Submitted:
23 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
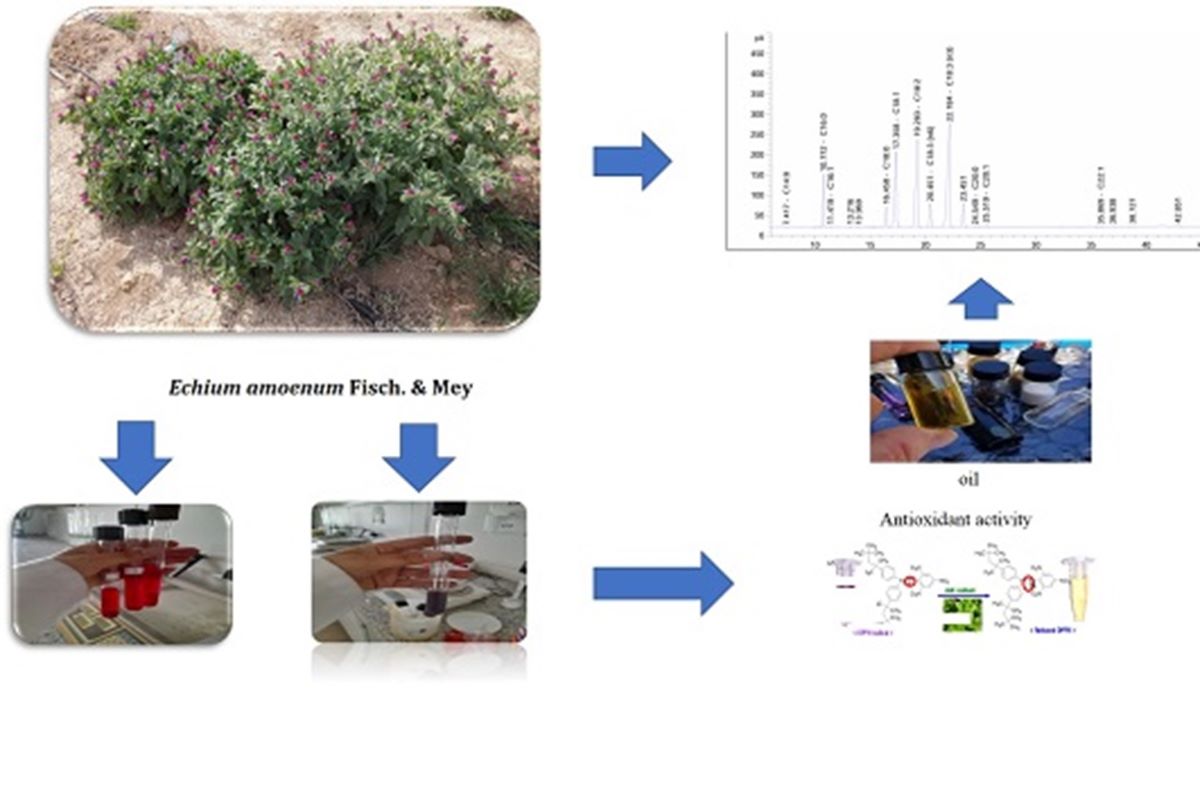
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Soil Chemical and Physical Analysis
2.4. Experimental Design and Treatments
2.5. Morphological Characteristics
2.6. Photosynthetic Pigments
2.7. Plant Extract
2.8. Determination of Total Phenol
2.9. Determination of Total Flavonoids
2.10. Total Anthocyanin Content
2.11. Determination of Antioxidant Properties of Extracts
2.12. Extraction of Fixed Oils
2.13. Fatty Acid Methyl Ester (Preparation)
2.14. Quantification of Fatty Acids
2.15. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Yield Characteristics
3.2. Photosynthetic Pigments
