1. Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as one of the most devastating global health crises in recent history[
1]. Since the first reported case in Wuhan, China, in December 2019, the virus has spread rapidly worldwide, resulting in over 760 million cases and 6.9 million deaths [
2]. The RNA virus has replicated repeatedly, leading to the emergence of new variants with genomic mutations associated with alterations in severity, transmissibility, and immune escape[
3].
The global response to the pandemic has been unprecedented, with researchers and pharmaceutical companies worldwide driving innovative efforts to develop effective vaccines and respond to emerging variants. In Africa, where COVID-19 vaccination rates have been among the lowest globally, seven types of COVID-19 vaccines have been introduced, including inactivated vaccines such as Sinopharm (BBIBP-CorV) and Sinovac (Coronovac) [
4].
Clinical trials and real-world studies have demonstrated promising results for these inactivated vaccines. Phase III data for Sinopharm, following two doses 21 days apart, reported vaccine efficacy of 78.1% (95% CI: 64.8-86.3%) against symptomatic disease[
5]. Sinovac's phase III trial in Brazil showed 50.7% efficacy (95% CI: 36-62%) against symptomatic disease, 100% (95% CI: 17-100%) against severe disease, after two doses given 14 days apart[
6]. Trials in Indonesia and Turkey demonstrated efficacies of 65% (95% CI: 20-85%) and 83.5% (95% CI: 65.2-92.1%) respectively against symptomatic disease[
7,
8].
Vaccine efficacy in Africa has shown considerable variation, ranging from 41% to 100% across different vaccine platforms, including mRNA, non-replicating viral vectors, and protein subunit vaccines[
9]. However, studies on the effectiveness of inactivated vaccines in Africa are limited. Vaccine effectiveness (VE) studies with inactivated vaccines in Egypt and Morocco demonstrated VE of 67% (95%CI 43-80%) and 64% (95% CI: 59-69) against symptomatic disease several months post vaccination respectively [
10,
11]. Another Moroccan study retrospectively evaluated real world effectiveness of the BBIBP-CorV (Sinopharm) vaccine, reporting a VE of 88.5% (95% CI: 85.8-90.7%) against severe disease[
12]. These studies were either in specialized populations e.g. healthcare workers, retrospective analyses, conducted shortly after vaccines were administered and within limited geographies e.g. North Africa predominantly.
Approximately 20% of vaccines acquired in Africa were inactivated vaccines (6.8% Sinovac and 13.6% Sinopharm), with countries such as Zimbabwe relying almost entirely on inactivated vaccines in their vaccination programs [
13]. While clinical trials provide important insights into efficacy, real world evidence of VE is critical for evaluating effectiveness particularly in key groups such as pregnant women, people living with HIV or comorbidities that may have been underrepresented in clinical trial cohorts. Additionally, with the continuous emergence of novel variants, monitoring VE and the impact of boosting is crucial for assessing the effectiveness of public health measures [
14]. In low- and middle-income countries (LMICs) with fragile health systems that lack robust record-keeping or large electronic health records (EHRs), prospective observational studies such as test-negative case-control designs offer a cost-effective and practical approach to assess vaccine effectiveness [
15,
16]. To provide critical real-world data, we conducted a test-negative case-control study in Zimbabwe to estimate the effectiveness of licensed inactivated COVID-19 vaccines against laboratory-confirmed symptomatic COVID-19 disease at a time when Omicron variants were dominant [
17].
2. Materials and Methods
We conducted a prospective test-negative case-control study among symptomatic adults at treatment centers across six Zimbabwean provinces from November 2022 to October 2023. Eligible participants were adults (≥18 years) presenting within 10 days of symptom onset, and meeting the WHO surveillance case definition for COVID-19 [
18]. We excluded individuals who had exclusively received non-inactivated vaccines. After obtaining written informed consent, we conducted a comprehensive questionnaire obtaining data on socio-demographic information, medical history, concomitant medications, and details of clinical presentation. Socioeconomic status (SES) was categorized based on monthly income, with three distinct tiers: low-income (less than
$200 per month), middle-income (
$200 to
$800 per month), and high-income (more than
$800 per month). High risk occupation was defined by jobs or roles where workers were at an elevated risk of exposure to the virus due to the nature of their work environment, the tasks they performed, or the people they interacted with e.g healthcare workers, transportation and food service workers.
