1. Introduction
The prerequisite for successful controlled reproduction is controlled broodstock farming, which ensures a good and stable physiological mode in the fish (homeostasis) that provides a predictable response to the control tool. Challenges with disease, wounds, and mortality in farmed lumpfish (
Cyclopterus lumpus) have often been reported [
1], indicating cases of poor farming conditions and chronic stress. In addition, there is a large spread in spawning time and fecundity within lumpfish spawning groups [
2,
3,
4,
5]. Early in sexual maturity, stress can lead to reallocation of energy where investment in offspring (gamete production) is given lower priority but at later stages stress can lead to prioritisation of egg production at the expense of somatic growth [
6]. Plasma cortisol is one of the most commonly measured indicators of stress and has a direct inhibitory effect on steroidogenesis [
7]. Schreck [
8] concluded that understanding the influence of stress and the stress response depends on where in the reproductive process the influence occurs and may be relevant for optimizing operating routines to ensure good reproductive fitness (reproductive yield, hatching rate and quality of offspring).
The mucosal barriers of skin and gills are the frontline protection in teleosts, containing multitudinous substances for communication with the external world, including microbia and the physical impacts. Such mucosal barriers are part of the external immune system and have acted as a communication layer of living cells between aquatic organisms and the environment for over half a billion years [
9]. The mucus layer of fish contains a number of immune-relevant compounds such as lectins, lysozymes, and immunoglobulins, which form a biochemical barrier that constitutes the fish’s first line of defence or external barrier against a number of pathogens. Several of these have also been detected in lumpfish [
10]. In general, the skin acts like a shield, reflecting the microbiome of surface contacts and impacts, while the gills act as a sentinel guard, comprising 50% of the surface area of the fish, and in constant dialogue with water quality, particles and waterborne microbia [
11,
12] and both can respond to various kinds of stress [
13].
Attempts to quantify the factories of skin, gill or gut mucus, the mucous cells, were hampered by descriptive results and traditional counting of number of cells per variable unit of measure until the development of quantitative mucosal mapping, now trademarked as Veribarr™, the verification of barriers [
13,
14,
15,
16,
17,
18]. The use of number alone as a response descriptor is particularly misleading for biological significance because of the effect of cell size on the functional potential of these cells (
Supplementary Table S1). The normal response to chronic, but not acute, stress in the fish skin [14-17] is reduced volumetric density of mucous cells in the epithelium (MCD, %), increased or exhausted mean area of mucous cells (MCA, μm
2) and reduced barrier strength and is associated with reduced robustness and welfare. Healthy gills, by contrast, have little need for mucous cells in the respiratory tissue of gill lamellae whereas the gill filament may secrete somatic substances through its mucous cell population which is both larger and more abundant than in the lamella [
16].
This paper aims to build on the work already conducted in optimising lumpfish broodstock temperature [
4], photoperiod [2-3] and nutrition [
19] by providing vital information on the stress relationship between mucosal barrier functions and sexual development.
2. Materials and Methods
2.1. Experimental Fish
Juvenile lumpfish (N=300, weight range =8-10 g) produced at Fjord Forsk Sogn, Sogndal, Norway (offspring of wild fish caught in the Sognefjord) were kept at Industrial Laboratory (ILAB) at the University of Bergen until November 2016 during simulated natural photoperiod for Bergen (60°N) on water pumped from 100 m depth and stable temperature (8.5-9.4 °C) and salinity (33.9-34.7 ‰). Oxygen saturation ranged from 85-94%. The fish group was kept in a 3 m tank of 7,000 litres and fed a commercial marine dry feed where pellet size was adjusted according to weight. The fish density was kept lower than 30 kg/m
3. The fish were raised here for the next 14 months until the start of this trial. The corresponding data on gonadal development of lumpfish in the present experiment are described by [
5].
2.2. Stress Induction
At the start of the experiment 5 January 2018 six undisturbed lumpfish (1587 g, SEM ± 704 g) from a base population of approx. 300 fish contained in a 6000 L tank were sampled for blood, skin tissue and gills. Thereafter, two groups of 20 lumpfish each were randomly collected from the base population. Before moving the two groups to separate tanks (2500 L), they were injected with 30 (“high”) and 15 mg (“low”) cortisol implants per kg fish, and group marked with different colours. The cortisol injection was done in order to test how the introduction of chronically elevated plasma cortisol (stress) affects the natural sexual maturation cycle. The cortisol implant (hydrocortisone, H4001 SIGMA, CAS Number 50-23-7) was diluted to 20 mg cortisol per ml vegetable fat (mixture of vegetable oil and hydrogenated fat in ratio 1:1) and injected intraperitoneally [
