Submitted:
23 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Fat Content of Milk per Breed/Region
2.2. Fatty Acid Content of Different Dairy Breeds
2.3. Different Types of Fatty Acids per Breeds/Region
2.4. Proportions of Different Types of Fatty Acids per Breed/Region
2.5. Protein Content per Breed/Region
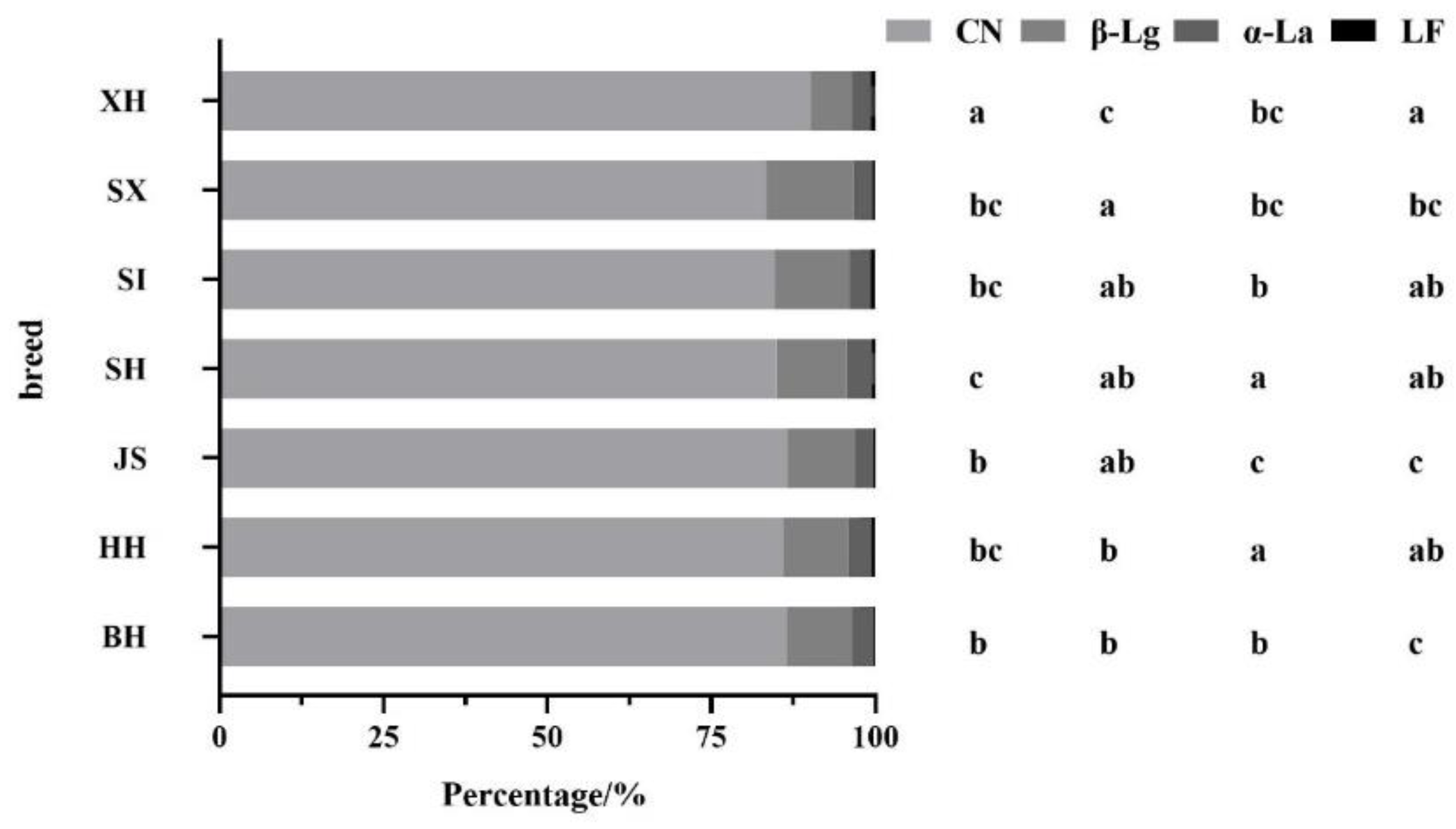
2.6. Proportions of Different Types of Proteins per Breeds/Region
3. Discussion
4. Materials and Methods
4.1. Milk Collection
4.2. Reagents
4.3. Milk Fat Measurement
4.4. Milk Fatty Acid Measurement
4.4.1. Methylation of Milk Fatty Acids
4.4.2. Milk Fatty Acid Measurement
4.5. Milk Protein Measurement
4.5.1. Extraction of Milk Proteins
4.5.2. Casein Content Measurement
4.5.3. Whey Protein Measurement
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, Y. W.; Haenlein, G. F. W.; Wendorff, W. L., Overview of Milk of Non-Bovine Mammals (Second Edition). In Handbook of Milk of Non-Bovine Mammals, 2017; pp 1-9.
