1. Introduction
Acute ischemic stroke (AIS) caused by large-vessel occlusion (LVO) is a significant cause of morbidity and mortality worldwide. Timely and effective intervention is critical to minimizing brain damage and improving patient outcomes. LVOs, characterized by blockages in the proximal intracranial vessels, account for approximately 24% to 46% of all acute ischemic strokes [
1]. While ischemic changes can occur within minutes, the extent of infarcted tissue is primarily influenced by the severity and duration of hypoperfusion, with collateral circulation playing a crucial role in stroke progression.
Due to advancements in neuroimaging and interventional devices, mechanical thrombectomy (MT) has become the standard treatment for AIS-LVO, allowing for the removal of the obstructing clot from the affected artery. Current guidelines advocate for intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rtPA) within 4.5 hours of symptom onset as the first-line therapy for eligible patients, significantly increasing the likelihood of a favorable outcome.
Despite its efficacy, the use of IVT prior to MT (bridging therapy, BT) in clinical practice raises concerns. BT may delay the initiation of MT and introduce additional risks, such as distal embolization and hemorrhagic transformation, potentially compromising reperfusion rates and complicating the procedure. Each hour of delay in starting MT reduces the chance of achieving functional independence by 5.3%, raising the question of whether omitting IVT in cases of AIS-LVO could optimize treatment workflows and improve clinical outcomes [
2]. Furthermore, individual patient characteristics are crucial in guiding treatment decisions, and tailoring therapies based on these variables may predict outcomes and mitigate unnecessary risks associated with IVT.
Several randomized controlled trials (RCTs) have provided conflicting evidence regarding the non-inferiority of direct mechanical thrombectomy (d-MT) compared to BT. However, these studies have exclusively focused on patients treated at 'mothership' hospitals, where all interventions are provided at a single comprehensive stroke center (CSC). To date, no RCTs have investigated the efficacy and safety of BT within the 'drip-and-ship' model, where IVT is administered at a primary stroke center (PSC) before transferring the patient to a thrombectomy-capable center, leaving uncertainty about the efficacy and safety of IVT in this context. Current guidelines recommend IVT before MT for eligible patients who are directly admitted to CSCs within 4.5 hours of symptom onset. However, there is limited evidence supporting the use of IVT in AIS-LVO patients arriving at PSCs without endovascular facilities, highlighting the need for further research to determine the optimal treatment approach for these patients.
In this study, we aimed to evaluate the efficacy and safety of IVT before MT compared to d-MT within 6 hours of symptom onset in patients with AIS-LVO who presented to our PSC, thereby contributing to the ongoing clinical debate.
2. Materials and Methods
Study Design and Patient Population
We conducted a retrospective study using data from our prospective Transzlációs Idegtudományi Nemzeti Laboratórium (TINL) STROKE-registry. This registry includes comprehensive demographic and clinical data such as age, sex, chronic comorbidities, cardiovascular risk factors, previous medications, premorbid functional status, imaging parameters, stroke characteristics, and functional and procedural outcomes.
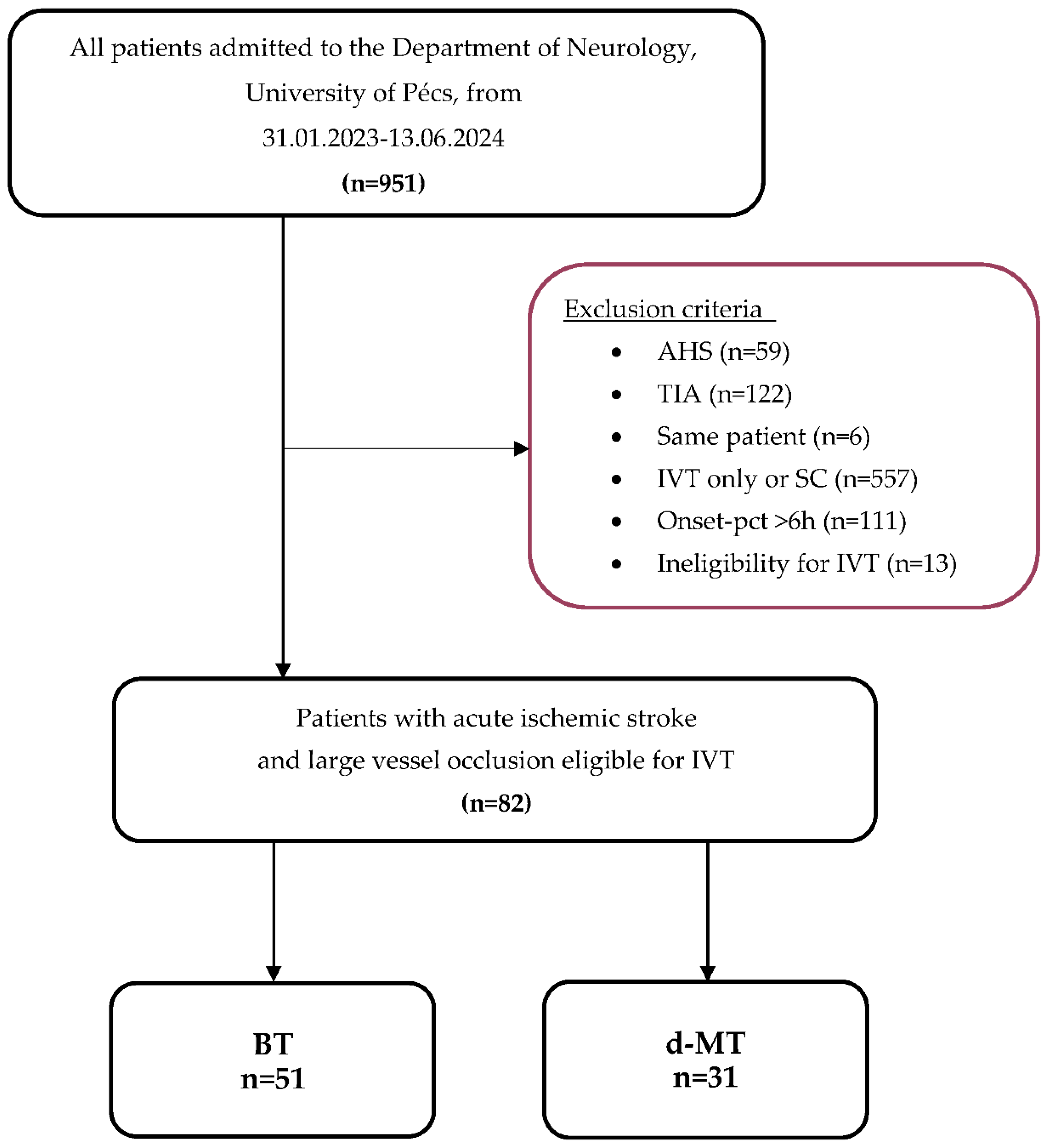
