Submitted:
25 September 2024
Posted:
27 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
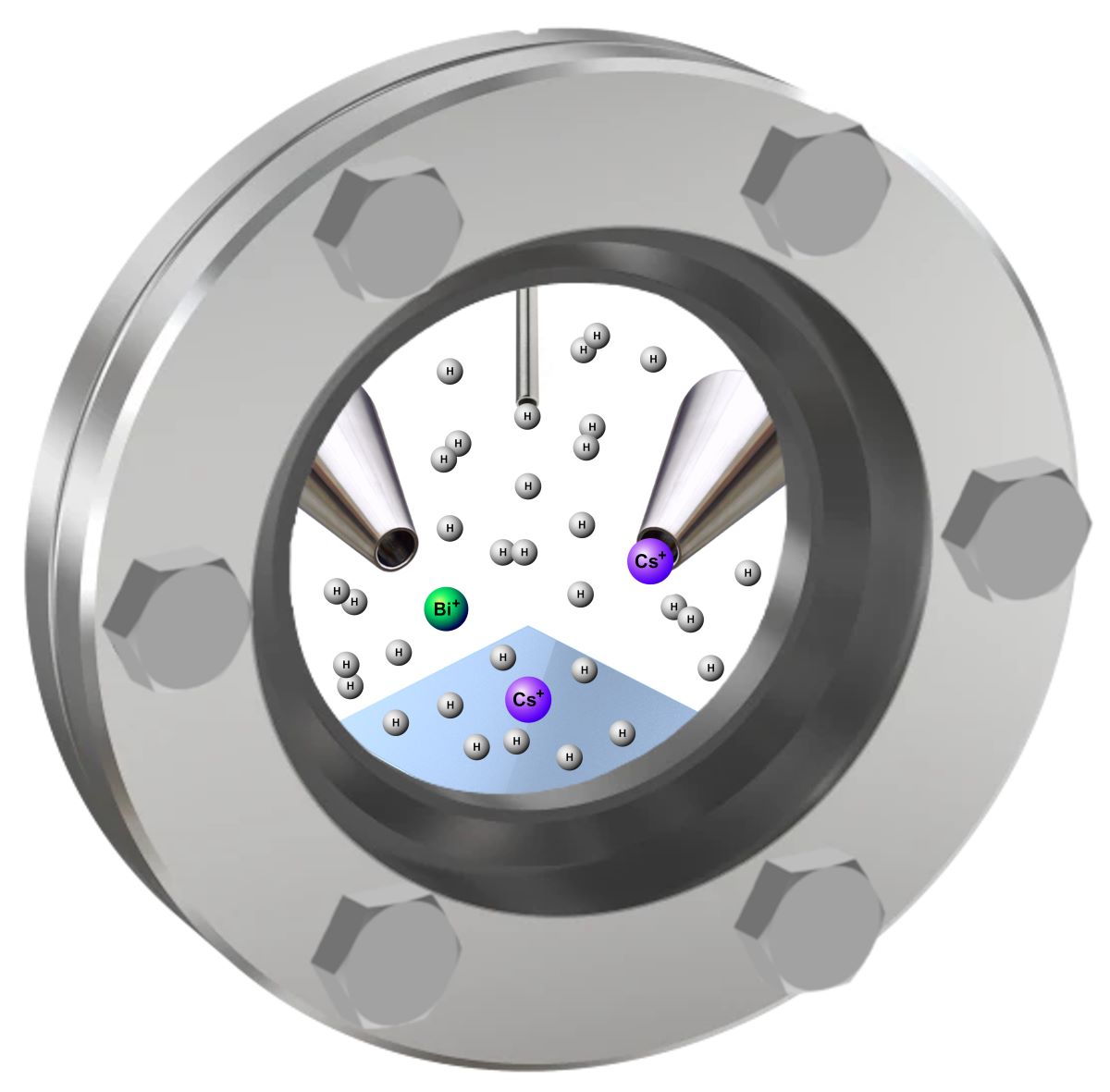
2.2. Secondary Ion Mass Spectrometry Measurements
