1. Background
Diseases caused by infections pose a serious risk to human health, and the prolonged use of antibiotics has contributed to the development of multidrug-resistant bacteria (Srivastava & Kim, 2022; Blair et al., 2015). Current wound dressings may contain glia, which can cause skin irritation or allergic reactions. Additionally, their limited ability to absorb large amounts of exudate can lead to infections or detachment from the wound. As a result, there is an urgent need to develop antibacterial materials to address these issues (Li et al., 2018).
Nypa fruticans Wurmb, commonly known as Nipa palm, is a mangrove palm species native to the coastal and estuarine regions of the Indian and Pacific Oceans, particularly in Southeast Asia, including the Philippines, Malaysia, and Indonesia. Historically, it played a key role in the Philippine economy, providing essential materials for constructing traditional homes before cement became widespread. Its fronds were used for roof thatching and wall partitioning, while its leaflets and midribs were crafted into brooms, baskets, mats, and sunhats. Additionally, dried parts of the plant served as fuel. Nipa palm sap, extracted from its inflorescence stalks, has long been used in Southeast Asia to produce treacle, sugar, alcohol, and vinegar. The fermented sap, known as "toddy" or "tuba" in the Philippines, is a popular local beverage (Hamilton and Murphy, 1988). The palm's white endosperm is also consumed as a snack, and various parts are used in traditional medicine, including treatments for ulcers, herpes, headaches, and toothaches.
Furthermore, nipa palm is also known to have antimicrobial properties which could potentially fight back infections when used as medical treatment. Nipa is a rich source of various biochemical compounds such as polyphenols which indicates excellent antibacterial activity. Studies have shown that aqueous and ethanolic extracts from various parts of the nipa palm exhibit good antimicrobial resistance against common pathogens (Ebana et al., 2015).
Interestingly, in a study conducted by Radi (2013) on the physicochemical and microbiological changes during fermentation and storage of nipa sap, results showed that various microbial species were present including Lacticaseibacillus paracasei which is a probiotic bacterium known for its health benefits and is commonly used in food, agriculture, and pharmaceutical industries. Studies from Hill et., (2018) and Jones (2017) report various benefits associated with L. paracasei strains, including antimicrobial and antibiofilm activity.
In addition to these uses and benefits, nipa palm has attracted renewed commercial interest due to its high cellulose content which are common by-products after the extraction of nipa palm flesh. Previous studies have reported that different parts of the nipa palm contain 28.9–48.2% α-cellulose, making them suitable raw materials for producing fuels and chemicals (Tamunaidu & Saka, 2011). Therefore, nipa palm fronds were considered for cellulose extraction for the formulation of hydrogel.
This study aimed to assess the potential of a cellulose hydrogel derived from nipa fronds, loaded with probiotics (ProbioGel) as an antimicrobial agent to prevent infections.
2. Methods
2.1. Phenotypic Characterization of Lacticaseibacillus paracasei BCRC-16100
Purified Lacticaseibacillus paracasei BCRC-16100 from fermented nipa sap was subjected to morphological test by observing its growth on de Man Rogosa Sharpe (MRS) agar plate and biochemical test particularly gram staining using Gram’s stain solutions. Furthermore, the potential pathogenicity of the isolate was assessed by examining its hemolytic activity through a hemolysin test using Blood agar. Results are categorized into alpha (α), beta (β), and gamma (γ) hemolysis. Alpha hemolysis (α) presents as partial hemolysis with a greenish discoloration around colonies, while beta hemolysis (β) manifests as complete lysis, creating a clear zone around colonies, often associated with pathogenic bacteria (McCaughey, E. J., et al, 2016;). Gamma hemolysis (γ) shows no lysis, indicating non-pathogenicity.