All participants underwent nasopharyngeal swab collection for SARS-CoV-2 PCR testing (USTAR Biotechnologies, Hangzhou, China), with positives classified as cases and negatives as controls. Blood samples were collected for immunology analyses, and HIV testing was offered. We documented hospitalization outcomes where applicable. Vaccination status was verified through multiple methods, including physical inspection of vaccination cards, review of digital card images, and examination of clinic vaccination registers. Participants were classified as having a verified status if they either verbally confirmed no vaccination history or, for those reporting vaccination, provided proof through one of the aforementioned verification methods. Vaccination status was categorized as follows: partially vaccinated if the participant had received the first vaccine dose within 14 days prior to enrolment, fully vaccinated if more than 14 days had passed since the second vaccine dose and boosted if the participant had received a third vaccine dose. Follow-up was conducted 2-8 weeks post-enrolment to assess symptom resolution.
Statistical Analysis
We calculated the sample size using WHO-recommended methods for test-negative case control vaccine effectiveness study design [
16]. Bivariate and multivariate logistic regression analyses were performed to investigate the relationships between various known COVID-19-associated demographic and health characteristics with vaccination status and with SARS-CoV-2 PCR positivity.
The primary outcome of interest was vaccine effectiveness at any time after receipt of at least one COVID-19 inactivated vaccine. In our secondary analyses, we estimated VE for each inactivated vaccine, boosting, time since vaccination, and enrolment location (clinic vs hospital setting). These analyses adjusted for covariates including age, sex, BMI, and various comorbidities such as diabetes, cardiovascular disease, HIV, tuberculosis, and cancers. Conditional logistic regression to assess the odds of testing SARS-CoV-2 PCR positive among the vaccinated vs. unvaccinated group was conducted.
We produced covariate-adjusted point estimates of vaccine effectiveness (VE), calculated as VE = (1 - aOR) × 100%. Conditional logistic regression models revealed the estimated vaccine effectiveness, expressed as odds ratios, along with corresponding confidence intervals. Our model adjusted for a comprehensive set of potential confounders including socio-demographic factors, lifestyle factors, comorbidities, and time since vaccination. Variable selection for the multivariate regression model involved identifying potential confounders based on existing literature, biological plausibility, and statistical significance in univariate analyses. For each VE estimate, we calculated 95% confidence intervals and used 2-sided 5% significance to identify statistical differences between cases and controls. Matching variables by age and statistical analysis was performed in STATA (18th Edition, College Station, Texas, US).
This study was conducted with ethical approval from the Medical Research Council of Zimbabwe (MRCZ/A/2914).
3. Results