2.3. Principles of the Hydrogen Atom Beam Source Operation
3. Results
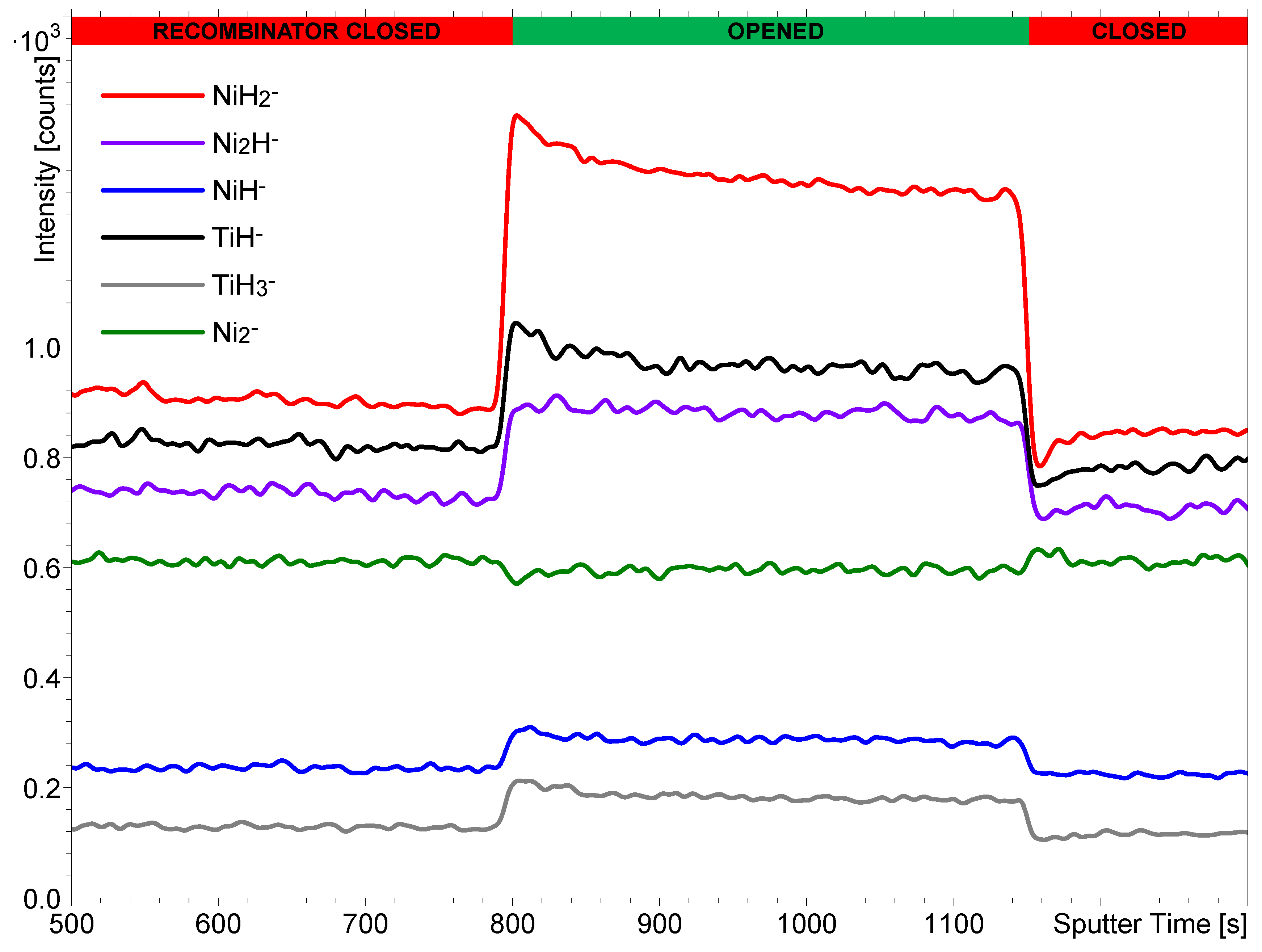
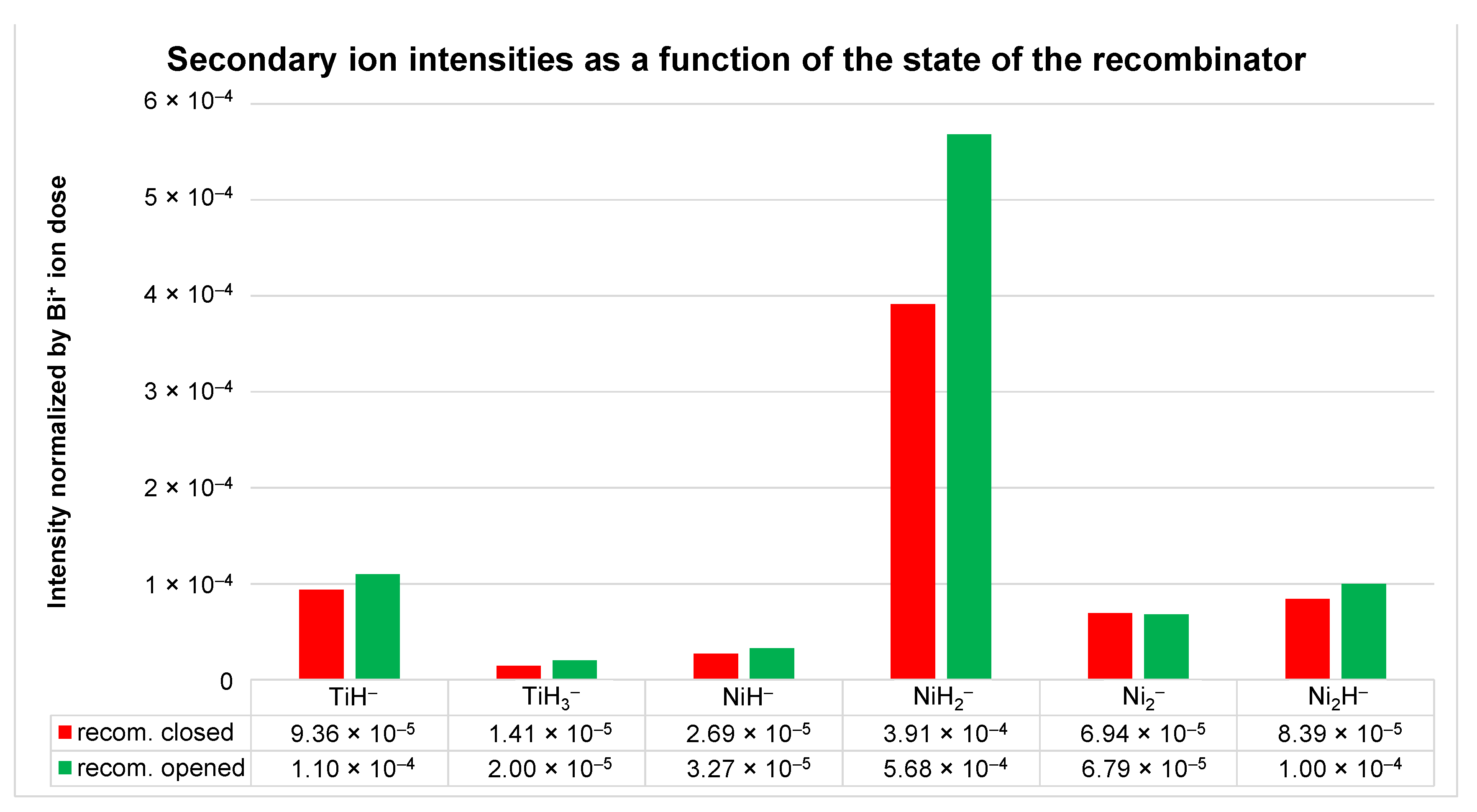
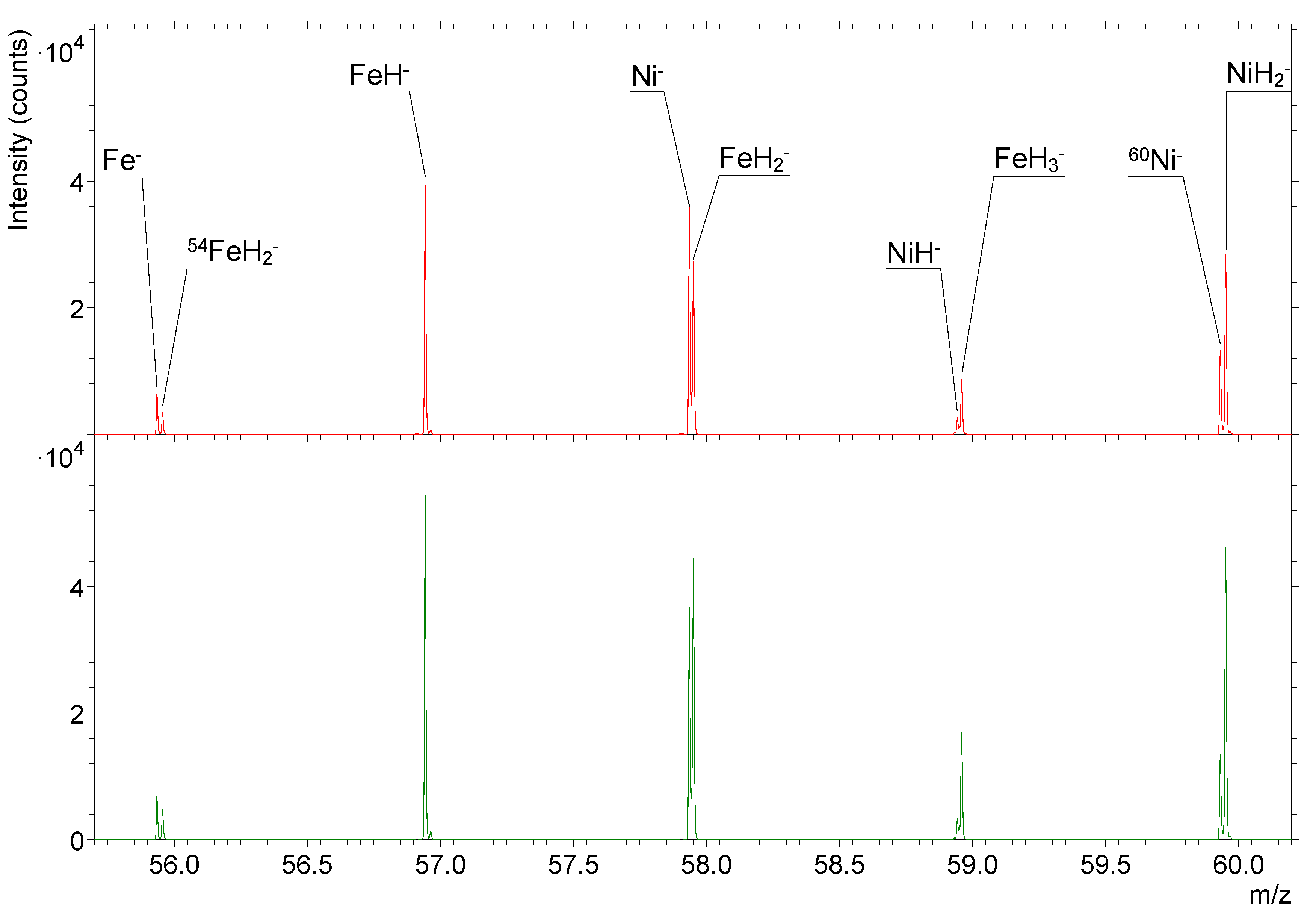
3.1. Comparison of the Effects of