20]. The fluorescent colour mark (Northwest Marine Technology Inc., WA, USA) was injected subcutaneously at the top of the head.
2.2.1. Pilot Trial
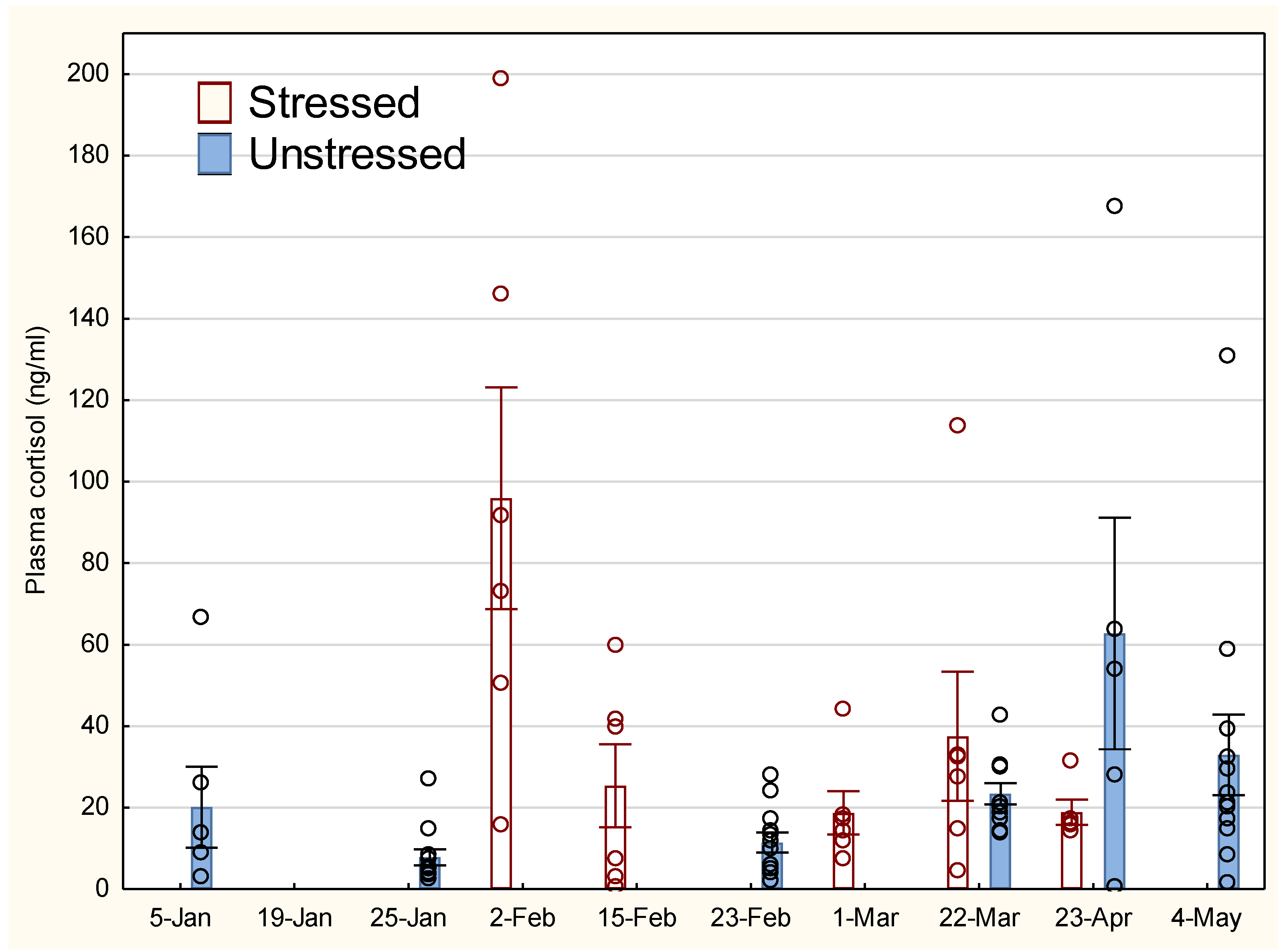
The dose-response was tested in a pilot trial between the two injected groups, to determine what cortisol dosage to use in the trial. Plasma cortisol measured on Jan 19 from 6 fish from each group showed the “high” group had 931.2 (SEM ± 204) while the “low” group had 320.3 (SEM ± 165) ng/ml plasma cortisol. These levels decreased to 95.9 (SEM ± 27.2) and 44.1 (SEM ± 20.4) on February 2 and further decreased to 25.4 (SEM ± 10.1) and 6.7 (SEM ± 2.0) on February 15, for “high” and “low” group respectively. The plasma cortisol levels in the two experimental groups were significantly different at all these time points (one-way ANOVA, P < 0.05). There was no mortality in any of the groups. Due to the higher and more prolonged stress level in the “high” group, this group was selected for further comparison with unstressed group, after which they were transferred to the base population under common garden conditions February 15. The “low” dose group was euthanized at a lethal dose of 500 mg/L Metacaine (MS222), followed by bleeding.
2.2.2. Experimental Groups
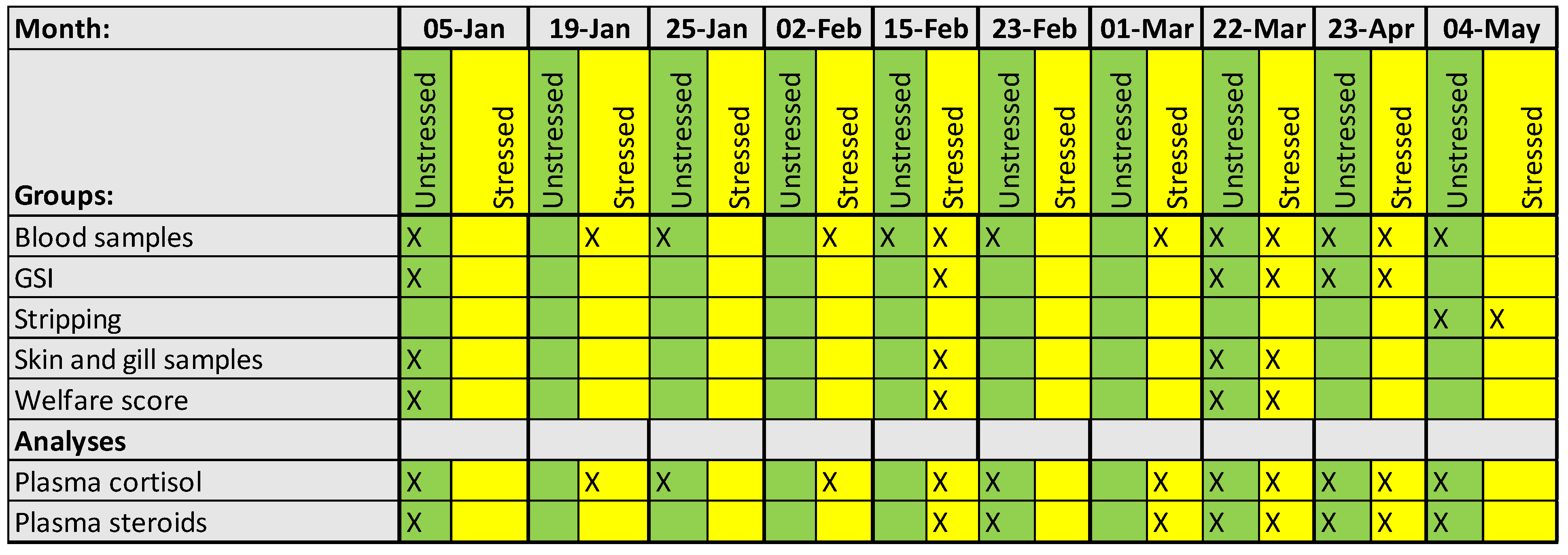
The unstressed base-population (reference group) was measured on 5 January, 25 January, 23 February and 22 March (
Table 1), and following plasma cortisol (ng/ml) values were measured: 20.16 (SEM ± 9.92), 7.9 (SEM ± 1.9), 11.5 (SEM ± 2.4), and 23.1 (SEM ± 2.7) respectively. These levels were generally significantly lower than the high dose group measured during this period, except for 23 February where there was no significant difference between plasma cortisol levels in the unstressed group compared to those of the “high” dose group on February 15 (
P=0.52).
To reduce the number of fish sacrificed and reduce the risk of stress due to handing, stressed and unstressed fish were, with a few exceptions, sampled at different dates (see
Table 1). The cortisol measurements from the reference fish were derived from blood sampled for the sex steroid analyses presented in [
5]. Their data show that stress was induced during mid to late vitellogenesis (ovarian stage 4 to 5) in the present study.
2.3. Sampling Protocol
During sampling 6 fish from each group (3 fish at a time) were netted from the tank to a 20 L tub of seawater with 200 mg/L Metacaine added for 2-5 minutes until the fish lost equilibrium and was apathetic. At each time of sampling, the fish were weighed, and length measured and approximately 1 ml blood was taken with 2 ml heparinised syringes ventrally from the caudal peduncle just behind the caudal aorta and transferred to Eppendorf tubes. The samples were then centrifuged (13000 rpm for approx. 5 min) and blood plasma pipetted into new Eppendorf tubes and stored on liquid nitrogen (-80 °C).
Some of the samples were also analyzed for steroids (E2 and 11-ketotestosterone). These are reported together with detailed information on the gonadal maturation process [
5] on fish from the present experiment. The schedule for blood samples and other analyses is given in
Table 1. Due to a limited number of fish in the experiment, there was a spartan withdrawal of lethal biopsies of skin and gills. Samples were collected for mucosal barrier analyses at three timepoints: January 5 from 6 unstressed reference group fish (U), 15 February from 6 stressed fish (S), and 22 March from 6 fish from both groups while on the final two dates (Apr 23 and May 4) 6 number of fish were sampled from each group for blood and gonad analyses. For each euthanized fish, a dorsal skin biopsy of 1.5 x 2.0 cm was taken from the right side between two rows of ossicles and second gill arch from the right gill was excised. The samples were fixed in 4% phosphate-buffered formalin prior to processing and analysis for mucosal barrier health by the standardized Veribarr™ method [
15,
17]. The same fish examined for mucosal barrier health were also examined for a set of external visual welfare indicators, according to an assessment index defined in [
21]. The parameters examined were deformities or ulcers on: Suction disc, Dorsal fin (comb), Caudal fin, Pectoral fin, fin, posterior dorsal fin, snout/mouth, Head, Gill lid, Eye (cataract, injuries/bleeding), Skin, left side, Skin, right side. When compiling and weighting, this gives a score from 1-4 for each indicator, depending on the extent and severity of each condition (1: good condition, 4: severe condition). The index has proven to be a good practical tool for assessing welfare.
