Submitted:
22 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
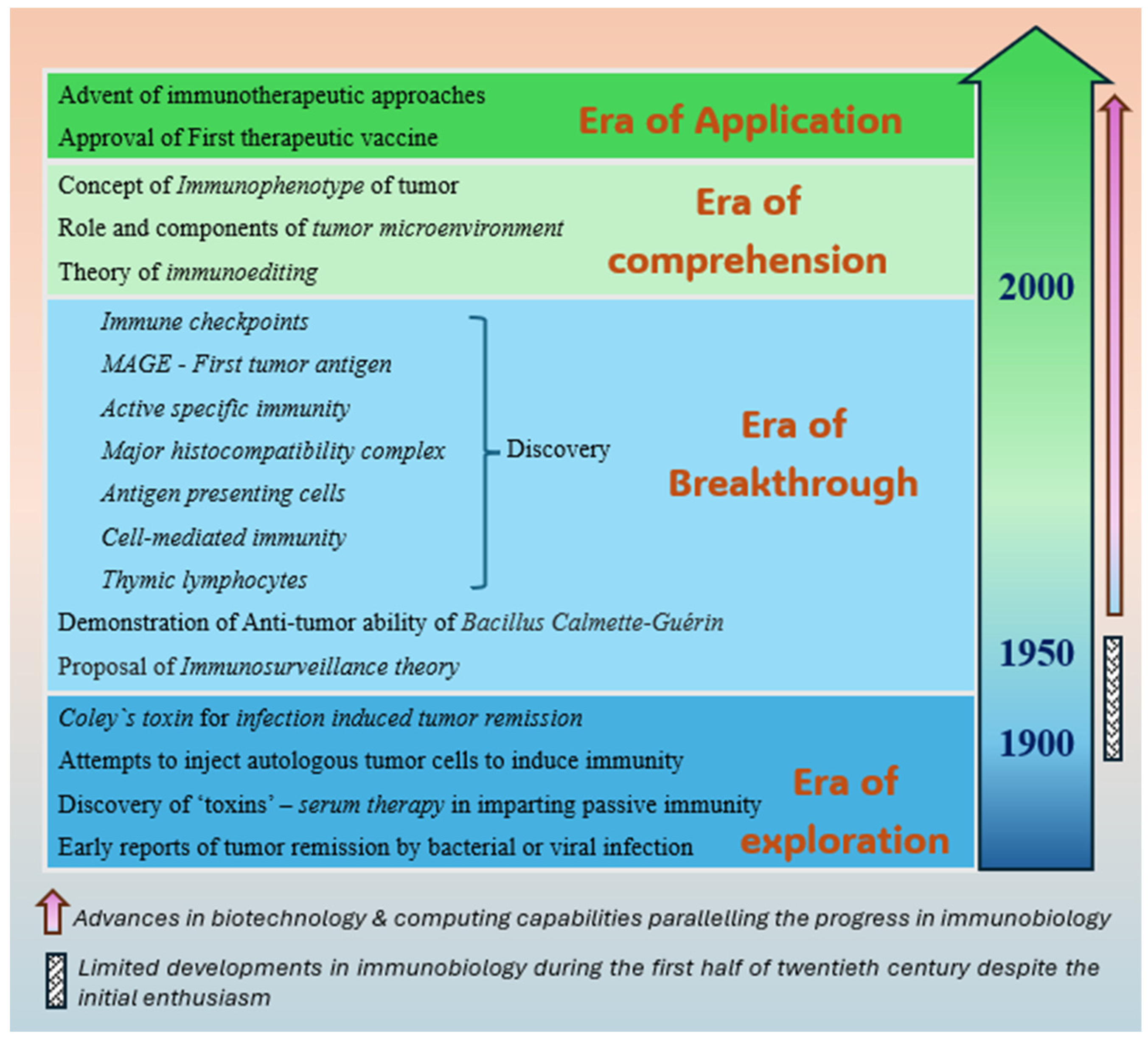
1. Introduction
2. Origin of the Concepts of Immunology at the Turn of Twentieth Century
2.1. First Reports of Infection-Induced Cancer Remission
2.2. Discovery of Antibodies
2.3. Coley's Toxin—The First Immunotherapeutic
2.4. Other Attempts to Induce Anti-Tumor Immunity
3. Advancement of Immunobiology in the Latter Half of the Twentieth Century
3.1. Establishment of a Dedicated Institute for Research on Cancer Immunology
3.2. Genesis of the Theory of Immune Surveillance
3.3. Discovery of T-Lymphocytes—The Dawn of the Era of Immunotherapy
3.4. Discovery of Other Key Apparatus of Immune Mechanisms
3.5. BCG—The First Therapeutic Vaccine with Definitive Indication
3.6. Central Mechanism of Therapeutic Vaccines
4. Explosion of Immunological Concepts—At the Turn of the Twenty-First Century
4.1. Discovery of Immune Tolerance Mechanisms—Checkpoints
4.2. Melacine—The First Therapeutic Vaccine to be Engineered
4.3. Enhanced Ability to Measure the Elicited ASIR
4.4. Isolation of DC
4.5. MAGE—The First Tumor Antigen to be Recognized
4.6. Theory of Immunoediting
4.7. Role of TME in the Process of Immunoediting
4.8. Immunophenotyping of Cancer
4.9. Provenge—The First FDA-Approved Therapeutic Vaccine
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat Rev Immunol 2020, 20, 651–668. [CrossRef]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat Rev Clin Oncol 2021, 18, 215–229. [CrossRef]
- Devaraja, K. Current Prospects of Molecular Therapeutics in Head and Neck Squamous Cell Carcinoma. Pharmaceut Med 2019, 33, 269–289. [CrossRef]