3.3. Total Phenol Content
3.4. Total Flavonoids Content (TFC)
3.5. Total Anthocyanins Content (TAC)
3.6. Antioxidant Capacity (DPPH Radical Scavenging Activity)
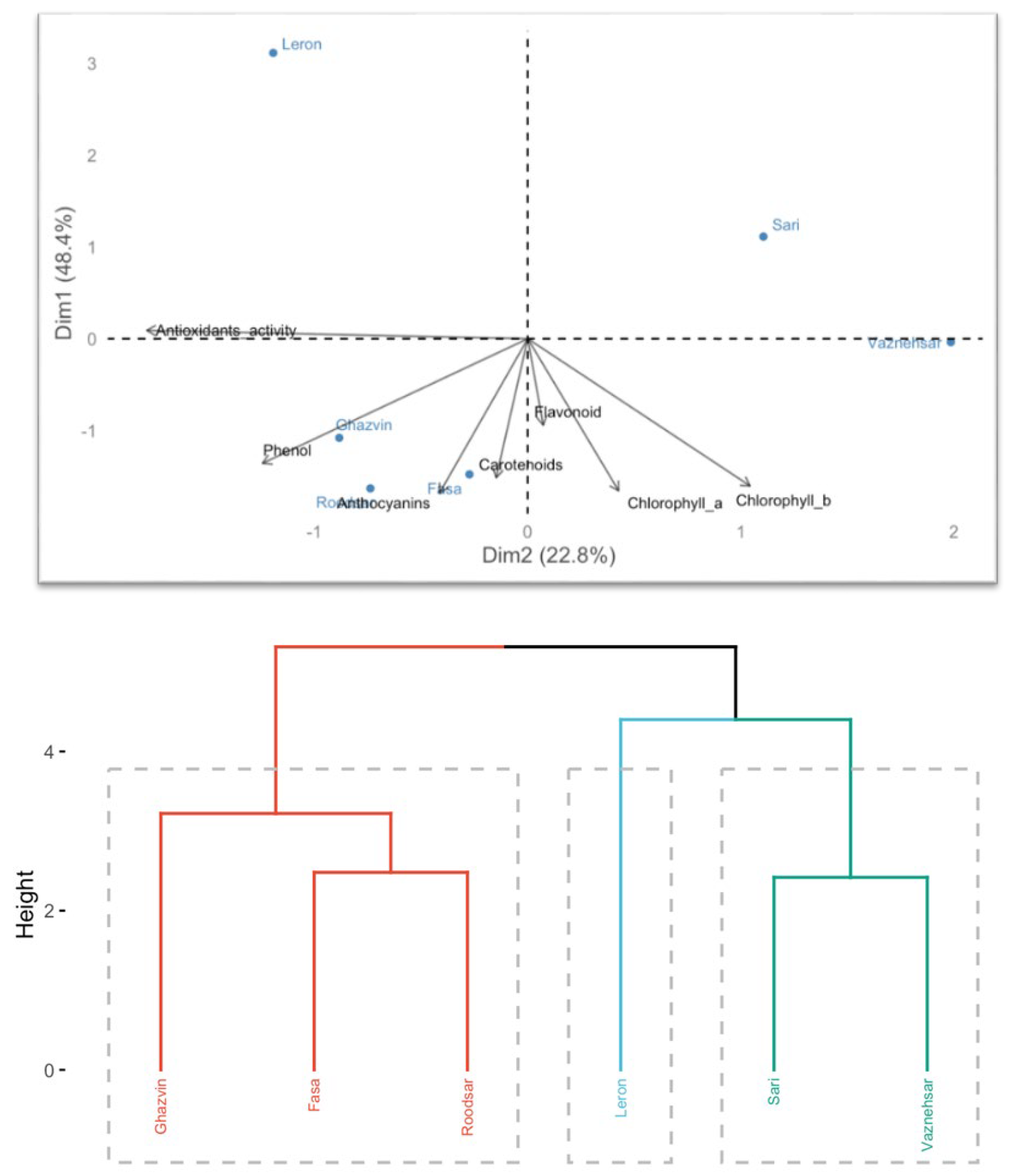
3.7. Multivariate Analysis of Antioxidants Attributes
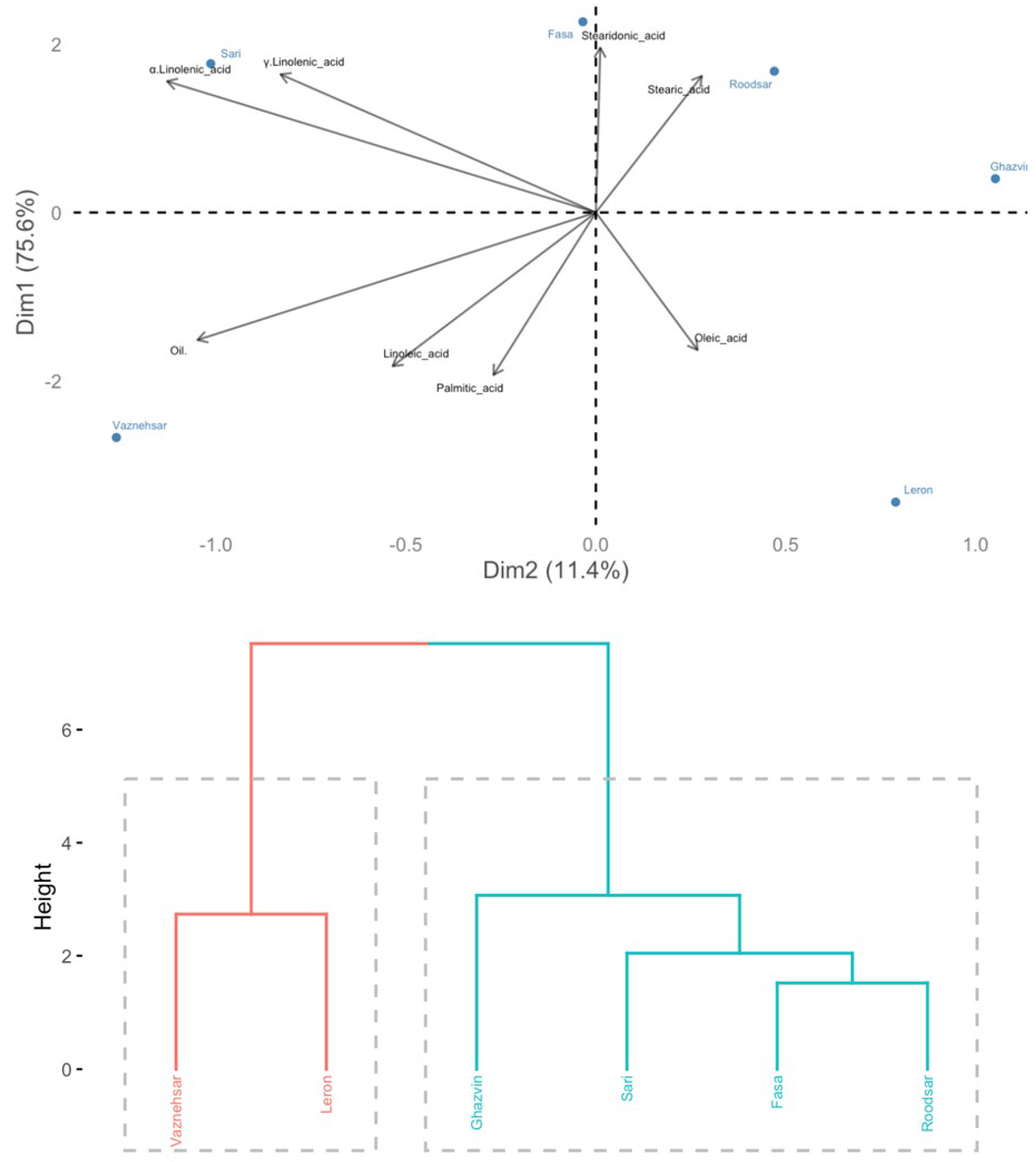
3.8. Oil Content and Fatty Acids Profile
4. Conclusions
Authorship Contribution
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, V. A pictorial dictionary of botany botanical taxonomy (Latin English French–Germany–Persian/complied). Farahang Moaser. 2008. [Google Scholar]
- Sayyah, M.; Boostani, H.; Pakseresht, S.; Malaieri, A. Efficacy of aqueous extract of Echium amoenum in treatment of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009, 33, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.; Ghassemi, N.; Sajjadi, S.E.; Ghannadi, A.; Shams-Ardakani, M. Main phenolic compounds of petals of Echium amoenum fisch. and C.A. Mey., a famous medicinal plant of Iran. Daru. 2005, 13, 65–69. [Google Scholar]
- Ghasemi Pirbalouti, A. Medicinal plants used in Chaharmahal and Bakhtyari districts, Iran. Herba Pol. 2009, 55, 69–75. [Google Scholar]
- Daneshfar, E.; Alirezalu, K.; Ahmadi Hoseini, M.; Naghavi, M.R.; Omidbaigi, R. Evaluation of oil content, fatty acid composition and physicochemical characteristics of some of Echium amoenum Fisch. Accessions. Iranian Journal of Medicinal and Aromatic Plants (IJMAPR). 2013, 28, 700–708.