Gonads were excised from 6 females and 6 males on 22 March and 23 April and weighed for gonadosomatic index (GSI) calculated as:
2.4. Analyses
Plasma was diluted 1:100 and analyzed in duplicate. Plasma cortisol (ng/ml) was analyzed with the DetectX
® Cortisol Enzyme Immunoassay Kit (Arbor Assays, USA). The standard curve with a range from 0 to 320 ng/ml was based on a standard with a known cortisol concentration. The detection limit has been tested at 0.045 ng/ml. Samples outside the lower limit of the standard curve were set at <0.05 ng/ml. Steroids (estrogen (E2) and testosterone (11-KT) were extracted from blood plasma according to method modified by [
22].
The formalin-fixed samples of skin and gills were purified in phosphate-buffered saline (PBS) and dehydrated in ethanol (50%, 70% and 80%), followed by paraffin-embedding, tangential sectioning [
15] and staining with Periodic Acid Schiff (PAS)-Alcian Blue [14-15]. High resolution digital scans of one section per tissue per fish were used to determine the mean size of mucous cells (MCA), the volumetric density of mucous cells in the epithelium (MCD), and from these measures derive the defence activity or barrier status (DA) of the tissue using the formula DA=(1/(MCA:MCD)) × 1000).
Analysis also revealed another dominant and large cell type in the skin, referred to as Q cells, which can have obvious internal structures [
23] or little or no content. These resemble club cells [
24] but differ in that they may have internal structures, they do not contain collagen or glycoproteins when staining with Massons trichrome and PAS-AB, and they can change location, size and volumetric density in the epithelium in response to treatment and habitat [
23]. These Q-cells were measured and registered using the same method as for mucous cells in the skin.
2.5. Statistical Analyses
Statistical tests were performed in STATISTICA
TM 14.0 (Tibco, 3307 Hillview Avenue, Palo Alto, California, USA) and Microsoft Excel. To assess normality of distributions a Kolmogorov-Smirnov test [
25] was used, and homogeneity of variances was tested using Levene’s F test [
26]. One-way ANOVA [
26] was used to investigate the effect of treatment. Significant ANOVA was followed by Student Newman-Keul’s post hoc test (SNK) to determine the differences between the groups. A significance level (α) of 0.05 was used if not stated otherwise.
3. Results
3.1. Stress Development
The development in stress response for the unstressed (U) and stressed group (S) is shown in
Figure 1, where S received elevated cortisol up to 15 February compared to U. There was a tendency towards increased cortisol levels in U later in the experiment. There was no significant difference between the groups on 22 March, but a tendency towards lower cortisol levels in S on 23 April.
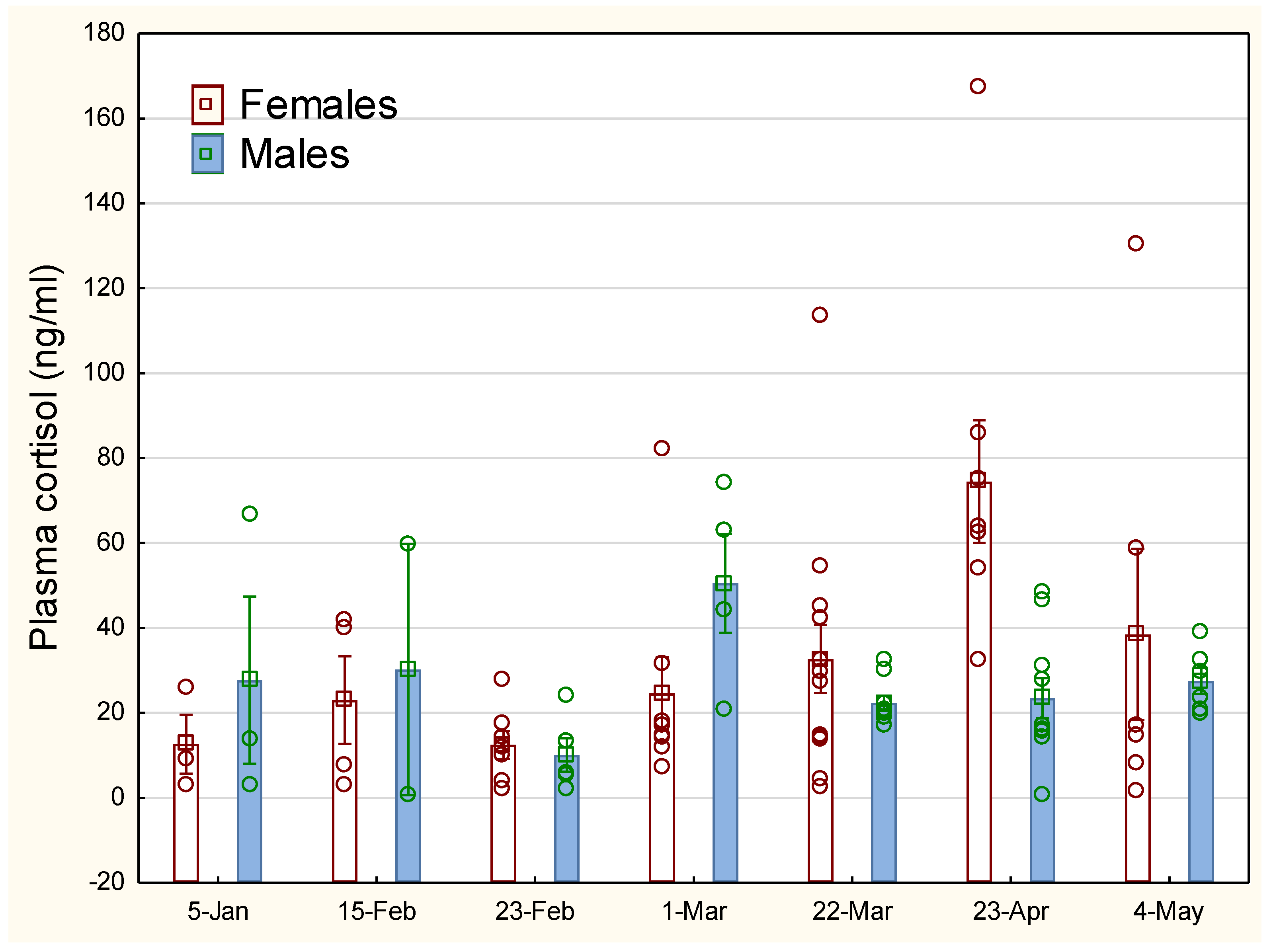
Gender-specific variation in cortisol for those measurement points where reliable information on gender is available shows little variation in stress levels between the sexes for pooled treatment groups (
Figure 2), except on 23 April when female fish had significantly (
P < 0.01) higher cortisol levels (74.5 ng/ml) compared to male fish (24.2 ng/ml).