- Devaraja, K.; Aggarwal, S.; Singh, M. Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma-A Review. Vaccines (Basel) 2023, 11, 634. [CrossRef]
- Levine, D.B. The Hospital for the Ruptured and Crippled: William Bradley Coley, Third Surgeon-in-Chief 1925-1933. HSS J 2008, 4, 1–9. [CrossRef]
- Hobohm, U. Fever and Cancer in Perspective. Cancer Immunol Immunother 2001, 50, 391–396. [CrossRef]
- Oiseth, S.J.; Aziz, M.S. Cancer Immunotherapy: A Brief Review of the History, Possibilities, and Challenges Ahead. Journal of Cancer Metastasis and Treatment 2017, 3, 250–261. [CrossRef]
- Suzuki, A.; Abe, S.; Koyama, K.; Suzuki, S.; Nagao, M.; Kobayashi, M.; Nomura, J.; Tsutsumi, T.; Takeda, T.; Oka, Y.; et al. Spontaneous Regression of Blastic Plasmacytoid Dendritic Cell Neoplasm Following Sepsis by Serratia Marcescens: A Case Report and Literature Review. Intern Med 2021, 60, 927–933. [CrossRef]
- Burnet, M. Cancer; a Biological Approach. I. The Processes of Control. Br Med J 1957, 1, 779–786. [CrossRef]
- Thomas, L.; Lawrence, H. Cellular and Humoral Aspects of the Hypersensitive States. New York: Hoeber-Harper 1959, 529–532.
- McCarthy, E.F. The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop J 2006, 26, 154–158.
- Busch, W. Aus Der Sitzung Der Medicinischen Section Vom 13 November 1867. Berl Klin Wochenschr 1868, 5, 137.
- Fehleisen, N. Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Uebertragbarkeit auf den Menschen. Dtsch Med Wochenschr 1882, 8, 553–554. [CrossRef]
- Bruns, P. von Die Heilwirkung Des Erysipels Auf Geschwulste. Beitr Klin Chir 1887, 3, 443–466.