- Abbaszadeh, S.; Radjabian, T.; Taghizadeh, M. Identification and determination of phytosterols in oilseeds of some populations from two Iranian Echium species. Iranian Journal of Medicinal and Aromatic Plants (IJMAPR). 2013, 28, 742–755. [Google Scholar]
- Naderi Hagy Bagher Candy, M.; Rezaee, M.B. Primory phytochemical investigation of Echium amoenium. Iranian Journal of Medicinal and Aromatic Plants (IJMAPR). 2004, 20, 377–383. [Google Scholar]
- Mehrabani, M.; Ghannadi, A.; Sajadi, E.; Ghasemi, N.; Ardakani, M.R. Toxic pyrrolozidine alkaloids of Echium amoenum fisch. & C.A. Mey, DARU. 2006, 14, 122–127.
- Mojab, F.; Behfar, A.; Kobarfard, F.; Nick avar, B.; Jafari, B. Investigating the composition of fatty acids in the seeds of Echium amoenum. Mey.et Fisch. J. Med. Plant Res. 2008, 29, 80–87. [Google Scholar]
- Sun, C.; Jia, L.; Xi, B.; Wang, L.; Weng, X. Natural variation in fatty acid composition of Sapindus spp. seed oils. Ind. Crops Prod. 2017, 102, 97–104. [Google Scholar] [CrossRef]
- Nayebpour, N.; Asadi-Gharneh, H.A. Variability of fatty acids composition of wild sumac (Rhus coriaria L). fruit. J. Med. Plants 2019, 3, 118–129. [Google Scholar] [CrossRef]
- Fei, X.; Qi, Y.; Lei, Y.; Wang, S.; Hu, H.; Wei, A. Transcriptome and metabolite analysis reveals key genes for melanin synthesis during the development of Zanthoxylum bungeanum seeds. Ind. Crops Prod. 2021, 165, 113419. [Google Scholar] [CrossRef]
- Yaghmaei, L.; Soltani, S.; Khodagholi, M. Bioclimatic classification of Isfahan province using multivariate statistical methods. Int. J. Climatol. 2009, 29, 1850–1861. [Google Scholar] [CrossRef]
- Page, A.L. (Eds.),. Methods of Soil Analysis. Part 2-Chemical and Microbiological Properties, Second edition. 1982.
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In: Banderis, A.D., Barter, D.H., Anderson, K. (Eds.), Agricultural and Advisor. U.S. Department of Agriculture Circular No. 1954, 939.
- Black, C.A. Methods of Soil Analysis, Part I-Physical and Mineralogical Properties Including Statistics of Measurement and Sampling. 1965.