3.1. Sexual Maturation
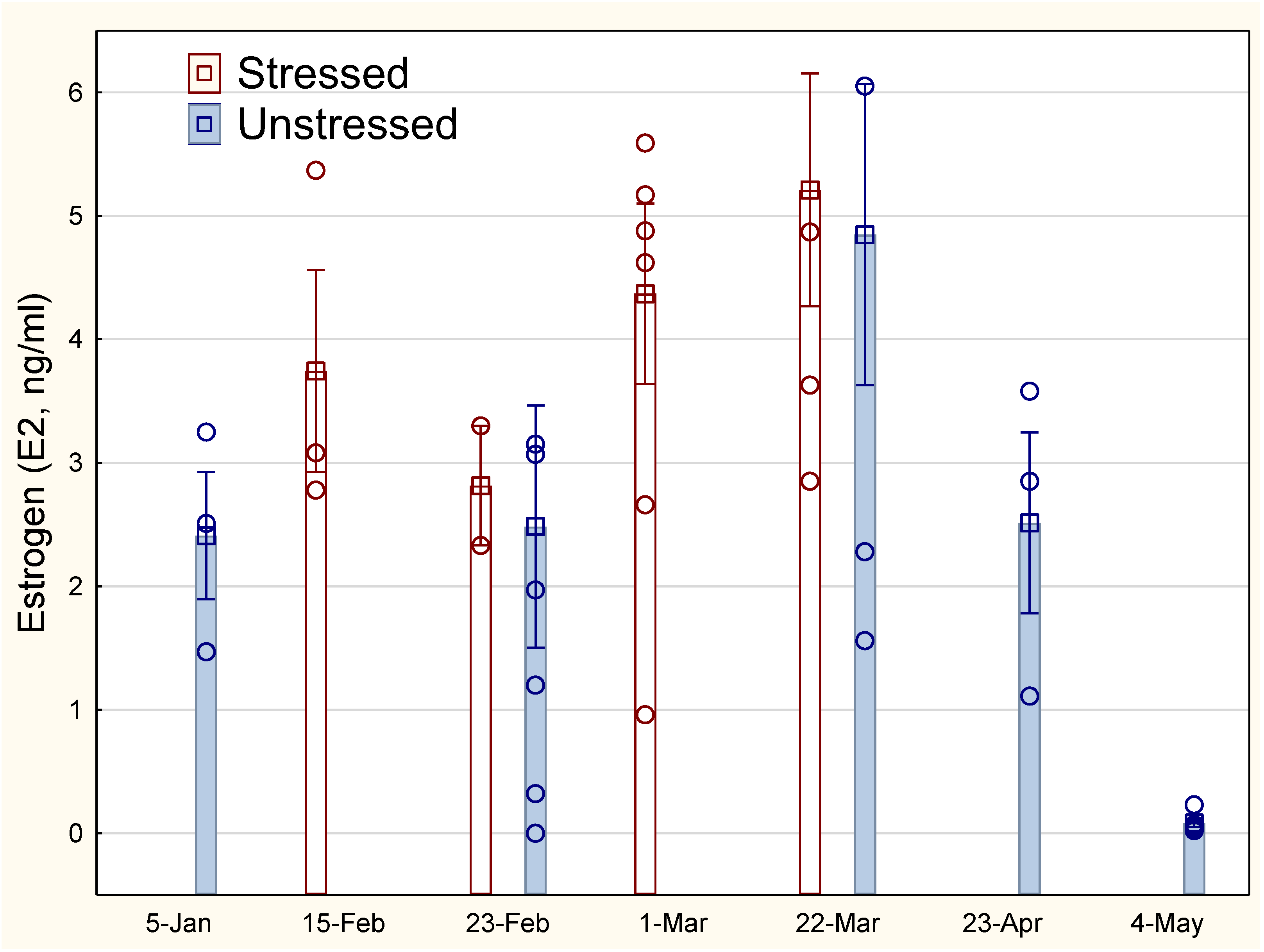
The sexual maturation development for female fish shown as the concentration of oestrogen (E2) in S and U is shown in
Figure 3, with an increase in oestrogen for all groups up to a peak around 22 March. There were no statistical differences in oestrogen between the groups and measurement points from February 23 to March 22. No weight differences were found between the females of the two experimental groups (one-way ANOVA,
P > 0.5).
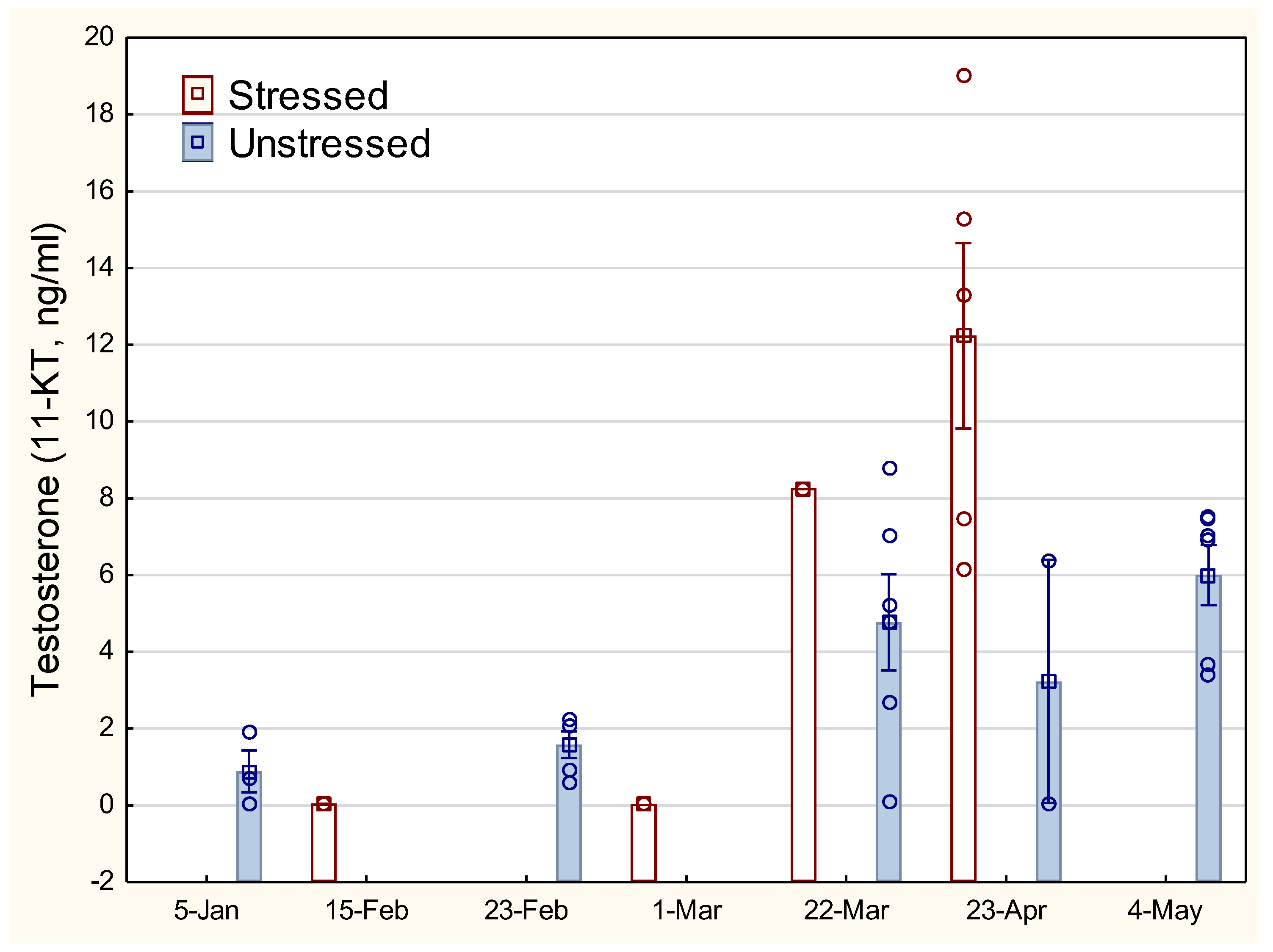
The development in 11-ketotestosterone (11-KT) for male fish increased from about March (
Figure 4). Large individual variation within each group and small number of male fish makes the analyses uncertain. Nevertheless, there is a clear tendency towards a higher and more limited peak in testosterone for stressed male fish in March-April, compared with unstressed males.