Hot H2 Molecules and H Atoms on the SIMS Signals
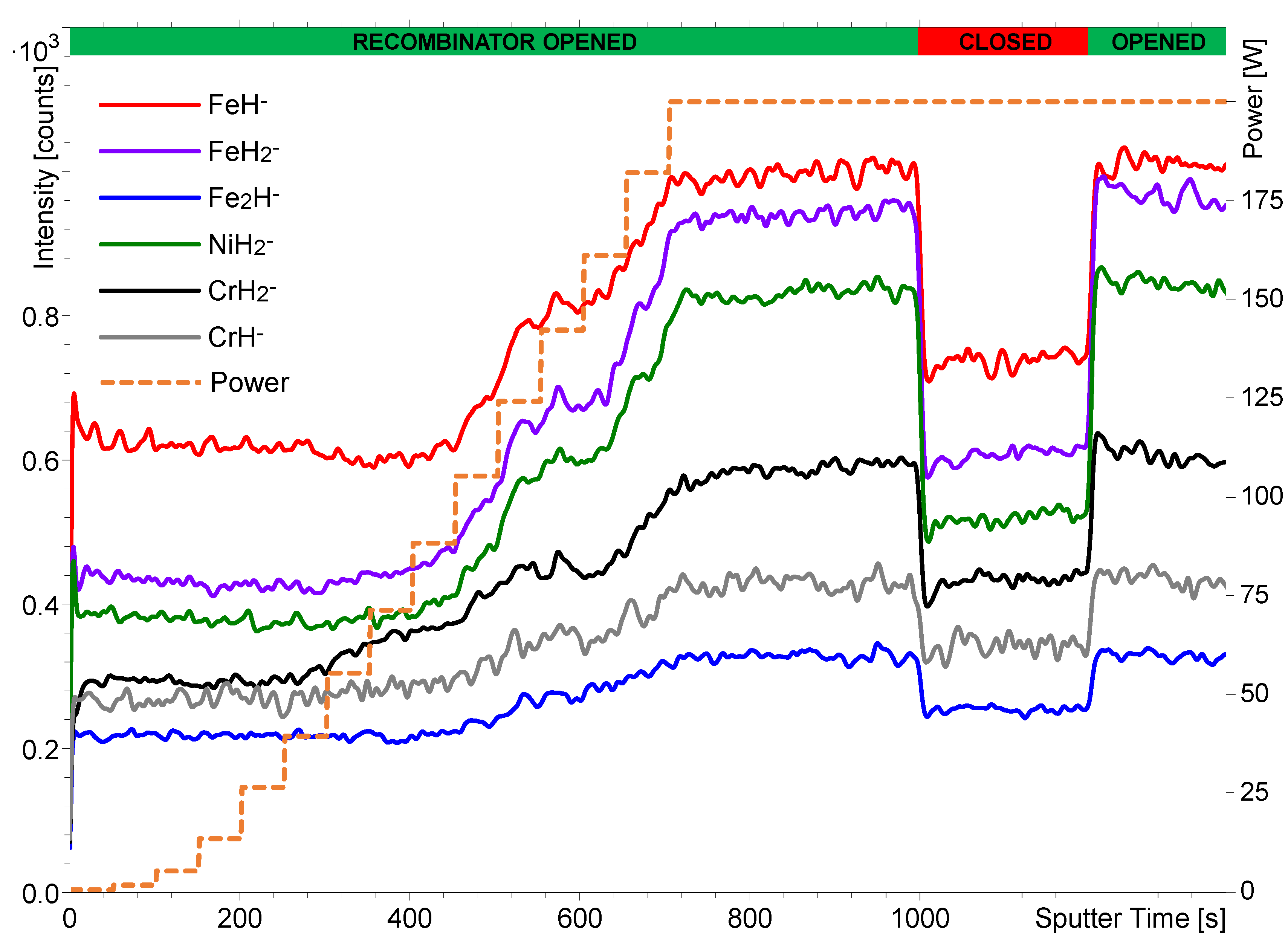
3.2. Effect of the Hydrogen Atom Beam Source Power on the Secondary Ion Intensity
4. Discussion
4.1. Adsorption Affinity of H2 and H
4.2. Mechanism of Metal Hydride Secondary Ion Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vickerman, J.C. Prologue: ToF-SIMS—An evolving mass spectrometry of materials. In ToF-SIMS: Materials Analysis by Mass Spectrometry, 2nd Ed., Editors D. Vickerman, J. C. & Briggs, Publisher: IM Publications LLP and SurfaceSpectra Limited, Chichester, Manchester, United Kingdom, 2013; pp. 1–37.