From February 2023 to June 2024, 951 consecutive adult patients were admitted to the Department of Neurology, University of Pécs. A total of 856 patients were excluded for the following reasons: acute hemorrhagic stroke (AHS) (n=59), transient ischemic attack (TIA) (n=122), treatment with only IVT or supportive care (SC) (n=557), onset-to-puncture time exceeding 6 hours (n=111), and absolute contraindications for IVT (n=13). Ultimately, 82 patients were included in the study and were divided into the BT group (n=51) and the d-MT group (n=31).
Inclusion criteria were: (1) acute ischemic stroke caused by large vessel occlusion, (2) triaged at the primary stroke center using multimodal CT imaging, including non-contrast computed tomography (NCCT), CT angiography (CTA), or CT perfusion (CTP), immediately after clinical evaluation, (3) occlusion located in the intracranial internal carotid artery (ICA), middle cerebral artery (M1, M2, and proximal M3 segments), the A1 segment of the anterior cerebral artery (ACA), or the P1 segment of the posterior cerebral artery (PCA), and (4) subsequent mechanical thrombectomy within 6 hours of symptom onset. The flow chart is shown in
Figure 1.
Data Collection and Measurements
Brain and vessel imaging findings included early ischemic signs assessed using the manual Alberta Stroke Program Early Computed Tomography Score (mASPECTS), the occlusion site, and the graded multiphase computed tomography angiography (mCTA) collateral score, evaluated by a radiologist or neuroradiologist. The stroke mechanism was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria. Treatment times were recorded for the intervals from onset to admission, door-to-needle, and door-to-puncture. Stroke severity was assessed by a stroke neurologist using the National Institute of Health Stroke Scale (NIHSS) score at admission and 72 hours post-treatment.
Final treatment decisions were made case-by-case at the discretion of the neurologist and neurointerventionalist on duty. The primary reasons for not administering IVT to eligible patients included exclusion criteria from previous guidelines, which are no longer present in current guidelines, such as a time window exceeding 4.5 hours (n=2), or relative contraindications like early CT signs of ischemia (n=7), known malignancy (n=6), or being near the 4.5-hour time window (n=4). All contraindications are detailed in
Table 1.
Patients in the BT group received rtPA at a dose of 0.9 mg/kg of body weight within 4.5 hours of symptom onset, in line with national and international stroke guidelines. Standard laboratory and clinical inclusion and exclusion criteria for IVT were applied. IVT was initiated in the CT suite immediately after confirming the absence of bleeding. MT was performed following digital subtraction angiography (DSA) via a femoral artery approach, under either general anesthesia or conscious sedation, by board-certified interventional neuroradiologists. The choice of thrombectomy device, including aspiration techniques and stent retrievers, was left to the operator’s discretion.
Recanalization success was assessed by a neurointerventionalist using the modified Thrombolysis in Cerebral Infarction (mTICI) scale, categorized as 2a (partial filling <50%), 2b (partial filling ≥50%), and 3 (complete perfusion). Repeated CT imaging was performed twenty-four hours post-treatment or in the event of deterioration to detect hemorrhagic transformation (HT). The type of intracerebral hemorrhage (ICH) was classified during hospitalization according to the European Cooperative Acute Stroke Study (ECASS) classification as hemorrhagic infarction (HI) and parenchymal hemorrhage (PH) or subarachnoid hemorrhage (SAH). Functional outcomes were evaluated using the modified Rankin Scale (mRS) through telephone interviews conducted 90 days after the intervention.