There was no correlation between the hormone status of male fish (11-KT, R2<0.01, P = 0.62, N=41) or female fish (E2, R2<0.001, P = 0.77, N=57) and stress levels (plasma cortisol).
There were no significant differences (one-way ANOVA, P > 0.55) in mean (±SEM) GSI for lumpfish females in the S-group (14.6 % ± 1.4) and the U-group (12.1 % ± 3.6) on either 22 March or 23 April (S-group = 16.9 % ± 2.2), U-group = 19.0 % ± 4.1).
3.3. Mucosal Barrier Responses
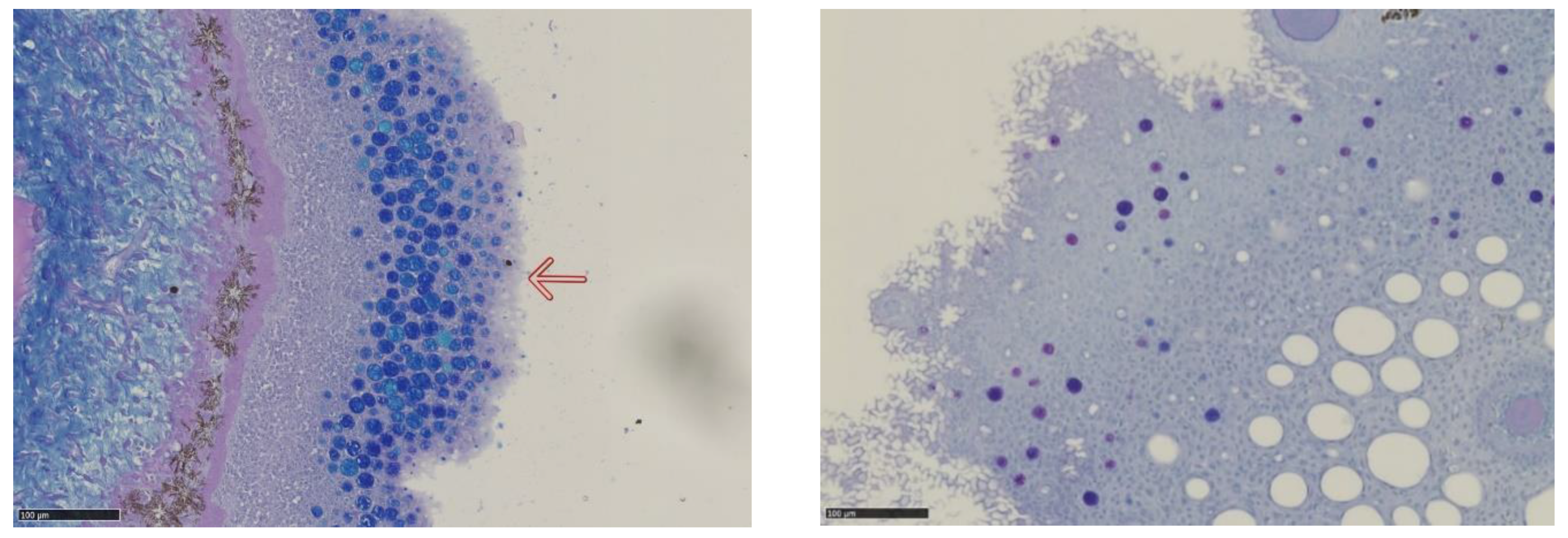
An example of tangentially sectioned lumpfish skin with a “fronded” cell structure on the surface, small mucus cells (small cells, coloured blue) distributed in the superficial layer of the skin surrounded by large “empty” Q cells (unstained large cells) is shown in
Figure 5. Q cells are found only in the skin, not on gill tissue.
3.3.1. Skin
Skin samples from males and females were combined, in order to achieve a sufficient number for analyses (N=12 for each group,
Table 2). There was an impact of stress on the size of the mucous cells, where stressed fish showed a significantly smaller MCA on 15 February compared with unstressed fish at the beginning of the experiment 5 January (133 μm
2 vs. 112 μm
2, one-way ANOVA,
P < 0.05). However, there were no significant differences in MCD or barrier strength at these early points and no differences between stressed and unstressed groups were found on the final date of March 22 (
Table 2), when all fish were in oocyte stage 7 (
Figure 3)
3.3.2. Gill Lamellae
The gill lamellae of stressed fish showed significantly smaller MCA (67 μm
2) on February 15 compared to the unstressed group (90 μm
2) on January 5 (one-way ANOVA,
P < 0.05,
Table 2), but no significant difference in MCD and barrier strength. There were no differences in MCA and MCD between the groups on March 22. There was a tendency towards somewhat reduced barrier strength over time.
3.3.3. Gill Filaments
On January 5, the unstressed fish gill filaments had significantly larger cells at greater density and barrier strength than did the stressed group 15 February (
Table 2). For the stressed group MCA showed a small reduction over time from 47 μm
2 to 41 μm
2 (
P<0.001), a small increase in MCD from 2.3% to 2.7% and an increase in barrier strength from 0.5 to 0.6. This contrasts with the significant reductions over time in the unstressed group filament cell size (MCA from 89 to 50 μm
2), in density (MCD from 10.6% to 3.5%) and in barrier strength (from 0.12 to 0.07).
3.4. Q Cells
When analysing the skin samples, the Q-cell appeared 2-10 times more abundant and 10-20 times larger than the mucous cells, and thus visually more dominant (
Table 2,
Figure 5). The size (QCA) of the Q-cells was significantly larger for stressed fish on 15 February (2136 μm
2) compared to unstressed fish on 5 January (1677 μm
2), and there was a significantly elevated QCA for stressed fish on 15 February (2136 μm
2) and 22 March (2666 μm
2) compared with unstressed fish (1677 μm
2) on January 5 (
P < 0.05).
The density (QCD) of the Q cells was initially higher in unstressed fish (46.2% vs 16.5% for stressed fish (P < 0.05), giving an increased number of small Q-cells in the skin mucus of unstressed lumpfish. There was a clear tendency towards reduced QCD over time for unstressed fish, which was significantly lower (23 %) on 22 March compared to initial values (46%; P < 0.01) and both groups had similar Q-cell densities on 22 March (23% and 22%).