- van der Heide, P. Sputtering and Ion Formation. In Secondary Ion Mass Spectrometry – An Introduction to Principles Practices, Publisher: John Wiley & Sons, Hoboken, New Jersey, USA, 2014; pp. 44–143.
- van der Heide, P. The Art of Measurement. In Secondary Ion Mass Spectrometry – An Introduction to Principles Practices, Publisher: John Wiley & Sons, Hoboken, New Jersey, USA, 2014; pp. 195–208.
- Niehuis, E.; Grehl, T. Depth profiling of inorganic materials. In ToF-SIMS: Materials Analysis by Mass Spectrometry, 2nd Ed., Editors D. Vickerman, J. C. & Briggs, Publisher: IM Publications LLP and SurfaceSpectra Limited, Chichester, Manchester, United Kingdom, 2013; pp. 613–635.
- van der Heide, P. Secondary Ion Yields. In Secondary Ion Mass Spectrometry – An Introduction to Principles Practices, Publisher: John Wiley & Sons, Hoboken, New Jersey, USA, 2014; pp. 93–121.
- Grasserbauer, M. Quantitative Secondary Ion Mass Spectrometry. J. Res. Natl. Bur. Stand. 1988, 93, 510–518. [Google Scholar] [CrossRef]
- Priebe, A.; Xie, T.; Bürki, G.; Pethö, L.; Michler, J. The matrix effect in TOF-SIMS analysis of two-element inorganic thin films. J. Anal. At. Spectrom. 2020, 35, 1156–1166. [Google Scholar] [CrossRef]
- Karki, V.; Singh, M. Quantitative depth distribution analysis of elements in high alloy steel using MCs+-SIMS approach. Int. J. Mass Spectrom. 2018, 430, 22–30. [Google Scholar] [CrossRef]
- Satoh, H.; Owari, M.; Nihei, Y. Relative sensitivity factors for submicron secondary ion mass spectrometry with gallium primary ion beam. Jpn. J. Appl. Phys. 1993, 32, 3616–3620. [Google Scholar] [CrossRef]
- Hongo, C.; Tomita, M.; Suzuki, M. Quantitative secondary ion mass spectrometry analysis of impurities in GaN and AlxGa1−xN films using molecular ions MCs+ and MCs2+. Appl. Surf. Sci. 1999, 144–145, 306–309. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, S.H.; Kim, K.J.; Kim, D.; Lee, Y. Comparison of quantitative analyses using SIMS, atom probe tomography, and femtosecond laser ablation inductively coupled plasma mass spectrometry with Si1−XGeX and Fe1−XNiX binary alloys. J. Vac. Sci. Technol. B. 2020, 38, 034009. [Google Scholar] [CrossRef]
- Wucher, A. Laser post-ionisation—fundamentals. In ToF-SIMS: Materials Analysis by Mass Spectrometry, 2nd Ed., Editors D. Vickerman, J. C. & Briggs, Publisher: IM Publications LLP and SurfaceSpectra Limited, Chichester, Manchester, United Kingdom, 2013; pp. 217–246.
