1. Introduction
Cardiovascular risk factors (CRFs), such as high blood pressure, hyperlipidemia, and diabetes mellitus, are highly prevalent among the elderly population [
1,
2]. These risk factors significantly contribute to the development of cognitive impairment and dementia [
3]. Moreover, CRFs have detrimental cerebral effects in not only dementia and mild cognitive impairment (MCI) but also in cognitively intact elderly individuals, owing to their association with cerebral small vessel disease (cSVD) [
4]. Diabetes mellitus, in particular, has been associated with a higher odds ratio for cSVD markers, such as periventricular and subcortical white matter lesions, and lacunar infarcts [
5]. Other CRFs, including elevated cholesterol and hypertension, have shown significant positive associations with cSVD burden [
6].
cSVD is characterized by structural abnormalities in the small arteries, arterioles, capillaries, and venules of the brain, encompassing a spectrum of intracranial vascular diseases with varied pathologies, clinical presentations, and neuroimaging markers. Notably, cSVD is defined by features such as cerebral microbleeds, lacunar infarcts, enlarged perivascular spaces, and White Matter Hyperintensity (WMH) [
7]. Among these, WMH is the most studied neuroimaging marker to classify cSVD extent [
8]. WMH can be classified into confluent (coalescing clusters) and punctate (non-confluent) types [
9,
10]. Confluent WMH is associated with greater cognitive decline and disruption to cerebral connectivity [
11,
12].
The blood-brain barrier (BBB) is a crucial structure for regulating exchanges between the neural milieu and circulatory system, ensuring neuronal function. It consists of endothelial cells, basement membrane, glial podocytes, and pericytes [
13]. BBB-forming endothelial cells are packed with tight junctions and adhesion molecule proteins, regulating diffusion. Influx and efflux transporters facilitate selective uptake of metabolites and waste removal [
14]. BBB dysfunction has been implicated in the pathogenesis of neurodegenerative diseases and cSVD [
15,
16], and is likely an early process in cognitive disorders. Mouse models have demonstrated BBB dysfunction preceding cognitive decline, with accelerated BBB permeability linked to cognitive deficits [
17]. Furthermore, dynamic contrast-enhanced MRI studies have shown that individuals with baseline BBB leakage exhibited increased parenchymal diffusivity over two years, particularly around perilesional WMH zones [
18].
The link between BBB dysfunction and cognitive impairment is of growing interest, suggesting a role in the exacerbation of cSVD markers on neuroimaging. To investigate BBB permeability, studies have employed transendothelial electrical resistance (TEER) and permeability assays, which are well-established methods for assessing BBB integrity [
19,
20]. Limited studies, however, have evaluated TEER changes in response to plasma from cSVD patients, a fundamental metric reflecting endothelial barrier integrity [
21]. TEER measurement depends on the formation of tight junctions between adherent cells, representing the ionic conductance of the paracellular pathway [
22]. This approach allows controlled manipulation of experimental conditions to assess BBB integrity [
23,
24].
This study examines the relationship between CRFs, such as blood glucose, blood pressure, and lipid profiles, with BBB integrity quantified via TEER. Additionally, the association between TEER and WMH was studied. We hypothesized a strong association between BBB dysfunction, as measured by TEER, with WMH volumes, and that TEER would correlate with CRFs.
2. Methods
2.1. Participants
One hundred and fifty-five participants from Dementia Research Centre (Singapore) were included in this study. Participants were recruited from the ongoing Biomarkers and Cognition Study, Singapore (BIOCIS) [
25]. The inclusion criteria for the BIOCIS study were individuals aged 30 to 95 years from the community with cognitive concerns but with intact mental capacity and without serious neurological, psychiatric, or systemic diseases. The exclusion criteria were illiteracy, clinical diagnosis of serious mental illness, contraindications to Magnetic Resonance Imaging (MRI), diagnosis of cancer within the last two years prior to screening, and pregnancy. Informed consent was obtained from participants according to the Declaration of Helsinki and local clinical research regulations. Furthermore, the procedures utilized in this study were in accordance with ethical guidelines and approved by the Nanyang Technological University Institutional Review Board (study 2021-1036).
2.2. Image Acquisition
MRI was performed using a 3T Prisma Fit system (Siemens, Erlangen, Germany). High resolution T1-weighted MPRAGE (Magnetization Prepared Rapid Gradient Echo: 176 continuous sagittal slices, TR/TE/TI = 2000/2.26/800ms, flip angle = 8°, FOV = 256x256mm2, matrix = 256x256, isotropic voxel size = 1.0x1.0x1.0mm3, bandwidth = 200 Hz/pixel) and FLAIR (Fluid Attenuated Inversion Recovery: 192 continuous sagittal slices, TR/TE/TI = 7000/394/2100ms, flip angle = 120°, FOV = 320x320mm2, matrix = 320x320, isotropic voxel size = 0.8x0.8x1.0mm3, bandwidth = 650 Hz/pixel) sequences were acquired. Scanned images were reviewed during acquisition, and participants with severe motion artifacts and overt pathological findings were excluded.
2.3. Visual Ratings of White Matter Hyperintensities
Two trained raters visually evaluated the FLAIR scans for WMH severity using the modified Fazekas scale, where periventricular WMH (PVH) and subcortical WMH (DWMH) were separately rated on a 0-3 point scale for each hemisphere [
9,
26]. The scoring criteria were as follows: For PVH, absence of any WMH = 0; presence of caps or pencil-thin lining = 1; smooth halo along the edges of the lateral ventricle = 2; irregular hyperintensities extending into the subcortical white matter = 3. For DWMH, absence of any WMH = 0; presence of nonconfluent foci of WMH in the subcortical region = 1; beginning of confluence of WMH foci = 2; presence of large confluent areas = 3. Discrepancies between the two raters in the MRI visual rating score were resolved by consensus. Subjects were additionally classified as having confluent or non-confluent WMH based on the Staals criteria [
10]: Subjects with a WMH rating of 3 in either periventricular and/or a rating of 2 or 3 in subcortical white matter regions in either hemisphere were assigned as having confluent WMH and the rest as non-confluent WMH.
