1. Introduction
The global transition to cleaner and more sustainable transportation technologies has accelerated in response to increasingly stringent emissions regulations and environmental concerns [1]. Vehicle electrification, driven by rechargeable lithium-ion battery (LIB) technology, has emerged as the dominant solution in this shift [2]. However, while LIBs offer numerous advantages, they face critical challenges, particularly in terms of safety and performance under demanding conditions [3]. The high flammability of the liquid electrolyte, combined with lithium’s propensity to form dendrites—needle-like structures that can cause internal short circuits—pose significant risks [4]. These safety concerns have prompted researchers and industries to explore alternatives that can enhance both the safety and energy density of energy storage systems.
The rechargeable solid-state battery (SSB) has emerged as a leading candidate to address these challenges, offering a safer, more efficient solution by replacing the flammable liquid electrolyte with a non-flammable solid electrolyte [5]. The industry expects this transition to provide significant benefits, including improved thermal stability, suppression of dendrite formation, and the ability to operate over a wider temperature range without significant degradation [6]. Furthermore, SSBs enable the direct use of lithium metal as an anode, unlocking the potential for higher energy densities, reduced weight, and longer ranges for electric vehicles and other applications [7]. These advancements make SSBs particularly appealing for sectors such as electric vehicles, drones, and grid storage, where high energy density, safety, and longevity are paramount.
Despite their potential, significant obstacles remain in the development of SSBs, particularly in the creation of stable, low-resistance interfaces between solid electrolytes and electrodes [8]. Unlike liquid electrolytes, solid-state materials cannot easily conform to electrode surfaces, making it difficult to achieve consistent, high-quality contact across the interface. Poor interface contact can lead to increased resistance, reduced ionic conductivity, and degraded battery performance. To overcome these hurdles, ongoing research has been focusing on optimizing solid electrolyte materials, improving interface design, and integrating advanced manufacturing techniques [9].
The goal of this paper is to provide a thematic and bibliometric analysis of the recent developments in SSB technology, emphasizing advancements in key areas such as electrolyte engineering, electrode engineering, battery architecture, and performance evaluation. By reviewing both experimental and theoretical studies, this paper identifies current trends, challenges, and future opportunities in SSB research. Specifically, the author highlights innovations that address the critical barriers to SSB commercialization, such as improving interface stability, enhancing ionic conductivity, and developing scalable, cost-effective manufacturing processes. The contributions of this work are insights that provide a deeper understanding of the trajectory of SSB research and highlight the growing momentum toward realizing solid-state batteries as the next-generation energy storage solution.
The organization of the rest of this paper is as follows:
Section 2 provides a primer on rechargeable batteries by using the lithium-ion architecture as a focus, reviews the solid-state design, active research areas, and topics in battery pack construction.
Section 3 describes the methodological workflow, including the methods developed to conduct the thematic and bibliometric analysis.
Section 4 presents the results, including visualization of the data.
Section 5 discusses the results, limitations of the work, and implications for future directions.
Section 6 concludes the research and suggests future work.
2. Literature Review
The following subsections provide a primer on the fundamentals of rechargeable battery operation, solid state designs, active research areas within SSB development, and battery pack development.
2.1. Rechargeable Battery Primer
The lithium-ion battery (LIB) has dominated over the previous generation nickel metal hydride (NiMH) type rechargeable battery because it can store more energy at a lower weight (gravimetric energy density) and volume (volumetric energy density), has a longer lifespan, and performs better at operating temperature extremes [10]. Modern rechargeable batteries utilize highly reactive lithium ions as the primary energy source. Although safer than NiMH, there are still concerns because LIBs have caused fires due to mechanical, electrical, and thermal abuse [11]. Moreover, the transportation industry is seeking higher energy density and safer batteries to increase electric vehicle (EV) driving range and to enable new transport modes such as electrified aircraft [12].
2.1.1. Basic Operation
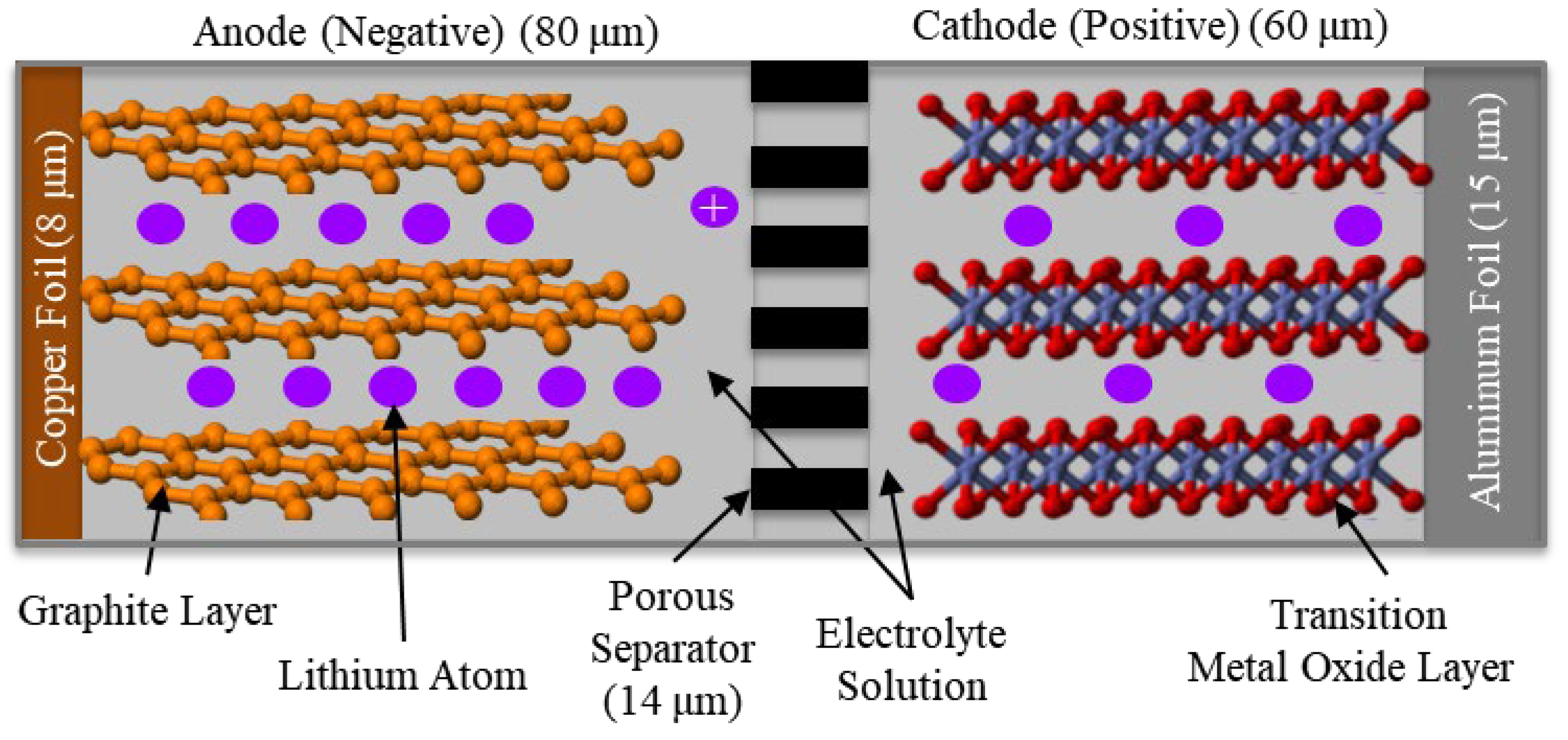
An LIB consists of anode and cathode materials immersed in a liquid electrolyte with a polymer material physically separating the electrodes, as illustrated in
Figure 1. The dimensions shown in the figure are typical, but they vary depending on the specific materials used and the design goals of the battery [13]. The desire is to make all layers as thin as possible without compromising safety or mechanical integrity, which helps to maximize energy density and minimize internal resistance [14].
The microporous polymer separator prevents the two electrodes from internally short circuiting [15]. The separator is an insulator that blocks the conduction of electrons while allowing the passage of ions through its electrolyte filled pores.
Figure 1.
Figure 1. The typical electrochemical structure of a LIB. ©Raj Bridgelall.
Figure 1.
Figure 1. The typical electrochemical structure of a LIB. ©Raj Bridgelall.
The anode material (negative electrode) is typically composed of graphite, a stable form of carbon atoms organized as a crystal [16]. The typical manufacturing process coats the anode and cathode materials onto thin copper and aluminum foils, respectively. These foils, also called current collectors, facilitate electron conduction through an external circuit. The cathode material (positive electrode) is usually a lithium metal oxide such as lithium cobalt oxide (a crystalline solid) or lithium iron phosphate (an inorganic compound) [17]. Anode and cathode materials store lithium atoms by inserting them within their layered atomic structure during the charge and discharge cycles, respectively, in a chemical process called intercalation. That is, lithium atoms intercalate into the anode structure during charging and return to the cathode structure as the battery discharges.
Both the anode and cathode materials are porous, containing gaps of various sizes that enable the liquid electrolyte to permeate the pores [18]. The porosity of these materials is a critical design factor, optimized to maximize the surface area and electrolyte permeation without compromising mechanical stability. That is, if the pores are too large or too numerous, the anode might become too weak or less efficient at storing lithium atoms. Conversely, if the pores are too small or sparse, the electrolyte might not penetrate deeply enough to facilitate ion transport. Ideally, the manufacturing process aims to distribute the electrolyte evenly throughout the porous structures. This ensures that lithium ions can reach every part of the anode and cathode materials, maximizing their utilization and enhancing the battery’s overall energy density.
When charged, the anode has a higher chemical potential relative to the cathode because of the higher concentration of lithium atoms [19]. During discharge, this concentration gradient creates a chemical potential difference that drives positively charged lithium ions (Li⁺) from the anode to a lower potential energy at the cathode. That is, Li⁺ have a natural tendency to move toward a state of lower chemical potential energy by giving up their single outer shell electron in an oxidization reaction.
Engineers design the composition of the cathode material to be at a higher positive electric potential (voltage) relative to the anode [17]. This potential difference, typically between 3 and 4 volts, generates an electric field with its positive end at the cathode and its negative end at the anode. During discharge, the negative end at the anode repels electrons from the lithium atoms, while the positive end on the cathode attracts electrons. Hence, when connected to an external circuit, electrons flow from the anode to the cathode. Electrons in the cathode attract the positive migrating Li⁺ with sufficient force to overcome the energy barriers associated with intercalation. Each electron in the cathode combines with a Li⁺ to form a lithium atom that intercalates within the cathode material to form a stable structure. Although the positive end of the electric field at the cathode produces a repulsive electrostatic force on Li⁺, the stronger chemical gradient between the electrodes overcomes that force. That is, the electric field primarily affects the electron flow in the external circuit rather than significantly hindering ion migration.
During discharge, the combined chemical and electrical potential energies, also known as the electrochemical potential, drive the movement of lithium ions through the electrolyte and electrons through the external circuit [20]. Electrochemical potential refers to the energy level at which electrons exist in a material relative to a standard such as lithium metal. This electrochemical process powers devices connected to the battery as they harness the flow of electrons as electric current. As electrons flow through various components in the circuit, they lose potential energy corresponding to the voltage drop across each component. Hence, having lost energy from doing work in the circuit, electrons enter the cathode at a lower energy state. The battery discharges until it reaches a specified lower voltage threshold, at which point external circuitry prevents further discharge to protect the battery.
When charging, the external electrical charger applies direct current (DC) to the battery terminals, maintaining a higher positive voltage at the cathode relative to the anode [21]. This voltage difference generates an electric field within the battery that initiates oxidation reactions at the cathode. The electric field repels the positively charged ions in the cathode and pushes them through the electrolyte toward the anode. This movement occurs against the natural tendency of the Li⁺ to move from the anode to the cathode during discharge. The electric field must be strong enough to overcome the chemical potential difference between the anode and cathode, effectively forcing Li⁺ back into a higher chemical potential energy state when they reach the anode for storage. The recombination of Li⁺ and electrons restores the lithium atoms within the anode material, effectively recharging the battery.
2.1.2. Anode Material
The anode in an LIB stores lithium atoms in graphite (a carbon crystal), though some designs use lithium titanate or silicon-based materials. Engineers choose graphite because it is inexpensive and abundant, conducts electricity with low resistance, and efficiently stores lithium ions [19]. Carbon is the smallest atom that can form the most covalent bonds, which is four. This characteristic enables it to form complex structures such as graphite and even DNA in living things. These bonds are strong because of the close proximity of carbon’s valence shell to its protons, resulting in high structural integrity. Hence, carbon is likely to remain an important material in battery construction.
Graphite has a layered structure that allows lithium ions to reversibly intercalate between its layers of graphene sheets and with low expansion. The manufacturing process coats a slurry form of the material onto a copper foil, dries it, and then compresses it to form a thin film current collector [22]. Manufacturing prepares the slurry by processing the graphite into a fine powder and mixing it with a binder and a solvent. Initially charging the battery introduces the lithium atoms to the graphite layers (lithiation) that holds them in place with electrostatic forces. This crystalline structure provides high capacity for lithium atom storage while maintaining structural stability over many cycles of charging and discharging. The ease of lithiation is due to graphite’s low electrochemical potential difference from lithium metal of close to 0.1V.
Modern rechargeable batteries often use metals from the alkali group (Group 1 of the periodic table) because these metals have a single electron in their outermost shell, requiring little energy to release them [23]. The metal atoms from this group achieve a stable electronic configuration after releasing an electron. Hence, this ease of electron release is why alkali metals are among the most reactive elements in nature. Lithium is the smallest and lightest metal in the alkali group. Its low atomic mass contributes to maximizing the energy density of batteries [24]. Its small ionic radius allows it to easily move through electrolytes and insert itself into the cathode material structure. Ease of ionic movement translates to higher power output and faster charging speeds. For these reasons, it is highly likely that lithium will continue to dominate in modern battery technologies.
2.1.3. Cathode Material
The cathode material must efficiently store lithium atoms as well as create a significant electrochemical potential difference with the anode. Manufacturers select cathode materials based on their standard electrode potentials [10]. That is, a compound with a higher positive potential is better at attracting electrons (undergoing reduction), while those with lower or negative potentials are better at losing electrons (undergoing oxidation). For example, lithium cobalt oxide (LiCoO2), a common cathode material, creates a higher electrical potential than the anode due to its high reduction potential, the stability of its layered structure that holds lithium atoms at high energy states, and the strong tendency of cobalt to reduce when intercalating lithium atoms. These properties result in a significant voltage difference between the cathode and the anode, which is the source of the battery’s energy density.
A common method for synthesizing cathode materials is a solid-state reaction. This process involves mixing lithium salts (such as lithium carbonate, Li2CO3) with transition metal oxides or precursors (such as cobalt oxide, iron phosphate, or manganese oxide) in a specific stoichiometric ratio [25]. Heating the mixture at high temperatures (usually 700°C–900°C) facilitates a chemical reaction that forms the desired intercalation compound such as lithium metal oxide. Hence, the compound contains lithium atoms within its crystal structure, ready to participate in electrochemical reactions.
The cathode material determines many of the battery’s characteristics, including energy density, power capability, and overall performance. Other common cathode materials include lithium iron phosphate (LiFePO₄) or LFP, lithium manganese oxide (LiMn₂O₄) or LMO, and various combinations of nickel, manganese, and cobalt (NMC), lithium nickel cobalt aluminum oxide (NCA), and lithium–titanate (LTO) [17]. Each material offers different trade-offs between energy density, power output, safety, cost, and cycle life. For example, LiCoO₂ provides high energy density but is expensive compared with alternatives and has safety concerns at high temperatures. LiFePO₄, on the other hand, is cobalt-free, offers excellent thermal stability and long cycle life but at the cost of lower energy density. The choice of cathode material often depends on the specific application requirements, such as high energy density for mobile devices or improved safety for electric vehicles.
2.1.4. Electrolyte Material
The electrolyte has a wide electrochemical stability window, meaning it does not break down at the operating voltages of the battery. However, the liquid electrolyte can decompose at high temperatures and ignite into a fire [26]. The liquid electrolyte in a commercial LIB is composed of a lithium salt dissolved in an organic solvent [27]. The most commonly used lithium salt is lithium hexafluorophosphate (LiPF6). Typical organic solvents include mixtures of ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC).
The solvent contains a salt of lithium. The solvent pulls the salt apart as it dissolves to form a mixture of positive and negative ions. Each solvent molecule exhibits a polarization due to the uneven distribution of electrons within it such that one end has a positive charge with respect to the other end. Li⁺ will attract the negative (or partially negative) end of a solvent molecule, surrounding it to form solvation shells. The size and structure of these shells can significantly affect the ion’s mobility and, consequently, the electrolyte’s conductivity. These shells lower the free energy of the system by stabilizing the ions. Therefore, manufacturers must balance the ionic conductivity and stability by carefully controlling the lithium salt concentration [25]. The solvent maintains a balanced charge of positive and negative ions. During charging, releasing a positive lithium ion into the electrolyte solution means that it must give up a positive lithium ion elsewhere in the solution to maintain a balanced charge. The system achieves this balance as electrons entering the anode combine with a positive lithium ion, pulling it from the salt solution.
When first charged in the factory, an additive to the salt solvent enables a reaction at the anode interface to form a solid protective film, called a solid electrolyte interface(SEI), on the graphite particles [28]. These reactions use from 5% to 10% of the lithium in the battery in the first charge cycle. This film is a stable surface that extends battery life. Lithium ions can pass through the SEI.
