1. Introduction
Hematopoietic cell transplant (HCT) is increasingly utilized as a curative treatment for malignant and non-malignant diseases. However, the rates of morbidity and mortality following HCT are high, not just due to disease relapse, but also secondary to complications arising from the treatment itself. Recently, fluid overload (FO) has been identified as a significant risk factor that increases mortality in critically ill children. Children post HCT are particularly at higher risk of FO. During the transplant course, patients often receive a significant volume of infusions from total parental nutrition (TPN), antimicrobial agents, immunosuppressive drugs, and blood product transfusions. In addition, complications following HCT contribute to fluid retention and overload. Cytokine release syndrome as encountered in sepsis or engraftment syndrome can lead to capillary leak and edema. In addition, other complications such as sinusoidal obstruction syndrome (SOS), and acute kidney injury (AKI) increase the risk of FO. In addition to its contribution to a higher risk of mortality, FO can worsen AKI and acute respiratory failure (ARF). This review highlights the effect of FO on outcomes in critically ill children following HCT. In addition, our review explores the impact of FO on common pathologic conditions such as ARF, AKI, and sepsis in this population.
2. FO Pathophysiology
Fluid management in critical care medicine is fundamental to patient care. Not unlike the use of vaccines in the 1900s, the appropriate use of fluids is pivotal to changing current outcomes in ICU populations. The dogma behind fluid management has evolved, with a focus on using fluid as a medication providing therapeutic benefit but realizing its potential adverse side effects. Conservative fluid use, closer monitoring of fluid status, and detailed attention to the physiologic effects of resuscitation have become the gold standard. The interplay of fluid overload within the body is multifaceted. When the volume of fluid in is greater than the volume of fluid out, the accumulation leads to fluid deposition in extracellular compartments. Eventually, the positive fluid status leads to fluid congestion and increased cardiac filling pressure, leading to clinical signs of fluid overload and end organ dysfunction [
1,
2]. Thus, while initial resuscitation may be needed, fluid may quickly go from being a supportive agent to negatively affecting the clinical status. Understanding where a patient’s fluid status sits on the spectrum can often be unclear and continuously changing. Fluid can be beneficial in restoring perfusion and end organ function, but it can quickly play a role in capillary leak, disruption of cell-to-cell interactions, metabolic diffusion, and ultimately, end organ dysfunction [
1]. Using appropriate assessment techniques allows for rapid assessment and intervention. Fluid balance should be evaluated frequently and closely to understand each individual patient’s fluid evolution throughout treatment. A well-rounded assessment includes a clinical examination and history, diagnostic testing to observe end-organ function, and evaluation of biomarkers.
3. FO Definition
FO/cumulative fluid accumulation is calculated mainly by 2 methods: Fluid intake/fluid output or weight-based method.
The formula using fluid balance is: Fluid input − Fluid output (L)/ weight (kg)×100
The formula using weight is: Current weight − admission weight (kg)/ weight (kg) ×100
The most commonly used weight in the denominator is the ICU admission weight, but other weights, such as the hospital admission weight or baseline weight have been used in some studies.
4. FO Assessment
The history and physical examination should be used to understand the patient’s status. Chest radiography is easily accessible and offers an initial insight into a patient’s fluid status. X-rays can show signs of volume overload such as cardiomegaly, interstitial edema, dilated pulmonary artery, pleural effusions, alveolar edema, or Kerly lines [
1]. Although radiography is a good tool for initial evaluation and insight, studies have shown that chest radiographs lack both sensitivity and specificity. Technique, status of the patient, positioning, and the use of portable x-rays can decrease the sensitivity even further [
1].
4.1. Ultrasound
Ultrasound offers a non-invasive and consequential look into fluid overload. Basic evaluation for FO on a cardiac ultrasound may include left ventricular ejection fraction based on wall mobility and size of the ventricle, presence of a pericardial effusion, or clear valvular dysfunction [
2]. More-advanced investigation of FO using cardiac ultrasound can include right ventricular enlargement with intraventricular septum flattening in only diastole as well as tricuspid regurgitation secondary to right ventricular enlargement. Furthermore, fluid responsiveness can be tested via monitoring of stroke volume and LV outflow tract velocities by using doppler [
2]. In addition, inferior vena cava (IVC) size and collapsibility can add to clinical assessment of fluid status and give a basic estimate of the right atrial pressure. An IVC size of < 1.5 cm signifies volume depletion status but a size of >2.5 cm suggests volume overload. In patients with appropriate fluid status, IVC size will be between 1.5 and 2.5 cm, and diameter will vary with inspiration and expiration [
1]. In addition, ultrasound can easily identify edema within the lung tissue by demonstrating A- and B-lines when interstitial thickening is present from, most notably, fluid. Increased B-lines correlate with increased pulmonary edema [
2]. Clinical assessment must be used in conjunction with the lung ultrasound findings because interstitial thickening can be due to factors other than fluid. Ultrasound can show other signs of increased fluid with bowel wall edema, ascites, and jugular vein pressure [
2,
3].