A change in the prevalence of Q-cells and mucous cells was observed, as a result of stress induction. Before stress, the mucous cells were confined to a thin band in the epidermis and the Q cells gathered in a deeper surface band, while after stress and over time the mucous cells were also distributed in the deeper epidermis, closely associated with the large Q-cells that had been reduced in abundance.
Correlations between skin mucosal epithelium cell state, gill lamellae and gill filament with sex hormone status (cortisol, estrogen, and 11-keto-testosterone) are shown in
Table 3. There was a significant negative correlation between skin cell density (MCD) and cortisol, and between skin barrier status and cortisol. There was a significant (
P < 0.05) negative correlation between the density of Q cells in male lumpfish skin (QCD) and 11-KT. In addition, there was a nearly significant (
P = 0.06) positive correlation between the size of Q cells (QCA) and plasma cortisol. Thus, the differential action of these two cell types to (chronic) increased cortisol seems to be to reduce mucous cell size and density but to increase the size of the Q-cells, as well as to mobilize a repositioning in the skin tissue. There was evidence of sex-dependent action of the Q-cells.
4. Discussion
4.1. Stress and Mobilization of Energy Reserves
Comparisons of stress responses between different farmed species [
27] have shown a general primary stress response with increased cortisol but these have also shown that there is a species-specific variation in patterns and degree of responses to stress and stress tolerance. In comparative stress trials with Atlantic salmon, Ballan wrasse (
Labrus bergylta) and lumpfish, lumpfish showed the lowest response to crowding stress (about 200 ng/ml cortisol), where salmon and Ballan wrasse had a maximum of 800-1000 and 500-700 ng/ml respectively [
28]. Compared with species such as salmon and Ballan wrasse, with a clear flight response to threats and where cortisol contributes to the rapid mobilization of energy stores, lumpfish have a more sedate lifestyle and another evolutionary defence strategy against threats, which does not require the mobilization of energy reserves [
29].
Reproduction is encompassed by some of the most energy-intensive processes, and great flexibility in terms of energy allocation gives the fish the opportunity to set priorities in relation to maintenance metabolism, immune defences, somatic growth, and gonad development [
30]. Chronic stress can therefore influence the fish’s sexual maturation strategy through its influence on maintenance metabolism of the broodstock and nutritional status of the oocyte and embryo [
30,
31]. If stress in lumpfish causes little mobilisation of energy stores, it is conceivable that stress has less impact on the course of sexual maturation compared to species where stress causes high energy mobilisation. This is consistent with the overall development in sex hormones in this trial.
4.2. Influence of Stress Induction on Maturation
While there is ambiguity in the spawning window of lumpfish within the North Atlantic [
4,
32,
33] the population of lumpfish in this study began to ovulate in March- April. A pattern of gradual increase in cortisol towards a peak around the time of ovulation in March-April for lumpfish [
5]. Such a development shows that cortisol is part of the natural endocrine control of reproduction and can be produced in female and male gonads [
34]. This is consistent with the biological effects of cortisol on mechanisms prior to ovulation, including changes in metabolic parameters, such as increased hepatic amino acid catabolism and increased release of glucose [
34]. For lumpfish in this study the higher levels of plasma cortisol for female fish before spawning (ovulation) at the end of April [5, present study] suggest that this metabolic regulation is more demanding for female fish than for male fish.
The absence of correlations between the levels of plasma cortisol and the two sex hormones (E2 and 11-KT) in present study supports the observations that increased stress did not affect sexual maturation in lumpfish late in their sexual maturation cycle. This may possible be related to lower energy mobilisation of stressed lumpfish and thus a lower vulnerability in relation to ensuring energy allocation for egg growth and sperm production under increased stress. Another possible explanation is that the effects of cortisol on sexual maturation are primarily associated with early stages of the sexual maturation cycle, and not to the maturation stage itself [
7].
4.3. Gill Health
In general, the gills of lumpfish responded to chronic cortisol (stress over time) by reducing lamellar mucous cell size, density and barrier strength. There was also a tendency towards a negative correlation between barrier status and chronic cortisol in gill filament, supporting the role of filamental mucous cells as a site of secretion of somatic substances through perhaps rapid turnover of increasingly smaller cells [
16].
4.4. Skin Health
Mucus-producing cells, called mucous cells or goblet cells, in barrier tissues of skin, gills and intestinal tracts can vary in abundance and size depending on environment, tissue, gender, life stage and physiological state [
35]. The sampling site for lumpfish skin samples based on the QuantiDoc method has been standardised. In this experiment with lumpfish, the observed reduction in mucus cell size in the skin after stress inducement is therefore an expected functional response to stress in healthy fish – small cells can be efficiently generated and can “wash off” surface irritants through rapid turnover [
36]. These results of reduced skin protection after stress somewhat parallel those of wild shorthorn sculpins exposed to environmental heavy metals, where skin barrier status was
reduced with increasing levels of background lead and zinc while in the gill filaments the mucous cell significantly
increased with increasing toxin levels and was positively correlated with liver lead levels [
16].When a stimulus overwhelms the constructive response, the skin (or “shield”) may either reduce mucous cell production in favour of literal shielding or increase the mucous cell size and abundance to increase production of immune substances but simultaneously weakening the biotensegrity [
37] of the skin barrier.
4.5. Gill Health
In general, the gills of lumpfish responded to chronic cortisol (stress over time) by decrease lamellar mucous cell size, density, and barrier strength, acting unlike shorthorn sculpin gills and thus suggesting a lumpfish-specific response [
16]. There was also a tendency towards a negative correlation between barrier status and chronic cortisol in gill filament, supporting the role of filamental mucous cells as a site of secretion of somatic substances through perhaps rapid turnover of increasingly smaller cells [
16].
In comparison with the data on large broodstock of lumpfish in this experiment, previous studies of smaller lumpfish, between 36 and 103 g [
38] have shown smaller and fewer mucus cells in the skin (43 – 98 μm
2, 0.7 – 13.1 %). However, in the gill filaments of small lumpfish, the mucous cells were of the same order of magnitude or larger than in this experiment (93 – 111 μm
2) and somewhat higher density (11.8 – 13.5 %). The gill lamellae of small lumpfish also had mucus cells that were larger than in this experiment (89 – 105 μm
2), but similar or somewhat higher density (4.3 – 7.6 %). This indicates that the broodstock in this experiment had potentially better welfare compared to previously analysed groups of small lumpfish and that lumpfish also respond to habitat conditions [
23]. In the present experiment, no size-related differences in skin mucus cell status were found.
It is conceivable that mucus cell status reflects generally good fish welfare based on good farming conditions in this experiment, regardless of treatment, with little handling stress and solid ground to rest on. Experiments with lumpfish in cages showed that lumpfish that did not get a substrate to attach to (hide) had larger skin mucous cells and lower cell density compared to lumpfish that were given a sitting substrate [
23].
4.6. Q Cells
The Q cells observed only in the skin were 10–15 times larger and had 3-10 times higher density than the mucous cells. This apparent dominance over the smaller mucus cells with more limited prevalence indicates a prominent role for the Q cells in the regulation of homeostasis and in the innate immune response. The change in the physical location of these Q cells in response to stress indicates a regulated mechanism of cell propagation and an involvement in communication between cells at the tissue level. Ytteborg et al. [
24] found that in immature lumpfish, mucous and club (Q-) cells exposed to hydrogen peroxide changed their relative positions and Q-cells appeared more in the superficial layers of the skin. The Q-cells in the present study were smaller (1295 – 1682 μm
2) than previously observed on lumpfish between 36 and 103 g [
38], but with a similar density of cells (16-40 %). In the study of Jonassen et al. [
38] groups of smaller lumpfish were transferred from nursery to sea cages and there was no clear association to stress (plasma cortisol). For the Q-cells, there was a systematic change after transfer to cages [
38], and it was concluded that the development in Q cells in lumpfish may be a useful response variable in relation to the environment, but that it is very context-specific, such that e.g., the background of the fish (environment, genetics, nutrition, etc.) is likely to influence the environmental response.