- Breuer, L.; Tian, H.; Wucher, A.; Winograd, N. Molecular SIMS Ionization Probability Studied with Laser Postionization: Influence of the Projectile Cluster. J. Phys. Chem. C. 2019, 123, 565–574. [Google Scholar] [CrossRef]
- Breuer, L.; Popczun, N.J.; Wucher, A.; Winograd, N. Reducing the Matrix Effect in Molecular Secondary Ion Mass Spectrometry by Laser Post-Ionization. J. Phys. Chem. C. 2017, 121, 19705–19715. [Google Scholar] [CrossRef]
- Kopnarski, M.; Jenett, H. Electron-Impact (EI) Secondary Neutral Mass Spectrometry (SNMS). In Surface and Thin Film Analysis, Publisher: Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011; pp. 161–177.
- Wilson, R.; Van Den Berg, J.A.; Vickerman, J.C. Quantitative surface analysis using electron beam SNMS: Calibrations and applications. Surf. Interface Anal. 1989, 14, 393–400. [Google Scholar] [CrossRef]
- Oechsner, H. Secondary Ion Mass Spectrometry SIMS V, Proceedings of the Fifth SIMS International Conference, Washington, DC, USA, September 30 – October 4, 1985, Publisher: Springer, Berlin, Germany, 1986.
- Debord, J.D.; Prabhakaran, A.; Eller, M.J.; Verkhoturov, S.V.; Delcorte, A.; Schweikert, E.A. Metal-assisted SIMS with hypervelocity gold cluster projectiles. Int. J. Mass Spectrom. 2013, 343–344, 28–36. [Google Scholar] [CrossRef]
- Dunham, S.J.B.; Comi, T.J.; Ko, K.; Li, B.; Baig, N.F.; Morales-Soto, N.; Shrout, J.D.; Bohn, P.W.; Sweedler, J.V. Metal-assisted polyatomic SIMS and laser desorption/ionization for enhanced small molecule imaging of bacterial biofilms. Biointerphases. 2016, 11, 02A325. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, A.; Tobe, T.; Akiba, S.; Owari, M. Metal-Assisted SIMS for three-dimensional analysis using shave-off section processing. Surf. Interface Anal. 2014, 46, 1215–1218. [Google Scholar] [CrossRef]
- Lanni, E.J.; Dunham, S.J.B.; Nemes, P.; Rubakhin, S.S.; Sweedler, J.V. Biomolecular imaging with a C60-SIMS/MALDI dual ion source hybrid mass spectrometer: Instrumentation, matrix enhancement, and single cell analysis. J. Am. Soc. Mass Spectrom. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Locklear, J.E.; Guillermier, C.; Verkhoturov, S.V.; Schweikert, E.A. Matrix-enhanced cluster-SIMS. Appl. Surf. Sci. 2006, 252, 6624–6627. [Google Scholar] [CrossRef]
- Fitzgerald, J.J.D.; Kunnath, P.; Walker, A.V. Matrix-enhanced secondary ion mass spectrometry (ME SIMS) using room temperature ionic liquid matrices. Anal. Chem. 2010, 82, 4413–4419. [Google Scholar] [CrossRef]
- Tian, H.; Wucher, A.; Winograd, N. Dynamic Reactive Ionization with Cluster Secondary Ion Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2016, 27, 285–292. [Google Scholar] [CrossRef]
- Tian, H.; Wucher, A.; Winograd, N. Reducing the Matrix Effect in Organic Cluster SIMS Using Dynamic Reactive Ionization. J. Am. Soc. Mass Spectrom. 2016, 27, 2014–2024. [Google Scholar] [CrossRef]