- Lin, T. T.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A., Bioactives in bovine milk: chemistry, technology, and applications. Nutrition Reviews 2021, 79, (2), 48-69. [CrossRef]
- Tzompa-Sosa, D. A.; Meurs, P. P.; van Valenberg, H. J. F., Triacylglycerol Profile of Summer and Winter Bovine Milk Fat and the Feasibility of Triacylglycerol Fragmentation. European Journal of Lipid Science and Technology 2018, 120, (3). [CrossRef]
- Khastayeva, A. Z.; Zhamurova, V. S.; Mamayeva, L. A.; Kozhabergenov, A. T.; Karimov, N. Z.; Muratbekova, K. M., Qualitative indicators of milk of Simmental and Holstein cows in different seasons of lactation. Vet World 2021, 14, (4), 956-963. [CrossRef]
- Rezagholivand, A.; Nikkhah, A.; Khabbazan, M. H.; Mokhtarzadeh, S.; Dehghan, M.; Mokhtabad, Y.; Sadighi, F.; Safari, F.; Rajaee, A., Feedlot performance, carcass characteristics and economic profits in four Holstein-beef crosses compared with pure-bred Holstein cattle. Livestock Science 2021, 244. [CrossRef]
- LIU, B., Comparative Analysis of Nutrient Contents, Fatty Acid Content and Composition of Raw Milk from Dairy Cattle of Different Breeds. Animal Husbandry and Feed Science 2022, 43, (5), 93-97.
- ZHANG, S. L.; SUN, D. X., Past, now, and future of Dairy breeding Industry. China Livestock Industry 2021, (15), 22-26.
- Huson, H. J.; Sonstegard, T. S.; Godfrey, J.; Hambrook, D.; Wolfe, C.; Wiggans, G.; Blackburn, H.; VanTassell, C. P., A Genetic Investigation of Island Jersey Cattle, the Foundation of the Jersey Breed: Comparing Population Structure and Selection to Guernsey, Holstein, and United States Jersey Cattle. Frontiers in Genetics 2020, 11. [CrossRef]
- Zhang, X. Y.; Wang, W.; Cao, Z. J.; Yang, H. J.; Wang, Y. J.; Li, S. L., Effects of altitude on the gut microbiome and metabolomics of Sanhe heifers. Frontiers in Microbiology 2023, 14. [CrossRef]
- Zhou, J. H.; Liu, L. Y.; Chen, C. J.; Zhang, M. H.; Lu, X.; Zhang, Z. W.; Huang, X. X.; Shi, Y. G., Genome-wide association study of milk and reproductive traits in dual-purpose Xinjiang Brown cattle. Bmc Genomics 2019, 20, (1). [CrossRef]
- Wei, C.; Luo, H. P.; Wang, Y. C.; Huang, X. X.; Zhang, M. H.; Zhang, X. X.; Wang, D.; Ge, J. J.; Xu, L.; Jiang, H.; Ju, X., Analyses of the genetic relationships between lactose, somatic cell score, and growth traits in Simmental cattle. Animal 2021, 15, (1). [CrossRef]
- FU, M. Z.; Wang, W.; Yi, J., New dairy cattle breed Shu Xuanhua Cattle. Rural Pundit 2023, (3), 35.
- Tvrzicka, E.; Kremmyda, L. S.; Stankova, B.; Zak, A., Fatty acids as biocompounds: their role in human metabolism, health and disease--a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2011, 155, (2), 117-30.
- Mohan, M. S.; O'Callaghan, T. F.; Kelly, P.; Hogan, S. A., Milk fat: opportunities, challenges and innovation. Crit Rev Food Sci Nutr 2021, 61, (14), 2411-2443.
- Sales-Campos, H.; de Souza, P. R.; Peghini, B. C.; da Silva, J. S.; Cardoso, C. R., An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini-Reviews in Medicinal Chemistry 2013, 13, (2), 201-210.