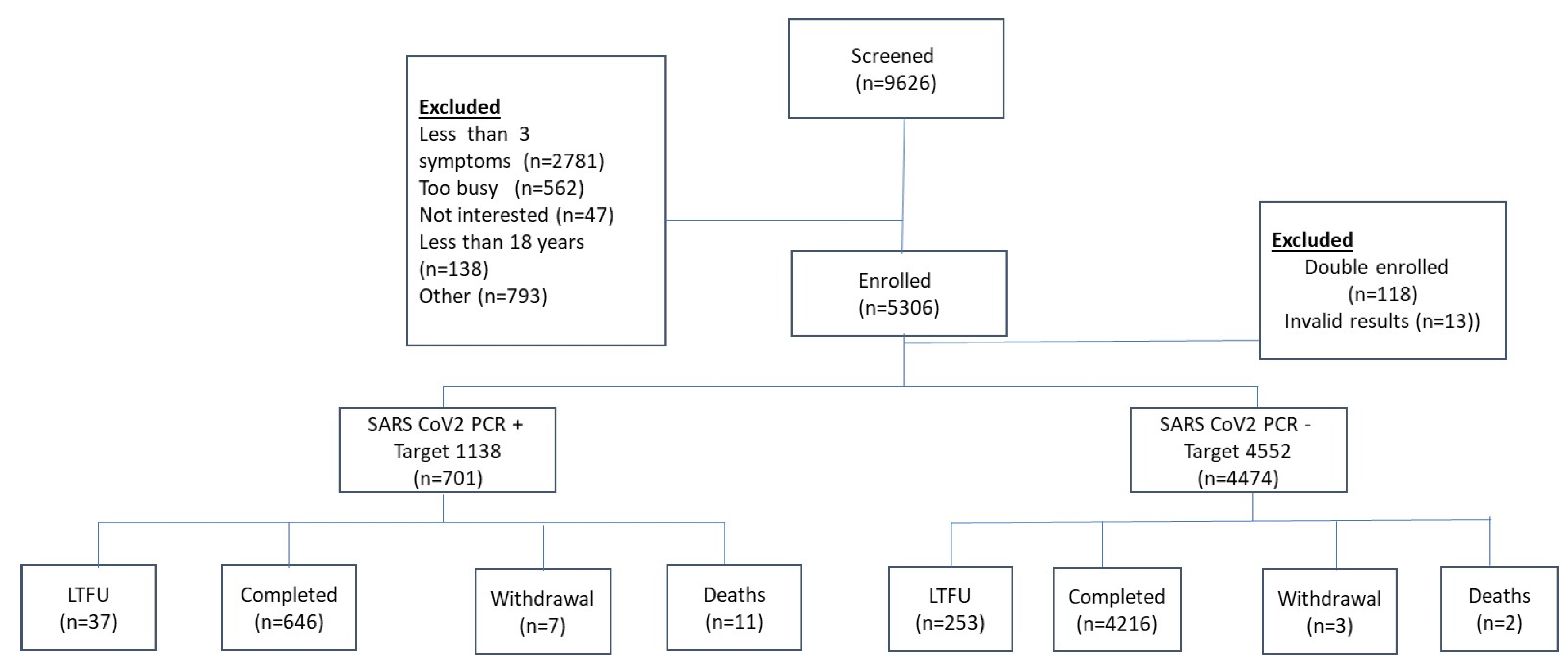
We screened 9626 individuals for eligibility at 22 health facilities across Zimbabwe, ranging from primary health care centers providing outpatient clinical care to district and provincial hospitals, including national referral hospitals. The majority of the exclusions were due to participants having fewer than 3 symptoms (n=2781) (
Figure 1). A total of 5306 participants were enrolled. Following further exclusions for double enrolment (n=118) and invalid laboratory results (n=13), the final analytic cohort consisted of 5175 participants. Of these, 701 tested positive for SARS-CoV-2 by PCR (cases) and 4474 tested negative for SARS-CoV-2 by PCR (controls). Other reasons for screen failure include individuals who refused to participate, failure to meet minimum age requirement for the study i.e., <18 years, and non-eligible vaccine type etc. (
Supplementary Table S1).
Demographic data was available on all 5175 enrolled participants. There was a slight, non-significant difference in gender distribution between cases and controls, with 71.5% of cases being female compared to 67.9% of controls (p=0.057) (
Table 1). The median age was modestly higher among cases (37 years, IQR 27-48) compared to controls (36 years, IQR 26-44, p=0.001). The distribution of age groups also showed a significant association with SARS-CoV-2 positivity, particularly in the 65+ age group (6.99% in cases vs. 2.55% in controls, p<0.001). The ethnic distribution was predominantly Black African. Socioeconomic status did not significantly differ between cases and controls (p=0.366). The majority of participants were enrolled from hospital-based outpatient clinic or emergency room settings (66.72%), with a significantly higher proportion of cases than controls among hospitalized inpatients (7.7% in cases vs. 0.38% in controls, p<0.001). Community outpatient clinic enrolment was more common among controls than cases (33.57% vs. 21.26%) (
Table 1).
Cases had a slightly higher median BMI (26 (IQR 23-30) vs. 25 (IQR 22-29), p=0.005). The burden of comorbid conditions differed significantly between cases and controls (
Table 2). Cases had a higher proportion of participants with hypertension (19.16% vs 14.15%, p=0.001), asthma (3.86% vs 2.51%, p=0.039), and tuberculosis (1.66% vs 0.78%, p=0.034) compared to controls. HIV prevalence was high in both groups but not significantly different (22.07% in cases vs 22.75% in controls, p=0.698). Notably, a significantly higher proportion of cases than controls were pregnant women (16.19% vs 8.68%, p<0.001) (
Table 2). The most common comorbid conditions across the entire study population were HIV (22.66%), hypertension (14.83%), and obesity (BMI≥30) (24.83%) (
Table 2). Among the HIV infected 83.4% were on ART (
Table 2).
Common symptoms like acute fever (50.4% vs 38%, p<0.001), muscle pain (37.7% vs 33.5%, p=0.031), general weakness (65.9% vs 57.9%, p<0.001) and altered mental state (4.6% vs 3.1%, p=0.042) were significantly more prevalent in cases compared to controls, and prior COVID-19 infection was also more common among cases (36.8% vs. 32.6%, p=0.030) (
Supplementary Table S2).