- Loeffler, F. Untersuchungen Über Die Bedeutung Der Mikroorganismen Für Die Entstehung Der Diphtherie Beim Menschen, Bei Der Taube Und Beim Kalbe; 1884;
- Winau, F.; Winau, R. Emil von Behring and Serum Therapy. Microbes Infect 2002, 4, 185–188. [CrossRef]
- Behring, E. von Über Die Ursache Der Immunität von Ratten Gegen Milzbrand. Drucke 19. Jh. 1888.
- Von Behring, E.; Kitasato, S. Uber Das Zustandekommen Der Diphtherie-Immunitat Und Der Tetanus-Immunitat Bei Thieren. Deut. Med. Wochenschr. 1890, 16, 1113–1114.
- Triendl, R. Translational Research in Immunology: Japanese Perspectives. Nat Rev Immunol 2004, 4, 72–76. [CrossRef]
- Winau, F.; Westphal, O.; Winau, R. Paul Ehrlich--in Search of the Magic Bullet. Microbes Infect 2004, 6, 786–789. [CrossRef]
- Ehrlich, P. Die Seitenkettentheorie Und Ihre Gegner. Münchner Med Wochenschr 1901, 52, 2123–2124.
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus Erysipelas and the Bacillus Prodigiosus). Proc R Soc Med 1910, 3, 1–48.
- Coley, W.B. II. Contribution to the Knowledge of Sarcoma. Ann Surg 1891, 14, 199–220. [CrossRef]
- Ehrlich, P. Ueber Den Jetzigen Stand Der Karzinomforschung; 1908;
- Miller, J.F. Immunological Function of the Thymus. Lancet 1961, 2, 748–749. [CrossRef]
- Miller, J.F.; Mitchell, G.F.; Weiss, N.S. Cellular Basis of the Immunological Defects in Thymectomized Mice. Nature 1967, 214, 992–997. [CrossRef]
- Burnet, M. Role of the Thymus and Related Organs in Immunity. Br Med J 1962, 2, 807–811. [CrossRef]
- Claman, H.N.; Chaperon, E.A.; Triplett, R.F. Thymus-Marrow Cell Combinations. Synergism in Antibody Production. Proc Soc Exp Biol Med 1966, 122, 1167–1171. [CrossRef]
- Wagner, H.; Röllinghoff, M.; Nossal, G.J. T-Cell-Mediated Immune Responses Induced in Vitro: A Probe for Allograft and Tumor Immunity. Transplant Rev 1973, 17, 3–36. [CrossRef]
- Carlson, R.D.; Flickinger, J.C.; Snook, A.E. Talkin’ Toxins: From Coley’s to Modern Cancer Immunotherapy. Toxins (Basel) 2020, 12, 241. [CrossRef]
- Currie, G.A. Eighty Years of Immunotherapy: A Review of Immunological Methods Used for the Treatment of Human Cancer. Br J Cancer 1972, 26, 141–153. [CrossRef]
- Von Leyden, V.; Blumenthal, F. Vorläufige Mittheilungen Über Einige Ergebnisse Der Krebsforshung Auf Der 1. Medizinschen Klinik. Dtsch. Med. Wschr. 1902, 28, 637–638.
- RISLEY, E.H. The Gilman–Coca Vaccine Emulsion Treatment of Cancer. The Boston Medical and Surgical Journal 1911, 165, 784–788.
- VAUGHAN, J.W. Cancer Vaccine and Anticancer Globulins as an Aid in the Surgical Treatment of Malignancy. Journal of the American Medical Association 1914, 63, 1258–1265.
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front Immunol 2019, 10, 2965. [CrossRef]
- Dock, G. The Influence of Complicating Diseases upon Leukaemia. The American Journal of the Medical Sciences (1827-1924) 1904, 127, 563.
- Larson, C.; Oronsky, B.; Scicinski, J.; Fanger, G.R.; Stirn, M.; Oronsky, A.; Reid, T.R. Going Viral: A Review of Replication-Selective Oncolytic Adenoviruses. Oncotarget 2015, 6, 19976–19989. [CrossRef]
- Jensen, C. Experimental Studies on Cancer in Mice (from German). Zentralblatt für Bacteriologie, Parasitenkunde und Infektionskrankheiten 1903, 34, 28–34.
