Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
HPLC Method Development
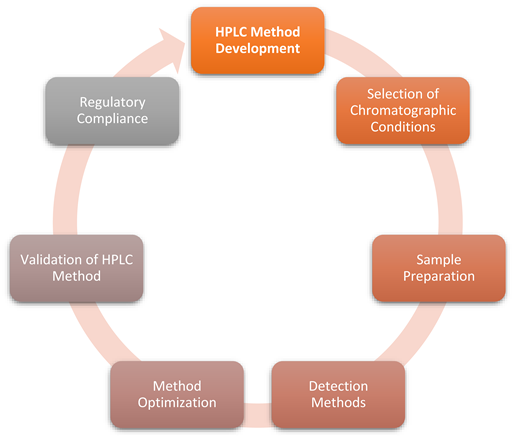
- Selection of Chromatographic Conditions.
- -
- Choosing appropriate solvents based on the polarity of analytes.
- -
- pH and buffer selection to control ionization and retention times.
- -
- Gradient elution vs. isocratic elution for optimizing separation efficiency.
- -
- Selection of column type (e.g., C18, C8, phenyl, cyano) based on analyte characteristics.
- -
- Particle size and pore size considerations for resolution and analysis time.
- -
- Control of column temperature to maintain consistent retention times and improve separation.
- 2.
-
Sample Preparation
- -
- Techniques such as filtration, centrifugation, and solid-phase extraction to remove matrix components and enhance analyte recovery.
- -
- Minimizing sample degradation and matrix effects to ensure accurate quantification.
- 3.
-
Detection Methods
- -
- Selection of appropriate detection techniques based on analyte properties (e.g., UV-Vis, fluorescence, mass spectrometry).
- -
- Optimizing detector settings for sensitivity, linearity, and specificity.
- 4.
-
Method Optimization
- -
- Resolution: Maximizing separation between analyte peaks.
- -
- Efficiency: Ensuring narrow peak widths for precise quantification.
- -
- Selectivity: Tailoring conditions to resolve closely eluting peaks.
- -
- Robustness: Testing method robustness against small variations in parameters like pH, temperature, and flow rate.
- -
- Speed: Balancing analysis time with method sensitivity and resolution.
- 5.
-
Validation of HPLC Method
- -
- Accuracy: Comparing measured values to true values or reference methods.
- -
- Precision: Assessing repeatability (intra-day) and reproducibility (inter-day).
- -
- Specificity: Ensuring analyte detection in the presence of matrix components.
- -
- Linearity: Establishing a linear relationship between analyte concentration and detector response.
- -
- Limit of Detection (LOD) and Limit of Quantification (LOQ): Determining the lowest concentration reliably detectable and quantifiable.
- -
- Range: Validating the upper and lower limits of the method's analytical range.
- -
- System Suitability: Evaluating chromatographic parameters to ensure consistent performance.
- 6.
-
Regulatory Compliance
- -
- Adhering to guidelines such as ICH Q2(R1) and USP <1225> for method validation.
- -
- Documenting and reporting validation results to demonstrate method reliability and compliance.
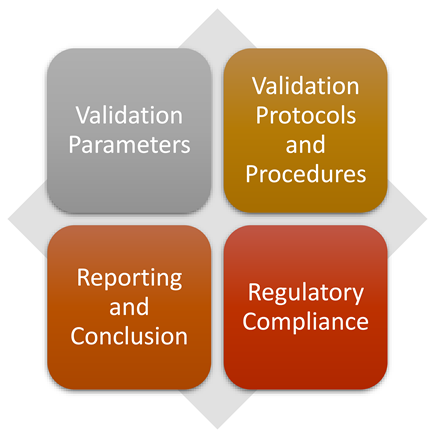
HPLC Method Validation
- 1.
-
Validation Parameters
- -
- Accuracy: Determines the closeness of test results to the true value. Accuracy is typically assessed by comparing the measured value to a known standard or reference material.
- -
-
Precision:
- -
- Repeatability: Also known as intra-day precision, evaluates the variation in results obtained within a short time period under the same conditions by the same analyst using the same equipment.
- -
- Intermediate Precision: Assesses the variation in results obtained within the same laboratory but under different conditions (e.g., different days, different analysts, different equipment).
- -
- Reproducibility: Evaluates the variation in results obtained in different laboratories using the same method.
- -
- Specificity: Determines the ability of the method to distinguish the analyte from other components in the sample matrix. Specificity is often assessed through peak purity tests and interference studies.
- -
-
Limit of Detection (LOD) and Limit of Quantification (LOQ):
- -
- LOD: The lowest concentration of analyte that can be reliably detected but not necessarily quantified.
- -
- LOQ: The lowest concentration of analyte that can be quantitatively determined with acceptable accuracy and precision.