4.2.Bioimpedance Analysis
Although not commonly used in PICUs, bioimpedance analysis examines alternating currents to measure body composition, including soft-tissue hydration [
1,
2]. This tool uses body impedance and resistance to estimate fluid status. Further studies are required to understand how applicable and useful this technique is in the critical care setting, but it has shown correlation with central venous pressure and may offer insight into fluid status on admission [
1].
4.3.Biomarkers
Biomarkers used in conjunction with other tools may help provide insight into fluid status and the status of underlying illness. B-type natriuretic peptide (BNP) has shown excellent negative predictive value in diagnosis of heart failure and shows down-trending in the setting of diuretic therapy for heart failure and cardiomyopathy but still requires further studies to drive therapy [
2]. Other biomarkers, such as lactate, hemoglobin, and hematocrit, are less specific to fluid overload but may provide insight into the adequacy of end organ perfusion and fluid effects such as hemodilution [
2].
Individually, all these tests give insight into fluid status but likely a combination of many of these tests and the creation of predictive tests will lead to optimal clinical decision making. An example of this is the recently reported Endothelial Activation and Stress Index (EASIX) [
4]. In states of hypervolemia, adult patients following HCT have increased amounts of endothelial glycocalyx layer, a part of the capillary permeability barrier as well as increased atrial natriuretic peptide. EASIX, a marker of endothelial dysfunction, is defined as lactate dehydrogenase (U/L) × creatinine (mg/dL)/platelets (10
9cells/L]. In adults post HCT, an EASIX score of > 4.4 at admission in the study cohort (145 patients, haploidentical) was a significant predictor of grade ≥2 FO (hazard ratio (HR) = 4.8,
P < .001) [
4]. Likewise, a high EASIX score on admission was associated with FO grade ≥2 in the validation cohort (449 patients, HLA matched), with a cutoff value of 4.3 (HR = 4.8,
P < .001). Future studies are needed to validate the association of this index with FO in the post-HCT pediatric population.
Table 1.
Diagnostic evaluation of FO.
Table 1.
Diagnostic evaluation of FO.
|
Diagnostic
|
Findings of FO
|
Other findings
|
Pros/Cons
|
| CXR |
Cardiomegaly, interstitial edema, dilated pulmonary artery, pleural effusion, alveolar edema, Kerly lines |
|
Easily obtained/ Good initial screening
Lacks sensitivity and specificity |
| Ultrasound |
|
|
|
| Cardiac |
Presence of pericardial effusion, poor LV ejection fraction, valvular dysfunction, right ventricular enlargement, intraventricular septum flattening in diastole, tricuspid regurgitation, LV outflow tract velocities with doppler |
|
Easily obtained/Good initial screening
Similar findings can be present for different etiologies |
| |
IVC collapsibility |
Diameter
<1.5 cm = volume depleted status
>2.5 cm = volume overload w/ no variability with breathing |
Lacks sensitivity and specificity |
| Abdominal |
Evaluate hepatic vein, portal vein, intrarenal artery for end organ perfusion due to increased right atrial pressure, bowel wall edema, ascites |
|
Easily obtained, lacks sensitivity and specificity |
| |
Venous congestion with doppler |
Diameter >2 cm indicates congestion in IVC
Edema is notable if B-lines present |
Requires more evidence |
| Bioimpedance Analysis |
Alternating currents to measure body composition and soft-tissue hydration |
|
Not easily accessible
Requires more evidence |
| Biomarker |
|
|
|
| BNP |
Secreted by cardiomyocytes under stretch condition |
Down trending during diuretic therapy in patients with heart failure |
Excellent negative predictive value in diagnosis of heart failure |
| Urinary NGAL |
Increased early in AKI
|
|
Reduced rate of FO ≥ 15% prior to CKRT and a shorter length of stay in PICU when combined with RAI |
| |
|
|
|
5. FO in the Transplant World
FO is highly prevalent in children during their post-HCT course. In a cohort of 484 children post HCT, FO>10% occurred in 46% of the total cohort and in 67% of those with severe AKI [
5]. In addition, FO is a strong predictor for PICU admission in these children [
6]. Benoit et al. followed up 87 children who underwent HCT and compared their maximal weight gain (WG) and cumulative fluid balance from the day of admission for transplant to those on the day of ICU admission (PICU group, 19 patients) or hospital discharge (non-PICU group, 68). WG > 10% was strongly associated with PICU admission and was encountered in 68.4% in the PCU group and 22.1% in the non-PICU group [
6]. Recently, Rondon et al. proposed the following grading system for fluid toxicity in patients post HCT: grade 1, WG <10% from baseline; might require decreasing IV fluids or occasional diuretics; grade 2, symptomatic and/or WG 10 to <20% from baseline; might require continuing diuretics; grade 3, WG ≥20% from baseline or not responding to diuretics; and grade 4, major organ dysfunction (renal, pulmonary, or cardiac dysfunction) or requiring intensive care [
7]. In their cohort of 145 adults post haploidentical HCT, FO grade ≥2 was an independent predictor of D +100 non-relapse related mortality (HR=13, p<0.001) [
7].