Overall, the majority of the population, 82.9% reported to have been vaccinated and only 877 (17.1%) of the population had not been vaccinated. Among those reporting their vaccination status, 42.1% of participants were fully vaccinated (
Table 3), and 33.4% received the booster doses. Partial vaccination was observed in 7.2% of the cohort. Younger individuals (18-49) had higher rates of partial and full vaccination, while older individuals (50+) have higher rates of booster vaccination, participants aged 50-64 (49.9%) and 65+ (51.9%) (
Table 4). Sinopharm was the most commonly utilized vaccine; 57.4% of the cohort had been vaccinated with Sinopharm, 39.5% with Sinovac, and 0.4% with Covaxin (
Table 3). Age-wise distribution showed a similar pattern, with Sinopharm being the dominant vaccine across all age groups.
The median time since the last vaccine dose for the overall vaccinated group was 434 days (IQR: 266-616) (
Table 4). Median time since the last booster dose was 310 days (IQR:205-409 days). This varied across age groups, with the longest duration observed in the 65+ group (median 368 days (IQR: 307-467 days) (
Table 4).
There are significant differences in median age, age group distribution, socio-economic status, and enrolment site, between vaccinated and unvaccinated individuals (
Supplementary Table S3). Gender (p=0.937) and ethnicity (p=0.773) distributions are similar across both groups with no significant differences noted. There were significantly less individuals with tuberculosis among the vaccinated compared to the unvaccinated individuals (0.7% vs 1.9%, p=0.002) (
Supplementary Table S4). We successfully verified the vaccination status of 3297/4292 (76.8%) of those reporting vaccination, primarily through vaccine card inspection and digital image submission of vaccination card. (
Supplementary Table S5).
In the analysis of participants with verified vaccination status within the study cohort, both bivariate and multivariate logistic regression models were used to assess the association between demographic variables, health indicators, and vaccination status (
Table 5). In the multivariate analysis, significant risk factors include high BMI (aOR=1.44, 95% CI 1.127-1.835), hospital enrolment (aOR=1.49, 95% CI 1.231-1.791), previous COVID diagnosis (aOR=2.85, 95% CI 2.051-3.952), hypertension (aOR=1.64, 95% CI 1.225-2.206), and working in a high-risk occupation (aOR=2.98, 95% CI 2.430-3.643). Protective factors include a low BMI (aOR=0.50, 95% CI 0.312-0.814), and active tuberculosis (aOR=0.44, 95% CI 0.211-0.904) (
Table 5).
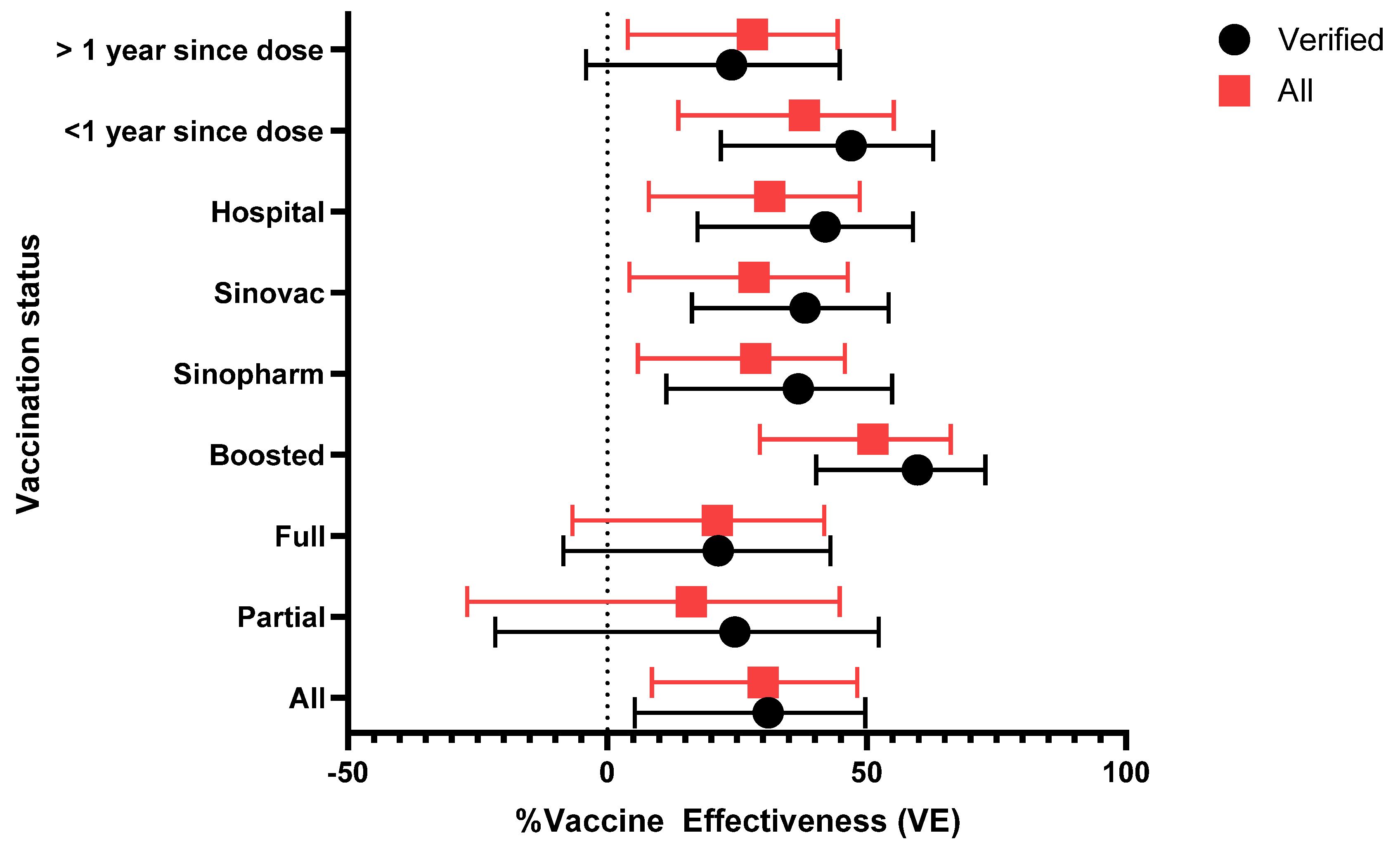