2.4. Image Pre-Processing & White Matter Hyperintensity Volume Derivation
The Computational Anatomy Toolbox (
http://dbm.neuro.uni-jena.de/cat12/) protocol was built into Statistical Parametric Mapping (SPM12) (
http://www.fil.ion.ucl.ac.uk/spm/) and utilized to process the T1-weighted images. The normalization of the above-mentioned 3D images used affine transformation and nonlinear registration with correction for bias field inhomogeneities. To obtain better precision in spatial normalization to the standard MNI template, Diffeomorphic Anatomic Registration Through Exponentiated Lie algebra algorithm (DARTEL) was used [
27]. A modulation step involving nonlinear deformation on the normalized segmented images was carried out to provide a comparison of the absolute amount of tissue corrected for individual differences in brain size. Finally, all segmented, normalized, and modulated Grey Matter and White Matter images were smoothed with an 8 mm full-width-half-maximum isotropic Gaussian smoothing kernel. To quantify WMH volumes, CAT12 method based on the 3D T1-weighted sequence and region-growing approach comparable to currently used T2/FLAIR-based methods was used [
28,
29].
2.5. Blood Collection, Processing & Analysis
Before venipuncture, blood pressure was measured to ensure safety and eligibility for blood collection. Subsequently, venous blood was collected from each participant in EDTA Becton Dickinson Vacutainer ® tubes. Plasma was immediately separated through centrifugation (2000g for 10 min at 4°C) and refrigerated at -80°C. Venous blood collected in Serum Separating Tube, EDTA and sodium fluoride tube were sent to an accredited commercial biochemistry laboratory (Innoquest Laboratories, Singapore) within 4 hours of the blood draw for blood-based cardiovascular risk analysis.
2.6. Transendothelial Electrical Resistance (TEER) Assay
Human brain microvascular endothelial cells (hBMEC from afirmus bioscience) were seeded in a 24 well transwell® plate (Corning Transwell 3470 Polystyrene Membrane Insert, Sterile, 6.5mm Membrane Diameter, 0.4μm Membrane Pore Size, 0.33cm2 Membrane Area) with a cell density of 3x105 cells/cm2 cultured in (EGMTM-2) Endothelial Cell Growth Medium-2 BulletKitTM (Lonza Bioscience) supplemented with 10% Heat-Inactivated Fetal Bovine Serum (HI-FBS from life technologies) for 8 hours. Following which, the hBMECs were starved with EGMTM-2 supplemented with 2% HI-FBS for 2 hours before challenging them with collected participant’s plasma for 6 hours. Finally, TEER measurements were performed using an EVOM2TM Epithelial Voltohmmeter and its STX2 chopstick electrode (World Precision Instruments), following the plasma challenge.
2.7. Statistical Analyses
2.7.1. Association of WMH Volume with TEER Values
A linear regression model was built with TEER as the dependent variable to assess the association between WMH severity and TEER. Natural logarithm transformed WMH volume data was designated as the independent variable and age at visit (stratified as “young” or “old” where “young” was defined as those aged below 65 and old as those aged 65 and above), sex and Total intracranial volume were included as covariates in the model. The statistically significant effect of WMH volume was based on a p-value threshold of p < 0.05 after False Discovery Rate (FDR) correction.
To identify differences in TEER between participants with low and high WMH burden, mean TEER values between subjects with confluent and non-confluent WMH were compared using analysis of covariance. Furthermore, the mean TEER values for low and high WMH burden in the periventricular and subcortical white matter regions were also similarly compared. Age and sex were included as covariates in this analysis. Statistical significance between groups was defined based on a p < 0.05 threshold after FDR correction.
2.7.2. Association between CRFs and TEER Values
To assess the correlation between blood glucose levels, lipid profile, blood pressure and TEER, a linear regression model was built with TEER as the dependent variable and the respective CRF variables (HBA1C, fasting blood glucose, total cholesterol, low-density lipoprotein (LDL), and triglyceride) as the independent variables of interest. Age, sex, and chronic medications used to target cardiovascular risk factors were included as covariates in the linear model. Statistical significance was defined as a p-value threshold of p < 0.05 after FDR correction.
All statistical analyses were conducted using R 4.2.3 (R CoreTeam, 2021) with RStudio (RStudio Team, 2023).
3. Results
The age range of the cohort was 31.89 to 84.18 years with a mean of 63.12 (SD = 9.70) years with majority of participants being female (60.0%). Of the 155 participants, 76 (49%) had low TEER defined as values below the mean split of the total TEER value (
Table 1). The mean split for TEER value was used to determine low and high TEER, as no standard or published threshold is available for such groupings thus far. Low TEER is indicative of greater transendothelial junction integrity disruption and thus a surrogate measure for the deterioration of BBB integrity disruption.
Participants with low TEER (BBB integrity disruption) had significantly higher WMH volume (5.1mL vs. 2.8mL, p = <0.001), significantly higher systolic BP (136.5mmHg vs 129.1mmHg, p = 0.00698), significantly higher diastolic BP (81.4mmHg vs. 77.0mmHg, p = 0.0163) and significantly higher BMI (24.7kg/m
2 vs 22.8kg/m
2, p = <0.001) compared to participants with high TEER (
Table 1). The tryglyceride levels demonstrated a trend towards being higher in the low TEER group.