These Q cells have also previously been referred to as vacuoles [
39] or club cells, and have been shown to vary in abundance, size, and distribution between individuals, where the typical feature was numerous vacuoles layered in the epidermis for small fry (< 5 g) and fewer, smaller, and more dispersed vacuoles in larger fish (> 50 g). Whether the observed variation in Q-cells was associated with stress and varying environmental conditions was not investigated in the study of Ytteborg et al. [
24]. Overall, these previous observations on juvenile lumpfish (> 50 g) are consistent with the picture from broodstock in the present study. The analyses of mucus cell status indicate that induced stress produced a positive functional response (stimulus) in lumpfish, through a gentle increase in the barrier strength of the skin, and reduced mucus cell size and increased density of mucus cells, resulting in a strengthening of the respiratory capacity of the gill vanes. The reduced density of “empty” cells (Q cells) after stress induction indicates that these cells may have an impact on the maintenance of homeostasis, but there is a need for further study of both the function and content of these cells.
5. Conclusions
The stress-induced cortisol implant group showed elevated plasma cortisol over a period of approximately one month, which coincided with early and middle vitellogenesis. This did not affect the sexual steroid development of the lumpfish. Mucous cell density and calculated barrier strength in the skin were significantly negatively correlated with plasma cortisol, while in the gill filaments of females there was a significant negative correlation between mucous cell density and estrogen levels. The density of skin Q-cells in males was significantly negatively correlated with 11-Ketotestosterone levels. Analyses of mucus cell density and distribution generally showed that the induced stress produced a positive functional response (stimulus) in lumpfish, through a gentle increase in the barrier strength of the skin, and reduced mucus cell size and reduced density of mucous cells. The reduced density of “empty” cells (Q cells) after stress induction indicates that these cells are important for the maintenance of homeostasis (physiological equilibrium).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1. The relationship between cell size, cell surface area, cell volume, number of cells and the resulting cell volume. The Table shows that that cell size and the volumetric density of a size of cell in the tissue are more important than the number of cells. Reporting only the number of cells can be misleading for biological functions, by ignoring the resultant surface area for transmembrane mucins and membrane receptors and the resultant volume of the cytosol for producing mucins and other proteins. Cell size and volumetric density in the tissue better describe the functioning of that tissue. Typical gill lamellae have mucous cells between 20-60 µ2, whereas typical skin mucous cells are from 150-300 µ2 and intestinal mucosa present intermediary cell sizes. Five cells of 300 µ2 have 15 times more surface area and 58 times more cytosol volume than 5 cells of 20 µ2.
Author Contributions
TMJ Writing – original draft, Writing – review & editing, Methodology, Investigation, Formal analysis, Funding acquisition, Conceptualization. AKDI: Writing – original draft, Writing – review & editing, Methodology, Data analysis. KP: Writing – review & editing, Methodology, Data analysis.
Funding
The project was funded by the Research council of Norway, project no. 269043 (STAMINA) and the Norwegian Seafood Research Fund, project no. 901798 (VEIEN).
Institutional Review Board Statement
The experiment described has been approved by the local responsible laboratory animal science specialist under the surveillance of the Norwegian Animal Research Authority (NARA) and registered by the Authority.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Peter Hovgaard at Fjord Forsk Sogn for contributing with fish, extensive hands-on experience on lumpfish and design for small scale incubators. We would also like to thank the industry partners Lerøy Aurora AS, Lumarine AS and Fjord Forsk Sogn AS.
Conflicts of Interest
There is no conflict of interest in relation to this study.
References
- Bornø, G.; Alarcón, M.; Linaker, M.L.; Colquhoun, D.; Nilsen, H.; Gu, J.; Gjerset, B.; Hansen, H.; Thoen, E.; Gulla, S.; Jensen, B.B.. Akutt dødelighet hos rognkjeks (Cyclopterus lumpus) i 2015. Veterinærinstituttets rapportserie – 2 – 2016, ISSN 1890-3290.
- Imsland, A.K.; Jonassen, T.M.; Hangstad, T.A.; Stefansson, S.O.; Elvegård, T.A.; Lemmens, S.C.A.; Urskog, T.C.; Nytrø, A.V.; Reynolds, P. The effect of continuous light and compressed photoperiods on growth and maturation in lumpfish Cyclopterus lumpus. Aquaculture 2018, 485, 166-172. [CrossRef]
- Imsland, A.K.D.; Hangstad, T.A.; Jonassen, T.M.; Stefansson, S.O.; Nilsen, T.O.; Hovgaard, P.; Elvegård, T.A.; Lindberg, K.S.; Mikalsen, B.; Urskog, T.C.; Norberg, B.; Andersson, E.; Spetland, F.; Reynolds, P. The use of photoperiods to provide year round spawning in lumpfish Cyclopterus lumpus. Comp. Biochem. Physiol. 2019, A228, 62-70. [CrossRef]
- Pountney, S.M.; Lein, I.; Migaud, H.; Davie, A. 2020. High temperature is detrimental to captive lumpfish (Cyclopterus lumpus L.) reproductive performance. Aquaculture 2020, 522, 735121 . [CrossRef]
- Andersson, E.; Denker, E.; Norberg, B.; Schulz, R.W.; Olausson, S.; Thorsen, A.; Stefansson, S.O.; Imsland, A.K.D. Lumpfish Cyclopterus lumpus reproduction: Pituitary gene expression, physiological and morphological changes accompanying gonadal maturation. Aquaculture 2023, 566, 739162. [CrossRef]
- Roff, D.A.; 1982. Reproductive strategies in flatfish: a first synthesis. Can. J. Fish. Aquat. Sci. 1982, 39, 1686–1698.