The adjusted vaccine efficacy (VE) against SARS-CoV-2 infection was evaluated across various subgroups within the study cohort (
Table 6). Overall, the adjusted VE was 31% (95% CI: 5.3% to 49.7%) among verified participants and 30% (95% CI: 8.6% to 48.2%) among all participants. Participants enrolled from hospitals showed a higher VE of 42% (95% CI: 17.4% to 58.9%) among verified participants. VE was similar in those that received the Sinopharm and Sinovac vaccine with adjusted VEs of 36.8% (95% CI: 11.4% to 54.9%) and 38.1% (95% CI: 16.3% to 54.2%), respectively, among verified participants (
Figure 2).
Individuals who had received booster doses demonstrated the highest vaccine effectiveness (VE) at 59.8% (95% CI: 40.3% to 72.9%) in the verified group; this compares to 21.4% (95% CI: -8.5% to 43%) for fully vaccinated individuals and 24.6% (95% CI: -21.6% to 52.3%) for partially vaccinated participants. Time since vaccination appeared to impact effectiveness. The median time post-vaccination was 434 days (IQR: 266 to 616 days) (
Table 4). Individuals who received their last vaccine dose within the past year demonstrated a higher VE of 47% (95% CI: 21.9% to 62.8%) compared to those vaccinated more than a year ago, where VE was no longer evident at 24% (95% CI: -4.1% to 44.8%).
A sensitivity analysis was conducted to compare adjusted vaccine effectiveness (VE) by vaccination status and various subgroups, focusing on all participants and those whose vaccination status was successfully verified through a rigorous process. The vaccination verification involved checking vaccination cards at enrolment or follow up, conducting phone calls to obtain vaccination details and digital pictures of the cards, and making home visits and verifying records at the clinics. Despite these efforts, only 79.2% of participants who reported being vaccinated were successfully verified (
Table 3). In the analysis verified participants consistently demonstrated higher VE across all subgroups compared to the broader group that included those reported vaccination but could not be verified. For instance, boosted individuals exhibited the highest VE, with 59.8% (95% CI: 40.3% to 72.9%) in the verified group, compared to a lower VE (51.2% (95% CI: 29.4% to 66.2%)) in all those that reported vaccination. Similarly, the effectiveness of Sinovac and Sinopharm vaccines was higher among verified participants (
Figure 2).
4. Discussion
This study provides valuable insights into the real-world effectiveness of inactivated COVID-19 vaccines in Zimbabwe, revealing a modest level of protection against symptomatic SARS-CoV-2 infection, with notable variations across different vaccination statuses and subgroups.
