Submitted:
23 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
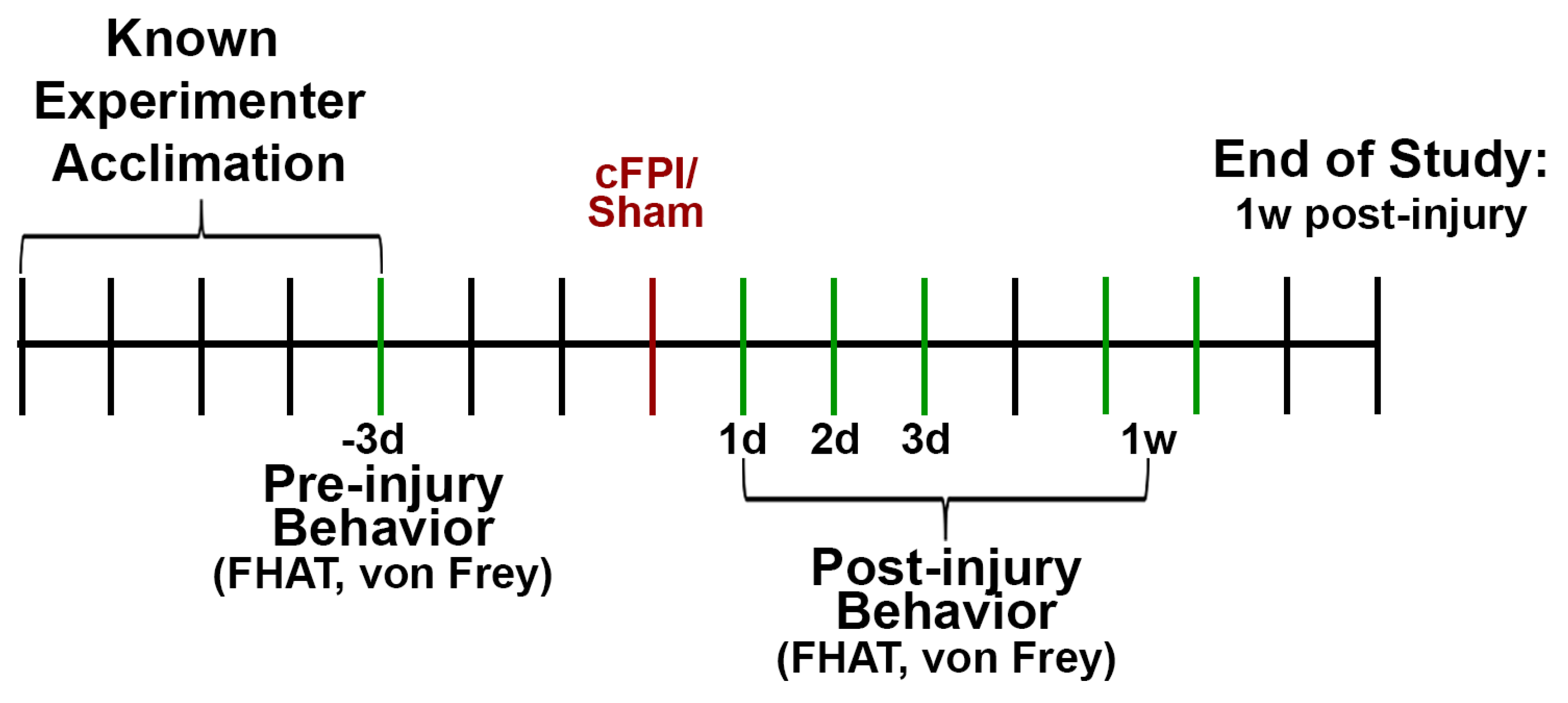
2. Materials and Methods
2.1. Animals
2.2. Surgical Preparation and Induction of Traumatic Brain Injury
2.3. Forced Human Approach Task (FHAT)
2.4. Von Frey Monofilament Tests
2.5. Statistical Analysis
3. Results
3.1. Forced Human Approach by an Unknown Experimenter Is Negatively Affected by cFPI in Female Minipigs
3.2. Von Frey Sensitivities Are Affected by TBI in Sex-Dependent Loci
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The Impact of Traumatic Brain Injuries: A Global Perspective. NeuroRehabilitation 2007, 22, 341–353. [CrossRef]
- Coronado, V.G.; Xu, L.; Basavaraju, S.V.; McGuire, L.C.; Wald, M.M.; Faul, M.D.; Guzman, B.R.; Hemphill, J.D.; Centers for Disease Control and Prevention (CDC) Surveillance for Traumatic Brain Injury-Related Deaths--United States, 1997-2007. Morb. Mortal. Wkly. Rep. Surveill. Summ. Wash. DC 2002 2011, 60, 1–32.
