Introduction
The San Francisco Estuary (estuary), including the Sacramento-San Joaquin Delta (Delta), Suisun Bay, and Suisun Marsh, has been experiencing the effects of global climate change for at least the past forty years. Each of the past four decades has been successively warmer than all previous decades and the 2010’s have been the warmest decade on record (Cloern et al. 2010, Goss et al. 2020, IPCC 2021). Climate change models predict a continuation of these trends, with a temperature increase of 2 to 4°C over the next century (Dettinger et al. 2016, Knowles et al. 2018, Pierce et al. 2018), more precipitation falling as rain instead of snow (Dettinger et al. 2016), increased precipitation variability (Swain et al. 2018), increased extreme precipitation (Polade et al. 2017, He 2022a), and increased saltwater intrusion due to a combination of reduced freshwater outflow and sea level rise (Dettinger et al. 2016, Polade et al. 2017, Knowles et al. 2018, Pierce et al. 2018, Swain et al. 2018, Ghalambor et al. 2021).
The impact of climate change on estuaries is of worldwide concern because estuaries provide key ecosystem services, including nursery habitat for fisheries, buffering development from storms, carbon sequestration, transportation, and recreation ((Barbier et al. 2011). However, estuaries are also frequently highly developed, and thus already threatened by human impacts in addition to climate change. A 12-year monitoring study of 166 estuaries in Australia showed an increase in temperature and acidification rate over that time span, with the final values for both exceeding the Intergovernmental Panel on Climate Change (IPCC) projection by 2100 (Scanes et al. 2020). The changes observed are significantly more extreme than the predictions from global models, which indicates a need for regional-scale estuarine models (Collins et al. 2012, Knowles et al. 2018, Scanes et al. 2020). Analysis of expected and potential impacts to estuaries in Australia, the United Kingdom and Chesapeake Bay found wide-ranging impacts to species from the locally predicted changes in precipitation, water temperature and chemistry, salinity, flow, primary productivity, turbidity, and geomorphology (Gillanders et al. 2011, Robins et al. 2016).
Within the estuary, there is considerable regional variability in measured water temperature (Bashevkin et al. 2022b). While water temperature is controlled primarily by air temperature (Vroom et al. 2017), inflow and precipitation also interact with seasonal and spatial water temperature patterns in the system (Bashevkin and Mahardja 2022, Bashevkin et al. 2022b). As with estuaries worldwide, the estuary will increase in temperatures with climate change, and local models have projected up to a 4°C increase in annual mean water temperature between 2020 and 2099, with greater increases in temperature predicted during the summer than the winter (He 2022b).
The estuary is home to many native fish species, including several that are listed as threatened or endangered under the state or federal Endangered Species Acts (hearafter - "listed species"; CDFW 2022). How water temperature affects these species on a regional scale is important for identifying climate change refugia and potential conservation actions for such listed species. This idea has been applied to many cold-water stream systems across the United States, where restoration of riparian vegetation, bank structure, and flows has been targeted to preserve habitat for sensitive fish species (Kurylyk et al. 2015, Ebersole et al. 2020), but has rarely been evaluated in estuaries. To enhance our understanding of ecological interactions and implications relative to water temperature, we examined spatial and seasonal water temperature trends in relation to a number of listed and unlisted species that were focused around 1) resource management (e.g., state or federally listed endangered or threatened species), 2) species that may negatively affect management-relevant species (e.g., non-native predators and competitors, benthic invertebrates that affect the lower trophic food web, aquatic vegetation, which may negatively impact habitat, and toxic cyanobacteria), and 3) other native fishes where information is less known.
Listed fishes in the estuary including Delta Smelt (Hypomesus transpacificus), Longfin Smelt (Spirinchus thaleichthys), spring run and winter run Chinook Salmon (Oncorhynchus tshawytscha), and Green Sturgeon (Acipenser medirostris) are particularly sensitive to warm water temperature (Mayfield and Cech 2004, Komoroske et al. 2014, Jeffries et al. 2016, Zillig et al. 2021). Each species has a limited temperature optimum (the temperature range for optimal performance) such that increasing water temperature may increase chronic temperature stress and decrease health, growth, and/or reproductive fitness (Fangue et al. 2020). Negative changes in physiological and behavioral performance can affect growth, survival, and recruitment and limit or exclude native species from the estuary (Lewis et al. 2021). For example, an analysis of the spawning window of the endangered Delta Smelt predicted that under most climate change scenarios, they may no longer be able to reproduce in the estuary (Brown et al. 2016, Hobbs et al. 2019), though an alternative model found their spawning window may expand, but shift earlier in the year (Huntsman 2024). Warming water temperature increases stressful days for juveniles and initiates earlier spawning which would decrease the maturation window and also likely have negative impacts on fitness (Brown et al. 2016). Therefore, it is important to identify when and where thermally suitable habitats occur for native species of concern to focus conservation and habitat restoration efforts.
Here, we use continuous (real-time) water temperature data and literature on fish physiology and field detections to address the following three objectives with the study goal to better understand how warming temperatures may impact aquatic species:
- (1)
Describe the inter-annual variation in water temperature in the estuary between 2010-2019 and determine whether spatial differences are detectable
- (2)
-
52152
- a.
Determine species thermal sensitivities based on cited literature and field data
- b.
Evaluate how thermal stress varies by species and Endangered Species Act (ESA) listed versus non-listed status
- (3)
Assess what parts of the estuary may provide thermal refuge at different temporal scales
We combined records from continuous temperature probes from the past 10 years with data on the thermal sensitivity of aquatic organisms to assess future impacts of warming on the Delta ecosystem. First we examined how temperature has varied temporally over the previous decade and how temperature varies regionally across the Delta. Next we conducted a literature review to describe thermal sensitivities of native and non-native fishes, invertebrates, and primary producers. We then combined thermal sensitivity data to establish thresholds for each taxon and examine how habitat suitability and thermal stress is likely to vary spatially and through time as the climate continues to warm.
Resource managers rely on the use of temperature thresholds and habitat suitability models to assess risk to listed species and make decisions for real-time water operations and inform potential locations for habitat improvements via restoration or flow augmentations. This information will help guide species management and conservation measures needed to lessen the impact that stressful thermal conditions will have due to continued drought periods and climate change.
Materials and Methods
Study Area
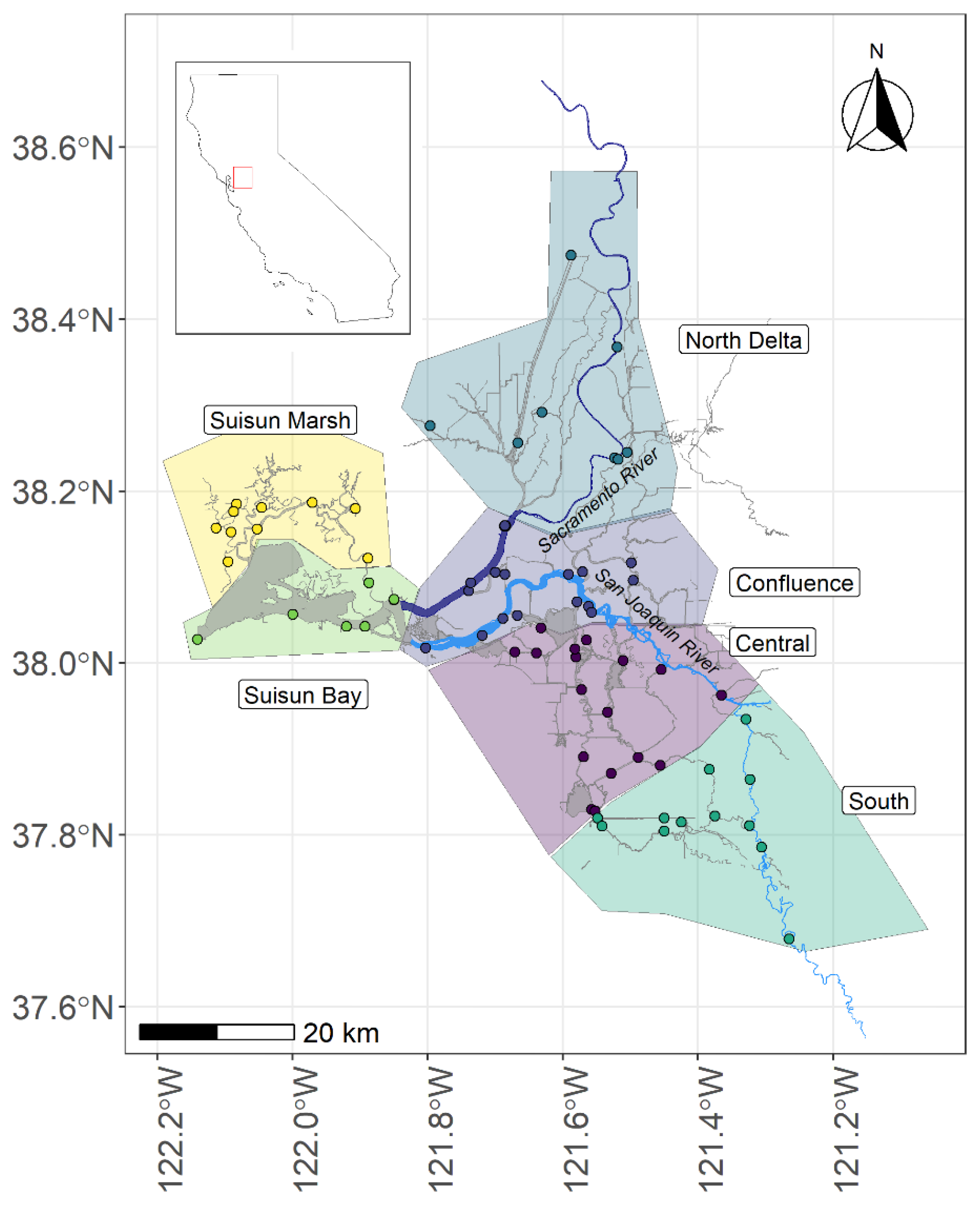
The study was conducted in the Upper San Francisco Estuary, including the Sacramento-San Joaquin Delta (Delta), Suisun Bay, and Suisun Marsh. The Delta is a tidal inland delta with fresh to brackish water in the Central Valley of California. The Sacramento River provides freshwater from the north, while the San Joaquin River provides freshwater from the south. From the Delta, water flows into Suisun Bay and Suisun Marsh before entering the San Franisco Bay on its way into the Pacific Ocean. The system plays an important role in water delivery to the state of California, and, as a result, has an extensive network of environmental monitoring stations (
Figure 1; Table S1).
Data Compilation, Cleaning, and Filtering
We compiled data from the California Data Exchange Center (CDEC;
https://cdec.water.ca.gov/), which hosts non quality-controlled real-time water quality data from several different monitoring programs. We downloaded all available event (15-minute) and hourly water temperature data in the estuary using the “CDECRetrieve” package (Rodriguez E 2022). We then standardized all data to hourly data by taking the first value of each hour. The full integrated dataset and associated metadata, including details on the sensors and data contacts, are available on the Environmental Data Initiative (EDI) (IEP et al. 2020).
We removed data that were of questionable quality based on the following criteria: 1) values outside a range of 1-40°C based on our knowledge of the system, 2) any day with fewer than 20 values (out of 24), 3) any day with 18 or more repeating values, 4) outliers (defined as values outside 3 times above or below the interquartile range) on the remainder component of the seasonally decomposed dataset, and 5) values where there was greater than a 5 degree change in temperature within an hour. Approximately 2.8% of values were removed due to these steps. Additional details and code for downloading data and QA/QC are available on EDI (IEP et al. 2020). We then removed 15 stations that were not representative of our study (non-contiguous stations, stations not in the Delta, some duplicate stations), and filtered to 2010-2019 as most of the stations initiated monitoring by 2010, removing 36.2% of remaining values. We then removed station-years with 275 days or fewer days per year and 10 months or fewer months of data per year to avoid biasing calculated summary statistics. Based on these filters, we then removed stations with fewer than 8 years of data. Approximately 26.9% of remaining data were removed due to these filters, with a final dataset composed of 6,149,782 values across 75 stations (Table A1; see
https://deltascience.shinyapps.io/ContinuousWaterTempQC/ for spatial representation of all stations originally considered).