The overall vaccine effectiveness (VE) of 31% (95% CI: 5.3-49.7%) for prevention of symptomatic disease among verified vaccinated individuals is lower than the efficacy reported in initial clinical trials[
5,
6,
7,
8]. In a VE study in Morocco that was conducted prior to emergence of Omicron variant observed a higher VE of 67% (95% CI: 43% to 80%) [
10]. Our data however is more closely aligned with a large VE study conducted in Shanghai, China that enrolled participants before and after the emergence of the Omicron variant. This study demonstrated a VE of 16.3% (95%CI: 15.4% to 17.2%) against symptomatic disease increasing to 88.6% (95%CI: 85.8% to 90.9%) for severe disease and 91.7% (95%CI: 86.95% to 94.5%) for death [
19]. In our study the relatively low VE for symptomatic disease is likely attributable to the predominance of Omicron variants during our study period. The omicron variant and its subvariants have demonstrated increased ability to evade vaccine-induced immunity[
17,
20]. The VE that we observed is slightly higher for symptomatic disease with Omicron variants than observed in the Shanghai, China cohort and a recent metanalysis (VE 16.4% (95%CI: 9.5 to 22.8% for omicron) [
21]. We hypothesize that this may reflect hybrid immunity induced by both vaccination and recent infection, given high levels of circulating virus[
22,
23].
We observed a higher VE of 42% (95% CI: 17.4-58.9%) among hospital-enrolled participants. This suggests stronger protection against more severe forms of COVID-19 requiring hospital care, consistent with findings from other countries[
11,
12,
19]. However, the low proportion of severely ill participants in our study (1.3% hospitalized) limited our ability to evaluate VE against severe disease directly. We conducted our study at a period when Omicron was dominant and had been associated with less severe disease on the continent[
24,
25].
The effectiveness of booster doses is a crucial finding, with VE increasing to 59.8% (95% CI: 40.3-72.9%) for boosted individuals. This underscores the importance of booster vaccination programs, especially in the context of emerging variants where homologous boosting with inactivated vaccines against the original variants, has been shown to confer protection against emergent Delta and Omicron variants[
26]. The relative VE of 27.4% (95% CI: 8.8%-42.8%) for boosted compared to fully vaccinated individuals further reflects the additional protection provided by booster doses.
Time since vaccination emerged as a critical factor influencing effectiveness. VE dropped significantly to 24% (95% CI: -4.1% to 44.8%) at ≥1-year post-vaccination, compared to 47% (95% CI: 21.9% to 62.8%) for those vaccinated within the past year. This waning effectiveness aligns with observations from other studies and vaccine platforms[
27] [
28].
We observed similar VE for Sinopharm (36.8%, 95% CI: 11.4-54.9%) and Sinovac (38.1%, 95% CI: 16.3-54.2%) vaccines, indicating comparable performance of these inactivated vaccines in the study population.
The vaccination rate in our study population (83%) was notably higher than the national average. In Zimbabwe the vaccine rollout initially targeted older age groups but expanded to everyone above 12 years by August 2021. As of 31 December 2023, national vaccination coverage stood at 51% for the first dose, 38% for the second dose and 15% for the booster shot (WHO COVID-19 Dashboard, Zimbabwe). This discrepancy likely reflects a combination of factors, including potential selection bias towards individuals more likely to seek healthcare and the higher socioeconomic status (SES) of our study population. Indeed, 46.3% of our participants fell into the middle or high SES categories, which is substantially higher than the national average where about 80% of the urban population has a monthly income below
$200[
29].
The study employed meticulous vaccination verification methods, achieving a verification rate of 79,2% among those reporting vaccination. Vaccination verification is challenging in many settings including Africa where records are not digitized, and cards may be easily lost. In addition, with COVID there were significant challenges due to card falsification. The verification rate that we achieved was relatively high [
30] due to the intense resources dedicated to following up on all study participants and documenting vaccination. However, despite this, we acknowledge the potential for misclassification bias, particularly among those reporting no vaccination, which we accepted at face value. We address this bias by sensitivity analysis for VE, by separating analysis of VE in those whose status was unverified as well as self-reported vaccination.
Several limitations should be considered when interpreting our results. The test-negative design, while practical for real-world effectiveness studies, is subject to potential biases, including differences in misclassification bias and the impact of healthcare-seeking behavior in the analyzed cohort. The study cohort, predominantly young and female, may limit generalizability but reflects common health system utilization trends in Africa[
31].
5. Conclusions
The study findings provide evidence of reduced effectiveness of inactivated COVID-19 vaccines against symptomatic Omicron infection in Zimbabwe, consistent with global trends. The increased effectiveness with boosters and the significant waning of protection over time underscore the need to consider potential annual boosting strategies. However, future boosters may require optimized vaccines that better match circulating strains. In resource-limited settings, special consideration must be given to the cost-effectiveness of vaccination programs, given the relatively low disease severity observed. This may necessitate a targeted approach, focusing on vaccinating the most high-risk groups. Larger studies will be required to define these high-risk populations in Africa that would benefit most from continued vaccination efforts.