2.2. Solid State Designs
Compared with the conventional batteries based on the liquid electrolyte, solid-state batteries are still less studied and face more challenges [19]. A solid-state battery (SSB) design replaces the flammable liquid electrolyte with a non-flammable or less-flammable solid to increase its inherent safety [29]. Engineers keep lithium metal in the anode because of its high theoretical capacity and low electrochemical potential. The solid-state electrolyte allows for the direct use of lithium metal without the graphite material in the anode, which further increases the battery’s energy density. During charging, lithium ions participate in a reduction reaction by gaining electrons from the external circuit, and the atoms deposit onto the lithium metal surface in the anode. Hence, the anode increases in mass and thickness as lithium atoms plate the lithium metal. These repeated volume changes induce mechanical stress within the anode. Over time, this stress can cause the formation of cracks or voids, which can degrade the battery’s performance and life span [30].
A common issue with lithium metal is dendrite formation from the repeated shuffling of lithium ions between the electrodes during charge-discharge cycles [7]. Dendrites are needle-like structures that can grow through an electrolyte during charging. Dendrites can pierce the electrolyte and cause short circuits, leading to potential safety risks such as thermal runaway. Solid electrolytes replace the electrode separator and also resist dendrite growth better than liquid electrolytes, but the challenge remains significant [6]. That is, uneven lithium deposition during charging can exacerbate dendrite growth.
The materials used for the cathode are similar to those used in liquid-electrolyte batteries, but they must be compatible with the solid electrolyte. The anode and cathode materials form a continuous direct interface with the solid electrolyte. These interfaces must be stable to prevent side reactions that could degrade the battery’s performance. The manufacturing process typically presses, sinters, or otherwise brings the solid electrolyte into intimate contact with the electrode materials [31]. This direct physical contact is essential for enabling lithium-ion transport between the electrodes. A poor contact can lead to high interfacial resistance that reduces the battery’s performance.
The solid electrolyte must be highly conductive to lithium ions while remaining electronically insulating to prevent short-circuiting between the anode and cathode. Lithium ions move through the solid electrolyte via diffusion mechanisms, which is different from the free flow of stabilized solvation shells in a liquid electrolyte [32]. The diffusion of ions in a solid material involves hopping between specific sites within the crystal structure of the solid material. This can reduce the ion conductivity relative to liquid electrolytes. Engineers form these sites by promoting defects or vacancies in the lattice of the solid electrolyte. Improving the ion conductivity in solid electrolytes is an active area of research.
2.3. Active Research Areas
Current LIB material choices are the result of decades of research and development, balancing performance, safety, cost, and manufacturability [33]. Ongoing research continues to explore new materials and combinations to further improve battery performance and address current limitations. In general, SSB research efforts focus on improving energy density, power output, safety, cost, and cycle life.
2.3.1. Electrolyte
Solid-state electrolytes promise to be a safer alternative to flammable liquid electrolytes [26]. Solid electrolytes use a crystalline structure to create low energy pathways for ions to move under the influence of the electric field. Manufacturers can make solid electrolytes from ceramics, polymers, or glassy materials that conduct lithium ions [6]. Ceramics have high ion conductivity, but they are brittle. Polymers are more flexible, but they have lower conductivity [34]. Glassy materials have high conductivity, but they are reactive with moisture. The industry is seeking solid electrolytes with high ionic conductivity, mechanical flexibility, and chemical stability.
2.3.2. Anode
Graphite has a lower lithium storage capacity compared with some alternative materials [35]. For instance, silicon can theoretically store up to 4.4 lithium ions per silicon atom, compared with only 1 lithium per 6 carbon atoms in traditional graphite anodes [19]. As a result, the theoretical capacity of silicon is about 4,200 mAh/g, which is more than an order of magnitude higher than that of graphite, which has a theoretical capacity of 372 mAh/g. However, silicon anodes face issues with large volume expansion of up to 300% when absorbing lithium ions during charging, which can lead to mechanical degradation and capacity fade. Silicon anodes can also form an unstable SEI layer, especially in solid-state systems, leading to increased resistance and reduced cycle life [28].
The repeated plating and stripping of lithium atoms can roughen the surface and increase the interfacial resistance with the solid electrolyte [36]. The incomplete stripping of lithium during discharge can lead to the formation of voids that can accumulate and disrupt the structural integrity of the anode, reducing its effective capacity. Hence, researchers are investigating ways to improve the performance and longevity of lithium metal anodes through surface coatings, pre- lithiation, and microstructure 3D architectures. Applying protective coatings to the lithium metal surface can help manage the interface with the solid electrolyte and reduce the risk of dendrite formation and excessive interphase layer growth. Pre-lithiation can stabilize the lithium metal anode and reduce the initial formation of the interphase layer, leading to better cycling stability. Designing lithium metal anodes with engineered microstructures, such as 3D architectures, can accommodate volume changes more effectively and reduce the likelihood of dendrite formation.
2.3.3. Cathode
Increasing the voltage difference relative to the anode, while retaining the energy storage capacity, increases the battery’s energy storage and delivery capability [9]. A key area of research is finding cathode materials that operate at higher potentials relative to lithium while storing more lithium per unit volume. Cathode materials must also be stable at high voltages and withstand repeated lithium insertion and extraction without significant structural degradation. This is crucial for the long-term stability and cycle life of the battery. Charge transfer kinetics, which is the rate that charged particles can intercalate, is important for high-power applications [37]. Cathode materials must also be stable when in contact with the electrolyte at high voltages.
2.3.4. Interfacial Integrity
The interface design must ensure that lithium ions can easily pass from the solid electrolyte into the electrode materials and vice versa [38]. The interface between the solid electrolyte and the electrode materials must maintain mechanical integrity during cycling. As the battery charges and discharges, volume changes in the electrode materials can create stress at the interface. However, achieving consistent and low resistance contact between the solid electrolyte and electrode materials is a significant challenge. Hence, scientists and engineers are investigating techniques such as interface coating, thermal treatment, and mechanical compression to optimize the interfaces.
During battery operation, a solid-state interphase (SSI) can form at the interface, similar to the SEI in liquid electrolytes [39]. This layer must be stable, thin, and conductive to lithium ions but insulating to electrons to prevent further reactions. The formation of a stable interphase is crucial for long-term battery performance as it protects the electrolyte and electrode from further unwanted reactions.
Solid-state batteries often require very thin layers of solid electrolyte to reduce internal resistance and ensure efficient ion transport. Creating these thin layers, especially in large-scale production, demands advanced techniques such as vapor deposition or sputtering, which are more complex and expensive than the processes used for traditional batteries [22]. The manufacturing process must precisely stack multiple thin layers of anode, electrolyte, and cathode material with high alignment accuracy. Any misalignment can lead to poor performance or short circuits. Ensuring uniform pressure and contact across the layers during assembly is challenging, as any air gaps or uneven pressure can degrade battery performance. The assembly equipment must carefully control the layering and compression to avoid damaging the materials or introducing defects. Improving yield while reducing defects is a critical issue in making solid-state batteries commercially viable. For solid-state batteries to become competitive, manufacturers must also reduce their costs significantly, which requires innovations in both materials and processes.
2.3.5. Battery Pack Development
The assembly of commercial lithium-ion batteries involves multiple stages, each contributing to the overall energy density and performance of the final battery. The assembly consists of three main levels: cell, module, and pack [15]. Cells can come in various formats, such as cylindrical, prismatic, and pouch cells. Prismatic and pouch cells can achieve higher energy densities due to better space utilization, as cylindrical cells often leave small gaps between them when arranged in a module [40].
Connecting multiple individual cells in series (to increase voltage) or parallel (to increase capacity) forms a module [41]. The arrangement depends on the application and the desired power and energy requirements. Modules often include cooling systems, protective casings, and safety features like thermal management and battery management systems. These additions help to prevent overheating, optimize performance, and ensure battery longevity. While cooling systems and safety features are essential for performance and safety, they can reduce the energy density at the module level by taking up space and adding weight. Efficient thermal management systems are crucial for minimizing this impact.
Multiple connected modules form a battery pack. The number of modules and their configuration (series or parallel) depend on the desired output voltage, capacity, and power of the battery. A sophisticated system monitors the health, temperature, and state of charge of each cell or module in the pack. The system ensures the safe operation of the battery, manages charging and discharging, and prevents overcharging, over-discharging, and overheating [42]. The arrangement of the pack casing and modules also affect the final energy density. In practice, the energy density at the pack level can be 10% to 25% lower than at the cell level due to the added weight and volume of these systems. Efforts to minimize the weight and size of the pack structure while ensuring safety and stability can help maximize the usable energy density of the pack.
Structural batteries are emerging as a manufacturing strategy that combines the functions of a battery and a structural component to significantly increase energy density [43]. The key idea is to make the battery itself a part of the structure of a device or vehicle, rather than a separate component that adds extra weight. Engineers can achieve this by using materials that can both store energy and bear mechanical loads. This approach will enable longer range ground vehicles and new modes of transportation such as electric vertical takeoff and landing (eVTOL) aircraft [44].
3. Methodology
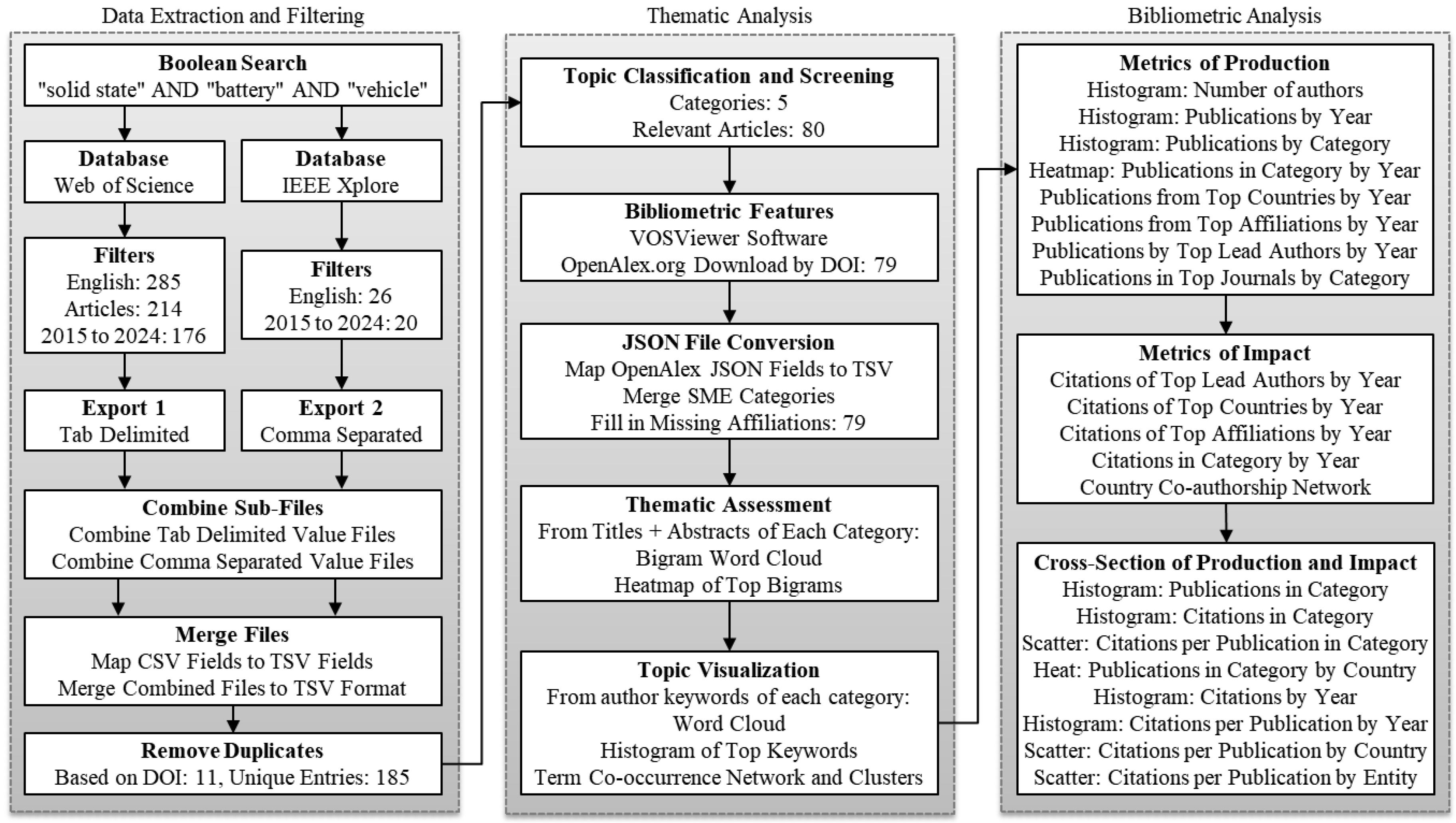
Figure 2 presents a detailed workflow illustrating the methodological approach used in the bibliometric and thematic analysis. The process begins with a Boolean search using the keywords “solid state,” “battery,” and “vehicle” across two databases: Web of Science (WOS) and IEEE Xplore. The selection of these as the primary databases for this analysis is based on their comprehensive coverage of high-impact, peer-reviewed research in the fields of science, technology, and engineering. The research community widely recognizes WOS for its multidisciplinary reach and robust citation tracking, while IEEE Xplore specializes in cutting-edge innovations in electrical engineering, computer science, and electronics. Scopus is another popular database used in literature reviews. However, the author excluded it due to institutional policy, given its considerable overlap with WOS and the need to manage research costs efficiently.
Selecting only a few keywords balanced the casting of a wide search with high relevance. That is, too few keywords such as only “battery” would result in an incredibly broad search, whereas using too many keywords would overly narrow the search. The author filtered the search results by language (English) and a decade of publications (2015–2024). This yielded 176 and 20 relevant articles from WOS and IEEE Xplore, respectively. The databases exported the results in different formats: tab-delimited for WOS and comma-separated for IEEE Xplore. The author wrote Python procedures to combine the sub-files, map fields from the different formats, and merged them into a unified tab-separated-value (TSV) format. The procedures removed duplicate records based on their document identification numbers (DOI), resulting in 185 unique entries.
Following the data collection, the author, a subject matter expert (SME), screened and categorized those articles into five themes by reviewing the titles and abstracts. This step resulted in 80 articles. Then using their DOI as a unique key, the VOSViewer software (version 1.6.20) downloaded their bibliometric features from the OpenAlex database [45]. The result was a JSON file with 79 of the articles located. After converting the JSON file to TSV format and merging back the categories that the SME identified, the author looked up and filled in any missing affiliations.
Thematic assessment involved analyzing titles and abstracts using natural language processing (NLP) techniques to generate bigram word clouds and heatmaps. The author wrote Python code to clean, analyze, and visualize the downloaded bibliometric data by producing word clouds, histograms, heatmaps, scatter plots, and term co-occurrence networks of the most frequent keywords. The visualization mapped the distribution of publications and citations across categories, authors, countries, and institutions. The author also carefully read the publications to offer some details and insights of research directions within each SME category. The workflow emphasizes a comprehensive, data-driven approach to analyzing and visualizing publication trends in the solid-state battery (SSB) field.
4. Results
The following subsections discuss the results of the thematic analysis, bibliometric analysis, and specific findings from selected works within each category. The SME-defined categories were Electrolyte Engineering, Electrode Engineering, Architecture Research, Multidisciplinary Review, and Performance Evaluation.
4.1. Thematic Analysis
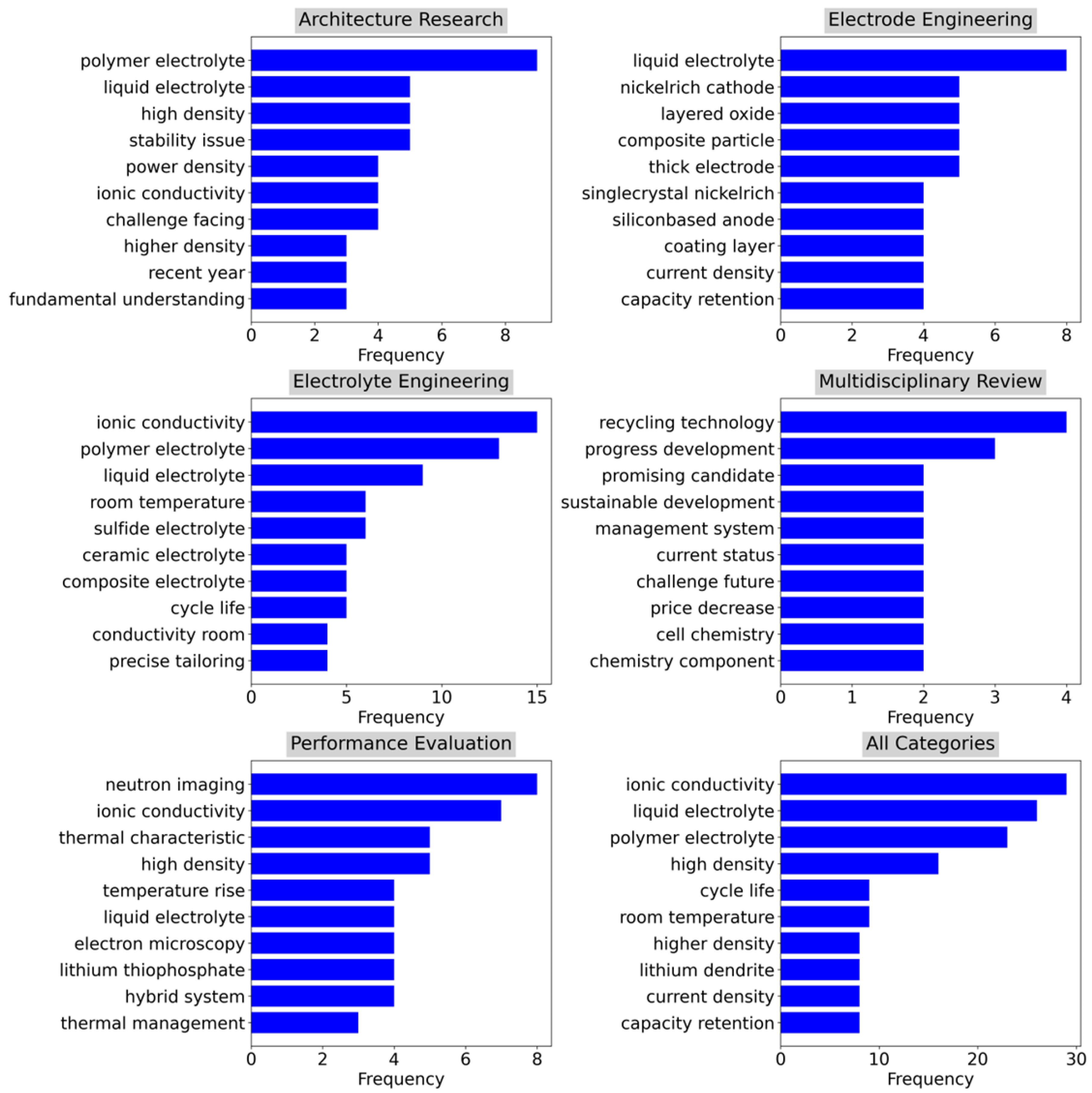
Figure 3 illustrates the thematic categorization of key research areas related to solid-state batteries in vehicles via bigram word clouds. Each word cloud in the category highlighted prominent research themes by the frequency of term occurrence.