Outcome Parameters
The primary endpoint of this study was functional independence at 90 days, defined as a mRS score of 0-2, assessed by a physician or a trained and certified neurology nurse. Secondary endpoints included clinical improvement at 72 hours, defined as a NIHSS score of ≤1 or an improvement from baseline (ΔNIHSS) of ≥4, and successful recanalization, defined as a mTICI score of ≥2b. Safety outcomes were evaluated based on thrombus migration and ICH. Missing NIHSS scores were retrospectively scored, and an mRS score of 0–5 at 30 days was considered missing.
Statistical Analyses
Data were analyzed using the Statistical Product and Service Solutions (SPSS) program (version 23). Independent continuous variables were assessed for normality using both descriptive and analytical criteria. Baseline characteristics were summarized using descriptive statistics. Continuous variables were described as mean ± standard deviation (SD) or median (with interquartile range [IQR]) values and compared using the Mann-Whitney-U test. Categorical variables were displayed as numbers, frequencies, or percentages and compared using Fisher’s exact test.
Differences between groups in demographic, clinical, imaging, and procedural characteristics were evaluated using Fisher's exact test for binary data. Multivariate linear and logistic regression analysis were conducted to evaluate predictors of functional and procedural outcomes, adjusting for baseline variables such as age, pre-mRS score, admission NIHSS score, mASPECTS, and mCTA collateral score. Additionally, logistic regression was performed to quantify the association of BT with mRS score when treated as a continuous variable to capture more subtle associations.
All statistical tests were two-tailed, and odds ratios (OR) with 95% confidence intervals (CI) were reported. A p-value of < 0.05 was considered statistically significant.
3. Results
Demographic and Clinical Characteristics
Eighty-two consecutive patients with AIS due to LVO were retrospectively reviewed. Among these, 51 patients (62.2%) received BT (47.1% male, median age 67 years [IQR, 33-89]), and 31 patients (37.8%) underwent d-MT who would have qualified for BT (35.5% male, median age 72 years [IQR, 44-93]). Clinical characteristics included a pre-mRS score of 0 (IQR, 0-5) for the BT group and 0 (IQR, 0-4) for the d-MT group, and an NIHSS score of 10 (IQR, 1-36) for the BT group and 9 (IQR, 0-39) for the d-MT group. Detailed demographic and clinical characteristics are presented in
Table 2.
Imaging and Stroke Characteristics
There was no significant difference in imaging characteristics between the groups, except for a higher median mASPECTS in the BT group (9 [IQR, 5-10]) compared to the d-MT group (8 [IQR, 5-10], p=0.027). Multivariate linear regression analysis revealed mCTA collateral score (p=0.032), plasma sodium level (p=0.008), and C-reactive protein (CRP) (p=0.028) as significant predictors of mASPECTS. Collateral scores showed only numerical differences; excellent: 4-5 (72.0% vs. 65.4%), good: 2-3 (26.0% vs. 34.6%), and poor: 0-1 (2.0% vs. 0.0%). Significant predictors of an excellent mCTA collateral score included mASPECTS (p=0.032), admission NIHSS score (p=0.035), plasma glucose (p=0.034), and sodium level (p=0.023). Median door-to-needle time for the BT group was 43 minutes (IQR, 25-195), while median door-to-puncture time was 126 minutes (IQR, 69-290) for BT and 112 minutes (IQR, 30-293) for d-MT. Detailed imaging and stroke characteristics are summarized in
Table 3.
