1. Introduction
Chronic diseases, characterized by their long-term progressive nature and ongoing physical, psychological, and social impact, impose a significant and ever-growing financial and emotional burden on individuals, families, communities, and healthcare systems worldwide [
1]. Among these chronic conditions, dementia—a progressive syndrome characterized by a decline in cognitive function that interferes with daily life—is poised to become a major social and economic challenge in the near future [
2]. With new cases emerging at an alarming rate, nearly one every 3 seconds according to Alzheimer’s Disease International (ADI), the World Health Organization estimates a staggering global total of 139 million people living with dementia by 2050. Notably, Alzheimer’s disease (AD), a neurodegenerative disorder characterized by the buildup of amyloid plaques and tau tangles in the brain, is believed to be responsible for a significant portion, with estimates ranging from 60-70% of all dementia cases [
3,
4]. This translates to millions of individuals worldwide grappling with memory loss, impaired judgment, and a decline in daily living activities, placing a tremendous strain on caregivers and healthcare systems.
However, a glimmer of hope emerges amidst this growing challenge. Artificial intelligence (AI) is making significant strides in the healthcare field, and its potential to predict Alzheimer’s disease earlier holds immense promise for alleviating the burden of this devastating disease [
5]. By enabling earlier detection and intervention, AI-powered solutions could significantly improve patient outcomes, potentially slow disease progression, and allow for better management of symptoms. This could enhance the quality of life for patients and their families and reduce the immense financial strain placed on healthcare systems globally.
FDG PET is the most sensitive modality for the early detection and investigation of AD [
6]. Therefore, quantitative analysis and understanding of imaging biomarkers can give more comprehensive information on an individual patient’s condition. Radiomics allows us to obtain imaging features that the naked eye cannot detect. Therefore, by analyzing these features (imaging biomarkers) it is possible to gain a deeper insight into the disease [
7]. By prioritizing explainability and transparency, radiomics helps to build trust in AI-driven decision-making, ultimately leading to better patient outcomes [
8,
9]. In our previous work, we showed that these biomarkers help us detect AD accurately, predict amyloid positivity, determine severity and stage, and monitor progress in time [
10,
11,
12]. In this way, a single test can give us a mine of information and understanding of the particular characteristics on an individual level.
In addition to the well-known pathological hallmarks of AD, amyloid-β (Aβ) and neurofibrillary tangles composed of tubulin-associated unit (Tau) protein, genetics plays another critical role in the underlying disease process [
13,
14,
15]. Since its discovery in 1993 [
16], the apolipoprotein ε4 allele (ApoE4) has consistently emerged as the single most potent genetic risk factor for AD across the entire human genome [
17]. The presence of the ApoE4 allele significantly elevates an individual’s risk of developing AD. Individuals with two copies of the ApoE4 allele (homozygous ApoE4) have approximately a 12-fold increased risk of developing AD compared to those with two copies of the ε3 allele [
18]. Those with one copy of the ApoE4 allele (heterozygotes ApoE4) have a lower risk, and individuals without the ApoE4 gene have a much lower risk of developing Alzheimer’s [
19].
The objective of this paper is to extend the characterization of AD-suspected patients by studying the association between imaging features and the ApoE4 allele. This can lead us to the following benefits: (i) To predict ApoE4 carrier status from FDG PET scan but more importantly (ii) to determine the significant brain regions and their calculated image features. This can help us not only have a better understanding of the disease but also point to new future research on a biological level for deciphering pathological mechanisms and discovering new targeted drugs.
2. Method
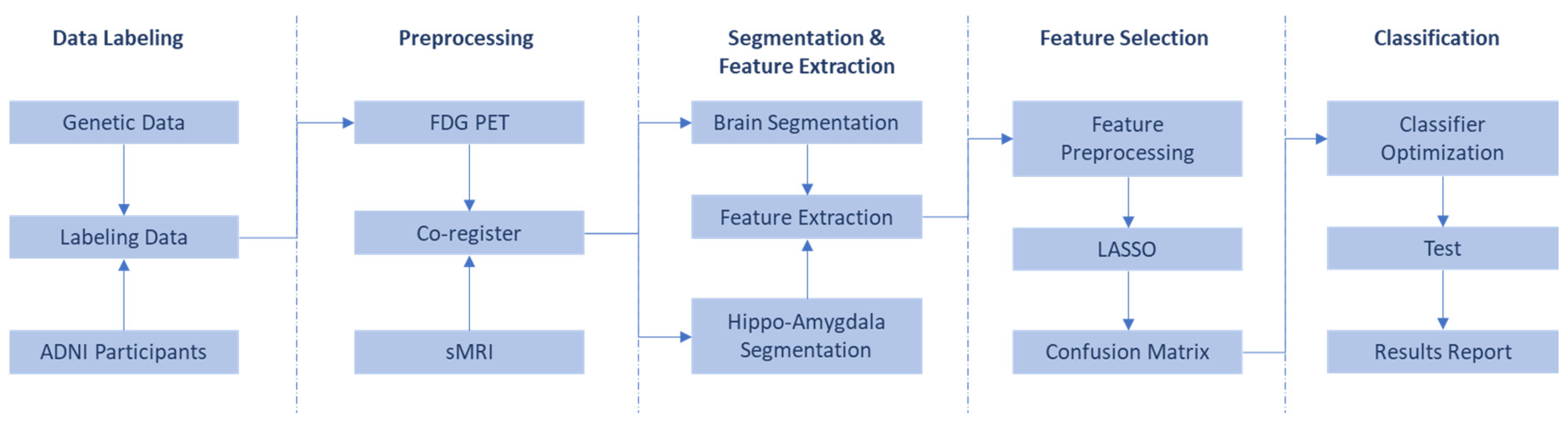
Our study proposes a radiomics-based approach to predict the presence of the homozygous ApoE4 genotype using FDG PET scans. Radiomics, a field extracting quantitative features from medical images, offers a rich data source for exploring biological underpinnings. Our meticulous workflow involves several key stages: data selection with labeling, co-registration for image alignment, segmentation to isolate brain regions, feature extraction to quantify image characteristics, feature selection to identify the most informative features, and finally, model building and evaluation (as detailed in
Figure 1). Rigorous metrics will assess the model’s performance and generalizability to unseen data. By following these steps, we aim to develop a robust and generalizable model for homozygous ApoE4 genotype prediction using FDG PET, with detailed explanations provided in subsequent sections.