Table 2 summarizes the results of published studies of FO in children following HCT.
Table 2.
Summary of FO studies in children following hematopoietic stem cell transplant.
Table 2.
Summary of FO studies in children following hematopoietic stem cell transplant.
| Author, year |
Population(n) |
Fluid balance |
Outcome |
Main findings |
Michael et al.
2004
|
Allogeneic (26)
Acute renal failure
Admitted to PICU (23) |
Cumulative fluid input and output |
Survival
Euvolemia (FO<10%) |
Survival rate was 42%
Survival rate in children who received KRT was 29%
All survivors were euvolemic |
Benoit et al.
2007
|
Allogeneic and Auto (87)
HCT to PICU admission (19)
HCT to hospital discharge (68) |
Weight-based
Cumulative fluid input and output |
PICU admission |
Weight gain >10% on admission to HCT is a risk factor for PICU admission |
Flores et al.
2008
|
Stem cell (51)
CKRT (51) |
Cumulative fluid input and output |
PICU survival |
FO was the most common reason for CRRT initiation
Survival to PICU discharge was 45%
|
Lombel et al.
2012
|
Stem cell (21)
CKRT (21) |
Weight-based
Cumulative fluid input and output |
PICU mortality
PELOD scores |
Survival to PICU discharge was 61%
FO% was not associated with PICU mortality
FO was predictive of higher subsequent PELOD scores |
Raymakers-Janssen et al.
2019
|
Cancer and HCT (68)
HCT (23)
CKRT (68) |
Weight-based |
PICU mortality |
Survival to PICU discharge was 45.6%
Survival of HCT patients to PICU discharge was 39.1%
FO and inotropic support at the start of CRRT were associated with PICU mortality |
Elbahlawan et al.
2020
|
Allogeneic and Auto (30)
ARF/IMV (30) |
Cumulative fluid input and output |
PICU survival |
Survival to PICU discharge was 80%
Lower cumulative FO% in survivors on D4 and D5 after start of IMV |
Mueller et al.
2020
|
Allogeneic and Auto (92)
|
Cumulative fluid input and output |
PICU mortality |
Fluid balance 24 h prior and 24 h post PICU admission was not associated with survival, ventilator- free days, or PICU-free days |
Sallee et al.
2021
|
Allogeneic (198)
ARF/IMV (198) |
Cumulative fluid input and output |
Effect of cumulative fluid balance on outcome |
Positive D3 CFB was associated with higher PICU mortality and a lower rate of extubation |
Bauer et al.
2023
|
Allogeneic and Autologous (484)
AKI (168) |
|
AKI |
FO>10% was present in 67% of patients with severe AKI
Risk of death at 1 year was higher in severe AKI than in those with no AKI (HR, 4.6) |
6. FO in the PICU World
Similarly, FO is encountered often in critically ill children and is associated with poor outcomes. A large metanalysis (44 studies,7507 children) reported a pooled median FO incidence of 33% in critically ill children (range, 10-83%) [
3]. A recent metanalysis of 44,682 critically ill children from 120 studies confirmed that fluid accumulation (FA) during PICU admission was strongly associated with mortality [
8]. Each 1% increase in the percentage of FA was associated with 6% increased odds of mortality. Importantly, FA within 24 h of PICU admission increased the risk of mortality significantly, with OR of 7.93 (95% CI, 2.81–22.39; p < 0.001) for FA>5% and OR of 8.77 (95% CI, 2.42–31.77; p < 0.001) for FA>10%. This is particularly important considering that almost half of children post HCT experience FO>10% [
5]. Therefore, early detection and management of FO in these children is crucial on the transplant unit and in the early stage of PICU admission.