Functional and Procedural Outcomes and Predictors
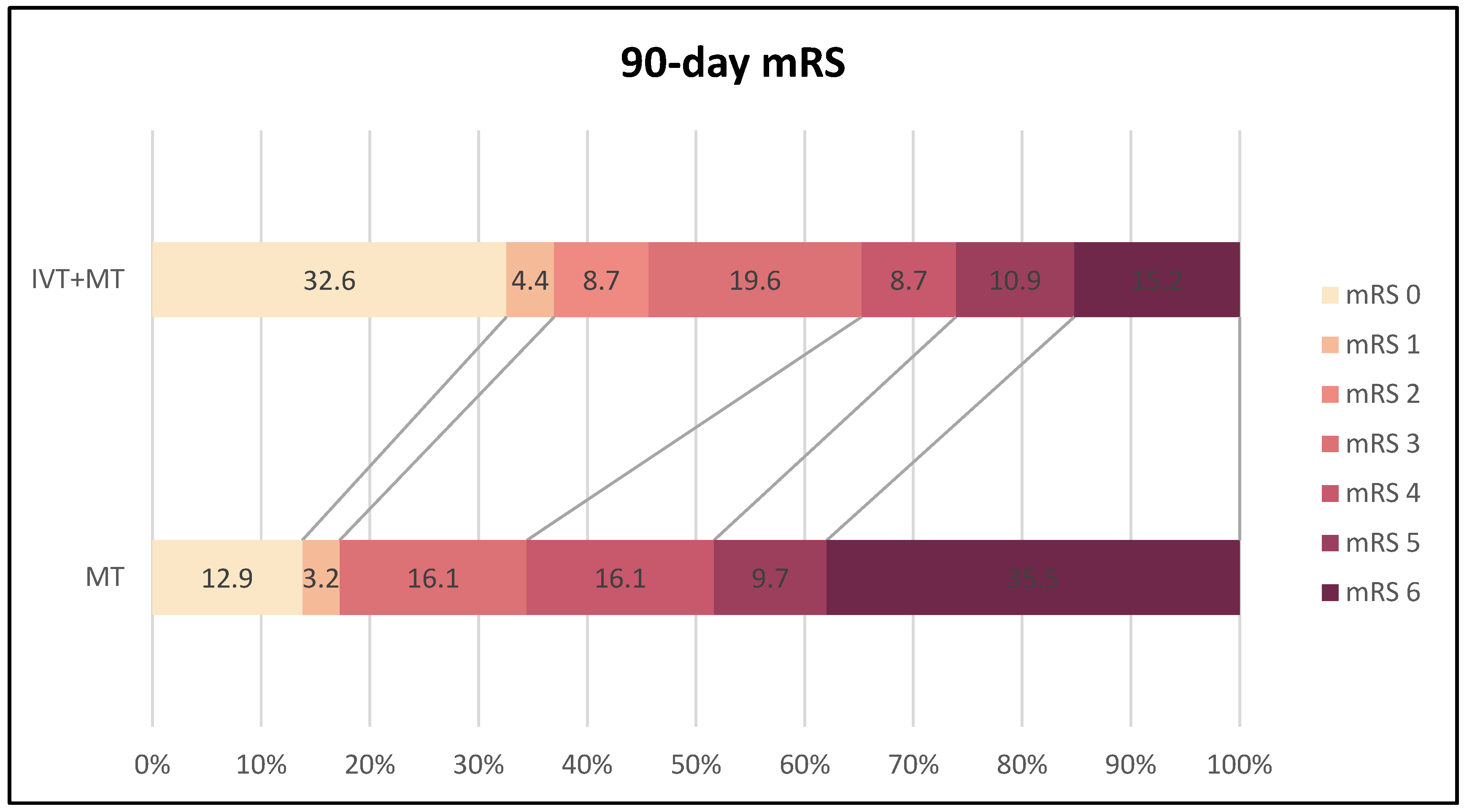
At the 90-day follow-up, a significantly higher proportion of patients in the BT group (n=46) achieved functional independence (mRS 0-2) compared to the d-MT group (n=29) (45.7% vs. 17.2%, p=0.014). Multivariate linear regression analysis identified BT (p=0.011), pre-mRS score (p=0.029), baseline NIHSS score (p=0.015), 72-hour NIHSS score (p<0.001), age (p=0.001), mASPECTS (p=0.042), and history of dyslipidemia (p=0.001) as significant predictors of favorable functional outcome. The 90-day mortality rate was significantly lower in the BT group (13.7% vs. 35.5%, p=0.029). Protective factors against death within the first three months included BT (p=0.021), higher mASPECTS (p=0.002), and better collateral status (p=0.010). In contrast, higher pre-mRS score (p=0.028), older age (p=0.004), higher NIHSS score at admission (p=0.003) and after 72 hours (p<0.001), and hyperglycemia (p=0.032) were associated with increased mortality. Logistic regression analysis indicated that preceding IVT was independently associated with a favorable functional outcome and lower mortality at 90 days, reducing the odds of mRS scores >2 by 74% (p=0.019) and death by 70.4% (p=0.028) compared to d-MT.
Figure 2 illustrates the distribution of 90-day mRS scores in both treatment groups.
The BT group showed a trend towards lower NIHSS scores at 72 hours, with a median score of 3 compared to 9 in the d-MT group (p=0.095). However, statistical significance was not reached, likely due to the small sample size. Multiple linear regression analysis revealed that better neurological outcomes at 72 hours were significantly associated with admission NIHSS score (p<0.001), mASPECTS (p<0.001), collateral status (p=0.014), number of passes during MT (p=0.019), history of dyslipidemia (p=0.001), and plasma sodium levels (p=0.047). Additionally, there was a trend towards better outcomes with preceding IVT (p=0.090) and successful recanalization (p=0.080).
There was no significant difference in clinical improvement at 72 hours between the BT and d-MT groups (NIHSS score of 0 or 1: 33.3% vs. 16.1%, p=0.124; ΔNIHSS ≥4: 45.1% vs. 29.0%, p=0.169).
The rates of successful recanalization, as determined by mTICI grading scales, were comparable between the BT and d-MT groups (86.3% vs. 93.6%, p=0.472). There was a numerical difference in thrombus migration and the incidence of ICH between the two groups, with the BT group showing a higher frequency of embolization (17.7% vs. 9.7%, p=0.521) but a lower rate of ICH (3.9% vs. 12.9%, p=0.193) compared to the d-MT group. Notably, both ICH events in the BT group were asymptomatic (aICH), whereas 2 out of 4 ICH in the d-BT group were symptomatic (sICH). Detailed functional and procedural outcomes and their predictors are summarized in
Table 4 and
Table 5.
