Submitted:
23 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Growth and Flowering Traits
2.2. Biomass production and Root Colonization
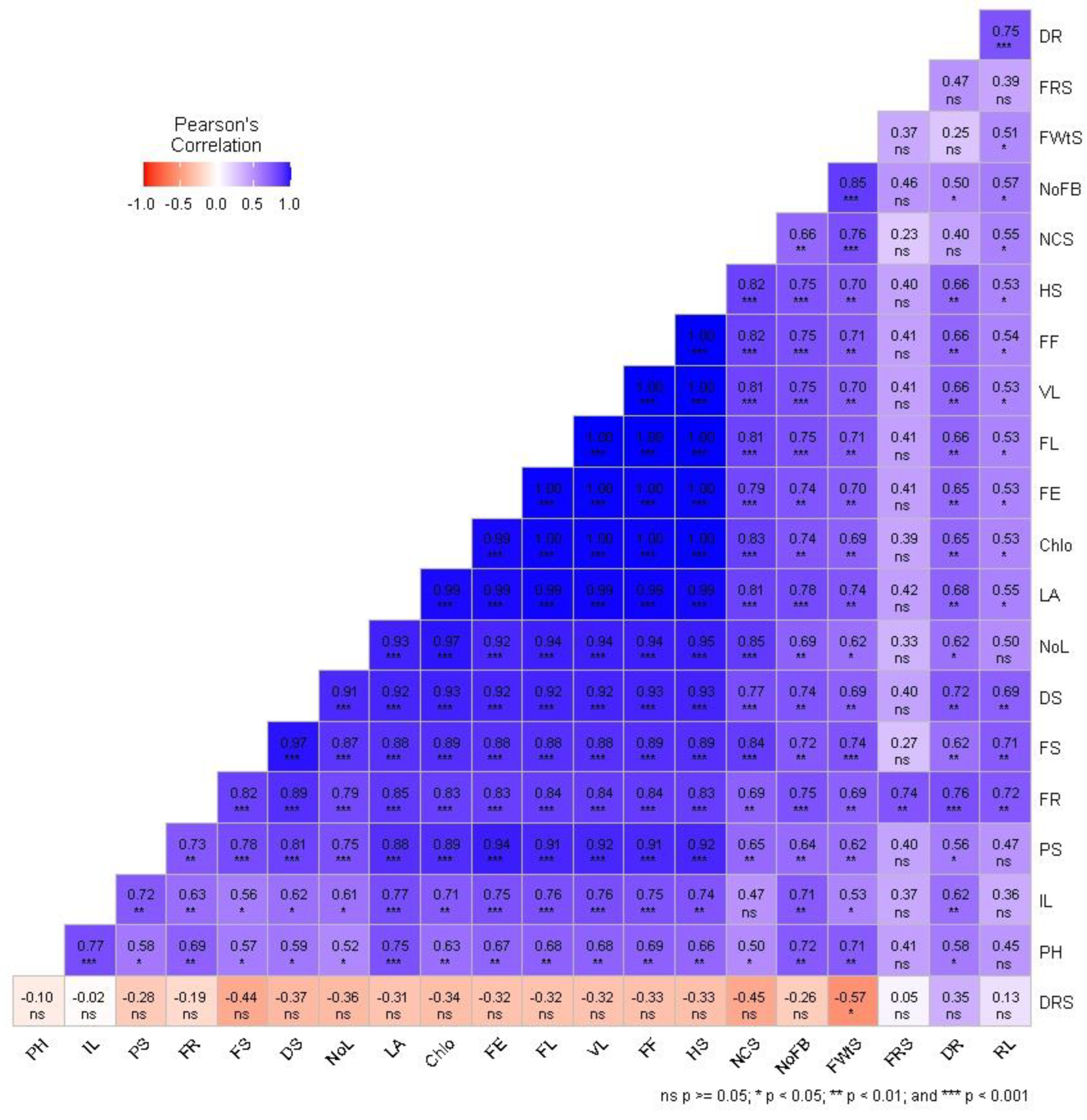
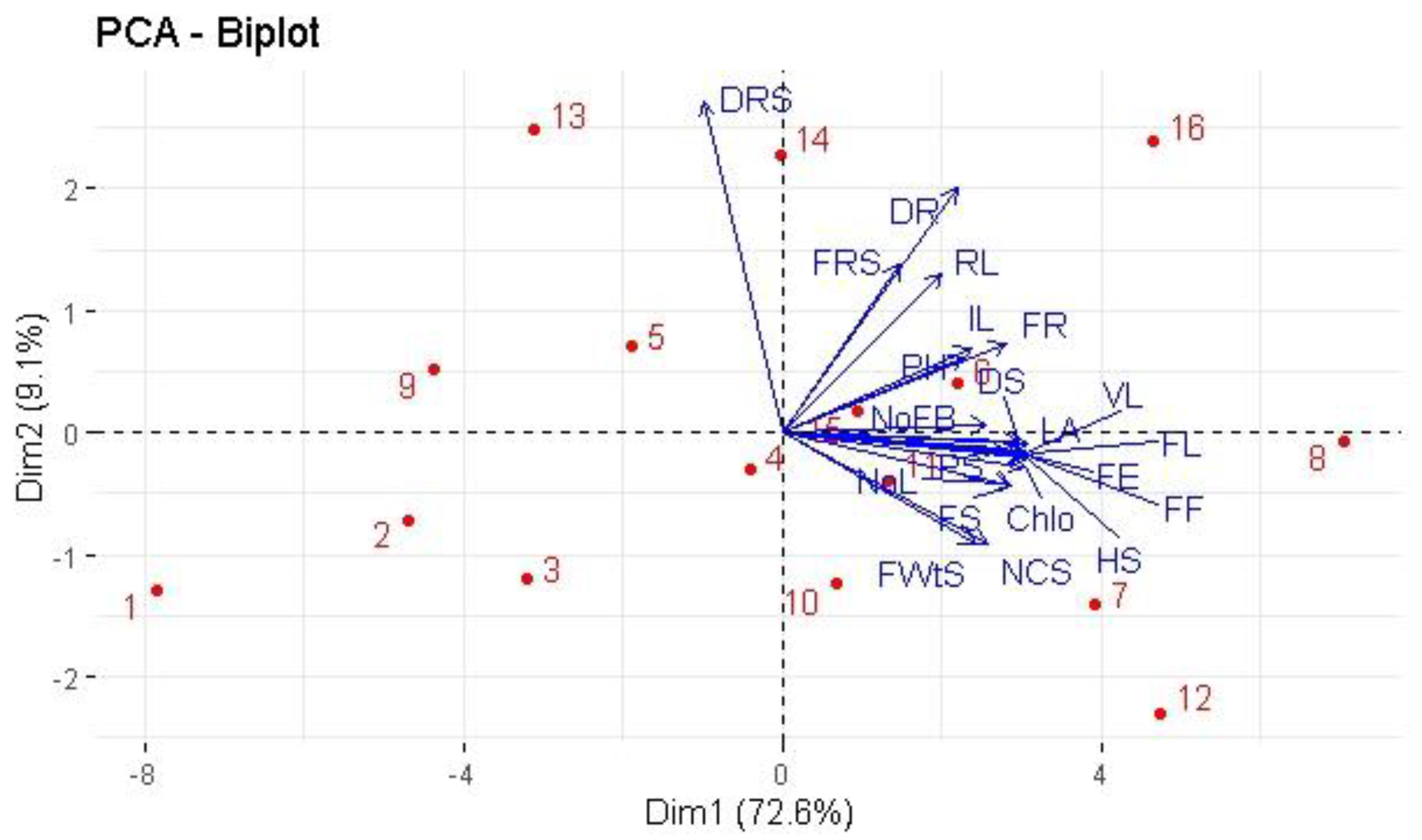
2.3. Correlation and Principal Component Analysis
3. Discussion
3.1. Growth and Vegetative Traits
3.2. Flowering and Quality Traits
3.3. Biomass Production and Root Colonization
4. Materials and Methods
4.1. Experimental Site
4.2. Plant Material
4.3. Experimental Design
4.4. Evaluations of Growth and Flowering Parameters
4.5. Determination of Mycorrhizal Spore Count and Mycorrhizal Colonization of Roots
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whipker, B.E.; Hammer, P.A. Growth and yield characteristics of field-grown Limonium sinuatum (L.). Hortic Sci. 1994, 29, 638-640.