- Miller, G.F.; DePadilla, L.; Xu, L. Costs of Nonfatal Traumatic Brain Injury in the United States, 2016. Med. Care 2021, 59, 451–455. [CrossRef]
- Dikmen, S.; Machamer, J.; Temkin, N. Mild Traumatic Brain Injury: Longitudinal Study of Cognition, Functional Status, and Post-Traumatic Symptoms. J. Neurotrauma 2017, 34, 1524–1530. [CrossRef]
- Cole, W.R.; Bailie, J.M. Neurocognitive and Psychiatric Symptoms Following Mild Traumatic Brain Injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; Frontiers in Neuroscience; CRC Press/Taylor and Francis Group: Boca Raton (FL), 2016 ISBN 978-1-4665-8491-4.
- Mott, T.F.; McConnon, M.L.; Rieger, B.P. Subacute to Chronic Mild Traumatic Brain Injury. Am. Fam. Physician 2012, 86, 1045–1051.
- Bryant, R.A.; O’Donnell, M.L.; Creamer, M.; McFarlane, A.C.; Clark, C.R.; Silove, D. The Psychiatric Sequelae of Traumatic Injury. Am. J. Psychiatry 2010, 167, 312–320. [CrossRef]
- Baguley, I.J.; Cooper, J.; Felmingham, K. Aggressive Behavior Following Traumatic Brain Injury: How Common Is Common? J. Head Trauma Rehabil. 2006, 21, 45.
- Benedictus, M.R.; Spikman, J.M.; van der Naalt, J. Cognitive and Behavioral Impairment in Traumatic Brain Injury Related to Outcome and Return to Work. Arch. Phys. Med. Rehabil. 2010, 91, 1436–1441. [CrossRef]
- Thielen, H.; Tuts, N.; Welkenhuyzen, L.; Huenges Wajer, I.M.C.; Lafosse, C.; Gillebert, C.R. Sensory Sensitivity after Acquired Brain Injury: A Systematic Review. J. Neuropsychol. 2023, 17, 1–31. [CrossRef]
- Boucher, O.; Turgeon, C.; Champoux, S.; Ménard, L.; Rouleau, I.; Lassonde, M.; Lepore, F.; Nguyen, D.K. Hyperacusis Following Unilateral Damage to the Insular Cortex: A Three-Case Report. Brain Res. 2015, 1606, 102–112. [CrossRef]
- Kumar, S.; Rao, S.L.; Nair, R.G.; Pillai, S.; Chandramouli, B.A.; Subbakrishna, D.K. Sensory Gating Impairment in Development of Post-Concussive Symptoms in Mild Head Injury. Psychiatry Clin. Neurosci. 2005, 59, 466–472. [CrossRef]
- Shultz, S.R.; McDonald, S.J.; Corrigan, F.; Semple, B.D.; Salberg, S.; Zamani, A.; Jones, N.C.; Mychasiuk, R. Clinical Relevance of Behavior Testing in Animal Models of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2381–2400. [CrossRef]
- Netzley, A.H.; Pelled, G. The Pig as a Translational Animal Model for Biobehavioral and Neurotrauma Research. Biomedicines 2023, 11, 2165. [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The Neuropathology of Traumatic Brain Injury. Handb. Clin. Neurol. 2015, 127, 45–66. [CrossRef]
- Elliott, M.B.; Oshinsky, M.L.; Amenta, P.S.; Awe, O.O.; Jallo, J.I. Nociceptive Neuropeptide Increases and Periorbital Allodynia in a Model of Traumatic Brain Injury. Headache J. Head Face Pain 2012, 52, 966–984. [CrossRef]
- Keizer, D.; van Wijhe, M.; Post, W.J.; Uges, D.R.A.; Wierda, J.M.K.H. Assessment of the Clinical Relevance of Quantitative Sensory Testing with Von Frey Monofilaments in Patients with Allodynia and Neuropathic Pain. A Pilot Study. Eur. J. Anaesthesiol. EJA 2007, 24, 658. [CrossRef]