We anticipate that the results from this study can inform public health decision-making in Zimbabwe and other sub-Saharan countries with similar demographic and epidemiological profiles. As the global community continues to navigate the challenges posed by COVID-19, real-world effectiveness data from diverse settings remains crucial for shaping equitable, cost-effective, and tailored pandemic responses. Future strategies should prioritize rapid deployment of effectiveness studies in target populations to better inform regional epidemic responses.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Azure Makadzange; Data curation, Azure Makadzange and Patricia Gundidza; Formal analysis, Azure Makadzange and Patricia Gundidza; Funding acquisition, Azure Makadzange; Investigation, Azure Makadzange and Kimberley Konono; Methodology, Azure Makadzange; Project administration, Azure Makadzange, Kimberley Konono and Chiratidzo Ndhlovu; Supervision, Azure Makadzange and Chiratidzo Ndhlovu; Writing – original draft, Azure Makadzange; Writing – review & editing, Patricia Gundidza, Kimberley Konono, Margaret Gurumani and Chiratidzo Ndhlovu.
Funding
This research was funded by Coalition for Epidemic Preparedness Innovations (CEPI).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Medical Research Council of Zimbabwe (MRCZ/A/2914 and date of approval: 20 July 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
The authors express their gratitude towards the personnel at the study sites: Parirenyatwa Group of Hospitals, Sally Mugabe Central Hospital, Chitungwiza Central Hospital, Marondera Provincial Hospital, Chinhoyi Provincial Hospital, Bindura Provincial Hospital, Collin Saunders Medical Centre, Hippo Valley Medical Centre, Victoria Chitepo Provincial Hospital, Norton Hospital, Arundel Hospital, Sakubva Polyclinic, Kuwadzana Polyclinic, Mabvuku Polyclinic, Glenview Polyclinic, Budiriro Polyclinic, Dzivarasekwa Polyclinic, Epworth Polyclinic, CIMAS private clinic, Rutsanana Polyclinic, Mbare Polyclinic. Furthermore, the authors express their sincere gratitude towards the study team members who were instrumental in ensuring the success of the study: Norest Beta, Eddy Makaha, Michelle Muzanenhamo, Sofia Muyemayema, Sinikiwe Mbanje, Victoria Changara, Gamuchirai Kamangira, Mandy Marume, Norest Mutukwa, Marvel Maswera, Ruvimbo Chigumbu, Faustine Mupoperi, Farai Chinoko, Takura Mangwiro, Naison Rupungu, Emelda Chikosha, Brenda Dube, Percy Ndhovu, Simon Mateko, Tongayi Matambanadzo, Gamuchirai Kavhumbura, Tawonga Chilundo, Anesu Muriritirwa, Zorodzai Tangwena, Rufaro Chivaura, Vinnie Koaumou, Xeshelihle Mhlanga, Cuthbert Karengwa, Fiona Chihuri, Fibion Mukwati, Yolanda Chihwehwete, Tashinga Chigodora, William Mangwa, Jafta Chikorowondo, Tendai Mutanga, Tanaka Mudimu and Palmer Mudare. The authors also wish to extend gratitude to colleagues from the University of Oxford who supported the work: Professor Merryn Vorsey, Andrew Pollard, John Bell and Romina Mariano.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bhadoria, P., G. Gupta, and A. Agarwal, Viral Pandemics in the Past Two Decades: An Overview. J Family Med Prim Care, 2021. 10(8): p. 2745-2750. [CrossRef]
- Organization, W.H. Coronavirus disease (COVID-19). 2023 9 August 2023; Available from: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(covid-19).
- Li, X., et al., Transmission dynamics and evolutionary history of 2019-nCoV. J Med Virol, 2020. 92(5): p. 501-511.