2.1. ADNI and Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD.
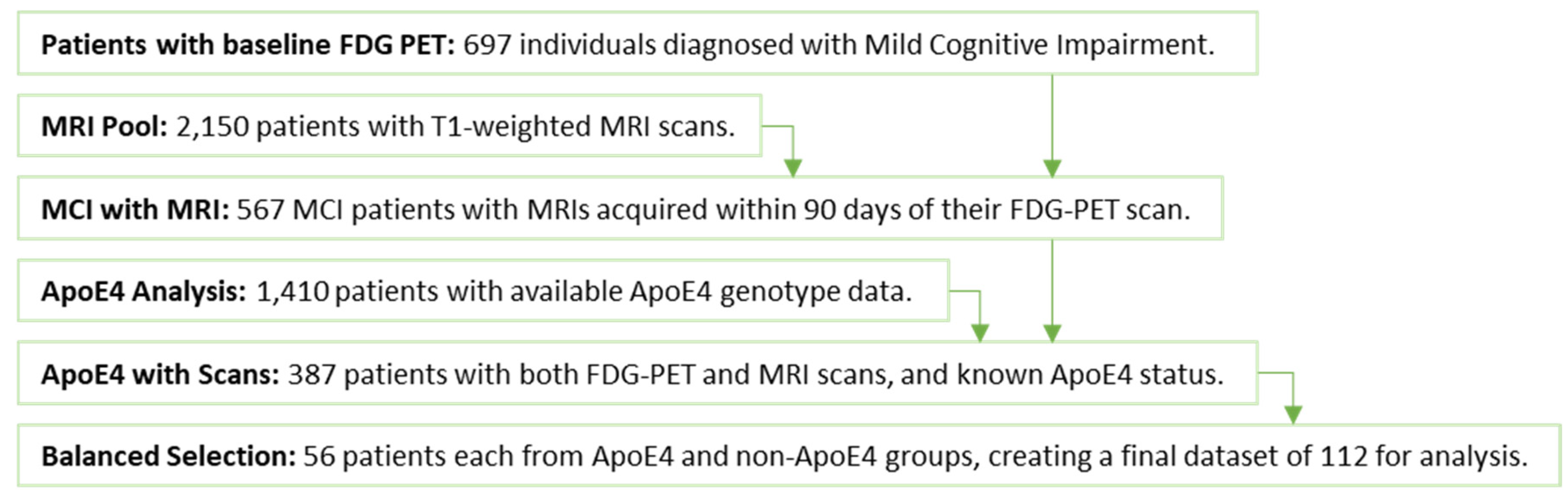
We studied 697 MCI patients with baseline FDG PET scans, selecting 567 structural MRI (sMRI) scans acquired within 90 days of their corresponding FDG PET scans. To investigate the relationship of brain metabolism and structure with the ApoE4 genotype, we focused on a subset of 387 patients who had both baseline FDG PET and sMRI scans along with ApoE4 genotype information. This group included 56 individuals with the homozygous ApoE4 genotype and 331 without (ApoE non-ε4 carriers). To create a balanced dataset for analysis, we randomly selected 56 patients with ApoE non-ε4 carriers. This resulted in a final group of 112 patients with a balanced number of individuals possessing the homozygous ApoE4 genotype and those lacking the ε4 allele entirely (refer to
Figure 2 for details on participant distribution). Additional clinical characteristics of the participants can be found in
Table 1.
2.2. ApoE Genotyping
ApoE genotyping was performed to assess AD risk in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study (adni.loni.usc.edu). While the main genotyping platform for ADNI utilized Illumina BeadChips (Illumina BeadStudio 3.2 software for initial analysis, later updated to Illumina GenomeStudio v2009.1), the specific ApoE alleles (ε2, ε3, and ε4) were not included on the Human610-Quad BeadChip used. Therefore, DNA was extracted from blood samples, and specific SNPs (rs429358 and rs7412) defining the ApoE alleles were genotyped using a separate method.
2.3. FDG PET Acquisition
Our study used FDG PET scans that involved injecting participants with 185 MBq (5 mCi) of FDG and then performing a dynamic 3D scan consisting of six 5-minute frames acquired 30-60 minutes post-injection. ADNI provides four processed formats for FDG PET scans, ensuring compatibility between scanners and simplifying analysis. We used 3rd type preprocessed FDG PET scans (CO-REG, AVG, STANDARDIZED IMAGE, AND VOXEL SIZE) which each subject co-registered averaged image from their baseline FDG PET scan is then reoriented into a standard 160×160×96 voxel image grid, having 1.5 mm cubic voxels. The image grid is aligned with the subject’s anterior-posterior axis parallel to the anterior and posterior commissure (AC-PC) line. Each FDG PET scan, including the baseline and all subsequent scans, is co-registered to this baseline image. An average image is created from the AC-PC co-registered frames and intensity is normalized using a subject-specific mask [
20].
2.4. MRI Acquisition
The ADNI project has collected a wide range of sMRI data across its phases (1/GO/2/3). While sMRI acquisition protocols have evolved, the initiative provides standardized datasets to address variations in sMRI scanners between sites and ensure consistency for research. ADNI 3 exclusively uses 3T scanners and offers two tiers (“Basic” and “Advanced”) to manage this variability. Our study employed standardized sMRI data, specifically T1-weighted 3D-MRI acquired with a sagittal MP-RAGE plane and a 1.2mm slice thickness. These datasets were developed to promote data analysis consistency and facilitate researcher use [
21].