- Macolino, C.M.; Daiutolo, B.V.; Albertson, B.K.; Elliott, M.B. Mechanical Allodynia Induced by Traumatic Brain Injury Is Independent of Restraint Stress. J. Neurosci. Methods 2014, 226, 139–146. [CrossRef]
- Moharić, M.; Vidmar, G.; Burger, H. Sensitivity and Specificity of von Frey’s Hairs for the Diagnosis of Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus. J. Diabetes Complications 2012, 26, 319–322. [CrossRef]
- Wattiez, A.-S.; Castonguay, W.C.; Gaul, O.J.; Waite, J.S.; Schmidt, C.M.; Reis, A.S.; Rea, B.J.; Sowers, L.P.; Cintrón-Pérez, C.J.; Vázquez-Rosa, E.; et al. Different Forms of Traumatic Brain Injuries Cause Different Tactile Hypersensitivity Profiles. PAIN 2021, 162, 1163. [CrossRef]
- Daiutolo, B.V.; Tyburski, A.; Clark, S.W.; Elliott, M.B. Trigeminal Pain Molecules, Allodynia, and Photosensitivity Are Pharmacologically and Genetically Modulated in a Model of Traumatic Brain Injury. J. Neurotrauma 2016, 33, 748–760. [CrossRef]
- Stelfa, G.; Svalbe, B.; Vavers, E.; Duritis, I.; Dambrova, M.; Zvejniece, L. Moderate Traumatic Brain Injury Triggers Long-Term Risks for the Development of Peripheral Pain Sensitivity and Depressive-like Behavior in Mice. Front. Neurol. 2022, 13. [CrossRef]
- Guide for the Care and Use of Laboratory Animals; Institute of Laboratory Animal Resources, National Research Council, Eds.; 8. ed.; National Academies Press: Washington, D.C, 2011; ISBN 978-0-309-15400-0.
- 2019; 24. Animal Welfare Act; 2019;
- 2024; 25. Animal Welfare Regulations; 2024;
- Pavlichenko, M.; Lafrenaye, A.D. The Central Fluid Percussion Brain Injury in a Gyrencephalic Pig Brain: Scalable Diffuse Injury and Tissue Viability for Glial Cell Immunolabeling Following Long-Term Refrigerated Storage. Biomedicines 2023, 11, 1682. [CrossRef]
- Filley, C.M.; Kelly, J.P. White Matter and Cognition in Traumatic Brain Injury. J. Alzheimers Dis. 2018, 65, 345–362. [CrossRef]
- Hoffe, B.; Holahan, M.R. The Use of Pigs as a Translational Model for Studying Neurodegenerative Diseases. Front. Physiol. 2019, 10. [CrossRef]
- Lafrenaye, A.D.; Todani, M.; Walker, S.A.; Povlishock, J.T. Microglia Processes Associate with Diffusely Injured Axons Following Mild Traumatic Brain Injury in the Micro Pig. J. Neuroinflammation 2015, 12, 186. [CrossRef]
- Lunney, J.K.; Goor, A.V.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021. [CrossRef]
- Prange, M.T.; Margulies, S.S. Regional, Directional, and Age-Dependent Properties of the Brain Undergoing Large Deformation. J. Biomech. Eng. 2002, 124, 244–252. [CrossRef]
- Ransom, B.R.; Goldberg, M.P.; Baltan, S. 8 - Molecular Pathophysiology of White Matter Anoxic-Ischemic Injury. In Stroke (Fifth Edition); Mohr, J.P., Wolf, P.A., Grotta, J.C., Moskowitz, M.A., Mayberg, M.R., von Kummer, R., Eds.; W.B. Saunders: Saint Louis, 2011; pp. 122–137 ISBN 978-1-4160-5478-8.