- Kumar, M.R.; Topno, S.E.; Kerketta, A. Effect of crop geometry and age of seedlings on its growth, flower yield and quality of statice (Limonium sinuatum L.) under Prayagraj agro climatic conditions. Int J Environ Clim Change, 2022,12, 700-708.
- Lledo, M.D.; Karis, P.O.; Crespo, M.B.; Fay, M.F.; Chase, M.W. Endemism and evolution in Macaronesian and Mediterranean Limonium taxa, in: Bramwell, D., Caujape-Castells, J. (Eds.), The Biology of Island Floras. Cambridge University Press, 2011; pp. 325-337.
- Grieve, C.M.; Poss, J.A.; Grattan, S.R.; Shouse, P.J.; Lieth, J.H.; Zeng, L. Productivity and mineral nutrition of Limonium species irrigated with saline waste waters. Hortic Sci. 2005, 40, 40654–40658. [Google Scholar]
- Galage, N.; Topno, S.E.; Parsad, V.M. Studies on the effect of different plant densities and levels of CRF on growth, yield and quality of flowers of statice (Limonium sinuatum L.). Int J Curr Microbiol Appl Sci, 2021, 10, 259-266.
- Mellesse, B.; Kassa, N.; Mohammed, A. Yield and quality of statice [Limonium sinuatum (L.) Mill.] as affected by cultivars and planting densities. Afr J Plant Sci. 2013, 7, 528-537.
- Kottayam, S.; Sartaj, A.; Ansari, H. Yield quality of statice [Limonium sinuatum (L.) Mill.] as affected by cultivars and planting densities. Int J Hortic Floric, 2014, 2, 089-097.
- Jain, R.; Singh, M.K.; Swaroop, K.; Reddy, M.V.; Janakiram, T.; Kumar, P.; Pinder, R. Optimization of spacing and nitrogen dose for growth and flowering of statice (Limonium sinuatum). Indian J Agric Sci. 2018, 88, 1108–1114. [Google Scholar] [CrossRef]
- Burchi, G.; Mercuri, A.; Bianchini, C.; Mercatelli, E.; Maletta, M.; Schiva, T. Results of a Breeding Activity on Limonium spp. Int Eucarpia Symp Orn Breed Beaut. 2006, 714, 43–50. [Google Scholar] [CrossRef]
- Nataraj, S. K.; Gangadharappa, P. M.; Reddy, B. S.; Naik, K. B.; Prashanth, S. J.; Prakash, D. P. Evaluation of annual statice (Limonium sinuatum L.) cultivars. J Hortic Sci. 2009, 4,184-186.
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agric. 2020, 10, 334. [Google Scholar] [CrossRef]
- Ajmal, M.; Ali, H.I.; Saeed, R.; Akhtar, A.; Tahir, M.; Mehboob, M.Z.; Ayub, A. Biofertilizer as an alternative for chemical fertilizers. J Agric Allied Sci. 2018, 7, 1–7. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and Sustainable Agriculture: Exploring Arbuscular Mycorrhizal Fungi. Appl Microbiol Biotechnol 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Johnson, D.; Martin, F.; Cairney, J.W.; Anderson, I.C. The importance of individuals: intraspecific diversity of mycorrhizal plants and fungi in ecosystems. New Phytol, 2012,194, 614-628.
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza. 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol, 2011, 62, 227–250.
- Patreze, C.M.; Moreira, M.; Tsai, S.M. Advances in molecular diversity of arbuscular mycorrhizal fungi (Phylum Glomeromycota) in forest ecosystems, in: Blanco, A.J., Lo, H.Y. (Eds.), Forest Ecosystems: More than Just Trees. In Tech, Rijeka, 2012, pp.53-80.
- Morton, J.B.; Benny, G.L. Revised Classification of Arbuscular Mycorrhizal Fungi (Zygomycetes): A New Order, Glomales, two new suborders, Glomineae and Gigasporineae and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon. 1990, 37, 471–491. [Google Scholar]
- Morton, J.B.; Bentivenga, S.P. Levels of diversity in endomycorrhizal fungi (Glomales, Zygomycetes) and their role in defining taxonomic and non-taxonomic groups. Plant Soil, 1994, 159, 47–59.
- Kim, K.Y.; Jordan, D.; McDonald, G.A. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol Fertil Soils, 1997, 26: 79-87.
- Davies Jr, F.T.; Potter, J.R.; Linderman, R.G. Drought resistance of mycorrhizal pepper plants independent of leaf P concentration response in gas exchange and water relations. Physiol Plant. 1993, 87, 45–53. [Google Scholar] [CrossRef]
- Azcon-Aguilar, C.; Padilla, I.G.; Encina, C.L.; Azcon, R.; Barea, J.M. Arbuscular mycorrhizal inoculation enhances plant growth and changes root system morphology in micropropagated Annona cherimola Mill. Agronomie J Genet Plant Breed. 1996, 16, 647–652. [Google Scholar] [CrossRef]
- Elsayed Abdalla, M.; Abdel-Fattah, G.M. Influence of the endomycorrhizal fungus Glomus mosseae on the development of peanut pod rot disease in Egypt. Mycorrhiza, 2000,10, 29-35.
- Abdel-Fattah, G.M.; Mankarios, A.T. Functional activity of vesicular-arbuscular fungus Glomus mosseae in the protection of soybean from infection by the pathogenic fungus Chalara elegans. Egypt J Microbiol. 1995, 30, 287–305. [Google Scholar]
- Sbrana, C.; Avio, L.; Giovannetti, M. Beneficial mycorrhizal symbionts affecting the production of health-promoting phytochemicals. Electrophoresis, 2014, 35, 1535–1546.
- Avio, L.; Turrini, A.; Giovannetti, M.; Sbrana, C. Designing the ideotype mycorrhizal symbionts for the production of healthy food. Front Plant Sci, 2018, 9, 1089.
- Sharma, M.; Delta, A.K.; Kaushik, P. Glomus mosseae and Pseudomonas fluorescens application sustains yield and promote tolerance to water stress in Helianthus annuus L. Stresses, 2021, 1, 305-316.
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci Total Environ. 2016, 566, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.A.F.; Rodrigues, A.F.; Marques, L.F. Long-term effects of alternative and conventional fertilization I: Effects on arbuscular mycorrhizal fungi community composition. Russ Agric Sci, 2015, 41; 454-461.
- Linderman, R.G.; Davis, E.A. Evaluation of commercial inorganic and organic fertilizer effects on arbuscular mycorrhizae formed by Glomus intraradices. HortTechnology, 2004, 14, 196-202.
- Sensoy, S.; Demir, S.; Turkmen, O.; Erdinc, C.; Savur, O. B. Responses of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Scientia Hort. 2007, 113, 92-95.
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol, 2008, 177, 779-789.