- Zárate, R.; el Jaber-Vazdekis, N.; Tejera, N.; Pérez, J. A.; Rodríguez, C., Significance of long chain polyunsaturated fatty acids in human health. Clinical and Translational Medicine 2017, 6. [CrossRef]
- D'Angelo, S.; Motti, M. L.; Meccariello, R., ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, (9). [CrossRef]
- Simopoulos, A. P., The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & Pharmacotherapy 2002, 56, (8), 365-379.
- Goulding, D. A.; Fox, P. F.; O'Mahony, J. A., Milk proteins: An overview. 2020; p 21-98.
- Pastuszka, R.; Barlowska, J.; Litwinczuk, Z., Allergenicity of milk of different animal species in relation to human milk. Postepy Higieny I Medycyny Doswiadczalnej 2016, 70, 1451–1459. [CrossRef]
- Layman, D. K.; Lönnerdal, B.; Fernstrom, J. D., Applications for α-lactalbumin in human nutrition. Nutrition Reviews 2018, 76, (6), 444-460. [CrossRef]
- Wang, B.; Timilsena, Y. P.; Blanch, E.; Adhikari, B., Lactoferrin: Structure, function, denaturation and digestion. Critical Reviews in Food Science and Nutrition 2019, 59, (4), 580-596.
- Antoshin, A. A.; Shpichka, A. I.; Huang, G.; Chen, K.; Lu, P.; Svistunov, A. A.; Lychagin, A. V.; Lipina, M. M.; Sinelnikov, M. Y.; Reshetov, I. V.; Timashev, P. S., Lactoferrin as a regenerative agent: The old-new panacea? Pharmacol Res 2021, 167, 105564.