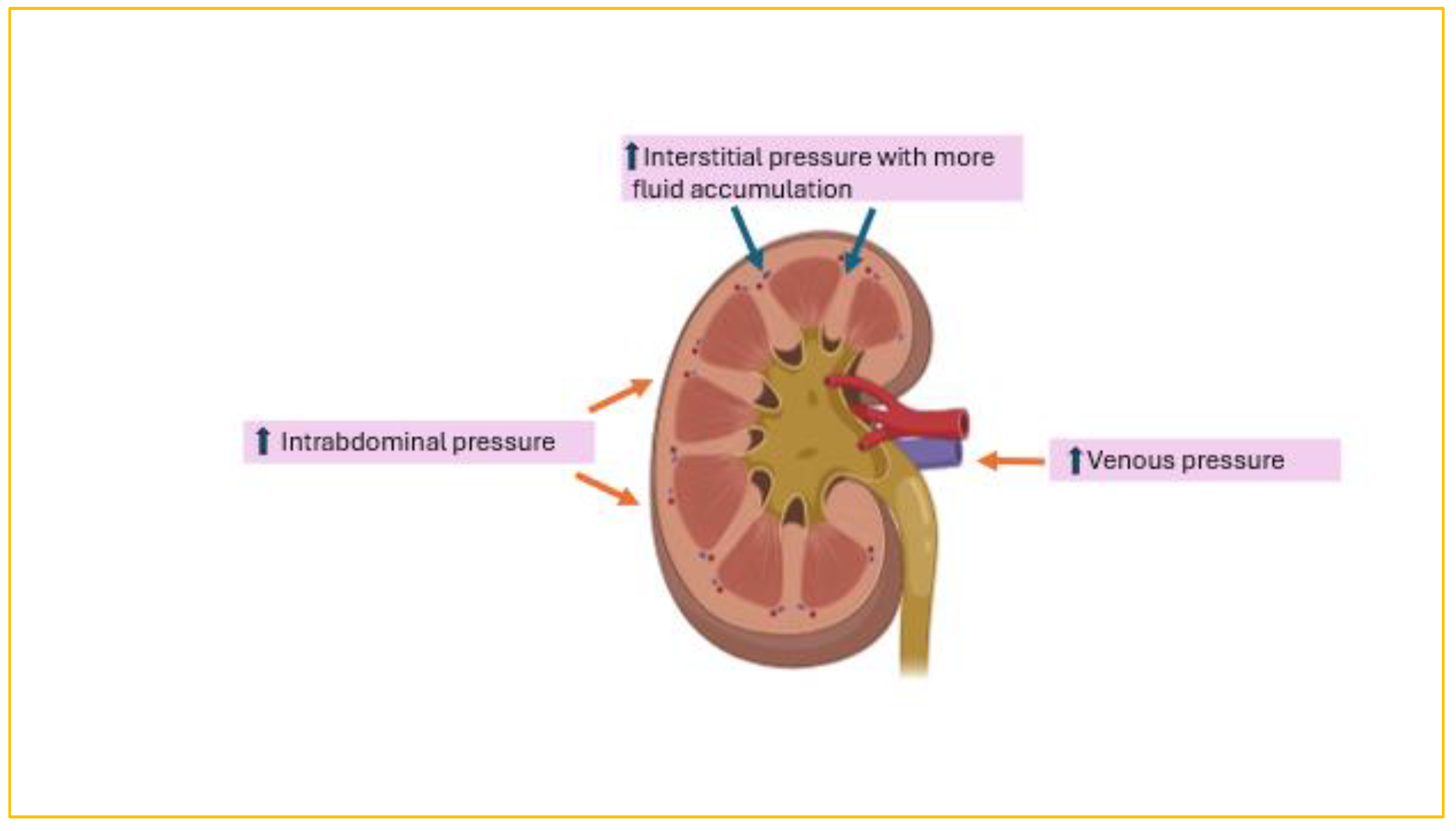
7. FO and AKI
AKI is common and encountered in half of children following HCT, with severe AKI occurring in 12% [
9,
10]. Most importantly, AKI reduces the 3-year overall survival rate from 79.6% in children with no AKI to 46.7% in children with stage 2 AKI and 25% in those with stage 3 AKI (
p = .025 and .002, respectively) [
11]. In a pediatric cohort of 484 post-HCT patients, AKI developed in 38%, 42% of whom experienced severe AKI [
5]. Moreover, FO>10% was present in 67% of children with severe AKI post HCT. Risk of death was significantly higher in patients with severe AKI than in those without AKI (HR, 4.6). The relationship between FO and AKI is complex (
Figure 1). FO can exacerbate kidney injury by worsening kidney venous hypertension from increased central venous pressure, impairing perfusion pressure capacity of the glomerular capillaries, or from raised intra-abdominal pressure. In addition, the kidney is an encapsulated organ; thus, FO will raise interstitial pressure, leading to compromised blood flow. Conversely, AKI itself can exacerbate FO secondary to the renal impairment itself. As a result, a vicious cycle is perpetuated whereby each condition exacerbates the other. The largest metanalysis to date (44,682 critically ill children) confirmed an increased risk of AKI (OR = 1.98 [95% CI,1.60–2.44]; p < 0.001) with fluid accumulation. Balancing the management of FO without exacerbating AKI, especially in the context of diminished intravascular volume from capillary leak or acute tubular necrosis due to renal hypoperfusion, is quite challenging. Fluid restriction and diuretics are usually the first lines of management. Subsequently, continuous kidney replacement therapy (CKRT) is initiated if these measures fail to improve FO. However, a more-systemic proactive approach to FO detection and management at the time of AKI, such as using the renal angina index (RAI) and urinary biomarkers such as neutrophil gelatinase-associated lipocalin (uNGAL), could reduce mortality. The RAI combines risk stratification and clinical signs of injury [
12]. Risk stratification elements include ICU admission, stem cell transplantation, and mechanical ventilation or inotropic support. Clinical signs of injury are based on changes in estimated creatinine clearance or % fluid overload (%FO). An RAI score of ≥ 8 indicates a higher risk of occurrence of AKI by day 3 of PICU admission [
13]. Goldstein et al tested the use of automated clinical decision in 286 critically ill children using the RAI and uNGAL levels [
14]. This approach resulted in a reduced rate of FA ≥ 15% prior to CKRT and an 11-day shorter length of stay in the ICU as well as higher survival rates to ICU discharge.
8. FO and ARF
ARF is a serious complication seen in 25% of children post HCT, with a high mortality rate of 42-64%% [
15,