- Sheikh-Assadi, M.; Khandan-Mirkohi, A.; Taheri, M.R.; Babalar, M.; Sheikhi, H.; Nicola, S. Arbuscular Mycorrhizae contribute to growth, nutrient uptake and ornamental characteristics of Statice (Limonium sinuatum [L.] Mill.) subject to appropriate inoculum and optimal phosphorus. Horticulturae. 2023, 9, 564.
- Gao, X.; Liu, Y.; Liu, C.; Guo, C.; Zhang, Y.; Ma, C.; Duan, X. Individual and combined effects of arbuscular mycorrhizal fungi and phytohormones on the growth and physiobiochemical characteristics of tea cutting seedlings. Front. Plant Sci. 2023, 14, 1140267. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: a Review. Environ Exp Bot, 2010, 68,14-25.
- Kadam, M.S.; Malshe, K.V.; Salvi, B.R.; Chavan, S.S. Effect of plant growth regulators on flowering and flower yield in gaillardia (Gaiilardia pulchella) cv. Local double. Int J Chem Stud, 2020, 8: 927-930.
- Luo, Y.; Liu, M.; Cao, J.; Cao, F.; Zhang, L. The role of salicylic acid in plant flower development. J For Res, 2022, 2, 14.
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress?. J Plant Physio, 2007, 164, 685-694.
- El-Tayeb, M.A. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul, 2005, 45, 215-224.
- Noreen, S.; Ashraf, M.; Hussain, M.; Jamil, A. Exogenous application of salicylic acid enhances antioxidative capacity in salt stressed sunflower (Helianthus annuus L.) plants. Pak J Bot, 2009, 41, 473-479.
- Abdelaal, K.A. Effect of salicylic acid and abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J Plant Prod, 2015, 6, 1771-1788.
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol, 2009, 47: 177-206.
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; Rawat, S.; Grover, A. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands; Norwell, MA, USA. 1995.
- Leslie, C.A.; Romani, R.J. Inhibition of ethylene biosynthesis by salicylic acid. Plant physiol, 1988, 88, 833-837.
- Racey, G.D. A com parison of planting stock characterisation with root area index, volume and dry weight. Forestry Chronicle. 1985, 61, 64–70. [Google Scholar] [CrossRef]
- Gardemann, J.W.; Nicholson, T. H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of British Mycol Society. 1963, 46, 235–44. [Google Scholar] [CrossRef]
- Porter, W.N. The most probable number method for enumerating infective propagules of VA-mycorrhizal fungi in soil. Austrailian J Soil Res. 1979, 17, 515–9. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res, 1989, 92, 486-488.
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol, 1980, 84, 489-500.
- Thakur, M.; Kumar, R. Foliar application of plant growth regulators modulates the productivity and chemical profile of Damask rose (Rosa damascena Mill.) under mid hill conditions of the western Himalaya, Ind Crop Prod. 2020, 158, 113024.
- Brejda, J.J.; Moorman, T.B.; Karlen, D.L.; Dao, T.H. Identification of regional soil quality factors and indicators I. Central and Southern High Plains. SSSAJ, 2000, 64, 2115-2124.
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010; p. 32. ISBN 0080559344. [Google Scholar]
- Nunes, C.E.P.; Stancato, G.C.; Da Silveira, A.P.D. Anthurium growth responses to phosphate fertilisation and inoculation with an arbuscular mycorrhizal fungus. J Hortic Sci Biotechnol, 2014, 89, 261-267.
- Turjaman, M.; Tamai, Y.; Santoso, E.; Osaki, M.; Tawaraya, K. Arbuscular mycorrhizal fungi increased early growth of two non timber forest product species Dyera polyphylla and Aquilaria filaria under greenhouse conditions. Mycorrhiza. 2006, 16, 459–464. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Wei, S.; Xiao, X.; Su, C.; Jiang, P.; Song, Z.; Wang, T.; Yu, Z. Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 2007, 52, 29–39. [Google Scholar] [CrossRef]
- Vosnjak, M.; Likar, M.; Osterc, G. The effect of mycorrhizal inoculum and phosphorus treatment on growth and flowering of Ajania (Ajania pacifica (Nakai) Bremer et Humphries) plant. Horticulturae, 2021, 7, 178.
- El-Mergawi, R.A.; Abdel-Wahed, M.S. Diversity in salicylic acid effects on growth criteria and different indole acetic acid forms among faba bean and maize. Egypt J Agron, 2004, 26, 49-61.
- Grown, B.A. Physiological role of salicylic acid in improving performance, yield and some biochemical aspects of sunflower plant grown under newly reclaimed sandy soil. Aust J Basic Appl Sci, 2012, 6, 82-89.
- Shahabivand, S.; Maivan, H. Z.; Goltapeh, E. M.; Sharifi, M.; Aliloo, A. A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol Biochem. 2012, 60, 53–58. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J Sci Food Agric. 2012, 95, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Palencia, P.; Martinez, F.; Pestana, M.; Oliveira, J. A.; Correia, P. J. Effect of Bacillus velezensis and Glomus intraradices on fruit quality and growth parameters in strawberry soilless growing system. Hortic. J. 2015, 84, 122–130. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Dai, Y.; Liu, Q.; Tang, J.; Bian, X.; Chen, X. Effects of arbuscular mycorrhizal fungi on plant growth depend on root system: a meta-analysis. Plant Soil. 2015, 389, 361–374. [Google Scholar] [CrossRef]
- Gutjahr, C.; Casieri, L.; Paszkowski, U. Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol, 2009, 182, 829–837.
- Vos, C.; Schouteden, N.; van Tuinen, D.; Chatagnier, O.; Elsen, A.; De Waele, D.; Panis, B.; Gianinazzi-Pearson, V. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol Biochem. 2013, 60, 45–54. [Google Scholar] [CrossRef]
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D., Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular Mycorrhizal Fungi contribute to phosphorous uptake and allocation strategies of Solidago canadensis in a phosphorous-deficient environment. Front Plant Sci. 2022, 13, 1-11.
- Grimoldi, A.A.; Kavanova, M.; Lattanzi, F.A.; Schnyder, H. Phosphorus nutrition-mediated effects of arbuscular mycorrhiza on leaf morphology and carbon allocation in perennial ryegrass. New Phytol. 2005, 168, 435–444. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol, 2003, 132, 272-281.
- Gharib, F.A.; Abed, F. Effect of salicylic acid on the growth, metabolic activities and oil content of basil and marjoram. Int J Agric Biol, 2006, 4, 485-492.
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul, 30.
- Traw, M.B.; Kim, J.; Enright, S.; Cipollini, D.F.; Bergelson, J. Negative cross-talk between salicylate-and jasmonate-mediated pathways in the Wassilewskija ecotype of Arabidopsis thaliana. Mol Ecol. 2003, 12, 1125–1135. [Google Scholar] [CrossRef]
- Basit, A.; Shah, K.; Rahman, M. Ur.; Xing, L.; Zuo, X.; Han, M.; Alam, N.; Khan, F. A. Salicylic acid an emerging growth and flower inducing hormone in marigold (Tagetes sp. L). Pure Appl Biol. 2018, 7:1301–1308.
- Goh, D.M.; Cosme, M.; Kisiala, A.B.; Mulholland, S.; Said, Z.M.; Spichal, L.; Emery, R.N.; Declerck, S.; Guinel, F.C. A stimulatory role for cytokinin in the arbuscular mycorrhizal symbiosis of pea. Front Plant Sci. 2019, 10, 262. [Google Scholar] [CrossRef]
- Swaroop, K.; Singh, K.P.; Raju, D.V.S. Vegetative growth, flowering and seed characters of African marigold (Tagetes erectaLinn.) as influenced by different growth substances during mild off seasons. J Ornam Hortic, 2007, 10, 268- 270.