The word cloud for All Categories aggregated themes across the entire dataset. In the category of Architecture Research, significant terms include “polymer electrolyte,” “ionic conductivity,” and “liquid electrolyte,” indicating a focus on foundational aspects of SSB architectures and their material properties. The category of Electrode Engineering emphasizes terms such as “nickel-rich cathode,” “layered oxide,” and “composite particle,” suggesting a focus on electrode materials and their functional optimization.
The category of Electrolyte Engineering highlights “ionic conductivity,” “liquid electrolyte,” and “polymer electrolyte,” pointing to research directed toward improving electrolyte performance, especially in terms of conductivity and stability. The Multidisciplinary Review category covers broad themes like “recycling technology,” “sustainable development,” and “price decrease,” suggesting a comprehensive assessment of the solid-state battery (SSB) landscape, including sustainability and future challenges. The category of Performance Evaluation highlighted “neutron imaging,” “ionic conductivity,” and “thermal characteristics,” indicating an emphasis on advanced diagnostic tools and performance metrics for batteries.
The word cloud for “All Categories” synthesizes the most common terms across all research areas, highlighting the recurring themes of “ionic conductivity,” “polymer electrolyte,” and “high density” as central to the field of SSB research. This visualization effectively highlights the thematic distribution and priority areas within the academic discourse on solid-state batteries.
Figure 4 complements the word cloud by showing the distribution of key research themes across different categories through bar charts representing the frequency of prominent bigrams. The frequency distributions provide a quantitative view of the research focus and the relative importance of the themes within each category.
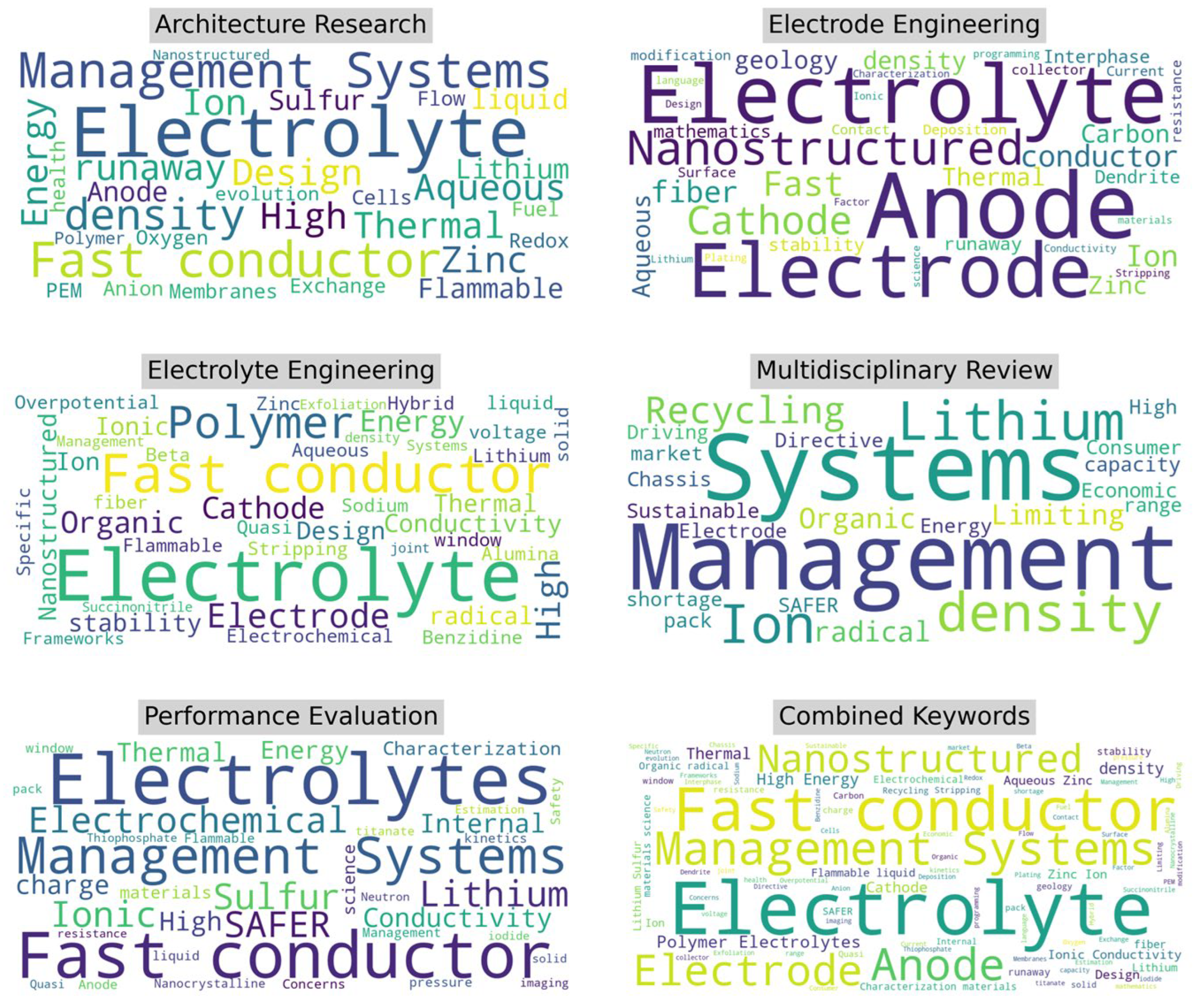
Figure 5 provides another view of the key themes from the perspective of the author-provided keywords across the five key research categories. In Architecture Research, “electrolyte,” “management systems,” and “fast conductor” are dominant terms. The emphasis on “fast conductor” and “density” points to efforts in improving the charging rates and energy density of battery architectures. For Electrode Engineering, the most prominent terms are “electrolyte,” “anode,” “cathode,” and “nanostructured,” highlighting a concentrated effort on electrode materials, their performance, and their functional properties.
This focus is consistent with the pursuit of enhancing battery performance through material innovations. In Electrolyte Engineering, the leading terms are “fast conductor,” “polymer,” “electrolyte,” and “organic,” suggesting an emphasis on developing advanced conductive materials and polymers for improved electrolyte performance. The Multidisciplinary Review category features “systems,” “management,” “lithium,” and “recycling,” which emphasizes a broad, systemic view of solid-state batteries, considering not only technical aspects but also sustainability and future management strategies.
The Performance Evaluation category highlights terms such as “fast conductor,” “electrochemical,” and “SAFER” with a focus on advanced diagnostics, safety concerns, and the overall system performance of batteries. The Combined Keywords word cloud synthesizes all the prominent terms across categories, with “fast conductor,” “electrolyte,” “management systems,” and “nanostructured” being the most significant, indicating central themes in the field. These visualizations provide a comprehensive overview of the focus areas within SSB research from title, abstract, and author keywords, highlighting both material innovations and systemic challenges.
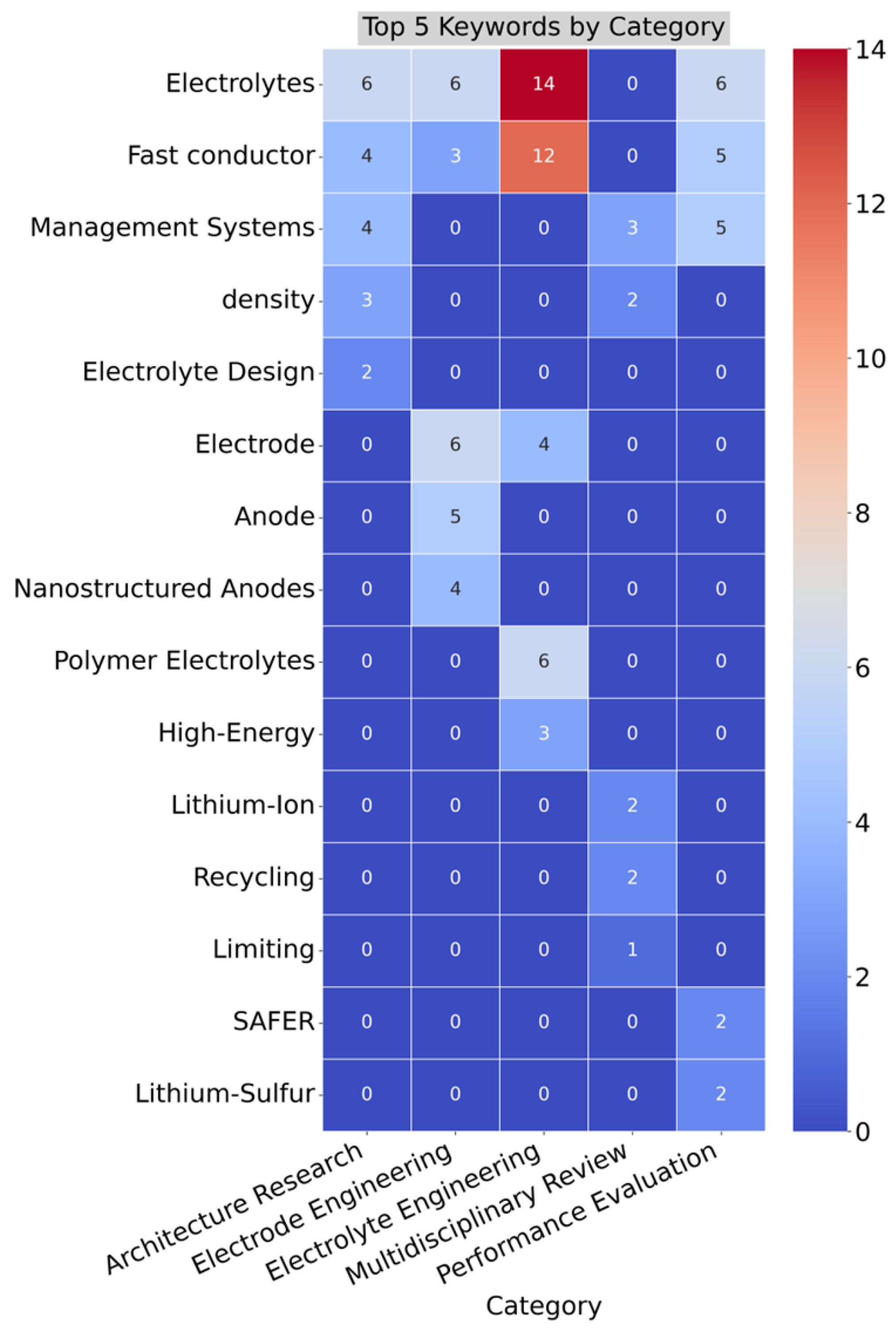
Figure 6 provides a cross-sectional visualization via a heatmap of the top five keywords by category. Each row represents a distinct author keyword, and each column corresponds to one of the five research categories. The color intensity indicates the frequency of keyword occurrences, with darker blue shades representing fewer occurrences and darker red shades indicating higher frequencies. The keyword “Electrolytes” appears prominently across most categories, especially in “Electrolyte Engineering” where it records the highest occurrence of 14 mentions. This indicates a strong focus on electrolyte materials across multiple research areas. The chart also features “Fast conductor” across several categories, most notably in “Electrolyte Engineering” (12 mentions), which aligns with the importance of conductivity improvements in electrolyte research.
Figure 4.
Bigram distribution of titles and abstracts within each category.
Figure 4.
Bigram distribution of titles and abstracts within each category.
Keywords such as “Management Systems” and “density” show notable frequencies in the Architecture Research and Multidisciplinary Review categories, reflecting a systems-oriented approach to battery performance and energy density. “Electrolyte Design” and “Polymer Electrolytes” are highly concentrated in Architecture Research and Electrolyte Engineering, where material design and polymer innovation are critical research topics. “Electrode” and “Anode” are dominant in Electrode Engineering, consistent with the category’s focus on electrode material and structure development.
“Recycling” and “Lithium-Ion” are key themes in Multidisciplinary Review, reflecting sustainability and material reuse concerns in the broader battery lifecycle. This heatmap provides a clear, comparative view of the distribution of specific research themes across different categories, offering insights into the dominant research focuses and emerging trends within the SSB field.
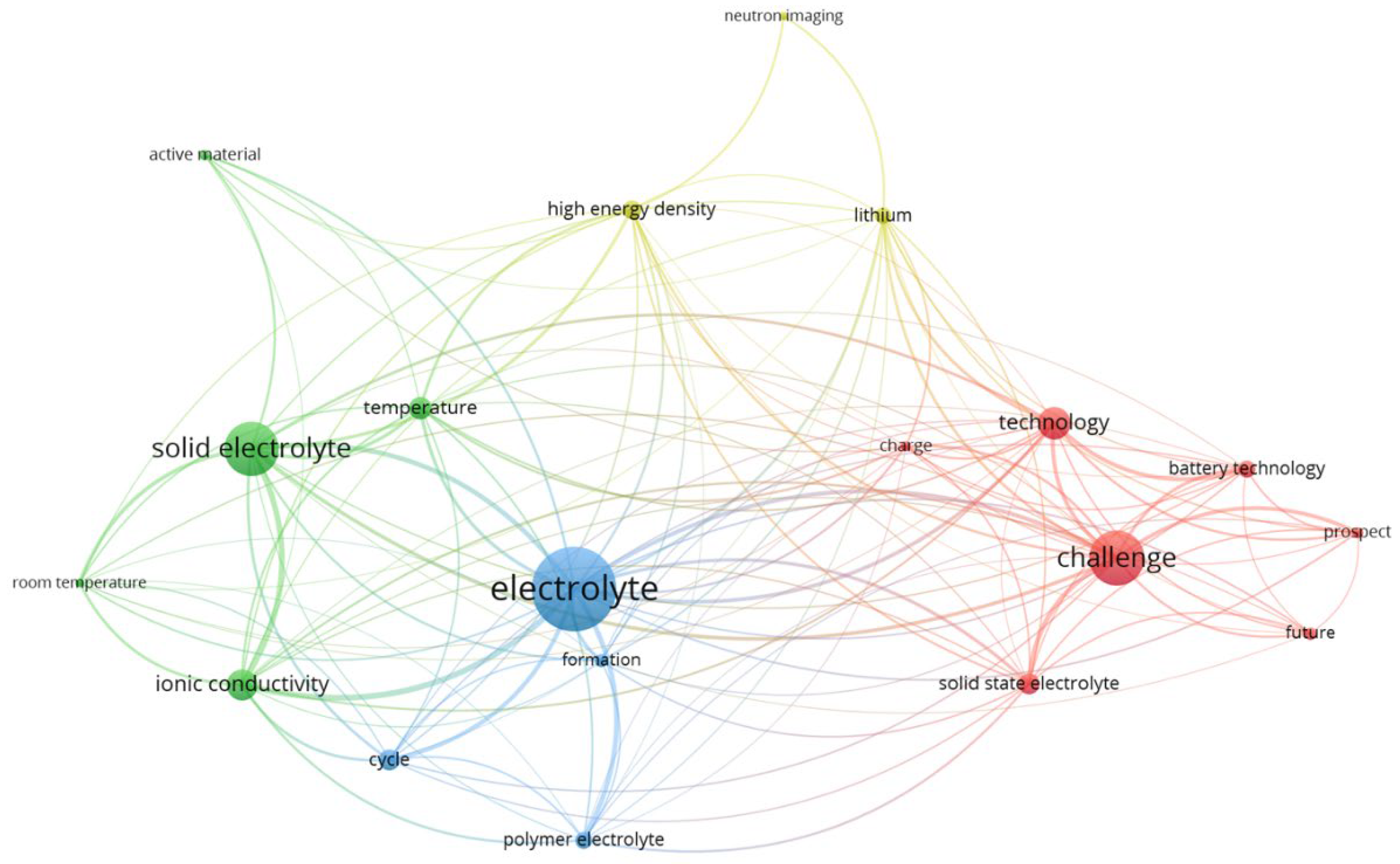
Figure 7 presents a term co-occurrence network generated using VOSViewer, illustrating the relationships between key terms within SSB research. Each node represents a specific term, and the size of the node correlates with its frequency of occurrence in the corpus. The lines (or edges) between nodes indicate co-occurrences, with the thickness of the lines representing the strength of the association between terms. The color coding reflects different clusters of closely related terms, indicating distinct thematic areas within the field. At the center of the network, the term “electrolyte” (blue cluster) forms the largest and most interconnected node, highlighting its central role in SSB research. Surrounding it are closely related terms such as “ionic conductivity,” “polymer electrolyte,” and “solid electrolyte,” emphasizing the focus on improving electrolyte materials for better performance. The green cluster centers around the term “solid electrolyte,” linking to terms such as “active material” and “ionic conductivity,” which further supports the emphasis on material innovations aimed at enhancing battery conductivity and stability at room temperature.