- GB5009.6-2016, National Food Safety Standards Determination of fat in food. The National Health and Family Planning Commission of the People's Republic of China and the State Food and Drug Administration: Beijing, 2016; pp 7-9.
- GB5009.168—2016, National Food Safety Standards Determination of Fatty Acids in Food. The National Health and Family Planning Commission of the People's Republic of China and the State Food and Drug Administration: Beijing, 2016; pp 8-11.
- HAN, Z. Q.; FANG, J. H., Technology of Extracting Casein from Milk in Laboratory. Animal Husbandry and Feed Science 2010, 31, (08), 83-85.
- Ostertag, F.; Schmidt, C. M.; Berensmeter, S.; Hinrichs, J., Development and validation of an RP-HPLC DAD method for the simultaneous quantification of minor and major whey proteins. Food Chemistry 2021, 342. [CrossRef]
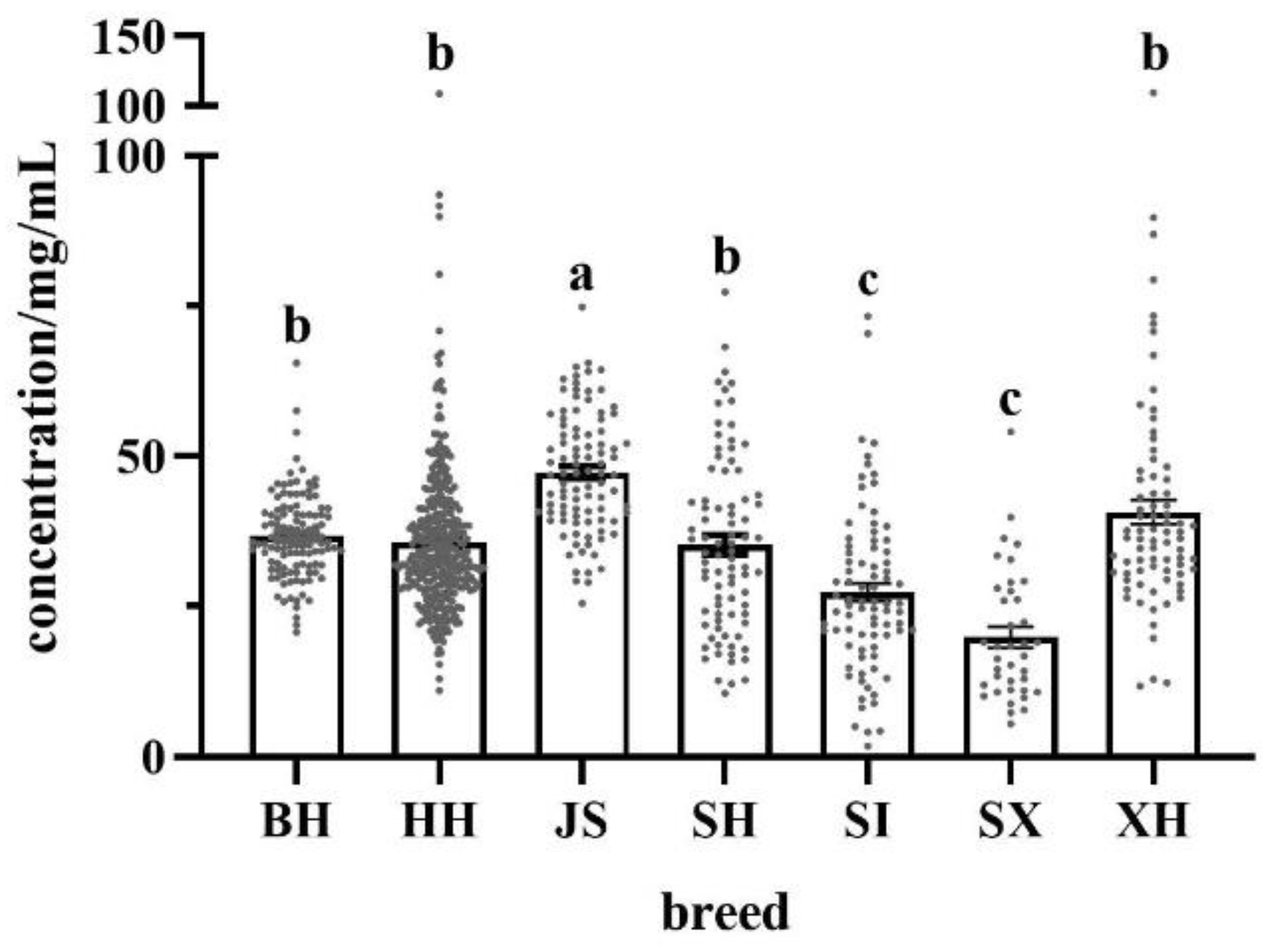
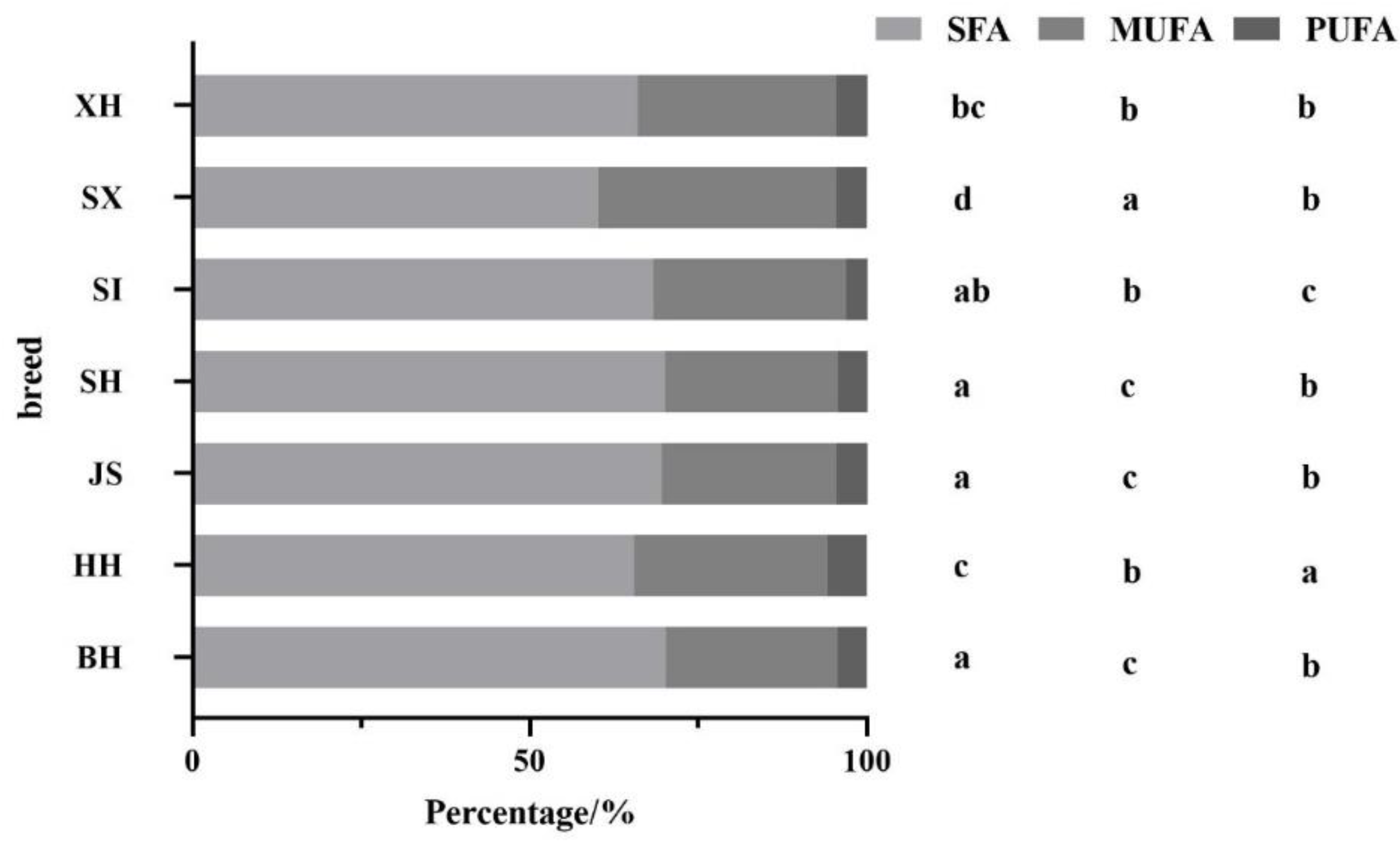
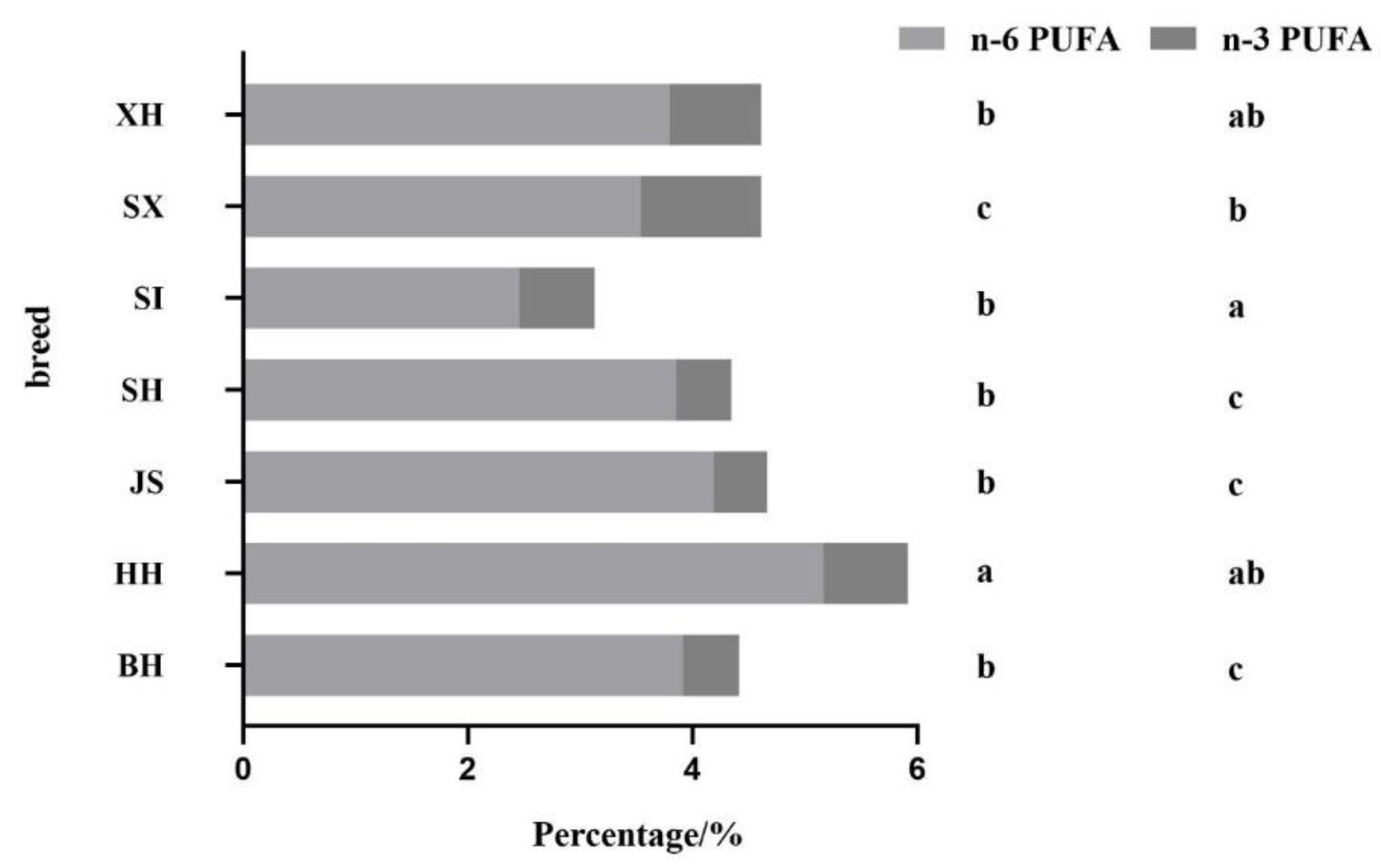
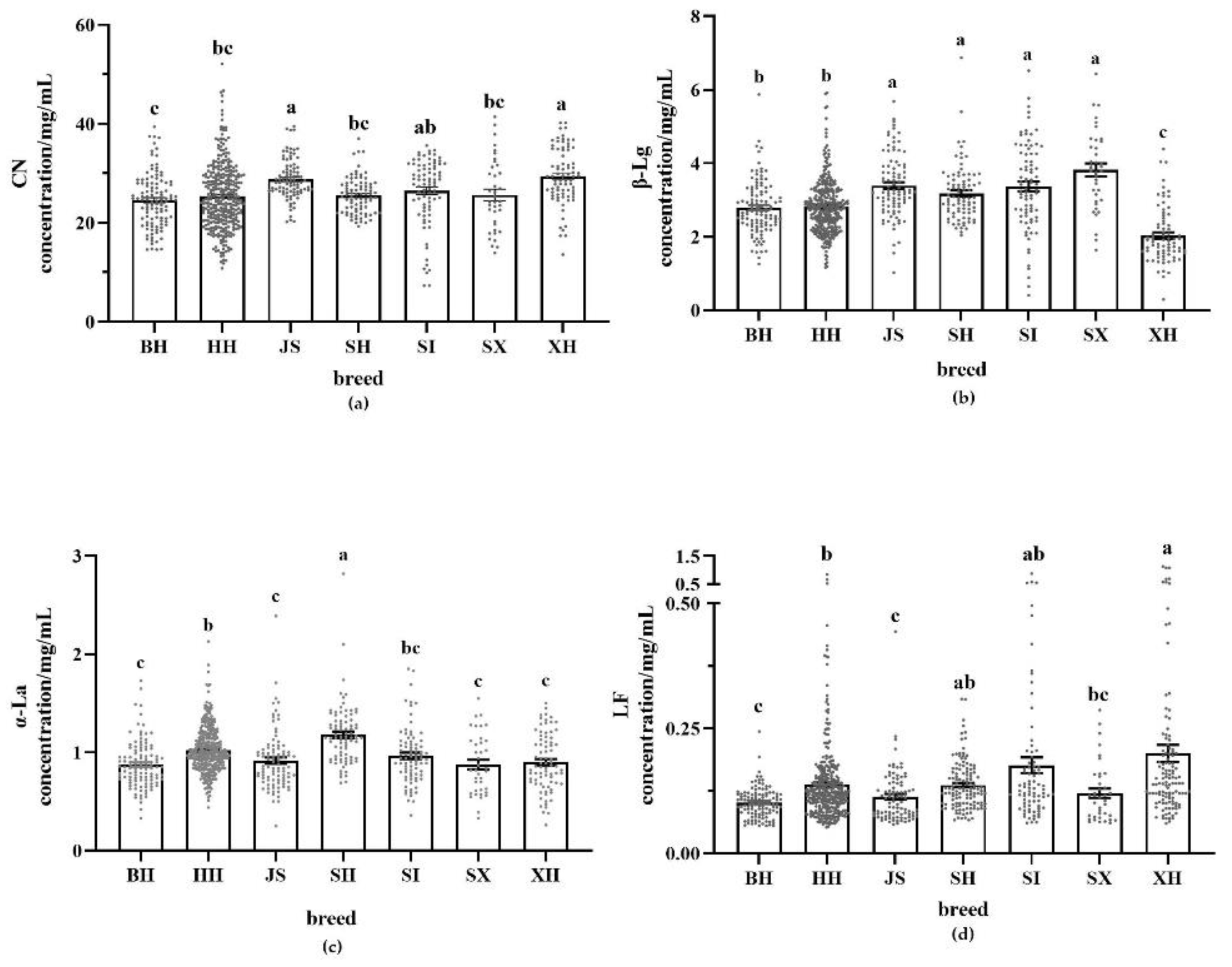
| Breed | Coefficient of variation/% |
|---|---|
| BH (n=115) | 19.19 |
| HH (n=348) | 33.73 |