4. Discussion
In this study, we evaluated the benefits and risks associated with administering IVT prior to MT. By excluding patients who were not eligible for IVT, our linear and logistic regression analyses confirmed a positive predictive association between IVT administration and favorable functional outcome and reduced mortality at 90 days, without an increased risk of thrombus migration or near-term hemorrhagic complications.
Current Knowledge on Bridging Therapy vs. Direct Mechanical Thrombectomy
The optimal treatment approach for patients with AIS due to LVO remains a subject of debate. Recent RCTs and meta-analyses have yielded mixed evidence regarding the efficacy of BT compared to d-MT.
Early trials, such as the DIRECT-MT and the DEVT trials in China, demonstrated the non-inferiority of d-MT compared to BT with alteplase in IVT-eligible patients [
3,
4]. However, these studies were limited by generous non-inferiority margins and extended door-to-IVT times [
5]. Similarly, the SKIP trial conducted in Asia failed to show the non-inferiority of d-MT compared to BT [
6].
The MR CLEAN-NO IV trial aimed to assess the superiority of d-MT over BT but found no significant difference in functional outcomes at 90 days, neither in terms of superiority or non-inferiority [
7]. The SWIFT-DIRECT and DIRECT-SAFE trials evaluated the non-inferiority of d-MT for ICA and M1 occlusions [
8,
9]. The DIRECT-SAFE trial uniquely included M2 and basilar artery occlusions,[
9] making it the only one of the six RCTs to address posterior circulation LVOs.
A data analysis of over 2,300 patients across the cited RCTs did not confirm the non-inferiority of MT alone compared to bridging therapy based on the predefined non-inferiority margin of 1.3% [
10]. As a result, the latest guidelines recommend IVT preceding MT over d-MT for AIS patients with anterior LVO within 4.5 hours of symptom onset who are directly admitted to a thrombectomy-capable center [
10].
This recommendation contrasts with findings from two major meta-analyses of observational studies, which suggested that BT is associated with higher rates of successful recanalization, better functional outcomes, and lower 90-day mortality without an increased risk of sICH [
11,
12]. Nonetheless, these results should be interpreted cautiously, as many d-MT patients were ineligible for IVT, potentially placing them at higher risk for unfavorable outcomes and hemorrhagic complications. Observational data from two small single-center studies involving patients eligible for BT but treated with MT alone indicate that d-MT is equally effective when patients receive immediate treatment at a stroke center with rapid access to interventional procedures [
13,
14].
The six recent RCTs investigating the impact of IVT with alteplase before MT focused exclusively on 'mothership' patients with anterior circulation LVOs who were eligible for both treatments, with IVT administered within 4.5 hours of stroke onset. These findings should not be generalized to patients receiving IVT at other centers ('drip-and-ship,' 'drip-and-drive,' or 'drip-and-fly') [
5].
Systematic reviews and meta-analyses of observational data support the current guideline recommending BT for all IVT-eligible LVO 'drip-and-ship' patients [
15,
16]. Although the quality of evidence supporting the recommendation to withhold IVT in MT-eligible patients arriving at PSCs without thrombectomy facilities is low, no RCTs address this specific question, and such studies are unlikely to be conducted due to the lack of support from trials involving patients directly admitted to thrombectomy-capable centers.[
5] However, direct access to MT is limited to a minority of LVO-patients [
17], and withholding IVT at PSCs may result in the denial of reperfusion therapy for some patients, especially those who reach CSCs outside the time window for endovascular therapies or those with unsuccessful MT [
18,
19].
Study Findings
A subgroup analysis of the SELECT cohort study revealed that patients with LVO treated with BT at PSCs before being transferred to MT-capable centers showed higher rates of excellent functional outcomes (mRS 0-1) compared to IVT-eligible patients receiving d-MT [
20]. Our study produced similar results, showing significantly higher rates of good functional outcome and lower 90-day mortality in the BT group compared to the d-MT group, contrasting with the findings of previous studies by Broeg-Morvay
et al. and Weber
et al. [
13,
14]. The sustained beneficial effect of IVT beyond the critical event of recanalization during thrombectomy may be attributed to the prolonged pharmacologic impact of rtPA on cerebral microcirculation. A randomized placebo-controlled clinical trial involving 121 LVO-patients found that intra-arterial administration of rtPA after thrombectomy with successful reperfusion increased the likelihood of achieving an excellent functional outcome at 90 days [
21].
Our study observed a trend toward lower median NIHSS scores in patients with BT at 72 hours post-treatment. This contrasts with Broeg-Morvay
et al., who reported a trend toward neurological improvement in NIHSS scores from baseline at 90 days in the d-MT group [
13]. One possible explanation could be that early recovery trends may change when observed over a longer time period.