- Lichtenthaler, H.K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, J.; Luyck, M.; Cazin, M. , Cazin, J. C, Bailleul, F., Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench). hulls and flour. J. Ethnopharmacol. 2000, 72, 35–40. [Google Scholar] [CrossRef]
- Wagner, G.J. Content and vacuole/extra vacuole distribution of neutral sugars free amino acids, and anthocyanins in protoplast. Plant. Physiol. 1979, 64, 88–93. [Google Scholar] [CrossRef]
- Wang, M.; Rangarajan, L.J.; Shao, M.; La Voie, E.J.; Huang, C.T.; Ho, C.T. Antioxidative phenolic compounds from sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind. Crops Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- ISO, 12966-4. Fatty acid methyl esters (FAME) in oil samples. Merck KGaA, Darmstadt, Germany. 2015.
- Kiani, M.; Alahdadi, I.; Soltani, E.; Boelt, B.; Benakashani, F. Variation of seed oil content, oil yield, and fatty acids profile in Iranian Nigella sativa L. landraces. Ind. Crops Prod. 2020, 2020. 149, 112367. [Google Scholar] [CrossRef]
- Wang, W.; Wei, L.; Li, H.; Xu, H.; Xu, Z.; Yan, C.; Wu, Y.; Ji, S.; Wang, T. Effects of sowing date on photosynthetic characteristics chlorophyll fluorescence and yield of different Echium plantagineum L. cultivars. Sci. Rep. 2023, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, N.; Chamani, E.; Shokouhian, A.A.; Ramezanpour, S.S.; Soltanlou, H. Assessment of genetic diversity and photosynthetic pigments among wild populations of Yellow Flag (Iris pseudacorus). J. Plant Mol. Breed. 2021, 9, 1–11. [Google Scholar]
- Taamalli, A.; Arráez Román, D.; Gómez Caravaca, A.M.; Zarrouk, M.; Segura Carretero, A. Geographical characterization of Tunisian olive tree leaves (cv. Chemlali) using HPLC-ESI-TOF and IT/MS fingerprinting with hierarchical cluster analysis. J. Anal. Chem. 2018, 6789704, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Pasković, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z. Determination of the variability of biophenols and mineral nutrients in olive leaves with respect to cultivar, collection period and geographical location for their targeted and well-timed exploitation. Plants. 2020, 9, 1–20. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; … Finglas, P. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. , Phenolic profile and comparison of the antioxidant, anti-Ageing, anti-Inflammatory, and protective activities of Borago officinalis extracts on skin cells. Molecules. 2021, 28, 1–19. [Google Scholar] [CrossRef]
- Ahmadi, H.; Babalar, M.; Sarcheshmeh, M.A.A.; Morshedloo, M.R.; Shokrpour, M. Effects of exogenous application of citrulline on prolonged water stress damages in hyssop (Hyssopus officinalis L.): Antioxidant activity, biochemical indices, and essential oils profile. Food Chem 2020, 333, 127433. [Google Scholar] [CrossRef]