- Little, C.C. A POSSIBLE MENDELIAN EXPLANATION FOR A TYPE OF INHERITANCE APPARENTLY NON-MENDELIAN IN NATURE. Science 1914, 40, 904–906. [CrossRef]
- Murphy, J.B.; Morton, J.J. The Lymphocyte as a Factor in Natural and Induced Resistance to Transplanted Cancer. Proc Natl Acad Sci U S A 1915, 1, 435–437. [CrossRef]
- The CRI Timeline Available online: https://www.cancerresearch.org/timeline (accessed on 26 July 2023).
- Everson, T.C.; Cole, W.H. Spontaneous Regression of Malignant Disease. J Am Med Assoc 1959, 169, 1758–1759. [CrossRef]
- Old, L.J.; Clarke, D.A.; Benacerraf, B. Effect of Bacillus Calmette-Guerin Infection on Transplanted Tumours in the Mouse. Nature 1959, 184(Suppl 5), 291–292. [CrossRef]
- Silverstein, A.M. The Curious Case of the 1960 Nobel Prize to Burnet and Medawar. Immunology 2016, 147, 269–274. [CrossRef]
- Liston, A. Immunological Tolerance 50 Years after the Burnet Nobel Prize. Immunol Cell Biol 2011, 89, 14–15. [CrossRef]
- Gowans, J.L.; McGREGOR, D.D.; Cowen, D.M. Initiation of Immune Responses by Small Lymphocytes. Nature 1962, 196, 651–655. [CrossRef]
- Miller, J.F.; Grant, G.A.; Roe, F.J. EFFECT OF THYMECTOMY ON THE INDUCTION OF SKIN TUMOURS BY 3,4-BENZOPYRENE. Nature 1963, 199, 920–922. [CrossRef]
- Miller, J.F. INFLUENCE OF THYMECTOMY ON TUMOR INDUCTION BY POLYOMA VIRUS IN C57BL MICE. Proc Soc Exp Biol Med 1964, 116, 323–327. [CrossRef]
- Miller, J.F. a. P. Events That Led to the Discovery of T-Cell Development and Function--a Personal Recollection. Tissue Antigens 2004, 63, 509–517. [CrossRef]
- Miller, J. The Early Work on the Discovery of the Function of the Thymus, an Interview with Jacques Miller. Cell Death Differ 2020, 27, 396–401. [CrossRef]
- Cerottini, J.C.; Nordin, A.A.; Brunner, K.T. In Vitro Cytotoxic Activity of Thymus Cells Sensitized to Alloantigens. Nature 1970, 227, 72–73. [CrossRef]
- Cerottini, J.C.; Nordin, A.A.; Brunner, K.T. Specific in Vitro Cytotoxicity of Thymus-Derived Lymphocytes Sensitized to Alloantigens. Nature 1970, 228, 1308–1309. [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a Novel Cell Type in Peripheral Lymphoid Organs of Mice. I. Morphology, Quantitation, Tissue Distribution. J Exp Med 1973, 137, 1142–1162. [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [CrossRef]
- Geissmann, F.; Gordon, S.; Hume, D.A.; Mowat, A.M.; Randolph, G.J. Unravelling Mononuclear Phagocyte Heterogeneity. Nat Rev Immunol 2010, 10, 453–460. [CrossRef]
- Zinkernagel, R.M.; Doherty, P.C. Restriction of in Vitro T Cell-Mediated Cytotoxicity in Lymphocytic Choriomeningitis within a Syngeneic or Semiallogeneic System. Nature 1974, 248, 701–702. [CrossRef]
- Zinkernagel, R.M.; Doherty, P.C. Immunological Surveillance against Altered Self Components by Sensitised T Lymphocytes in Lymphocytic Choriomeningitis. Nature 1974, 251, 547–548. [CrossRef]
