Submitted:
24 September 2024
Posted:
25 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain, Media and Chemicals
2.2. Anthraquinone (AQ) Substitutions (Figure 2)
2.3. Optical Measurements
2.4. Models of the Electrostatic Interactions
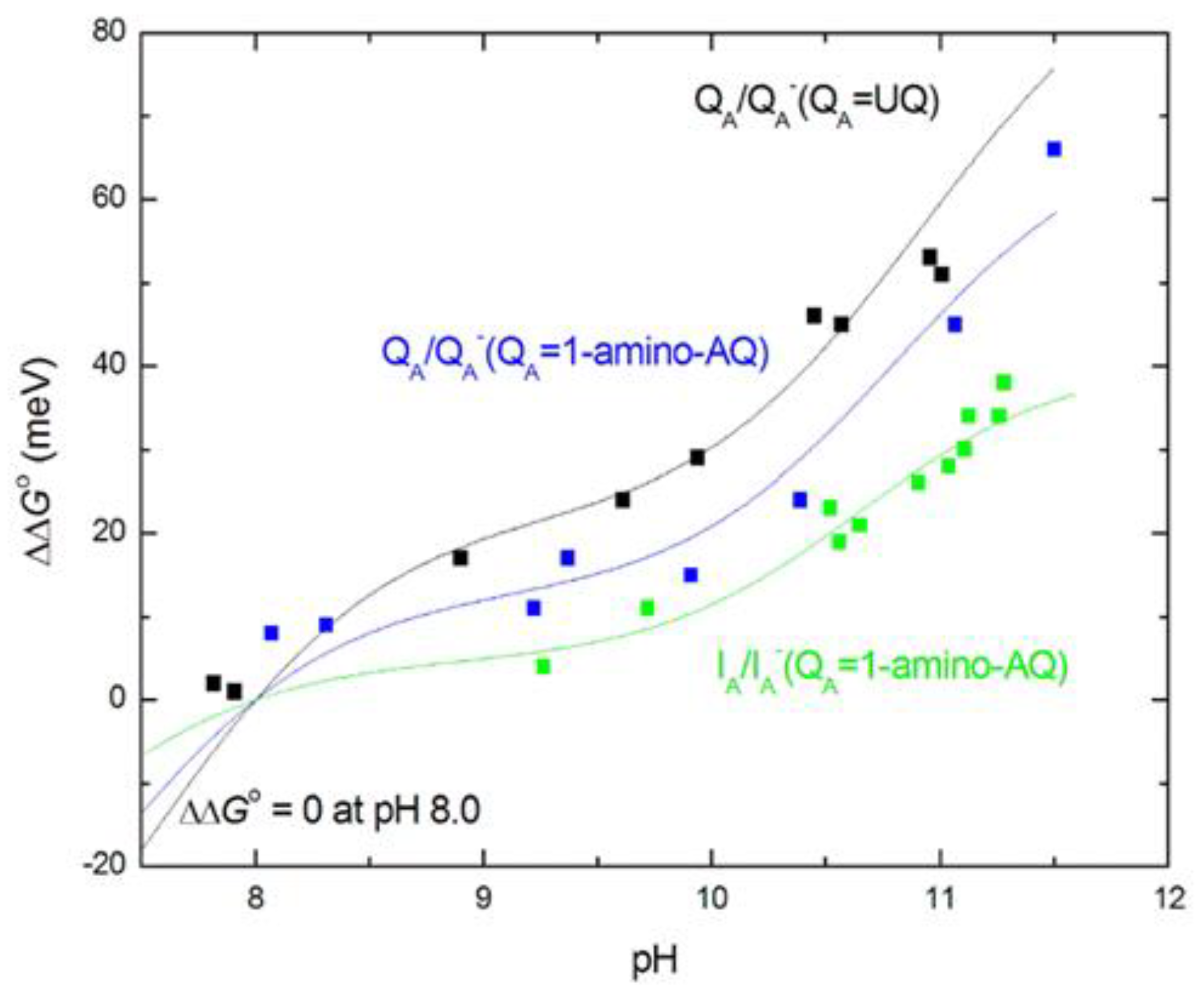
2.4.1. Calculation of the pH-Dependence of the Free Energy Change due to QA/QA‒ and IA/IA‒ Transitions
2.4.2. Screening of the Electrostatic Interaction between Charge Pairs in the RC
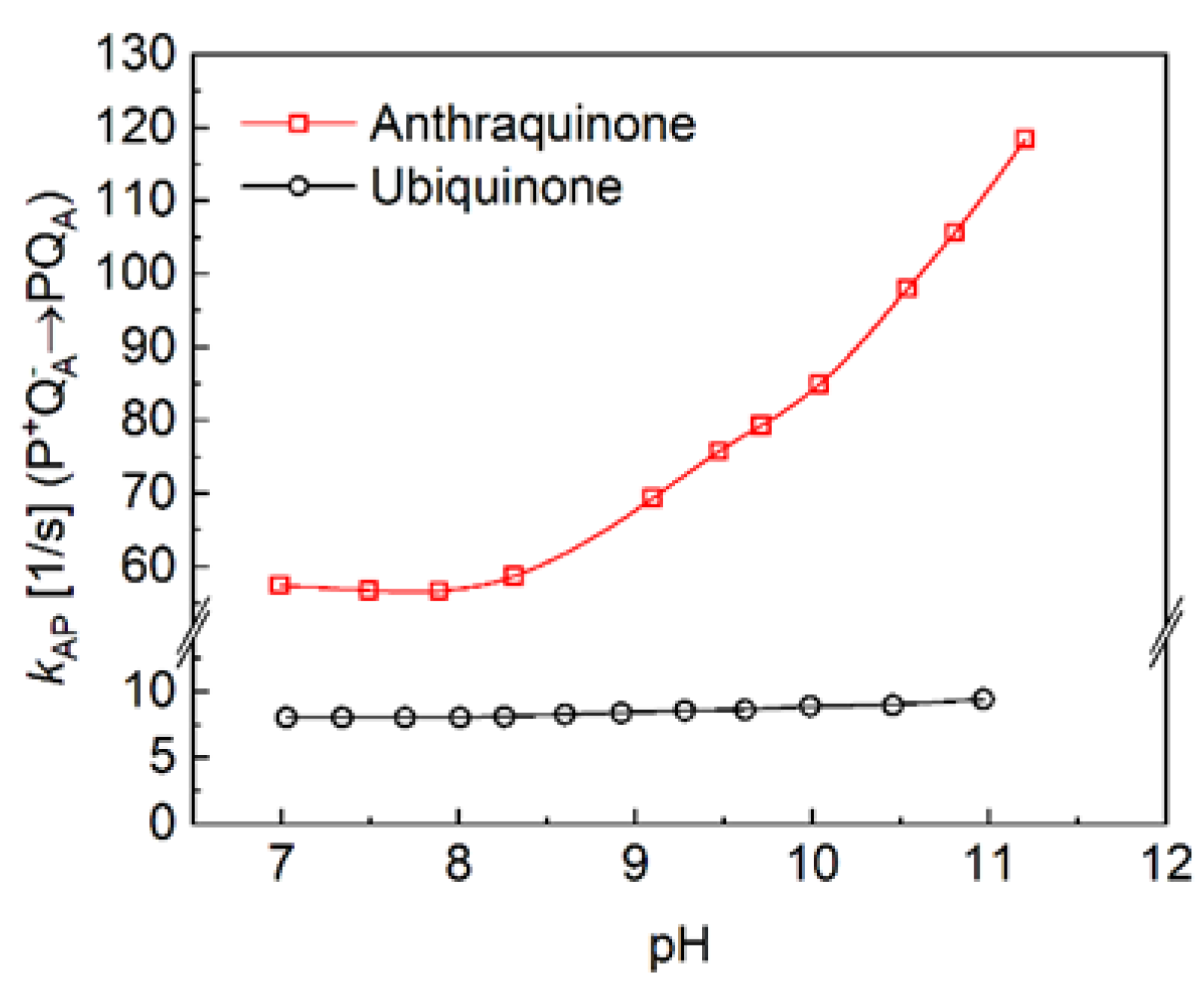
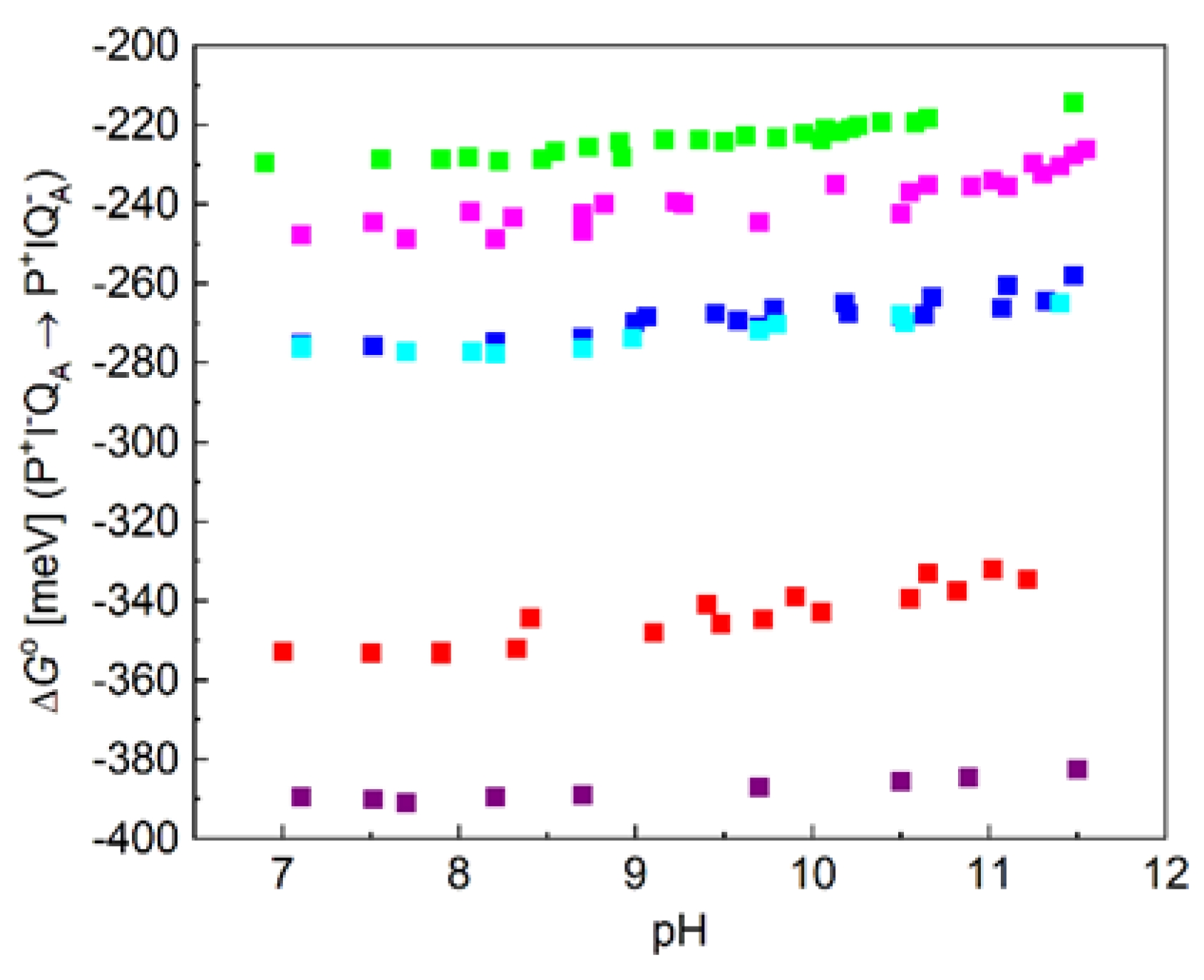
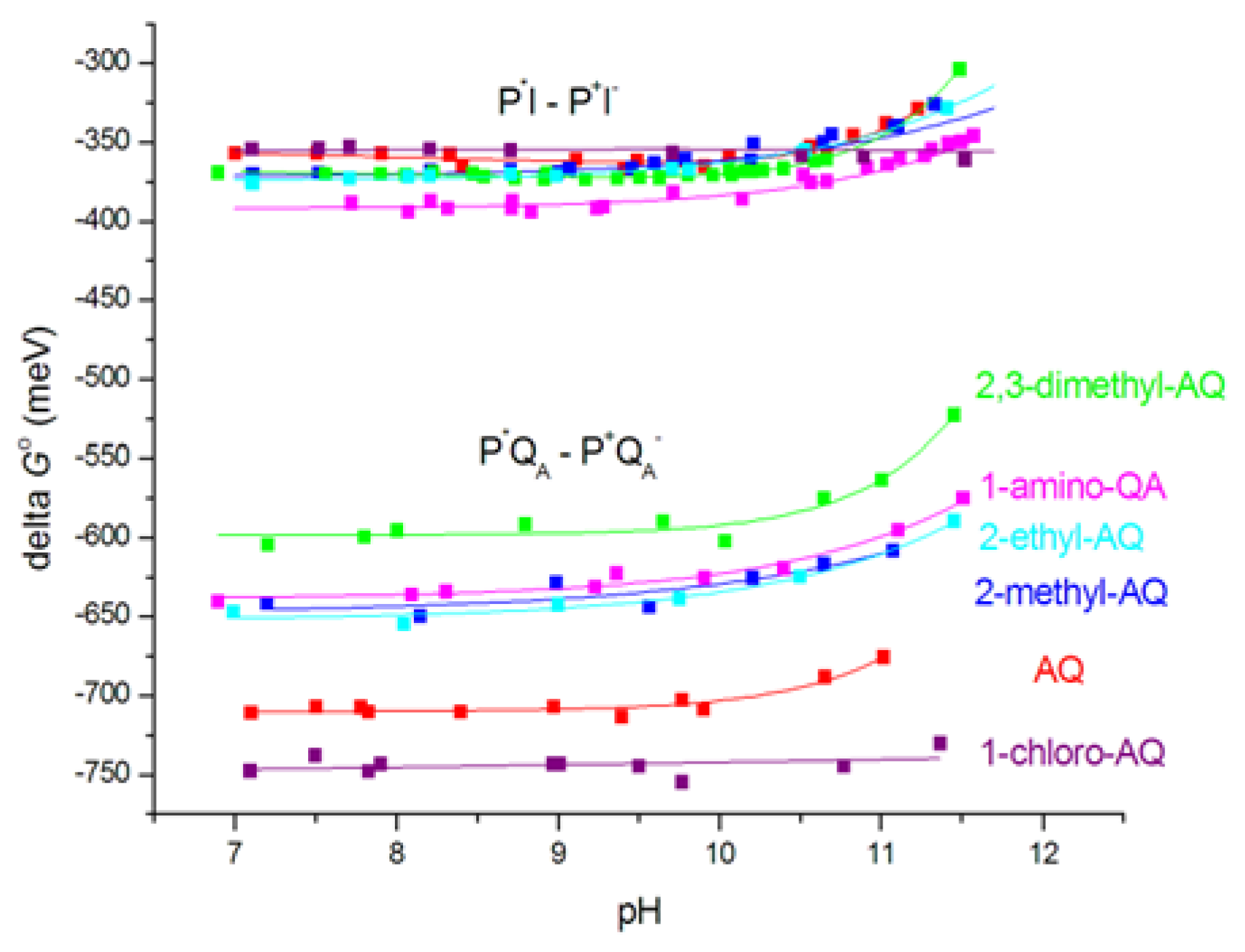
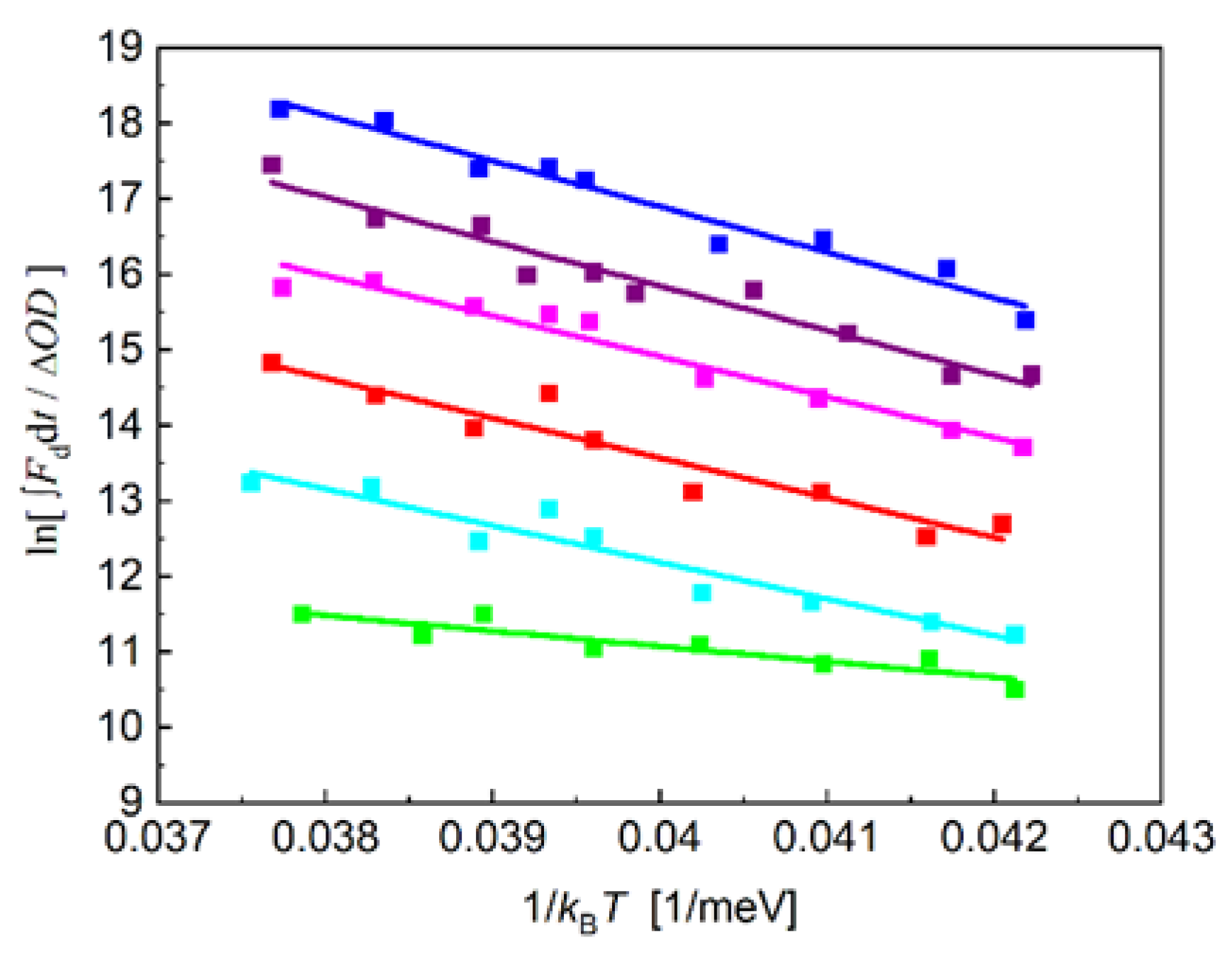
3. Results
4. Discussion
4.1. Interaction of the P+IA− State with the Acidic Cluster at QB
4.1.1. Comparison of the Measured and Calculated Free Energies of QA/QA‒ and IA/IA‒ at High pH
4.1.2. Role of Protein in Determination of the Interaction Energies
4.1.3. Dielectric Relaxation of the Free Energy of the P+IA− State
4.2. Thermodynamics of P+QA─ and P+IA− Formation.
4.3. Comparison of the Rates of Protonation and Electron Transfer Back Reaction
5. Summary
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