2.5. Co-Registration
Our study addressed the challenge of aligning information from FDG PET and sMRI scans using the Advanced Normalization Tools (ANTs), a free and open-source software toolkit. ANTs’ tool is highly effective for image registration, correcting spatial discrepancies between scans. We specifically used ANTs’ rigid registration to address basic misalignments such as translation and rotation, ensuring precise spatial matching between the FDG PET and sMRI scans[
22].
2.6. Segmentation
Our study leveraged FreeSurfer’s (FS) extensive segmentation capabilities to analyze various brain regions. FS version 7.1 provided automated segmentation of the whole brain into 95 regions using the DKT-Atlas [
23]. Additionally, it performed segmentation for the hippocampus subfields and amygdala nuclei. The hippocampal segmentation employed a probabilistic atlas built from high-resolution ex vivo scans and standard-resolution in vivo images. This method classified voxels within the hippocampus into 19 subfields, including CA1, dentate gyrus, and the fimbria. Similarly, the amygdala segmentation utilized an atlas derived from high-resolution ex vivo data to parcellate the amygdala into 9 subnuclei [
24,
25].
2.7. Feature Extraction
To address the challenge of obtaining standard features in radiomics, we utilized PyRadiomics an open-source platform that aligns with the International Biomarker Standardization Initiative (IBSI) [
26]. This platform simplifies the extraction of a wide variety of features from medical images. PyRadiomics provides dedicated tools for analyzing first-order statistics, shapes, and textures using methods like gray-level co-occurrence matrices, gray-level run-length matrices, and gray-level size zone matrices. Importantly, it allows feature extraction from both individual slices (2D) and entire volumes (3D) across various imaging modalities. This standardized workflow within PyRadiomics promotes consistency and reproducibility, enabling researchers to effectively compare findings across different studies [
27].
2.8. Feature Selection
Feature redundancy is a significant issue in radiomics that leads to overfitting in classification models. To address this, we implemented a comprehensive feature selection approach involving both preprocessing and advanced selection methods. Initially, we reduced the dimensionality by eliminating constant, quasi-constant, and duplicated features. Subsequently, we applied the LASSO (Least Absolute Shrinkage and Selection Operator) [
28] method, an embedded feature selection technique, which utilizes the cost function:
where is
the number of data points,
represents the true values,
denotes the predicted values based on features,
are the feature coefficients, and
is the regularization parameter. LASSO effectively shrinks the coefficients of irrelevant features towards zero
, thus removing them. By exploring different
values, we controlled the level of shrinkage.
From the top features identified by LASSO, we further refined our selection using the correlation method, a filter technique. This method evaluates the Pearson correlation coefficient:
where
and
are the features,
and
are individual feature values, and
and
are the feature means. This step helps eliminate redundant information by selecting a representative feature from highly correlated pairs. Our combined approach as the hybrid method leverages LASSO’s capacity to handle a large number of features and the correlation method’s focus on linear dependencies, resulting in a robust and informative feature set for radiomics classification tasks.
2.9. Classification
In this study, we used a HistGradientBoosting (HGB) Classifier to classify individuals based on their ApoE4 status. To ensure performance and generalizability, we implemented several key improvements. We used stratified K-Fold cross-validation to prevent bias, ensuring each fold had a similar distribution of homozygous ApoE4 carriers and non-ε4 carriers. Grid search was employed for hyperparameter tuning, systematically evaluating parameter combinations to find the optimal configuration. Additionally, we applied StandardScaler to standardize feature values, enhancing training stability and potentially improving performance.
3. Results
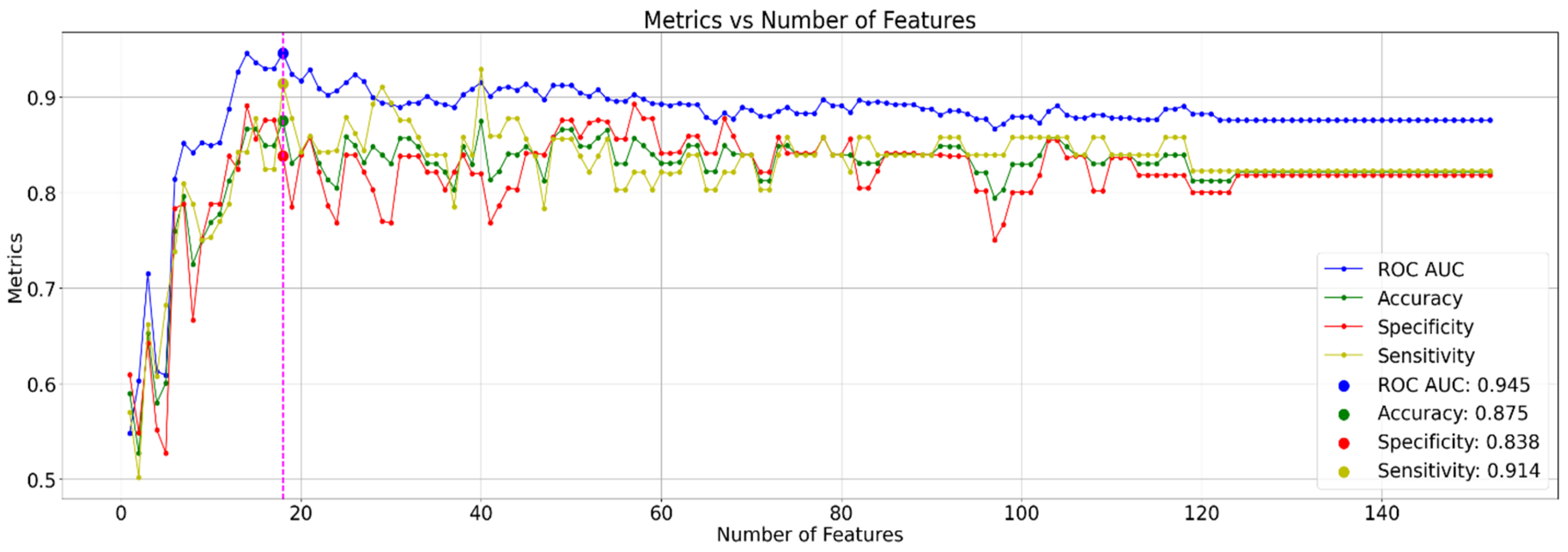
This study employed a radiomics-based approach to develop a predictive model for identifying the ApoE4 genotype. Quantitative image features were extracted from segmented regions of interest and subjected to LASSO regression for feature selection. A rank-based strategy, as illustrated in
Figure 3, was implemented to incrementally incorporate features into the model based on the absolute magnitude of their LASSO coefficients. The performance of each feature subset was assessed using stratified five-fold cross-validation.