- Fereidoonfar, H.; Salehi-Arjmand, H.; Khadivi, A.; Akramian Safdari, L. Chemical variation and antioxidant capacity of sumac (Rhus coriaria L.). Ind. Crops Prod. 2019, 139, 111518. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants. 2020, 9, 1098. [Google Scholar]
- Zemmouri, H.; Ammar, S.; Boumendjel, A.; Messarah, M.; El Feki, A.; Bouaziz, M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria. Arab. J. Chem. 2019, 12, 1954–1963. [Google Scholar] [CrossRef]
- Mumivand, H.; Babalar, M.; Tabrizi, L.; Craker, L.; Shokrpour, M.; Hadian, J. Antioxidant properties and principal phenolic phytochemicals of Iranian tarragon (Artemisia dracunculus L.) accessions. HORTIC ENVIRON BIOTE. 2017, 58, 414–422. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ibrahim, S.A.; Koca, I. Extraction of Anthocyanins from Borage (Echium amoenum) flowers using choline chloride and a glycerol-based, deep eutectic solvent: Optimization, antioxidant activity, and in vitro bioavailability. Molecules. 2022, 27, 134. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Khorami, S.; Safarabadi, M.; Shahmoradi, A.; Malekirad, A.A.; Vakilian, K.; Mandegary, A.; Abdollahi, M. Antioxidant activity of Iranian Echium amoenum Fisch & C.A. Mey flower decoction in humans: A cross-sectional before/after clinical trial Evid. -Based Complement. J Evid Based Complementary Altern Med. 2006, 3, 469–473. [Google Scholar]
- Sakhr, K.; Sami, E. Physiochemical properties and medicinal, nutritional and industrial applications of Lebanese Sumac (Syrian Sumac-Rhus coriaria): A review. Heliyon. 2020, 6, e03207. [Google Scholar] [CrossRef]
- Guil-Guerreroa, J.L.; Gómez-Mercadob, F.; Ramos-Buenoai, R.P.; González-Fernándeza, M.J.; Urrestarazucii, J.B.; Bélair, G. Fatty acid profiles and sn-2 fatty acid distribution of γ-linolenic acid-rich Borago species. J. Food Compos. Anal. 2017, 66, 74–80. [Google Scholar] [CrossRef]
- Tsegay, G.; Redi-Abshiro, M.; Chandravanshi, B.S.; Ele, E.; Mohammed, A.M.; Mamo, H. Effect of altitude of coffee plants on the composition of fatty acids of green coffee beans. BMC Chemistry. 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Gazave, E.; Tassone, E.E.; Baseggio, M.; Cyder, M.; Byriel, K.; Oblath, E.; … Pauli, D. Genome-wide association study identifies acyl lipid metabolism candidate genes involved in the genetic control of natural variation for seed fatty acid traits in Brassica napus L. Ind. Crops Prod. 2020, 145, 1–12. [Google Scholar] [CrossRef]
- Zhu, D.; Le, Y.; Zhang, R.; Li, X.; Lin, Z. . A global survey of the gene network and key genes for oil accumulation in cultivated tetraploid cottons. Plant Biotechnol. 2021, 19, 1170–1182. [Google Scholar] [CrossRef]
| N | Area of sampling collection | Populations | Longitude | Latitude | Altitude (m) |
Mean annual rainfall (mm) |
Mean annual temperature (◦C) |
Habitat |