In the red cluster, terms like “challenge” and “technology” dominate, reflecting the ongoing challenges in the development and future prospects of SSB technology. Terms such as “battery technology,” “solid-state electrolyte,” and “future” are strongly associated with this cluster, pointing to the future-oriented research focus on overcoming technological hurdles.
Figure 5.
Categorical word clouds of author keywords.
Figure 5.
Categorical word clouds of author keywords.
Figure 6.
Cross sectional analysis of author keywords and research categories.
Figure 6.
Cross sectional analysis of author keywords and research categories.
Figure 7.
Keyword co-occurrence and cluster analysis.
Figure 7.
Keyword co-occurrence and cluster analysis.
Finally, the yellow cluster highlights terms such as “high energy density” and “lithium,” emphasizing efforts to increase energy density in SSBs, a key driver for their application in electric vehicles and other high-demand technologies. Overall, this network visualization provides a comprehensive overview of the interconnection of different research themes and challenges, offering insights into the dominant topics and their relationships within the SSB research community.
4.2. Bibliometric Analysis
Figure 8 provides a comprehensive analysis of the publication trends in SSB research through four distinct visualizations.
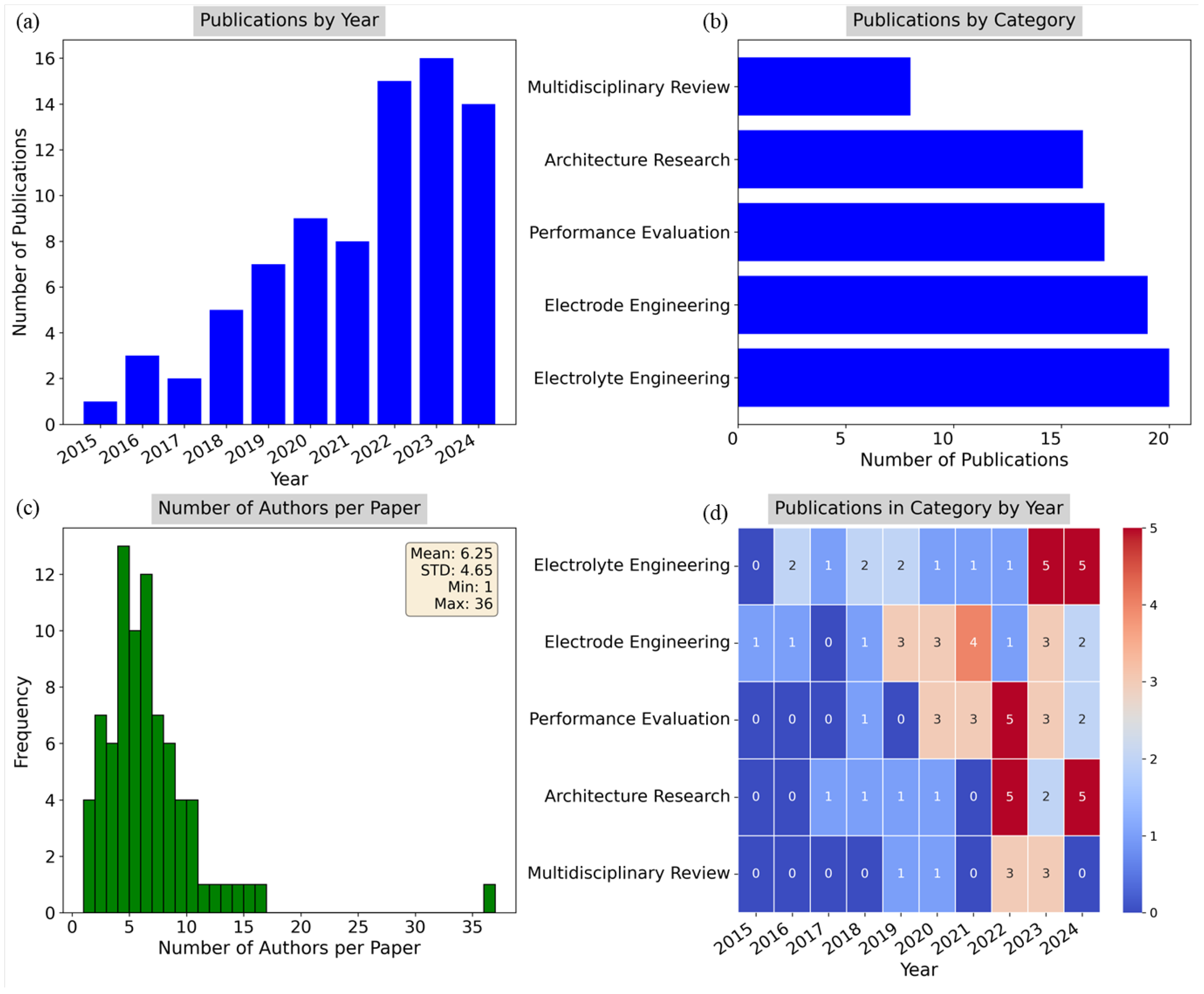
Figure 8a presents the number of publications by year, showcasing a steady increase in scholarly output from 2015 through 2024. The growth is particularly notable after 2018, reflecting the rising interest and advancements in SSB technologies. The year 2023 saw the highest number of publications, with 2024 expected to continue this upward trend.
Figure 8b depicts the number of publications by category, indicating the distribution of research across the five thematic areas. Electrolyte Engineering leads with the highest number of publications, followed closely by Electrode Engineering and Performance Evaluation. Multidisciplinary Review has the fewest, suggesting a more focused approach in specific technical domains rather than broader reviews.
Figure 8c displays a histogram of the number of authors per paper, showing that most publications have between three and 10 authors, with the mean number of authors being 6.25 with a standard deviation of 4.65. Pasta et al. (2020) was an outlier with 36 authors, reflecting a large-scale collaborative effort with several universities and institutions in the United Kingdom [46].
Figure 8d is a heatmap representing the distribution of publications in each category by year. The color intensity indicates the number of publications within each category, with Electrolyte Engineering and Electrode Engineering maintaining steady growth over the years. Other categories, such as Performance Evaluation and Architecture Research, have shown intermittent publication activity, with peaks in 2022 and 2024. These visualizations collectively highlight the increasing focus on solid-state batteries over the last decade, with a particular emphasis on electrolyte and electrode innovations. The interdisciplinary nature of the field is also evident in the large number of authors per paper on average, reflecting the collaborative efforts driving this area of research.
Figure 9 presents a set of heatmaps detailing the distribution of publications across several key bibliometric dimensions, including lead authors, countries, affiliations, and journals.
Figure 9a shows publications by the top 10 lead authors, revealing that authors such as Jin Yi (7 co-authors) and Rui Yang (13 co-authors) have contributed most actively to the SSB field. The heatmap highlights that these authors began publishing more frequently after 2020, with a few outliers making significant contributions earlier in 2017.
Figure 9b illustrates publications by the top 10 countries of lead authors, with China and the United States leading in output. In particular, China has shown a continuous rise in the number of publications since 2020, while the U.S. peaked in 2024. Germany and Japan also had a notable presence, with consistent contributions across the years.
Figure 9c focuses on publications by the top 10 affiliations of lead authors, showcasing prominent institutions such as Tsinghua University, University of Maryland, and Argonne National Laboratory. The heatmap demonstrates that these affiliations have been particularly active between 2020 and 2024, indicating strong institutional interest in SSB research during this period.
Figure 9d details publications in the top 10 journals by category, highlighting the primary outlets for research in each thematic category. Advanced Energy Materials, Advanced Materials, and Energy Storage Materials have the highest representation, particularly for Electrode Engineering and Electrolyte Engineering. The categories Performance Evaluation and Multidisciplinary Review have fewer dedicated publications in top journals, suggesting more specialized or niche outlets for these areas. These heatmaps provide a clear overview of the geographical, institutional, and journal-level trends in SSB research, offering insights into the key contributors and publication channels driving the field forward.
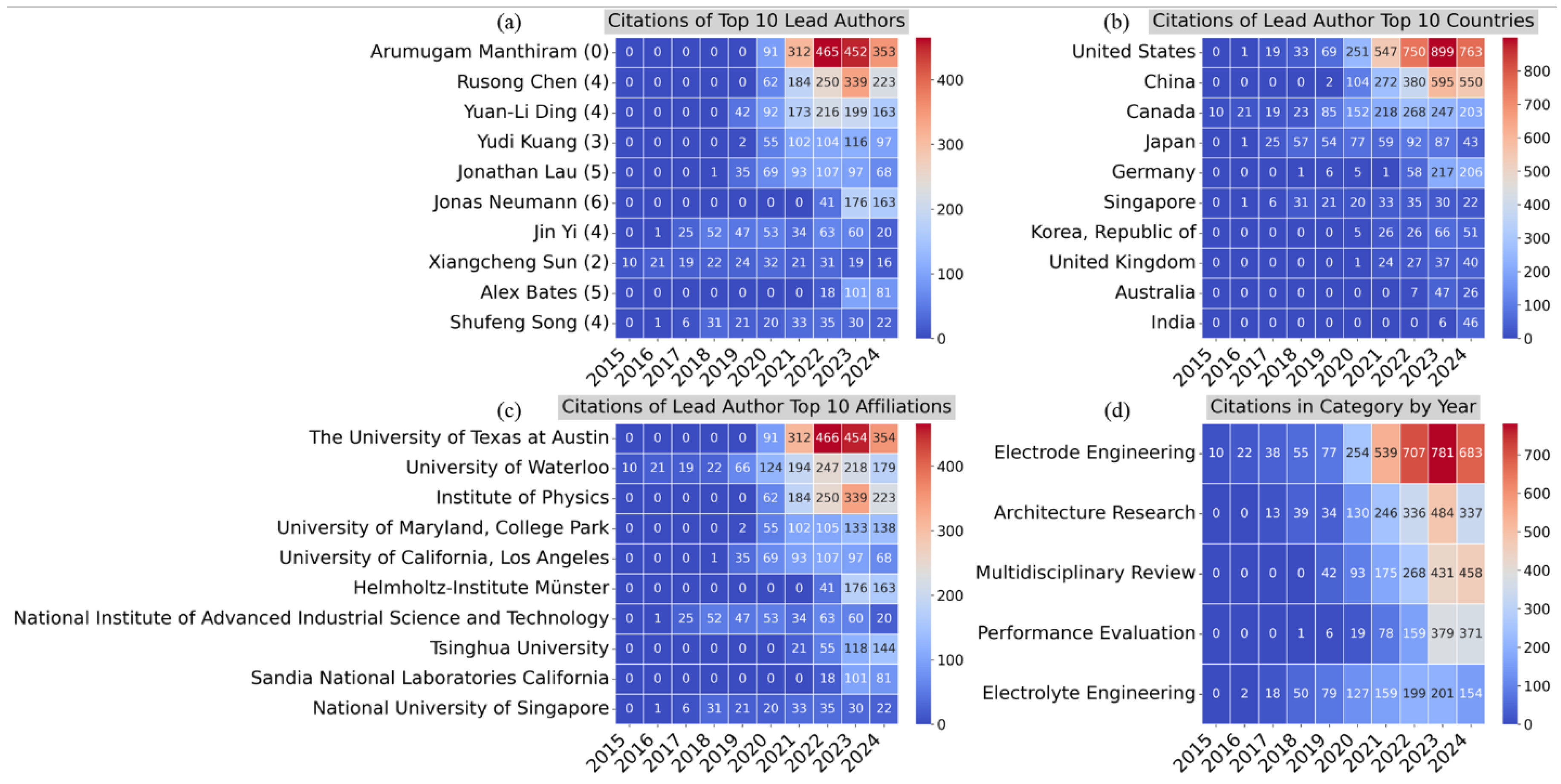
Figure 10 presents a series of heatmaps analyzing citation trends in SSB research. The figure categorizes the data by lead authors, countries, affiliations, and research categories, providing insights into the impact and influence of different contributors and regions within the field.
Figure 10a displays the citations of the top 10 lead authors by year. Arumugam Manthiram stands out with a significant increase in citations beginning in 2021, reaching over 450 citations in 2023. Other authors, such as Rusong Chen, Yuan-Li Ding, and Jonathan Lau, have experienced steady growth in citations, reflecting their growing influence in the field over time.
Figure 10b highlights the citations of lead authors from the top 10 countries. The United States leads with the highest number of citations, particularly from 2020 to 2023, peaking at about 900 citations in 2022. China follows closely, with a significant rise starting in 2020. Other countries, such as Canada, Japan, and Germany, also contribute notable citation counts, though at a smaller scale compared with the U.S. and China.
Figure 10c focuses on the citations of lead authors by the top 10 affiliations. The University of Texas at Austin and the University of Waterloo show the most substantial citation impact, particularly from 2021 to 2023. The Institute of Physics and University of Maryland also demonstrate growing citation influence, while institutions such as Helmholtz-Institute Münster and Tsinghua University showed more recent growth in impact.
Figure 10d presents the citations by research category over the years. Electrode Engineering leads with the highest number of citations, particularly from 2021 onward, peaking at 781 citations in 2023. Architecture Research and Multidisciplinary Review categories have also seen increasing citation counts, reflecting their rising importance in recent years. Electrolyte Engineering shows steady but moderate citation growth, while Performance Evaluation remains consistent but comparatively lower in citation impact. Overall, these heatmaps highlight the increasing influence of certain authors, institutions, and countries within the SSB research community. The growth in citations reflects the evolving importance of specific research themes, with Electrode Engineering and Architecture Research emerging as particularly high-impact areas in recent years.
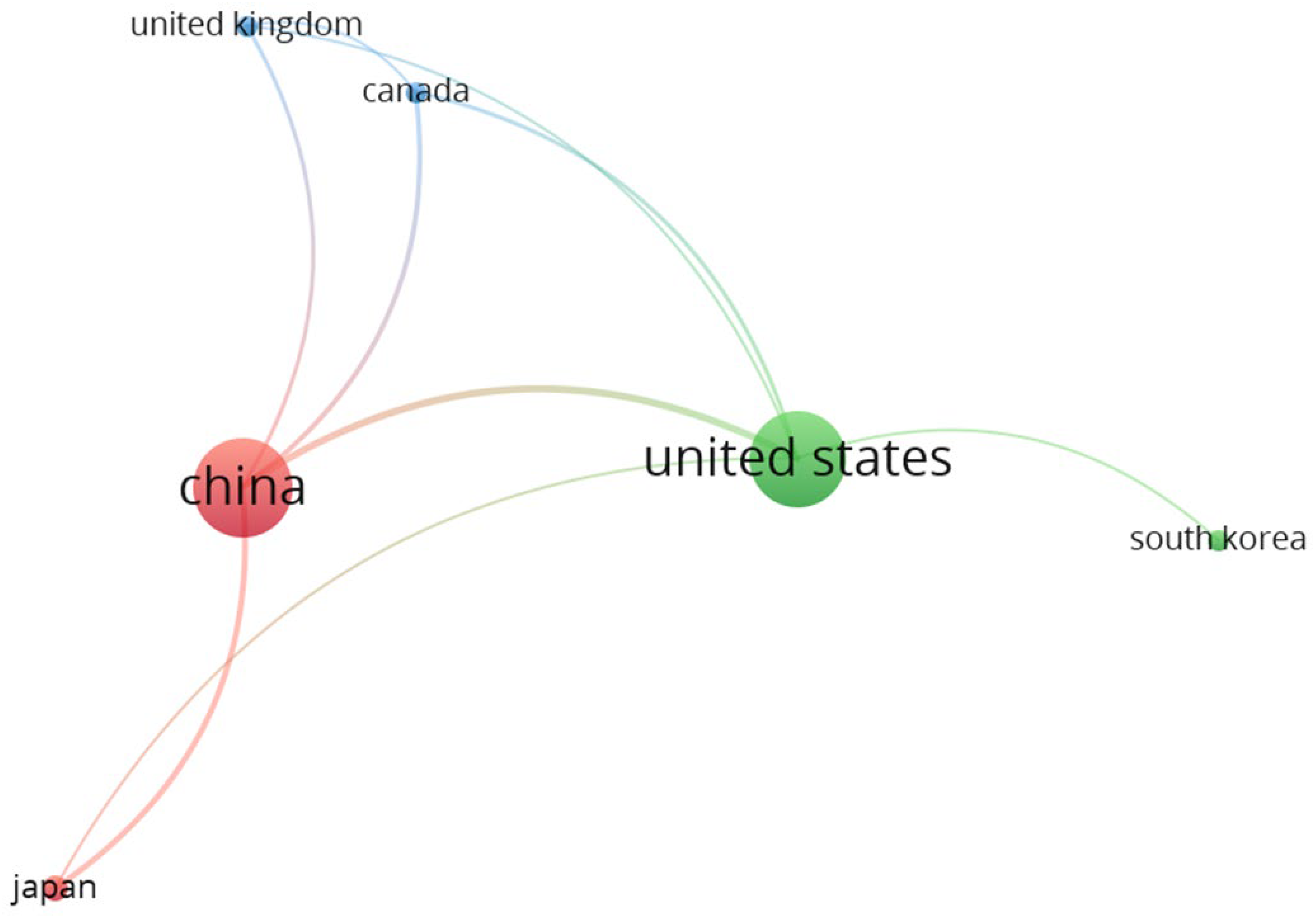
Figure 11 illustrates the country co-authorship network for SSB research, focusing on the six countries that have contributed to at least five publications.
The network diagram shows the collaborative relationships between these countries, with node size indicating the number of documents, and the thickness of the connecting lines representing the strength of collaboration (link strength) between countries.
