Submitted:
25 September 2024
Posted:
25 September 2024
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
DISCUSSION
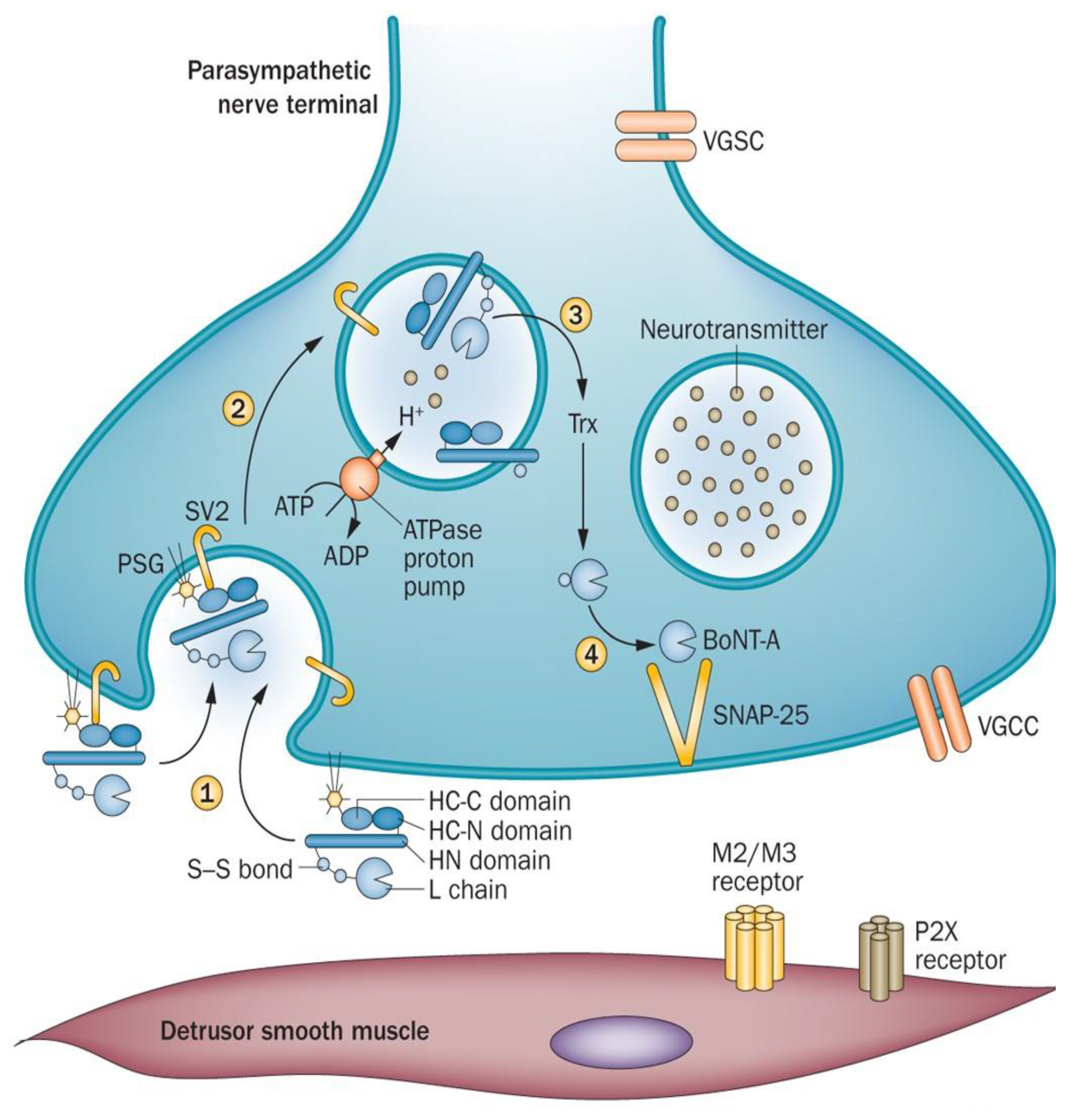
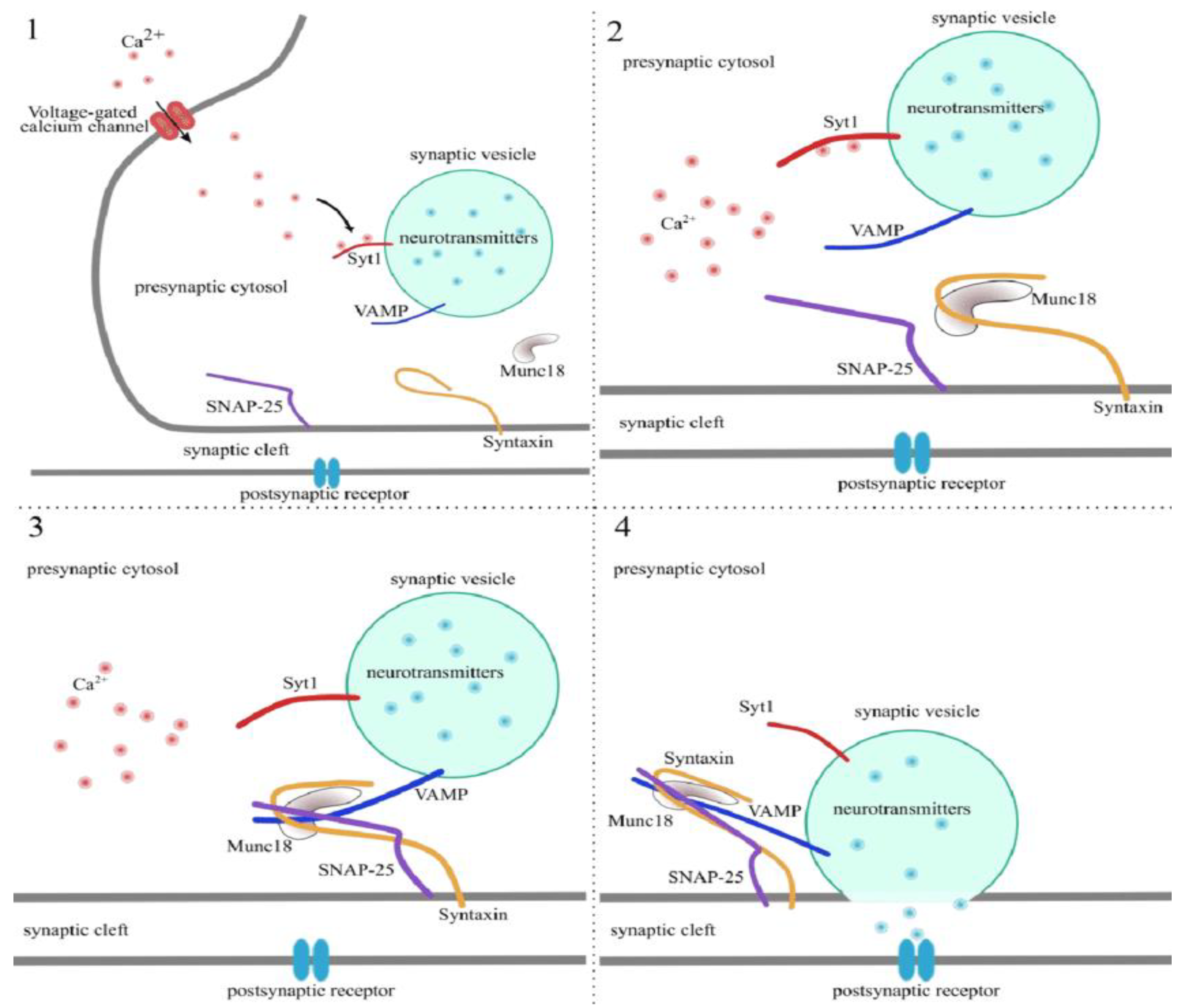
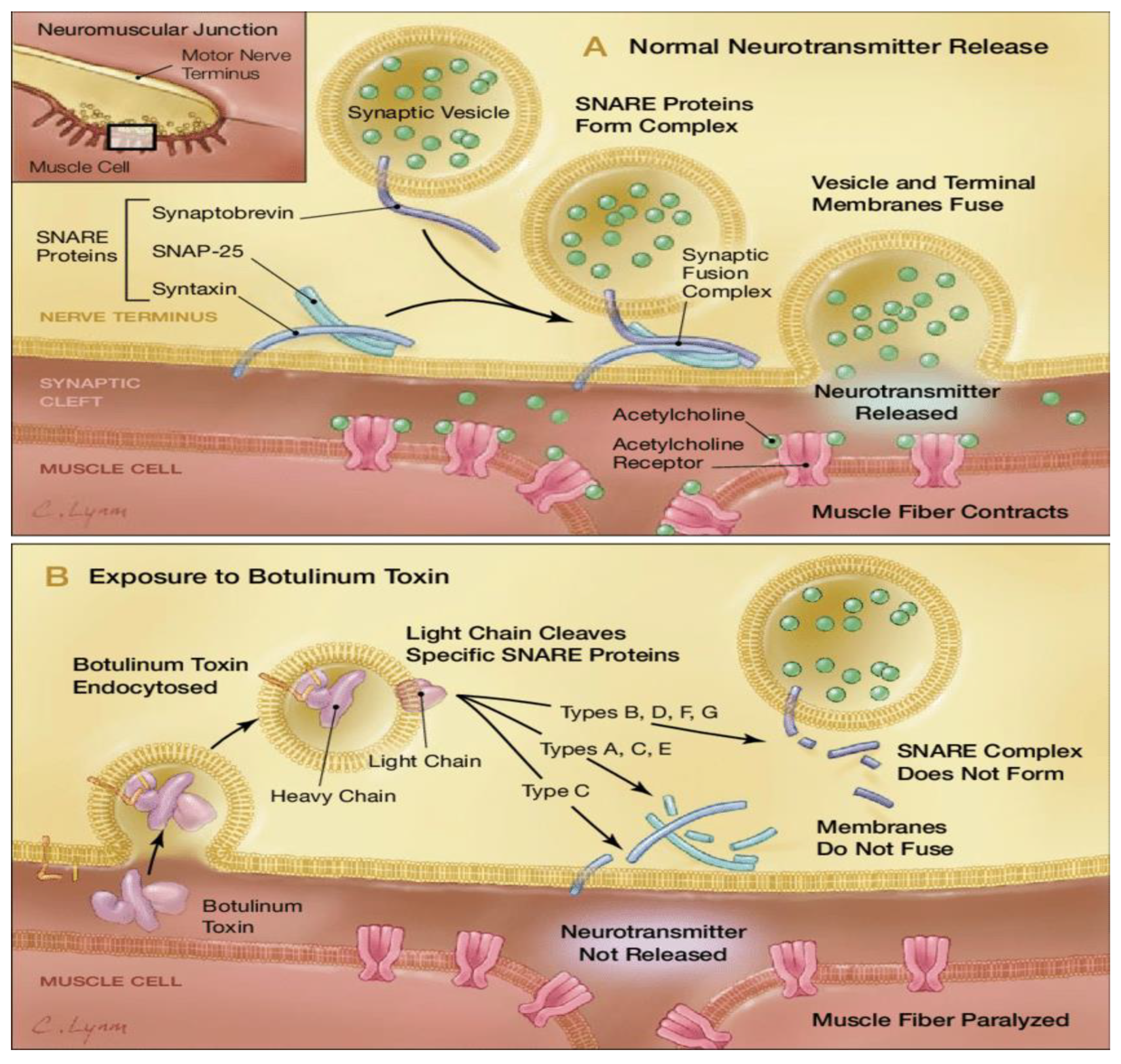
Molecular Characterization and Mechanism of Action
Therapeutic Uses in Neuromuscular Disorders.