2.2. Genome Sequencing, Promoter Analysis and Production of ProbioGel
Identification of the Isolate by Capillary Sequencing. Genomic DNA (gDNA) of BCRC-1600 was extracted using the Quick-DNA fungal/Bacterial Miniprep Kit (Zymo Research, USA) according to manufacturer’s protocol. PCR amplicons were subjected to purification using AMPure XP beads (Cat. No. 163881). One microliter of the purified PCR amplicons was loaded into 1% agarose gel run at 120 V for 45 min, with Invitrogen 1kb Plus DNA Ladder. Capillary sequencing involved the incorporation of fluorescently labeled chain terminator ddNTPs. The reaction components include amplicons, corresponding primers, and the ABI BigDye® Terminator v3.1 Cycle Sequencing Kit (Cat No. 4337455). The cycling parameters on the thermal cycler were as follows: pre-hold at 4 °C; 96 °C for 1 min; 25 cycles at 96°C for 10 s, 50°C for 5 s, 62°C for 4 min; and hold at 4 °C. Ethanol precipitation was performed to remove unincorporated ddNTPs, excess primers, and primer dimers. Capillary electrophoresis was carried out on the ABI 3730xl DNA Analyzer using a 50cm 96-capillary array, POP7 Polymer (Cat No. 4393714), and 3730xl Data Collection Software v3.1. Base calling was performed using the Sequencing Analysis Software v5.4.
Whole Genome Sequencing. Library preparation was performed using the TruSeq DNA Nano Kit (Illumina, USA), and sequencing was conducted using an Illumina MiSeq instrument and a paired end read format of 2 x 150bp for 300 cycles at the Philippine Genome Center, Quezon City, Philippines.
Prediction of Promoter Elements. BPROM and BLAST were used to predict the promoter elements involved in the expression of genes associated with the antimicrobial activity of BCRC-16100. The upstream regions of these genes were extracted from their whole genome sequences (WGS) and subjected to analysis in the BPROM website where results show possible – 10 and –35 boxes of predicted promoters, their positions in the submitted sequence along with possible transcription factors.
2.3. Production of Cellulose Hydrogel from Nipa Frond
Methods for the isolation and purification of α-cellulose from nipa fronds were adapted from the study conducted by Cariaga et al., (2019), while the synthesis of the hydrogel was based on the procedures developed by the study of Domingo et al., (2022).
Derivatization of Carboxymethylcellulose (CMC) from Cellulose. Synthesis of Carboxymethylcellulose followed an established procedure described by Asl et al., (2017). Nine grams of α-cellulose powder isolated from nipa fronds, 30 ml of 40% NaOH, and 270 ml of solvent isopropanol were stirred together in a beaker and let stand for 30 minutes at room temperature. Afterward, 10.8g of sodium mono chloroacetate was added and stirred in a beaker using a magnetic hotplate stirrer. The beaker was covered with aluminum foil and was kept at 55°C for 3 hours with constant stirring at 1200rpm. After 3 hours, the resulting mixture was let stand for a few minutes to divide the upper phase and sedimentary phase. The upper phase was discarded, and sedimentary phases were suspended in 70% methanol and were 35 neutralized using glacial acetic acid. After neutralization, it was then filtered and washed five times with 70% ethanol using vacuum filtration. Lastly, it was washed again with absolute methanol and filtered. The obtained CMC was then air-dried.
Loading of Lacticaseibacillus paracasei into the formulated Nipa Hydrogel. First, pure culture of L. paracasei was inoculated into a 250 ml Erlenmeyer flask containing MRS broth and incubated at 35-37°C for 48 hours. After turbidity was observed in the flask, the formulation of CMC Nipa Hydrogel began using the methods developed by Domingo et al., (2022). First, 7% NaOH, 12% Urea, and 81% Distilled water were mixed in an Erlenmeyer flask. It was then pre-cooled at -12.6°C. 1g of carboxymethylcellulose was then added to the solution and stirred for 10 mins at 1500 rpm. The solution then underwent centrifugation at 8000 rpm for 20 mins. After centrifugation, the solution was transferred to a beaker and neutralized using 10% sulfuric acid. CMC solutions were then autoclaved, and all glassware was sterilized in a hot air oven at 160°C for 2 hours and UV-treated for 30 minutes along with the carbomer powder before use. To ensure a sterile environment and promote the growth of only Lacticaseibacillus paracasei, the following steps were taken. First, 50 mL of CMC solution was aseptically transferred to a beaker placed on a magnetic hotplate stirrer. Carbomer powder was then gradually incorporated into the CMC solution while stirring continuously until a homogeneous, clump-free mixture was achieved. One drop of triethanolamine was subsequently added once gelation began, followed by 50 mL of MRS broth pre-inoculated with L. paracasei. Finally, the solution, having reached the target viscosity, was transferred into a sterile petri dish, and stored at 4°C.