16]. FO has been implicated in previous studies to associate with higher mortality rate, fewer ventilator-free days, and oxygen deficits in critically ill children. The pooled data from 12 pediatric studies with 1819 patients confirmed the association of FO with a prolonged duration of mechanical ventilation (weighted mean difference = 38.1 h [95% CI, 19.35–56.84]; p < 0.001) [
8]. FO also affects outcome in children with ARF post HCT. In a cohort of 198 post-HCT intubated children with ARF, the cumulative fluid balance on d 3 of their invasive mechanical ventilation [IMV) course was significantly associated with mortality [
17]. Each 1% increase of cumulative fluid balance (CFB) on d 3 increased the odds of PICU mortality by 3% (OR 1.03, 95% CI 1.00-1.07). In addition, higher CFB on d 3 was associated with a lower rate of extubation at d 28 and d 60 [
17]. In another cohort of 30 intubated post-HCT children with acute respiratory failure secondary to engraftment syndrome, lower median cumulative FO% on d 4 and d 5 after the start of IMV was observed in survivors (2.8 vs. 14.0 mL/kg,
p = 0.038 on day 4, and 1.8 vs. 14.9 mL/kg,
p = 0.044 on day 5, respectively) [
18]. Similar findings are reported in critically ill children with pediatric acute respiratory distress syndrome. In a large cohort of 723 children with ARDS, a positive CFB on days 5 through 7 was associated with a higher mortality rate[
19]. In addition, a higher CFB on days 4 to 7 was associated with a lower probability of successful extubation.
9. FO and CKRT
CKRT is used to manage FO when other measures such as fluid restriction and diuretics have failed. FO is the main indication for initiation of CKRT in children post HCT [
20]. In a large metanalysis that examined the effect of FA in critically ill children, FA at the time of initiation of kidney replacement therapy (KRT) was associated with a higher mortality rate. In children with FA%>10% and FA>20%, the pooled OR for mortality was 2.96 (95% CI, 1.85–4.73; p < 0.001; I 2 = 80%; n = 2488) and 2.91 (95% CI, 1.82–4.63; p < 0.001; I 2 = 68%; n = 1991), respectively [
8]. The same deleterious effect of FO at the time of initiation of CKRT was observed in a pediatric oncology cohort (total number of patients=68, post-HCT=23) from 8 PICUs in the Netherlands [
21]. Children with FO > 10% at CKRT initiation were 6.16 times more likely to die than were those with FO ≤ 10%. Thus, the timing of initiation of CKRT is critical and should be considered before FA exceeds 10%. Unfortunately, the mortality of children post HCT receiving CKRT continues to be high (52–65%). Risk factors other than FO that are associated with the higher mortality rate include vasoactive support and mechanical ventilation [
21,
22]. The management of FO with CKRT typically involves a slow and consistent rate of net fluid removal over an extended period. This approach makes CKRT more tolerable from a hemodynamic standpoint. Aggressive and fast fluid removal during CKRT may affect not only blood pressure but also survival. Studies of adults suggest that higher net ultrafiltration rates (>1.75 ml/kg/h) increase the risk of mortality and reduce the probability of kidney recovery in patients receiving CKRT [
23,
24].