3.1. Association between WMH and TEER
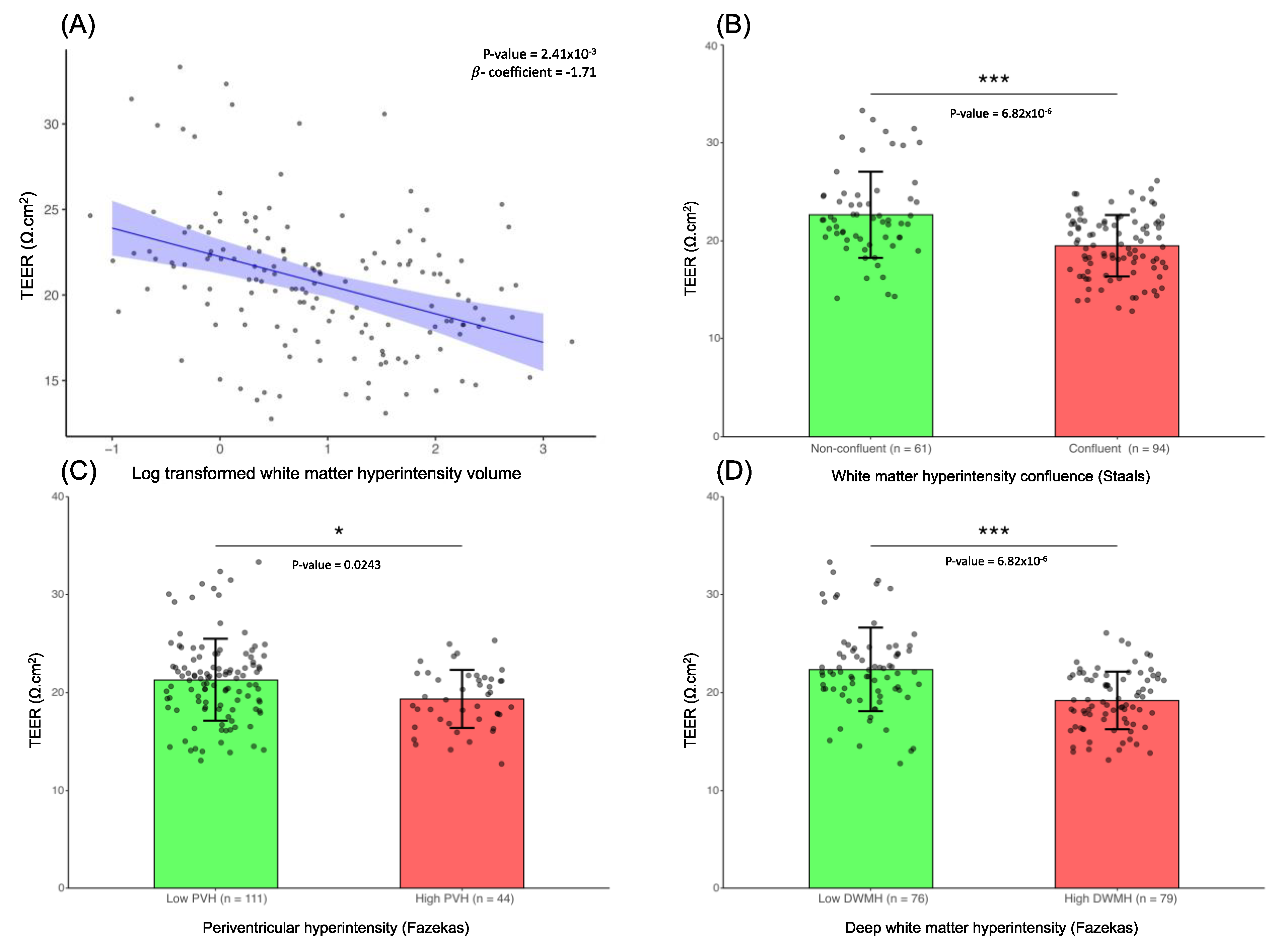
Based on the linear regression model built for WMH and TEER, we observed that lower TEER values were related to greater WMH volume (β = -1.71, FDR-corrected
p = 2.41x10
-3) (
Figure 1A). This suggests that there is a direct association between increase in WMH volume, and disruption in the barrier integrity of the hBMEC (as observed by the lower TEER). Additionally, TEER measurements were significantly lower in the confluent WMH group than in the non-confluent WMH group (FDR-corrected
p = 6.82x10
-6) (
Figure 1B). High periventricular WMH and high subcortical WMH were also associated with significantly lower TEER values in comparison to low WMH burden in the respective regions (FDR-corrected
p = 0.0243 and FDR-corrected
p = 6.82x10
-6 respectively) (
Figure 1C and
Figure 1D).
3.2. Association between Blood Glucose and TEER
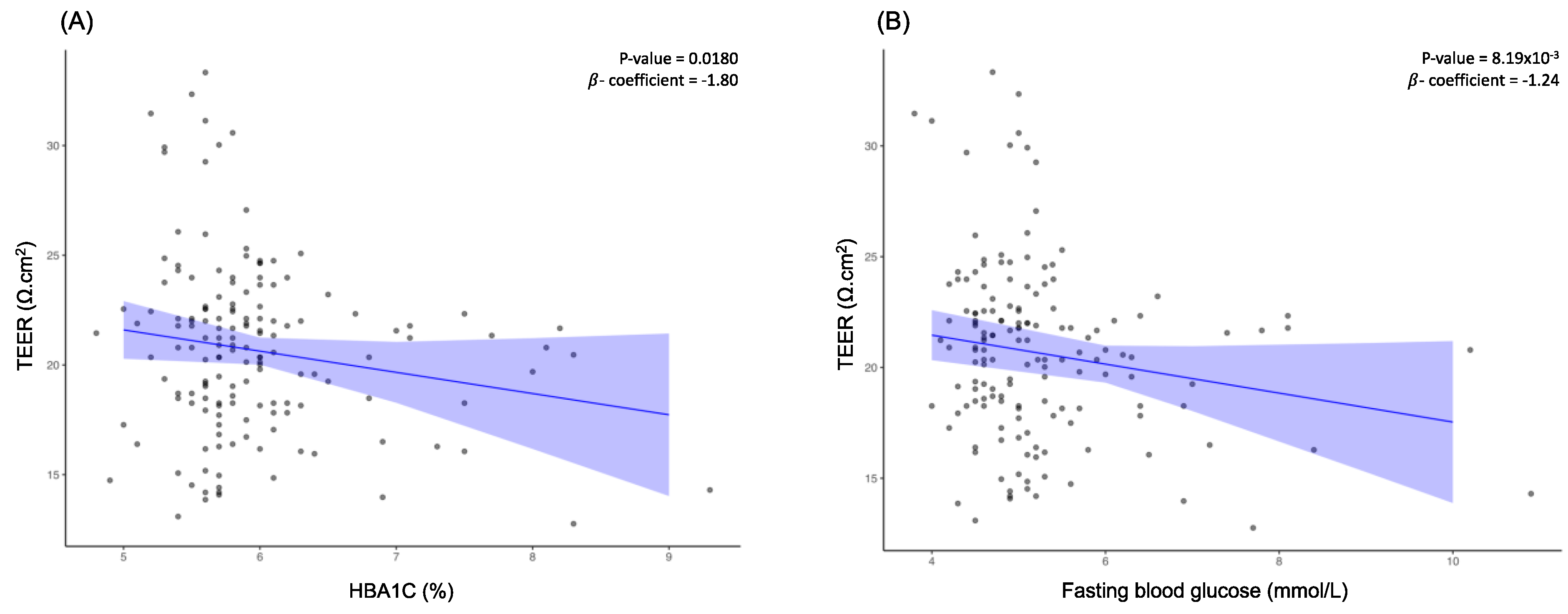
Examination of the relationship between blood glucose levels and TEER indicated that TEER decreased with increasing blood glucose levels. To understand whether long-term or short-term increases in blood glucose levels contribute more to the reduction in TEER value, both HBA1C levels and fasting blood glucose levels were evaluated against TEER. Based on this analysis, we observed that both HBA1C (β = -1.80, FDR-corrected
p = 0.0147) and fasting blood glucose levels (β = -1.24, FDR-corrected
p = 6.70x10
-3) were negatively associated with TEER (
Figure 2A and
Figure 2B).