2.4. Microbiological Assay Using Kirby-Bauer Method
The antimicrobial potency of the nipa hydrogel loaded with probiotics and their components was tested against the three most common pathogenic bacteria that proliferate in wounds and are the primary causes of delayed healing and infection, these are Staphylococcus aureus, Escherichia coli, and S. epidermidis. Moreover, these were also tested against Candida albicans, a fungus that is frequently found in wounds and can lead to complications in healing. Lacticaseibacillus paracasei was subjected to antibacterial and antifungal assay using Mueller-Hinton Agar and Potato Dextrose Agar, respectively. Both agar types were autoclaved at 121°C for 15 minutes. After cooling, the agar was poured into sterile petri dishes with a depth of 4-5mm and was allowed to solidify before use.
Preparation of Inoculum. Preparation of inoculum such as the S. aureus, E. coli, S. epidermidis, and C. albicans was done inside a Level 2 Biosafety cabinet. A sterile wire loop was flamed to achieve sterilization. Using the sterilized wire loop, four or five colonies were taken from a pure bacterial culture of S. aureus. The colonies of S. aureus were then immersed into a test tube with 10ml of 0.85% saline solution, that was tightly covered. The same procedure was followed with the other microorganisms to be utilized. All test tubes were incubated at 35°C until it achieved the turbidity of a 0.5 MacFarland standard. These microorganisms served as the bacterial and fungal inocula throughout the experiment.
Inoculation of Plates and Placement of Antimicrobial Discs. A sterile cotton swab was first dipped into the standardized bacterial suspension, and this was inoculated into the agar by streaking. The plates were rotated by 60°, and the rubbing procedure was repeated three times to ensure an even distribution of the inoculum. The same procedure was done for the antifungal study. A total of 10 plates were used for the antimicrobial study. Filter paper discs were sterilized by placing it inside a beaker, covered with foil, and subjected to autoclaving at 121°C for 15 minutes. The sterilized paper discs of 6mm were soaked in the formulated nipa hydrogel treatments for about 24-48 hours. After impregnation, they were placed at the surface 38 of the inoculated and dried plate using sterile forceps by pressing them down lightly to ensure complete contact between the disk and agar surface. Discs were positioned such that the minimum center-center distance was 24 mm and no closer than 10-15mm from the edge of the petri dish. The plates were then incubated in an inverted position at 30°C for 24, 48, and 72 hours to further observe the growth of the microorganisms.
Measurement of Zones of Inhibition and Interpretation. Following incubation, zone sizes were measured edge to edge across the zone of inhibition over the center of the disk using a ruler or a digital vernier caliper. The value of the inhibitory zone diameters served as criteria to assess the antimicrobial activity, which is based on Manurung et al., (2018). The classification was as follows: strong activity was indicated by inhibitory zone diameters of ≥ 20mm, diameters ranging from 10 to 20mm were indicated as active, while diameters of the inhibitory zones ranging from 5 to 10mm were considered moderately active, and diameters of inhibitory zones that were invisible or ≤ 5mm were considered not active.