Safety Profile Considerations Between Different BoNT Formulations
Comparative Compositional Analysis of BonT-A Formulations
CONCLUSION
ACKNOWLEDGEMENT
References
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J Dermatol 2010, 55, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jeffery IA, Karim S. “Botulism.,” StatPearls. Treasure Island (FL): StatPearls Publishing (2024) http://www.ncbi.nlm.nih.gov/books/NBK459273/ [Accessed September 23, 2024].
- Tiwari A, Nagalli S. “Clostridium botulinum Infection.,” StatPearls. Treasure Island (FL): StatPearls Publishing (2024) http://www.ncbi.nlm.nih.gov/books/NBK553081/ [Accessed September 24, 2024].
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol Rev 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Whitemarsh, R.C.M.; Tepp, W.H.; Bradshaw, M.; Lin, G.; Pier, C.L.; Scherf, J.M.; Johnson, E.A.; Pellett, S. Characterization of botulinum neurotoxin A subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect Immun 2013, 81, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D. Therapeutic Use of Botulinum Toxin in Neurorehabilitation. J Toxicol 2012, 2012, 802893. [Google Scholar] [CrossRef]
- Park, M.Y.; Ahn, K.Y. Scientific review of the aesthetic uses of botulinum toxin type A. Arch Craniofac Surg 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Tehran, D.A.; Pirazzini, M. Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip. Toxins 2018, 10, 190. [Google Scholar] [CrossRef]
- Brin, M.F.; Burstein, R. Botox (onabotulinumtoxinA) mechanism of action. Medicine (Baltimore) 2023, 102, e32372. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Liao, C.-H.; Kuo, H.-C. Current and potential urological applications of botulinum toxin A. Nat Rev Urol 2015, 12, 519–533. [Google Scholar] [CrossRef]
- Ayyar, B.V.; Aoki, K.R.; Atassi, M.Z. The C-terminal heavy-chain domain of botulinum neurotoxin a is not the only site that binds neurons, as the N-terminal heavy-chain domain also plays a very active role in toxin-cell binding and interactions. Infect Immun 2015, 83, 1465–1476. [Google Scholar] [CrossRef]
- M; M Translocation of botulinum neurotoxin light chain protease by the heavy chain protein-conducting channel. Toxicon : official journal of the International Society on Toxinology 2009, 54. [CrossRef]
- Fj, L.; Rz, C.; U, M.; R, S.; Br, S.; M, A. The zinc-dependent protease activity of the botulinum neurotoxins. Toxins 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Schjeide, B.-M. Entwicklung und Charakterisierung des MoN-Light BoNT-Tests zur Bestimmung der Toxizität von Botulinum-Neurotoxin in Motorneuronen, die aus CRISPR-modifizierten induzierten pluripotenten Stammzellen differenziert wurdenDevelopment and characterization of the MoN-Light BoNT assay to determine the toxicity of botulinum neurotoxin in motor neurons differentiated from CRISPR-modified induced pluripotent stem cells, Universität Potsdam, 2021. [CrossRef]
- J, M.; J, W.; M, S.; Jo, D. TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Scientific reports 2016, 6. [Google Scholar] [CrossRef]