- Krugmann, K.; Warnken, F.; Krieter, J.; Czycholl, I. Are Behavioral Tests Capable of Measuring Positive Affective States in Growing Pigs? Anim. Open Access J. MDPI 2019, 9, 274. [CrossRef]
- Czycholl, I.; Menke, S.; Straßburg, C.; Krieter, J. Reliability of Different Behavioural Tests for Growing Pigs On-Farm. Appl. Anim. Behav. Sci. 2019, 213, 65–73. [CrossRef]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.-V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the Human–Animal Relationship in Farmed Species: A Critical Review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [CrossRef]
- Hartlage, L.C.; Durant-Wilson, D.; Patch, P.C. Persistent Neurobehavioral Problems Following Mild Traumatic Brain Injury. Arch. Clin. Neuropsychol. 2001, 16, 561–570. [CrossRef]
- Ponsford, J.; Alway, Y.; Gould, K.R. Epidemiology and Natural History of Psychiatric Disorders After TBI. J. Neuropsychiatry Clin. Neurosci. 2018, 30, 262–270. [CrossRef]
- Kelly, G.; Brown, S.; Todd, J.; Kremer, P. Challenging Behaviour Profiles of People with Acquired Brain Injury Living in Community Settings. Brain Inj. 2008, 22, 457–470. [CrossRef]
- Camerlink, I.; Ursinus, W.W. Tail Postures and Tail Motion in Pigs: A Review. Appl. Anim. Behav. Sci. 2020, 230, 105079. [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of Positive and Negative Emotions and Emotional Contagion in Pigs. Physiol. Behav. 2013, 109, 42–50. [CrossRef]