| JS (n=94) | 21.11 |
| SH (n=84) | 41.79 |
| SI (n=82) | 49.37 |
| SX (n=38) | 53.28 |
| XH (n=78) | 42.41 |
| Fatty acid | Breed | ||||||
|---|---|---|---|---|---|---|---|
| BH (n=115) |
HH (n=348) |
JS (n=94) |
SH (n=84) |
SI (n=82) |
SX (n=38) |
XH (n=78) |
|
| C6:0 | 0.467±0.013b | 0.384±0.010c | 0.590±0.016a | 0.553±0.026ab | 0.236±0.015d | 0.143±0.014d | 0.555±0.030ab |
| C8:0 | 0.410±0.014c | 0.422±0.010c | 0.668±0.019a | 0.610±0.028ab | 0.340±0.019c | 0.154±0.013d | 0.634±0.036a |
| C10:0 | 0.686±0.025c | 0.640±0.017c | 1.190±0.045a | 0.942±0.046b | 0.417±0.027d | 0.210±0.020d | 0.995±0.060ab |
| C12:0 | 0.902±0.029c | 0.877±0.026c | 1.699±0.069a | 1.268±0.066b | 0.553±0.037d | 0.293±0.028d | 1.340±0.087b |
| C14:0 | 2.392±0.063c | 2.739±0.078c | 4.437±0.109a | 3.369±0.148b | 1.760±0.113d | 1.072±0.113d | 3.720±0.218b |
| C14:1 | 0.196±0.006b | 0.396±0.010a | 0.431±0.024a | 0.468±0.023a | 0.159±0.011b | 0.120±0.015b | 0.459±0.032a |
| C15:0 | 0.194±0.006b | 0.275±0.010a | 0.335±0.014a | 0.357±0.018a | 0.189±0.013b | 0.158±0.022b | 0.309±0.019a |
| C16:0 | 7.120±0.194c | 9.786±0.242b | 10.913±0.281a | 9.277±0.429b | 5.758±0.365cd | 3.971±0.397d | 9.547±0.503b |