- Lamptey, E., et al., COVID-19 vaccines development in Africa: a review of current situation and existing challenges of vaccine production. Clin Exp Vaccine Res, 2022. 11(1): p. 82-88. [CrossRef]
- Al Kaabi, N., et al., Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. Jama, 2021. 326(1): p. 35-45. [CrossRef]
- Palacios, R., et al., Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN Electronic Journal, 2021. [CrossRef]
- Tanriover, M.D., et al., Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet, 2021. 398(10296): p. 213-222. [CrossRef]
- Fadlyana, E., et al., A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: An interim analysis in Indonesia. Vaccine, 2021. 39(44): p. 6520-6528. [CrossRef]
- Mengstu, S. and A. Beyene Berha, Safety and Efficacy of COVID-19 Vaccine in Africa: Systematic Review. Infect Drug Resist, 2023. 16: p. 3085-3100. [CrossRef]
- Ashmawy, R., et al., Effectiveness and Safety of Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) among Healthcare Workers: A Seven-Month Follow-Up Study at Fifteen Central Hospitals. Vaccines (Basel), 2023. 11(5). [CrossRef]
- Belayachi, J., et al., Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero Cells) against COVID-19 associated severe and critical hospitalization in Morocco. PLoS One, 2022. 17(12): p. e0278546. [CrossRef]
- Zhang, Y., et al., Real-world study of the effectiveness of BBIBP-CorV (Sinopharm) COVID-19 vaccine in the Kingdom of Morocco. BMC Public Health, 2022. 22(1): p. 1584. [CrossRef]
- Control, A.C.f.D. Proportion of Vaccine Type Acquired by Member States in Africa. 2021 [cited 2024; Available from: https://africacdc.org/covid-19/covid-19-vaccination/.
- Flores-Vega, V.R., et al., SARS-CoV-2: Evolution and Emergence of New Viral Variants. Viruses, 2022. 14(4). [CrossRef]
- Dean, N.E., J.W. Hogan, and M.E. Schnitzer, Covid-19 Vaccine Effectiveness and the Test-Negative Design. New England Journal of Medicine, 2021. 385(15): p. 1431-1433. [CrossRef]
- Organization, W.H. Evaluation of COVID-19 vaccine effectiveness. 2021; Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1.
- Viana, R., et al., Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature, 2022. 603(7902): p. 679-686. [CrossRef]
- Organziation, W.H. WHO COVID-19 Case definition. 2022 [cited 2024; Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1.
- Huang, Z., et al., Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med, 2022. 20(1): p. 400. [CrossRef]
- Rana, R., et al., Omicron variant: Current insights and future directions. Microbiol Res, 2022. 265: p. 127204. [CrossRef]
- Xu, S., et al., Real-world effectiveness and factors associated with effectiveness of inactivated SARS-CoV-2 vaccines: a systematic review and meta-regression analysis. BMC Med, 2023. 21(1): p. 160. [CrossRef]
- Madhi, S.A., et al., Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N Engl J Med, 2022. 386(14): p. 1314-1326. [CrossRef]
- Madhi, S.A., et al., Durability of ChAdOx1 nCoV-19 (AZD1222) vaccine and hybrid humoral immunity against variants including omicron BA.1 and BA.4 6 months after vaccination (COV005): a post-hoc analysis of a randomised, phase 1b-2a trial. Lancet Infect Dis, 2023. 23(3): p. 295-306. [CrossRef]
- Davies, M.-A., et al., Outcomes of laboratory-confirmed SARS-CoV-2 infection during resurgence driven by Omicron lineages BA.4 and BA.5 compared with previous waves in the Western Cape Province, South Africa. International Journal of Infectious Diseases, 2023. 127: p. 63-68. [CrossRef]
- Abdullah, F., et al., Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int J Infect Dis, 2022. 116: p. 38-42. [CrossRef]
- Tang, L., et al., Relative vaccine effectiveness against Delta and Omicron COVID-19 after homologous inactivated vaccine boosting: a retrospective cohort study. BMJ Open, 2022. 12(11): p. e063919. [CrossRef]
- Menni, C., et al., COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis, 2022. 22(7): p. 1002-1010. [CrossRef]
- Ferdinands, J.M., et al., Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. Bmj, 2022. 379: p. e072141. [CrossRef]
- Trust, F. FinScope Consumer Survey Report. 2022 [cited 2024; Available from: https://www.rbz.co.zw/documents/BLSS/2022/Zimbabwe_FinScope_Consumer_2022_Survey_Report.pdf.
- Hamisu, M., et al., Microplanning verification and 2017/2018 measles vaccination campaign in Nigeria: Lessons learnt. Vaccine, 2021. 39 Suppl 3: p. C46-c53. [CrossRef]
- Yeatman, S., S. Chamberlin, and K. Dovel, Women's (health) work: A population-based, cross-sectional study of gender differences in time spent seeking health care in Malawi. PLoS One, 2018. 13(12): p. e0209586. [CrossRef]
Note
| 1 |
Total represents that total number with reference indication included in the model. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).