References
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 3rd ed.; John Wiley & Sons: 2021.
- Croce, R.; van Grondelle, R.; van Amerongen, H.; van Stokkum, I. (Eds.) Light Harvesting in Photosynthesis; CRC Press: 2018.
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 2016, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Maróti, P.; Kovács, I.A.; Kis, M.; Smart, J.L.; Iglói, F. Correlated clusters of closed reaction centers during induction of intact cells of photosynthetic bacteria. Nature Sci Reports 2020, 10, 14012. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Orcutt, K.; Cook, R.L.; Sabines-Chesterking, J.; Tong, A.L.; Schlau-Cohen, G.; Zhang, X.; Fleming, G.R.; Whaley, K.B. Single-photon absorption and emission from a natural photosynthetic complex. Nature 2023, 619, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Laible, P.D.; Hanson, D.K.; Buhrmaster, J.C.; Tira, G.A.; Faries, K.M.; Holten, D.; Kirmaier, C. Switching sides - Reengineered primary charge separation in the bacterial photosynthetic reaction center. Proc Natl Acad Sci U S A. 2020, 117, 865–871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Cheng, Y.C.; Fleming, G.R. Coherence dynamics in photosynthesis: Protein protection of excitonic coherence. Science 2007, 316, 1462–1465. [Google Scholar] [CrossRef]
- Strümofer, J.; Schulten, K. Excited state dynamics in photosynthetic reaction center and light harvesting complex 1. J. Chem. Phys. 2012, 137. [Google Scholar] [CrossRef]
- Zhu, J.; van Stokkum, I.H.; Paparelli, L.; Jones, M.R.; Groot, M.L. Early bacteriopheophytin reduction in charge separation in reaction centers of Rhodobacter sphaeroides. Biophys. J. 2013, 104, 2493–2502. [Google Scholar] [CrossRef]
- Dubas, K.; Szewczyk, S.; Białek, R.; Burdziński, G.; Jones, M.R.; Gibasiewicz, K. Antagonistic Effects of Point Mutations on Charge Recombination and a New View of Primary Charge Separation in Photosynthetic Proteins. J. Phys. Chem. B. 2021, 125, 8742–8756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parson, W.W.; Warshel, A. Mechanism of Charge Separation in Purple Bacterial Reaction Centers. In: The Purple Phototrophic Bacteria, Eds.: Hunter, C.N.; Daldal, F.; Thurnauer, M.C.; Beatty, J.T. Springer-Verlag, Dordrecht, 2009, pp 355–377.