3. Results
3.1. Phenotypic Characterization of Lacticaseibacillus paracasei BCRC-16100
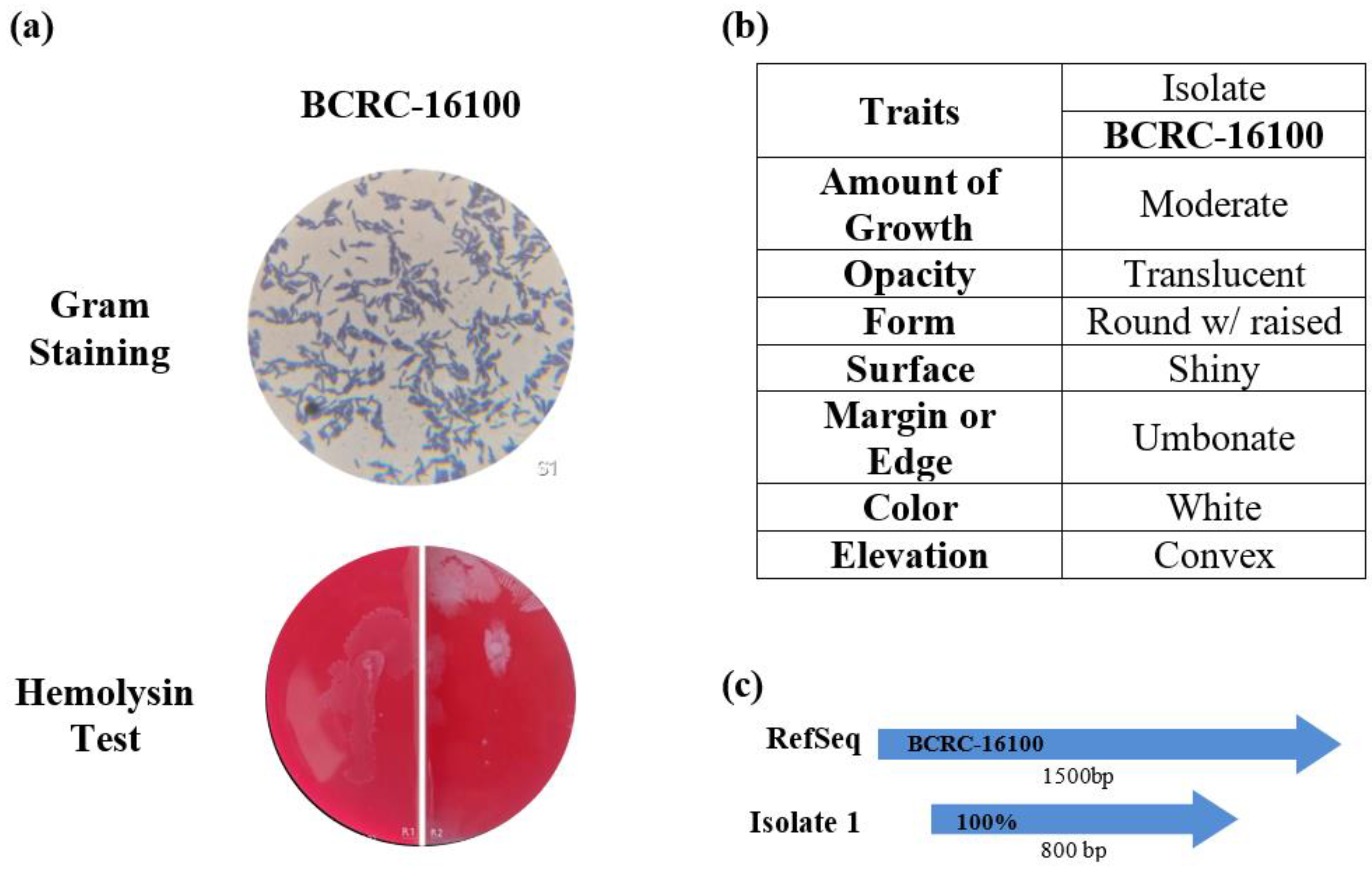
The isolated BCRC-16100 from fermented Nipa was subjected to phenotypic and genotypic characterization. The bacterial isolate was gram-positive with bacillus shape. Furthermore, its colony morphology has translucent opacity with shiny surface. It also exhibited round with raised form, umbonate margin, white color and convex elevation (see
Figure 1b). In hemolysin test, findings indicate that
L. paracasei BCRC-16100 exhibited gamma hemolysis (γ), suggesting its non-pathogenic nature regarding red blood cells (see
Figure 1a). These results align with previous studies reporting gamma-hemolytic behavior in non-pathogenic bacteria, reinforcing the safety of these microorganisms in the context of hemolytic activity (Smith, J. K., & Jones, R. S., 2018; Chen, Y., & Wang, Y., 2020). Furthermore, the isolate was identified to be
Lacticaseibacillus paracasei through 16sRNA (see
Figure 1c).