10. FO and Sepsis
Sepsis is associated with a higher risk of mortality in children post HCT, particularly increasing the risk of hospital mortality to four-fold that of septic children with no history of HCT [25). In the early phase of septic shock, fluid resuscitation is key to restoring adequate tissue perfusion. The Surviving Sepsis Campaign guidelines published in 2021 recommend that 30 mL/kg of IV crystalloid fluid be given within the first 3 h of resuscitation [
26]. Additional fluid needs should be guided by the patient’s hemodynamic status and responsiveness to fluid boluses. The Surviving Sepsis Campaign guidelines did not have recommendations for either restrictive or liberal fluid management in the first 24 h of resuscitation after the initial fluid bolus. Furthermore, in the FEAST Trial, which included 3141 children with sepsis in Africa, fluid boluses significantly increased the 48-h mortality rate in critically ill children with impaired perfusion [
27]. Therefore, it is prudent to continuously evaluate the patient’s status and fluid responsiveness as fluid administration may become more harmful beyond the initial state of resuscitation.
11. Other Transplant-Related Complications and FO
Hepatic sinusoidal obstruction syndrome (SOS), also known as veno-occlusive disease of the liver, is a condition that is well known to be associated with FO. The pathophysiology stems from prep regimen–related injury to the sinusoidal endothelium of the liver, ultimately leading to embolization of the centrilobular vein and subsequent post-sinusoidal obstruction [
28,
29]. This, in turn, leads to the classic presentation of ascites, weight gain, abdominal pain, and jaundice. Furthermore, FO is exacerbated by accompanying acute renal injury and frequent platelet transfusions triggered by SOS-related consumptive thrombocytopenia. Fluid accumulation in SOS is extravascular; thus, diuresis can only partially correct fluid status. Defibrotide, approved by the FDA in 2016 for the treatment of SOS, promotes endothelial cell proliferation and partial revascularization of the sinusoid, leading to lower mortality rates than achieved by supportive treatment only [
28,
29]. Despite treatment with defibrotide, most patients still require additional supportive measures, such as fluid restriction and diuretic therapy, and some will have progressive acute renal injury, with poor urine output requiring renal replacement therapy.
The constellation of fever, rash, and weight gain around the time of neutrophil recovery in the early post-HCT period characterizes engraftment syndrome. This could be accompanied by any combination of liver dysfunction, renal dysfunction, or encephalopathy [
30]. The release of cytokines, including IL-1, TNF-alpha, and IFN-gamma, during engraftment leads to capillary leak and third space fluid loss that is responsible for the weight-related, pulmonary, and renal components of engraftment syndrome. Similar to SOS, the intravascular volume status is not repleted, making it difficult to correct the fluid balance state with diuretics alone. Although engraftment syndrome can be self-limited in some patients, requiring no therapy, patients with severe disease require intervention. This includes patients with high fevers and oxygen requirements due to pulmonary edema. These patients may be started on intravenous methyl prednisone at 1 mg/kg/day and weaned over a few days according to response. Diuresis can also be considered to aid fluid and weight management.
12. Management
Prevention of FO is the cornerstone of management. In children following HCT, FO prevention should start on the ward before PICU admission. Initially on PICU admission, fluid may be needed to restore end organ perfusion, but it is crucial to reduce fluid administration once it starts to become detrimental. Even maintenance fluids can contribute to gradual fluid accumulation due to “fluid creep”. Fluid creep is the sum of the volumes of electrolytes, the small volumes to keep venous lines open (saline or glucose 5%), and the total volume used as a vehicle for medication and accounts for nearly one-third of the mean daily fluid volume [
31,
32]. Therefore, to avoid reducing nutritional intake, it is important to minimize the volume of fluid creep. The ReLiSCh Trial compared restrictive fluid therapy (40% of maintenance fluids) with usual/liberal fluid therapy (70-80% of maintenance fluids) in mechanically ventilated children. The restricted group had significantly more ventilator-free days (23 vs. 17 d, p = 0.008) and PICU-free days (19 vs. 15 d, p = 0.007). These findings are comparable to those in the earlier FACTT-trial, which included 1000 adult patients with acute lung injury. A restrictive fluid strategy was associated with a shorter duration of mechanical ventilation and ICU-length of stay but not with decreased mortality [
33]. Although the debate about whether to use restrictive or non-restrictive fluid administration continues, a proactive approach to prevent FO is crucial. This includes accurately monitoring daily fluid intake and output, restricting volume (particularly fluid creep), and administering intermittent or continuous infusion of diuretics as needed. CKRT should be considered if FO approaches 10% despite other treatments.