Region Assignments
To identify regions within the study area with similar thermal regimes, we calculated the daily mean temperature (Tavg) for each station for each day of the year over the entire time frame of the dataset (2010-2019). We then used hierarchical cluster analysis on the Euclidian distance between the Tavg for each day of the year (function ‘hclust’ in R version 4.1.1; R Core Team 2021) with Ward’s minimum variance method. We based geographic regions on cluster assignments, but did adjust assignments slightly based on spatial proximity.
Water Temperature Patterns
To address objective 1, we used our compiled water temperature dataset and tested for statistically significant trends in average maximum (T
max) and minimum (T
min) water temperature. Due to the limited temporal scale of our dataset and the known variability between years, we did not aim to detect a trend over time, but instead looked at differences between individual years and regions-seasons combinations. To reduce temporal auto-correlation, we calculated monthly average T
max and T
min and ran mixed effects models with maximum likelihood as an estimation method. We conducted analyses in R Version 4.2.1 (R Development Core Team 2021). We used the “lme4” package to run mixed effects models (Hothorn et al. 2008, Wood 2017, Lenth et al. 2022). We included the fixed effects of water year (October 1 of calendar year-1 to September 30 of calendar year), season, and region, with an interaction term between season and region. We also included station and month as random effects. We conducted post-hoc tests using the Bonferroni correction and because there was some temporal auto-correlation, we used a more conservative alpha = 0.01 to distinguish differences between pairs.
where
Temp = average monthly maximum or average monthly minimum water temperature (°C),
WY = water year as a factor, where WYi = October 1i-1 to September 30i
Season = season as a factor,
where wet season = October-April and dry season = May-September
Region = region as a factor, as defined by
Figure 1,
Station = station as a factor, included as a random effect on the intercept (denoted by ‘(1|…)’) to account for spatial autocorrelation
Month = month as a factor, included as a random effect on the intercept (denoted by `(1|…)`).
Species Temperature Thresholds
To evaluate the biological implications of water temperature patterns and address objective 2a, we selected key native and non-native fishes, invertebrates, and primary producers relevant to the estuary. We then conducted a literature review of available temperature thresholds, and summarized long-term field survey data to develop a single integrated table of temperature thresholds. We describe different temperature threshold terms in
Table 1.
For each species and life stage (where possible) we included two documented or estimated temperature thresholds: suboptimum temperature (T
sopt) and maximum temperature tolerance (T
tol). We defined T
sopt as the temperature value outside documented thermal optima at which physiology ‘turns for the worse’ and performance decreases (Fangue et al. 2020). Suboptimal temperatures are considered moderately stressful and can reduce energy diverted to growth, reproduction, and activity due to the increased energy demand for basic maintenance mechanisms (Sokolova et al. 2012). For this study, we focused only on T
sopt when temperatures are warmer than optimal, not T
sopt when temperatures are cooler than optimal. The second species threshold, T
tol, we described as the upper temperature limit for survival. We used combined literature on critical thermal maxima, similar end-point measures, and mortality to assign T
tol thresholds, thus we list a range of threshold values found in
Table 2.
As a quality check of the T
sopt and T
tol assignments, many of which came from laboratory studies, we compared threshold values to temperatures associated with fish field detections (i.e. the field detection temperatures should not be greater than the tolerances obtained from literature). We calculated the mean, 75% quantile, and maximum water temperature at which species were detected in field surveys using data from the “deltafish” package (Clark and Bashevkin 2022), which accesses an integrated dataset of 9 long-term fish monitoring surveys in the estuary from 1959 to 2021 (Bashevkin et al. 2022a). While some species have distinct life stage nomenclature (e.g. salmonid alevins, fry, etc.), given variation in life-stage designations within the literature and among monitoring surveys, we standardized all species into three life stages (larvae, juveniles, adults) for consistency and generalization of analyses. We subsequently use species thresholds to inform the following analysis of heat stress in the estuary and species sensitivity to estuary temperature (
Table 2). Additional details about threshold and life stage determinations have been published in Davis et al. (2022).
Thermal Stress and Suitability
We compared water temperature data to species thresholds to determine how thermal stress varied among different species and life stages (Objective 2a), and to determine which areas of the estuary might provide thermally suitable habitat or refuge (Objective 3). These analyses all use measured continuous water temperature data to examine data at different scales.
Regional and Seasonal Vulnerability
We calculated a ‘temperature margin’ (T
mar) for species-life stages by season and region to indicate how close species were to experiencing temperatures above their thresholds. We first filtered seasonal and regional presence for each species-life stage of interest using fish monitoring data. We used the “deltafish” package (Clark and Bashevkin 2022) to access the integrated fish dataset (Bashevkin et al. 2022a), plus additional fish datasets from fish salvage counts collected at the State Water Project pumping facility (
https://filelib.wildlife.ca.gov/Public/salvage/), from the Yolo Bypass Fish Monitoring Program (Interagency Ecological Program et al. 2022), and from CDFW Trammel Net Survey (Stompe and Hobbs 2023) to determine regional and seasonal presence of fishes. Presence of species and life stages was determined by there being >1 detection of that species-life stage combination in the given region and season during the entire duration of the dataset based on life stage-length designations specified in Davis et al. (2022). Thus, a species-life stage classified as “present” in a region and season may not currently or commonly be present in a region where it is labeled as “present,” but there is some historical precedence for its presence there. It is also important to note that cases in which species were classified as “not present,” do not mean species are absent in the region, but that they were not detected in the particular surveys we used.
We calculated Tmar as the difference between a species’ Ttol (lower value if there was a range of values) and the Tmax (mean maximum temperature for a given station, month, and water year averaged across season and region; Figure S7), which is a slightly modified approach to add ecological relevance to fish presence. This was in contrast to previous temperature margin models that calculate the difference between a species tolerance and maximum field temperature (Deutsch et al. 2008, Davis et al. 2019). We then binned the Tmar values to visually assess level of vulnerability across species and life-stages where possible. Tmar bins from most vulnerable to least vulnerable included <0°C, 0-3°C, 3-6°C, and >6°C. Season was designated as follows: Winter = January-March, Spring = April-June, Summer = July-September, Fall = October-December.
Thermal Stress by Species Life Stages
We calculated the number of stressful days experienced per year based on T
sopt and T
tol thresholds for a range of species and life stages (
Table 2). Species and life stages that lacked T
sopt and T
tol were not included in analyses. To reduce inflation of stressful days, for each species-life stage, water temperature data were filtered to season-region combinations where the species-life stage was present, based on fish monitoring data. T
sopt and T
tol were compared to daily T
max at each station to determine whether the observed temperature was above the species threshold. To be moderately conservative with our estimates of suitability, within each region, if >30% of stations in a region were above the water temperature threshold (T
sopt or T
max) for a particular day, that entire region was considered to be above the threshold for that day. For each species-life stage, we summed days exceeding the water temperature thresholds for the suboptimum (E
sopt) or tolerance (E
max) by water year, and created boxplots to show the range of days exceedance across the 10 years of the dataset. We were not able to acquire both kinds of thresholds for all species of interest and life stages, hence not all species were included in exceedance analysis, but threshold information is provided in
Table 2. If there was a range of thresholds reported in the literature, we used the lower threshold value for our visualizations, and reported results for lower and upper thresholds.
Thermal Stress by Listing Status
To assess whether there were differences in E
max by species ESA listing status (i.e. listed or non-listed), we filtered the datasets to the adult life stages of fishes and repeated the model for both the upper and lower range values of T
tol. We did not conduct analyses for T
sopt due to a lack of available threshold values in the literature. We ran a mixed effects models with a binomial distribution to represent proportional data using the “lme4” package (Bates et al. 2016), with the fixed effect of listed status and the random effect of species:
where
pTOL = proportion of days per year exceeding tolerance threshold,
status = listed or unlisted, and
(1|species) = random effect of species
We assessed residuals for assumptions of homogeneity of variance and normality.
Daily Thermal Stress by Station
We visualized the proportion of each day that temperatures were >22°C. While other analyses have focused on mean and maximum daily temperatures, these visualizations allows us to better understand, on multiple scales, when and where certain species may be able to recover. We are also able to note differences within a region to identify particularly warm or cool stations.
We used the continuous water temperature dataset to calculate, for each station and day of the year between April and September, the proportion of each day that was >22°C. We selected 22°C to represent the T
sopt of Delta Smelt, which is a species for which management actions occur in the summer, when temperatures are warmest. However, this 22°C also represents temperatures that are suboptimal for Green Sturgeon, White Sturgeon, Sacramento Suckers, Prickly Sculpin, Tule Perch,
O. mykiss, Chinook Salmon, Sacramento Splittail, Sacramento Pikeminnow, and Longfin Smelt (see T
sopt in
Table 2). Temperatures at each station were averaged across the dataset for each calendar day. To further put the analysis in context of warming temperatures, we also examined how potential recovery temperatures might differ between the whole dataset and warm years by comparing the same metric for the whole dataset (2010-2019) and the three warmest (2014, 2015, 2016) years in our dataset.
Results
Region Assignments
After clustering stations into six thermal regimes based on Tavg across the year (Figure S1), we saw the thermal regimes frequently fit into geographic regions (Figure S2). Most stations in the far south of the Delta had a similar thermal regime (Cluster 3), the Central Delta also had a similar thermal regime (Cluster 2), Suisun Marsh were mostly in the same thermal regime (Cluster 4), as was Suisun Bay (Cluster 5). The Lower San Joaquin River and the Lower Sacramento River had two clusters interspersed (Custers 1 and 6), but were combined into a single ‘Confluence’ region due to geographic proximity. The Cache Slough and Sacramento River in the northern Delta also had stations representing a several thermal regimes, but were combined for ease of analysis.