3.2. Genomic Characterization and Analysis of Lacticaseibacillus paracasei BCRC-16100
To support the inclusion and further utilization of Lacticaseibacillus paracasei BCRC-16100 into the hydrogel, promoter analysis was needed to be conducted first to determine the different promoter elements involved in the expression of genes linked with antimicrobial activity of the L. paracasei BCRC-16100.
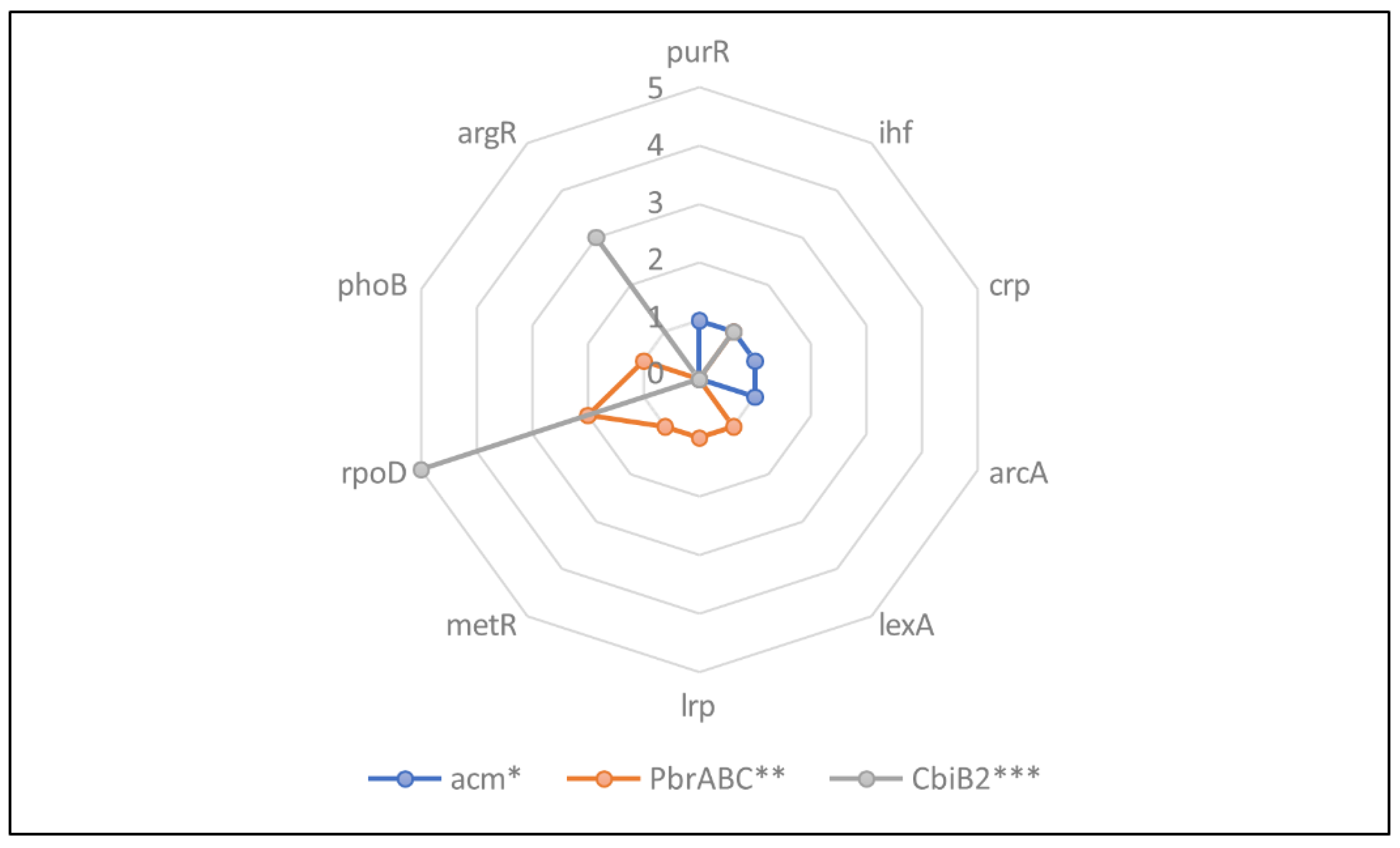
The different transcription frequencies were found to be associated with the antimicrobial activity of the
Lacticaseibacillus paracasei BCRC-16100 (see
Figure 2). Acm and CbiB2 were identified to encode lysozyme and putative carnobacteriocin B2, which exhibit distinct yet complementary antimicrobial mechanisms. Acm encodes the enzyme lysozyme, which disrupts the peptidoglycan layer of bacterial cell walls, leading to osmotic lysis and cell 47 death. In contrast, CbiB2 encodes for putative carnobacteriocn B2, which is an antimicrobial peptide that directly targets and kills specific bacteria, potentially through membrane disruption or inhibition of essential cellular processes. This can be attested by the research of Kiousi et al., (2022) regarding the genomic insight of another strain,
L. paracasei SP5, which also identified putative bacteriocin biosynthesis clusters, suggesting its potential antimicrobial activity. Previous findings also indicate that
L. paracasei can present antibacterial and antifungal activity in situ (Plessas et al., 2021). PbrABC gene, which codes for fatty acid ABC transporter ATP binding, is involved in the transport of fatty acids across cell membranes. These genes exhibited various transcription frequencies with 4 for acm gene (purr, ihf, crp and arcA), 7 (ihf, lexA, lrp, metR, rpoD, and phoB) for PbrABC and 9 (ihf, rpoD and argR) for CbiB2. The most dominant transcription factor was rpoD, followed by ihf and argR across all three genes, which suggests their potential role in core regulatory process governing the expression of these antimicrobial factors in BCRC-16100.
Overall, the presence of these genes indicates that the Lacticaseibacillus paracasei BCRC-16100 has a good potential to be a therapeutic tool for antimicrobial activity. The identified antimicrobial peptides and enzymes proved to be very essential in the microbiological assay of the ProbioGel.