- Woodbury, N.W.; Parson, W.W.; Gunner, M.R.; Prince, R.C.; Dutton, P.L. Radical-pair energetics and decay mechanisms in reaction centers containing anthraquinones, naphthoquinones or benzoquinones in place of ubiquinone. Biochim. Biophys. Acta 1986, 851, 6–22. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Nishikawa, G.; Sugo, Y.; Saito, K.; Ishikita, H. Absence of electron-transfer-associated changes in the time-dependent X-ray free-electron laser structures of the photosynthetic reaction center. eLife 2023, 12, RP88955. [Google Scholar] [CrossRef] [PubMed]
- Müh, F.; Williams, J.C.; Allen, J.P.; Lubitz, W. A conformational change of the photoactive bacteriopheophytin in reaction centers from Rhodobacter sphaeroides. Biochemistry 1998, 37, 13066–13074. [Google Scholar] [CrossRef] [PubMed]
- Dods, R.; Båth, P.; Morozov, D.; Gagnér, V.A.; Arnlund, D.; Luk, H.L.; Kübel, J.; Maj, M.; Vallejos, A.; Wickstrand, C.; Bosman, R.; Beyerlein, K.R.; Nelson, G.; Liang, M.; Milathianaki, D.; Robinson, J.; Harimoorthy, R.; Berntsen, P.; Malmerberg, E.; Johansso, L.; et al. Ultrafast structural changes within a photosynthetic reaction centre. Nature 2021, 589, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.B.; Lin, C.-Y.; Faries, K.M.; Mathews, I.; Russi, S.; Holten, D.; Kirmaier, C.; Boxer, S. Photosynthetic Reaction Center Variants Made via Genetic Code Expansion Show Tyr at M210 Tunes the Initial Electron Transfer Mechanism. Proc. Nat. Acad. Sci. 2021, 118, e2116439118. [Google Scholar] [CrossRef]
- Saggu, M.; Fried, S.D.; Boxer, S.G. Local and Global Electric Field Asymmetry in Photosynthetic Reaction Centers. J. Phys. Chem. B 2019. [Google Scholar] [CrossRef]
- Williams, J.C.; Allen, J.P. Directed modification of reaction centers from purple bacteria. In: The Purple Phototrophic Bacteria, Eds.: Hunter, C.N.; Daldal, F.; Thurnauer, M.C.; Beatty, J.T., Springer-Verlag, Dordrecht, 2009, pp 337–353.
- Jones, M.R. Structural plasticity of reaction centers from purple bacteria. In: The Purple Phototrophic Bacteria, eds.: Hunter, C.N.; Daldal, F.; Thurnauer, M.C.; Beatty, J.T., Springer-Verlag, Dordrecht, The Netherlands, 2009, pp 295–321.
- Alden, R.; Parson, W.; Chu, Z.; Warshel, A. Orientation of the OH Dipole of Tyrosine (M)210 and its Effect on Electrostatic Energies in Photosynthetic Bacterial Reaction Centers. J. Phys. Chem. 1996, 100, 16761–16770. [Google Scholar] [CrossRef]
- Saggu, M.; Carter, B.; Zhou, X.; Faries, K.; Cegelski, L.; Holten, D.; Boxer, S.G.; Kirmaier, C. Putative hydrogen bond to tyrosine M208 in photosynthetic reaction centers from Rhodobacter capsulatus significantly slows primary charge separation. J. Phys. Chem. B 2014, 118, 6721–6732. [Google Scholar] [CrossRef]
- Pan, J.; Saer, R.G.; Lin, S.; Guo, Z.; Beatty, J.T.; Woodbury, N.W. The Protein Environment of the Bacteriopheophytin Anion Modulates Charge Separation and Charge Recombination in Bacterial Reaction Centers. J. Phys. Chem. B 2013, 117, 7179–7189. [Google Scholar] [CrossRef]
- Pan, J.; Saer, R.; Lin, S.; Beatty, J.T.; Woodbury, N.W. Electron Transfer in Bacterial Reaction Centers with the Photoactive Bacteriopheophytin Replaced by a Bacteriochlorophyll through Coordinating Ligand Substitution. Biochemistry 2016, 55, 4909–4918. [Google Scholar] [CrossRef]
- Sun, C.; Carey, A.M.; Gao, B.R.; Wraight, C.A.; Woodbury, N.W.; Lin, S. Ultrafast Electron Transfer Kinetics in the LM Dimer of Bacterial Photosynthetic Reaction Center from Rhodobacter sphaeroides. J. Phys. Chem. B 2016, 120, 5395–5404. [Google Scholar] [CrossRef] [PubMed]
- Maróti, P.; Wraight, C.A. Flash-induced H+ binding by bacterial photosynthetic reaction centers - comparison of spectrophotometric and conductimetric methods. Biochim. Biophys. Acta 1988, 934, 314–328. [Google Scholar] [CrossRef]
- Kálmán, L.; Maróti, P. Conformation-activated protonation in reaction centers of the photosynthetic bacterium Rhodobacter sphaeroides. Biochemistry 1997, 36, 15269–15276. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.S.; Williams, J.C.; Allen, J.P.; Kálmán, L. Light-induced conformational changes in photosynthetic reaction centers: Dielectric relaxation in the vicinity of the dimer. Biochemistry 2011, 50, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.S.; Kálmán, L. Tuning the redox potential of the primary electron donor in bacterial reaction centers by manganese binding and light-induced structural changes. Biochim. Biophys. Acta - Bioenergetics 2020, 1861, 148285. [Google Scholar] [CrossRef]
- Maróti, P.; Wraight, C.A. Kinetics of H+ ion binding by the P+QA- state of bacterial photosynthetic centers: Rate limitation within the protein. Biophys. J. 1997, 73, 367–381. [Google Scholar] [CrossRef]
- Sebban, P. pH effect on the biphasicity of the P+QA− charge recombination kinetics in the reaction centers from Rhodobacter sphaeroides, reconstituted with anthraquinones. Biochim. Biophys. Acta 1988, 936, 124–132. [Google Scholar] [CrossRef]
- Miksovska, J.; Schiffer, M.; Hanson, D.K.; Sebban, P. Proton uptake by bacterial reaction centers: The protein complex responds in a similar manner to the reduction of either quinone acceptor. P. Natl. Acad. Sci. USA 1999, 96, 14348–14353. [Google Scholar] [CrossRef]
- Wraight, C.A. Proton and electron transfer in the acceptor quinone complex of bacterial photosynthetic reaction centers. Frontiers Biosci. 2004, 9, 309–327. [Google Scholar] [CrossRef]
- Wraight, C.A.; Gunner, M.R. The Acceptor Quinones of Purple Photosynthetic Bacteria - Structure and Spectroscopy. In: The Purple Phototrophic Bacteria, Eds.: Hunter, C.N.; Daldal, F.; Thurnauer, M.C.; Beatty, J.T. Springer-Verlag, Dordrecht, 2009, pp 379-405.