- Noonan, G.J.; Rand, J.S.; Priest, J.; Ainscow, J.; Blackshaw, J.K. Behavioural Observations of Piglets Undergoing Tail Docking, Teeth Clipping and Ear Notching. Appl. Anim. Behav. Sci. 1994, 39, 203–213. [CrossRef]
- Khanna, R.; Moutal, A.; White, K.A.; Chefdeville, A.; Negrao de Assis, P.; Cai, S.; Swier, V.J.; Bellampalli, S.S.; Giunta, M.D.; Darbro, B.W.; et al. Assessment of Nociception and Related Quality-of-Life Measures in a Porcine Model of Neurofibromatosis Type 1. PAIN 2019, 160, 2473. [CrossRef]
- Harting, M.T.; Jimenez, F.; Adams, S.D.; Mercer, D.W.; Cox, C.S. Acute, Regional Inflammatory Response after Traumatic Brain Injury: Implications for Cellular Therapy. Surgery 2008, 144, 803–813. [CrossRef]
- Chang, T.T.-L.; Ciuffreda OD, P., Kenneth Joseph; Kapoor, N. Critical Flicker Frequency and Related Symptoms in Mild Traumatic Brain Injury. Brain Inj. 2007, 21, 1055–1062. [CrossRef]
- Chorney, S.R.; Suryadevara, A.C.; Nicholas, B.D. Audiovestibular Symptoms as Predictors of Prolonged Sports-Related Concussion among NCAA Athletes. The Laryngoscope 2017, 127, 2850–2853. [CrossRef]
- Schrupp, L.E.; Ciuffreda, K.J.; Kapoor, N. Foveal versus Eccentric Retinal Critical Flicker Frequency in Mild Traumatic Brain Injury. Optom. - J. Am. Optom. Assoc. 2009, 80, 642–650. [CrossRef]