3.1. Feature Extraction
To address the high-dimensional nature of the dataset, which included 14,760 features (95 regions of interest * 120 radiomic features for the whole brain, 19 hippocampal subfields * 120 features, and 9 amygdala subnuclei * 120 features), we implemented a multi-step feature selection process to reduce the risk of overfitting during the classification stage. The initial step involved data pre-processing to eliminate irrelevant features like constant, quasi-constant, and duplicates. This initial cleaning reduced the feature set to a more manageable size of 6,278. Following pre-processing, we employed the LASSO method for further feature selection. LASSO works by shrinking coefficient values toward zero, effectively removing features with minimal predictive power. This technique significantly reduced the feature set to a more focused set of 152 features likely to be the most informative for our classification task. The LASSO implementation involved data scaling, a technique to ensure all features are on a similar scale, and setting a high maximum number of iterations (10,000) to guarantee the model converges on a solution. Finally, we optimized the model parameters using grid search with a range of alpha values (a key parameter in LASSO) logarithmically spaced between 10⁻⁴ and 10² and 10-fold cross-validation with ROC AUC scoring to evaluate model performance.
3.2. Classification
Building upon the feature selection process, we employed a HGB classifier for classification. HGB classifier is a powerful ensemble learning method that iteratively builds decision trees, focusing on improving classification for previously misclassified instances. This approach allows the model to learn from its mistakes and refine its predictions over time. The HGB classifier utilized the selected set of 152 features by LASSO for classification. Notably, we employed a rank-based scoring method that emphasized features with higher absolute LASSO coefficients. These features were likely identified as having a stronger association with the target variable (ApoE4 genotype) during the feature selection step. This approach of feature selection and HGB classification achieved excellent results in differentiating MCI patients with homozygous ApoE4 carriers and non-ε4 carriers. The average Area Under the ROC Curve (AUC) was 0.945, with a 95% confidence interval for ROC AUC ranging from 0.868 to 0.926, indicating a very strong ability for the model to distinguish between the two patient groups (
Figure 3).
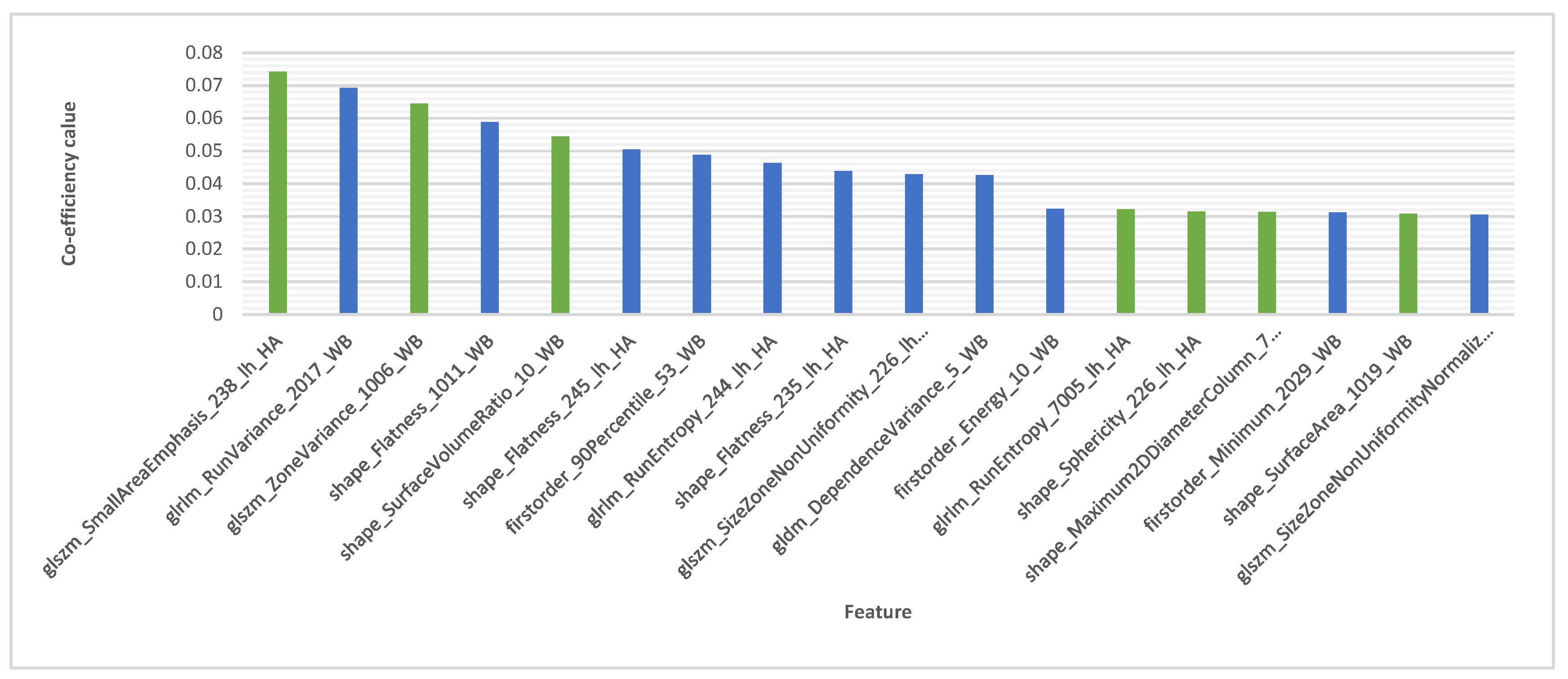
Additionally, the model achieved an average accuracy of 0.875, signifying a high proportion of patients were correctly classified. Furthermore, the model demonstrated exceptional sensitivity (0.914) for detecting individuals with homozygous ApoE4, highlighting its effectiveness in identifying this specific patient group. The model also maintained good specificity (0.838) in correctly identifying MCI patents without the ApoE4 genotype. It’s important to note that achieving this level of accuracy with only 18 features, selected for their highest coefficients as shown in
Figure 4, highlights the model’s efficiency and its potential for clinical applications. This suggests that a smaller set of highly informative features can be sufficient for accurate classification, leading to a more streamlined and potentially more interpretable model.