Water Temperature Patterns
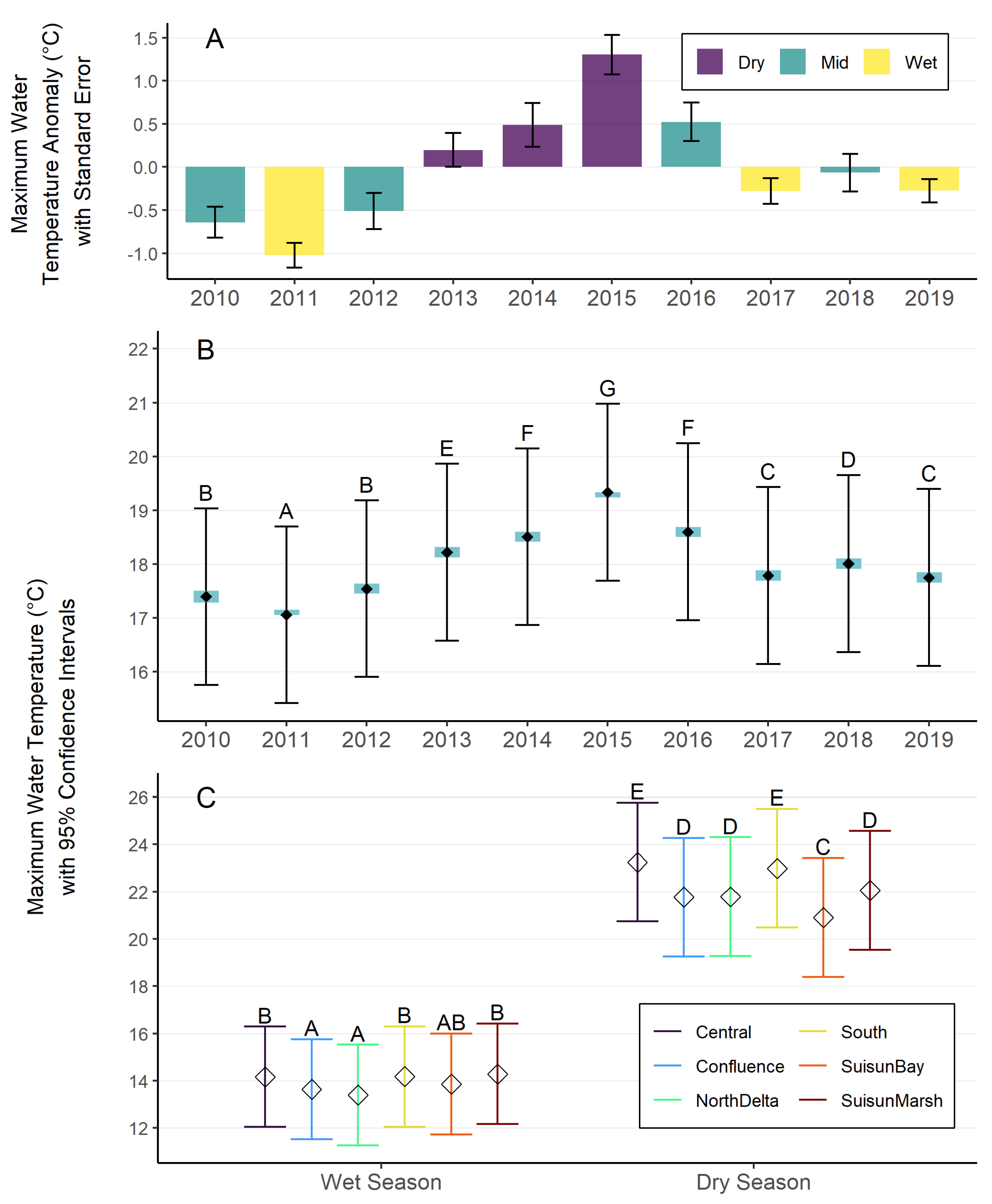
We ran a mixed effects model on maximum and minimum water temperature to statistically test for variation among years, regions, and seasons. We found significant differences between year, region, season, and the interaction between region and season. Monthly average T
max and T
min in 2015 were particularly high (95% CI: 17.7 – 21.0°C for maximum temperature; 16.4 – 20.0°C for minimum temperature), with 2014 and 2016 following as the next hottest years (
Figure 2B; See Supplemental Materials for additional results and model validation). For our comparison of region by wet and dry season, we found that during the dry season (May-September), the Central and South Delta experienced the highest average maximum temperatures, and Suisun Bay experienced the lowest average maximum temperatures (
Figure 2C). During the wet season (October-April), there were less differences in temperature between regions. Suisun Marsh, the South, and the Central Delta experienced the warmest average maximum temperatures, and the North Delta and Confluence experienced the coolest average maximum water temperatures. The wet years had negative anomalies of maximum daily temperatures, whereas most of the dry years had positive anomalies, with 2015 experiencing the highest anomaly (
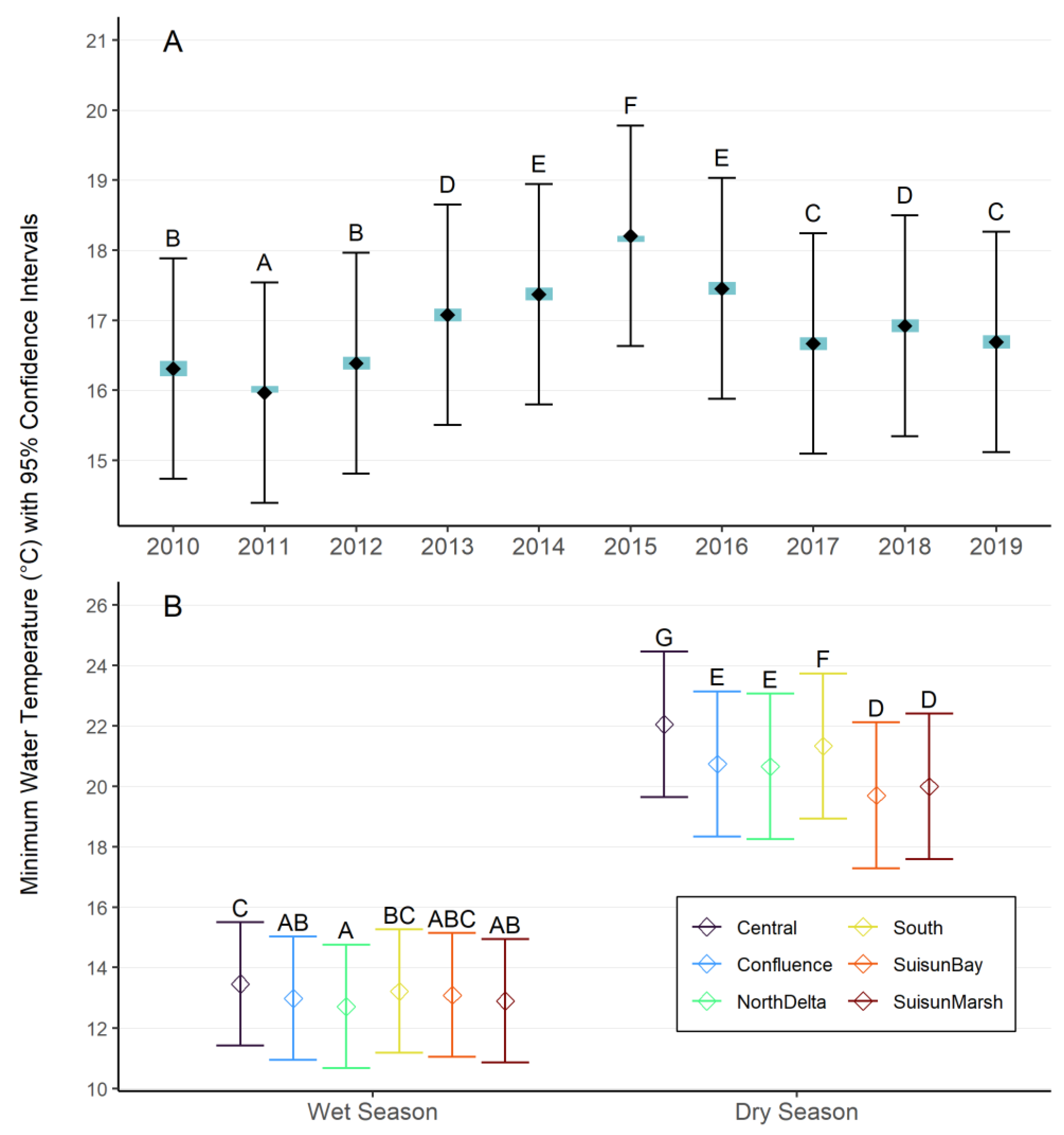
Figure 2A). Trends in model results were generally similar for mean minimum water temperatures, with the exception that mean minimum temperatures in Suisun Marsh were cooler than those in Suisun Bay, reflecting the larger range that the region can experience (
Figure 3A,
Figure 3B; see Supplemental Materials for additional results and model validation).
Thermal Stress and Suitability
Regional and Seasonal Vulnerability
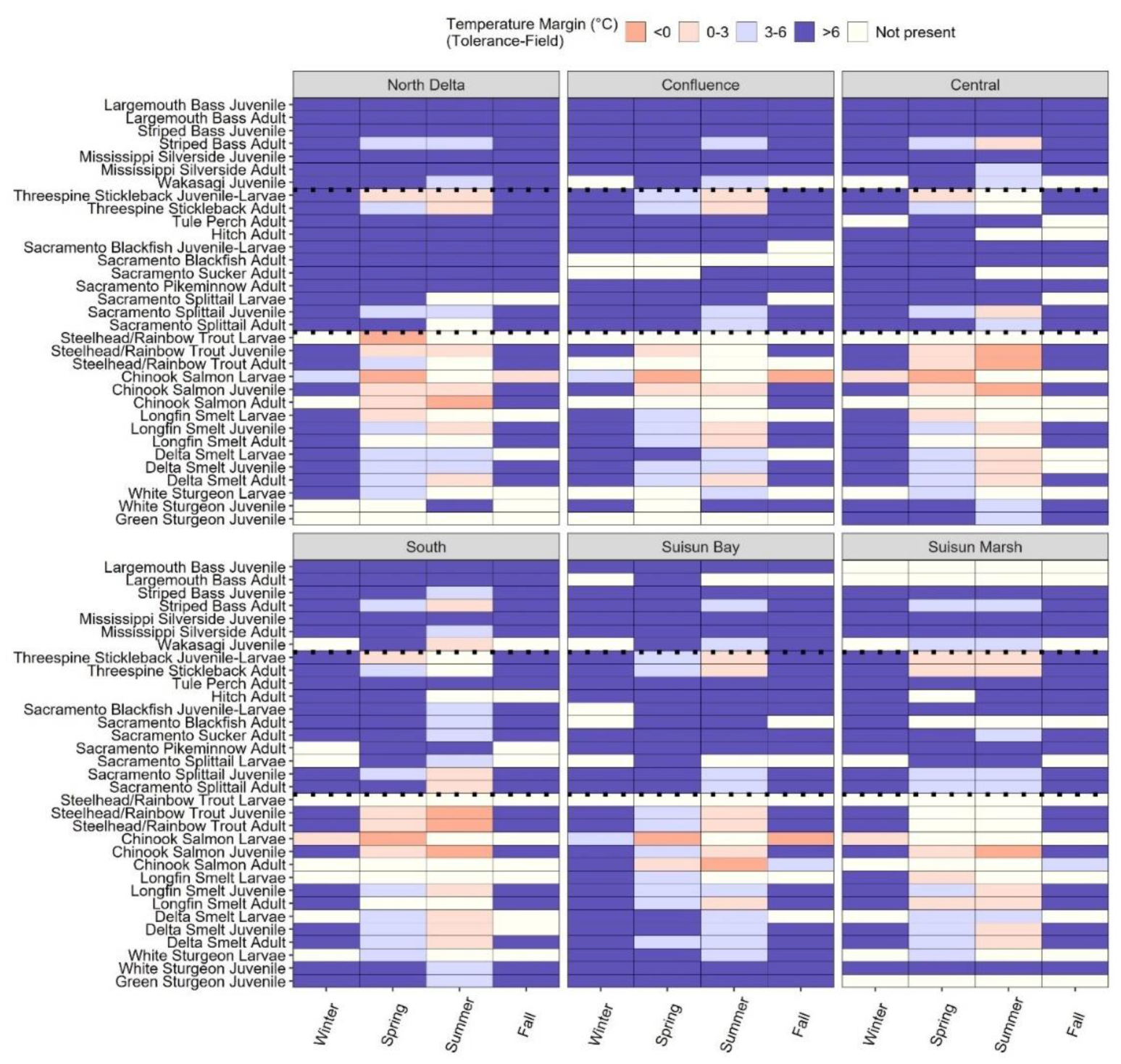
Estimated T
mar varied broadly across species and life stages. Several species, particularly ESA-listed fishes, were within 3°C of their T
tol or were detected in regions that exceeded their T
tol (
Figure 4). Most species had wider T
mar in fall (October-December; >3°C), and all species had robust margins in the winter (January – March; 3-6°C or >6°C). We found negative T
mar (i.e., habitat temperatures were warmer than species thresholds in the <0°C category) for all life stages of Rainbow Trout/Steelhead and Chinook Salmon during spring and summer in at least some of the regions in which they were detected. Species in the 0-3°C T
mar bin included listed salmonids and osmerids, and additional unlisted species such as Striped Bass (adult), Sacramento Splittail (juvenile), and Threespine Stickleback. Sturgeon, Tule Perch, Sacramento Blackfish, Sacramento Sucker, Sacramento Pikeminnow, and most of the non-native fishes also had relatively robust temperature margins. The North Delta, Central Delta, and South Delta had the greatest number of species-life stage combinations in which T
mar was negative or in the 0-3°C bin, and the Confluence and Suisun Bay had the fewest species-life stage combinations that might experience negative or small T
mar.