3.3. Microbiological Assay Using Kirby-Bauer Method
The ProbioGel was subjected to antimicrobial assay against common microorganisms present in mammalian skin such as
Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, and
Candida albicans. After incubation, the resulting inhibition zones (ZOI) of the ProbioGel were measured (see
Table 1).
The biggest inhibition zone of the ProbioGel after 24 hours of observation was against C. albicans, while the least ZOI was seen in S. aureus. After 48 hours, all inhibition zones increased significantly with E. coli having the highest ZOI, followed by S. epidermidis, C. albicans, and S. aureus, respectively. The observed increase in the inhibition zones of ProbioGel against all tested bacteria after 48 hours also suggests a possible bactericidal effect.
4. Discussion
Phenotypic characterization and genomic analysis combined with antimicrobial assay provide an approach to studying the antimicrobial potency of ProbioGel. Throughout the years, various treatment methods and modalities have been used for treating infections including antibiotics. However, the use of antibiotics is not 100% effective and therefore it is still considered an unmet clinical need.
In the present study, the efficacy of cellulose-based hydrogels from Nipa fronds loaded with probiotics as antimicrobial agents in wound treatment was evaluated. The Lacticaseibacillus paracasei BCRC-16100 isolated from fermented Nipa sap was phenotypically characterized which involved morphological observation and hemolysin test prior to its identification. L. paracasei displayed gamma hemolysis, suggesting its non-pathogenic activity and reinforcing its safety. Whole genome sequencing and analysis of L. paracasei was done to provide molecular biology clues for its potential beneficial effects.
The antimicrobial properties of ProbioGel was evaluated using the Kirby-Bauer method against Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, and Candida albicans. The observed increase in inhibition zones against these common pathogens indicates that ProbioGel exhibits strong antagonistic activity. This is a particularly important trait for antimicrobial agents used in therapeutic applications, as it suggests a lasting impact on microbial populations, reducing the risk of infections.