3.3. Additional Feature Refinement
Following the initial LASSO feature selection that yielded 152 features, we conducted a more granular analysis to identify the most relevant features specifically related to ApoE4. This additional step focused on the top 18 features listed in
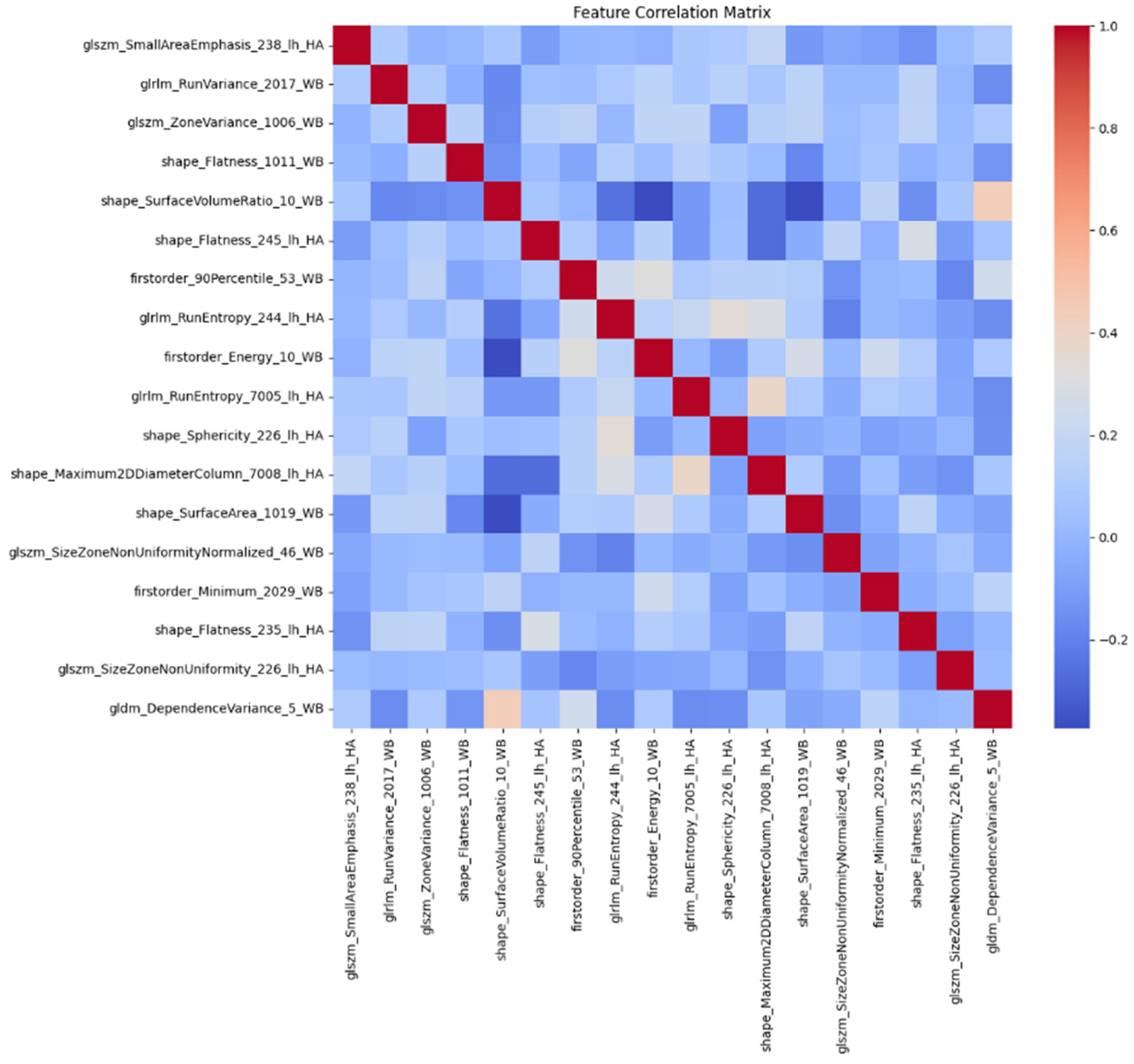
Table 2 identified by LASSO, which were presumed to have the strongest predictive power. We computed Pearson correlation coefficients to assess the linear relationships between these top features. A high correlation value (positive or negative) indicated a significant overlap in information between features. Utilizing a correlation matrix (shown in
Figure 5), we removed features with high correlations to avoid redundancy. We focused on the absolute value of the correlation coefficient to identify the strength of the relationship, regardless of direction. Self-correlations were filtered out, and only the pairs with the weakest correlations were retained.
This process led to the selection of seven features from the top 18 LASSO features, categorized into three distinct groups: glszm (SmallAreaEmphasis, ZoneVariance) related to Gray Level Size Zone Matrix, shape (SurfaceVolumeRatio, Sphericity, Maximum2DDiameterColumn, SurfaceArea) related to shape characteristics, and glrlm (RunEntropy) related to Gray Level Run Length Matrix.
These seven key features were identified in specific brain regions of the left hemisphere, including the hippocampus, entorhinal cortex, amygdala, thalamus, and pars orbitalis. This approach ensured that the final set of features was informative and non-redundant, providing a solid basis for further investigating the link between ApoE4 and MCI.