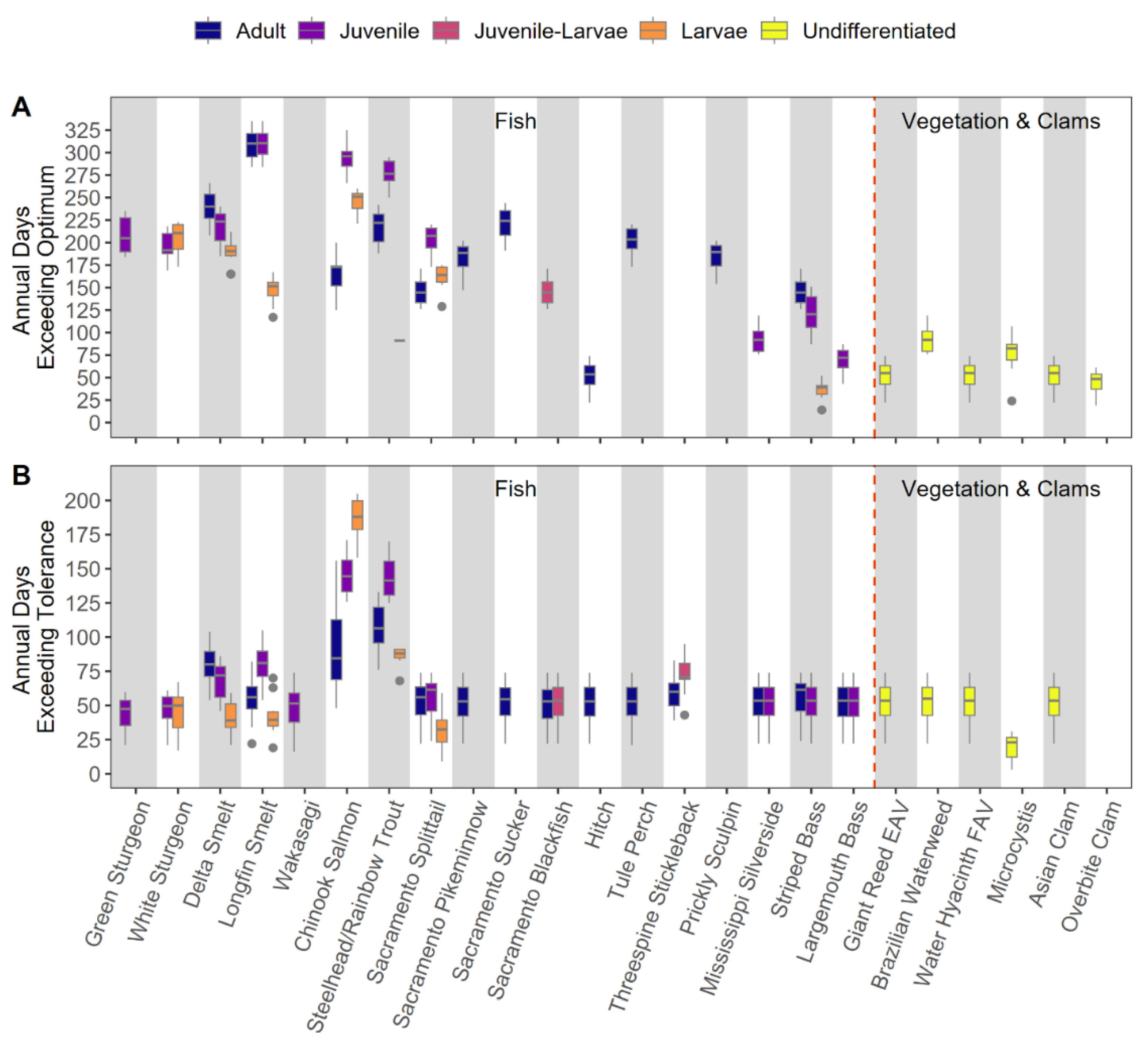
Thermal Stress by Species Life Stages
The mean number of exceedance days across all fish species and life stages in the last ten water years was 138 days for E
sopt and 52 days for E
tol, based on the temperature thresholds selected from the literature (
Figure 5). Many of the listed species, such as osmerids and salmonids, have lower T
sopt and T
tol relative to other species (
Table 3) and thus exhibited greater vulnerability compared with non-native fishes and heat-tolerant native fishes. The fishes with the lowest T
tol, adult Steelhead/Rainbow Trout and juvenile Longfin Smelt, had the greatest vulnerability (mean 81-107 E
tol), and all other species had fairly similar T
max, and thus, similar E
tol (33-59 E
max). When assessing vulnerability by T
sopt, adult and juvenile Longfin Smelt had the greatest E
sopt (309-310 days), followed by juvenile Chinook Salmon, adult Sacramento Sucker, adult Steelhead/Rainbow Trout, adult Tule Perch, and larval White Sturgeon (>200 days E
sopt) (
Figure 5;
Table 3). While E
tol were relatively similar among fishes, certain lifestages of Sacramento Splittail, Hitch, Striped Bass, Largemouth Bass, and Mississippi Silverside had lower E
sopt, suggesting they may better withstand warmer water temperature compared with other species and life stages of fishes. Adult fishes tended to have higher E
sopt and E
tol than juvenile and larval fishes (
Figure 5,
Table 3). Among nuisance species,
Microcystis only exceeded its T
tol a few days each year (mean 19 days E
tol). Aquatic vegetation and non-native clams also had high T
tol, with only a mean of 52 days per year that exceeded threshold values (
Figure 5,
Table 3).
Thermal Stress by Listing Status
For Etol, ESA-listed species experienced a significantly greater proportion of days exceeding the lower range of adult Ttol, with listed species experiencing stressful days for 18.1% of the year (95% CI: 16.3-20.2%) and unlisted species experiencing stressful days for 12.7% of the year (95% CI: 11.7-13.6%), though there was no difference detected between listed and unlisted species in the Etol based on upper range of adult Ttol values. See Supplemental Materials for addditional model results.
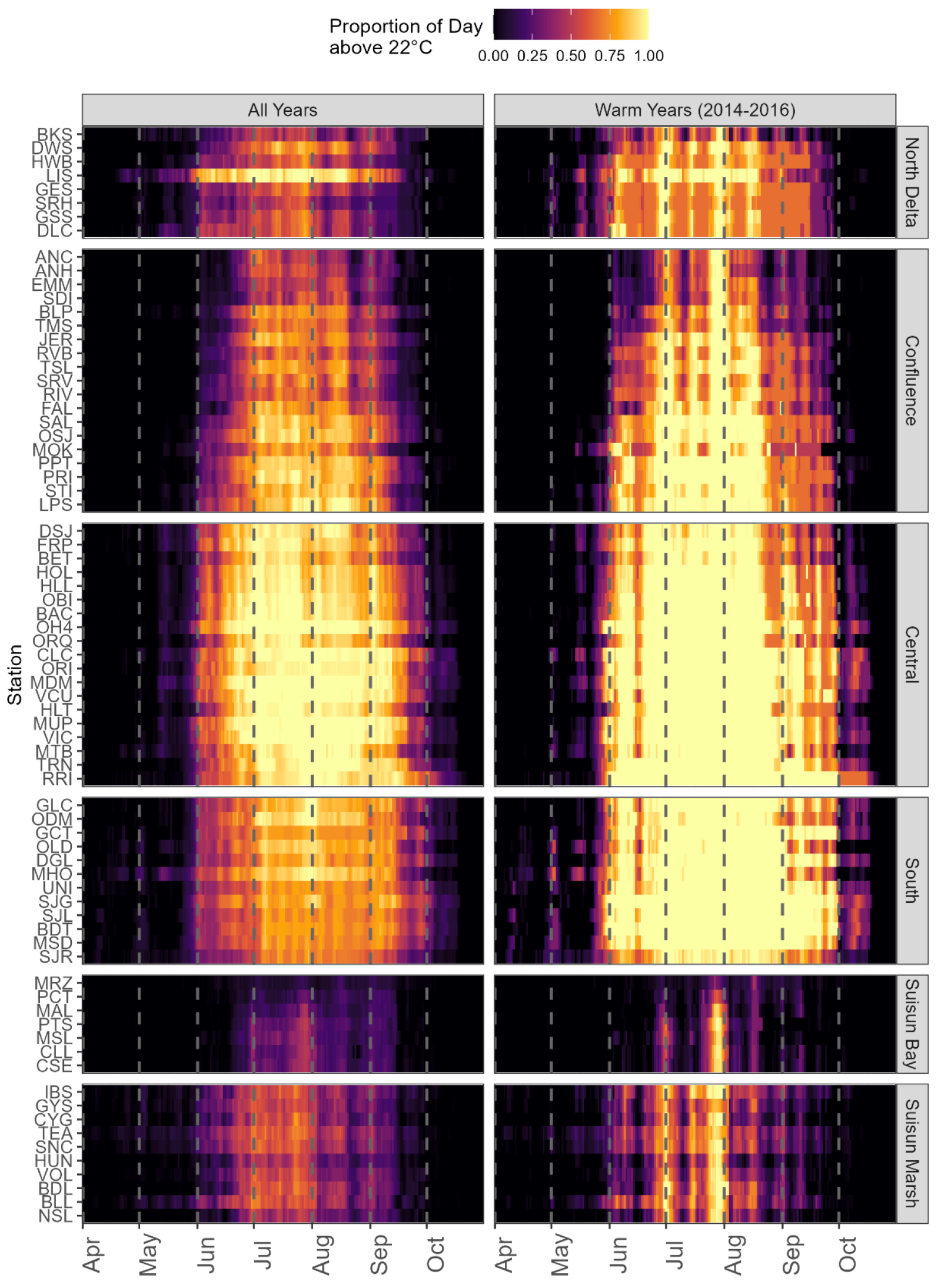
Daily Thermal Stress by Station
The days exceeding 22°C have ranged from April to October, and primarily occur between June and September (
Figure 6). Across years and stations, the Central Delta clearly exceeds 22°C for the majority of the day between mid-June and mid-September across stations, allowing for little recovery. Although Suisun Bay reaches 22°C during most of July and August, on average, this is for less than 50% of the day, potentially allowing for periods of recovery.
Comparing a particularly warm period, which was also a drought period, with the entire dataset, the period of daily stress increases to incorporate more stations, notably in the Central and South Delta, as well as many of the more eastward stations of the Confluence, and more days extending through the rest of June and September (
Figure 6). Much of the Confluence, Central, and South Delta provide suboptimal temperatures for all of July and most of August, with no periods for recovery during the day.
Discussion
With growing evidence of climate change impacts on the San Francisco Estuary, we show how spatial and seasonal patterns in water temperature may affect select special status species, as well as other native, non-native, and nuisance species. We found that across all ten years and all regions of our study, late spring, summer, and early fall water temperatures may cause sublethal and lethal impacts on listed and unlisted native species, severely limiting the habitat they can occupy. Many native fishes in the estuary are already living near the edge of their thermal limits, and increasing temperatures favor non-native fishes, invertebrates, and aquatic weeds which have the potential to further threaten native fishes through altered predator-prey interactions, competition, and food availability.