- Okamura, M.Y.; Paddock, M.L.; Graige, M.S.; Feher, G. Proton and electron transfer in bacterial reaction centers, Biochim. Biophys. Acta 2000, 1458, 148–163. [Google Scholar] [CrossRef]
- Maróti, P. Chemical rescue of H+ delivery in proton transfer mutants of reaction center of photosynthetic bacteria. Biochim. Biophys. Acta 2019, 1860, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Maróti, P. Thermodynamic view of proton activated electron transfer in the reaction center of photosynthetic bacteria. J. Phys. Chem. B 2019, 123, 5463–5473. [Google Scholar] [CrossRef] [PubMed]
- Maróti, P. Govindjee Energy conversion in photosynthetic bacteria. Photosynth. Res. 2016, 127, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Nabedryk, E.; Breton, J. Coupling of electron transfer to proton uptake at the Q(B) site of the bacterial reaction center: A perspective from FTIR difference spectroscopy. Biochim. Biophys. Acta 2008, 1777, 1229–1248. [Google Scholar] [CrossRef]
- Sugo, Y.; Saito, K.K.; Ishikita, H.H. Mechanism of the formation of proton transfer pathways in photosynthetic reaction centers. Proc. Natl. Acad. Sci. USA 2021, 118, e2103203118. [Google Scholar] [CrossRef]
- Tiede, D.M.; Hanson, D.K. Protein Relaxation Following Quinone Reduction in Rhodobacter capsulatus - Detection of Likely Protonation-Linked Optical Absorbency Changes of the Chromophores. In: Photosynthetic Bacterial Reaction Center II, Eds.: Breton, J.; Verméglio, A., vol 237. NATO ASI Series (Series A: Life Sciences). Springer, Boston, MA, 1992, pp 341-350. [CrossRef]
- Sipka, G.; Maróti, P. Photoprotection in intact cells of photosynthetic bacteria: Quenching of bacteriochlorophyll fluorescence by carotenoid triplets. Photosynth. Res. 2018, 136, 17–30. [Google Scholar] [CrossRef]
- Okamura, M.Y.; Isaacson, R.A.; Feher, G. Primary acceptor in bacterial photosynthesis: Obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. P. Natl. Acad. Sci. USA 1975, 72, 3491–3495. [Google Scholar] [CrossRef]
- Liu, B.L.; Yang, L.H.; Hoff, A.J. On the depletion and reconstitution of both QA and metal in reaction centers of the photosynthetic bacterium Rb. sphaeroides R-26. Photosynth. Res. 1991, 28, 51–58. [Google Scholar] [CrossRef]
- Kálmán, L.; Maróti, P. Stabilization of reduced primary quinone by proton uptake in reaction centers of Rhodobacter sphaeroides. Biochemistry 1994, 33, 9237–9244. [Google Scholar] [CrossRef]
- Xu, Q.; Gunner, M.R. Temperature dependence of the free energy, enthalpy, and entropy of P+QA‒ charge recombination in Rhodobacter sphaeroides R-26 reaction centers. J. Phys. Chem. B, 2000, 104, 8035–8043. [Google Scholar] [CrossRef]
- Turzó, K.; Laczkó, G.; Filus, Z.; Maróti, P. Quinone-dependent delayed fluorescence from the reaction center of photosynthetic bacteria. Biophys. J. 2000, 79, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Rinyu, L.; Martin, E.W.; Takahashi, E.; Maróti, P.; Wraight, C.A. Modulation of the free energy of the primary quinone acceptor (QA) in reaction centers from Rhodobacter sphaeroides: Contributions from the protein and protein-lipid(cardiolipin) interactions. Biochim. Biophys. Acta-Bioenergetics 2004, 1655, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Onidas, D.; Sipka, G.; Asztalos, E.; Maróti, P. Mutational control of bioenergetics of bacterial reaction center probed by delayed fluorescence. Biochim. Biophys. Acta 2013, 1827, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.; Maróti, P. Delayed fluorescence in anoxygenic photosynthesis. Photochemistry, 2024, 52, 329–343. [Google Scholar]
- Maróti, P. Bacteriochlorophyll Fluorescence as a Monitor of Electronic States and Processes in Photosynthetic Bacteria, In: Handbook of Photosynthesis, ed. Pessarakli, M., CRC Press, Boca Raton, 2025, pp. 359–387.
- Filus, Z.; Laczkó, G.; Wraight, C.A.; Maróti, P. Delayed fluorescence from the photosynthetic reaction center measured by electronic gating of the photomultiplier. Biopolymers 2004, 74, 92–95. [Google Scholar] [CrossRef]
- Arata, H.; Parson, W.W. Delayed fluorescence from Rhodopseudomonas sphaeroides reaction centers: Enthalpy and free energy changes accompanying electron transfer from P870 to quinones. Biochim. Biophys. Acta 1981, 638, 201–209. [Google Scholar] [CrossRef]
- Maróti, Á.; Wraight, C.A.; Maróti, P. The rate of second electron transfer to QB− in bacterial reaction center of impaired proton delivery shows hydrogen-isotope effect. Biochim. Biophys. Acta 2015, 1847, 223–230. [Google Scholar] [CrossRef]
- Kaur, D.; Khaniya, U.; Zhang, Y.; Gunner, M.R. Protein Motifs for Proton Transfers That Build the Transmembrane Proton Gradient. Front. Chem. 2021, 9, 660954. [Google Scholar] [CrossRef]
- Khaniya, U.; Mao, J.; Wie, R.; Gunner, M.R. Characterizing protein protonation microstates using Monte Carlo sampling, Biorxiv. org 2022. [Google Scholar] [CrossRef]
- Maróti, Á.; Wraight, C.A.; Maróti, P. Protonated rhodosemiquinone at the QB binding site of the M265IT mutant reaction center of photosynthetic bacterium Rhodobacter sphaeroides. Biochemistry 2015, 54, 2095–2103. [Google Scholar] [CrossRef]
- Cheap, H.; Tandori, J.; Derrien, V.; Benoit, M.; de Oliveira, P.; Köpke, J.; Lavergne, J.; Maróti, P.; Sebban, P. Evidence for delocalized anticooperative flash induced proton binding as revealed by mutants at the M266His iron ligand in bacterial reaction centers. Biochemistry 2007, 46, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- Gilson, M.K.; Rashin, A.; Fine, R.; Honig, B. On the Calculation of Electrostatic Interactions in Proteins. J. Mol. Biol. 1985, 183, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Bossa, G.V.; May, S.S. Integral Representation of Electrostatic Interactions inside a Lipid Membrane. Molecules 2020, 25, 3824. [Google Scholar] [CrossRef] [PubMed]
- Feher, G.; Arno, T.R.; Okamura, M.Y. The Effect of an Electric Field on the Charge Recombination Rate of D+Q−A → DQA in Reaction Centers from Rhodobacter sphaeroides R-26. In: The Photosynthetic Bacterial Reaction Center (Eds.: Breton, J. et al.) 1988, pp 271-287. [CrossRef]
- Gunner, M.R.; Dutton, P.L. Temperature and ‒ΔGo dependence of the electron transfer from BPh‒ to QA in reaction center protein from Rhodobacter sphaeroides with different quinones as QA. J. Am. Chem. Soc. 1989, 11, 3400–3412. [Google Scholar] [CrossRef]
- Mccomb, J.C.; Stein, R.R.; Wraight, C.A. Investigations on the Influence of Headgroup Substitution and Isoprene Side-Chain Length in the Function of Primary and Secondary Quinones of Bacterial Reaction Centers. Biochim. Biophys. Acta 1990, 1015, 156–171. [Google Scholar] [CrossRef]
- Schelvis, J.P.M.; Liu, B.L.; Aartsma, T.J.; Hoff, A.J. The Electron-Transfer Rate from Bpha‒ to Qa in Reaction Centers of Rhodobacter-Sphaeroides R-26 - Influence of the H-Subunit, the Qa and Fe2+ Cofactors, and the Isoprene Tail of Qa. Biochim. Biophys. Acta 1992, 1102, 229–236. [Google Scholar] [CrossRef]
- Kleinfeld, D.; Okamura, M.Y.; Feher, G. Charge recombination kinetics as a probe of protonation of the primary acceptor in photosynthetic reaction centers. Biophys. J. 1985, 48, 849–852. [Google Scholar] [CrossRef]
- Maróti, P.; Wraight, C.A. The redox midpoint potential of the primary quinone of reaction centers in chromatophores of Rhodobacter sphaeroides is pH independent. Eur. Biophys. J. 2008, 37, 1207–1217. [Google Scholar] [CrossRef]
- Woodbury, N.W.; Parson, W.W. Nanosecond fluorescence from isolated photosynthetic reaction centers of Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta 1984, 767, 345–361. [Google Scholar] [CrossRef]
- Peloquin, J.M.; Williams, J.C.; Lin, X.; Alden, R.G.; Taguchi, A.K.W.; Allen, J.P.; Woodbury, N.W. Time-Dependent Thermodynamics During Early Electron-Transfer in Reaction Centers from Rhodobacter Sphaeroides. Biochemistry 1994, 33, 8089–8100. [Google Scholar] [CrossRef]
- Woodbury, N.; Peloquin, J.M.; Alden, R.G.; Lin, X.; Lin, S.; Taguchi, A.K.W.; Williams, J.; Allen, J. Relationship between Thermodynamics and Mechanism during Photoinduced Charge Separation in Reaction Centers from Rhodobacter sphaeroides. Biochemistry, 1994, 33, 8101–8112. [Google Scholar] [CrossRef] [PubMed]
- Chamorovsky, C.S.; Chamorovsky, S.K.; Semenov, A.Y. Dielectric and photoelectric properties of photosynthetic reaction centers. Biochemistry (Moscow) 2005, 70, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, C.R.D.; Michel, H. The coupling of light-induced electron transfer and proton uptake as derived from crystal structures of reaction centres from Rhodopseudomonas viridis modified at the binding site of the secondary quinone, QB. Structure 1997, 5, 1339–1359. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.J.; Zhang, Y.; Mao, J.; Kaur, D.; Khaniya, U.; Gunner, M.R. Comparison of Proton Transfer Paths to the QA and QB Sites of the Rb. sphaeroides Photosynthetic Reaction Centers. Photosynth. Res. 2022. [Google Scholar] [CrossRef]
- Fritzsch, G.; Ermler, U.; Michel, H. The water chains around QA and QB and other structural aspects of the reaction center from Rb. sphaeroides. In: Photosynthesis: From Light to Biosphere. Ed., Mathis, P., Kluwer Academic Publishers, Dordrecht, 1995, pp 599-602.
