linked epilepsy, Pallister-Hall syndrome, schizophrenia, ASD, epilepsy |
| KCND2 | CNV | chr7:120,272,908-120,750,337 | Potassium voltage-gated channel, mediates transmembrane potassium transport in the brain | Early Myoclonic Encephalopathy |
| CACNA1E | CNV | chr1:181,317,690-181,813,262 | Voltage-Dependent Calcium Channel Alpha 1E Subunit; involved in the modulation of calcium mediated hormone secretion | Spastic tetraplegia, cerebral cortical atrophy, Developmental regression |
| SHANK3 | Deletionmethylation | chr22:50,672,823-50,733,212 | Multidomain scaffold proteins of the postsynaptic density that connect neurotransmitter receptors, ion channels, and other membrane proteins to the actin cytoskeleton and G-protein-coupled signaling pathways. Also plays a role in synapse formation and dendritic spine maturation. | Social communication, behavior, language, synaptic transmission, motor delay, delayed CNS myelination, seizures |
| MecP2 | methylation | chrX:154,021,573-154,137,103 | Chromosomal protein that binds to methylated DNA the corepressor SIN3A |
Cooperation with other genes, repressor synapse development Severe neonatal onset encephalopathy with microcephaly |
| UBE3A | methylation | chr15:25,333,728-25,439,056 | encodes an E3 ubiquitin-protein ligase, part of the ubiquitin protein degradation system | Loss of imprinting, motor delay, intellectual disability, delayed speech and language development |
| KCTD13 | Deletionduplication | chr16:29,905,012-29,926,236 | Contributes to ubiquitin-protein transferase activity. | Reduced NSC proliferation and maturation, ASD, Intellectual Disability Syndrome |
| KMT2C | CNV | chr7:152,134,922-152,436,644 | Possesses histone methylation activity and is involved in transcriptional coactivation | Cerebellar hypoplasia, Motor delay, Delayed speech and language development, Global development delay, Seizures |
| GABRB3 | CNV | chr15:26,543,552-26,939,539 | Oncoprotein acts as multi-subunit chloride channel that serves as the receptor for gamma-aminobutyric acid, a major inhibitory neurotransmitter | Epilepsy, childhood absence epilepsy, Lennox-Gastaut Syndrome |
| BDNF | CNV | chr11:27,654,893-27,722,058 | Encodes a nerve growth factor family of proteins. During development, promotes the survival and differentiation of selected neuronal populations of the peripheral and central nervous systems.Participates in axonal growth, pathfinding and in the modulation of dendritic growth and maturation. |
Cognitive impairment, seizure, intellectual disability, neuroblastoma, ganglioneuroblastoma |
| PCDDH7, PCDHB1 | CNV | chr5:141,051,374-141,059,346 | Potential calcium-dependent cell-adhesion protein.May be involved in the establishment and maintenance of specific neuronal connections in the brain. | Rett syndrome, schizophrenia, ASD |
| FOXP2 | deletion | 7q.31.1 | Plays role in developing neural, gastrointestinal and cardiovascular tissues.Plays a role in synapse formation by regulating SRPX2 levels.Involved in neural mechanisms mediating the development of speech and language. | Mental retardation, many organ development Delayed speech and language development Abnormal basal ganglia morphology Deficit in grammar Skeletal muscle atrophy |
| IMMP2L | CNV | chr7:110,662,644-111,562,5177q31.1 | Catalyzes the removal of transit peptides required for the targeting of proteins from the mitochondrial matrix, across the inner membrane, into the inter-membrane space.Known to process the nuclear encoded protein DIABLO | ASD |
| RELN | CNV | chr7:103,471,381-103,989,658 | This gene encodes a large secreted extracellular matrix protein thought to control cell-cell interactions critical for cell positioning and neuronal migration during brain development. | Cerebellar hypoplasia Global development delay Seizures Thick cerebral cortex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