Water Temperature Patterns
With available water temperature data in the estuary, we identified the existing range of water temperatures observed in the past decade and examined if and how water temperatures differed between years, regions, and seasons. We found there was ~1.3°C (modeled mean annual maximum) and ~2.2°C (modeled mean annual minimum) difference between the warmest and coolest years in the dataset. The warmest period we observed (2014-2016) overlapped with the extended and extreme drought that occurred between 2012 to 2016, which in addition to elevated water temperature, was associated with low streamflow (Swain et al. 2018).
We found that the regional variation was more pronounced in the dry season, with ~2.3°C difference between the warmest and coolest regions for both maximum and minimum water temperatures, though these differences are likely greater when looking at individual stations and days within regions and seasons. The warmest temperatures during the dry season occurred in the Central and South Delta and coolest temperatures occurred in Suisun Bay and Marsh (
Figure 2). The measured continuous temperature dataset indicates that maximum temperatures have reached 24°C at all stations in our dataset over the decade, and up to 30°C at several stations in the South region (Table S7). Climate change modeling has indicated that these extreme water temperatures will become more common in the next few decades during the summer (Huntsman et al. 2024), as evidenced in July 2024, which was the hottest July on record globally and in California (NOAA 2024a, NOAA 2024b).
Comparison of Stress by Species
While several studies have demonstrated that increases in water temperature will affect ESA- listed fishes in the estuary such as Delta Smelt and Chinook Salmon (Cloern et al. 2011, Brown et al. 2016, Mahardja et al. 2022), we wanted to apply observed temperature patterns to a range of species and examine both sublethal and lethal effects. There was variation in which species were identified as most vulnerable or stressed depending on the type of threshold used (lethal versus suboptimal, as well as the particular Ttol or Tsopt metric considered).
Across analyses, we found that listed species such as native osmerids and salmonids are more vulnerable to increased temperatures associated with climate change and experience significantly more days of thermal stress compared to unlisted species (particularly if behavioral movement to cooler habitat is limited). Meanwhile, many of the unlisted native species, such as Sacramento Blackfish, adult Hitch, adult Prickly Sculpin, adult Sacramento Pikeminnow, adult Sacramento Sucker, adult Tule Perch, and larval Sacramento Splittail, have Ttol >30°C and are thus likely more resilient to current and future temperatures. However, some of these unlisted native species as well as non-native species have lower suboptimal thresholds (adult Sacramento Sucker, adult Tule Perch, adult Striped Bass) comparable to those of native salmonids and osmerids, and thus may be vulnerable to continued increases in water temperature.
In contrast to many of the listed and some unlisted native fishes, non-native fishes such as Mississippi Silversides and Largemouth Bass may be less vulnerable to warming estuary temperatures, and continue to thrive, demonstrated by lower Esopt, Etol, and larger temperature margins. These findings may explain Silverside population increases in recent years, particularly during drought conditions (Mahardja et al. 2016). The increase of non-native fishes with increasing water temperature may intensify predation on and competition with the native, endangered species such as Delta Smelt (Baerwald et al. 2012) and Chinook Salmon (McInturf et al. 2022), especially with availability of more suitable predator habitat and increased bioenergetic demands of predators with warmer conditions (Nobriga et al. 2021).
In addition to non-native fishes, warming temperatures are likely to promote harmful cyanobacteria and aquatic weed growth, as well as a continuation or increase in invasive clam populations due to the higher temperature thresholds and lower Esopt and Etol of these species. Microcystis blooms begin to appear in the water column when water temperature reaches 19°C and peak in abundance at 25°C in the estuary (Lehman et al. 2013, Lehman et al. 2021, Lehman et al. 2022). Microcystis blooms and their toxins have been linked to decreased health and survival for native fish and zooplankton (Ger et al. 2009, Ger et al. 2010, Acuna et al. 2012a, Acuna et al. 2012b, Kurobe et al. 2018, Acuña et al. 2020), as well as change in phytoplankton community composition in the estuary (Lehman et al. 2010, Lehman et al. 2021). The invasion of aquatic weeds, particularly E. densa, into subtidal habitat can also permanently affect native fish communities by creating slower, clearer water (Hestir et al. 2016, Work et al. 2020), promoting alien sunfishes and Largemouth Bass over native fishes (Brown 2003, Brown and Michniuk 2007, Conrad et al. 2016). The low abundance of Microcystis and E. densa in Suisun Marsh and Suisun Bay have partly been attributed to high salinity and turbidity in these regions (Moisander et al. 2009, Borgnis and Boyer 2015, Durand et al. 2016). Our analysis suggests that lower temperatures may also limit their growth in Suisun Marsh and Suisun Bay compared with the Central and South regions.
Non-native clams, such as the Overbite Clam (
Potamocorbula amurensis), and particularly the Asian Clam (
Corbicula fluminea), have high temperature tolerances (
Table 2), although it should be noted that temperature tolerance for
Potamocorbula amurensis is based on a congener as lab studies have not been conducted on this species. These clams have been implicated in decreasing primary productivity in the estuary and thus food availability for estuarine fishes (Kimmerer & Thompson 2014; Cloern & Jassby 2012). Both spatially and temporally, much of the estuary is suitable for both species of non-native clams.
Stress by Region and Season
For all listed species besides sturgeon, the Central and South Delta are already above or within 0-3°C of their lethal T
tol during the summer, and salmonids are also vulnerable in most regions during the spring (
Figure 4), similar to findings in Mahardja et al. 2022. In some seasons, fish species may be able to find cooler temperatures, though fish behavior and the ability to adapt can vary by and within species (Cocherell et al. 2012; Myrick and Cech, 2000). For example, juvenile Chinook salmon and
O. mykiss T
mar in most regions in spring are 0-3°C, such that additional increases in warming may exacerbate mortalities directly or indirectly from predation, disease, respiratory stress, or other factors. However, we found Chinook juvenile temperature margins are more robust in the Suisun Bay in the spring (3-6°C), potentially allowing them to find suitable thermal habitat before they migrate to cooler temperatures in the San Francisco Bay. Similar refugia may occur for Delta and Longfin Smelt in the summer if they are able to access it.
Species comparisons with T
sopt indicate the entire study area exceeds thresholds for optimal physiological performance throughout the entirety of the summer for many listed species. The modeled average T
max during the dry season (May-September) ranged 20.9-23.3°C (95% CI: 18.1-20.4°C to 23.8-26.1°C) across regions during the dry season, exceeding Delta Smelt, Green Sturgeon T
sopt, and Steelhead/Rainbow Trout, Longfin Smelt, and Chinook Salmon T
sopt and T
tol, and leading to potentially >146 days and >33 days of sublethal and lethal stress, respectively, across native species (
Table 3). Furthermore, we found that summer temperatures in certain regions exceeded T
sopt for the entire day during July and August, limiting a species’ ability to recover over the course of a day. These cumulative stress days occurred across the estuary, with the greatest frequency in the Central Delta, and with spatial extent extending to the Lower San Joaquin River portion of the Confluence and the South during warmer years (
Figure 6). Chronic stress from prolonged exposure to higher temperatures may result in long-term changes to stress responses, diminished ability to cope with other stressors, and increased energy demands when compared with acute temperature stress (Alfonso et al. 2021).
Study Limitations
This study relied on the usage of available data published on data repositories and in the literature. Thus, there were some limitations related to both available data and standardization of data across studies. For example, while compiling temperature thresholds, we included various metrics and types of studies, but they were not always available or consistent across all species and life stages (Davis et al. 2022). There was also variability in temperatures and metrics reported for species thresholds, thus our selection could bias our results, depending on the value selected. For determining where and when species-life stages were present, surveys were not equally sampled across all regions, habitats, seasons, species, and life stages. Thus, we may have over- or under-estimated the number of stressful days for certain species and life stages depending on the particular study we used to represent a threshold, and depending on how representative sampling methods were of particular species and life stages.
During our calculations of T
mar, we observed some fish were detected in field surveys at temperatures higher than the physiological T
tol thresholds recorded in literature (Figure S8;
Table 2). This was contrary to other studies that have found lower field tolerances than laboratory tolerances (Eaton et al. 1995). We attribute these to either life stage cutoffs being slightly different between studies and our literature, fish being dead or in very poor condition when caught, or some level of field or local acclimation/adaptation that is either different from what is experienced in the laboratory or has changed over time and with warming temperatures (McCullough et al. 2009). In particular, acclimating fishes to variable temperatures can result in higher thermal tolerances (Schaefer and Ryan 2006), therefore, the highly variable field temperatures may produce greater thermal plasticity than that seen in a laboratory settings. More research is warranted describing species ability to acquire temperature tolerance in variable and complex field conditions versus laboratory conditions.
Temperature Effects and Interactions
Increased temperatures are expected to exacerbate compounding stressors that are hypothesized to have led to declines in native species. Increases in water temperature can increase rates of metabolic processes and therefore energetic demands (Davis et al. 2019, Hammock et al. 2020); If these energetic demands are not matched with an increase in energy supply due to low food availability, stress levels may worsen, and growth and reproduction can be affected (Lusardi et al. 2020). Food limitation is thought to be one of many important stressors contributing to the Pelagic Organism Decline (Mac Nally et al. 2010). Zooplankton, which comprise the major food source for pelagic fishes, shift to smaller body size or smaller species when temperatures warm, limiting available food resources (Richardson 2008). High water temperatures can also cause osmoregulatory difficulties and, since increased salinization of the estuary is expected to coincide with climate change, there is the potential for synergistic impacts to fish and invertebrate physiology (Davis et al. 2019, Ghalambor et al. 2021). Increased temperatures can also increase the toxicity of certain contaminants, which may contribute to native fish and invertebrate declines (Fong et al. 2016, DeCourten and Brander 2017, DeCourten et al. 2019).
The resilience and adaptability of a fish, as well as its degree of dependence on specific habitats and environmental conditions, will also greatly impact how a species responds to future conditions. Studies by Moyle et al. (2013) and (Mahardja et al. 2021) evaluated climate change vulnerability and drought resilience of fishes in the estuary, respectively. Similar to this study, both studies found that salmonids and osmerids were highly vulnerable to climate change and drought, and that heat-tolerant non-native species, such as Mississippi Silversides, sunfishes, and Largemouth Bass, fared better with climate change and drought conditions, with littoral fishes faring better than pelagic species.
Potential for Refugia
For this study, we wanted to identify what parts of the estuary were particularly stressful for fish, and where thermal refugia might exist. Based on our analyses, the only region to provide consistently suitable temperatures is Suisun Bay. However suitable thermal habitat largely exists in the western and Sacramento River portions of the Confluence, Suisun Bay, and Suisun Marsh, as well as some locations in the North Delta, which is consistent with Brown et al. (2016). This cool water temperature corridor corresponds with the “North Delta Arc of Native Fish Habitat” (Durand 2013), which is primarily where Delta Smelt have been detected in recent years (Hobbs et al. 2017), and have been found to have seasonally lower warming trends (Bashevkin et al. 2022b). The North Delta Arc corridor has been identified as an area to target for tidal wetland restoration (Durand 2013, Hobbs et al. 2017), and has been the key location for recent releases of cultured Delta Smelt into the estuary to support the population (USFWS 2021). The higher diversity and abundance of native fish in this region has been attributed to relatively high turbidity, hydrodynamic complexity (Bever et al. 2016), and tidal wetland area (Sommer and Mejia 2013). However, our research demonstrates that cool water temperature in this region may also be a reason why this area is beneficial for native fishes, many of which have lower temperature tolerances (Davis et al. 2022). It is important to note, however, that salinity is strongly correlated to the distribution of several estuary fishes (Feyrer et al. 2010, Sommer and Mejia 2013). Suisun Bay, Marsh, and parts of the Confluence (especially during dry years in the summer and fall) are prone to higher salinities, which limits the habitat to euryhaline taxa, and may decrease the amount of suitable habitat for certain species of concern and/or have indirect effects on the lower trophic food web (Ghalambor et al. 2021).