Table 1 provides additional data on the number of documents, citations, and total link strength (TLS) for each country.
China and the United States dominate the network, with the largest nodes and the strongest co-authorship links. They show significant collaboration with each other as well as with other countries like Canada, Japan, and the United Kingdom. China led with 27 publications, 2,617 citations, and a total link strength of 15 while the United States followed closely with 26 publications, 4,561 citations, and a total link strength of 11. Japan ranked third in publications with seven documents and 534 citations, but a much lower total link strength of 5, indicating a lesser degree of international collaboration compared with China and the United States. The United Kingdom, South Korea, and Canada exhibit moderate contributions to the field, each with six publications and varying degrees of collaboration. Overall, the network highlights the global nature of SSB research, with the United States and China acting as central hubs for international collaboration and driving much of the citation impact in the field.
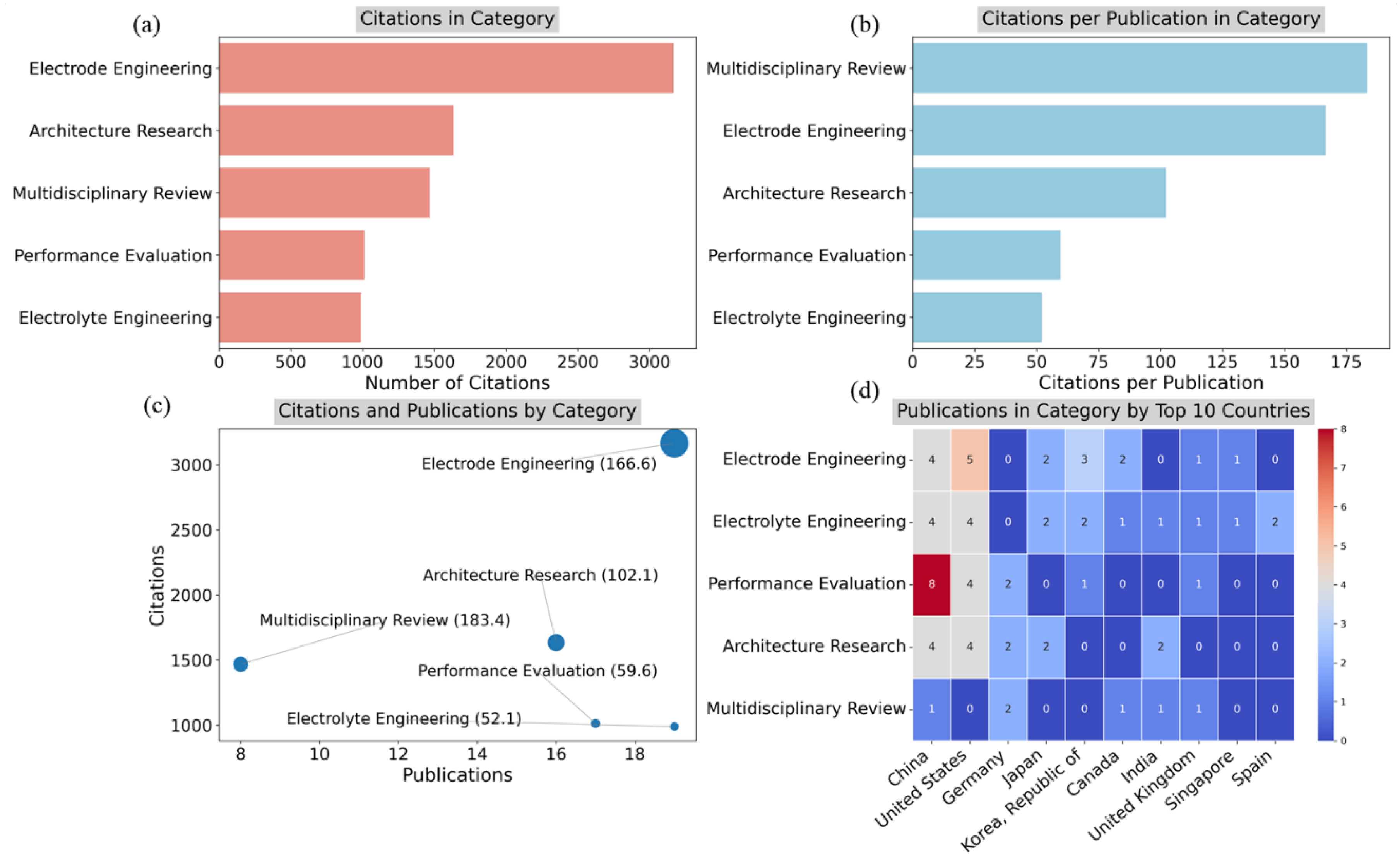
Figure 12 provides a detailed analysis of citation patterns across the research categories, including the number of citations, citation rates per publication, and publications distribution by country.
Figure 12a shows the citations in each category, highlighting that Electrode Engineering garnered the highest number of citations (more than 3,000), followed by Architecture Research and Multidisciplinary Review, each with more than 1,500 citations. Electrolyte Engineering and Performance Evaluation have received fewer citations overall.
Figure 12b examines the citations per publication in each category, revealing that Multidisciplinary Review has the highest citation rate per publication, with more than 175 citations per paper. Electrode Engineering and Architecture Research also had a high influence, each averaging over 100 citations per paper, while Performance Evaluation and Electrolyte Engineering have lower citation rates.
Figure 12c provides a scatter plot of citations versus publications by category, where the size of the bubble represents the average number of citations per publication. Electrode Engineering stands out with both the highest number of publications and the highest citation count, averaging 166.6 citations per paper. Multidisciplinary Review has fewer publications but a high citation average (183.4), indicating significant influence despite fewer contributions. Architecture Research and Performance Evaluation fall in the middle range, while Electrolyte Engineering has the lowest citation impact, averaging 52.1 citations per publication.
Figure 12d presents a heatmap showing the distribution of publications in each category by the top 10 countries. China and the United States dominate across categories, especially in Performance Evaluation and Electrolyte Engineering. Germany shows a weak presence in both electrode and electrolyte engineering, while Japan, South Korea, and other nations contribute more moderately across the various research categories. Overall,
Figure 12 emphasizes the dominance of Electrode Engineering in both citation impact and publication volume, while also showcasing the importance of Multidisciplinary Review for high-impact research despite its smaller number of publications. The distribution across countries highlights China and the United States as key players in the field.
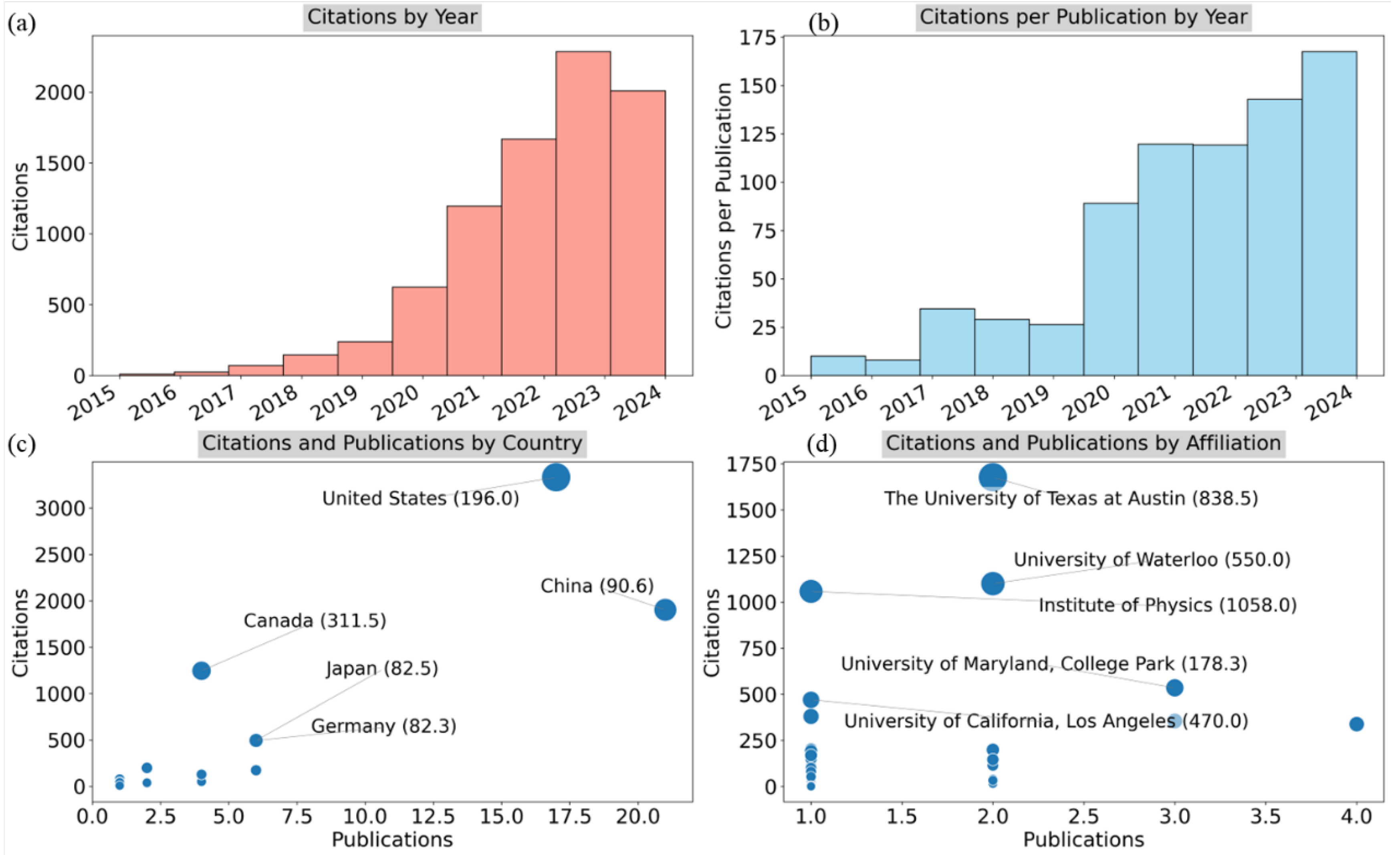
Figure 13 provides an in-depth look at citation trends in SSB research, focusing on citation patterns by year, country, and institutional affiliation.
Figure 13a illustrates the citations by year, showing a dramatic rise in citations starting in 2020, with a sharp increase through 2023. This trend reflects the growing attention to SSB research, with citations more than doubling between 2020 and 2023. The data as of September 2024 indicate a continued high impact of this field.
Figure 13b examines the citations per publication by year, which mirrors the pattern in citations by year. The average number of citations per publication has steadily increased since 2018, with a notable peak up to September 2024 when each publication averaged more than 150 citations. This indicates that recent studies are gaining significant traction in the academic community.
Figure 13c presents a scatter plot of citations versus publications by country, with the size of each bubble representing the average number of citations per publication. The United States stands out with the highest number of citations and publications, averaging 196 citations per paper.
China follows, though its citation rate per publication is slightly lower at just over 90. Canada has fewer publications but a comparatively high citation impact, with an average of just over 311 citations per publication. Japan and Germany also contribute notably but with fewer citations and publications overall.
Figure 13d provides a scatter plot of citations versus publications by institutional affiliation, with the University of Texas at Austin, the University of Waterloo, and the Institute of Physics standing out for their high citation impact. The University of Texas at Austin has a citation average of just over 838, while the Institute of Physics leads with 1,058 citations per paper, indicating a substantial influence in the field. The University of Waterloo and University of Maryland also show strong citation performance, reflecting their importance in the global research landscape for SSB research.
Overall,
Figure 13 highlights the rapid rise in citation impact for SSB research in recent years, with the United States leading in both publication volume and citation count. Institutions such as the University of Texas and the University of Waterloo are emerging as key contributors to this growing body of literature.
4.3. Specific Developments
4.3.1. Electrolyte Engineering
Electrolyte engineering continues to make significant strides in advancing SSB performance, especially in areas such as ionic conductivity, mechanical stability, and compatibility with lithium-metal anodes. Research in this field focuses on synthesizing innovative electrolyte materials, both solid and hybrid, which offer improved conductivity and stability at room temperature, as well as enhanced electrochemical performance under high-voltage conditions. The development of these materials addresses the key challenges of lithium dendrite formation, low conductivity at ambient temperatures, and poor interfacial contact between the electrolyte and electrode materials.
Table 2 summarizes key findings from recent studies in electrolyte engineering.
4.3.2. Electrode Engineering
Electrode engineering is pivotal in enhancing the performance, energy density, and durability of solid-state batteries. Recent innovations have focused on developing electrodes that are structurally robust, capable of high energy transfer, and effectively integrated with solid-state electrolytes. These advancements aim to address the challenges related to energy barriers, electrode material distribution, and interfacial stability, which are critical for optimizing battery performance and life cycle.
Table 3 summarizes notable contributions from research in this area while
Table 4 and
Table 5 focus separately on specific innovations in cathode and anode engineering, respectively.
Architecture Research
Table 6 is a summary of recent key developments in SSB architectural research. This domain covered innovations in battery structure, materials, and performance. Architectural advancements aim to address challenges such as scalability, mechanical stability, fast charging, and the integration of novel materials into battery cell, module, and pack designs.
The focus is often on improving the energy density, structural integrity, and overall efficiency of batteries through innovative design concepts like bipolar stacking, nanoscale materials, and structural batteries.
4.3.3. Performance Evaluation
Performance evaluation plays a critical role in advancing SSB technology by identifying key limitations and areas for improvement. Researchers utilize various techniques to analyze battery performance, focusing on factors such as ionic conductivity, interfacial stability, structural integrity, and thermal management. These evaluations help to address challenges in battery degradation, mechanical stress, and heat dissipation, all of which are crucial for optimizing SSB efficiency and longevity.
Table 7 is a summary of recent key findings in SSB performance evaluations.
4.3.4. Multidisciplinary Reviews
Multidisciplinary reviews play a crucial role in providing a holistic perspective on the technological, economic, and environmental aspects of SSBs in contrast to LIBs. These reviews not only covered the advancements in battery technology but also assessed the broader market trends, regulatory impacts, and recycling challenges, offering valuable insights into the future direction of the industry.
Table 8 highlights key findings from relevant multidisciplinary reviews, particularly focusing on the intersection of technology development, market forces, and environmental concerns.
5. Discussion
LIBs, widely used in portable electronics and electric vehicles, owe their efficiency and popularity to the advancement in cathode materials. These materials have allowed for higher energy density compared with other battery systems. Three main categories of oxide cathodes have driven the progress of modern LIBs: layered oxides, spinel oxides, and polyanion oxides [10]. Layered oxides (e.g., LiCoO2) are known for their high voltage close to 4 V and fast charge/discharge rates. Despite their structural stability and high conductivity, they have low capacities and utilize expensive, resource constrained materials like cobalt. Spinel oxides (e.g., LiMn2O4) offer cost advantages and higher thermal stability. However, they suffer from issues like Mn dissolution, which affects long-term cycling performance. Polyanion oxides (e.g., LiFePO4) offer improved safety and thermal stability at the cost of lower energy density. Their potential lies in the tunability of operating voltage through various chemistries, like phosphates and sulfates.
This literature review found that research has focused on developing low-cost, high-energy cathodes that maintain performance over long cycling periods. Hence, the industry must continue to explore novel chemistries and improve manufacturing processes for large-scale applications like electric vehicles and grid storage. As environmental sustainability becomes a priority, finding solutions to minimize the use of cobalt and other scarce materials will be critical. The next subsections discuss the specific developments summarized in the tables in the results section.
5.1. Electrolyte Engineering
Advancements in electrolyte engineering for SSBs have centered on enhancing ionic conductivity, mechanical stability, and compatibility with electrodes through innovative material design and processing techniques. A prominent strategy involves creating composite electrolytes that synergistically combine different materials to optimize performance. For example, Thomas et al. (2024) engineered a nanocomposite gel electrolyte by exfoliating kaolinite into nanoplatelets and integrating them with a succinonitrile liquid electrolyte, achieving high ionic conductivity and thermal stability beyond 100°C [49]. Similarly, Martinez et al. (2019) [52] and Yi et al. (2017) [54] developed hybrid polymer-ceramic electrolytes, blending polymers with ceramic materials to improve ionic transport and mechanical properties, demonstrating efficient interfacial contact and substantial energy densities.
Another approach focused on doping and modifying solid electrolytes to tailor ionic pathways and enhance mechanical properties. Zhu et al. (2024) doped LATP ceramic electrolytes with zinc, strengthening mechanical attributes while optimizing lithium-ion channels, leading to 180 cycles of battery life [48]. Li et al. (2023) utilized a halide solid electrolyte (Li3InCl6) to create supersonic conducting grain boundaries, reducing ion-hopping energy barriers and suppressing dendrite growth, which resulted in 2,000 cycles and 93.7% capacity retention [30]. Zhang et al. (2023) developed a dual-halogen solid electrolyte (Li3YCl6), significantly increasing ionic conductivity and retaining 80% capacity after 1,000 cycles at low temperatures [50].
Innovation in electrolyte materials was also evident in the development of novel organic and inorganic compounds. Lee et al. (2023) introduced an organic solid electrolyte based on a diethylene glycol-modified pyridinium covalent organic framework, achieving high ionic conductivity and effective dendrite suppression [27]. Choi et al. (2024) synthesized a solid electrolyte through a novel separation process, post high-energy ball milling, enhancing ionic conductivity and lithium metal compatibility at room temperature [47]. Swathi et al. (2023) incorporated calcium hydroxide nanofillers derived from natural seashells into a polymer electrolyte, improving ionic conductivity and highlighting a trend toward utilizing abundant natural resources [34].