3.3. Association between Blood Lipid Profile and TEER
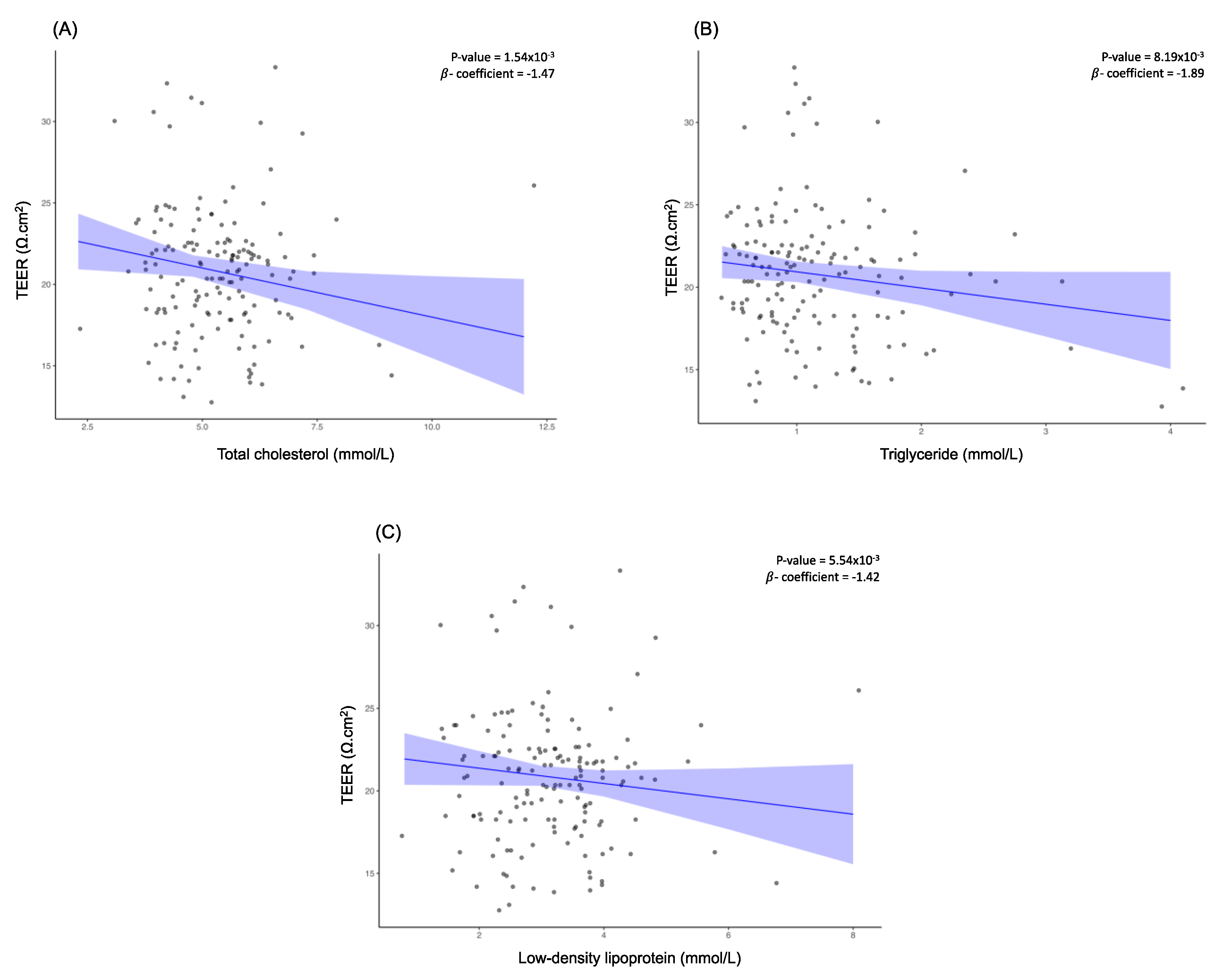
Upon assessment of the relationship between blood lipid profile and TEER, higher total cholesterol level was associated with lower TEER (β = -1.47, FDR-corrected
p = 1.26x10
-3) (
Figure 3A). This trend was also observed for triglycerides (β = -1.89, FDR-corrected
p = 6.70x10
-3) and LDL (β = -1.42, FDR-corrected
p = 4.53x10
-3) (
Figure 3B and
Figure 3C).