- Shepherd, D.; Landon, J.; Kalloor, M.; Theadom, A. Clinical Correlates of Noise Sensitivity in Patients with Acute TBI. Brain Inj. 2019, 33, 1050–1058. [CrossRef]
- Wehling, E.; Naess, H.; Wollschlaeger, D.; Hofstad, H.; Bramerson, A.; Bende, M.; Nordin, S. Olfactory Dysfunction in Chronic Stroke Patients. BMC Neurol. 2015, 15, 199. [CrossRef]
- Sandercock, D.A.; Gibson, I.F.; Rutherford, K.M.D.; Donald, R.D.; Lawrence, A.B.; Brash, H.M.; Scott, E.M.; Nolan, A.M. The Impact of Prenatal Stress on Basal Nociception and Evoked Responses to Tail-Docking and Inflammatory Challenge in Juvenile Pigs. Physiol. Behav. 2011, 104, 728–737. [CrossRef]
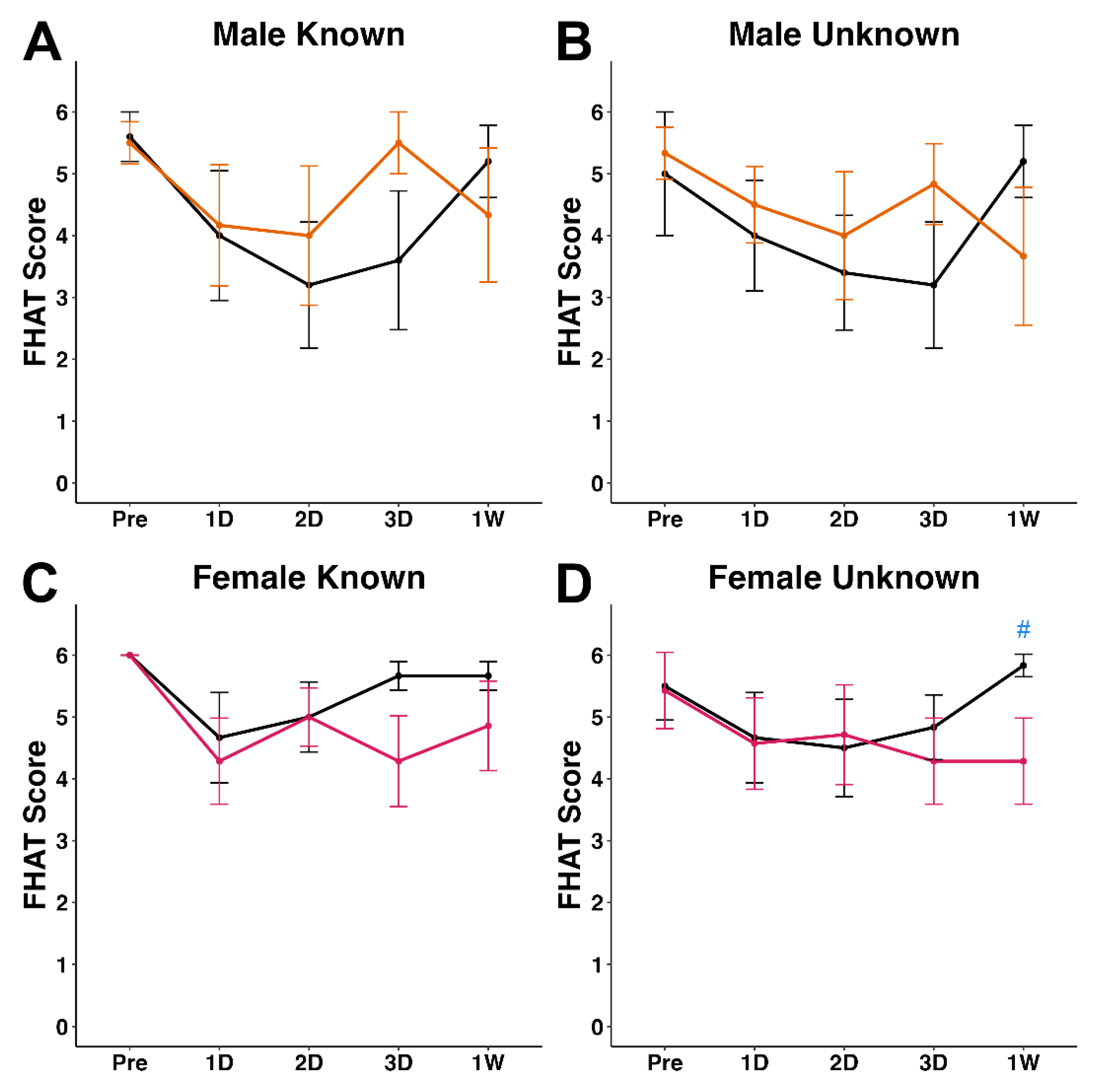
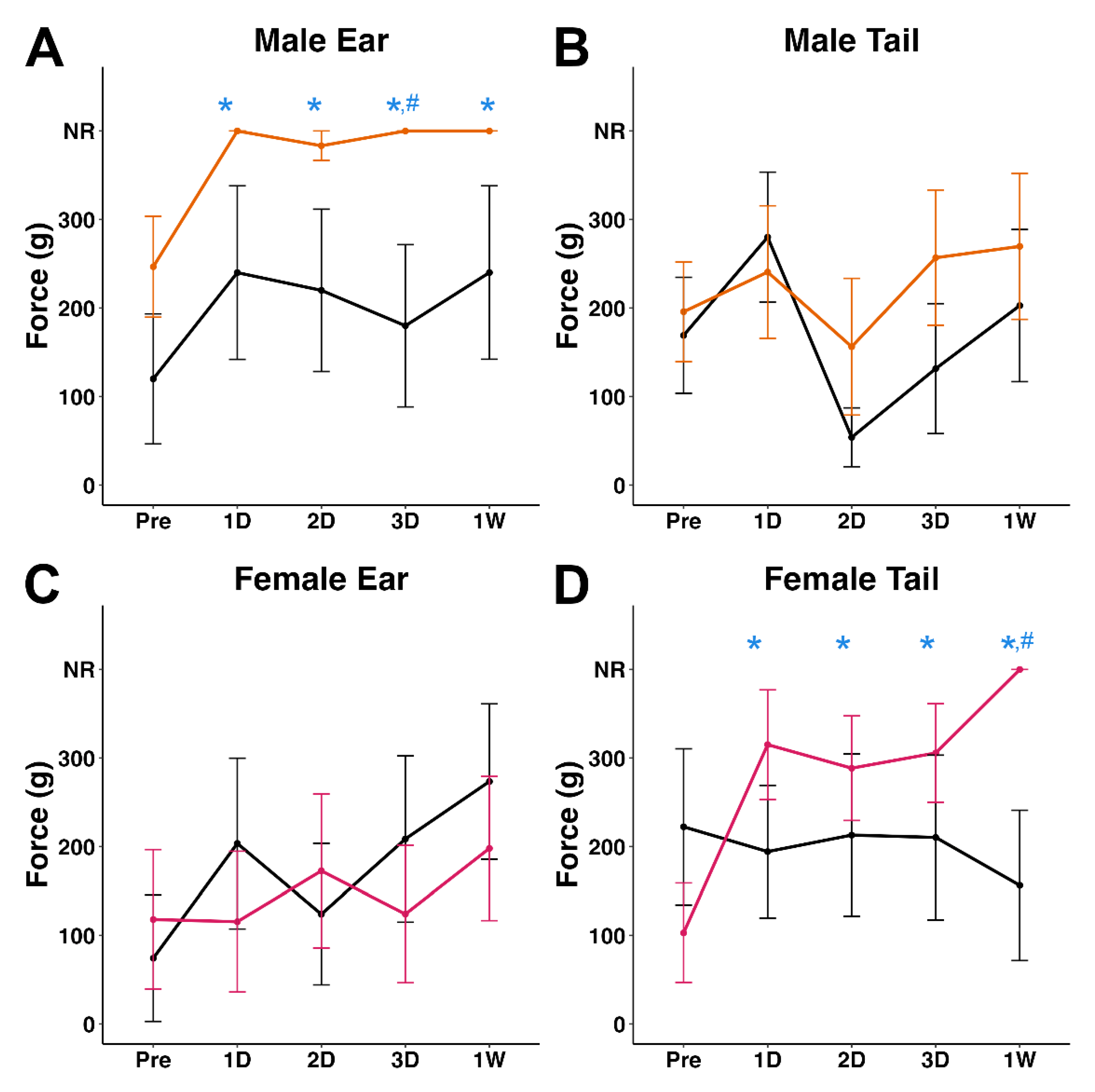
| FHAT Score | Minipig Behavioral Description |
| 0 | Unable to interact with or move away from experimenter |
| 1 | Avoidance of any contact and/or display of defensive behaviors (e.g., growling) |
| 2 | Withdrawal upon any physical contact |
| 3 | Withdrawal upon physical contact on more than one locus |
| 4 | Tensing/shying upon physical contact on certain loci, no withdrawal |
| 5 | No withdrawal or tensing upon physical contact, no interaction with experimenter |
| 6 | No withdrawal or tensing upon physical contact, interaction with experimenter (e.g., voluntarily approaching experimenter, rubbing body against experimenter’s legs, chewing shoelaces/shoe covers) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