- Tandori, J.; Maróti, P.; Alexov, E.; Sebban, P.; Baciou, L. Key role of proline L209 in connecting the distant quinone pockets in the reaction center of Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. USA 2002, 99, 6702–6706. [Google Scholar] [CrossRef]
- Johnson, E.T.; Parson, W.W. Electrostatic interactions in an integral membrane protein. Biochemistry 2002, 41, 6483–6494. [Google Scholar] [CrossRef]
- Maróti, P.; Hanson, D.K.; Baciou, L.; Schiffer, M. ; Sebban, Proton conduction within the reaction centers of Rhodobacter capsulatus: The electrostatic role of the protein. P. Proc. Natl. Acad. Sci. USA 1994, 91, 5617–5621. [Google Scholar] [CrossRef]
- Hienerwadel, R.; Grzybek, S.; Fogel, C.; Kreutz, W.; Okamura, M.Y.; Paddock, M.L.; Breton, J.; Nabedryk, E.; Mantele, W. Protonation of Glu L212 following QB‒ formation in the photosynthetic reaction center of Rhodobacter sphaeroides: Evidence from time-resolved infrared spectroscopy. Biochemistry 1995, 34, 2832–2843. [Google Scholar] [CrossRef]
- Zhu, Z.; Gunner, M.R. Energetics of quinone-dependent electron and proton transfers in Rhodobacter sphaeroides photosynthetic reaction centers. Biochemistry 2005, 44, 82–96. [Google Scholar] [CrossRef]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Research, 2018, 46, (W1), pp W363–W367. [CrossRef]
- Parson, W.W.; Chu, Z.T.; Warshel, A. Electrostatic Control of Charge Separation in Bacterial Photosynthesis. Biochim Biophys Acta 1990, 1017, 251–272. [Google Scholar] [CrossRef]
- Hou, H.J.M. Enthalpy, Entropy, and Volume Changes of Electron Transfer Reactions in Photosynthetic Proteins. In: Application of Thermodynamics to Biological and Materials Science, Ed.: Tadashi, M. 2011, InechOpen, pp.93-110.