3.4. Association between Blood Pressure and TEER
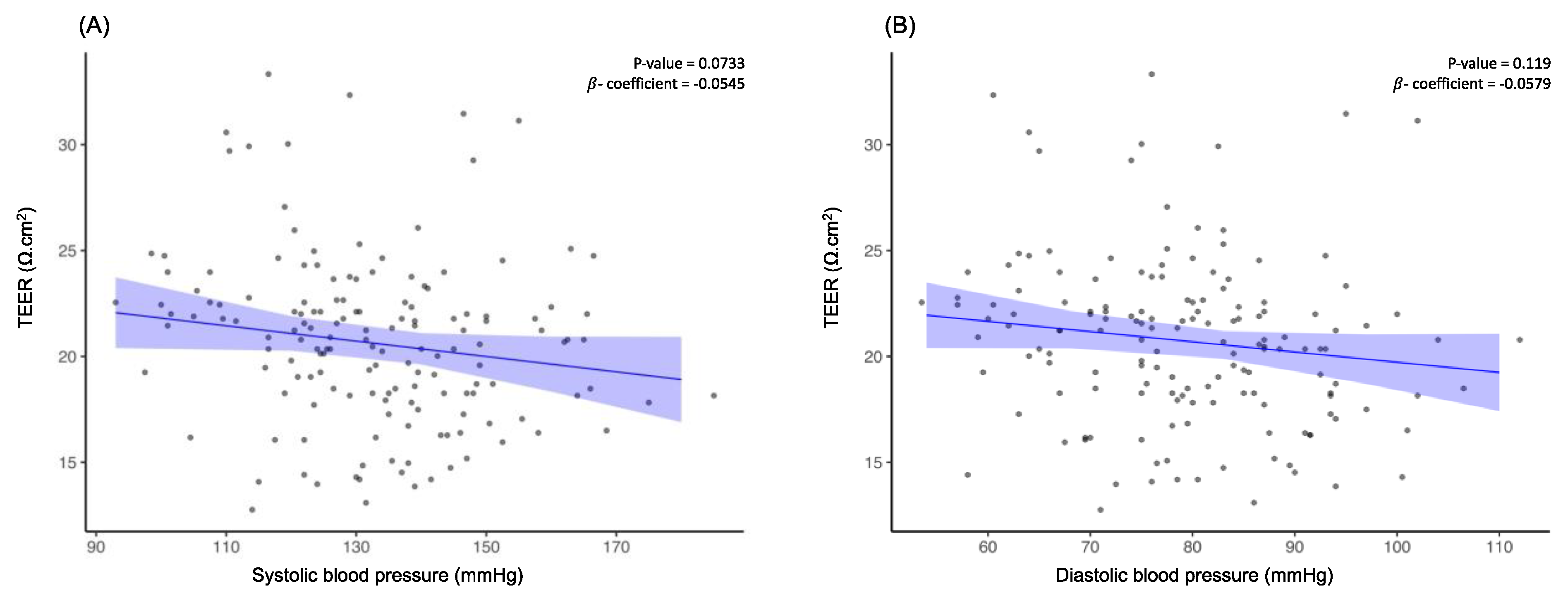
Upon evaluating the relationship between systolic and diastolic blood pressure with TEER (β = -0.0545, FDR-corrected
p = 0.0733 and β = -0.0579, FDR-corrected
p = 0.119 respectively) (
Figure 4A and
Figure 4B), it was determined that there was no association between the two variables and TEER although participants with low TEER had significantly higher systolic and diastolic BP.
4. Discussion
In this study, we examined the influence of BBB integrity disruption with both WMH and CRF using plasma of individuals with MRI quantified WMH. We demonstrate that the exposure of blood plasma from individuals with high WMH, both in the periventricular and subcortical white matter regions, to be associated with a deterioration in the junction integrity of hBMECs. Furthermore, the higher systolic BP, diastolic BP and BMI in individuals with BBB integrity disruption as well as the observed regression-based association between elevated blood sugar levels and altered lipid profiles with reduced hBMEC TEER value supports our hypothesis that CRFs are strongly associated with the occurrence of BBB integrity disruption.
We have discovered that the blood plasma of individuals with WMH illustrates a detrimental impact on TEER across hBMECs. Specifically, a higher WMH load was found to be correlated with lower TEER, indicating increased BBB junction integrity compromise. Such derogatory associations of WMH on BBB junction integrity are consistent with previous findings of higher BBB permeability in participants with cognitive impairment and cSVD [
30,
31]. The consistency in the observation, spanning both the periventricular and subcortical white matter regions, may imply that increased BBB integrity disruption is present in regions of both periventricular and subcortical WMH. Hypothesized characteristics of this mechanism include alterations in intercellular structures, decreased expression of transendothelial carriers, the activation of various vasoactive mediators, and the involvement of both astroglia and monocytes/macrophages [
32]. It is thus of utmost importance to conduct further longitudinal research to definitively establish the mechanism by which WMH and BBB function are interconnected.
Consistent with previous research, our investigation revealed a positive association between elevated blood glucose levels and BBB junction integrity deterioration [
33]. It is postulated that elevated blood glucose levels contribute to increased BBB permeability through the mechanism of metabolic overload and its associated systemic low-grade inflammation. Specific to metabolic overload, it impairs BBB function by increasing paracellular permeability and decreasing TEER. These are changes attributable to the loss of tight junctions, occludin, claudin-5 and vessel basal lamina [
33]. Low-grade inflammation can also have a detrimental impact on the function of the BBB by disrupting the balance between matrix metalloproteinases and tissue inhibitors of metalloproteinases activities. During such prolonged inflammation, matrix metalloproteinases levels increase while tissue inhibitors of metalloproteinases levels decrease, leading to a compromised BBB [
33,
34,
35,
36]. Prior research has shown that human brain endothelial cells (hCMEC/D3) exhibit lower TEER values when exposed to serum obtained from patients with type 2 diabetes as compared to healthy controls, which is attributed to the elevated levels of tumor necrosis factor - and interleukin-6 in the serum of type 2 diabetes patients [
33]. Therefore, it is crucial to conduct additional research into the relationship between metabolic overload, low-grade inflammation, and BBB dysfunction, given the lack of clarity regarding the mechanisms involved. This is particularly important in the context of individuals with elevated glucose levels, as it may shed light on the downstream effects of such conditions on BBB function.