13. Conclusions
In conclusion, reducing complications after HCT enhances the success of this therapy and improves survival. Prompt detection and treatment of fluid accumulation should be fundamental in caring for these children upon hospitalization or PICU admission. FO not only raises the risk of mortality but also exacerbates the severity and likelihood of other complications, such as AKI and ARF. Hospitals should implement a systematic approach for early recognition and management, including electronic health record alerts, to ensure early detection.
Author Contributions
“Conceptualization, LE; resources, LE, AQ, RM, AS; writing-original draft preparation, LE, AQ, RM, AS; writing-review and editing, LE, AQ, RM, AS. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was supported by ALSAC.
Acknowledgments
Special thanks to Cherise Guess, PhD, ELS, for editing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016, 17, 109. [Google Scholar]
- Koratala A, Ronco C, Kazory A. Diagnosis of Fluid Overload: From Conventional to Contemporary Concepts. Cardiorenal Med. 2022, 12, 141–54. [Google Scholar] [CrossRef] [PubMed]
- Alobaidi R, Morgan C, Basu RK, Stenson E, Featherstone R, Majumdar SR, et al. Association Between Fluid Balance and Outcomes in Critically Ill Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 257–68. [Google Scholar] [CrossRef] [PubMed]
- Varma A, Rondon G, Srour SA, Chen J, Ledesma C, Champlin RE, et al. Endothelial Activation and Stress Index (EASIX) at Admission Predicts Fluid Overload in Recipients of Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2020, 26, 1013–20. [Google Scholar] [CrossRef]
- Bauer A, Carlin K, Schwartz SM, Srikanthan M, Thakar M, Burroughs LM, et al. Risk factors for severe acute kidney injury after pediatric hematopoietic cell transplantation. Pediatr Nephrol. 2023, 38, 1365–72. [Google Scholar] [CrossRef]
- Benoit G, Phan V, Duval M, Champagne M, Litalien C, Merouani A. Fluid balance of pediatric hematopoietic stem cell transplant recipients and intensive care unit admission. Pediatr Nephrol. 2007, 22, 441–7. [Google Scholar] [CrossRef]
- Rondón G, Saliba RM, Chen J, Ledesma C, Alousi AM, Oran B, et al. Impact of Fluid Overload as New Toxicity Category on Hematopoietic Stem Cell Transplantation Outcomes. Biol Blood Marrow Transplant. 2017, 23, 2166–71. [Google Scholar] [CrossRef]
- Lintz VC, Vieira RA, Carioca FL, Ferraz IS, Silva HM, Ventura AMC, et al. Fluid accumulation in critically ill children: a systematic review and meta-analysis. EClinicalMedicine. 2024, 74, 102714. [Google Scholar] [CrossRef]
- Li Z, Liu J, Jing B, Shen W, Liu P, Liu Y, et al. Incidence of acute kidney injury after hematopoietic stem cell transplantation in children: a systematic review and meta-analysis. Eur J Pediatr. 2023, 182, 3511–7. [Google Scholar] [CrossRef]
- James V, Angelo J, Elbahlawan L. Kidney Injury in Children after Hematopoietic Stem Cell Transplant. Curr Oncol. 2023, 30, 3329–43. [Google Scholar] [CrossRef]
- Matsuoka D, Hirabayashi K, Murase T, Saito S, Nakazawa Y. Impact of acute kidney injury on overall survival in children and young adults undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2021, 68, e29167. [Google Scholar] [CrossRef] [PubMed]
- Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol. 2010, 5, 943–9. [Google Scholar] [CrossRef] [PubMed]
- Soliman ASA, Al-Ghamdi HS, Abukhatwah MW, Kamal NM, Dabour SA, Elgendy SA, et al. Renal angina index in critically ill children as an applicable and reliable tool in the prediction of severe acute kidney injury: Two tertiary centers' prospective observational study from the Middle East. Medicine (Baltimore). 2023, 102, e36713. [Google Scholar] [CrossRef] [PubMed]
- Goldstein SL, Krallman KA, Roy JP, Collins M, Chima RS, Basu RK, et al. Real-Time Acute Kidney Injury Risk Stratification-Biomarker Directed Fluid Management Improves Outcomes in Critically Ill Children and Young Adults. Kidney Int Rep. 2023, 8, 2690–700. [Google Scholar] [CrossRef]