- Doherty, P.C. Challenged by Complexity: My Twentieth Century in Immunology. Annu Rev Immunol 2007, 25, 1–19. [CrossRef]
- Raju, T.N. The Nobel Chronicles. 1996: Peter Charles Doherty (b 1940) and Rolf M Zinkernagel (b 1944). Lancet 2000, 356, 172. [CrossRef]
- Volchenkov, R.; Sprater, F.; Vogelsang, P.; Appel, S. The 2011 Nobel Prize in Physiology or Medicine. Scand J Immunol 2012, 75, 1–4. [CrossRef]
- Chen, J.; Gao, L.; Wu, X.; Fan, Y.; Liu, M.; Peng, L.; Song, J.; Li, B.; Liu, A.; Bao, F. BCG-Induced Trained Immunity: History, Mechanisms and Potential Applications. J Transl Med 2023, 21, 106. [CrossRef]
- Pearl, R. On the Pathological Relations Between Cancer and Tuberculosis. Proceedings of the Society for Experimental Biology and Medicine 1928, 26, 73–75. [CrossRef]
- Morales, A.; Eidinger, D. Bacillus Calmette-Guerin in the Treatment of Adenocarcinoma of the Kidney. J Urol 1976, 115, 377–380. [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J Urol 1976, 116, 180–183. [CrossRef]
- Hanna, M.G.; Peters, L.C. Specific Immunotherapy of Established Visceral Micrometastases by BCG-Tumor Cell Vaccine Alone or as an Adjunct to Surgery. Cancer 1978, 42, 2613–2625. [CrossRef]
- Grant, R.M.; Mackie, R.; Cochran, A.J.; Murray, E.L.; Hoyle, D.; Ross, C. Results of Administering B.C.G. to Patients with Melanoma. Lancet 1974, 2, 1096–1100. [CrossRef]
- Donaldson, R.C. Chemoimmunotherapy for Cancer of the Head and Neck. Am J Surg 1973, 126, 507–512. [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An Endotoxin-Induced Serum Factor That Causes Necrosis of Tumors. Proc Natl Acad Sci U S A 1975, 72, 3666–3670. [CrossRef]
- Old, L.J. Cancer Immunology. Sci Am 1977, 236, 62–70, 72–73, 76, 79. [CrossRef]
- Ashley, M.P.; Zbar, B.; Hunter, J.T.; Rapp, H.J.; Sugimoto, T. Adjuvant-Antigen Requirements for Active Specific Immunotherapy of Microscopic Metastases Remaining after Surgery. Cancer Res 1980, 40, 4197–4203.
- Sukumar, S.; Hunter, J.T.; Terata, N.; Rapp, H.J. Eradication of Microscopic Hepatic Metastases by Active Specific Immunization. Cancer Immunol Immunother 1983, 14, 151–154. [CrossRef]
- Bier, H.; Armonat, G.; Bier, J.; Schirrmacher, V.; Ganzer, U. Postoperative Active-Specific Immunotherapy of Lymph Node Micrometastasis in a Guinea Pig Tumor Model. ORL J Otorhinolaryngol Relat Spec 1989, 51, 197–205. [CrossRef]
- Morton, D.L.; Foshag, L.J.; Hoon, D.S.; Nizze, J.A.; Famatiga, E.; Wanek, L.A.; Chang, C.; Davtyan, D.G.; Gupta, R.K.; Elashoff, R. Prolongation of Survival in Metastatic Melanoma after Active Specific Immunotherapy with a New Polyvalent Melanoma Vaccine. Ann Surg 1992, 216, 463–482. [CrossRef]