Understanding how temperature regimes vary in different regions and seasons of the estuary will help inform restoration and/or reinforcement efforts for native species. For example, certain parts of the North Delta may be too warm in the summer, such that benefits of restored or enhanced habitat and food enhancement actions may not outweigh the negative impacts associated with warming water temperature (e.g., increased energy demands, increased non-native predators, etc.). Having unsuitable temperatures in the summer does not necessarily mean habitat restoration is not valuable in a given location, however, as much of the estuary experiences suitable temperatures during the rest of the year such that restoration can target life stages present in those seasons, and can also produce food and benefit other species (e.g. mammals, waterfowl). Targeted protection of climate refugia has been a useful tool for conservation in other systems (Kurylyk et al. 2015, Justice et al. 2017), but connectivity, flow management, and alignment of stakeholder objectives need to be taken into account for it to be effective (Ebersole et al. 2020).
Climate Change Effects
Recent modeling studies based on historical data have found average increases in temperature on the order of 0.017°C per year, with specific regions and months exhibiting greater than 0.15°C increases per year, and more recent periods exhibiting significant increases in March-June across much of the Upper estuary (Bashevkin et al. 2022). Heatwave incidents have been prevalent in many recent years (Bashevkin et al., in review) and climate change modeling further indicates significant temperature increases in the next few decades across the estuary (Huntsman 2024). Comparisons of the 10-year temperature dataset with the warmest three years of the dataset indicate a large increase of stations and days in the Confluence, Central and South regions that become stressful for native species for entire months with no nighttime recovery to temperatures below thresholds (
Figure 6, Figure S12). Warmer temperatures in the spring may provide benefits in certain cases, such as potentially providing longer spawning periods for certain species (Huntsman et al. 2024). However, given existing conditions and predicted increases, the next few decades will likely lead to further narrowing of temperature margins across the estuary and shifting towards higher numbers of E
tol and E
sopt, especially of the coldwater native species of species.
Because most life stages of fish are mobile, it is possible fish species may find additional refuge in unsampled parts of the estuary, such as deeper parts of the estuary (Mahardja et al. 2022). Some species may also be able to acquire additional thermal tolerance, shift the distribution and timing of their migration/residence depending on temperature, or compensate by finding additional food resources (Lusardi et al. 2020, Alfonso et al. 2021). However, rapid shifts in the composition and timing of food resources may make adaptation difficult as climate change and non-native species drive phenological mismatches between predators and prey (Merz et al. 2016, Renner and Zohner 2018).
Acknowledgments
The views expressed are those of the authors and do not represent the views of the respective agencies. We would like to thank staff from various monitoring programs who collected the continuous water temperature data we used in our analyses, including staff from the California Department of Water Resources, United States Geological Survey, California Department of Fish and Wildlife, and Bureau of Reclamation. We would like to thank Sam Bashevkin, Brian Schreier, and Brian Mahardja for providing expertise on analysis techniques and/or fish distribution. We would also like to thank Sam Bashevkin, Elissa Buttermore, Lauren Damon, Karen Gehrts, Daphne Gille, Josh Israel, Nicole Kwan, Laurel Larsen, Dean Messer, Shaun Philippart, and JohnFranco Saraceno for reviewing and providing valuable comments on the manuscript.
References
- Acuña, S., D. Baxa, P. Lehman, F.-C. Teh, D.-F. Deng, and S. Teh. 2020. Determining the Exposure Pathway and Impacts of Microcystis on Threadfin Shad, Dorosoma petenense, in San Francisco Estuary. Environmental Toxicology and Chemistry. [accessed 2023 Jan 12]. 39 (4):787-798. [CrossRef]
- Acuna, S., D. Baxa, and S. Teh. 2012a. Sublethal dietary effects of microcystin producing Microcystis on threadfin shad, Dorosoma petenense. Toxicon. [accessed 2023 Jun 22]. 60:1191-1202. [CrossRef]
- Acuna, S., D. F. Deng, P. Lehman, and S. Teh. 2012b. Sublethal dietary effects of Microcystis on Sacramento splittail, Pogonichthys macrolepidotus. Aquatic Toxicology. [accessed 2023 Feb 6]. 110-111:1-8. [CrossRef]
- Alfonso, S., M. Gesto, and B. Sadoul. 2021. Temperature increase and its effects on fish stress physiology in the context of global warming. Journal of Fish Biology. 98 (6):1496-1508. [CrossRef]
- Baerwald, M. R., B. M. Schreier, G. Schumer, and B. May. 2012. Detection of threatened delta smelt in the gut contents of the invasive Mississippi silverside in the San Francisco Estuary using TaqMan assays. Transactions American Fisheries Society. [accessed 2023 Jun 22]. 141:1600-1607. [CrossRef]
- Barbier, E. B., S. D. Hacker, C. Kennedy, E. W. Koch, A. C. Stier, and B. R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs. [accessed 2014/08/15]. 81 (2):169-193. 10.1890/10-1510.
- Bashevkin, S. M., J. W. Gaeta, T. X. Ngyuen, L. Mitchell, and S. Khanna. 2022a. Fish abundance in the San Francisco Estuary (1959-2021), an integration of 9 monitoring surveys. [accessed. [CrossRef]
- Bashevkin, S. M., and B. Mahardja. 2022. Seasonally variable relationships between surface water temperature and inflow in the upper San Francisco Estuary. Limnology and Oceanography. [accessed 2023 Jan 4]. 67:684-702. [CrossRef]
- Bashevkin, S. M., B. Mahardja, and L. R. Brown. 2022b. Warming in the upper San Francisco Estuary: Patterns of water temperature change from 5 decades of data. Limnology & Oceanography. [accessed 2023 Jan 3]. 67 (5):1065-1080. [CrossRef]
- Bashevkin, S.M., B. Mahardja, C. Pien, S. Khanna, D. Pearson, B. Davis, and R. Basu. In review. Heatwaves and Rising Temperatures in the Upper San Francisco Estuary: Trends and Impacts on Ecosystems and Humans.
- Bates, D., M. Maechler, B. Bolker, and S. Walker. 2016. lme4: Linear Mixed-Effects Models using 'Eigen' and S4. The Comprehensive R Archive Network (CRAN). [accessed https://github.com/lme4/lme4/ http://lme4.r-forge.r-project.org.
- Beakes, M. P., C. Graham, J. L. Conrad, J. R. White, M. Koohafkan, J. Durand, and T. Sommer. 2021. Large-Scale Flow Management Action Drives Estuarine Ecological Response. North American Journal of Fisheries Management. [accessed 2023 Jun 22]. 41 (1):64-77. [CrossRef]
- Bever, A. J., M. L. MacWilliams, B. Herbold, L. R. Brown, and F. V. Feyrer. 2016. Linking hydrodynamic complexity to Delta Smelt (Hypomesus transpacificus) distribution in the San Francisco Estuary, USA. San Francisco Estuary and Watershed Science. [accessed 2023 Jan 4]. 14 (1). [CrossRef]
- Borgnis, E., and K. E. Boyer. 2015. Salinity tolerance and competition drive distributions of native and invasive submerged aquatic vegetation in the upper San Francisco Estuary. Estuaries and Coasts. [accessed 2023 Jun 22]. 39 (3):1-11. [CrossRef]
- Brown, L. R. 2003. Will tidal wetland restoration enhance populations of native fishes? San Francisco Estuary and Watershed Science. [accessed 2024 Apr 18]. 1 (1):43 pages. [CrossRef]
- Brown, L. R., L. M. Komoroske, R. W. Wagner, T. Morgan-King, J. T. May, R. E. Connon, and N. A. Fangue. 2016. Coupled Downscaled Climate Models and Ecophysiological Metrics Forecast Habitat Compression for an Endangered Estuarine Fish. PLoS One. 11 (1):e0146724. 10.1371/journal.pone.0146724.
- Brown, L. R., and D. Michniuk. 2007. Littoral fish assemblages of the alien-dominated Sacramento-San Joaquin Delta, California, 1980-1983 and 2001-2003. Estuaries and Coasts. 30 (1):186-200.
- Clark, J., and S. M. Bashevkin. 2022. deltafish: Accesses an INtegrated Fish Count and Length Dataset from the San Francisco Delta. [accessed,.
- Cloern, J. E., K. A. Hieb, T. Jacobson, B. Sansó, E. Di Lorenzo, M. T. Stacey, J. L. Largier, W. Meiring, W. T. Peterson, T. M. Powell, M. Winder, and A. D. Jassby. 2010. Biological communities in San Francisco Bay track large-scale climate forcing over the North Pacific. Geophysical Research Letters. 37 (21):n/a-n/a. [CrossRef]
- Cloern, J. E., N. Knowles, L. R. Brown, D. Cayan, M. D. Dettinger, T. L. Morgan, D. H. Schoellhamer, M. T. Stacey, M. van der Wegen, R. W. Wagner, and A. D. Jassby. 2011. Projected Evolution of California's San Francisco Bay-Delta-River System in a Century of Climate Change. PLOS ONE. 6 (9):e24465. [CrossRef]
- Cocherell SA, Chun SN, Cocherell DE, Thompson LC, Klimley AP, Cech JJ. 2012. A lateral-displacement flume for fish behavior and stranding studies during simulated pulsed flows. Environmental biology of fishes. 93:143-50.
- Collins, M., R. E. Chandler, P. M. Cox, J. M. Huthnance, J. Rougier, and D. B. Stephenson. 2012. Quantifying future climate change. Nature Climate Change. 2 (6):403-409. [CrossRef]
- Conrad, J. L., A. J. Bibian, K. L. Weinersmith, D. De Carion, M. J. Young, P. Crain, E. L. Hestir, M. J. Santos, and A. Sih. 2016. Novel species ineractions in a highly modified estuary: Association of Largemouth Bass with Brazilian waterweed Egeria densa. Transactions American Fisheries Society. [accessed 2023 Jan 3]. 145:249-263. [CrossRef]
- Daniels, M. E., and E. M. Danner. 2020. The Drivers of River Temperatures Below a Large Dam. Water Resources Research. 56 (5):e2019WR026751. [CrossRef]
- Davis, B., E. Bush, P. Lehman, and C. Pien. 2022. Temperature Thresholds for Aquatic Species in the Sacramento San-Joaquin Delta. ver 2. Environmental Data Initiative. [accessed 2023 Feb 13]. [CrossRef]
- Davis, B. E., D. E. Cocherell, N. A. Fangue, A. E. Todgham, T. Sommer, R. D. Baxter, and T.-C. Hung. 2019. Sensitivities of an endemic, endangered California smelt and two non-native fishes to serial increases in temperature and salinity: implications for shifting community structure with climate change. Conservation Physiology. [accessed 2019 Apr 4]. 7 (1). [CrossRef]
- DeCourten, B. M., and S. M. Brander. 2017. Combined effects of increased temperature and endocrine disrupting pollutants on sex determination, survival, and development across generations. Sci Rep. 7 (1):9310. [CrossRef]
- DeCourten, B. M., R. E. Connon, and S. M. Brander. 2019. Direct and indirect parental exposure to endocrine disruptors and elevated temperature influences gene expression across generations in a euryhaline model fish. PeerJ. 7:e6156. [CrossRef]
- Dettinger, M., J. Anderson, M. Anderson, L. Brown, D. Cayan, and E. Maurer. 2016. Climate change and the Delta. San Francisco Estuary and Watershed Science. [accessed 2023 Jan 12]. 14 (3). [CrossRef]
- Deutsch, C. A., J. J. Tewksbury, R. B. Huey, K. S. Sheldon, C. K. Ghalambor, D. C. Haak, and P. R. Martin. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences. [accessed 2023 Jun 22]. 105 (18):6668-6672. [CrossRef]
- Durand, J. 2013. 'North Delta Arc' lifts hope for recovery of native fish.in U. D. C. f. W. Sciences, editor. California Water Blog. [accessed,.