Furthermore, the addition of L. paracasei further aided its antagonistic activity as this probiotic bacterium has an innate ability to stimulate the immune system, in which macrophages, neutrophils, and dendritic cells provide primary protective effect via direct antifungal activities, including phagocytosis and release of antimicrobial peptides (VazquezMunoz & Dongari-Bagtzoglou, 2021). This is attested by the studies of Rossoni et al., (2017) and Pimentel et al., (2018) where L. paracasei 28.4 upregulated genes that encode the antifungal peptides galiomicin and gallerymicin, and negatively regulating TEC1 and UME6 genes, essential to produce hyphae. The results of the two studies both led to a significant decrease in the number of fungal cells. In terms of antibacterial activity, studies have demonstrated L. paracasei’s ability to inhibit pathogens and the formation of biofilm, exhibiting greater inhibitory activity on Gram-positive bacteria such as S. aureus (Shahverdi et al., 2023: Tsai et al., 2021: Brandi et al., 2020). Findings of this antimicrobial activity can also be inferred from the promoter analysis conducted, BCRC-16100 exhibits antimicrobial activity through identified genes that encode for antimicrobial peptides such as carnobacteriocin B2 and the enzyme lysozyme.
5. Conclusions
In conclusion, this study highlights the potential of ProbioGel as a promising antimicrobial agent. This is reinforced by the identification of genes in the probiotic bacteria that encode antimicrobial peptides, including putative carnobacteriocin B2, and enzymes linked to antimicrobial activity. These findings suggest that ProbioGel could serve as an effective alternative for preventing infections in wounds.
Authors Contributions
A.S.C.: Performed the antimicrobial activity and drafted the original manuscript. A.G.D.: Responsible for conceptualization, methodology, and genome analysis. J.F.C.: Handled cellulose extraction and hydrogel production. F.A.G.: Conducted the isolation and characterization of the bacteria. A.C.S.C.: Carried out the antimicrobial analysis. A.N.Q.P.: Involved in data curation and software analysis. J.Z.P.F.: Conducted the safety evaluation and microorganism identification. P.J.I.G.: Contributed to investigation, resources, and genome analysis. B.S.S.: Managed data curation and secured funding. S.C.A.: Provided supervision and acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funding from the National Bioenergy Research and Innovation Center.
Data Availability Statement
The corresponding author will make the datasets generated and/or analyzed in this study available upon reasonable request.
Acknowledgements
We express our gratitude to the Philippine Genome Center and the Genomics and Genetic Engineering Laboratory for their support and contributions to this research. We also extend special thanks to the Commission on Higher Education (CHED)-LAKAS program of the Philippines.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brandi, J., Cheri, S., Manfredi, M., Claudia Di Carlo, Virginia Vita Vanella, Federici, F., Cecconi, D. (2020). Exploring the wound healing, anti-inflammatory, antipathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Scientific Reports, 10(1). [CrossRef]
- Blair, J. M. A., Webber, M. A., Baylay, A. J., Ogbolu, D. O., & Piddock, L. J. V. (2014). Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology, 13(1), 42–51. [CrossRef]
- Cariaga, J. F., Domingo, A. G., Santos, B. S., & Agrupis, S. C. (2023). Isolation of a - Cellulose from Nipa (Nypa fruticans Wurmb) Frond using Physico-Chemical Treatment. 16(23), 1754–1759. [CrossRef]
- Domingo AG, Cariaga JF, Santos BS, Agrupis SC (2023) Production of Cellulose Hydrogel from Nipa (Nypa fruticans Wurmb) Frond. Indian Journal of Science and Technology 16(21): 1-8. [CrossRef]
- Ebana, R. U. B., Etok, C. A., & Edet, U. O. (2015). Phytochemical Screening and Antimicrobial Activity of Nypa fruticans Harvested from Oporo River in the Niger Delta Region of Nigeria. International Journal of Innovation and Applied Studies, 10(4), 1120–1124. http://www.ijias.issrjournals.org/abstract.php?article=IJIAS-14-345-02.