| C16:1 | 0.329±0.010b | 0.630±0.017a | 0.518±0.017a | 0.536±0.028a | 0.261±0.017b | 0.302±0.046b | 0.537±0.031a |
| C18:0 | 1.995±0.057cd | 2.402±0.081c | 3.869±0.126a | 2.119±0.110cd | 1.744±0.136d | 1.467±0.127d | 3.208±0.169b |
| C18:1 | 4.571±0.127c | 6.510±0.169b | 7.742±0.242a | 5.821±0.348b | 4.140±0.283bc | 4.046±0.392c | 7.934±0.458a |
| C18:2 | 0.738±0.020c | 1.298±0.031a | 1.356±0.051a | 0.923±0.039bc | 0.345±0.022d | 0.379±0.033d | 1.055±0.051b |
| C18:3 | 0.094±0.002c | 0.194±0.006ab | 0.148±0.005b | 0.116±0.005c | 0.099±0.007c | 0.124±0.016c | 0.231±0.015a |
| C20:1 | 0.044±0.001c | 0.071±0.002b | 0.086±0.004a | 0.068±0.004b | 0.052±0.005c | 0.061±0.009bc | 0.091±0.005a |
| C20:4 | 0.0458±0.0018b | 0.0710±0.0020a | 0.0821±0.004a | 0.0712±0.0039a | 0.0308±0.0029c | 0.0411±0.0058bc | 0.0820±0.0046a |
| C20:5 | 0.0040±0.0002c | 0.0047±0.0002c | 0.0085±0.0008b | 0.0060±0.0005b | 0.0022±0.0003d | 0.0044±0.0010cd | 0.0121±0.0009a |
| C22:6 | 0.0019±0.0001d | 0.0030±0.0002bc | 0.0028±0.0002bcd | 0.0034±0.0003b | 0.0030±0.0002bc | 0.0028±0.0007cd | 0.0047±0.0003a |
| Fatty acid | Breed | ||||||
|---|---|---|---|---|---|---|---|
| BH (n=115) |
HH (n=348) |
JS (n=94) |
SH (n=84) |
SI (n=82) |
SX (n=38) |