- Sable, P.B.; Ransingh, U.R.; Waskar, D.P. Effect of foliar application of plant growth regulators on growth and flower quality of gladiolus cv.‘HB Pitt’. J Hortic, 2015, 2, 141-143.
- Pawar, A.; Chopde, N.; Nikam, B. Thiourea and salicylic acid influences growth, yield and quality of gladiolus. J Pharmacogn Phytochem, 2018, 7, 970-972.
- Karlidag, H.; Yildirim, E.; Turan, M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci Agric, 2009, 66, 180-187.
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol, 2003, 160, 485-492.
- Szepesi, A. Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt-and osmotic stress. Acta Biol Szeged, 2005, 49, 123-125.
- Garg, N.; Bharti, A. Salicylic acid improves arbuscular mycorrhizal symbiosis, and chickpea growth and yield by modulating carbohydrate metabolism under salt stress. Mycorrhiza, 2018, 28, 727-746.
- Sohn, B.K.; Kim, K.Y.; Chung, S.J.; Kim, W.S.; Park, S.M.; Kang, J.G.; Rim, Y.S.; Cho, J.S.; Kim, T.H.; Lee, J.H. Effect of the different timing of AMF inoculation on plant growth and flower quality of chrysanthemum. SciHortic. 2003, 98, 173–183. [Google Scholar] [CrossRef]
- Prasad, K.; Aggarwal, A.; Yadav, K.; Tanwar, A. Impact of different levels of superphosphate using arbuscular mycorrhizal fungi and Pseudomonas fluorescens on Chrysanthemum indicum L. J Soil Sci Plant Nutr, 2012, 12, 451–462.
- Liang, J.F.; An, J.; Gao, J.Q.; Zhang, X.Y.; Yu, F.H. Effects of arbuscular mycorrhizal fungi and soil nutrient addition on the growth of Phragmites australis under different drying-rewetting cycles. Public Lib Sci, 2018, 13, 0191999.
- Adeyemi, N.O.; Atayese, M.O.; Olubode, A.A.; Akan, M.E. Effect of commercial arbuscular mycorrhizal fungi inoculant on growth and yield of soybean under controlled and natural field conditions. J Plant Nutr. 2020, 43: 487-499.
- Rousseau, J.V.D.; Reid, C.P.P. Effects of phosphorus and ectomycorrhizas on the carbon balance of loblolly pine seedlings. For Sci, 1990, 36, 101-112.
- Monticelli, S.; Puppi, G.; Damiano, C. Effects of in vivo mycorrhization on micropropagated fruit tree rootstocks. Appl Soil Ecol, 2000, 15, 105-111.
- Nowak, J.; Nowak, J.S. CO2 enrichment and mycorrhizal effects on cutting growth and some physiological traits of cuttings during rooting. Acta Sci Pol Hortorum Cultus, 2013, 12, 67-75.
- Long, L.K.; Yao, Q.; Huang, Y.H.; Yang, R.H.; Guo, J.; Zhu, H.H. Effects of arbuscular mycorrhizal fungi on Zinnia and the different colonization between Gigaspora and Glomus. World J Microbiol Biotechnol. 2010, 26, 1527–1531. [Google Scholar] [CrossRef]
- Hassoon, A.S.; Abduljabbar, I.A. Review on the role of salicylic acid in plants, in: Hasanuzzaman, M., Fujita, M., Filho, T.M.C.M., Nogueira, R.A.T., Galindo, S.F. (Eds.), Sustainable crop production. London, U.K, pp. 2019, 61-64.
- Rawi, A.T.A.; Ghani, E.T.A. The Effect of Salicylic Acid on the Growth Features of Sunflower Crops in Iraqi Middle-area Conditions. In: IOP Conf Ser Earth Environ Sci, 2023, 1755-1307.
- Asrar, A.A.; Abdel-Fattah, G.M.; Elhindi, K.M. Improving growth, flower yield and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica, 2012, 50, 305-316.
- Gaur, A., Adholeya, A. Diverse response of five ornamental plant species to mixed indigenous and single isolate arbuscular-mycorrhizal inocula in marginal soil amended with organic matter. J Plant Nutr, 2005, 28, 707-723.
- Aboul-Nasr, A. Effects of Vesicular-Arbuscular Mycorrhiza on Tagetes erecta and Zinnia elegans. Mycorrhiza, 1995, 6, 61–64.
- Serek, M. Does salicylic acid affect the postharvest characteristics of Campanula carpatica? Gartenbauwissenschaft, 1992, 57, 112- 114.
- Kumar, S.; Nanda, K.K. Gibberellic acid-and salicylic acid-caused formation of new proteins associated with extension growth and flowering of Impatiens balsamina. Biol Plant, 1981, 23, 321-327.
- Dawood, M.G.; Sadak, M.S.; Hozayen, M. Physiological role of salicylic acid in improving performance, yield and some biochemical aspects of sunflower plant grown under newly reclaimed sandy soil. Aust J Basic Appl Sci, 2012, 6: 82-89.
- Martin-Mex, R.; Nexticapan-Garcez, A.; Villanueva-Couoh, E.; Uicab-Quijano, V.; Vergara-Yoisura, S.; Larque-Saavedra, A. Salicylic acid stimulates flowering in micropopagated gloxinia plants. Rev Fitotec Mex. 2015, 38, 115–118. [Google Scholar] [CrossRef]
- Mackay, W.A.; Sankhla, N.; Sankhla, D.; Davis, T.D. Postharvest performance of Lupinus havardii Wats. , a new cut flower crop. Lupin, an ancient crop for the new millennium Proceedings of the 9th International Lupin Conference, Klink/Muritz, Germany, 20-24 June 1999, 2000, 330–332. [Google Scholar]
- Bayat, H.; Aminifard, M.H. Salicylic acid treatment extends the vase life of five commercial cut flowers. Electron J Biol, 2017, 13, 67-72.
- Hatamzadeh, A.; Hatami, M.; Ghasemnezhad, M. Efficiency of salicylic acid delay petal senescence and extended quality of cut spikes of Gladiolus grandiflora cv ‘Wing’s Sensation’. Afr J Agric Res, 2012, 7, 540-545.
- Pellegrino, E.; Opik, M.; Bonari, E.; Ercoli, L. Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biol Biochem, 2015, 84, 210-217.
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuca, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64: 283-289.
- Wu, S.; Shi, Z.; Chen, X.; Gao, J.; Wang, X. Arbuscular mycorrhizal fungi increase crop yields by improving biomass under rainfed condition: a meta-analysis. J Life Environ Sci, 2022, 10, 2861.
- Hazzoumi, Z.; Moustakime, Y.; Hassan Elharchli, E.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs and yield of essential oil in basil (Ocimum gratissimum L). Chem Biol Technol Agric. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Puschel, D.; Janouskova, M.; Hujslova, M.; Slavikova, R.; Gryndlerova, H.; Jansa, J. Plant–fungus competition for nitrogen erases mycorrhizal growth benefits of Andropogon gerardii under limited nitrogen supply. Ecol Evol. 2016, 6, 4332–4346. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza, 2003, 13, 309-317.
- Zhang, F.; Wang, P.; Zou, Y.N.; Wu, Q.S.; Kuca, K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch Agron Soil Sci, 2019, 65, 1316-1330.
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric Ecosyst Envirot, 2006, 113, 17-35.
- Jia-Dong, H.; Tao, D.; Hui-Hui, W.; Ying-Ning, Z.; Qiang-Sheng, W.; Kamil, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci Hortic, 2019, 243, 64-69.
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D.; Zhou, X. Arbuscular mycorrhizae improve Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ. 2012, 58(4), 186–191. [Google Scholar] [CrossRef]
- Singh, B.; Usha, K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul, 2003, 39, 137-141.