A 2021 multicenter retrospective cohort study found significantly higher rates of successful recanalization in IVT-eligible d-MT patients compared to BT patients (92.0% vs. 81.9%) [
11]. Other studies have also shown a trend towards mTICI 2b in d-MT patients eligible for both treatments [
22,
23]. In contrast, Broeg-Morvay
et al. reported no significant difference in recanalization rates between the groups [
13]. Similarly, our study demonstrated comparable rates of successful recanalization in both BT and d-MT patients, which may reflect consistent procedural standards and similar patient characteristics within our cohort.
Various studies have reported different incidences of ICH following BT and d-MT. Broeg-Morvay
et al. reported higher aICH rates in the BT group [
13], while Kurminas
et al. and Kolahchi
et al. observed significantly higher sICH incidences in BT patients [
24,
25], contrasting with Kass-Hout
et al., who found no difference in hemorrhagic complications between the groups [
26]. In our study, the d-MT group had numerically higher ICH rates, particularly sICH. This increased rate could be related to anticoagulation therapy, as d-MT patients were more likely to be anticoagulated on admission (16.1% vs. 7.8%, p=0.288). However, patients in both groups were not under the effect of anticoagulants during MT, and there is no current evidence suggesting an association between anticoagulation and bleeding in d-MT patients.
Advantages and Disadvantages of Bridging Therapy
There are several considerations in favor of administering IVT before MT. Pretreatment with IVT can alter the composition of the clot, making it more susceptible to MT, potentially reducing the number of stent retriever passes required [
27,
28,
29,
30], and even achieving early reperfusion, which could negate the need for MT altogether [
31]. Moreover, IVT may address distal microemboli that are difficult to reach with MT or residual occlusions after MT, benefiting patients where MT is delayed, not feasible, or insufficient to achieve complete reperfusion [
32].
Conversely, the primary theoretical benefits of withholding IVT for LVO patients revolve around concerns about efficacy and safety. Evidence suggests that IVT is ineffective in the majority of LVO patients, with recanalization rates as low as 4-20% [
33,
34], and often does not result in significant symptom improvement [
35]. Safety concerns include the risk of bleeding and embolization associated with IVT [
31]. Additionally, logistic delays in transferring patients to a CSC and economic considerations must be taken into account [
36,
37].
Predictors for Administering IVT before MT
Recent studies suggest that baseline variables could guide the decision to administer IVT before MT, with factors such as stroke severity, mASPECTS, and collateral status influencing the potential benefit [
38]. For example, the limited efficacy of IVT in patients with higher NIHSS scores is likely due to the correlation between elevated NIHSS scores and major vessel occlusion, as IVT is three times more effective for M2 than ICA occlusions [
39]. Contrasting findings, such as the ETIS study, reported higher functional independence at 90 days, better early neurologic improvement, and successful reperfusion in BT patients with large infarct cores (mASPECTS 0-5), suggesting even patients with substantial initial infarct burden might benefit from BT [
40]. However, whether these characteristics reliably predict the harm or benefit of IVT in MT-eligible patients remains uncertain, underlining the need for ongoing research to refine treatment protocols and optimize outcomes for AIS-LVO patients.
Limitations
This study has several limitations, including a relatively small sample size from a single center and the non-randomized nature of the comparison, which introduces potential bias. Our observation of improved functional outcomes following BT was derived from a retrospective analysis of a prospective registry with partially imbalanced groups. Despite efforts to adjust for baseline differences, unmeasured confounding variables may still influence the outcomes. Treatment allocation was based on clinical judgment, leading to potential confounding by indication, which could skew the results toward a poorer prognosis.
5. Conclusions
In conclusion, our study findings indicate that BT is independently associated with improved functional outcomes and reduced 90-day mortality rates compared to d-MT. The lack of significant differences in recanalization success or safety measures between the groups implies that the benefits of BT are likely due to the additional therapeutic effects of IVT administered prior to MT. These results align with current guidelines recommending BT for IVT-eligible AIS-LVO patients undergoing MT within 6 hours of symptom onset. Considering our findings and previous studies, further research in the form of RCTs is necessary to determine whether d-MT could be as effective as BT in specific patient populations. Such research could refine treatment protocols and enhance decision-making in the management of acute ischemic stroke.