3.4. Classification with Reduced Feature Set
Leveraging these seven selected features, we built a classification model using the HGB model. To ensure model reliability and generalizability, we employed Stratified five-fold cross-validation. Hyperparameter tuning with grid search was performed to identify the optimal configuration for the HGB model. This involved searching through a range of specified parameters, including learning rates of 0.01, 0.1, and 0.2, maximum tree depths of 3, 5, and 7, training iterations of 100, 200, and 300, and minimum samples per leaf node of 20, 50, and 100. The tuning aimed to maximize the model’s performance based on ROC AUC score, a key metric for evaluating binary classifiers in medical applications.
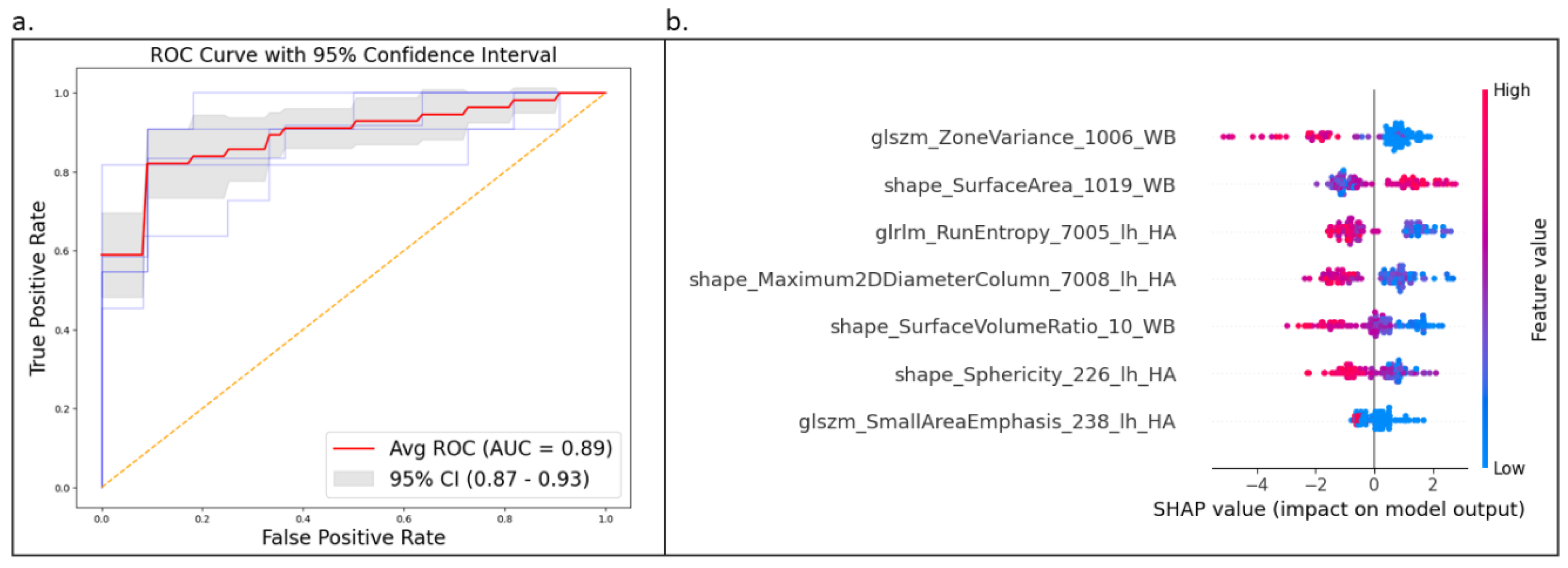
Using grid search to determine the optimal hyperparameters, the HistGradientBoosting (HGB) classifier was evaluated with five-fold stratified cross-validation. The model achieved an average performance with ROC AUC of 0.889, accuracy of 0.796, specificity of 0.768, and sensitivity of 0.821, with a 95% confidence interval between 0.868 and 0.926 as shown in
Figure 6a.
The proposed model, trained on a reduced feature set of seven variables (
Table 2, *), demonstrated robust and balanced classification performance for the ApoE4 genotype (p < 0.05). Notably, this model effectively discriminated between individuals with and without the homozygous ApoE4 genotype, despite the small number of input features.
4. Discussion
4.1. Findings
Our investigation presents an approach to exploring FDG PET’s potential to identify individuals with the homozygous ApoE4 genotype, a known genetic risk factor for Alzheimer’s. It also attempts to find a few brain regions and features that are associated with this genotype to open new promising ways for a better understanding of the disease mechanisms. The results underscore the importance of integrating advanced genetic analysis and neuroimaging techniques to further elucidate the complex interactions between genetics and brain structure and activity.
We analyzed FDG PET images and genetic data from 112 individuals diagnosed with various subtypes of Mild Cognitive Impairment (MCI). To account for class imbalance, we included equal numbers of participants with homozygous ApoE4 carriers and non-ε4 carriers. We employed two separate segmentation techniques to isolate the whole brain and hippo-amygdala, resulting in 123 distinct regions for analysis. We hypothesized that a radiomics approach using FDG PET imaging could be used to predict the homozygous ApoE4 genotype. To achieve this, we implemented a hybrid feature selection process. This involved dimensionality reduction using the LASSO method on preprocessed features, followed by correlation analysis to pinpoint the most informative ones. To enhance model performance and generalizability, we employed a nested framework for training and hyperparameter tuning. This approach utilized stratified five-fold cross-validation, where grid search was applied within each fold to optimize hyperparameters through cross-validation. Importantly, this approach ensured robustness against overfitting by consistently employing stratified K-fold cross-validation throughout the process [
29]. Furthermore, maintaining a consistent random state across all steps fostered the reproducibility of our results [
30].