- Böhle, A.; Brandau, S. Immune Mechanisms in Bacillus Calmette-Guerin Immunotherapy for Superficial Bladder Cancer. J Urol 2003, 170, 964–969. [CrossRef]
- de Jong, W.H.; Teppema, J.S.; Wagenaar, S.S.; Paques, M.; Steerenberg, P.A.; Ruitenberg, E.J. Histological Evaluation of Immunologically Mediated Tumor Regression of the Line 10 Guinea Pig Hepatocarcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol 1986, 50, 249–269. [CrossRef]
- Miyazaki, J.; Onozawa, M.; Takaoka, E.; Yano, I. Bacillus Calmette-Guérin Strain Differences as the Basis for Immunotherapies against Bladder Cancer. Int J Urol 2018, 25, 405–413. [CrossRef]
- Klein, E. Lymphocyte-Mediated Lysis of Tumor Cells in Vitro. Antigen-Restricted Clonal and Unrestricted Polyclonal Effects. Springer Semin Immunopathol 1982, 5, 147–159. [CrossRef]
- Stauss, H.J.; Van Waes, C.; Fink, M.A.; Starr, B.; Schreiber, H. Identification of a Unique Tumor Antigen as Rejection Antigen by Molecular Cloning and Gene Transfer. J Exp Med 1986, 164, 1516–1530. [CrossRef]
- Cole, W.H. Efforts to Explain Spontaneous Regression of Cancer. J Surg Oncol 1981, 17, 201–209. [CrossRef]
- Thomas, L. On Immunosurveillance in Human Cancer. Yale J Biol Med 1982, 55, 329–333.
- Burnet, M. Donn. Br Med Bull 1964, 20, 154–158. [CrossRef]
- Ribatti, D. The Concept of Immune Surveillance against Tumors. The First Theories. Oncotarget 2017, 8, 7175–7180. [CrossRef]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A New Member of the Immunoglobulin Superfamily--CTLA-4. Nature 1987, 328, 267–270. [CrossRef]
- Linsley, P.S.; Wallace, P.M.; Johnson, J.; Gibson, M.G.; Greene, J.L.; Ledbetter, J.A.; Singh, C.; Tepper, M.A. Immunosuppression in Vivo by a Soluble Form of the CTLA-4 T Cell Activation Molecule. Science 1992, 257, 792–795. [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc Natl Acad Sci U S A 2002, 99, 12293–12297. [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat Med 2002, 8, 793–800. [CrossRef]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [CrossRef]
- Chen, Y.-S.; Shen, C.-R. Immune Checkpoint Blockade Therapy: The 2014 Tang Prize in Biopharmaceutical Science. Biomed J 2015, 38, 5–8. [CrossRef]
- Huang, P.-W.; Chang, J.W.-C. Immune Checkpoint Inhibitors Win the 2018 Nobel Prize. Biomed J 2019, 42, 299–306. [CrossRef]
- Mitchell, M.S.; Kan-Mitchell, J.; Kempf, R.A.; Harel, W.; Shau, H.Y.; Lind, S. Active Specific Immunotherapy for Melanoma: Phase I Trial of Allogeneic Lysates and a Novel Adjuvant. Cancer Res 1988, 48, 5883–5893.
- Mitchell, M.S.; Harel, W.; Kempf, R.A.; Hu, E.; Kan-Mitchell, J.; Boswell, W.D.; Dean, G.; Stevenson, L. Active-Specific Immunotherapy for Melanoma. J Clin Oncol 1990, 8, 856–869. [CrossRef]
- Mitchell, M.S. Perspective on Allogeneic Melanoma Lysates in Active Specific Immunotherapy. Semin Oncol 1998, 25, 623–635.