| 1 | Fars Province | Fasa | 53°38′ | 28° 56′ | 1370 | 380 | 20 | Arid and Semi-arid |
| 2 | Gilan Province | Roodsar | ′ 28°50 | 37°13′ | -19 | 1178 | 15.8 | Mountain-Humid |
| 3 | Mazandaran Province | Sari | 5° 53′ | 36°39′ | 11 | 790 | 15.5 | Mountain-Humid |
| 4 | Ghazvin Province | Ghazvin | 57′ °49 | 36°18′ | 1314 | 318 | 14 | Mountain-Cold temperate |
| 5 | Gilan Province | Vazneh sar | 48°90′ | 37°80′ | 54 | 1300 | 16 | Mountain-Humid |
| 6 | Gilan Province | Leron | 48°80′ | 37°40′ | 60 | 1200 | 17 | Mountain-Humid |
| First Year | |||||||||||
| Seed weight per plant | 1000-seed weight | Leaf width | Leaf length | Number of Lateral Branch | Flower number | Canopy diameter | Plant Height | Accession names | Accessions number | ||
| 3.00±0.72b | 1.30±0.17a | 2.10±0.76ab | 10.60±0.60b | 88.30±31.75a | 311.70±155.4a | 53.30±5.03a | 38.30±7.57a | Fasa | 1 | ||
| 4.37±0.94ab | 1.20±0.25ab | 2.60±0.12ab | 6.90±0.40c | 55.70±13.00ab | 196.70±56.19c | 48.30±7.63ab | 44.30±9.07a | Roodsar | 2 | ||
| 3.24±1.30b | 1.20±0.09ab | 3.00±0.05a | 4.80±0.15d | 64.30±35.70ab | 211.70±73.20b | 46.00±6.92abc | 48.00±18.35a | Ghazvin | 3 | ||
| 6.00±1.86a | 1.00±0.09bc | 2.40±1.24ab | 6.00d±0.25c | 37.70±26.80bc | 175.70±111.36d | 50.00±0.01ab | 44.30±6.02a | Sari | 4 | ||
| 0.87±0.04c | 0.70±0.04c | 2.95±1.13a | 9.506±1.28b | 10.70±3.54c | 31.70±18.92c | 32.00±3.78c | 31.70±2.88a | Vazneh | 5 | ||
| 0.52±0.08c | 0.70±0.06c | 1.80±0.47b | 12.80±1.25a | 13.70±5.85c | 29.00±7.93f | 29.00±9cd | 30.50±0.57a | Leron | 6 | ||
| Second year | |||||||||||
| 3.83±0.27b | 4.50±0.50d | 2.70±1.07a | 11.50±0.50b | 181.30±7.63a | 373.70±25.10a | 55.15±0.53a | 67.70±2.51a | Fasa | 1 | ||
| 4.80±1.00ab | 10.30±1.52a | 2.95±0.05a | 7.60±0.52c | 79.00±11.01c | 251.70±20.81c | 53.00±0.68a | 50.00±0.87c | Roodsar | 2 | ||
| 3.70±1.82b | 3.60±0.52d | 3.43±0.51a | 5.30±0.57d | 152.00±7.54b | 315.30±13.61b | 48.34±0.68b | 49.80±4.64c | Ghazvin | 3 | ||
| 6.50±2.36a | 6.90±1.12b | 2.90±1.15a | 6.50±0.51cd | 161.00±15.09b | 321.30±20.00b | 51.54±0.41ab | 59.00±7.00b | Sari | 4 | ||
| 0.86±0.11c | 4.83±0.76cd | 3.50±1.57a | 10.20±1.58b | 23.16±10.61d | 53.30±15.27d | 33.00±0.59c | 41.60±0.34d | Vazneh sar | 5 | ||
| 0.64±0.20c | 6.50±0.50bc | 2.23±0.49a | 13.70±1.52a | 27.33±15.37d | 26.70±2.88d | 23.00±5.56d | 28.90±0.62e | Leron | 6 | ||
| First Year | |||||||||
| Oil (%) |
Antioxidant activity (%) | Anthocyanins (mg g-1) |
Flavonoids (mg g-1) |
Phenol (mg g-1) |
Carotenoids (mg g-1) |
Chb (mg g-1) |
Cha (mg g-1) |
Accession names | Accessions number |
| 17.60±0.17 | 69.20±0.76a | 30.50±1.32a | 4.90±0.60c | 18.90±0.31b | 1.50±0.07b | 2.80±0.13d | 3.40±0.20c | Fasa | 1 |
| 16.20±0.06 | 58.20±1.04d | 30.10±0.76a | 3.00±1.19d | 31.25±1.91a | 2.30±0.10b | 3.50±0.37c | 4.40±0.26bc | Roodsar | 2 |