- -
- Linearity: Evaluates the relationship between analyte concentration and detector response over a specified range. Linearity is assessed by analyzing multiple calibration standards and plotting a calibration curve.
- -
- Range: Defines the concentration range over which the method demonstrates acceptable linearity, accuracy, and precision.
- -
- Robustness: Determines the reliability of the method with respect to small variations in method parameters such as pH, mobile phase composition, flow rate, and column temperature.
- -
- System Suitability: Ensures that the HPLC system is capable of providing adequate resolution, sensitivity, and reproducibility for the intended analysis. System suitability parameters include retention time, resolution between peaks, and column efficiency.
- 2.
-
Validation Protocols and Procedures
- -
- Design of Experiments (DoE): Statistical techniques such as factorial designs used to systematically evaluate and optimize method parameters during validation.
- -
- Validation Plan: Detailed document outlining the validation objectives, acceptance criteria, experimental protocols, and responsibilities of personnel involved in the validation process.
- -
- Validation Samples: Prepared to evaluate specificity, accuracy, precision, linearity, and robustness. Samples may include standard solutions, placebo samples, and spiked samples at different concentrations.
- -
- Documentation: Comprehensive documentation of validation results, including raw data, calculations, chromatograms, and a final validation report summarizing all findings.
- 3.
-
Regulatory Compliance
- -
- ICH Guidelines (Q2(R1)) and USP <1225>: International guidelines providing a framework for method validation in pharmaceutical analysis. These guidelines outline specific requirements for validation parameters, acceptance criteria, and documentation.
- -
- Good Laboratory Practices (GLP): Adherence to GLP principles ensures that the validation process is well-documented, controlled, and reproducible.
- 4.
-
Reporting and Conclusion
- -
- Validation Report: A detailed summary of validation results, conclusions, and recommendations for the use of the validated method in routine analysis.
- -
- Conclusion: Validation confirms that the HPLC method is fit for purpose, providing reliable and accurate results for its intended application. It ensures confidence in the method's performance and compliance with regulatory standards.
Summary of Methodology Enhancement
Method Validation Essentials
Regulatory Compliance and Quality Assurance
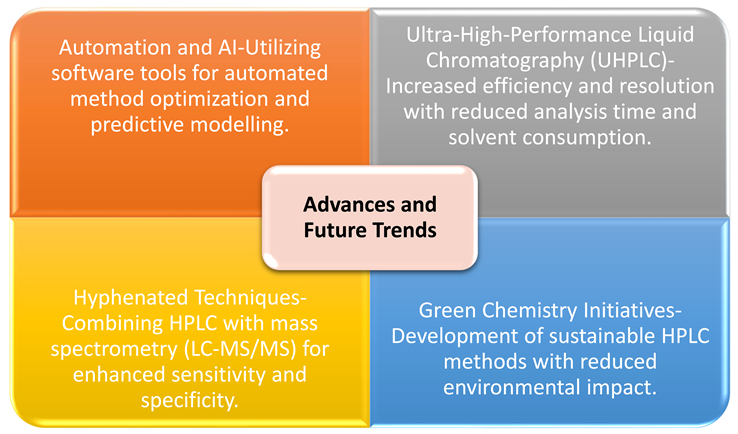
Progress and Emerging Pathways
Prospective Vision
Conclusions
Acknowledgments
References
- Snyder, L. R. , Kirkland, J. J., & Dolan, J. W. (2010). Introduction to Modern Liquid Chromatography. John Wiley & Sons.
- Skoog, D. A. , West, D. M., Holler, F. J., & Crouch, S. R. (2013). Fundamentals of Analytical Chemistry. Cengage Learning.
- Ahuja, S. , & Dong, M. (2006). Handbook of Pharmaceutical Analysis by HPLC. Elsevier.
- Kazakevich, Y. V. , & LoBrutto, R. (2007). HPLC for Pharmaceutical Scientists. Wiley-Interscience.
- Tiwari, S. , & Talreja, S. (). Thin Layer Chromatography (TLC) VS. Paper Chromatography: A Review. Acta Scientific Pharmaceutical Sciences (ISSN: 2581-5423).
- International Conference on Harmonisation (ICH). (2005). Validation of Analytical Procedures: Text and Methodology Q2(R1).
- United States Pharmacopeia (USP). (2019). General Chapter Validation of Compendial Procedures.
- Swartz, M. E. , & Krull, I. S. (2017). Analytical Method Development and Validation. CRC Press.
- Talreja, S. , & Tiwari, S. From One to Millions: The Revolution of Combinatorial Chemistry. Journal of Analytical Techniques and Research 2024, 6, 37–42. [Google Scholar]
- European Pharmacopoeia (Ph. Eur.). (2020). Chapter 2.2.46. Chromatographic Techniques.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