- Flores, M.; Savitsky, A.; Paddock, M.L.; Abresch, E.C.; Dubinskii, A.A.; Okamura, M.Y.; Lubitz, W.; Mobius, K. Electron-nuclear and electron-electron double resonance spectroscopies show that the primary quinone acceptor Q(A) in reaction centers from photosynthetic bacteria Rhodobacter sphaeroides remains in the same orientation upon light induced reduction. J. Phys. Chem. B 2010, 114, 16894–16901. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Gupta, K.B.; Alia, A.; Buda, F.; de Groot, H.J.M.; Matysik, J. Bacteriopheophytin a in the Active Branch of the Reaction Center of Rhodobacter sphaeroides Is Not Disturbed by the Protein Matrix as Shown by 13C Photo-CIDNP MAS NMR. J. Phys. Chem. B 2013, 117, 3287–3297. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Isaacson, R.; Abresch, E.; Calvo, R.; Lubitz, W.; Feher, G. Protein-cofactor interactions in bacterial reaction centers from Rhodobacter sphaeroides R-26: II. Geometry of the hydrogen bonds to the primary quinone QA.− by 1H and 2H ENDOR spectroscopy. Biophys. J. 2006, 90, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Kuglstatter, A.; Ermler, U.; Michel, H.; Baciou, L.; Fritzsch, G. Structure of the photosynthetic reaction center from Rhodobacter sphaeroides reconstituted with anthraquinone as primary quinone QA. FEBS Letter 2000, 472, 114–116. [Google Scholar] [CrossRef]
- Krishtalik, L.I. Role of the protein's low dielectric constant in the functioning of the photosynthetic reaction center. Photosynth. Res. 1999, 60, 241–246. [Google Scholar] [CrossRef]