- Lu, B. The destructive effect of botulinum neurotoxins on the SNARE protein: SNAP-25 and synaptic membrane fusion. PeerJ 2015, 3, e1065. [Google Scholar] [CrossRef]
- Rizo, J. Mechanism of neurotransmitter release coming into focus. Protein Sci 2018, 27, 1364–1391. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef]
- Brideau-Andersen, A.; Dolly, J.O.; Brin, M.F. Botulinum neurotoxins: Future innovations. Medicine (Baltimore) 2023, 102, e32378. [Google Scholar] [CrossRef]
- Chf, C.; Hag, T. Use of botulinum toxin for movement disorders. Drugs in context 2019, 8. [Google Scholar] [CrossRef]
- Tilton, A.H. Injectable neuromuscular blockade in the treatment of spasticity and movement disorders. J Child Neurol 2003, 18 Suppl 1, S50–66. [Google Scholar] [CrossRef]
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Kumar, H. Botulinum Toxin: An Update on Pharmacology and Newer Products in Development. Toxins (Basel) 2021, 13, 58. [Google Scholar] [CrossRef]
- Bentivoglio, A.R.; Del Grande, A.; Petracca, M.; Ialongo, T.; Ricciardi, L. Clinical differences between botulinum neurotoxin type A and B. Toxicon 2015, 107, 77–84. [Google Scholar] [CrossRef]
- Dressler, D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord 2004, 19 Suppl 8, S92–S100. [Google Scholar] [CrossRef]
- D, D.; F, A.S. Botulinum toxin: mechanisms of action. European neurology 2005, 53. [Google Scholar] [CrossRef]
- Walker, T.J.; Dayan, S.H. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol 2014, 7, 31–39. [Google Scholar] [PubMed]
- Frevert, J. Content of Botulinum Neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D 2010, 10, 67–73. [Google Scholar] [CrossRef]
- Field, M.; Splevins, A.; Picaut, P.; van der Schans, M.; Langenberg, J.; Noort, D.; Foster, K. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) Neurotoxin Content and Potential Implications for Duration of Response in Patients. Toxins (Basel) 2018, 10, 535. [Google Scholar] [CrossRef]
- Scaglione, F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins (Basel) 2016, 8, 65. [Google Scholar] [CrossRef]
- Thomas, A.J.; Larson, M.O.; Braden, S.; Cannon, R.B.; Ward, P.D. Effect of 3 Commercially Available Botulinum Toxin Neuromodulators on Facial Synkinesis. JAMA Facial Plast Surg 2018, 20, 141–147. [Google Scholar] [CrossRef]
- Pasricha, P.J.; Rai, R.; Ravich, W.J.; Hendrix, T.R.; Kalloo, A.N. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology 1996, 110, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Campanati A, Martina E, Gregoriou S, Kontochristopoulos G, Paolinelli M, Diotallevi F, Radi G, Bobyr I, Marconi B, Gualdi G, et al. Botulinum Toxin Type A for Treatment of Forehead Hyperhidrosis: Multicenter Clinical Experience and Review from Literature. Toxins (Basel) 2022, 14, 372. [Google Scholar] [CrossRef]
- Chow, P.-M.; Kuo, H.-C. Botulinum Toxin A Injection for Autonomic Dysreflexia—Detrusor Injection or Urethral Sphincter Injection? Toxins (Basel) 2023, 15, 108. [Google Scholar] [CrossRef]