XH (n=78) |
|
| C6:0 | 30.80 | 47.06 | 26.19 | 43.08 | 57.41 | 59.67 | 48.11 |
| C8:0 | 37.92 | 44.30 | 28.11 | 41.73 | 51.80 | 53.48 | 49.88 |
| C10:0 | 38.34 | 48.69 | 37.06 | 45.14 | 59.31 | 57.81 | 53.68 |
| C12:0 | 34.46 | 54.81 | 39.50 | 47.53 | 60.10 | 60.01 | 57.31 |
| C14:0 | 28.47 | 53.39 | 23.72 | 40.26 | 58.02 | 64.80 | 51.80 |
| C14:1 | 34.53 | 47.37 | 54.34 | 45.11 | 64.21 | 76.27 | 60.78 |
| C15:0 | 32.75 | 66.13 | 41.54 | 47.54 | 63.65 | 86.47 | 53.31 |
| C16:0 | 29.25 | 46.05 | 24.94 | 42.36 | 57.35 | 61.68 | 46.55 |
| C16:1 | 33.91 | 51.78 | 32.58 | 47.40 | 59.17 | 93.26 | 51.47 |
| C18:0 | 30.57 | 63.14 | 31.55 | 47.79 | 70.59 | 53.29 | 46.55 |
| C18:1 | 29.86 | 48.31 | 30.29 | 54.72 | 61.94 | 59.69 | 51.03 |
| C18:2 | 28.36 | 45.02 | 36.12 | 38.67 | 56.71 | 52.89 | 42.50 |
| C18:3 | 26.08 | 57.98 | 29.82 | 41.24 | 59.58 | 79.85 | 57.38 |
| C20:1 | 30.96 | 58.60 | 45.64 | 53.66 | 84.96 | 93.24 | 49.59 |
| C20:4 | 42.68 | 52.85 | 47.01 | 50.58 | 84.69 | 87.75 | 49.81 |
| C20:5 | 58.94 | 73.95 | 96.20 | 74.30 | 109.70 | 144.81 | 62.17 |
| C22:6 | 53.67 | 95.30 | 75.64 | 90.62 | 69.73 | 158.64 | 54.37 |
| Fatty acid | Breed | |||||||
|---|---|---|---|---|---|---|---|---|