- Jeyakumar, P.; Velu, G.; Rajendran, C.; Amutha, R.; Savery, M.A.J.R.; Chidambaram, S. Varied responses of black gram (Vigna mungo) to certain foliar applied chemicals and plant growth regulators. Legum Res An Int J. 2008, 31, 105–109. [Google Scholar]
- Gorni, P.H.; Pacheco, A.C.; Moro, A.L.; Silva, J.F.A.; Moreli, R.R.; de Miranda, G.R.; Pelegrini, J.M.; Spera, K.D.; Junior, J.L.B.; da Silva, R.M.G. Salicylic acid foliar application increases biomass, nutrient assimilation, primary metabolites and essential oil content in Achillea millefolium L. Sci Hortic. 2020, 270: 109436.
- Del Blanco, I.A.; Rajaram, S.; Kronstad, W.E.; Reynolds, M.P. Physiological performance of synthetic hexaploid wheat-derived populations. Crop Sci. 2000, 40, 1257–1263. [Google Scholar] [CrossRef]
- Rivas-San Vicente; M., Plasencia, J. Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot, 2011, 62, 3321-3338.
- Ibrahim, A.M.; Abd El-Mageed, T.A.; Abdou, N.M.; Abohamid, Y.M. Effect of salicylic acid on growth, physiological responses and yield of maize grown under drought stress. J Agric Res Dev. 2019, 33, 18–30. [Google Scholar]
- Ganadevi, G.; Haripriya, K. Studies on screening efficient VAM fungi for chrysanthemum. South Indian Hortic. 1999, 47(1/6), 325–6.
| Treatments | Plant height (cm) | Number of leaves per plant | Plant spread (cm) | Leaf area (cm2) |
Chlorophyll content (SPAD value) |
|---|---|---|---|---|---|
| Arbuscular Mycorrhizal Fungi (AMF) | |||||
| Un-inoculated Control (A0) | 59.04 ± 5.91 b | 51.12 ± 8.84 b | 48.03 ± 6.55c | 11.66 ± 2.35 c | 48.59 ± 4.85 b |
| Glomus mossae (A1) | 68.61 ± 4.57 a | 61.47 ± 11.98 a | 56.39 ± 4.55 a | 25.51 ± 3.75 a | 52.90 ± 4.34 a |
| Gigaspora margarita (A2) | 67.69 ± 4.14 a | 61.27 ± 11.84 a | 50.95 ± 5.86 bc | 18.19 ± 4.24 b | 52.48 ± 4.36 ab |
| Glomus mossae + Gigaspora margarita (A3) | 66.66 ± 5.25 a | 58.69 ± 8.51 a | 52.46 ± 3.87 b | 18.86 ± 3.96 b | 51.99 ± 2.69 ab |
| Significance | ** | ** | ** | ** | * |
| Salicylic Acid (SA) | |||||
| Control (S0) | 62.67 ± 7.97 b | 46.82 ± 4.38 c | 46.56 ± 5.99 c | 15.03 ± 5.51 c | 48.46 ± 4.87 b |
| SA @ 100 mg L-1 (S1) | 63.53 ± 4.83 b | 55.72 ± 5.70 b | 51.83 ± 5.52 b | 16.99 ± 5.04 b | 50.58 ± 3.39 ab |
| SA @ 150 mg L-1 (S2) | 67.70 ± 5.91 a | 59.40 ± 8.15 b | 52.42 ± 4.81 b | 20.54 ± 3.72 a | 53.01 ± 4.12 a |
| SA @ 200 mg L-1 (S3) | 68.09 ± 3.99 a | 70.60 ± 8.72 a | 57.01 ± 1.75 a | 21.66 ± 7.58 a | 53.91 ± 3.04 a |
| Significance | ** | ** | ** | ** | ** |
| AMF × SA | |||||
| A0 × S0 | 51.20 ± 2.27 c | 44.27 ± 2.34 f | 42.89 ± 4.95 e | 9.70 ± 0.71 f | 44.77 ± 5.55 a |
| A0 × S1 | 59.93 ± 0.54 bc | 49.20 ± 8.4 def | 44.67 ± 5.37 cde | 10.89 ± 0.91 ef | 48.33 ± 4.22 a |
| A0 × S2 | 61.29 ± 5.04 abc | 52.87 ± 11.32 cdef | 47.56 ± 1.83 bcde | 15.32 ± 0.66 de | 50.35 ± 6.14 a |
| A0 × S3 | 63.72 ± 5.25 ab | 58.13 ± 8.47 bcdef | 57.00 ± 1.32 a | 10.71 ± 0.75 ef | 50.91 ± 2.89 a |
| A1 × S0 | 70.73 ± 1.70 a | 46.07 ± 5.83 ef | 52.50 ± 7.88 abcd | 23.46 ± 2.49 b | 49.83 ± 5.54 a |
| A1 × S1 | 65.78 ± 7.06 ab | 56.93 ± 0.80 bcdef | 57.44 ± 2.00 a | 23.60 ± 1.99 b | 51.23 ± 2.88 a |
| A1 × S2 | 67.20 ± 5.71 ab | 66.80 ± 1.11 abc | 58.55 ± 3.12 a | 24.25 ± 2.80 b | 54.89 ± 5.05 a |
| A1 × S3 | 70.72 ± 1.11a | 76.07 ± 2.24 a | 57.05 ± 2.42 a | 30.72 ± 2.17 a | 55.66 ± 1.84 a |
| A2 × S0 | 67.11 ± 2.52 ab | 45.07 ± 1.00 ef | 43.28 ± 2.85 de | 13.73 ± 1.61 def | 46.65 ± 2.03 a |
| A2 × S1 | 64.89 ± 6.03 ab | 59.67 ± 1.92 bcde | 52.56 ± 2.26 abcd | 15.00 ± 1.54 de | 53.49 ± 2.41 a |
| A2 × S2 | 71.23 ± 3.99 a | 64.00 ± 2.83 abcd | 49.78 ± 1.43 abcde | 22.17 ± 1.30 bc | 53.78 ± 3.39 a |
| A2 × S3 | 67.52 ± 2.17 ab | 76.33 ± 3.20 a | 58.17 ± 1.24 a | 21.87 ± 1.80 bc | 55.99 ± 3.13 a |
| A3 × S0 | 61.66 ± 3.10 abc | 51.87 ± 3.44 cdef | 47.56 ± 3.46 bcde | 13.25 ± 1.29 ef | 52.59 ± 3.38 a |
| A3 × S1 | 63.51 ± 3.48 ab | 57.07 ± 3.44 bcdef | 52.67 ± 1.92 abcd | 18.45 ± 1.52 cd | 49.29 ± 2.83 a |
| A3 × S 2 | 71.08 ± 4.56 a | 53.93 ± 2.27 cdef | 53.78 ± 2.67 abc | 20.40 ± 0.95 bc | 53.01 ± 1.32 a |
| A3 × S3 | 70.40 ± 2.57 ab | 71.87 ± 1.36 ab | 55.83 ± 1.93 ab | 23.35 ± 0.63 bc | 53.06 ± 1.97 a |
| Significance | * | * | * | ** | ns |