- Romanov A, Pokushalov E, Ponomarev D, Bayramova S, Shabanov V, Losik D, Stenin I, Elesin D, Mikheenko I, Strelnikov A, et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: Three-year follow-up of a randomized study. Heart Rhythm 2019, 16, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.; Clark, J.D.; Mackey, S. Sympathetic Block with Botulinum Toxin to Treat Complex Regional Pain Syndrome. Ann Neurol 2009, 65, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Lawson, O.; Sisti, A.; Konofaos, P. The Use of Botulinum Toxin in Raynaud Phenomenon: A Comprehensive Literature Review. Ann Plast Surg 2023, 91, 159–186. [Google Scholar] [CrossRef]
- Geoghegan, L.; Rodrigues, R.; Harrison, C.J.; Rodrigues, J.N. The Use of Botulinum Toxin in the Management of Hidradenitis Suppurativa: A Systematic Review. Plast Reconstr Surg Glob Open 2022, 10, e4660. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, K.; Wang, Y.; Fang, R.; Sun, Q. Use of Botulinum Toxin in Treating Rosacea: A Systematic Review. Clin Cosmet Investig Dermatol 2021, 14, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Brisinda, G.; Chiarello, M.M.; Crocco, A.; Bentivoglio, A.R.; Cariati, M.; Vanella, S. Botulinum toxin injection for the treatment of chronic anal fissure: uni- and multivariate analysis of the factors that promote healing. Int J Colorectal Dis 2022, 37, 693–700. [Google Scholar] [CrossRef]
- González, C.; Franco, M.; Londoño, A.; Valenzuela, F. Breaking paradigms in the treatment of psoriasis: Use of botulinum toxin for the treatment of plaque psoriasis. Dermatol Ther 2020, 33, e14319. [Google Scholar] [CrossRef]
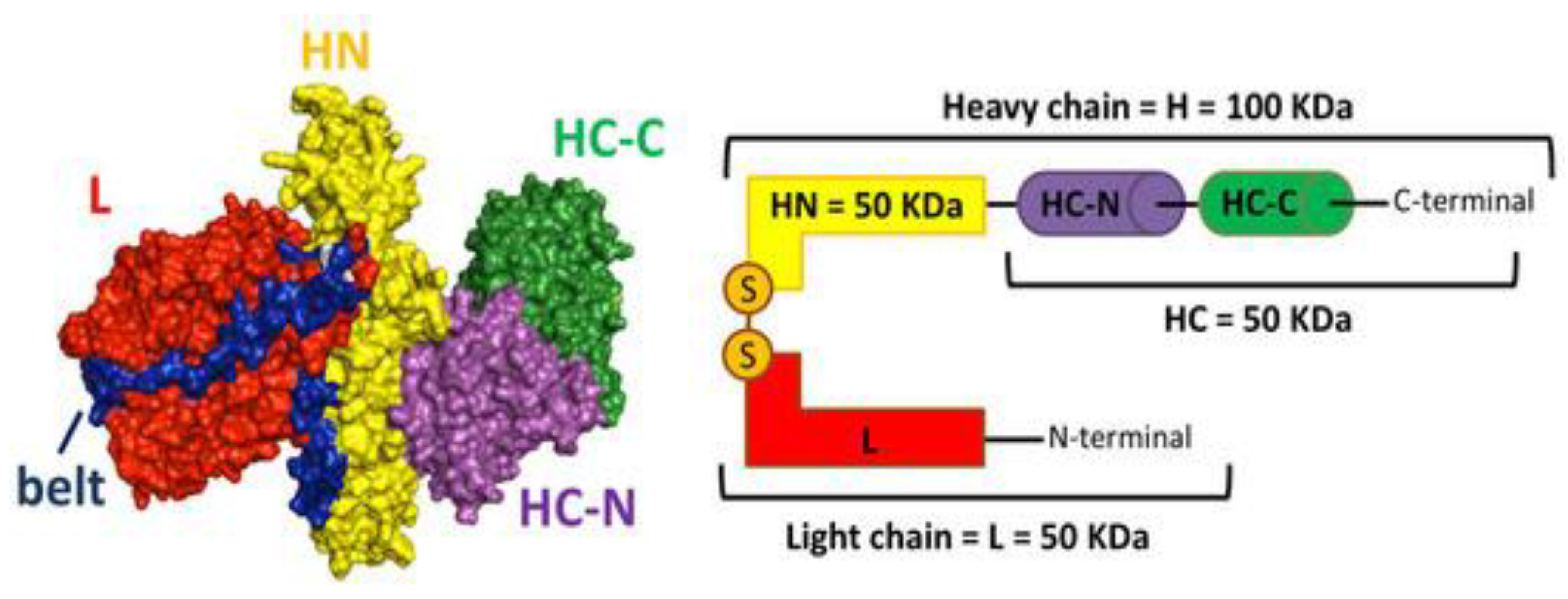
| Serial No. | Disorder Specification | Symptoms Experienced (27–30) |
|---|---|---|
| 1. | Upper Limb Spasticity | Muscles in the arms become stiff, flexed, & painful. |
| 2. | Lower Limb Spasticity | Muscles in the legs become stiff, flexed, & painful. |
| 3. | Cervical Dystonia | Abnormalities in head and neck posture with severe pain. |
| 4. | Chronic Migraine Prophylaxis | Recurring throbbing and pulsating sensations that impair daily activity. |
| 5. | Blepharospasm and Strabismus | Uncontrolled, spasmodic facial or eye muscle movement. |
| 6. | Bladder (detrusor) Dysfunction | Hyperactivity leading to increased urinary incontinence, urgency, and frequency. |
| 7. | Primary Axillary Hyperhidrosis | Over-stimulation of sweat glands that causes excessive perspiration. |
| 8. | Cosmetics | Glabellar/frown lines - vertical wrinkles from corrugator and procerus overactivity Crow’s Feet - lateral canthal lines from orbicularis oculi overactivity Forehead lines - horizontal wrinkles from frontalis overactivity. |
| Serial No. | Disorder Specification | Symptoms Experienced (27–30) |
|---|---|---|
| 1. | Cervical Dystonia | Abnormalities in head and neck posture with severe pain. |
| 2. | Upper Limb Spasticity | Muscles in the arms become stiff, flexed, & painful. |