Comprehensive reviews by Ha et al. (2022) [51] and Lau et al. (2018) [53] on sulfide-based solid electrolytes highlight ongoing challenges and opportunities in wet chemical synthesis and material optimization. Collectively, these studies highlight a trend toward multifunctional electrolyte materials that address key challenges such as enhancing ionic conductivity, mechanical robustness, dendrite suppression, and compatibility with various electrode materials, moving closer to the realization of high-performance solid-state batteries.
5.2. Electrode Engineering
Advancements in electrode engineering for SSBs have primarily targeted enhancing interfacial contact, mechanical stability, and energy density through innovative material design and interface optimization. A central theme involved improving the electrode-electrolyte interface to reduce internal resistance and facilitate efficient ionic and electronic transport. Mills et al. (2024) optimized the molecular weight of binders in flexible, durable solid-state electrodes to achieve structural integrity and low grain boundary resistance, resulting in an impressive energy density of 500 Wh/kg [14]. Similarly, Hayakawa et al. (2023) enhanced contact between electrode materials and solid electrolytes by coating them with conductive additives like acetylene black and vapor-grown carbon fiber, effectively reducing internal resistance [8].
Material modifications have been pivotal in addressing challenges such as dendrite formation and mechanical degradation. Alexander et al. (2023) introduced a single-phase mixed ion- and electron-conducting garnet for both electrodes, significantly increasing energy transfer and potentially extending battery life to 3,700 charge cycles [7]. To suppress dendrite growth and ensure uniform lithium deposition, Choi et al. (2021) [31] and Lee et al. (2020) [59] incorporated silver-based interlayers and nanocomposite layers, respectively, which formed silver-lithium alloys during cycling. These strategies resulted in enhanced stability over numerous cycles, with Lee et al. achieving stability over 1,000 cycles.
Efforts to increase energy density have also led to the exploration of thick electrode designs and silicon-based anodes. Kuang et al. (2019) reviewed the potential of thick electrodes to store more active material while reducing inactive components, aiming for higher energy densities without altering battery chemistry [18]. Mills et al. (2024) applied this concept by developing thick electrodes that maintained flexibility and durability [14]. On the anode front, Liu et al. (2023) surveyed silicon-based anodes, highlighting their high lithium storage capacity due to alloy formation [19]. However, silicon’s volume expansion poses challenges. Keles et al. (2020) addressed this by creating superlattice-structured anode films with alternating layers of silicon, molybdenum, and copper, which distributed stress evenly and prevented damage, maintaining a capacity of over 150 cycles [61].
Cathode engineering has also seen significant advancements through surface modifications and interface engineering to enhance performance and stability. Li et al. (2021) improved nickel-rich cathodes by coating them with nanoscale solid electrolyte particles, enhancing ionic conductivity and stabilizing the interface, resulting in 80% capacity retention after 400 cycles [57]. Huang et al. (2024) outlined strategies for improving single-crystal nickel-rich layered cathode materials, including element doping and surface-interface modification, linking structural design to electrochemical performance [37].
Overall, the research trends in electrode engineering emphasize the critical role of interface optimization, material innovations, and structural design to overcome challenges inherent to SSBs. These collective advancements contribute to enhanced battery performance, safety, and longevity, moving the technology closer to practical, high-energy-density applications.
5.3. Architecture Research
Advancements in solid-state battery (SSB) architecture have increasingly focused on integrating multifunctional materials and innovative designs to enhance performance, scalability, and sustainability. A notable trend is the development of structural batteries that combine energy storage with mechanical functions, aiming to reduce overall system weight and improve efficiency. Chaudhary et al. (2024) demonstrated a structural LIB using carbon fiber electrodes coated with LiFePO4, achieving 30 Wh/kg energy density, high cyclic stability up to 1,000 cycles, and an elastic modulus of 76 GPa [65]. Similarly, Iyer et al. (2024) developed a structural sodium-ion battery by incorporating glass fiber within a solid-state composite electrolyte based on polyethylene oxide, resulting in a high-tensile-strength electrolyte and an energy density of 23 Wh/kg [43]. Fu et al. (2022) proposed a structural battery based on modified fiber metal laminates, where metal sheets serve dual roles of impact resistance and electrical conduction, exemplifying the multifunctional approach [68].
Researchers also explored innovative architectural designs to address inherent challenges in SSBs. Sharma et al. (2024) reviewed anode-free configurations utilizing bipolar architectures, which can simplify battery design and potentially enhance energy density [29]. Li et al. (2022) proposed bipolar stacking of solid-state cells using a non-flammable ionogel between solid particles, aiming to improve safety and performance [67]. Yamamoto et al. (2018) introduced a binder-free sheet-type battery construction employing removable volatile binders based on polypropylene carbonate, achieving a 2.6-fold increase in energy density over previous designs, highlighting the benefits of architectural innovation [69].
The application of nanotechnology and advanced materials is another prominent theme. Kumar et al. (2024) reviewed the use of nanoscale technologies in electric vehicle components, including battery materials incorporating nanotubes, graphene, and metal oxides, which can enhance conductivity and structural properties [16]. Xu et al. (2019) developed a catalyst and membrane for zinc-air batteries using a hybrid of cobalt and manganese oxides anchored on carbon nanotubes, significantly improving catalytic activity and battery performance [23].
Comprehensive reviews by Kazyak et al. (2024) [64], Ma et al. (2023) [38], and Lee et al. (2024) [9] have identified ongoing challenges such as scalability, interfacial resistances, and mechanical instabilities at high currents. These works emphasize the need for sustainable processing methods and highlight areas where further research is critical. The exploration of alternative chemistries, as discussed by Whang et al. (2023) in the context of transition metal sulfide conversion for stationary energy storage, indicates a broadening of research efforts to find suitable materials and designs for various applications [66].
Overall, architectural research in solid-state batteries is trending toward integrating multifunctional materials, innovative designs, and nanotechnology to overcome current limitations. The focus on structural batteries and novel architectures highlights a holistic approach to battery development, simultaneously optimizing mechanical properties and energy storage capabilities. These advancements represent essential steps toward realizing SSBs that are not only high performing but also scalable and adaptable to diverse applications.
5.4. Performance Evaluation
Advancements in SSB performance evaluations have primarily focused on understanding and mitigating factors that limit their efficiency, safety, and longevity. A significant area of research addressed the thermal management challenges posed by the higher internal resistance of SSBs compared with traditional LIBs. Yang et al. (2024) highlighted that this increased resistance leads to greater heat generation, necessitating more capable thermal management systems [3]. To tackle this issue, Yang et al. (2024) developed a micro heat pipe array with air cooling at the battery pack level, effectively enhancing thermal regulation [41].
Another critical focus was the investigation of interfacial degradation mechanisms between solid electrolytes and electrodes, which are pivotal for battery performance and stability. Advanced characterization techniques have been employed to gain insights into these interfacial phenomena. Singh et al. (2023) used in-situ electrochemical scanning electron microscopy to examine the anode interface between lithium metal and a solid electrolyte (Li6PS5Cl) [28]. They discovered that a nanocrystalline lithium structure facilitates a stable interface through Coble creep deformation and supports high currents exceeding 150 mA/cm². Similarly, Zhang et al. (2019) utilized in-situ electrochemical impedance and Raman spectroscopy to reveal that lithium-ion migration significantly contributes to interfacial reactions that deteriorate electrochemical performance [72].
Researchers have advocated characterization methods like neutron imaging and in-situ transmission electron microscopy (TEM) to visualize and understand internal processes within SSBs. Cao et al. (2023) promoted neutron imaging to assess interfacial compatibility, reaction mechanisms, and structural stability [4]. Guo et al. (2022) employed in-situ TEM with a heating device to determine that lithium-ion conductivity, rather than electronic conductivity, dictates the performance of lithium-selenium SSBs at varying temperatures [71].
Efforts to enhance ionic conductivity and interfacial contact through material engineering have also been prominent. Yang et al. (2022) reviewed strategies such as doping, defect engineering, microstructure tuning, and interface modification to improve ionic conductivity in SSBs [32]. Gutierrez-Pardo et al. (2018) compared ceramic and polymer-ceramic composite electrolytes, finding that the composite performed better at elevated temperatures due to enhanced interfacial contact without the need for coatings [73].
Additionally, studies have identified vulnerabilities of certain solid electrolytes to temperature-induced degradation. Yoon et al. (2022) evaluated Li6PS5Cl and found it susceptible to degradation at temperatures as low as 70°C, emphasizing the need for thermally stable electrolyte materials [70]. Li et al. (2022) summarized experimental observations and modeling efforts to understand factors influencing cell degradation, including mechanical origins and structural changes, to forecast the evolution of SSB performance [67].
Collectively, these studies highlight the importance of thermal management, interface stability, and material optimization in advancing SSB technology. The utilization of advanced in-situ characterization techniques has been instrumental in uncovering underlying mechanisms affecting battery performance, guiding the development of strategies to enhance efficiency and longevity. The research trends highlight a comprehensive approach to addressing thermal effects and interfacial phenomena, which are crucial for the practical implementation of high-performance SSBs.
5.5. Multidisciplinary Review
Multidisciplinary reviews in SSB research have illuminated critical challenges and trends in recycling, market dynamics, and material resource management that influence the future of battery technology and electric vehicles (EVs). A significant theme involved the complexities of recycling SSBs compared with traditional lithium-ion batteries (LIBs). Wu et al. (2023) [5] and Neumann et al. (2022) [75] explored these challenges, emphasizing that existing recycling practices—including material collection, sorting, transportation, and regulatory compliance—must adapt to accommodate the unique properties of solid electrolytes and evolving battery chemistries. These studies underscore the necessity for developing efficient recycling processes to ensure the sustainable integration of SSBs into the market.
Market trends and economic drivers form another critical focus area. Boretti (2022) observed that environmental concerns, rather than purely economic factors, are propelling the shift toward battery EVs [74]. This suggests that policy interventions and consumer awareness are pivotal in driving market adoption. Bajolle et al. (2022) forecasted LIB price trends under various scenarios, highlighting the potential for rapid price stabilization due to raw material supply constraints or market disruption through the introduction of SSBs [1]. Fichtner (2021) analyzed trends in LIB storage capacity and cost reductions, noting that only a fraction of improvements stem from better chemical compositions [2]. The reviews increasingly attributed advancements to innovations in battery architecture, such as cell-to-pack and cell-to-chassis designs, which enable the use of more abundant and lower-cost materials like lithium iron phosphate (LiFePO4) over less sustainable cobalt-based compounds.
Researchers have thoroughly reviewed the evolving requirements for active battery materials and their implications. Ball et al. (2020) discussed the changing demands for active materials in automotive LIB applications, anticipating industry responses to these shifts [76]. Ding et al. (2019) focused on the challenges related to critical element resources, the evolution of the EV market, and the associated LIB costs and performance [77]. These studies collectively highlight the pressing need to identify alternative materials that balance performance, cost, and environmental impact, ensuring the sustainability of battery technologies.
Overall, these multidisciplinary reviews highlight a trend toward integrating sustainability considerations across all facets of battery development—from recycling and resource management to architectural innovations and market strategies. The collective insights emphasize that the future success of SSBs and the broader adoption of EVs will depend not only on technological advancements but also on addressing environmental, economic, and regulatory challenges to achieve long-term viability and market penetration.
5.6. Limitations and Future Research
While this study provides a comprehensive thematic and bibliometric analysis of recent SSB research, the author acknowledges several limitations. While the study highlights key advancements in battery materials and architecture and their performance evaluation, it does not delve deeply into the experimental nuances, manufacturing barriers, cost trends, and policy implications. The analysis also primarily focuses on developments within the recent decade, which may limit the historical perspective needed to fully contextualize the progress in SSB technology.
Future research should not only continue to explore material and interface optimization but also address the scalability and manufacturability challenges that remain critical barriers to commercial deployment. More targeted studies on the interplay between electrolyte-electrode interfaces and long-term SSRB stability are necessary, as well as greater emphasis on developing cost-effective, scalable production techniques. Enhanced diagnostic tools and deeper interdisciplinary collaboration will be essential for overcoming these challenges and realizing the full potential of SSRBs. Collaborative efforts across material science, engineering, and manufacturing will be essential to drive the next wave of innovation in SSB technology.
6. Conclusions
Solid-state rechargeable batteries (SSRBs), particularly those using lithium as the charge carrier, represent a transformative advancement in energy storage technology. They offer potential solutions to the safety, energy density, and longevity challenges faced by conventional lithium-ion batteries. This research has examined recent developments in SSB research through a thematic and bibliometric analysis, focusing on key areas such as electrolyte engineering, electrode engineering, battery architecture, and performance evaluation. The findings from these areas highlight both the progress made and the significant challenges that remain in advancing SSRBs toward commercial viability.
In electrolyte engineering, innovations such as doping, hybrid electrolyte designs, and improved synthesis techniques have contributed to increased ionic conductivity and thermal stability, addressing critical limitations in solid-state electrolyte performance. The development of materials like Li3InCl6 and LATP-based composites illustrates the potential for enhanced dendrite suppression and cycle stability, marking crucial steps forward in ensuring long-term performance and safety.
Electrode engineering has also seen substantial progress, particularly in the design of flexible, durable electrode materials that integrate better with solid electrolytes. Advances in binder optimization, interfacial coatings, and mixed-conducting materials have demonstrated improvements in energy transfer efficiency, cycling stability, and structural integrity. However, achieving seamless, low-resistance interfaces between solid electrolytes and electrodes remains a key challenge that the industry must address through further research.
The innovations in battery architecture have pushed the boundaries of what SSRBs can achieve, with developments in bipolar stacking, nanoscale technologies, and structural batteries showcasing the potential for scalable, high-energy-density designs. These architectural advancements aim to improve both the mechanical strength and electrochemical performance of SSRBs, with applications extending beyond electric vehicles to grid storage and stationary energy solutions.
Performance evaluation remains critical in identifying SSBs’ practical limitations. The use of advanced imaging and diagnostic techniques, such as neutron imaging, in situ electrochemical microscopy, and thermal management systems, has provided valuable insights into the degradation mechanisms, ion transport limitations, and thermal challenges faced by SSRBs. These evaluations emphasize the need for improved thermal management systems, stable interfacial contact, and scalable processing techniques to fully realize the potential of SSRBs. Despite these advancements, the transition to commercially viable SSRBs requires overcoming significant material, manufacturing, and integration hurdles. This work highlights the ongoing efforts to address these challenges through multidisciplinary research that integrates material science, engineering, and performance evaluation. The collaborative focus on improving solid electrolyte performance, electrode-electrolyte interfaces, and scalable battery designs reflects the industry’s strong belief in the potential of SSBs, particularly those using lithium to revolutionize energy storage. The ongoing focus on materials optimization, interface stability, and scalable architecture will be critical in pushing SSBs from the lab to the market, bringing society closer to a safer, more efficient, and sustainable energy future.
Funding
This research received funding from the United States Department of Transportation, Center for Transformative Infrastructure Preservation and Sustainability (CTIPS), Funding Number 69A3552348308.