| Treatments | Days to flower emergence | Days to first flowering | Days to harvesting of flower stem | Inflorescence length (cm) | Flower longevity (days) |
|---|---|---|---|---|---|
| Arbuscular Mycorrhizal Fungi (AMF) | |||||
| Un-inoculated Control (A0) | 86.37 ± 5.42 b | 118.18 ± 3.51 b | 129.83 ± 3.59 b | 4.35 ± 0.67 c | 25.83 ± 5.35 b |
| Glomus mossae (A1) | 90.48 ± 4.31 ab | 118.88 ± 2.51 ab | 134.75 ± 2.07 a | 4.92 ± 0.11 a | 30.47 ± 4.51 a |
| Gigaspora margarita (A2) | 90.81 ± 4.00 ab | 119.03 ± 3.13 ab | 132.78 ± 2.96 ab | 4.74 ± 0.35 b | 31.64 ± 2.47 a |
| Glomus mossae + Gigaspora margarita (A3) | 95.50 ± 5.12 a | 120.88 ± 2.32 a | 133.08 ± 2.88 ab | 4.92 ± 0.15 a | 30.61 ± 2.25 a |
| Significance | ** | ** | ** | ** | ** |
| Salicylic Acid (SA) | |||||
| Control (S0) | 94.14 ± 4.82 a | 120.93 ± 2.45 a | 131.45 ± 3.88 a | 4.26 ± 0.66 c | 27.36 ± 4.82 b |
| SA @ 100 mg L-1 (S1) | 90.37 ± 3.97 a | 120.25 ± 2.59 a | 132.17 ± 2.75 a | 4.84 ± 0.15 b | 29.17 ± 4.36 ab |
| SA @ 150 mg L-1 (S2) | 89.52 ± 6.96 a | 118.07 ± 2.60 b | 133.06 ± 3.80 a | 4.85 ± 0.24 b | 30.22 ± 3.72 ab |
| SA @ 200 mg L-1 (S3) | 89.13 ± 5.60 a | 117.73 ± 3.23 b | 133.78 ± 2.74 a | 4.97 ± 0.07 a | 31.80 ± 3.82 a |
| Significance | * | ** | ns | ** | * |
| AMF × SA | |||||
| A0 × S0 | 91.27 ± 3.52 ab | 120.27 ± 1.72 abc | 129.00 ± 2.64 a | 3.29 ± 0.21 f | 21.78 ± 0.99 b |
| A0 × S1 | 88.13 ± 3.90 ab | 120.33 ± 2.41 abc | 129.44 ± 3.40 a | 4.74 ± 0.26 cd | 24.00 ± 4.58 ab |
| A0 × S2 | 81.33 ± 3.36 b | 117.87 ± 3.20 bcd | 130.11 ± 6.30 a | 4.48 ± 0.18 d | 27.00 ± 5.56 ab |
| A0 × S3 | 84.73 ± 6.34 ab | 114.27 ± 3.49 d | 130.78 ± 3.20 a | 4.88 ± 0.02 abc | 30.55 ± 6.31 ab |
| A1 × S0 | 94.07 ± 4.19 ab | 121.67 ± 2.01 abc | 133.45 ± 3.34 a | 4.83 ± 0.14 abc | 26.89 ± 6.45 ab |
| A1 × S1 | 89.93 ± 4.77 ab | 119.20 ± 1.38 abcd | 134.33 ± 0.66 a | 4.87 ± 0.11 abc | 29.67 ± 3.09 ab |
| A1 × S2 | 88.80 ± 3.81 ab | 116.60 ± 2.42 cd | 135.33 ± 0.33 a | 4.93 ± 0.05 abc | 30.33 ± 2.52 ab |
| A1 × S3 | 89.13 ± 4.56 ab | 118.07 ± 1.51 bcd | 135.89 ± 2.54 a | 5.03 ± 0.02 a | 35.00 ± 1.84 a |
| A2 × S0 | 93.09 ± 5.65 ab | 118.07 ± 0.75 bcd | 131.67 ± 4.97 a | 4.17 ± 0.05 e | 30.56 ± 2.09 ab |
| A2 × S1 | 90.93 ± 3.91 ab | 122.60 ± 3.66 ab | 132.00 ± 2.0 a | 4.86 ± 0.12 abc | 32.11 ± 2.96 ab |
| A2 × S2 | 91.33 ± 3.50 ab | 117.13 ± 0.64 bcd | 133.33 ± 3.33 a | 4.99 ± 0.07 abc | 32.89 ± 1.97 a |
| A2 × S3 | 87.87 ± 2.73 ab | 118.33 ± 3.58 abcd | 134.11 ± 1.57 a | 4.93 ± 0.07 abc | 31.00 ± 3.39 ab |
| A3 × S0 | 98.13 ± 5.31 a | 123.73 ± 0.30 a | 131.67 ± 4.97 a | 4.77 ± 0.22 bc | 30.22 ± 2.48 ab |
| A3 × S1 | 92.47 ± 4.31 ab | 118.87 ± 1.72 abcd | 132.89 ± 2.58 a | 4.87 ± 0.07 abc | 30.89 ± 2.79 ab |
| A3 × S 2 | 96.60 ± 6.8 a | 120.67 ± 2.44 abc | 133.45 ± 2.67 a | 5.01 ± 0.08 ab | 30.67 ± 2.93 ab |
| A3 × S3 | 94.80 ± 4.97 ab | 120.27 ± 1.33 abc | 134.33 ± 1.20 a | 5.03 ± 0.02 ab | 30.67 ± 2.23 ab |
| Significance | ns | ** | ns | ** | ns |
| Treatments | Number of flower bunches per stem | Weight of cut stem (g) | Number of cut stem per plant | Vase life (days) |
|---|---|---|---|---|
| Arbuscular Mycorrhizal Fungi (AMF) | ||||
| Un-inoculated Control (A0) | 50.25 ± 11.90 b | 27.17 ± 3.63 d | 8.98 ± 1.08 c | 9.08 ± 1.70 a |
| Glomus mossae (A1) | 67.42 ± 10.18 a | 43.42 ± 5.80 a | 11.55 ± 2.38 a | 12.33 ± 2.65 a |
| Gigaspora margarita (A2) | 66.67 ± 6.55 a | 40.67 ± 4.71 b | 10.19 ± 1.76 b | 10.92 ± 2.96 ab |
| Glomus mossae + Gigaspora margarita (A3) | 61.75 ± 4.37 a | 32.17 ± 6.05 c | 9.10 ± 0.97 c | 11.92 ± 3.89 a |
| Significance | ** | ** | ** | ** |
| Salicylic Acid (SA) | ||||
| Control (S0) | 52.67 ± 10.37 c | 29.67 ± 6.02 c | 8.39 ± 0.64 c | 9.00 ± 1.07 b |
| SA @ 100 mg L-1 (S1) | 64.61 ± 8.99 ab | 35.92 ± 8.47 b | 9.19 ± 1.08 c | 11.08 ± 3.04 b |
| SA @ 150 mg L-1 (S2) | 61.19 ± 7.45 b | 39.33 ± 4.67 a | 10.60 ± 1.49 b | 9.83 ± 1.32 b |
| SA @ 200 mg L-1 (S3) | 67.61 ± 11.49 a | 38.50 ± 9.90 a | 11.64 ± 2.20 a | 14.33 ± 3.25 a |
| Significance | ** | ** | ** | ** |
| AMF × SA | ||||
| A0 × S0 | 36.78 ± 6.04 e | 23.00 ± 1.0 g | 8.11 ± 0.69 f | 8.00 ± 1.0 d |
| A0 × S1 | 51.89 ± 5.10 de | 26.33 ± 1.15 efg | 8.44 ± 0.83 ef | 8.00 ± 0.83 d |