- Elbahlawan L, Srinivasan A, Morrison RR. A Critical Care and Transplantation-Based Approach to Acute Respiratory Failure after Hematopoietic Stem Cell Transplantation in Children. Biol Blood Marrow Transplant. 2016, 22, 617–26. [Google Scholar] [CrossRef]
- Rowan CM, McArthur J, Hsing DD, Gertz SJ, Smith LS, Loomis A, et al. Acute Respiratory Failure in Pediatric Hematopoietic Cell Transplantation: A Multicenter Study. Crit Care Med. 2018, 46, e967–e74. [Google Scholar] [CrossRef]
- Sallee CJ, Smith LS, Rowan CM, Heckbert SR, Angelo JR, Daniel MC, et al. Early Cumulative Fluid Balance and Outcomes in Pediatric Allogeneic Hematopoietic Cell Transplant Recipients With Acute Respiratory Failure: A Multicenter Study. Front Oncol. 2021, 11, 705602. [Google Scholar] [CrossRef]
- Elbahlawan L, Morrison R, Li Y, Huang S, Cheng C, Avent Y, et al. Outcome of Acute Respiratory Failure Secondary to Engraftment in Children After Hematopoietic Stem Cell Transplant. Frontiers in Oncology. 2020, 10. [Google Scholar]
- Black CG, Thomas NJ, Yehya N. Timing and Clinical Significance of Fluid Overload in Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med. 2021, 22, 795–805. [Google Scholar] [CrossRef]
- Flores FX, Brophy PD, Symons JM, Fortenberry JD, Chua AN, Alexander SR, et al. Continuous renal replacement therapy (CRRT) after stem cell transplantation. A report from the prospective pediatric CRRT Registry Group. Pediatr Nephrol. 2008, 23, 625–30. [Google Scholar] [CrossRef]
- Raymakers-Janssen P, Lilien MR, Tibboel D, Kneyber MCJ, Dijkstra S, van Woensel JBM, et al. Epidemiology and Outcome of Critically Ill Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients Requiring Continuous Renal Replacement Therapy: A Retrospective Nationwide Cohort Study. Crit Care Med. 2019, 47, e893–e901. [Google Scholar] [CrossRef] [PubMed]
- Elbahlawan L, Bissler J, Morrison RR. Continuous Renal Replacement Therapy: A Review of Use and Application in Pediatric Hematopoietic Stem Cell Transplant Recipients. Front Oncol. 2021, 11, 632263. [Google Scholar] [CrossRef] [PubMed]
- Murugan R, Kerti SJ, Chang CH, Gallagher M, Neto AS, Clermont G, et al. Association between Net Ultrafiltration Rate and Renal Recovery among Critically Ill Adults with Acute Kidney Injury Receiving Continuous Renal Replacement Therapy: An Observational Cohort Study. Blood Purif. 2022, 51, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Sansom B, Udy A, Presneill J, Bellomo R. Early Net Ultrafiltration during Continuous Renal Replacement Therapy: Impact of Admission Diagnosis and Association with Mortality. Blood Purif. 2024, 53, 170–80. [Google Scholar] [CrossRef] [PubMed]
- Lindell RB, Gertz SJ, Rowan CM, McArthur J, Beske F, Plunkett A, et al. High Levels of Morbidity and Mortality Among Pediatric Hematopoietic Cell Transplant Recipients With Severe Sepsis: Insights From the Sepsis PRevalence, OUtcomes, and Therapies International Point Prevalence Study. Pediatr Crit Care Med. 2017, 18, 1114–25. [Google Scholar] [CrossRef] [PubMed]
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021, 49, 1974–82. [Google Scholar] [CrossRef]
- Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011, 364, 2483–95. [Google Scholar] [CrossRef]
- Bonifazi F, Barbato F, Ravaioli F, Sessa M, Defrancesco I, Arpinati M, et al. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2020, 11, 489. [Google Scholar] [CrossRef]
- Corbacioglu S, Jabbour EJ, Mohty M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol Blood Marrow Transplant. 2019, 25, 1271–80. [Google Scholar] [CrossRef]
- Spitzer, TR. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015, 50, 469–75. [Google Scholar] [CrossRef]
- Langer T, D'Oria V, Spolidoro GCI, Chidini G, Scalia Catenacci S, Marchesi T, et al. Fluid therapy in mechanically ventilated critically ill children: the sodium, chloride and water burden of fluid creep. BMC Pediatr. 2020, 20, 424. [Google Scholar]
- Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018, 44, 409–17. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006, 354, 2564–75. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).