Author Contributions
Conceptualization, J.S. and B.C.; methodology, J.S. and L.Z.; validation, Z.K. and E.B.; formal analysis, J.S.; data curation, J.S.; writing – original draft preparation, J.S. and B.C.; writing – review and editing, Z.K. and E.B.; visualization, J.S.; supervision, L.Z.; project administration, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Scientific and Research Ethics Committee of the Medical Research Council of the University of Pécs (RRF-2.3.1-21-2022-00011).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
List of abbreviations
| IVT |
intravenous thrombolysis |
| MT |
mechanical thrombectomy |
| AIS |
acute ischemic stroke |
| LVO |
large vessel occlusion |
| BT |
bridging therapy |
| d-MT |
direct mechanical thrombectomy |
| TINL |
Transzlációs Idegtudományi Nemzeti Laboratórium |
| mRS |
modified Rankin Scale |
| NIHSS |
National Institute of Health Stroke Scale |
| mTICI |
modified Thrombolysis in Cerebral Infarction |
| rtPA |
recombinant tissue plasminogen activator |
| RCT |
randomized controlled trial |
| CSC |
comprehensive stroke center |
| PSC |
primary stroke center |
| AHS |
acute hemorrhagic stroke |
| TIA |
transient ischemic attack |
| SC |
standard care |
| NCCT |
non-contrast computed tomography |
| CTA |
CT angiography |
| CTP |
CT perfusion |
| ICA |
internal carotid artery |
| ACA |
anterior cerebral artery |
| PCA |
posterior cerebral artery |
| mASPECTS |
manual Alberta Stroke Program Early CT Score |
| mCTA |
multiphase computed tomography angiography |
| TOAST |
Trial of Org 10172 in Acute Stroke Treatment |
| DSA |
digital subtraction angiography |
| HT |
hemorrhagic transformation |
| ICH |
intracranial hemorrhage |
| ECASS |
European Cooperative Acute Stroke Study |
| HI |
hemorrhagic infarction |
| PH |
parenchymal hemorrhage |
| SAH |
subarachnoidal hemorrhage |
| SPSS |
Statistical Product and Service Solutions |
| SD |
standard deviation |
| IQR |
interquartile range |
| OR |
odds ratio |
| CI |
confidence interval |
| aICH |
asymptomatic intracranial hemorrhage |
| sICH |
symptomatic intracranial hemorrhage |
| CRP |
C-reactive protein |
References
- Rennert RC, Wali AR, Steinberg JA, et al. Epidemiology, Natural History, and Clinical Presentation of Large Vessel Ischemic Stroke. Neurosurgery. 2019;85:S4–8. [CrossRef]
- Fischer U, Kaesmacher J, Mendes Pereira V, et al. Direct Mechanical Thrombectomy Versus Combined Intravenous and Mechanical Thrombectomy in Large-Artery Anterior Circulation Stroke. Stroke. 2017;48:2912–8. [CrossRef]
- Yang P, Zhang Y, Zhang L, et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. New England Journal of Medicine. 2020;382:1981–93. [CrossRef]
- Zi W, Qiu Z, Li F, et al. Effect of Endovascular Treatment Alone vs Intravenous Alteplase Plus Endovascular Treatment on Functional Independence in Patients With Acute Ischemic Stroke. JAMA. 2021;325:234. [CrossRef]
- Widimsky P, Snyder K, Sulzenko J, et al. Acute ischaemic stroke: recent advances in reperfusion treatment. Eur Heart J. 2023;44:1205–15. [CrossRef]
- Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of Mechanical Thrombectomy Without vs With Intravenous Thrombolysis on Functional Outcome Among Patients With Acute Ischemic Stroke. JAMA. 2021;325:244. [CrossRef]
- LeCouffe NE, Kappelhof M, Treurniet KM, et al. A Randomized Trial of Intravenous Alteplase before Endovascular Treatment for Stroke. New England Journal of Medicine. 2021;385:1833–44. [CrossRef]
- Fischer U, Kaesmacher J, Strbian D, et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. The Lancet. 2022;400:104–15. [CrossRef]
- Mitchell PJ, Yan B, Churilov L, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. The Lancet. 2022;400:116–25. [CrossRef]
- Turc G, Tsivgoulis G, Audebert HJ, et al. European Stroke Organisation – European Society for Minimally Invasive Neurological Therapy expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischaemic stroke and anterior circulation large vessel occlusion. Eur Stroke J. 2022;7:I–XXVI. [CrossRef]
- Wang Y, Wu X, Zhu C, et al. Bridging Thrombolysis Achieved Better Outcomes Than Direct Thrombectomy After Large Vessel Occlusion. Stroke. 2021;52:356–65. [CrossRef]
- Katsanos AH, Malhotra K, Goyal N, et al. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol. 2019;86:395–406. [CrossRef]
- Broeg-Morvay A, Mordasini P, Bernasconi C, et al. Direct Mechanical Intervention Versus Combined Intravenous and Mechanical Intervention in Large Artery Anterior Circulation Stroke. Stroke. 2016;47:1037–44. [CrossRef]
- Weber R, Nordmeyer H, Hadisurya J, et al. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerv Surg. 2017;9:229–33. [CrossRef]
- Du H, Lei H, Ambler G, et al. Intravenous Thrombolysis Before Mechanical Thrombectomy for Acute Ischemic Stroke: A Meta-Analysis. J Am Heart Assoc. 2021;10. [CrossRef]