| A0 × S2 | 57.67 ± 15.60 bcd | 32.33 ± 1.52 de | 9.78 ± 1.01 bcdef | 8.67 ± 0.63 cd |
| A0 × S3 | 54.67 ± 9.20 cd | 27.00 ± 1.0 efg | 9.59 ± 1.07 cdef | 11.67 ± 0.37 abcd |
| A1 × S0 | 58.33 ± 3.84 bcd | 36.33 ± 1.52 cd | 8.67 ± 0.33 ef | 10.00 ± 0.55 abcd |
| A1 × S1 | 69.22 ± 2.79 abcd | 45.67 ± 3.21 ab | 10.67 ± 0.33 bcde | 12.67 ± 0.50 abcd |
| A1 × S2 | 60.00 ± 2.95 bcd | 41.33 ± 3.05 bc | 12.08 ± 0.82 b | 11.00 ± 1.0 abcd |
| A1 × S3 | 82.11 ± 1.95 a | 50.33 ± 1.52 a | 14.78 ± 0.84 a | 15.67 ± 3.09 a |
| A2 × S0 | 59.78 ± 1.67 bcd | 34.00 ± 2.0 d | 8.22 ± 0.50 f | 9.00 ± 1.0 cd |
| A2 × S1 | 73.67 ± 3.18 ab | 41.33 ± 3.21 bc | 9.11 ± 0.69 def | 9.67 ± 0.33 bcd |
| A2 × S2 | 62.44 ± 3.17 bcd | 42.67 ± 2.08 b | 11.44 ± 1.17 bcd | 10.00 ± 2.0 abcd |
| A2 × S3 | 70.78 ± 4.07 abc | 44.67 ± 2.51 ab | 12.00 ± 0.33 bc | 15.00 ± 3.0 ab |
| A3 × S0 | 55.78 ± 4.6 cd | 25.33 ± 1.52 fg | 8.55 ± 1.07 ef | 9.00 ± 1.0 cd |
| A3 × S1 | 63.66 ± 1.52 bcd | 30.33 ± 1.52 def | 8.55 ± 0.69 ef | 14.00 ± 4.0 abc |
| A3 × S 2 | 64.67 ± 1.33 abcd | 41.00 ± 2.0 bc | 9.11 ± 0.69 def | 9.67 ± 0.37 bcd |
| A3 × S3 | 62.89 ± 2.50 bcd | 32.00 ± 1.0 de | 10.20 ± 0.65 bcdef | 15.00 ± 5.0 ab |
| Significance | * | ** | ** | ns |
| Treatments | Fresh weight of shoot (g) | Fresh weight of root (g) | Dry weight of shoot (g) | Dry weight of root (g) | Root: shoot ratio (Dry weight basis) | Root: shoot ratio (Fresh weight basis) |
|---|---|---|---|---|---|---|
| Arbuscular Mycorrhizal Fungi (AMF) | ||||||
| Un-inoculated Control (A0) | 328.42 ± 37.27 c | 9.50 ± 1.44 d | 88.67 ± 16.24 d | 3.81 ± 1.03 d | 0.041 ± 0.0067 b | 0.029 ± 0.0036 c |
| Glomus mossae (A1) | 499.00 ± 141.80 a | 18.73 ± 4.22 a | 155.58 ± 50.28 a | 5.95 ± 1.28 b | 0.042 ± 0.0083 b | 0.039 ± 0.0073 a |
| Gigaspora margarita (A2) | 423.92 ± 69.17 b | 14.85 ± 2.52 c | 136.08 ± 34.46 c | 4.87 ± 0.66 c | 0.038 ± 0.0094 b | 0.035 ± 0.0042 b |
| Glomus mossae + Gigaspora margarita (A3) | 430.25 ± 76.88 b | 16.77 ± 5.67 b | 144.50 ± 37.05 b | 7.64 ± 1.32 a | 0.056 ± 0.01 a | 0.038 ± 0.0076 ab |
| Significance | ** | ** | ** | ** | ** | ** |
| Salicylic Acid (SA) | ||||||
| Control (S0) | 309.83 ± 24.44 d | 10.45 ± 2.30 c | 83.92 ± 9.95 d | 4.55 ± 1.53 c | 0.052 ± 0.014 a | 0.034 ± 0.0075 b |
| SA @ 100 mg L-1 (S1) | 400.83 ± 55.19 c | 15.52 ± 3.74 b | 129.00 ± 27.74 c | 5.23 ± 1.75 b | 0.043 ± 0.0106 b | 0.038 ± 0.0049 a |
| SA @ 150 mg L-1 (S2) | 460.75 ± 102.56 b | 14.68 ± 3.28 b | 139.50 ± 38.74 b | 5.54 ± 1.15 b | 0.042 ± 0.0072 b | 0.032 ± 0.0057 b |
| SA @ 200 mg L-1 (S3) | 510.17 ± 98.98 a | 19.21 ± 6.13 a | 172.42 ± 38.09 a | 6.95 ± 1.88 a | 0.041± 0.0079 b | 0.037 ± 0.0082 a |
| Significance | ** | ** | ** | ** | ** | ** |
| AMF × SA | ||||||
| A0 × S0 | 280.00 ± 11.93 l | 7.33 ± 0.74 g | 70.00 ± 6.82 i | 2.51 ± 0.06 i | 0.033 ± 0.0058 cd | 0.026 ± 0.0035 g |
| A0 × S1 | 313.33 ± 8.76 k | 10.35 ± 0.10 f | 83.33 ± 2.69 gh | 3.58 ± 0.26 hi | 0.043 ± 0.0058 bcd | 0.033 ± 0.0006 defg |
| A0 × S2 | 350.00 ± 13.85 hi | 9.52 ± 0.42 fg | 90.00 ± 3.24 gh | 3.91 ± 0.18 h | 0.043 ± 0.0058 bcd | 0.027 ± 0.0035 fg |
| A0 × S3 | 370.34 ± 6.07 h | 10.80 ± 0.25 ef | 111.33 ± 6.94 f | 5.23 ± 0.44 efg | 0.043 ± 0.0058 bcd | 0.029 ± 0.0012 efg |
| A1 × S0 | 299.33 ± 5.99 kl | 13.25 ± 0.61 de | 82.33 ± 1.77 h | 4.45 ± 0.16 fgh | 0.053 ± 0.0058 ab | 0.044 ± 0.0031 abc |
| A1 × S1 | 450.00 ± 5.71 e | 20.41 ± 0.57 b | 143.33 ± 1.77 d | 5.37 ± 0.33 ef | 0.040 ± 0.0 bcd | 0.045 ± 0.0015 ab |
| A1 × S2 | 610.00 ± 9.99 b | 17.31± 1.63 c | 192.00 ± 3.21 b | 6.25 ± 0.51 de | 0.033 ± 0.0058 cd | 0.028 ± 0.0021 fg |
| A1 × S3 | 636.67 ± 11.01 a | 23.96 ± 1.28 a | 204.67 ± 3.36 a | 7.71 ± 0.24 bc | 0.040 ± 0.0 bcd | 0.038 ± 0.0021 bcde |
| A2 × S0 | 340.00 ±7.91 ij | 11.01 ± 1.13 ef | 89.67 ± 2.21 gh | 4.62 ± 0.54 fgh | 0.050 ± 0.0 bc | 0.032 ± 0.0046 defg |
| A2 × S1 | 430.00 ± 5.71 ef | 15.32 ± 0.63 cd | 147.67 ± 3.44 d | 4.09 ± 0.30 gh | 0.030 ± 0.0 d | 0.036 ± 0.0015 cdef |
| A2 × S2 | 402.33 ± 4.64 g | 16.23 ± 0.89 c | 126.67 ± 3.25 e | 5.42 ± 0.22 def | 0.043 ± 0.0058 bcd | 0.040 ± 0.0021 bcd |
| A2 × S3 | 523.34 ± 7.77 c | 16.86 ± 1.04 c | 180.33 ± 1.66 c | 5.35 ± 0.45 ef | 0.030 ± 0.0 d | 0.032 ± 0.0021 defg |