The study achieved promising performance metrics—an ROC AUC of 0.89, accuracy of 0.80, specificity of 0.77, and sensitivity of 0.82—using a minimal set of seven carefully chosen features. This simplification resulted from a meticulous feature selection process, leveraging a hybrid approach that combined various techniques (preprocessing, LASSO, correlation) to identify the most informative features for predicting the homozygous ApoE4 genotype. This method pinpointed seven key features within the only five critical brain sub-regions, leading to improved interpretability and reliability. The HGB Classifier, optimized via grid search, demonstrated high performance in a 70/30 training-test split, with a 95% confidence interval for ROC AUC ranging from 0.868 to 0.926, indicating acceptable confidence and precision in the model’s performance.
4.2. Explanation of the Relationship between Regions and APOE Genotype
Our study pinpointed five brain regions—the hippocampus, entorhinal cortex, amygdala, thalamus, and pars orbitalis with four of these demonstrating a strong association with the ApoE4genotype according to recent studies. Notably, the hippocampus shows accelerated shrinkage and dysfunction in APOE4 carriers [
31], which may contribute to memory decline in AD [
32]. The entorhinal cortex also exhibits earlier tau protein accumulation and neurodegeneration in these individuals, potentially linked to early memory issues [
33,
34]. Additionally, research suggests that the amygdala in ApoE4 carriers may experience reduced volume and altered activity [
35], potentially impacting emotional regulation and memory changes observed in some AD patients. Finally, the thalamus, a crucial relay center, displays structural and functional alterations in ApoE4 carriers, indicating a potential connection between this genetic risk factor and thalamic dysfunction [
36]. Crucially, the features extracted from these five brain regions (highlighted with an asterisk in
Table 2) exhibited a statistically significant association with the APOE4 genotype (p<0.05), providing further evidence for the involvement of these areas in the pathophysiology of AD among ApoE4 carriers.
4.3. Features Explanation
Our findings identified seven distinct features from different categories, with four being shape-based. These shape-based features in the thalamus, hippocampus, and amygdala are consistent with recent studies showing shape differences, atrophy, and shrinkage in these regions in individuals with homozygous APOE4. Therefore, these shape-based features can be effectively used to analyze and interpret the shape differences or shrinkage of these regions in relation to ApoE4, enhancing the interpretability of the final model results. Additionally, GLSZM features provide information about the distribution of gray levels within specific image regions, which can be useful in identifying abnormalities in texture or homogeneity. Furthermore, GLRLM features capture information about the spatial arrangement of pixels with similar gray levels, helping to detect patterns or irregularities in tissue texture.
4.4. Interpretability
To enhance model interpretability, we used SHAP (SHapley Additive exPlanations) values to understand feature importance and contributions to predictions. The SHAP analysis identified glszm_ZoneVariance of the entorhinal cortex as the most influential feature in differentiating ApoE4 carriers, followed by shape_SurfaceArea of parsorbitalis. While glrlm_RunEntropy of the central nucleus and shape_Maximum2DDiameterColumn of the accessory-basal nucleus also contributed to the model, their impact was less pronounced. The SHAP summary plot (
Figure 6. b) revealed a consistent pattern: higher values of glszm_ZoneVariance of the entorhinal cortex were associated with increased model output, whereas higher values of shape_SurfaceArea of parsorbitalis were associated with decreased model output. This comprehensive approach, encompassing data preprocessing, feature selection, model development, evaluation, and interpretability, enhances the model’s reliability and trustworthiness.
4.5. Clinical Significance
Early Detection: FDG PET can detect changes in brain metabolism associated with AD before cognitive symptoms become apparent. This early detection can allow for earlier intervention and potentially delay disease progression. Detecting imaging features that are associated with the different hallmarks and risk factors of AD may enhance the diagnostic, characterization, monitoring, and prognostic capability of FDG PET. This comprehensive information may lead to the development of more targeted treatment approaches.
4.6. Limitations
Our study encountered several limitations, primarily related to data availability. A larger dataset would strengthen the model’s reliability and adaptability. Moreover, the sensitivity of radiomic features to image acquisition and processing techniques can affect the consistency of our findings.
4.7. Future study
To address these issues, standardized imaging procedures and advanced image preprocessing techniques are needed. Using data from multi-centers should also be performed.
Our study unveiled a novel pars orbitalis region associated with the ApoE4 genotype. This newly discovered area, akin to other ApoE-linked brain regions, presents significant opportunities for clinical and scientific investigation into the ApoE4 variant. By identifying imaging features associated with ApoE4, clinicians might be able to:
i) Improve Diagnostic Accuracy: FDG PET could become a more accurate tool for diagnosing AD, especially in the early stages. Ii) Predict Disease Progression: Imaging features might help predict the rate of cognitive decline in ApoE4 carriers. These benefits should be investigated further.
5. Conclusions
In conclusion, the APoE-4 gene allele can be accurately predicted from FDG PET scans using a radiomics approach. The involved brain regions and associated image features can shed further light on the mechanism of AD in relation to genetic factors. This is likely to enhance the interpretation of FDG PET scans for AD-suspected patients. Further research is needed to expand this understanding in terms of these regions and features.
Acknowledgments
We gratefully acknowledge the financial support provided by the Bogazici University Research Fund (BAP) for this research under project code 19774. Their support was instrumental in carrying out the experiments and analyzing the data. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (
www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- Grover, A.; Joshi, A. An Overview of Chronic Disease Models: A Systematic Literature Review. Glob J Health Sci. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.P.; Williams, S.M.; Strine, T.W.; Anda, R.F.; Moore, M.J. Dementia and its implications for public health. Prev Chronic Dis. 2006, 3, A34. [Google Scholar]
- World Health Organization. Dementia: Key facts’. [Online]. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 23 July 2024).
- ‘Dementia, vs. Alzheimer’s Disease: What Is the Difference? [Online]. Available online: https://www.alz.org/ (accessed on 23 July 2024).