- Hamilton, L. S., & Murphy, D. H. (1988). Use and management of Nipa palm (Nypa fruticans, arecaceae): a review. Economic Botany, 42(2), 206–213. [CrossRef]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei Group: History and Health Related Applications. Front. Microbiol. 2018, 9, 2107. [CrossRef]
- Jones, R.M. The Use of Lactobacillus casei and Lactobacillus paracasei in Clinical Trials for the Improvement of Human Health. In The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics, and Dysbiosis; Academic Press: Cambridge, MA, USA, 2017; pp. 99–108. ISBN 9780128040249.
- Kiousi D., Efstathiou, C., Tegopoulos K., Mantzourani I., Alexopoulos, A., Plessas S., Galanis, A. (2022). Genomic Insight into Lacticaseibacillus paracasei SP5, Reveals Genes and Gene Clusters of Probiotic Interest and Biotechnological Potential. Frontiers in Microbiology, 13. [CrossRef]
- Li, S., Dong, S., Xu, W., Tu, S., Yan, L., Zhao, C., Ding, J., & Chen, X. (2018). Antibacterial hydrogels. Advanced Science, 5(5). [CrossRef]
- Manurung, H., Nugroho, R., & Marina, E. (2018). Phytochemical Screening and Antibacterial Activity of Leaves Extract Balangla (Litsea cubeba (Lour) Pers.) from Malinau, East Borneo. Retrieved August 3, 2023, from http://eprints.undip.ac.id/62385/1/04._Hetty_Manurung_et_al.pdf.
- Pimentel, P., Scorzoni, L., Felipe, Ruano, L., Beth Burgwyn Fuchs, Eleftherios Mylonakis, Rodnei Dennis Rossoni. (2018). Lactobacillus paracasei 28.4 reduces in vitro hyphae formation of Candida albicans and prevents the filamentation in an experimental model of Caenorhabditis elegans. Microbial Pathogenesis, 117, 80–87. [CrossRef]
- Plessas, S., Kiousi, D. E., Rathosi, M., Alexopoulos, A., Kourkoutas, Y., Mantzourani, I., et al. (2020). Isolation of a Lactobacillus paracasei strain with probiotic attributes from kefir grains. Biomedicines 8, 1–15. [CrossRef]
- Radi, N. A. (2013, June 1). Physico-chemical and microbiological changes during fermentation and storage of nipa sap (Nypa fruticans wurmb). Psasir.upm.edu.my. http://psasir.upm.edu.my/id/eprint/42828/.
- Rossoni R., Fuchs B., Pimentel, P., Velloso, M., Jorge, A., Junqueira, J., & Mylonakis E. (2017). Lactobacillus paracasei modulates the immune system of Galleria mellonella and protects against Candida albicans infection. PloS One, 12(3), e0173332–e0173332. [CrossRef]
- Shahverdi S., Barzegari A., Bakhshayesh R., & Nami, Y. (2023). In-vitro and in-vivo antibacterial activity of potential probiotic Lactobacillus paracasei against Staphylococcus aureus and Escherichia coli. Heliyon, 9(4), e14641–e14641. [CrossRef]
- Srivastava, P., & Kim, K. (2022). Membrane Vesicles Derived from Gut Microbiota and Probiotics: Cutting-Edge Therapeutic Approaches for Multidrug-Resistant Superbugs Linked to Neurological Anomalies. Pharmaceutics, 14(11), 2370. [CrossRef]
- Tamunaidu P, Matsui N, Okimori Y, Saka S. (2013). Nipa (Nypa fruticans) sap as a potential feedstock for ethanol production. Biomass Bioenergy 52: 96-102. [CrossRef]
- Tsai, W.-H., Chou, C.-H., Huang, T.-Y., Wang, H.-L., Chien, P.-J., Chang, W.-W., & Lee, H.-T. (2021). Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds. Cells, 10(11), 3264–3264. [CrossRef]
- Vazquez-Munoz, R., & Dongari-Bagtzoglou, A. (2021). Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Frontiers in Oral Health, 2. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).