| A3 × S0 | 320.00 ± 5.06 jk | 10.21 ± 0.28 f | 93.67 ± 6.94 f | 6.60 ± 0.26 cd | 0.070 ± 0.0 a | 0.032 ± 0.001 defg |
| A3 × S1 | 410.00 ± 10.0 fg | 16.00 ± 0.33 c | 141.67 ± 3.34 d | 7.87 ± 0.36 b | 0.057 ± 0.057 ab | 0.039 ± 0.0025 bcd |
| A3 × S 2 | 480.67 ± 4.46 d | 15.65 ± 0.55 cd | 149.33 ± 4.92 d | 6.57 ± 0.80 cd | 0.047 ± 0.0058 bcd | 0.032 ± 0.0012 defg |
| A3 × S3 | 510.34 ± 9.07 c | 25.24 ± 1.20 a | 193.33 ± 3.79 b | 9.53 ± 0.20 a | 0.050 ± 0.0 bc | 0.049 ± 0.0031 a |
| Significance | ** | ** | ** | ** | ** | ** |
| Treatments | Root length (cm) | Spore count per 20 of soil | Root colonization percentage |
|---|---|---|---|
| Arbuscular Mycorrhizal Fungi (AMF) | |||
| Un-inoculated Control (A0) | 11.10 ± 1.59 c | 41.46 ± 3.86 d | 30.23 ± 1.55 d |
| Glomus mossae (A1) | 23.05 ± 6.50 a | 162.95 ± 4.45 a | 54.73 ± 3.34 a |
| Gigaspora margarita (A2) | 16.20 ± 1.72 b | 123.61 ± 8.48 c | 48.21 ± 1.52 c |
| Glomus mossae + Gigaspora margarita (A3) | 22.22 ± 1.94 a | 143.58 ± 8.55 b | 52.21 ± 1.72 b |
| Significance | ** | ** | ** |
| Salicylic Acid (SA) | |||
| Control (S0) | 17.17 ± 4.57 b | 108.84 ± 46.91 d | 44.4 ± 9.33 c |
| SA @ 100 mg L-1 (S1) | 17.18 ± 5.65 b | 117.25 ± 47.96 c | 45.87 ± 9.87 bc |
| SA @ 150 mg L-1 (S2) | 18.00 ± 5.69 b | 120.48 ± 49.30 b | 46.92 ± 10.02 bc |
| SA @ 200 mg L-1 (S3) | 20.22 ± 7.88 a | 125.03 ± 49.38 a | 48.18 ± 11.25 a |
| Significance | ** | ** | ** |
| AMF × SA | |||
| A0 × S0 | 13.20 ± 1.37 fgh | 36.2 ± 1.95 k | 29.33 ± 2.15 f |
| A0 × S1 | 9.70 ± 0.71 h | 41.64 ± 1.12 j | 30.14 ± 1.41 f |
| A0 × S2 | 10.70 ± 0.76 gh | 42.08 ± 1.79 j | 30.45 ± 1.17 f |
| A0 × S3 | 10.80 ± 1.07 gh | 45.91 ± 1.37 j | 31.00 ± 1.76 f |
| A1 × S0 | 13.70 ± 1.21 fgh | 156.58 ± 1.59 c | 50.45 ± 1.92 cde |
| A1 × S1 | 23.60 ± 1.99 b | 162.57 ± 1.42 b | 54.13 ± 1.56 abc |
| A1 × S2 | 24.20 ± 1.78 b | 165.07 ± 1.96 ab | 55.71 ± 1.06 ab |
| A1 × S3 | 30.70 ± 1.68 a | 167.58 ± 0.73 a | 58.65 ± 1.30 a |
| A2 × S0 | 18.40 ± 1.61 cde | 111.33 ± 0.85 i | 46.98 ± 1.21 e |
| A2 × S1 | 15.00 ± 1.54 efg | 122.45 ± 1.69 h | 47.76 ± 1.90 e |
| A2 × S2 | 15.30 ± 0.70 ef | 127.34 ± 1.07 g | 48.45 ± 0.63 de |
| A2 × S3 | 16.10 ± 0.39 def | 133.33 ± 0.67 f | 49.65 ± 1.23 cde |
| A3 × S0 | 23.40 ± 2.58 b | 131.26 ± 1.21 fg | 50.87 ± 1.14 cde |
| A3 × S1 | 20.40 ± 0.95 bcd | 142.35 ± 2.28 e | 51.45 ± 1.64 bcde |
| A3 × S 2 | 21.80 ± 1.91 bc | 147.43 ± 0.70 d | 52.54 ± 0.97 bcd |
| A3 × S3 | 23.30 ± 0.72 b | 153.31 ± 1.13 c | 53.98 ± 1.75 bc |
| Significance | ** | ** | * |
| Principal components | Eigen value | Percentage of variance | Cumulative percentage of variance |
| Comp 1 | 15.24 | 72.58 | 72.58 |
| Comp 2 | 1.91 | 9.12 | 81.69 |
| Comp3 | 1.08 | 5.16 | 86.85 |
| Comp 4 | 0.98 | 4.69 | 91.54 |
| Comp 5 | 0.73 | 3.47 | 95.01 |
| Comp 6 | 0.32 | 1.50 | 96.52 |
| Comp 7 | 0.28 | 1.34 | 97.86 |
| Comp 8 | 0.24 | 1.16 | 99.02 |
| Comp 9 | 0.13 | 0.64 | 99.67 |
| Comp 10 | 0.04 | 0.18 | 99.85 |
| Comp 11 | 0.02 | 0.09 | 99.94 |
| Comp 12 | 0.01 | 0.04 | 99.99 |
| Comp 13 | 0.002 | 0.01 | 100.00 |
| Comp 14 | 0.00 | 0.002 | 100.00 |
| Comp 15 | 0.00 | 0.00 | 100.00 |
| Parameters | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| PH | 3.53 | 2.01 | 1.06 | 10.93 | 11.93 |
| NoL | 5.56 | 0.98 | 4.54 | 0.66 | 0.95 |
| PS | 5.13 | 0.08 | 2.08 | 3.15 | 1.83 |
| LA | 6.40 | 0.05 | 4.61 | 1.03 | 0.00 |
| Chlo | 6.25 | 0.40 | 2.66 | 0.12 | 0.67 |
| FE | 6.27 | 0.17 | 1.67 | 1.02 | 0.49 |
| FF | 6.36 | 0.19 | 1.50 | 0.60 | 0.25 |
| HS | 6.32 | 0.25 | 1.94 | 0.48 | 0.46 |
| IL | 3.80 | 2.58 | 6.78 | 24.91 | 4.45 |
| FL | 6.35 | 0.16 | 1.61 | 0.79 | 0.26 |
| VL | 6.34 | 0.15 | 1.68 | 0.90 | 0.26 |
| FS | 5.54 | 1.05 | 2.94 | 8.63 | 1.85 |
| FR | 5.32 | 2.88 | 3.73 | 2.63 | 5.78 |
| DS | 5.92 | 0.03 | 6.31 | 4.56 | 0.09 |
| DR | 3.22 | 21.51 | 4.02 | 1.36 | 0.54 |
| FRS | 1.47 | 10.11 | 1.99 | 0.01 | 49.12 |
| DRS | 0.65 | 39.28 | 4.83 | 0.54 | 2.99 |
| NoFB | 4.43 | 0.02 | 1.31 | 0.33 | 5.04 |
| FWtS | 3.94 | 4.45 | 2.36 | 0.20 | 4.12 |
| NCS | 4.51 | 4.47 | 1.37 | 5.74 | 1.24 |
| RL | 2.67 | 9.17 | 1.04 | 31.39 | 7.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