In this current study, we demonstrate the deleterious effects of elevated lipid profile levels on the function of the BBB. Specifically, this was observed for lipid profile constituents such as LDL and triglycerides. Consistent with prior
in vivo studies using mice fed with hypercholesterolemic diet, BBB disruption was observed in the prefrontal cortex and hippocampus, and there was a decrease in hippocampal claudin-5 and occludin mRNA levels [
37]. Furthermore, it was observed that mice with experimentally induced familial hypercholesterolemia (LDL receptor
-/-) displayed impaired BBB function when fed a normal diet, and this impairment was further exacerbated when they were fed a high-cholesterol diet [
37]. Although the precise mechanism by which LDL impairs BBB function remains unclear, it is well established that the accumulation of LDL in the bloodstream significantly increases the likelihood of LDL oxidation, which in turn triggers oxidative stress and inflammation. Given this information, it has been documented in prior research that
in vitro treatment of neural precursor cells with LDL has a profound effect on astrocyte morphology, which is intricately associated with the astrogliosis process. [
38]. The neuroinflammatory process is frequently associated with vascular alterations in the brain, including the reconfiguration of microvessels and the entry of circulating cells into the brain parenchyma, which exacerbates neuroinflammation and impairs the BBB [
39,
40]. Similar to elevated levels of LDL, studies have demonstrated that elevated triglycerides, particularly their lipolysis products, contribute to an increase in the BBB transfer coefficient and induce astrocytic proinflammatory and stress-related gene expression [
41,
42,
43]. Thus, it is suggested that chronic elevated levels of LDL and triglyceride levels may have indirect consequences on BBB dysfunction. This may be attributed to the accumulation of their metabolic byproducts, which subsequently triggers the activation of neuroinflammation and stress-related genes, ultimately resulting in cell death. Further investigation is necessary in future studies to elucidate the relationship between this phenomenon and BBB dysfunction, as well as its potential contribution to cognitive impairment.
It is perplexing that we did not observe a direct correlation between TEER and both levels of systolic and diastolic BP, although a significantly higher systolic and diastolic BP was observed in individuals and
in vivo rodent models with high BBB dyspermeability [
44,
45]. While we do not have a clear explanation, a potential mechanism for the association between BP and BBB permeability could be the chronicity of elevated BP. Studies have demonstrated that hypertension in mid-life but not late-life to be associated with dementia and hence it may not be the current status of BP but the duration of BP that may influence TEER values [
46,
47]. Unfortunately, we lack data on duration of elevated BP which hopefully can be addressed in future studies.
Based on the points discussed above, our study indicates that changes in the components of blood plasma, potentially due to increased blood glucose levels or hyperlipidemia, could contribute to BBB dysfunction, especially in individuals with WMH.
Our study has several limitations. The data utilized in our analyses were cross-sectional and derived from a limited sample size, which warrants further validation through longitudinal datasets and larger sample sizes. Additionally, the inflammatory, amyloid beta, and tau status of the participants remained unknown. Finally, permeability measurement using non-electrolyte tracers could be conducted to further demonstrate the impact of BBB junction integrity on its permeability. Future biomarker studies are expected to provide greater insight into the underlying mechanism of BBB impairment and its implications on the development of WMH and their association with elevated glucose levels and lipid profiles.
5. Conclusions
In summary, the present study provides early evidence that BBB integrity disruption in hBMECs can be induced by exposure to blood plasma from individuals with a high WMH load. Additionally, the study suggests that CRFs specifically elevated glucose levels and lipid profiles may play a significant role in BBB dysfunction, possibly through indirect means. This study underscores the significance of CRFs as precursory warning signs of BBB deterioration, which has been implicated in the development and progression of dementia.
Author Contributions
James Xiao Yuan Chen conceptualized and designed the study, carried out data analyses and drafted the manuscript and figures. Ashwati Vipin, Ee Soo Lee, Cheung Christine and Nagaendran Kandiah contributed to the study design, data analyses and drafting of the manuscript and carried out the final review. Gurveen Kaur Sandhu, Yi Jin Leow, Fatin Zahra Zailan, Pricilia Tanoto and Khang Leng Lee contributed to the acquisition of data.
Funding
The work is supported by the Ministry of Education Academic Research Funds (RG88/21), Vascular Research Initiative from the Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Strategic Academic Initiative grant from the Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, and National Medical Research Council, Singapore under its Clinician Scientist Award (MOH-CSAINV18nov-0007).
Institutional Review Board Statement
The study was approved by the Nanyang Technological University Institutional Review Board (Reference 2021-1036). All participants provided informed consent in accordance with the Declaration of Helsinki and local clinical research regulations.
Informed Consent Statement
Not applicable.
Availability of Data and Materials
The investigators attest that all data supporting the study's findings are contained within the paper. Additional information and requests for resources and reagents should be communicated with and will be provided by the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We extend our gratitude to all participants and clinical coordinators who have contributed to this study through the Dementia Research Centre, Singapore. Special thanks to Ms Hannah Wee Su-Ann for experimental assistance.