| 17.70±0.05 | 66.80±2.23b | 24.70±2.08b | 11.80±0.66a | 32.90±1.71a | 4.80±1.49a | 6.30±0.64a | 7.20±1.71a | Ghazvin | 3 |
| 16.50±0.1 | 61.00±1.11c | 24.30±2.08b | 5.80±0.58c | 8.00±0.58d | 3.80±1.48a | 2.70±0.23d | 4.00±1.25c | Sari | 4 |
| 20.60±0.09 | 51.10±1.31e | 19.00±1.00c | 7.60±0.44b | 8.80±0.38d | 4.60±0.46a | 2.60±0.41d | 4.80±1.38bc | Vazneh sar | 5 |
| 22.40±0.10 | 60.60d±1.80c | 15.30±1.52d | 3.90±0.45d | 11.80±0.68c | 4.60±0.36a | 4.50±0.40b | 5.77±1.23b | Leron | 6 |
| Second year | |||||||||
| 22.65±0.17 | 61.60±0.24c | 30.80±0.11b | 19.30±0.26a | 33.40±0.17b | 5.80±0.22b | 7.60±0.20a | 15.60±1.62a | Fasa | 1 |
| 21.50±0.13 | 63.60±0.56b | 31.25±0.17a | 19.50±0.20a | 33.00±0.13bc | 6.80±0.13a | 5.90±0.41b | 10.70±0.59b | Roodsar | 2 |
| 22.20±0.12 | 52.00±0.28f | 28.40±0.24d | 18.20±0.08c | 34.10±0.21a | 2.20±0.12e | 6.80±0.11ab | 7.50±0.25 c | Ghazvin | 3 |
| 25.50±0.12 | 58.10±0.63d | 29.30±0.24c | 18.60±0.11b | 32.70±0.46c | 3.20±0.17d | 2.80±0.09c | 6.00±0.18d | Sari | 4 |
| 24.00±0.21 | 54.90±2.25e | 28.20±0.21de | 17.30±0.21d | 32.87±0.53bc | 3.60±0.03c | 7.40±2.08ab | 10.84±0.26b | Vazneh sar | 5 |
| 21.00±1.0 | 70.90±0.59a | 27.90±0.04e | 17.20±0.18d | 33.62±0.18c | 1.80±0.21f | 1.40±0.14c | 3.20±0.11e | Leron | 6 |
| Stearidonic acid. (C18:4) |
α-Linolenic acid (C18:3) |
γ-Linolenic acid (C18:3) |
Linoleic acid (C18:2) |
Oleic acid (C18:0) |
Stearic acid (C18:0) |
Palmitic acid (C16:0) |
Accession names | Accessions number | ||
| 5.40±1.02a | 33.92±1.04b | 5.00±0.10a | 23.80±0.15d | 19.30±0.16ab | 4.60±0.13b | 6.30±0.10cde | Fasa | 1 | ||
| 5.00±1.04b | 33.90±0.97b | 4.80±0.07c | 24.30±0.11cd | 18.80±0.13c | 4.70±0.06ab | 6.60±0.05cd | Roodsar | 2 | ||
| 5.00±0.89b | 33.20±1.50c | 4.94±0.06b | 24.40±0.20cd | 19.40±0.13a | 4.80±0.07a | 6.80±0.06c | Ghazvin | 3 | ||
| 5.00±1.03b | 34.20±1.00a | 4.90±0.13b | 24.90±0.07c | 18.35±0.13cd | 4.70±0.07ab | 6.60±0.02cd | Sari | 4 | ||
| 4.60±0.95d | 32.30±1.10de | 4.70±0.07cd | 26.20±0.09a | 19.10±0.13ab | 4.25±0.02bc | 7.30±0.08a | Vazneh sar | 5 | ||
| 4.90±0.74c | 32.70±1.09d | 4.90±0.11b | 25.50±0.09b | 19.20±0.08ab | 4.10±0.09bcd | 7.00±0.10ab | Leron | 6 | ||
| 6.80±0.21a | 37.40±0.11a | 5.70±0.23a | 21.55±0.54e | 16.44±0.12d | 4.60±0.05ab | 5.70±0.18cd | Fasa | 1 | ||
| 6.40±0.15ab | 36.95±0.05b | 5.50±0.08bc | 22.20±0.17de | 16.55±0.11d | 4.50±0.13b | 5.90±0.10c | Roodsar | 2 | ||
| 5.60±0.05c | 34.92±0.05c | 5.40±0.12bcd | 22.69±0.13c | 17.94±0.10c | 4.97±0.34a | 6.10±0.15b | Ghazvin | 3 | ||
| 6.60±0.33ab | 37.30±0.06ab | 5.65±0.07ab | 22.30±0.07d | 15.80±0.07e | 4.52±0.30b | 5.90±0.08c | Sari | 4 | ||
| 5.00±0.03cd | 37.30±0.03ab | 5.65±0.20ab | 24.50±0.15ab | 19.30±0.16a | 4.64±0.10ab | 7.30±0.20a | Vazneh sar | 5 | ||
| 4.80±0.14de | 33.95±0.10d | 4.20±0.06de | 24.70±0.32a | 18.30±0.19b | 4.48±0.14bc | 7.30±0.07a | Leron | 6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