| BH (n=115) |
HH (n=348) |
JS (n=94) |
SH (n=84) |
SI (n=82) |
SX (n=38) |
XH (n=78) |
||
| SFA | 14.165±0.354c | 17.523±0.435b | 23.701±0.552a | 18.494±0.819b | 10.997±0.699d | 7.467±0.698d | 20.308±1.074b | |
| MUFA | 5.139±0.139d | 7.607±0.191b | 8.777±0.269a | 6.893±0.385c | 4.612±0.311d | 4.530±0.438d | 9.022±0.517ab | |
| PUFA | 0.884±0.023c | 1.571±0.038a | 1.598±0.056a | 1.119±0.046b | 0.480±0.030d | 0.551±0.051cd | 1.384±0.067ab | |
| n-6 PUFA | 0.784±0.021d | 1.369±0.033ab | 1.438±0.053a | 0.994±0.042c | 0.376±0.024e | 0.420±0.037e | 1.137±0.055bc | |
| n-3 PUFA | 0.100±0.002c | 0.201±0.006b | 0.160±0.005b | 0.125±0.006c | 0.105±0.007c | 0.131±0.016c | 0.247±0.016a | |
| n-6/n-3 | 7.8 | 7.5 | 9.4 | 8.2 | 3.8 | 4.1 | 5.0 | |
| Protein | Milk type | ||||||
|---|---|---|---|---|---|---|---|
| BH (n=112) |
HH (n=341) |
JS (n=93) |
SH (n=84) |
SI (n=82) |
SX (n=38) |
XH (n=78) |
|
| CN | 21.98 | 26.08 | 13.91 | 14.17 | 25.59 | 27.86 | 19.42 |
| β-Lg | 26.13 | 25.66 | 25.22 | 23.91 | 35.23 | 27.56 | 36.82 |
| α-La | 27.18 | 22.39 | 32.96 | 26.03 | 29.59 | 34.44 | 32.05 |
| LF | 29.92 | 59.80 | 46.55 | 36.30 | 79.02 | 48.07 | 91.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