- Sondak, V.K.; Sosman, J.A. Results of Clinical Trials with an Allogenic Melanoma Tumor Cell Lysate Vaccine: Melacine. Semin Cancer Biol 2003, 13, 409–415. [CrossRef]
- Sondak, V.K.; Liu, P.-Y.; Tuthill, R.J.; Kempf, R.A.; Unger, J.M.; Sosman, J.A.; Thompson, J.A.; Weiss, G.R.; Redman, B.G.; Jakowatz, J.G.; et al. Adjuvant Immunotherapy of Resected, Intermediate-Thickness, Node-Negative Melanoma with an Allogeneic Tumor Vaccine: Overall Results of a Randomized Trial of the Southwest Oncology Group. J Clin Oncol 2002, 20, 2058–2066. [CrossRef]
- Morton, D.L.; Mozzillo, N.; Thompson, J.F.; Kelley, M.C.; Faries, M.; Wagner, J.; Schneebaum, S.; Schuchter, L.; Gammon, G.; Elashoff, R. An International, Randomized, Phase III Trial of Bacillus Calmette-Guerin (BCG) plus Allogeneic Melanoma Vaccine (MCV) or Placebo after Complete Resection of Melanoma Metastatic to Regional or Distant Sites. JCO 2007, 25, 8508–8508. [CrossRef]
- Apostólico, J. de S.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J Immunol Res 2016, 2016, 1459394. [CrossRef]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. Methods Mol Biol 2022, 2412, 145–178. [CrossRef]
- Czerkinsky, C.C.; Nilsson, L.A.; Nygren, H.; Ouchterlony, O.; Tarkowski, A. A Solid-Phase Enzyme-Linked Immunospot (ELISPOT) Assay for Enumeration of Specific Antibody-Secreting Cells. J Immunol Methods 1983, 65, 109–121. [CrossRef]
- Slota, M.; Lim, J.-B.; Dang, Y.; Disis, M.L. ELISpot for Measuring Human Immune Responses to Vaccines. Expert Rev Vaccines 2011, 10, 299–306. [CrossRef]
- Ranieri, E.; Netti, G.S.; Gigante, M. CTL ELISPOT Assay and T Cell Detection. Methods Mol Biol 2021, 2325, 65–77. [CrossRef]
- Saxena, M.; Bhardwaj, N. Turbocharging Vaccines: Emerging Adjuvants for Dendritic Cell Based Therapeutic Cancer Vaccines. Curr Opin Immunol 2017, 47, 35–43. [CrossRef]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of Large Numbers of Dendritic Cells from Mouse Bone Marrow Cultures Supplemented with Granulocyte/Macrophage Colony-Stimulating Factor. J Exp Med 1992, 176, 1693–1702. [CrossRef]
- Young, J.W.; Szabolcs, P.; Moore, M.A. Identification of Dendritic Cell Colony-Forming Units among Normal Human CD34+ Bone Marrow Progenitors That Are Expanded by c-Kit-Ligand and Yield Pure Dendritic Cell Colonies in the Presence of Granulocyte/Macrophage Colony-Stimulating Factor and Tumor Necrosis Factor Alpha. J Exp Med 1995, 182, 1111–1119. [CrossRef]
- De Plaen, E.; Lurquin, C.; Van Pel, A.; Mariamé, B.; Szikora, J.P.; Wölfel, T.; Sibille, C.; Chomez, P.; Boon, T. Immunogenic (Tum-) Variants of Mouse Tumor P815: Cloning of the Gene of Tum- Antigen P91A and Identification of the Tum- Mutation. Proc Natl Acad Sci U S A 1988, 85, 2274–2278. [CrossRef]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A Gene Encoding an Antigen Recognized by Cytolytic T Lymphocytes on a Human Melanoma. Science 1991, 254, 1643–1647. [CrossRef]
- Boon, T.; van der Bruggen, P. Human Tumor Antigens Recognized by T Lymphocytes. J Exp Med 1996, 183, 725–729. [CrossRef]
- Wang, R.F.; Rosenberg, S.A. Human Tumor Antigens for Cancer Vaccine Development. Immunol Rev 1999, 170, 85–100. [CrossRef]
- Devaraja, K.; Aggarwal, S.; Verma, S.S.; Gupta, S.C. Clinico-Pathological Peculiarities of Human Papilloma Virus Driven Head and Neck Squamous Cell Carcinoma: A Comprehensive Update. Life Sci 2020, 245, 117383. [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour Antigens Recognized by T Lymphocytes: At the Core of Cancer Immunotherapy. Nat Rev Cancer 2014, 14, 135–146. [CrossRef]
- Zarour, H.M.; DeLeo, A.; Finn, O.J.; Storkus, W.J. Categories of Tumor Antigens. Holland-Frei Cancer Medicine. 6th edition 2003.