- Gibasiewicz, K.; Pajzderska, M.; Białek, R.; Jones, M.R. Temperature dependence of nanosecond charge recombination in mutant Rhodobacter sphaeroides reaction centers: Modelling of the protein dynamics. Photochemical & Photobiological Sciences 2021, 20, 913–922. [Google Scholar] [CrossRef]
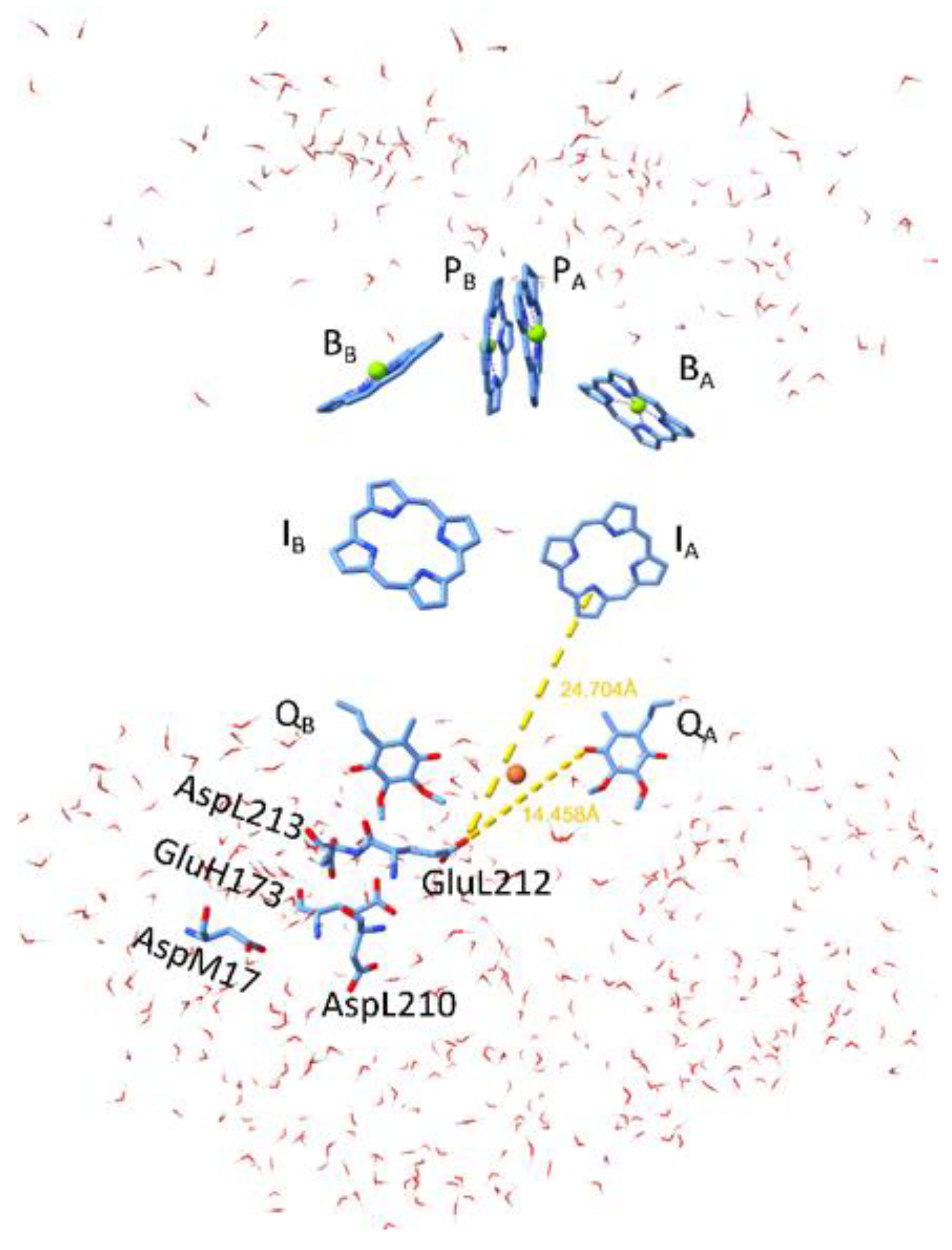
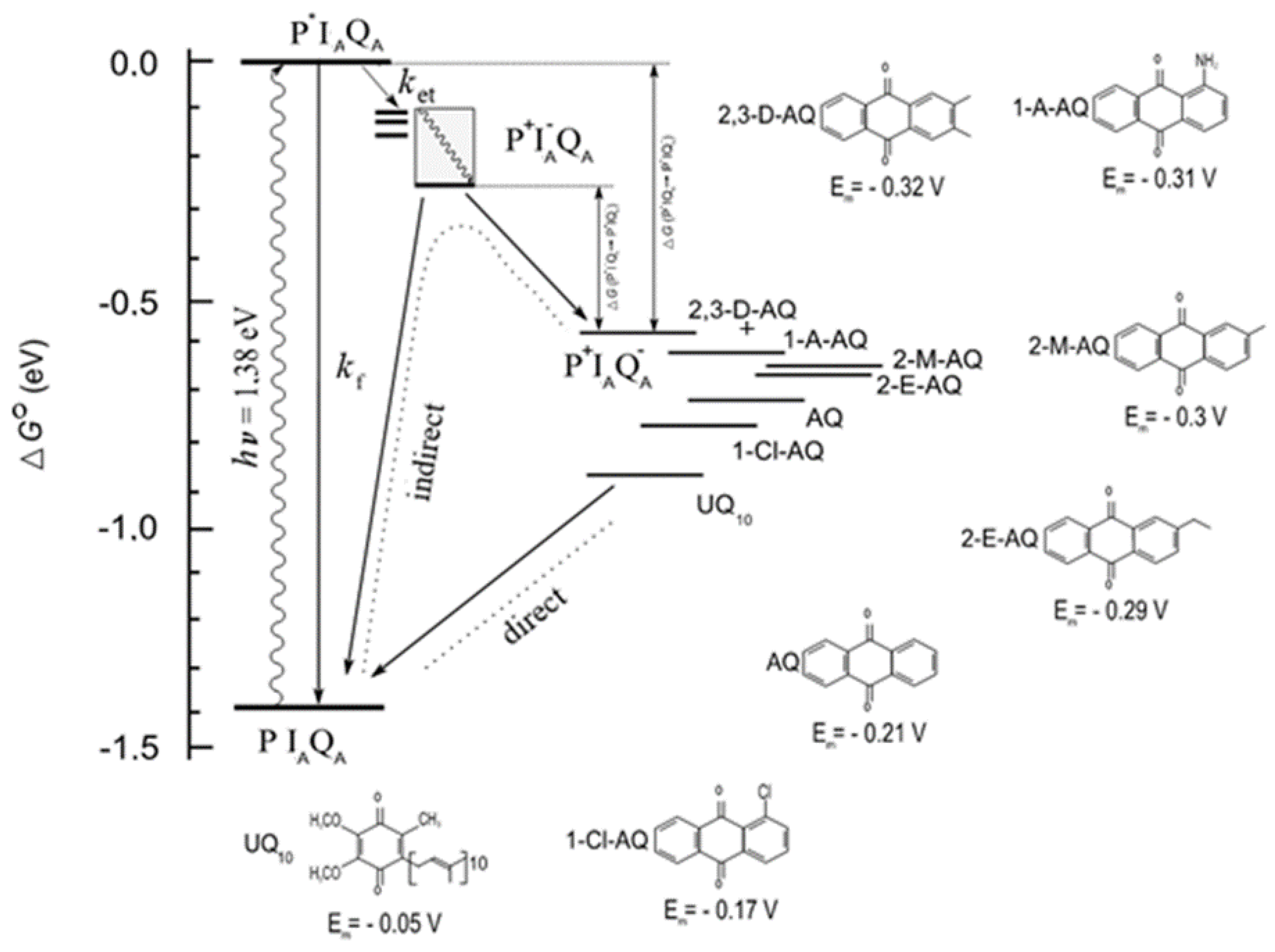
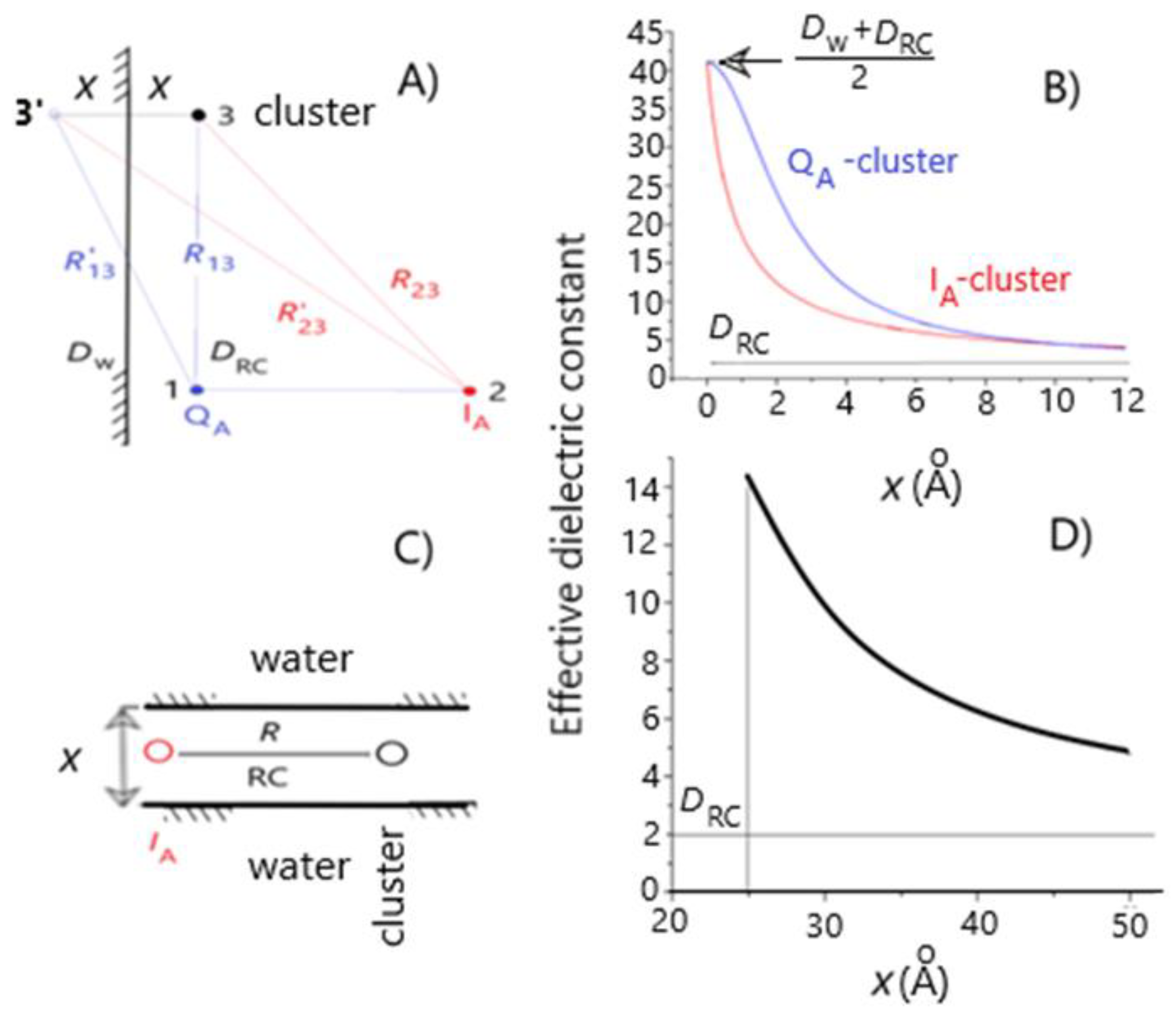
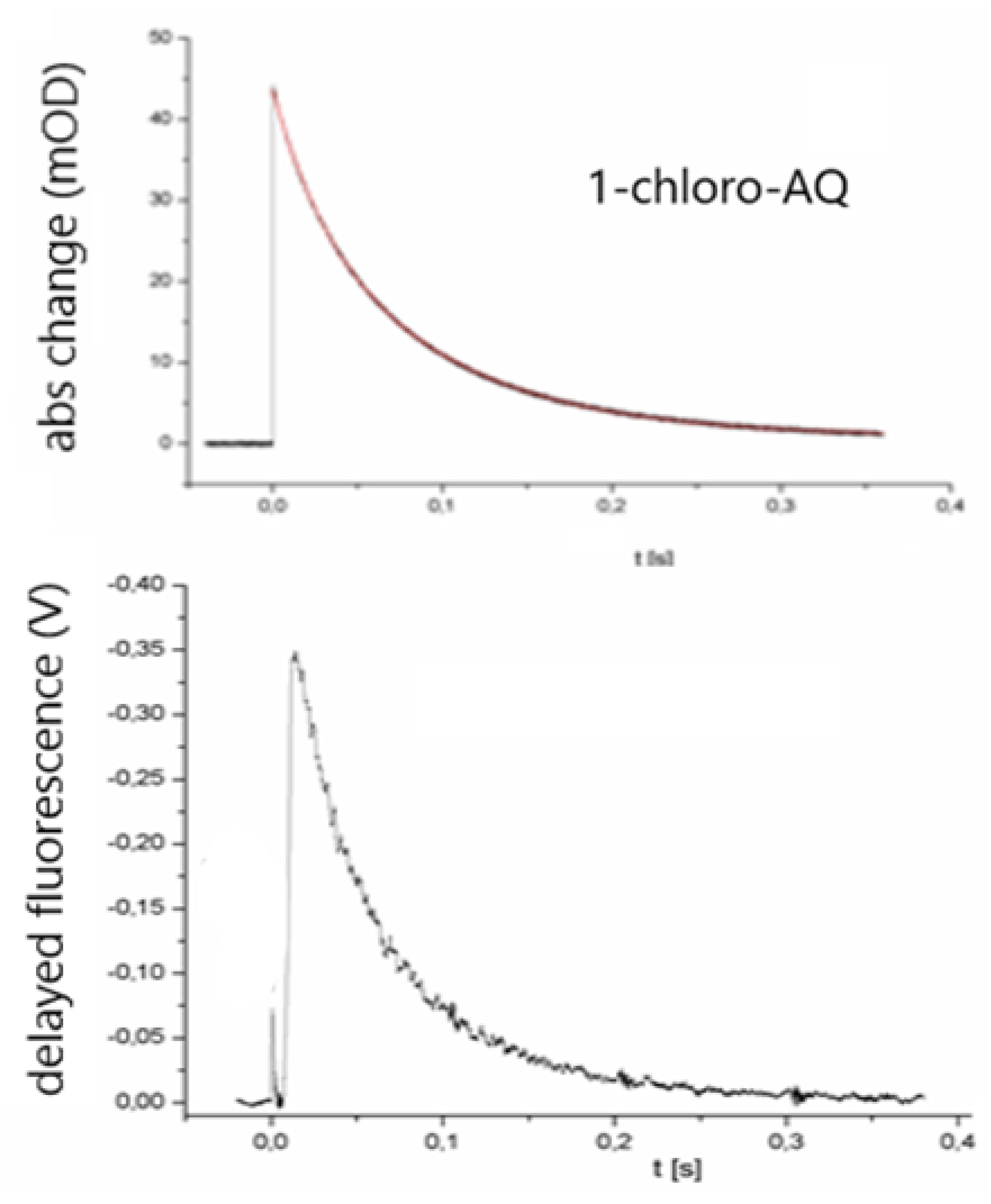
| P* → P+QA− | IA‒QA → IAQA− | |||||
|---|---|---|---|---|---|---|
| QA | ∆Go (meV) | ∆Ho (meV) | T∙∆So (meV) | ∆Go (meV) | ∆Ho (meV) | T∙∆So (meV) |
| Ubiquinone10 | -910 | -830 | +80 | |||
| 2-Methyl-AQ | -625 | -605 | +20 | -357 | -330 | +27 |
| 1-Amino-AQ | -625 | -535 | +90 | -390 | -273 | +17 |
| AQ | -700 | -585 | +115 | -360 | -267 | +93 |
| 1-Chloro-AQ | -750 | -600 | +150 | -363 | -410 | ‒55 |
| 2-Ethyl-AQ | -635 | -485 | +150 | -368 | -188 | +180 |
| 2,3-Dimethyl-AQ | -585 | -205 | +380 | -365 | -72 | +293 |
| GluL212 | AspL213 | AspM17 | GluH173 | ||
| GluL212 | ‒ | (9.65) | (13.81) | (8.56) | |
| AspL213 | ‒168 | ‒ | (8.46) | (10.0) | |
| AspM17 | ‒118 | ‒192 | ‒ | (9.28) | |
| GluH173 | ‒190 | ‒162 | ‒174 | ‒ | |
| UQ | QA | (14.46) | (22.42) | (25.69) | (16.74) |
| ‒96 | ‒61 | ‒54 | ‒83 | ||
| BPheoA | (25.20) | (30.91) | (35.63) | (27.11) | |
| ‒ | ‒ | ‒ | ‒ | ||
| 1-amino-AQ | QA | ‒75 | ‒48 | ‒42 | ‒65 |
| BPheoA | ‒41 | ‒34 | ‒30 | ‒38 | |
| 2-3 dimethyl-AQ | QA | ‒84 | ‒54 | ‒47 | ‒72 |
| BPheoA | ‒14 | ‒11 | ‒10 | ‒13 | |
| AQ | QA | ‒48 | ‒31 | ‒27 | ‒41 |
| BPheoA | ‒45 | ‒37 | ‒32 | ‒41 | |
| 2-methyl-AQ | QA | ‒84 | ‒54 | ‒47 | ‒72 |
| BPheoA | ‒55 | ‒45 | ‒39 | ‒51 | |
| 2-ethyl-AQ | QA | ‒87 | ‒56 | ‒49 | ‒75 |
| BPheoA | ‒53 | ‒44 | ‒38 | ‒49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