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591-625. [CrossRef]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 2001, 197, 3-24. [CrossRef]
- Xu, H.; Yang, M.; Qiu, W.; Pan, C.; Wu, M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ. Toxicol. Chem. 2013, 32, 1793–1799. [CrossRef]
- Patel, D.M.; Brinchmann, M.F. Skin mucus protein of lumpsucker (Cyclopterus lumpus). Comp. Biophys. Rep. 2017, 9, 217-225. [CrossRef]
- Minich, J.J.; Petrus, S.; Michael, J.D.; Michael, T.P.; Knight, R.; Allen, E.E. Temporal, environmental, and biological drivers of the mucosal microbiome in a wild marine fish, Scomber japonicus. mSphere 2020, 5,3. [CrossRef]
- Minich, J.J.; Poore, G.D.; Jantawongsri, K.; Johnston, C.; Bowie, K.; Bowman, J.; Knight, R.; Nowak, B.; Allen, E.E. Microbial ecology of Atlantic salmon (Salmo salar) hatcheries: Impacts of the built environment on fish mucosal microbiota. Appl. Environ. Microbiol. 2020, 86, 20. [CrossRef]
- Lazado, C.C.; Haddeland, S.; Timmerhaus, G.; Berg, R.S.; Merkin, G.; Pittman, K.; Pedersen, L.-F. Morphomolecular alterations in the skin mucosa of Atlantic salmon (Salmo salar) after exposure to peracetic acid-based disinfectant. Aquaculture Rep. 2020, 17, 100368. [CrossRef]
- Pittman, K.; Sourd, P.; Ravnøy, B.; Espeland, Ø.; Fiksdal, I.U.; Oen, T.; Pittman, A.; Redmond, K.; Sweetman, J. Novel method for quantifying salmonid mucous cells: Quantification of salmonid mucous cells. J. Fish Dis. 2011, 34, 931–936. [CrossRef]
- Pittman, K.; Pittman, A.; Karlson, S.; Cieplinska, T.; Sourd, P.; Redmond, K.; Ravnøy, B.; Sweetman, E. Body site matters: an evaluation and application of a novel histological methodology on the quantification of mucous cells in the skin of Atlantic salmon, Salmo salar L. J. Fish Dis. 2013, 36, 115-127. [CrossRef]
- Dang, M.; Pittman, K.; Bach, L.; Sonne, C.; Hansson, S.; Søndergaard, J.; Stride, M.; Nowak, B. Mucous cell responses to contaminants and parasites in shorthorn sculpins (Myoxocephalus scorpius) from a former lead-zinc mine in West Greenland. Sci. Total Env. 2019, 678, 207-2016. [CrossRef]
- Dang, M.; Pittman, K.; Sonne, C.; Hansson, S.; Bach, L.; Søndergaard, J.; Stride, M.; Nowak, B. Histological mucous cell quantification and mucosal mapping reveal different aspects of mucous cell responses in gills and skin of shorthorn sculpins (Myoxocephalus scorpius). Fish Shellfish Immunol. 2020, 100, 334–344. [CrossRef]
- Haddeland, S.; Lazado, C.C.; Merkin, G.V.; Myre, O.J.; Okubamichael, M.A.; Pedersen, L-F.; Pittman, K. Dynamic morphometrics of mucous cells reveal the minimal impact of therapeutic doses of peracetic acid on Atlantic salmon gill health. Aquaculture 2020, 534, 736315. [CrossRef]
- Pountney, S.M.; Lein, I.; Selly, S.L.C.; Migaud, H.; Davie, A. Comparative proximate analysis of wild and captive lumpfish (Cyclopterus lumpus) eggs show deficiencies in captive eggs and possible quality determinants. Aquaculture 2022, 557, 738356. [CrossRef]
- Specker, J.L.; Portesi, D.M.; Cornell, S.C.; Veillette, P.A. Methodology for implanting cortisol in Atlantic salmon and effects of chronically elevated cortisol on osmoregulatory physiology. Aquaculture 1994, 121, 181–193. [CrossRef]
- Rabadan, C.G.; Spreadbury, C.; Consuegra, S.; Garcia de Leaniz, C. Development, validation and testing of an Operational Welfare Score Index for farmed lumpfish Cyclopterus lumpus L. Aquaculture 2021, 531, 735777 . [CrossRef]
- Pankhurst, N.W.; Carragher, J.F. Oocyte maturation and changes in plasma steroid levels in snapper Pagrus (= Chrysophrys) auratus (Sparidae) following treatment with human chorionic gonadotropin. Aquaculture 1992, 101, 337– 347. [CrossRef]
- Svendsen, F.S.M. A pilot study of lumpfish (Cyclopterus lumpus) skin health, reared with three different treatments in land-based facilities and commercial net-pens. MSc thesis University of Bergen, 2021, Norway, 152 pp. https://bora.uib.no/bora-xmlui/handle/11250/2762635.
- Ytteborg, E.; Hansen, Ø.J.; Høst, V.; Afanasyev, S.; Vieweg, I.; Nahrgang, J.; Krasnov, A. Morphology, transcriptomics and in vitro model of skin from Polar cod (Boreogadus saida) and Atlantic cod (Gadus morhua). Fishes 2020, 5, 34. [CrossRef]
- Brown, M.B., Forsythe, A.B. Robust tests for the equality of variances. J. Am. Stat. Ass. 1974, 69, 364-367. [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc., Englewood Cliffs, N.J., 1984; 718 pp.
- Fanouraki, E.; Mylonas, C.C.; Papandroulakis, N.; Pavlidis, M. Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen. Comp. Endocrinol. 2021, 173, 313–322. [CrossRef]
- Espmark, Å.M.; Noble, C.; Kolarevic, J.; Berge, G.M.; Aas, G.H.; Tuene, S.; Iversen, M.H.; Wergeland, H.; Johansen, L.-H.; Burgerhout, E.; Gjerde, B.; Lein, I. Velferd hos rensefisk – operative velferdsindikatorer (OVI) – RENSVEL, Nofima rapport 12/2019, 114 pp. (In Norwegian with Abstract in English).
- Hvas, M.; Folkedal, O.; Imsland, A.K.; Oppedal, F. Metabolic rates, swimming capabilities, thermal niche and stress response of the lumpfish, Cyclopterus lumpus. Biology Open 2018, 7, bio036079. [CrossRef]
- Campell, J.H.; Dixon, B.; Whitehouse, L.M. The intersection of stress, sex and immunity in fishes. Immunogenetics 2021, 73, 111-129. [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549-556. [CrossRef]
- Davenport, J. Synopsis of biological data of the lumpsucker Cyclopterus lumpus (L 1758). FAO 1985, Fisheries synopsis No. 147. (31 pp.).
- Kennedy, J. Oocyte size distribution reveals ovary development strategy, number and relative size of egg batches in lumpfish (Cyclopterus lumpus). Polar Biol. 2018, 41, 1091–1103. [CrossRef]
- Milla, S.; Terrien, X.; Sturm, A.; Ibrahim, F.; Giton, F.; Fiet, J.; Prunet, P.; Le Gac, F. Plasma 11-deoxycorticosterone (DOC) and mineralocorticoid receptor testicular expression during rainbow trout Oncorhynchus mykiss spermiation: implication with 17alpha, 20beta-dihydroxyprogesterone on the milt fluidity? Reprod. Biol. Endocrinol. 2008, 6, 19. [CrossRef]
- Esteban, M.A. An overview of the immunological defences in fish skin. ISRN Immunol. 2012, 2012, 1–29. [CrossRef]
- Torrecillas, S.; Montero, D.; Caballero, M.J.; Pittman, K.; Campo, A.; Custodio, M.; Sweetman, J.; Izquierdo, M.S. Dietary mannan oligosaccharides: Counteracting the side effects of soybean oil inclusion on European sea bass (Dicentrarchus labrax) gut health? Front. Immunol. 2015, 6, 1 . [CrossRef]
- Ingber, D.E. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008, 97, 163-179. [CrossRef]
- Jonassen, T.M.; Foss, A.; Remen, M.; Watts, E.J.; Hangstad, T.A. Toleranse for transportstress og miljøoverganger hos berggylt og rognkjeks, Akvaplan-niva rapport nr. 9081, 2019, 62 pp (In Norwegian with Abstract in English).
- Klingenberg, O. Normalhistologi hos oppdrettet rognkjeks (Cyclopterus lumpus L.). Med fokus på organene hud, gjeller, hjerte, nyre, milt, pankreas og lever. MSc thesis, 2019, University of Tromsø, Norway, 113 pp. https://hdl.handle.net/10037/16133.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).