- Durand, J., W. Fleenor, R. McElreath, M. J. Santos, and P. Moyle. 2016. Physical controls on the distribution of the submersed aquatic weed Egeria densa in the Sacramento–San Joaquin Delta and implications for habitat restoration. San Francisco Estuary and Watershed Science. [accessed 2023 Jun 22]. 14 (1). http://www.escholarship.org/uc/item/85c9h479.
- Eaton, J. G., J. H. McCormick, B. E. Goodno, D. G. O'Brien, H. G. Stefany, M. Hondzo, and R. M. Scheller. 1995. A Field Information-based System for Estimating Fish Temperature Tolerances. Fisheries. [accessed 2023 Jun 22]. 20 (4):10-18. [CrossRef]
- Ebersole, J. L., R. M. Quiñones, S. Clements, and B. H. Letcher. 2020. Managing climate refugia for freshwater fishes under an expanding human footprint. Frontiers in Ecology and the Environment. 18 (5):271-280. [CrossRef]
- Enright, C., S. Culberson, and J. Burau. 2013. Broad timescale forcing and geomorphic mediation of tidal marsh flow and temperature dynamics. Estuaries and Coasts. [accessed 2024 Apr 18]. 36 (6):1319-1339. [CrossRef]
- Fangue, N. A., A. E. Todgham, and P. M. Schulte. 2020. Thermal Biology. Pages 91-104 in S. Currie and D. H. Evans, editors. The Physiology of Fishes. CRC Press., Boca Raton, FL.
- Feyrer, F., J. Hobbs, and T. Sommer. 2010. Salinity inhabited by age-0 splittail (Pogonichthys macrolepidotus) as determined by direct field observation and retrospective analyses with otolith chemistry. San Francisco Estuary and Watershed Science. [accessed 2023 Jun 22]. 8 (2). [CrossRef]
- Fong, S., S. Louie, I. Werner, J. Davis, and R. E. Connon. 2016. Contaminant Effects on California Bay–Delta Species and Human Health. San Francisco Estuary and Watershed Science. [accessed 2023 Jun 22]. 14 (4). [CrossRef]
- Fuller, M. R., P. Leinenbach, N. E. Detenbeck, R. Labiosa, and D. J. Isaak. 2022. Riparian vegetation shade restoration and loss effects on recent and future stream temperatures. Restoration Ecology. 30 (7):e13626. [CrossRef]
- Ger, K. A., S. J. Teh, D. V. Baxa, S. Lesmeister, and C. R. Goldman. 2010. The effects of dietary Microcystis aeruginosa and microcystin on the copepods of the upper San Francisco Estuary. Freshwater Biology. [accessed 2023 Jun 22]. 55:1548-1559. [CrossRef]
- Ger, K. A., S. J. Teh, and C. R. Goldman. 2009. Microcystin-LR toxicity on dominant copepods Eurytemora affinis and Pseudodiaptomus forbesi of the upper San Francisco Estuary. Science of the Total Environment. [accessed 2023 Jun 22]. 407:4852-4857. [CrossRef]
- Ghalambor, C. K., E. S. Gross, E. D. Grosholtz, K. M. Jeffries, J. K. Largier, S. D. McCormick, T. Sommer, J. Velotta, and A. Whitehead. 2021. Ecological Effects of Climate-Driven Salinity Variation in the San Francisco Estuary: Can We Anticipate and Manage the Coming Changes? San Francisco Estuary and Watershed Science. [accessed 2023 Jan 4]. 19 (2). [CrossRef]
- Gillanders, B. M., T. S. Eldson, I. A. Halliday, G. P. Jenkins, J. B. Robins, and F. J. Valesini. 2011. Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review. Marine & Freshwater Research. 62:1115-1131. [CrossRef]
- Goss, M., D. L. Swain, J. T. Abatzoglou, A. Sarhadi, C. A. Kolden, A. P. Williams, and N. S. Diffenbaugh. 2020. Climate change is increasing the likelihood of extreme autumn wildfire conditions across California. Environmental Research Letters. [accessed 2023 Jun 22]. 15 (9):094016. [CrossRef]
- Greenberg, J. A., E. L. Hestir, D. Riano, G. J. Scheer, and S. L. Ustin. 2012. Using LiDAR Data Analysis to Estimate Changes in Insolation Under Large-Scale Riparian Deforestation1. JAWRA Journal of the American Water Resources Association. 48 (5):939-948. [CrossRef]
- Hammock, B. G., W. F. Ramírez-Duarte, P. A. Triana Garcia, A. A. Schultz, L. I. Avendano, T.-C. Hung, J. R. White, Y.-T. Bong, and S. J. Teh. 2020. The health and condition responses of Delta Smelt to fasting: A time series experiment. Plos ONE. [accessed 2023 Jun 22]. 15 (9):e0239358. [CrossRef]
- He, M. 2022a. Assessing Changes in 21st Century Mean and Extreme Climate of the Sacramento–San Joaquin Delta in California. Climate. 10 (2). [CrossRef]
- He, M. 2022b. Assessing Changes in 21st Century Mean and Extreme Climate of the Sacramento - San Joaquin Delta in California. Climate. [accessed 2023 Feb 13]. 10 (2):16. [CrossRef]
- Herbold, B., D. M. Baltz, L. Brown, R. Grossinger, W. Kimmerer, P. Lehman, P. B. Moyle, M. Nobriga, and C. A. Simenstad. 2014. The Role of Tidal Marsh Restoration in Fish Management in the San Francisco Estuary. San Francisco Estuary and Watershed Science. [accessed 2022-04-13T20:01:13]. 12 (1). [CrossRef]
- Hestir, E. L., D. H. Schoellhamer, J. Greenberg, T. Morgan-King, and S. L. Ustin. 2016. The effect of submerged aquatic vegetation expansion on a declining turbidity trend in the Sacramento-San Joaquin River Delta. Estuaries and Coasts. [accessed 2023 Jan 4]. 39 (4):1100-1112. [CrossRef]
- Hobbs, J., P. B. Moyle, N. Fangue, and R. E. Connon. 2017. Is extinction inevitable for Delta Smelt and Longfin Smelt? An opinion and recommendations for recovery. San Francisco Estuary and Watershed Science. [accessed 2023 Jan 3]. 15 (2). [CrossRef]
- Hobbs, J. A., C. Denney, L. Lewis, M. Willmes, W. Xieu, A. Schultz, and O. T. Burgess. 2019. Environmental and Ontogenetic Drivers of Growth in a Critically Endangered Species. Pages 124-146 in A. A. Schultz, editor. Directed Outflow Project: Technical Report 1. U.S. Bureau of Reclamation, Bay-Delta Office, Mid-Pacific Region, Sacramento, CA.
- Hothorn, T., F. Bretz, and P. Westfall. 2008. Simultaneous Inference in General Parametric Models. Biometrical Journal. 50 (3):346-363. [CrossRef]
- Huntsman, B. B., Larry R.;Wulff, Marissa;Knowles, Noah;Wagner, R. Wayne;Feyrer, Frederick. 2024. Climate Change Scenarios for Air and Water Temperatures in the Upper San Francisco Estuary: Implications for Thermal Regimes and Delta Smelt. San Francisco Estuary and Watershed Science. 22 (2). [CrossRef]
- IEP, C. Pien, J. Hamilton, R. Hartman, M. Nelson, J. F. Saraceno, B. M. Schreier, and B. Davis. 2020. Hourly water temperature from the San Francisco Estuary, 1986-2019. [accessed. [CrossRef]
- Interagency Ecological Program, I., C. Pien, and N. K. . 2022. Interagency Ecological Program: Fish catch and water quality data from the Sacramento River floodplain and tidal slough, collected by the Yolo Bypass Fish Monitoring Program, 1998-2021. ver 3. Environmental Data Initiative. [accessed 2023 Jun 21]. [CrossRef]
- IPCC. 2021. Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change.
- Jeffries, K. M., R. E. Connon, B. E. Davis, L. M. Komoroske, M. T. Britton, T. Sommer, A. E. Todgham, and N. A. Fangue. 2016. Effects of high temperatures on threatened estuarine fishes during periods of extreme drought. The Journal of Experimental Biology. [accessed 2023 Jan 3]. 219 (11):1705-1716. [CrossRef]
- Justice, C., S. M. White, D. A. McCullough, D. S. Graves, and M. R. Blanchard. 2017. Can stream and riparian restoration offset climate change impacts to salmon populations? Journal of Environmental Management. [accessed 2023 Jun 22]. 188:212-227. [CrossRef]
- Knowles, N., C. Cronkite-Ratcliff, D. W. Pierce, and D. R. Cayan. 2018. Responses of Unimpaired Flows, Storage, and Managed Flows to Scenarios of Climate Change in the San Francisco Bay-Delta Watershed. Water Resources Research. [accessed 2023 Jun 22]. 54 (10):7631-7650. [CrossRef]
- Komoroske, L. M., R. E. Connon, J. Lindberg, B. S. Cheng, G. Castillo, M. Hasenbein, and N. A. Fangue. 2014. Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conservation Physiology. [accessed 2023 Jun 22]. 2:13. [CrossRef]
- Kurobe, T., P. W. Lehman, M. E. Haque, T. Sedda, S. Lesmeister, and S. Teh. 2018. Evaluation of water quality during successive severe drought years within Microcystis blooms using fish embryo toxicity tests for the San Francisco Estuary, California. Science of the Total Environment. [accessed 2023 Jan 6]. 610-611:1029-1037. [CrossRef]
- Kurylyk, B. L., K. T. B. MacQuarrie, T. Linnansaari, R. A. Cunjak, and R. A. Curry. 2015. Preserving, augmenting, and creating cold-water thermal refugia in rivers: concepts derived from research on the Miramichi River, New Brunswick (Canada). Ecohydrology. [accessed 2023 Jun 22]. 8 (6):1095-1108. [CrossRef]
- Lehman, P., K. Marr, G. Boyer, S. Acuna, and S. Teh. 2013. Long-term trends and causal factors associated with Microcystis abundance and toxicity in San Francisco Estuary and implications for climate change impacts. Hydrobiologia. [accessed 2023 Jan 4]. 718:141-158. [CrossRef]
- Lehman, P., S. Teh, G. Boyer, M. Nobriga, E. Bass, and C. Hogle. 2010. Initial impacts of Microcystis aeruginosa blooms on the aquatic food web in the San Francisco Estuary. Hydrobiologia. [accessed 2023 Feb 23]. 637 (1):229-248. [CrossRef]
- Lehman, P. W., T. Kurobe, K. Huynh, S. Lesmeister, and S. J. Teh. 2021. Covariance of Phytoplankton, Bacteria, and Zooplankton Communities Within Microcystis Blooms in San Francisco Estuary. Frontiers in Microbiology. [accessed 2023 Jun 22]. 12 (1184). [CrossRef]
- Lehman, P. W., T. Kurobe, and S. J. Teh. 2022. Impact of extreme wet and dry years on the persistence of Microcystis harmful algal blooms in San Francisco Estuary. Quaternary International. [accessed 2023 Jan 4]. 621:16-25. [CrossRef]
- Lenth, R. V., P. Buerkner, M. Herve, J. Love, H. Riebl, and H. Singmann. 2022. Package 'emmeans': Estimated Marginal Means, aka Least-Squares Means. Version 1.8.1. Comprehensive R Archive Network, CRAN. [accessed 2021 Aug 16], https://cran.r-project.org/web/packages/emmeans/index.html.