- Kosaka, A.; Yajima, Y.; Hatayama, M.; Ikuta, K.; Sasaki, T.; Hirai, N.; Yasuda, S.; Nagata, M.; Hayashi, R.; Harabuchi, S.; et al. A Stealth Antigen SPESP1, Which Is Epigenetically Silenced in Tumors, Is a Suitable Target for Cancer Immunotherapy. Cancer Sci 2021, 112, 2705–2713. [CrossRef]
- Yajima, Y.; Kosaka, A.; Ishibashi, K.; Yasuda, S.; Komatsuda, H.; Nagato, T.; Oikawa, K.; Kitada, M.; Takekawa, M.; Kumai, T.; et al. A Tumor Metastasis-Associated Molecule TWIST1 Is a Favorable Target for Cancer Immunotherapy Due to Its Immunogenicity. Cancer Sci 2022, 113, 2526–2535. [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin Cancer Res 2009, 15, 5323–5337. [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat Immunol 2002, 3, 991–998. [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [CrossRef]
- Song, Q.; Zhang, C.-D.; Wu, X.-H. Therapeutic Cancer Vaccines: From Initial Findings to Prospects. Immunol Lett 2018, 196, 11–21. [CrossRef]
- Shibata, H.; Xu, N.; Saito, S.; Zhou, L.; Ozgenc, I.; Webb, J.; Fu, C.; Zolkind, P.; Egloff, A.M.; Uppaluri, R. Integrating CD4+ T Cell Help for Therapeutic Cancer Vaccination in a Preclinical Head and Neck Cancer Model. Oncoimmunology 2021, 10, 1958589. [CrossRef]
- Wei, C.; Ma, Y.; Wang, F.; Liao, Y.; Chen, Y.; Zhao, B.; Zhao, Q.; Wang, D.; Tang, D. Igniting Hope for Tumor Immunotherapy: Promoting the “Hot and Cold” Tumor Transition. Clin Med Insights Oncol 2022, 16, 11795549221120708. [CrossRef]
- Smyth, M.J.; Godfrey, D.I.; Trapani, J.A. A Fresh Look at Tumor Immunosurveillance and Immunotherapy. Nat Immunol 2001, 2, 293–299. [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [CrossRef]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res 2016, 22, 1865–1874. [CrossRef]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical Roles of the Immune System during Cancer Development. Nat Rev Cancer 2006, 6, 24–37. [CrossRef]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.-A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of Cancer-Associated Immune Cells in Human Solid Tumors. Elife 2018, 7, e36967. [CrossRef]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The Immune Phenotype of Tongue Squamous Cell Carcinoma Predicts Early Relapse and Poor Prognosis. Cancer Med 2020, 9, 8333–8344. [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [CrossRef]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated Data from 2 Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trials of Active Cellular Immunotherapy with Sipuleucel-T in Advanced Prostate Cancer. Cancer 2009, 115, 3670–3679. [CrossRef]
- Burch, P.A.; Croghan, G.A.; Gastineau, D.A.; Jones, L.A.; Kaur, J.S.; Kylstra, J.W.; Richardson, R.L.; Valone, F.H.; Vuk-Pavlović, S. Immunotherapy (APC8015, Provenge) Targeting Prostatic Acid Phosphatase Can Induce Durable Remission of Metastatic Androgen-Independent Prostate Cancer: A Phase 2 Trial. Prostate 2004, 60, 197–204. [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med 2010, 363, 411–422. [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin Cancer Res 2011, 17, 3520–3526. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