Abbreviations
| CRF: Cardiovascular Risk Factors |
| cSVD: Cerebral Small Vessel Disease |
| WMH: White Matter Hyperintensity |
| BBB: Blood-Brain Barrier |
| BIOCIS: Biomarkers and Cognition Study, Singapore |
| TEER: Transendothelial Electrical Resistance |
| hBMEC: human brain microvascular endothelial cells |
| MCI: Mild Cognitive Impairment |
| MRI: Magnetic Resonance Imaging |
| MPRAGE: Magnetization Prepared Rapid Gradient Echo |
| TR: Repetition Time |
| TE: Time to Echo |
| TI: Inversion Time |
| FOV: Field-Of-View |
| FLAIR: Fluid Attenuated Inversion Recovery |
| PVH: Periventricular White Matter Hyperintensity |
| DWMH: Subcortical White Matter Hyperintensity |
| SPM12: Statistical Parametric Mapping |
| CAT12: Computational Anatomy Toolbox |
| DARTEL: Diffeomorphic Anatomic Registration Through Exponentiated Lie algebra algorithm |
| EDTA: Ethylenediaminetetraacetic acid |
| EGMTM-2: Endothelial Cell Growth Medium-2 |
| HI-FBS: Heat-Inactivated Fetal Bovine Serum |
| FDR: False Discovery Rate |
| HBA1C: Glycated Hemoglobin |
| LDL: Low-Density Lipoprotein |
| SD: Standard Deviation |
| BP: Blood Pressure |
| BMI: Body mass index |
References
- Nguyen, H.N.; Fujiyoshi, A.; Abbott, R.D.; Miura, K. Epidemiology of Cardiovascular Risk Factors in Asian Countries. Circ. J. 2013, 77, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, W.; Manolio, T.A.; Arnold, A.; Burke, G.L.; Bryan, N.; Jungreis, C.A.; Enright, P.L.; O’Leary, D.; Fried, L. Clinical Correlates of White Matter Findings on Cranial Magnetic Resonance Imaging of 3301 Elderly People: The Cardiovascular Health Study. Stroke 1996, 27, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Mortamais, M.; Artero, S.; Ritchie, K. Cerebral White Matter Hyperintensities in the Prediction of Cognitive Decline and Incident Dementia. Int. Rev. Psychiatry 2013, 25, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhai, F.-F.; Wang, Q.; Zhou, L.-X.; Ni, J.; Yao, M.; Li, M.-L.; Zhang, S.-Y.; Cui, L.-Y.; Jin, Z.-Y. Prevalence and Risk Factors of Cerebral Small Vessel Disease in a Chinese Population-Based Sample. J. Stroke 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.J.; Prins, N.D.; Vrooman, H.A.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Progression of Cerebral Small Vessel Disease in Relation to Risk Factors and Cognitive Consequences: Rotterdam Scan Study. Stroke 2008, 39, 2712–2719. [Google Scholar] [CrossRef]
- Wong, F.C.C.; Yatawara, C.; Low, A.; Foo, H.; Wong, B.Y.X.; Lim, L.; Wang, B.; Kumar, D.; Ng, K.P.; Kandiah, N. Cerebral Small Vessel Disease Influences Hippocampal Subfield Atrophy in Mild Cognitive Impairment. Transl. Stroke Res. 2021, 12, 284–292. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.-E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S. Neuroimaging Standards for Research into Small Vessel Disease—Advances since 2013. Lancet Neurol. 2023, 22, 602–618. [Google Scholar] [CrossRef]
- Mustapha, M.; Nassir, C.M.N.C.M.; Aminuddin, N.; Safri, A.A.; Ghazali, M.M. Cerebral Small Vessel Disease (CSVD)–Lessons from the Animal Models. Front. Physiol. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. Am. J. Neuroradiol. 1987, 8, 421–426. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke Subtype, Vascular Risk Factors, and Total MRI Brain Small-Vessel Disease Burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Heng, L.C.; Lim, S.H.; Foo, H.; Kandiah, N. Confluent White Matter in Progression to Alzheimer Dementia. Alzheimer Dis. Assoc. Disord. 2021, 35, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Vipin, A.; Wong, B.; Ng, K.P.; Kandiah, N. Differential Effects of Confluent and Nonconfluent White Matter Hyperintensities on Functional Connectivity in Mild Cognitive Impairment. Brain Connect. 2020, 10, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Dotiwala, A.K.; McCausland, C.; Samra, N.S. Anatomy, Head and Neck, Blood Brain Barrier. 2018. [Google Scholar]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-Brain Barrier: More Contributor to Disruption of Central Nervous System Homeostasis than Victim in Neurological Disorders. Front. Neurosci. 2020, 14, 764. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Zhang, C.E.; Wong, S.M.; van de Haar, H.J.; Staals, J.; Jansen, J.F.; Jeukens, C.R.; Hofman, P.A.; van Oostenbrugge, R.J.; Backes, W.H. Blood–Brain Barrier Leakage Is More Widespread in Patients with Cerebral Small Vessel Disease. Neurology 2017, 88, 426–432. [Google Scholar] [CrossRef]
- Takechi, R.; Lam, V.; Brook, E.; Giles, C.; Fimognari, N.; Mooranian, A.; Al-Salami, H.; Coulson, S.H.; Nesbit, M.; Mamo, J.C. Blood-Brain Barrier Dysfunction Precedes Cognitive Decline and Neurodegeneration in Diabetic Insulin Resistant Mouse Model: An Implication for Causal Link. Front. Aging Neurosci. 2017, 9, 399. [Google Scholar] [CrossRef]
- Kerkhofs, D.; Wong, S.M.; Zhang, E.; Staals, J.; Jansen, J.F.; van Oostenbrugge, R.J.; Backes, W.H. Baseline Blood-Brain Barrier Leakage and Longitudinal Microstructural Tissue Damage in the Periphery of White Matter Hyperintensities. Neurology 2021, 96, e2192–e2200. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Erickson, M.A.; Wilson, M.L.; Banks, W.A. In Vitro Modeling of Blood–Brain Barrier and Interface Functions in Neuroimmune Communication. Fluids Barriers CNS 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Zucco, F.; Batto, A.-F.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G. An Inter-Laboratory Study to Evaluate the Effects of Medium Composition on the Differentiation and Barrier Function of Caco-2 Cell Lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R. Transepithelial/Transendothelial Electrical Resistance (TEER) to Measure the Integrity of Blood-Brain Barrier. Blood-Brain Barrier 2019, 99–114. [Google Scholar]