Data Availability Statement
This article includes the data presented in the study.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Bajolle, H.; Lagadic, M.; Louvet, N. The Future of Lithium-Ion Batteries: Exploring Expert Conceptions, Market Trends, and Price Scenarios. Energy Res. Soc. Sci. 2022, 93, 102850. [Google Scholar] [CrossRef]
- Fichtner, M. Recent Research and Progress in Batteries for Electric Vehicles. Batter. Supercaps 2021, 5. [Google Scholar] [CrossRef]
- Yang, R.; Xie, Y.; Li, K.; Tran, M.-K.; Fowler, M.; Panchal, S.; Deng, Z.; Zhang, Y. Comparative Study on the Thermal Characteristics of Solid-State Lithium-Ion Batteries. IEEE Trans. Transp. Electrification 2023, 10, 1541–1557. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, Y.; Ji, T.; Zhu, H. In operando neutron imaging characterizations of all-solid-state batteries. MRS Bull. 2023, 48, 1257–1268. [Google Scholar] [CrossRef]
- Wu, X.; Ji, G.; Wang, J.; Zhou, G.; Liang, Z. Toward Sustainable All Solid-State Li–Metal Batteries: Perspectives on Battery Technology and Recycling Processes. Adv. Mater. 2023, 35, e2301540. [Google Scholar] [CrossRef]
- Razali, A.A.; Norazli, S.N.; Sum, W.S.; Yeo, S.Y.; Dolfi, A.; Srinivasan, G. State-of-the-Art of Solid-State Electrolytes on the Road Map of Solid-State Lithium Metal Batteries for E-Mobility. ACS Sustain. Chem. Eng. 2023, 11, 7927–7964. [Google Scholar] [CrossRef]
- Alexander, G.V.; Shi, C.; O’neill, J.; Wachsman, E.D. Extreme lithium-metal cycling enabled by a mixed ion- and electron-conducting garnet three-dimensional architecture. Nat. Mater. 2023, 22, 1136–1143. [Google Scholar] [CrossRef]
- Hayakawa, E.; Nakamura, H.; Ohsaki, S.; Watano, S. Design of active-material/solid-electrolyte composite particles with conductive additives for all-solid-state lithium-ion batteries. J. Power Sources 2022, 555. [Google Scholar] [CrossRef]
- Lee, J.; Zhao, C.; Wang, C.; Chen, A.; Sun, X.; Amine, K.; Xu, G.-L. Bridging the gap between academic research and industrial development in advanced all-solid-state lithium–sulfur batteries. Chem. Soc. Rev. 2024, 53, 5264–5290. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2. [Google Scholar] [CrossRef]
- Bridgelall, R. Aircraft Innovation Trends Enabling Advanced Air Mobility. Inventions 2024, 9, 84. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Iv, W.J.E.; Deng, D.Z.; Yang, J.; Xiao, J. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems. J. Mater. Chem. A 2016, 4, 15266–15280. [Google Scholar] [CrossRef]
- Mills, A.; Kalnaus, S.; Tsai, W.-Y.; Su, Y.-F.; Williams, E.; Zheng, X.; Vaidyanathan, S.; Hallinan, D.T.; Nanda, J.; Yang, G. Elucidating Polymer Binder Entanglement in Freestanding Sulfide Solid-State Electrolyte Membranes. ACS Energy Lett. 2024, 9, 2677–2684. [Google Scholar] [CrossRef]
- York, M.; Larson, K.; Harris, K.C.; Carmona, E.; Albertus, P.; Sharma, R.; Noked, M.; Strauss, E.; Ragones, H.; Golodnitsky, D. Recent advances in solid-state beyond lithium batteries. J. Solid State Electrochem. 2022, 26, 1851–1869. [Google Scholar] [CrossRef]
- Kumar, P.; Channi, H.K.; Babbar, A.; Kumar, R.; Bhutto, J.K.; Khan, T.M.Y.; Bhowmik, A.; Razak, A.; Wodajo, A.W. A Systematic Review of Nanotechnology for Electric Vehicles Battery. Int. J. Low-Carbon Technol. 2024, 19, 747. [Google Scholar] [CrossRef]
- Pimlott, J.L.; Street, R.J.; Down, M.P.; Banks, C.E. Electrochemical Overview: A Summary of ACoxMnyNizO2 and Metal Oxides as Versatile Cathode Materials for Metal-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2107761. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsch, D.J.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2022, 55, 244–263. [Google Scholar] [CrossRef]
- Mageto, T.; Bhoyate, S.; Souza, F.M.D.; Mensah-Darkwa, K.; Kumar, A.; Gupta, R.K. Developing Practical Solid-State Rechargeable Li-Ion Batteries: Concepts, Challenges, and Improvement Strategies. J. Energy Storage 2022, 55, 105688. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Zhang, X.; Zhu, Y. Strategies to Improve the Performance of Li Metal Anode for Rechargeable Batteries. Front. Chem. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Park, J.-W.; Kim, B.G.; Lee, Y.-J.; Ha, Y.-C.; Lee, S.-M.; Baeg, K.-J. Facile fabrication of solution-processed solid-electrolytes for high-energy-density all-solid-state-batteries by enhanced interfacial contact. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, Y.; Wang, M.; Fan, X.; Zhang, T.; Peng, L.; Zhou, X.-D.; Qiao, J. High-performing rechargeable/flexible zinc-air batteries by coordinated hierarchical Bi-metallic electrocatalyst and heterostructure anion exchange membrane. Nano Energy 2019, 65, 104021. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, K.; Pei, P.; Zuo, Y.; Wei, M.; Wang, H.; Zhang, P.; Shang, N. Advances in electrolyte safety and stability of ion batteries under extreme conditions. Nano Res. 2022, 16, 2311–2324. [Google Scholar] [CrossRef]
- Yu, D.; Min, J.; Lin, F.; Madsen, L.A. Mechanically and Thermally Robust Gel Electrolytes Built from A Charged Double Helical Polymer. Adv. Mater. 2024, 36, e2312513. [Google Scholar] [CrossRef]
- Shen, H.; Yi, E.; Cheng, L.; Amores, M.; Chen, G.; Sofie, S.W.; Doeff, M.M. Solid-state electrolyte considerations for electric vehicle batteries. Sustain. Energy Fuels 2019, 3, 1647–1659. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, H.; Lee, J.W.; Kang, T.W.; Park, J.H.; Shin, J.-H.; Lee, H.; Majhi, D.; Lee, S.U.; Kim, J.-H. Multicomponent Covalent Organic Framework Solid Electrolyte Allowing Effective Li-Ion Dissociation and Diffusion for All-Solid-State Batteries. ACS Nano 2023, 17, 17372. [Google Scholar] [CrossRef]
- Singh, D.K.; Fuchs, T.; Krempaszky, C.; Mogwitz, B.; Janek, J. Non-Linear Kinetics of The Lithium Metal Anode on Li6PS5Cl at High Current Density: Dendrite Growth and the Role of Lithium Microstructure on Creep. Adv. Sci. 2023, 10, e2302521. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, K.; Narayanan, K. A Review on Conventional to Bipolar Design of Anode Less All Solid-State Batteries. Energy Adv. 2024, 3, 1222. [Google Scholar] [CrossRef]
- Li, W.; Quirk, J.A.; Li, M.; Xia, W.; Morgan, L.M.; Yin, W.; Zheng, M.; Gallington, L.C.; Ren, Y.; Zhu, N.; King, G.; Feng, R.; Li, R.; Dawson, J.A.; Sham, T.-K.; Sun, X. Precise Tailoring of Lithium-Ion Transport for Ultralong-Cycling Dendrite-Free All-Solid-State Lithium Metal Batteries. Adv. Mater. 2023, 36. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, D.W.; Park, J.-W.; Park, J.-H.; Lee, Y.; Ha, Y.-C.; Lee, S.-M.; Yoon, S.Y.; Kim, B.G. In Situ Formed Ag-Li Intermetallic Layer for Stable Cycling of All-Solid-State Lithium Batteries. Adv. Sci. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2019, 120, 6820–6877. [Google Scholar] [CrossRef] [PubMed]
- Swathi, P.; V., S.O.; Panneerselvam, T.; Murugan, R.; Ramaswamy, A.P. Cross-Linked Polymer Composite Electrolyte Incorporated with Waste Seashell Based Nanofiller for Lithium Metal Batteries. Energy Fuels 2023, 37, 6186–6196. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M. Connecting battery technologies for electric vehicles from battery materials to management. iScience 2022, 25, 103744. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Dalapati, G.K.; Reddy, M.V.V.; Zaghib, K.; Chellappan, V.; Ramakrishna, S. Progress and Complexities in Metal–Air Battery Technology. Energy Technol. 2024. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, H.; Qin, N.; Wang, C.; Wang, L.; Lu, J. Single-Crystal Nickel-Rich Cathode Materials: Challenges and Strategies. Acta Phys. Chim. Sin. 2023, 40. [Google Scholar] [CrossRef]
- Ma, Y.; Shang, R.; Liu, Y.; Lake, R.; Ozkan, M.; Ozkan, C.S. Enabling fast-charging capability for all-solid-state lithium-ion batteries. J. Power Sources 2023, 559. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Sun, Q.; Li, X.; Liu, Y.; Liang, J.; Li, X.; Lin, X.; Li, R.; Adair, K.R.; et al. Stabilizing interface between Li10SnP2S12 and Li metal by molecular layer deposition. Nano Energy 2018, 53, 168–174. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, R.; Jiang, X.; Yin, M.; Zhang, D.; Xie, Z. An electrochemical-mechanical coupled multi-scale modeling method and full-field stress distribution of lithium-ion battery. Appl. Energy 2023, 347. [Google Scholar] [CrossRef]
- Yang, R.; Xie, Y.; Li, K.; Li, W.; Hu, X.; Fan, Y.; Zhang, Y. Thermal characteristics of solid-state battery and its thermal management system based on flat heat pipe. Appl. Therm. Eng. 2024, 252. [Google Scholar] [CrossRef]
- Waseem, M.; Ahmad, M.; Parveen, A.; Suhaib, M. Battery Technologies and Functionality of Battery Management System for EVs: Current Status, Key Challenges, and Future Prospectives. J. Power Sources 2023, 580, 233349. [Google Scholar] [CrossRef]
- Iyer, V.; Petersen, J.; Geier, S.; Wierach, P. Development and Multifunctional Characterization of a Structural Sodium-Ion Battery Using a High-Tensile-Strength Poly(ethylene oxide)-Based Matrix Composite. ACS Appl. Energy Mater. 2024, 7, 3968–3982. [Google Scholar] [CrossRef]
- Bridgelall, R.; Corcoran, M. Conformal Body Capacitors Suitable for Vehicles. United States of America Patent 9,966,790, 8 May 2018.
- N. J. v. Eck and L. Waltman, "VOSviewer," Leiden University, 1 July 2024. [Online]. Available: vosviewer.com. [Accessed 30 June 2024].
- Pasta, M.; Armstrong, D.; Brown, Z.L.; Bu, J.; Castell, M.R.; Chen, P.; Cocks, A.; Corr, S.A.; Cussen, E.J.; Darnbrough, E.; et al. 2020 roadmap on solid-state batteries. J. Phys. Energy 2020, 2, 032008. [Google Scholar] [CrossRef]
- Choi, Y.J.; Hwang, Y.J.; Kim, S.-I.; Kim, T. Performance Improvement of Argyrodite Solid Electrolyte for All-Solid-State Battery Using Wet Process. ACS Appl. Energy Mater. 2024, 7, 4421–4428. [Google Scholar] [CrossRef]
- Zhu, Q.-C.; Ma, J.; Huang, J.-H.; Mao, D.-Y.; Wang, K.-X. Realizing long-cycling solid-state Li–CO2 batteries using Zn-doped LATP ceramic electrolytes. Chem. Eng. J. 2024, 482. [Google Scholar] [CrossRef]
- Thomas, C.M.; Zeng, D.; Huang, H.C.; Pham, T.; Torres-Castanedo, C.G.; Bedzyk, M.J.; Dravid, V.P.; Hersam, M.C. Earth-Abundant Kaolinite Nanoplatelet Gel Electrolytes for Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2024, 16, 34913–34922. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, X.; Zeng, Z.; Zhang, Y.; Du, Y. Fast-charging batteries based on dual-halogen solid-state electrolytes. Mater. Chem. Front. 2023, 7, 4961–4970. [Google Scholar] [CrossRef]
- Ha, Y.-C. A Review on the Wet Chemical Synthesis of Sulfide Solid Electrolytes for All-Solid-State Li Batteries. J. Korean Electrochem. Soc. 2022, 25, 95–104. [Google Scholar]
- Martinez, A.I.P.; Aguesse, F.; Otaegui, L.; Schneider, M.; Roters, A.; Llordés, A.; Buannic, L. The Cathode Composition, A Key Player in the Success of Li-Metal Solid-State Batteries. J. Phys. Chem. C 2019, 123, 3270–3278. [Google Scholar] [CrossRef]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Yi, J.; Liu, Y.; Qiao, Y.; He, P.; Zhou, H. Boosting the Cycle Life of Li-O2 Batteries At Elevated Temperature By Employing A Hybrid Polymer-Ceramic Solid Electrolyte. ACS Energy Lett. 2017, 2, 1378. [Google Scholar] [CrossRef]
- Song, S.; Duong, H.M.; Korsunsky, A.M.; Hu, N.; Lu, L. A Na+ Superionic Conductor for Room-Temperature Sodium Batteries. Sci. Rep. 2016, 6, 32330. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.K.; Wada, Y.; Hayashi, K.; Kawamura, G.; Muto, H.; Matsuda, A. Fabrication of An All-Solid-State Zn-Air Battery using Electroplated Zn on Carbon Paper and KOH-ZrO2 Solid Electrolyte. Appl. Surf. Sci. 2019, 487, 343. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Huang, Z.; Zhao, B.; Zhang, G.; Li, S.; Cui, Y.; Dong, Z.; Liu, H. Nanoscale Operation of Ni-Rich Cathode Surface By Polycrystalline Solid Electrolytes Li3.2Zr0.4Si0.6O3.6 Coating. Chem. Eng. J. 2021, 417, 129217. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Wang, L.; Feng, X.; Ren, D.; Li, Y.; Rui, X.; Wang, Y.; Han, X.; Xu, G.-L.; et al. Development of cathode-electrolyte-interphase for safer lithium batteries. Energy Storage Mater. 2021, 37, 77–86. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y. Designing electrolyte additives for lithium metal batteries through multi-factor principle. J. Energy Chem. 2021, 61, 147–148. [Google Scholar] [CrossRef]
- Keles, O.; Karahan, B.D.; Eryilmaz, L.; Amine, R.; Abouimrane, A.; Chen, Z.; Zuo, X.; Zhu, Z.; Al-Hallaj, S.; Amine, K. Superlattice-structured films by magnetron sputtering as new era electrodes for advanced lithium-ion batteries. Nano Energy 2020, 76, 105094. [Google Scholar] [CrossRef]
- Park, S.W.; Oh, G.; Park, J.; Ha, Y.; Lee, S.; Yoon, S.Y.; Kim, B.G. Graphitic Hollow Nanocarbon as a Promising Conducting Agent for Solid-State Lithium Batteries. Small 2019, 15, e1900235. [Google Scholar] [CrossRef]
- Sun, X.; Radovanovic, P.V.; Cui, B. Advances in Spinel Li4Ti5O12 Anode Materials for Lithium-Ion Batteries. New J. Chem. 2015, 39, 38. [Google Scholar] [CrossRef]
- Kazyak, E.; García-Méndez, R. Recent progress and challenges for manufacturing and operating solid-state batteries for electric vehicles. MRS Bull. 2024, 49, 717–729. [Google Scholar] [CrossRef]
- Chaudhary, R.; Xu, J.; Xia, Z.; Asp, L.E. Unveiling the Multifunctional Carbon Fiber Structural Battery. Adv. Mater. 2024, e2409725. [Google Scholar] [CrossRef] [PubMed]
- Whang, G.; Zeier, W.G. Transition Metal Sulfide Conversion: A Promising Approach to Solid-State Batteries. ACS Energy Lett. 2023, 8, 5264–5274. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Su, Q.; Wu, M.; Que, X.; Liu, H. High-Power Bipolar Solid-State Batteries Enabled by In-Situ-Formed Ionogels for Vehicle Applications. ACS Appl. Mater. Interfaces 2022, 14, 5402–5413. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, Y.; Yu, X.; Zhou, L. Fiber metal laminated structural batteries with multifunctional solid polymer electrolytes. Compos. Sci. Technol. 2022, 230. [Google Scholar] [CrossRef]
- Yamamoto, M.; Terauchi, Y.; Sakuda, A.; Takahashi, M. Binder-free sheet-type all-solid-state batteries with enhanced rate capabilities and high energy densities. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, H.; Han, S.; Chan, T.-S.; Ko, K.-H.; Jo, S.; Park, J.; Kim, S.; Lee, S.; Noh, J.; et al. Detrimental effect of high-temperature storage on sulfide-based all-solid-state batteries. Appl. Phys. Rev. 2022, 9, 031403. [Google Scholar] [CrossRef]
- Guo, B.; Chen, J.; Wang, Z.; Su, Y.; Li, H.; Ye, H.; Zhang, L.; Tang, Y.; Huang, J. In situ TEM studies of electrochemistry of high temperature lithium-selenium all-solid-state batteries. Electrochimica Acta 2021, 404, 139773. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, C.; Li, L.; Xia, Y.; Huang, H.; Gan, Y.; Liang, C.; He, X.; Tao, X.; Zhang, W. Unraveling the Intra and Intercycle Interfacial Evolution of Li6PS5Cl-Based All-Solid-State Lithium Batteries. Adv. Energy Mater. 2019, 10. [Google Scholar] [CrossRef]
- Gutiérrez-Pardo, A.; Martinez, A.I.P.; Otaegui, L.; Schneider, M.; Roters, A.; Llordés, A.; Aguesse, F.; Buannic, L. Will the competitive future of solid state Li metal batteries rely on a ceramic or a composite electrolyte? Sustain. Energy Fuels 2018, 2, 2325–2334. [Google Scholar] [CrossRef]
- Boretti, A. Battery energy storage in electric vehicles by 2030. Energy Storage 2022, 5. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12. [Google Scholar] [CrossRef]
- Ball, S.; Clark, J.; Cookson, J. Battery Materials Technology Trends and Market Drivers for Automotive Applications. Johns. Matthey Technol. Rev. 2020, 64, 287–297. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
Figure 2.
The workflow developed for the thematic and bibliometric analysis.
Figure 2.
The workflow developed for the thematic and bibliometric analysis.
Figure 3.
Bigram word cloud of titles and abstracts within each category.
Figure 3.
Bigram word cloud of titles and abstracts within each category.
Figure 8.
Publications by a) year, b) category, c) author distribution, d) category and year.
Figure 8.
Publications by a) year, b) category, c) author distribution, d) category and year.
Figure 9.
Publications by a) lead authors, b) countries, c) affiliations, and d) category and journal.
Figure 9.
Publications by a) lead authors, b) countries, c) affiliations, and d) category and journal.
Figure 10.
Citations by a) lead authors, b) countries, c) affiliations, and d) category and journal.
Figure 10.
Citations by a) lead authors, b) countries, c) affiliations, and d) category and journal.
Figure 11.
Country co-authorship network.
Figure 11.
Country co-authorship network.
Figure 12.
Citations a) across research categories, b) per publication within categories, c) rates in categories, and d) publications in category by top 10 countries.
Figure 12.
Citations a) across research categories, b) per publication within categories, c) rates in categories, and d) publications in category by top 10 countries.
Figure 13.
Citations a) by year, b) per publication by year, c) rates by country, and d) rates by affiliation.
Figure 13.
Citations a) by year, b) per publication by year, c) rates by country, and d) rates by affiliation.
Table 1.
Co-authored publications by countries.
Table 1.
Co-authored publications by countries.
| Country |
Documents |
Citations |
TLS |
| China |
27 |
2,617 |
15 |
| United States |
26 |
4,561 |
11 |
| Canada |
6 |
1,282 |
6 |
| Japan |
7 |
534 |
5 |
| United Kingdom |
6 |
333 |
4 |
| South Korea |
6 |
177 |
1 |
Table 2.
A sample of specific developments in electrolyte engineering.
Table 2.