- Battista, P.; Salvatore, C.; Berlingeri, M.; Cerasa, A.; Castiglioni, I. Artificial intelligence and neuropsychological measures: The case of Alzheimer’s disease. Neurosci Biobehav Rev. 2020, 114, 211–228. [Google Scholar] [CrossRef]
- Collin-Castonguay, P.; Gourdeau, D.; Potvin, O.; Duchesne, S. Synthetic FDG-PET hypometabolism sensitivity validation in AD. Alzheimer’s & Dementia 2023, 19. [Google Scholar] [CrossRef]
- Pereira, H.R.; Ferreira, H.A. Exploring the Potential of Radiomics Features of the Hippocampus in Alzheimer’s Disease Considering Standard versus Parallel Imaging. Alzheimer’s & Dementia 2023 2023, 19. [Google Scholar] [CrossRef]
- Varriano, G.; Guerriero, P.; Santone, A.; Mercaldo, F.; Brunese, L. Explainability of radiomics through formal methods. Comput Methods Programs Biomed. 2022, 220, 106824. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.; et al. Radiomics: from qualitative to quantitative imaging. Br J Radiol 2020, 93. [Google Scholar] [CrossRef] [PubMed]
- Rasi, R.; Guvenis, A. Predicting amyloid positivity from FDG-PET images using radiomics: A parsimonious model. Comput Methods Programs Biomed 2024, 247, 108098. [Google Scholar] [CrossRef]
- Yuksel, C.; Rasi, R.; Guvenis, A. A New Method for Diagnosing Alzheimer’s Disease and Monitoring Its Severity Using FDG-PET. In 2022 Medical Technologies Congress (TIPTEKNO); IEEE, 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Rasi, R.; Güvenis, A. A Platform for the Radiomic Analysis of Brain FDG PET Images: Detecting Alzheimer’s Disease. In Proceedings of the Bioinformatics and Biomedical Engineering—10th International Work-Conference, IWBBIO 2023, Meloneras, Proceedings, Part I. Gran Canaria, Spain, 12–14 July 2023; Rojas, I., Valenzuela, O., Ruiz, F.R., Herrera, L.J., Ortuño, F.M., Eds.; Lecture Notes in Computer Science 1319. Springer; pp. 244–255. [Google Scholar] [CrossRef]
- Yi, L.X.; Zeng, L.; Wang, Q.; Tan, E.K.; Zhou, Z.D. Reelin links Apolipoprotein E4, Tau, and Amyloid-β in Alzheimer’s disease. Ageing Res Rev. 2024, 98, 102339. [Google Scholar] [CrossRef]
- Hampel, H.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Koutsodendris, N.; Nelson, M.R.; Rao, A.; Huang, Y. Apolipoprotein E and Alzheimer’s Disease: Findings, Hypotheses, and Potential Mechanisms. Annual Review of Pathology: Mechanisms of Disease 2022, 17, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Corder, E.H.; et al. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science (1979) 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Hyman, B.T.; Serrano-Pozo, A. Multifaceted roles of APOE in Alzheimer disease. Nat Rev Neurol 2024. [CrossRef] [PubMed]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.; et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 2011, 16, 903–907. [Google Scholar] [CrossRef]
- Jagust, W.J.; et al. The Alzheimer’s Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimer’s & Dementia 2015, 11, 757–771. [Google Scholar] [CrossRef]
- Wyman, B.T.; et al. Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimer’s & Dementia 2013, 9, 332–337. [Google Scholar] [CrossRef]
- Avants, B.; Epstein, C.; Grossman, M.; Gee, J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008, 12, 26–41. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Kahhale, I.; Buser, N.J.; Madan, C.R.; Hanson, J.L. Quantifying numerical and spatial reliability of hippocampal and amygdala subdivisions in FreeSurfer. Brain Inform. 2023, 10, 9. [Google Scholar] [CrossRef]
- Sämann, P.G.; et al. <scp>FreeSurfer</scp> -based segmentation of hippocampal subfields: A review of methods and applications, with a novel quality control procedure for <scp>ENIGMA</scp> studies and other collaborative efforts. Hum Brain Mapp 2022, 43, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Hatt, M.; Vallieres, M.; Visvikis, D.; Zwanenburg, A. IBSI: an international community radiomics standardization initiative. Soc Nuclear Med. 2018.
- van Griethuysen, J.J.M.; et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J R Stat Soc Series B Stat Methodol 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Wieczorek, J.; Guerin, C.; McMahon, T. K -fold cross-validation for complex sample surveys. Stat 2022, 11. [Google Scholar] [CrossRef]
- Impagliazzo, R.; Lei, R.; Pitassi, T.; Sorrell, J. Reproducibility in learning. In Proceedings of the 54th Annual ACM SIGACT Symposium on Theory of Computing; ACM: New York, NY, USA, 2022; pp. 818–831. [Google Scholar] [CrossRef]
- O’Dwyer, L.; et al. Reduced Hippocampal Volume in Healthy Young ApoE4 Carriers: An MRI Study. PLoS ONE 2012, 7, e48895. [Google Scholar] [CrossRef]
- Pievani, M.; Galluzzi, S.; Thompson, P.M.; Rasser, P.E.; Bonetti, M.; Frisoni, G.B. APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer’s disease. Neuroimage 2011, 55, 909–919. [Google Scholar] [CrossRef]
- Miranda, A.M.; et al. Effects of APOE4 allelic dosage on lipidomic signatures in the entorhinal cortex of aged mice. Transl Psychiatry 2022, 12, 129. [Google Scholar] [CrossRef]
- La Joie, R.; et al. Association of APOE4 and Clinical Variability in Alzheimer Disease With the Pattern of Tau- and Amyloid-PET. Neurology 2021, 96. [Google Scholar] [CrossRef]
- Young, C.B.; et al. APOE effects on regional tau in preclinical Alzheimer’s disease. Mol Neurodegener 2023, 18, 1. [Google Scholar] [CrossRef]
- Novellino, F.; López, M.E.; Vaccaro, M.G.; Miguel, Y.; Delgado, M.L.; Maestu, F. Association Between Hippocampus, Thalamus, and Caudate in Mild Cognitive Impairment APOEε4 Carriers: A Structural Covariance MRI Study. Front Neurol 2019, 10. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).