- Hwang, J.S.; Cha, E.-H.; Ha, E.; Park, B.; Seo, J.H. GKT136901 Protects Primary Human Brain Microvascular Endothelial Cells against Methamphetamine-Induced Blood-Brain Barrier Dysfunction. Life Sci. 2020, 256, 117917. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Dohgu, S.; Takata, F.; Iwao, T.; Kimura, I.; Tomohiro, M.; Aono, K.; Kataoka, Y.; Yamauchi, A. Serum Amyloid A-Induced Blood-Brain Barrier Dysfunction Associated with Decreased Claudin-5 Expression in Rat Brain Endothelial Cells and Its Inhibition by High-Density Lipoprotein in Vitro. Neurosci. Lett. 2020, 738, 135352. [Google Scholar] [CrossRef] [PubMed]
- Soo, S.A.; Kumar, D.; Leow, Y.J.; Koh, C.L.; Saffari, S.E.; Kandiah, N. Usefulness of the Visual Cognitive Assessment Test in Detecting Mild Cognitive Impairment in the Community. J. Alzheimers Dis. 2023, 93, 755–763. [Google Scholar] [CrossRef]
- Vipin, A.; Satish, V.; Saffari, S.E.; Koh, W.; Lim, L.; Silva, E.; Nyu, M.M.; Choong, T.-M.; Chua, E.; Lim, L. Dementia in Southeast Asia: Influence of Onset-Type, Education, and Cerebrovascular Disease. Alzheimers Res. Ther. 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Ashburner, J. A Fast Diffeomorphic Image Registration Algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Dahnke, R.; Ziegler, G.; Gaser, C. Detection of White Matter Hyperintensities in T1 without FLAIR. Mult. Scler. 2019, 59, 3774–3783. [Google Scholar]
- Mangin, J.-F.; Frouin, V.; Bloch, I.; Régis, J.; López-Krahe, J. From 3D Magnetic Resonance Images to Structural Representations of the Cortex Topography Using Topology Preserving Deformations. J. Math. Imaging Vis. 1995, 5, 297–318. [Google Scholar] [CrossRef]
- Zhang, C.E.; Wong, S.M.; Uiterwijk, R.; Backes, W.H.; Jansen, J.F.; Jeukens, C.R.; van Oostenbrugge, R.J.; Staals, J. Blood–Brain Barrier Leakage in Relation to White Matter Hyperintensity Volume and Cognition in Small Vessel Disease and Normal Aging. Brain Imaging Behav. 2019, 13, 389–395. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Zhang, X.; Shi, Q.; Yang, S.; Fan, H.; Qin, W.; Yang, L.; Yuan, J.; Jiang, T. Higher Blood–Brain Barrier Permeability Is Associated with Higher White Matter Hyperintensities Burden. J. Neurol. 2017, 264, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Walker, L.; Rauchmann, B.; Perneczky, R. Dysfunction of the Blood–Brain Barrier in Alzheimer’s Disease: Evidence from Human Studies. Neuropathol. Appl. Neurobiol. 2022, 48, e12782. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.H.; Errede, M.; d’Amati, A.; Khan, N.Q.; Fanti, S.; Loiola, R.A.; McArthur, S.; Purvis, G.S.; O’Riordan, C.E.; Ferorelli, D. Impact of Metabolic Disorders on the Structural, Functional, and Immunological Integrity of the Blood-Brain Barrier: Therapeutic Avenues. Faseb J. 2021, 36. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Ghorpade, A. Tissue Inhibitor of Metalloproteinase (TIMP)-1: The TIMPed Balance of Matrix Metalloproteinases in the Central Nervous System. J. Neurosci. Res. 2003, 74, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, A.; Persidskaia, R.; Suryadevara, R.; Che, M.; Liu, X.J.; Persidsky, Y.; Gendelman, H.E. Mononuclear Phagocyte Differentiation, Activation, and Viral Infection Regulate Matrix Metalloproteinase Expression: Implications for Human Immunodeficiency Virus Type 1-Associated Dementia. J. Virol. 2001, 75, 6572–6583. [Google Scholar] [CrossRef]
- Lewandowski, K.C.; Banach, E.; Bieńkiewicz, M.; Lewiński, A. Matrix Metalloproteinases in Type 2 Diabetes and Non-Diabetic Controls: Effects of Short-Term and Chronic Hyperglycaemia. Arch. Med. Sci. 2011, 7, 294–303. [Google Scholar] [CrossRef]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; Dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.; de Bem, A.F. High Cholesterol Diet Exacerbates Blood-Brain Barrier Disruption in LDLr–/–Mice: Impact on Cognitive Function. J. Alzheimers Dis. 2020, 78, 97–115. [Google Scholar] [CrossRef]
- Engel, D.F.; Grzyb, A.N.; de Oliveira, J.; Pötzsch, A.; Walker, T.L.; Brocardo, P.S.; Kempermann, G.; de Bem, A.F. Impaired Adult Hippocampal Neurogenesis in a Mouse Model of Familial Hypercholesterolemia: A Role for the LDL Receptor and Cholesterol Metabolism in Adult Neural Precursor Cells. Mol. Metab. 2019, 30, 1–15. [Google Scholar] [CrossRef]
- Andjelkovic, A.; Nikolic, B.; Pachter, J.; Zecevic, N. Macrophages/Microglial Cells in Human Central Nervous System during Development: An Immunohistochemical Study. Brain Res. 1998, 814, 13–25. [Google Scholar] [CrossRef]
- Chen, X.; Ghribi, O.; Geiger, J.D. Caffeine Protects against Disruptions of the Blood-Brain Barrier in Animal Models of Alzheimer’s and Parkinson’s Diseases. J. Alzheimers Dis. 2010, 20, S127–S141. [Google Scholar] [CrossRef]
- Lee, L.L.; Aung, H.H.; Wilson, D.W.; Anderson, S.E.; Rutledge, J.C.; Rutkowsky, J.M. Triglyceride-Rich Lipoprotein Lipolysis Products Increase Blood-Brain Barrier Transfer Coefficient and Induce Astrocyte Lipid Droplets and Cell Stress. Am. J. Physiol.-Cell Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S. Inflammation and Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Gerontology 1997, 43, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L. Inflammation and Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Friis, T.; Wikström, A.-K.; Acurio, J.; León, J.; Zetterberg, H.; Blennow, K.; Nelander, M.; Åkerud, H.; Kaihola, H.; Cluver, C. Cerebral Biomarkers and Blood-Brain Barrier Integrity in Preeclampsia. Cells 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, M.M.; Ahn, S.J.; Lane, D.; Faraco, G.; Garcia-Bonilla, L.; Racchumi, G.; Poon, C.; Schaeffer, S.; Segarra, S.G.; Körbelin, J. Endothelium-Macrophage Crosstalk Mediates Blood-Brain Barrier Dysfunction in Hypertension. Hypertension 2020, 76, 795–807. [Google Scholar] [CrossRef]
- Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Yoshida, D.; Doi, Y.; Hata, J.; Kanba, S.; Iwaki, T.; Kiyohara, Y. Midlife and Late-Life Blood Pressure and Dementia in Japanese Elderly: The Hisayama Study. Hypertension 2011, 58, 22–28. [Google Scholar] [CrossRef]
- Launer, L.J.; Ross, G.W.; Petrovitch, H.; Masaki, K.; Foley, D.; White, L.R.; Havlik, R.J. Midlife Blood Pressure and Dementia: The Honolulu–Asia Aging Study☆. Neurobiol. Aging 2000, 21, 49–55. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).