- Lewis, L. S., C. Denney, M. Willmes, W. Xieu, R. A. Fichman, F. Zhao, B. G. Hammock, A. Schultz, N. Fangue, and J. A. Hobbs. 2021. Otolith-based approaches indicate strong effects of environmental variation on growth of a Critically Endangered estuarine fish. Marine Ecology Progress Series. 676:37-56. [CrossRef]
- Lusardi, R. A., B. G. Hammock, C. A. Jeffres, R. A. Dahlgren, and J. D. Kiernan. 2020. Oversummer growth and survival of juvenile coho salmon (Oncorhynchus kisutch) across a natural gradient of stream water temperature and prey availability: an in situ enclosure experiment. Canadian Journal of Fisheries and Aquatic Sciences. 77 (2):413-424.
- Mac Nally, R., J. R. Thomson, W. J. Kimmerer, F. Feyrer, K. B. Newman, A. Sih, W. A. Bennett, L. Brown, E. Fleishman, S. D. Culberson, and G. Castillo. 2010. Analysis of pelagic species decline in the upper San Francisco Estuary using multivariate autoregressive modeling (MAR). Ecological Applications. [accessed 2023 Jan 3]. 20 (5):1417-1430. [CrossRef]
- Mahardja, B., S. M. Bashevkin, C. Pien, M. Nelson, B. E. Davis, and R. Hartman. 2022. Escape from the heat: thermal stratification in a well-mixed estuary and implications for fish species facing a changing climate. Hydrobiologia. [accessed 2023 Jan 4]. [CrossRef]
- Mahardja, B., J. L. Conrad, L. Lusher, and B. Schreier. 2016. Abundance Trends, Distribution, and Habitat Associations of the Invasive Mississippi Silverside (Menidia audens) in the Sacramento–San Joaquin Delta, California, USA. San Francisco Estuary and Watershed Science. [accessed 2023 Jan 3]. 14 (1). [CrossRef]
- Mahardja, B., V. Tobias, S. Khanna, L. Mitchell, P. Lehman, T. Sommer, L. Brown, S. Culberson, and J. L. Conrad. 2021. Resistance and resilience of pelagic and littoral fishes to drought in the San Francisco Estuary. Ecological Applications. [accessed 2023 Jan 3]. 31 (2):e02243, 02216 p. [CrossRef]
- Mayfield, R. B., and J. J. J. Cech. 2004. Temperature effects on Green Sturgeon bioenergetics. Transactions of the American Fisheries Society. [accessed 2023 Jun 22]. 133:961-970. [CrossRef]
- McCullough, D. A., J. M. Bartholow, H. I. Jager, R. L. Beschta, E. F. Cheslak, M. L. Deas, J. L. Ebersole, J. S. Foott, S. L. Johnson, K. R. Marine, M. G. Mesa, J. H. Petersen, Y. Souchon, K. F. Tiffan, and W. A. Wurtsbaugh. 2009. Research in Thermal Biology: Burning Questions for Coldwater Stream Fishes. Reviews in Fisheries Science. [accessed 2023 Jun 22]. 17 (1):90-115. [CrossRef]
- McInturf, A. G., K. W. Zillig, K. Cook, J. Fukumoto, A. Jones, E. Patterson, D. E. Cocherell, C. J. Michel, D. Caillaud, and N. A. Fangue. 2022. In hot water? Assessing the link between fundamental thermal physiology and predation of juvenile Chinook salmon. Ecosphere. [accessed 2023 Jun 22]. 13 (11):e4264. [CrossRef]
- Merz, J. E., P. S. Bergman, J. L. Simonis, D. Delaney, J. Pierson, and P. Anders. 2016. Long-term seasonal trends in the prey community of Delta Smelt (Hypomesus transpacificus) within the Sacramento-San Joaquin Delta, California. Estuaries and Coasts. [accessed 2021 Sep 07]. 39 (5):1526-1536. [CrossRef]
- Moisander, P., P. Lehman, M. Ochiai, and S. Corum. 2009. Diversity of Microcystis aeruginosa in the Klamath River and San Francisco Bay delta, California, USA. Aquatic Microbial Ecology. [accessed 2023 Jun 22]. 57:19-31. [CrossRef]
- Moyle, P. B., J. D. Kiernan, P. K. Crain, and R. M. Quinones. 2013. Climate change vulnerability of native and alien freshwater fishes of California: a systematic assessment approach. Plos ONE. [accessed 2023 Jun 22]. 8 (5):e63883. [CrossRef]
- Myrick CA, Cech JJ. 2000. Swimming performances of four California stream fishes: temperature effects. Environmental biology of fishes. 58:289-95.
- Nobriga, M. L., C. J. Michel, R. C. Johnson, and J. D. Wikert. 2021. Coldwater fish in a warm water world: Implications for predation of salmon smolts during estuary transit. Ecology and Evolution. [accessed 2023 Jan 3]. 11 (15):10381-10395. [CrossRef]
- NOAA. National Centers for Environmental Information, Monthly National Climate Report for July 2024, published online August 2024, retrieved on September 8, 2024 from https://www.ncei.noaa.gov/access/monitoring/monthly-report/national/202407.
- NOAA. Earth just had its warmest July on record, published online August 2024, retrieved on September 8, 2024 from https://www.noaa.gov/news/earth-just-had-its-warmest-july-on-record.
- Pierce, D. W., J. F. Kalansky, and D. R. Cayan. 2018. Climate, drought, and sea level rise scenarios for California’s fourth climate change assessment. California Energy Commission and California Natural Resources Agency. [accessed 2023 Jun 22]. https://www.energy.ca.gov/sites/default/files/2019-11/Projections_CCCA4-CEC-2018-006_ADA.pdf.
- Polade, S. D., A. Gershunov, D. R. Cayan, M. D. Dettinger, and D. W. Pierce. 2017. Precipitation in a warming world: Assessing projected hydro-climate changes in California and other Mediterranean climate regions. Scientific Reports. [accessed 2023 Jun 22]. 7 (1):10783. [CrossRef]
- R Development Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [accessed,.
- Renner, S. S., and C. M. Zohner. 2018. Climate Change and Phenological Mismatch in Trophic Interactions Among Plants, Insects, and Vertebrates. Annual Review of Ecology, Evolution, and Systematics. 49 (Volume 49, 2018):165-182. [CrossRef]
- Richardson, A. J. 2008. In hot water: zooplankton and climate change. ICES Journal of Marine Science. [accessed 2020 Jul 14]. 65 (3):279-295. [CrossRef]
- Robins, P. E., M. W. Skov, M. J. Lewis, L. Giménez, A. G. Davies, S. K. Malham, S. P. Neill, J. E. McDonald, T. A. Whitton, S. E. Jackson, and C. F. Jago. 2016. Impact of climate change on UK estuaries: A review of past trends and potential projections. Estuarine, Coastal and Shelf Science. [accessed 2023 Jun 22]. 169:119-135. [CrossRef]
- Rodriguez E, C. E. R. p. 2022. CDECRetrieve: Retrieve Data from the California Data Exchange Center. version 0.1.5. GitHub. [accessed 2023 Jun 29]. https://github.com/FlowWest/CDECRetrieve.
- Scanes, E., P. R. Scanes, and P. M. Ross. 2020. Climate change rapidly warms and acidifies Australian estuaries. Nature Communications. [accessed 2023 Jun 22]. 11 (1):1803. [CrossRef]
- Schaefer, J., and A. Ryan. 2006. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. Journal of Fish Biology. [accessed 2023 Jun 22]. 69 (3):722-734. [CrossRef]
- Sherman, S., R. Hartman, and D. Contreras. 2017. Effects of Tidal Wetland Restoration on Fish: A Suite of Conceptual Models. Interagency Ecological Program Technical Report 91. Department of Water Resources, Sacramento, CA. [accessed 2024 Apr 18]. https://cadwr.app.box.com/v/InteragencyEcologicalProgram/file/571038692179.
- Sokolova, I. M., M. Frederich, R. Bagwe, G. Lannig, and A. A. Sukhotin. 2012. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Marine environmental research. 79:1-15. [CrossRef]
- Sommer, T. 2020. How to Respond? An Introduction to Current Bay-Delta Natural Resources Management Options. San Francisco Estuary and Watershed Science. [accessed 2023 Feb 28]. 18 (3). [CrossRef]
- Sommer, T., R. Hartman, M. Koller, M. Koohafkan, J. L. Conrad, M. MacWilliams, A. Bever, C. Burdi, and M. P. Beakes. 2020. Evaluation of a large-scale flow manipulation to the upper San Francisco Estuary: Response of habitat conditions for an endangered native fish. Plos ONE. [accessed 2023 Jan 4]. 15 (10). [CrossRef]
- Sommer, T., and F. Mejia. 2013. A place to call home: a synthesis of Delta Smelt habitat in the upper San Francisco Estuary. San Francisco Estuary and Watershed Science. [accessed 2023 Jan 3]. 11 (2):25. [CrossRef]
- Sommer, T., Mount, J., Gray, B., Grenier, L., Harder, J., Sencan, G. 2024. Climate-Smart Tools to Protect California's Freshwater Biodiversity. https://www.ppic.org/publication/climate-smart-tools-to-protect-californias-freshwater-biodiversity/.
- Stompe, D. K., and J. A. Hobbs. 2023. California Department of Fish and Wildlife Adult Sturgeon Study, Sacramento-San Joaquin Watershed 1954-2022 ver 1. Environmental Data Initiative. [CrossRef]
- Swain, D. L., B. Langenbrunner, J. D. Neelin, and A. Hall. 2018. Increasing precipitation volatility in twenty-first-century California. Nature Climate Change. [accessed 2023 Jan 4]. 8 (5):427-433. [CrossRef]
- United States Fish and Wildlife Service (USFWS), California Department of Fish and Wildlife, California Department of Water Resources, United State Bureau of Reclamation, United States Geologic Survey, and University of California Davis. 2021. Delta smelt Experimental Release Study Plan, Draft 5; Sept 2021.
- Vroom, J., M. van der Wegen, R. Martyr-Koller, and L. Lucas. 2017. What Determines Water Temperature Dynamics in the San Francisco Bay-Delta System? Water Resources Research. [accessed 2023 Jan 4]. 53 (11):9901-9921. [CrossRef]
- Wildlife), C. C. D. o. F. a. 2022. California Natural Diversity Database. [accessed ^https://wildlife.ca.gov/Data/CNDDB/Plants-and-Animals.
- Wood, S. 2017. Generalized Additive Models: An Introduction with R, 2 edition. Chapman and Hall/CRC. https://cran.r-project.org/package=mgcv.
- Work, P. A., M. Downing-Kunz, and J. Z. Drexler. 2020. Trapping of Suspended Sediment by Submerged Aquatic Vegetation in a Tidal Freshwater Region: Field Observations and Long-Term Trends. Estuaries and Coasts. [accessed 2023 Jun 22]. [CrossRef]
- Yates, D., H. Galbraith, D. Purkey, A. Huber-Lee, J. Sieber, J. West, S. Herrod-Julius, and B. Joyce. 2008. Climate warming, water storage, and Chinook salmon in California’s Sacramento Valley. Climatic Change. [accessed 2023 Jun 22]. 91 (3):335. [CrossRef]
- Zillig, K. W., R. A. Lusardi, P. B. Moyle, and N. A. Fangue. 2021. One size does not fit all: variation in thermal eco-physiology among Pacific salmonids. Reviews in Fish Biology and Fisheries. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).