| 3. | Lower Limb Spasticity | Muscles in the legs become stiff, flexed, & painful. |
| 4. | Glabellar/Frown Lines | Moderate-severe vertical wrinkles from corrugator and procerus overactivity. |
| Serial No. | Disorder Specification | Symptoms Experienced (27–30) |
|---|---|---|
| 1. | Cervical Dystonia | Abnormalities in head and neck posture with severe pain. |
| 2. | Upper Limb Spasticity | Muscles in the arms become stiff, flexed, & painful. |
| 3. | Blepharospasm | Abnormal contractions that cause squeezing and twitching of the eyelid muscles. |
| 4. | Chronic Sialorrhea | Excessive drooling or salivation from poor orofacial muscle control. |
| 5. | Glabellar Lines | Moderate-severe vertical wrinkles from corrugator and procerus overactivity. |
| Serial No. | Disorder Specification | Mechanism of action | Symptoms Experienced |
|---|---|---|---|
| 1. | Achalasia | Relieves chronically contracted LES | Motor disorder of distal esophageal sphincter involving degeneration of inhibitory NO-producing neurons with resultant aperistalsis and hypertonia causing dysphagia to liquids and solids (31). |
| 2. | Palmar/Craniofacial Hyperhidrosis | Interrupt neural transmission of nicotinic and muscarinic receptors destined for eccrine and apocrine sweat glands. | Idiopathic excessive sweat production in other than axillary areas (32). |
| 3. | Autonomic Dysreflexia | Blunts reflexive sympathetic spinal responses. | Autonomic dysregulation often arising from spinal cord injury, leading to sympathetic control imbalances (33). |
| 4. | Atrial Fibrillation | Injection into epicardial fat pads suppresses ectopy. | Electrical abnormalities within the cardiac circuit that do not terminate spontaneously (34). |
| 5. | Complex Regional Pain Syndrome | Blockade of cholinergic preganglionic fibers with sympathetic ganglion block. | Disrupted relationship between sympathetic nervous system and nociceptive transmission (35). |
| 6. | Raynaud’s Phenomenon | Restoration of arterial flow through vasodilation. | Exaggerated vasoregulatory constriction of distal extremities in response to certain stressors (36). |
| 7. | Hidradenitis Suppurativa | Suppresses release of follicular contents into dermis. | Persistent, painful nodules, abscesses, and fistulas in the skin folds of the axilla, groin, gluteal, and perineal regions (37). |
| 8. | Rosacea | Modulation of Ach, substance P, CGRP, and VIP. | Erythema, telangiectasia, papules and pustules in and around the face, forehead, nose, cheeks, and chin (38). |
| 9. | Anal Fissures | Relieves painful spasms of internal anal sphincter and regulates autonomic control. | Tears in the epithelial lining of the anal canal distal to dentate line, sensitizing somatic nerve fibers (39). |
| 10. | Plaque Psoriasis | Intradermal injection demonstrates complete remission from targeted lesional eruption. | Erythematous, scaly papules and thick plaques typically on the scalp, trunk, elbows, knees, and gluteal folds (40). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