- Zhang J, Chen S, Shi S, et al. Direct endovascular treatment versus bridging therapy in patients with acute ischemic stroke eligible for intravenous thrombolysis: systematic review and meta-analysis. J Neurointerv Surg. 2022;14:321–5. [CrossRef]
- Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: A survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J. 2019;4:13–28. [CrossRef]
- Southerland AM, Johnston KC, Molina CA, et al. Suspected Large Vessel Occlusion. Stroke. 2016;47:1965–7. [CrossRef]
- Milne MSW, Holodinsky JK, Hill MD, et al. Drip ‘n Ship Versus Mothership for Endovascular Treatment. Stroke. 2017;48:791–4. [CrossRef]
- Sarraj A, Grotta J, Albers GW, et al. Clinical and Neuroimaging Outcomes of Direct Thrombectomy vs Bridging Therapy in Large Vessel Occlusion. Neurology. 2021;96. [CrossRef]
- Renú A, Millán M, San Román L, et al. Effect of Intra-arterial Alteplase vs Placebo Following Successful Thrombectomy on Functional Outcomes in Patients With Large Vessel Occlusion Acute Ischemic Stroke. JAMA. 2022;327:826. [CrossRef]
- Liu M, Li G. Is Direct Endovascular Treatment as an Alternative of Bridging Therapy in Acute Stroke Patients with Large Vessel Occlusion? Journal of Stroke and Cerebrovascular Diseases. 2019;28:531–41. [CrossRef]
- Kaesmacher J, Mordasini P, Arnold M, et al. Direct mechanical thrombectomy in tPA-ineligible and -eligible patients versus the bridging approach: a meta-analysis. J Neurointerv Surg. 2019;11:20–7. [CrossRef]
- Kurminas M, Berūkštis A, Misonis N, et al. Intravenous r-tPA Dose Influence on Outcome after Middle Cerebral Artery Ischemic Stroke Treatment by Mechanical Thrombectomy. Medicina (B Aires). 2020;56:357. [CrossRef]
- Kolahchi Z, Rahimian N, Momtazmanesh S, et al. Direct Mechanical Thrombectomy Versus Prior Bridging Intravenous Thrombolysis in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Life. 2023;13:185. [CrossRef]
- Kass-Hout T, Kass-Hout O, Mokin M, et al. Is Bridging with Intravenous Thrombolysis of Any Benefit in Endovascular Therapy for Acute Ischemic Stroke? World Neurosurg. 2014;82:e453–8. [CrossRef]
- Chandra R V, Leslie-Mazwi TM, Mehta BP, et al. Does the use of IV tPA in the current era of rapid and predictable recanalization by mechanical embolectomy represent good value? J Neurointerv Surg. 2016;8:443–6. [CrossRef]
- Fischer U, Kaesmacher J, Molina CA, et al. Primary Thrombectomy in tPA (Tissue-Type Plasminogen Activator) Eligible Stroke Patients With Proximal Intracranial Occlusions. Stroke. 2018;49:265–9. [CrossRef]
- Angermaier A, Michel P, Khaw A V., et al. Intravenous Thrombolysis and Passes of Thrombectomy as Predictors for Endovascular Revascularization in Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases. 2016;25:2488–95. [CrossRef]
- Guedin P, Larcher A, Decroix J-P, et al. Prior IV Thrombolysis Facilitates Mechanical Thrombectomy in Acute Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases. 2015;24:952–7. [CrossRef]
- Mueller L, Pult F, Meisterernst J, et al. Impact of intravenous thrombolysis on recanalization rates in patients with stroke treated with bridging therapy. Eur J Neurol. 2017;24:1016–21. [CrossRef]
- Rozes C, Maier B, Gory B, et al. Influence of prior intravenous thrombolysis on outcome after failed mechanical thrombectomy: ETIS registry analysis. J Neurointerv Surg. 2022;14:688–92. [CrossRef]
- Bhatia R, Hill MD, Shobha N, et al. Low Rates of Acute Recanalization With Intravenous Recombinant Tissue Plasminogen Activator in Ischemic Stroke. Stroke. 2010;41:2254–8. [CrossRef]
- Seners P, Turc G, Maïer B, et al. Incidence and Predictors of Early Recanalization After Intravenous Thrombolysis. Stroke. 2016;47:2409–12. [CrossRef]
- Rai AT, Carpenter JS, Raghuram K, et al. Endovascular therapy yields significantly superior outcomes for large vessel occlusions compared with intravenous thrombolysis: is it time to randomize? J Neurointerv Surg. 2013;5:430–4. [CrossRef]
- Ospel JM, McDonough R, Kunz WG, et al. Is concurrent intravenous alteplase in patients undergoing endovascular treatment for large vessel occlusion stroke cost-effective even if the cost of alteplase is only US$1? J Neurointerv Surg. 2022;14:568–72. [CrossRef]
- Menon BK, Almekhlafi MA, Pereira VM, et al. Optimal Workflow and Process-Based Performance Measures for Endovascular Therapy in Acute Ischemic Stroke. Stroke. 2014;45:2024–9. [CrossRef]
- Broocks G, Heit JJ, Kuraitis GM, et al. Benefit of Intravenous Alteplase before Thrombectomy Depends on ASPECTS. Ann Neurol. 2022;92:588–95. [CrossRef]
- Anadani M, Marnat G, Consoli A, et al. Endovascular therapy with or without intravenous thrombolysis in acute stroke with tandem occlusion. J Neurointerv Surg. 2022;14:314–20. [CrossRef]
- Derraz I, Moulin S, Gory B, et al. Endovascular Thrombectomy Outcomes with and without Intravenous Thrombolysis for Large Ischemic Cores Identified with CT or MRI. Radiology. 2023;309. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).