A sample of specific developments in electrolyte engineering.
| Source |
Key findings and/or contributions |
| Choi et al. (2024) |
Synthesized solid electrolyte by separating the precipitate and removing the supernatant after high-energy ball milling to increase the ionic conductivity and compatibility with lithium metal at room temperature [47]. |
| Zhu et al. (2024) |
Demonstrated that doping a lithium-aluminum-titanium-phosphate (LATP) solid-state ceramic electrolyte with zinc strengthened its mechanical properties while providing an optimized network channel for lithium-ion transport at room temperature [48]. They achieved 180 cycles of battery life. |
| Thomas et al. (2024) |
Proposed creating a nanocomposite electrolyte from kaolinite, an earth-abundant clay variety, by overcoming strong interlayer forces that have historically precluded their efficient exfoliation (separation) into thin sheets [49]. They used a special liquid process to break apart the kaolinite into very thin, tiny pieces (nanoplatelets) and combined those with a succinonitrile liquid electrolyte to form a nanocomposite gel electrolyte. They achieved a desired ionic conductivity at room temperature, high mechanical strength, a wide electrochemical stability window of 4.5 volts, and thermal stability beyond 100 degrees Celsius. |
| Yu et al. (2024) |
Developed a gel polymer electrolyte by mixing lithium salt and poly (ethylene glycol) dimethyl ether [25]. This resulted in tiny fibers formed from a rigid double helical structure. Combined with a lithium anode and a LiFePO4 cathode, the battery cell exhibited strong mechanical properties, a wide operating temperature range up to 120 degrees Celsius, high charge cycles, and high-capacity retention. |
| Li et al. (2023) |
Demonstrated the use of a halide solid electrolyte (Li3InCl6) to precisely tailor the movement of lithium-ion both within and between the grains of this material [30]. Their process led to the formation of unexpected supersonic conducting grain boundaries that lowered the energy required for ion hopping and suppressed lithium dendrite growth. They achieved 2,000 cycles of battery life and 93.7% capacity retention. |
| Lee et al. (2023) |
Developed an organic solid electrolyte based on a diethylene glycol-modified pyridinium covalent organic framework that exhibited high ion conductivity (1.71×10-4 S/cm) at room temperature, 99% coulombic efficiency, and effective suppression of lithium dendrite formation [27]. |
| Swathi et al. (2023) |
Incorporated a nanofiller made with calcium hydroxide derived from natural seashells into a polymer electrolyte (PEO + LiClO4 salt), yielding improved ionic conductivity of 4.12×10-5 S/cm at 25 degrees Celsius [34]. |
| Zhang et al. (2023) |
Developed a dual-halogen solid-state electrolyte (Li3YCl6) that increased ionic conductivity by more than one order of magnitude and retained capacity at 80% after 1,000 cycles at 4 degrees Celsius [50]. |
| Ha et al. (2022) |
Reviewed the literature on wet chemical synthesis of sulfide solid electrolytes, summarizing the reaction mechanism between the raw materials in the solvent [51]. |
| Martinez et al. (2019) |
Created a hybrid solid electrolyte by combining both polymer and ceramic materials to improve ionic conductivity and mechanical stability [52]. Blending the solid electrolyte with the cathode material (LiFePO4) at 30% to 40% by volume yielded good interfacial contact between the components and efficient ionic transport throughout the cell. They calculated theoretical gravimetric and volumetric energy densities of 185 Wh/kg and 345 Wh/L, respectively. |
| Lau et al. (2018) |
Reviewed the challenges and opportunities for sulfide-based solid electrolytes [53]. |
| Yi et al. (2017) |
Developed a hybrid polymer-ceramic electrolyte for a lithium-air battery, achieving 3.2×10-4 S/cm ionic conductivity at room temperature, and 350 cycles at 50 degrees Celsius [54]. |
| Song et al. (2016) |
Developed a solid electrolyte based on a sodium-zirconium-magnesium-silicon-phosphorus-oxide material and achieved ionic conductivity of 3.5×10-3 S/cm at room temperature [55]. The prototype used sodium and sulfur in the anode and cathode, respectively. |
Table 3.
A sample of specific developments in electrode engineering.
Table 3.
A sample of specific developments in electrode engineering.
| Source |
Key findings and/or contributions |
| Mills et al. (2024) |
Developed flexible and durable sheets of 30 micrometer-thick solid-state electrodes, bounded to a sulfide solid-state electrolyte [14]. They found that optimizing the molecular weight of the binder can achieve the best combination of structural integrity and grain boundary resistance. The prototype achieved an energy density of 500 Wh/kg. |
| Ramasubramanian et al. (2024) |
Explored strategies for mitigating energy barriers of the electrodes for metal-air batteries [36]. Strategies included 3D electrodes, oxygen-selective membranes, solid-state electrolytes, hybrid polymer electrodes, conductive electrocatalysts, and artificial solid-electrolyte interfaces. |
| Hayakawa et al. (2023) |
Coated the electrode materials with a conductive additive (acetylene black and vapor-grown carbon fiber), yielding improved contact with the solid electrolyte material and reduced internal resistance [8]. |
| Alexander et al. (2023) |
Proposed a single-phase mixed ion- and electron-conducting garnet for both electrodes to address the issues of limited plate stripping rates and dendrite growth in lithium metal anodes [7]. They observed six times more energy transfer than state-of-the-art batteries that can potentially last for 3,700 charge cycles. |
| Kim et al. (2020) |
Proposed two methods to ensure that the solid electrolyte material distributed evenly within the porous structure of the electrode [22]. Discovered that infiltration of the solid electrolyte into the electrode improved at higher processing temperatures, particularly above the boiling point of ethanol, due to the solvent vaporizing. Found that mixing active materials of different particle sizes helped fill the capillary pores in the electrode, resulting in a denser structure. Achieved initial discharge capacity of 177 mAh/g. |
| Tan et al. (2019) |
Developed a solid-state zinc-air battery by electroplating a zinc anode onto carbon paper to improve electron flow [56]. The cathode was oxygen from air, which increased the theoretical energy density. The prototype achieved a 30 mAh/g discharge capacity. |
| Kuang et al. (2019) |
Reviewed the principles, challenges, and potential of thick electrodes that can store more active materials and fewer inactive components to achieve higher energy density without fundamentally altering the battery chemistry [18]. |
| Wang et al. (2018) |
Utilized a molecular layer deposition method to develop an inorganic-organic hybrid interlayer (alucone) at the interface between the Li metal anode and the solid electrolyte [39]. The alucone served as an artificial SEI to suppress unwanted reactions at the interface by blocking electron transport. The battery achieved a high initial capacity of 120 mAh/g and a retention capacity of 60 mAh/g after 150 cycles. |
| Wu et al. (2016) |
Reviewed problems and solutions to the interfacial issues between electrodes and electrolytes of different types [13]. They concluded that despite the progress made in developing solid electrolytes, significant challenges remain in forming intimate contact at its anode and cathode interfaces. |
Table 4.
A sample of specific developments focused on cathode electrodes.
Table 4.
A sample of specific developments focused on cathode electrodes.
| Source |
Key findings and/or contributions |
| Huang et al. (2024) |
Reviewed common failure issues in both polycrystal and single-crystal cathode structures [37]. Outlined strategies for improving single-crystal nickel-rich layered cathode materials, including synthesis regulation, element doping, and surface-interface modification. Explored the connection between structural design and electrochemical performance. |
| Razali et al. (2023) |
Reviewed developments in solid state electrolytes (ceramic, polymer, soft sulfur-based, and their hybrid combinations) and concluded that lithium dendrite growth remains the key bottleneck in SSB development [6]. |
| Li et al. (2021) |
Tackled the problem that although nickel rich cathodes exhibit high energy storage capacity, they have low cycling stability. Found that coating the NMC cathode material with nanoscale polycrystalline particles of a solid electrolyte improved ionic conductivity, reduced unwanted reactions during cycling, and stabilized the interface with the solid electrolyte [57]. The battery retained 80% of its capacity after 400 cycles at a moderate charging rate. |
| Wu et al. (2021) |
Reviewed advancements in cathode-electrolyte interface formation in situ by conventional carbonate-based, fluorinated, concentrated, and solid-state electrolytes to improve thermal stability [58]. |
| Pimlott et al. (2021) |
Reviewed the state-of-the-art in lithium-based materials for cathode materials, highlighting their challenges and opportunities [17]. |
Table 5.
A sample of specific developments focused on anode electrodes.
Table 5.
A sample of specific developments focused on anode electrodes.
| Source |
Key findings and/or contributions |
| Liu et al. (2023) |
Surveyed the opportunities of silicon-based anode due to their abundance and ability to store much more lithium ions than carbon by forming alloys with lithium [19]. Found that silicon-based nanostructures or composite materials with higher structural stability can tackle the issue of anode failure due to silicon’s volume expansion during cycling. However, these approaches introduce a myriad of other challenges. |
| Choi et al. (2021) |
Used a roll pressing method to yield the in-situ formation of an intermetallic layer of silver-lithium alloy that mitigated uneven lithium deposition [31]. This approach enhanced the stability of the electrolyte (Li6PS5Cl) interface with the anode. They achieved 94.3% capacity retention after 140 cycles of high current rates. They reported that cells without the silver layer short circuited after only 30 cycles. |
| Lee et al. (2020) |
Utilized a silver-carbon nanocomposite layer to improve the uniformity of lithium deposition by forming a silver-lithium alloy during cycling [59]. This resulted in dense and uniform lithium deposits that enhanced stability over 1,000 cycles. The carbon component acted as a mechanical support and separator, suppressing lithium dendrites penetration into the solid electrolyte. |
| Xu et al. (2021) |
Used an electrolyte additive (potassium perfluorohexyl sulfonate) to form a stable SEI layer that protects the battery during lithium plating and stripping at the anode [60]. |
| Keles et al. (2020) |
Used a magnetron sputtering process to create a superlattice structured anode film comprised of alternating layers of silicon, molybdenum, and copper [61]. The alternating layers distributed the stress from silicon’s expansion evenly across the material, preventing damage. Achieved stable performance and maintained capacity over 150 cycles. |
| Park et al. (2019) |
Found that chemical attachments to carbon (functional groups like hydroxyl creating oxygen and hydrogen bonds) can participate in unwanted reactions at the solid electrolyte interface, leading to insulating byproducts that increase internal resistance [62]. Developed a graphitized hollow nanocarbon with fewer functional groups that reduced side reactions. |
| Sun et al. (2015) |
Explored the research directions for spinet (Li4Ti5O12) anodes based on their promise of good performance, ease of fabrication, and low cost [63]. |
Table 6.
A sample of specific developments in architecture research.
Table 6.
A sample of specific developments in architecture research.
| Source |
Key findings and/or contributions |
| Kazyak et al. (2024) |
Summarized recent progress and ongoing challenges in developing SSBs, emphasizing the need for scalable and sustainable processing of battery components [64]. |
| Sharma et al. (2024) |
Reviewed anode-free arrangements through a bipolar architecture [29]. |
| Kumar et al. (2024) |
Reviewed trends in using nanoscale technologies to make various electric vehicle parts, including battery materials that employ nanotubes, graphene, and metal oxides [16]. |
| Chaudhary et al. (2024) |
Demonstrated a structural LIB with the electrodes made of carbon fiber material [65]. They coated the cathode with lithium iron phosphate (LiFePO4) and used lithium bis (trifluoro methane) sulfonamide (LiTFSI) dissolved in ethyl-methylimidazolium tetrafluoroborate for the solid electrolyte. The prototype exhibited 30 Wh/kg, cyclic stability up to 1,000 cycles with just under 100% Coulombic efficiency, and elastic modulus of 76 GPa. |
| Lee et al. (2024) |
Reviewed the inherent challenges in cell chemistries within lithium-sulfur based SSBs, aiming to inspire the battery community to advance this technology toward practical applications [9]. |
| Iyer et al. (2024) |
Developed a structural sodium-ion battery by sandwiching a glass fiber between thin solid-state poly(ethylene oxide)-based composite electrolyte layers [43]. This approach yielded a high-tensile-strength structural electrolyte. They formed the structural cathode by heat pressing carbon fibers against the structural electrolyte. The resulting structural battery had a tensile strength of just under 41 MPa. It provided a typical energy density of 23 Wh/kg, 4.5 volts, ionic conductivity of 1.02×10-4 S/cm at 60 degrees Celsius, 500 cycles of lifetime, and retained 80% capacity until 225 cycles. |
| Ma et al. (2023) |
Reviewed issues that impede fast charging of SSBs [38]. These included low ionic and electronic conductivities in solid-state electrolytes (SSEs), electrode/electrolyte interfacial resistances, and mechanical instabilities at high currents. |
| Whang et al. (2023) |
Explored the opportunity space for transition metal sulfide conversion chemistries for stationary energy storage applications [66]. |
| Li et al. (2022) |
Proposed a bipolar stacking of two solid-state cells by forming a non-flammable gel (ionogel) between solid particles [67]. |
| Fu et al. (2022) |
Proposed a structural battery based on modified fiber metal laminates with added energy storage capability [68]. Metal sheets supporting the battery core served the dual purpose of resisting impact and conducting electricity. Demonstrated a prototype car frame with 11 mAh capacity. |
| Xu et al. (2019) |
Developed a catalyst and membrane that significantly improved the performance of rechargeable solid-state zinc-air batteries [23]. They used a hydrothermal process (water at high temperature and pressure) to create the hybrid material by combining cobalt oxide (Co3O4) anchored on manganese oxide (MnO2), mixed with carbon nanotubes. The hybrid structure and interaction between the cobalt and manganese oxides better catalyzed the chemical reactions with oxygen. |
| Yamamoto et al. (2018) |
Proposed a binder-free sheet-type battery construction by using removable volatile poly(propylene carbonate)-based binders [69]. Achieved a 2.6-fold increase in energy density over previously reported sheet-type batteries. |
| Yi et al. (2017) |
Reviewed the development of polymer electrolytes for lithium-air batteries [54]. |
Table 7.
A sample of key findings in performance evaluation.
Table 7.
A sample of key findings in performance evaluation.
| Source |
Key findings and/or contributions |
| Yang et al. (2024) |
The SSB evaluated has a higher internal resistance than LIB, causing it to generate more heat, thus requiring a more capable thermal management system [3]. |
| Yang et al. (2024) |
Developed a micro heat pipe array with air cooling to improve thermal management at the SSB pack level [41]. |
| Cao et al. (2023) |
Advocated for using a neuron imaging technique to visualize SSB operation and evaluate issues such as interfacial compatibility, reaction mechanisms, structural stability, ion transport constraints, and lithium dendrite inhibition [4]. |
| Singh et al. (2023) |
Utilized in-situ electrochemical scanning electron microscopy to examine the degradation mechanism at the anode interface between lithium metal and a solid electrolyte (Li6PS5Cl) during battery operation. They found that a nanocrystalline structure of lithium can help create a stable interface by allowing for a specific type of deformation (Coble creep) and uniform removal of lithium [28]. Also, they found that flaw-free surfaces can support high currents of more than 150 mA per square-centimeter. |
| Yang et al. (2022) |
Reviewed the literature on ionic conductivity in SSB and concluded that some strategies for improvement include doping, defect engineering, microstructure tuning, and interface modification [32]. |
| Yoon et al. (2022) |
Evaluated the solid electrolyte Li6PS5Cl and found that it is vulnerable to elevated-temperature storage as low as 70 degrees Celsius [70]. |
| Li et al. (2022) |
Summarized experimental observations and modeling efforts to understand the factors influencing LIB cell degradation due to mechanical origins, structural changes, and electrochemical changes to forecast the evolution of SSB performance [67]. |
| Guo et al. (2022) |
Investigated the electrochemistry of lithium-selenium SSBs at different temperatures using in-situ transmission electron microscopy equipped with a microelectromechanical systems heating device [71]. They found that lithium-ion conductivity and not electronic conductivity dictates the performance of this battery type. |
| Pasta et al. (2020) |
Highlighted that the main challenges in developing SSBs lie in the interfaces between electrolytes and electrodes, mechanical properties of the device, and scalability of processing, rather than in the ionic conductivity of solid electrolytes [46]. |
| Zhang et al. (2019) |
Utilized in-situ electrochemical impedance and Raman spectroscopy to study the degradation mechanisms in SSBs [72]. They found that lithium-ion migration is a significant cause of interfacial reactions that deteriorate the interfacial structure and electrochemical performance. |
| Gutierrez-Pardo et al. (2018) |
Compared the performance of ceramic and polymer-ceramic composite electrolytes without coating to improve interfacial contacts with the electrodes [73]. They found that the composite electrolyte performed better at 70 degrees Celsius due to its enhanced interfacial contact. |
Table 8.
A sample of key findings in multidisciplinary reviews.
Table 8.
A sample of key findings in multidisciplinary reviews.
| Source |
Key findings and/or contributions |
| Wu et al. (2023) |
Explored the challenges, technologies, and concepts for recycling SSBs relative to LIBs [5]. |
| Boretti (2022) |
Reviewed the battery market and found that despite significant technological improvements, environmental concerns rather than economics have been driving the shift to battery electric vehicles [74]. |
| Neumann et al. (2022) |
Explored current practices in recycling, including material collection, sorting, transportation, handling, regulations, and new battery directive demands to predict challenges in recycling future SSBs [75]. |
| Bajolle et al. (2022) |
Forecasted LIB price trends for scenarios ranging from rapid price stabilization due to insufficient raw material supply to market disruption by the introduction of SSBs [1]. |
| Fichtner (2021) |
Reviewed trends in LIB storage capacity and price reduction [2]. Found that only a fraction of the overall improvement was due to better chemical compositions. Predicted that further improvements will be due to innovations in cell-to-pack and cell-to-chassis designs, allowing for the replacement of less sustainable, albeit more energy dense, cobalt-based active materials with more earth abundant and lower-cost materials such as LiFePO4. |
| Ball et al. (2020) |
Reviewed the changing requirements for active materials in automotive LIB applications, anticipating responses from science and industry [76]. |
| Ding et al. (2019) |
Discussed recent advances and challenges of electric vehicles, focusing on resources of critical elements, EV market evolution, and LIB cost and performance [77]. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).