- Priebe, A.; Utke, I.; Pethö, L.; Michler, J. Application of a Gas-Injection System during the FIB-TOF-SIMS Analysis - Influence of Water Vapor and Fluorine Gas on Secondary Ion Signals and Sputtering Rates. Anal. Chem. 2019, 91, 11712–11722. [Google Scholar] [CrossRef]
- Kudriatsev, Y.; Villegas, A.; Gallardo, S.; Ramirez, G.; Asomoza, R.; Mishurnuy, V. Cesium ion sputtering with oxygen flooding: Experimental SIMS study of work function change. Appl. Surf. Sci. 2008, 254, 4961–4964. [Google Scholar] [CrossRef]
- Williams, P.; Franzreb, K.; Sobers, R.C.; Lörinčík, J. On the effect of oxygen flooding on the detection of noble gas ions in a SIMS instrument. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms. 2010, 268, 2758–2765. [Google Scholar] [CrossRef]
- Ekar, J.; Kos, S.; Kovač, J. Quantitative aspects of ToF-SIMS analysis of metals and alloys in a UHV, O2 and H2 atmosphere. Surfaces and Interfaces 2024, 49, 104408. [Google Scholar] [CrossRef]
- Ekar, J.; Panjan, P.; Drev, S.; Kovač, J. ToF-SIMS Depth Profiling of Metal, Metal Oxide, and Alloy Multilayers in Atmospheres of H2, C2H2, CO, and O2. J. Am. Soc. Mass Spectrom. 2022, 33, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Ekar, J.; Kovač, J. AFM Study of Roughness Development during ToF-SIMS Depth Profiling of Multilayers with a Cs+ Ion Beam in a H2 Atmosphere. Langmuir 2022, 38, 12871–12880. [Google Scholar] [CrossRef]
- Kozlica, D.K.; Ekar, J.; Kovač, J.; Milošev, I. Roles of Chloride Ions in the Formation of Corrosion Protective Films on Copper. J. Electrochem. Soc. 2021, 168, 031504. [Google Scholar] [CrossRef]
- Abe, Y.; Komatsu, M.; Okuhira, H. Estimation of ToF-SIMS information depth in micro-corrosion analysis. Appl. Surf. Sci. 2003, 203–204, 859–862. [Google Scholar] [CrossRef]
- Kia, A.M.; Haufe, N.; Esmaeili, S.; Mart, C.; Utriainen, M.; Puurunen, R.L.; Weinreich, W. ToF-SIMS 3D Analysis of Thin Films Deposited in High Aspect Ratio Structures via Atomic Layer Deposition and Chemical Vapor Deposition. Nanomater. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Cwil, M.; Konarski, P.; Ciosek, J. Ion mass interferences and matrix effects on SIMS depth profiling of thin Ti/Si multilayer films induced by hydrogen, carbon and oxygen contaminations. Int. J. Mass Spectrom. 2007, 263, 54–58. [Google Scholar] [CrossRef]
- Kovač, J.; Ekar, J.; Čekada, M.; Zajíčková, L.; Nečas, D.; Blahová, L.; Wang, J.Y.; Mozetič, M. Depth profiling of thin plasma-polymerized amine films using GDOES in an Ar-O2 plasma. Appl. Surf. Sci. 2022, 581, 152292. [Google Scholar] [CrossRef]
- Veith, L.; Böttner, J.; Vennemann, A.; Breitenstein, D.; Engelhard, C.; Meijer, J.; Estrela-Lopis, I.; Wiemann, M.; Hagenhoff, B. Detection of ZrO2 Nanoparticles in Lung Tissue Sections by Time-of-Flight Secondary Ion Mass Spectrometry and Ion Beam Microscopy. Nanomater. 2018, 8, 44. [Google Scholar] [CrossRef]
- Radnik, J.; Kersting, R.; Hagenhoff, B.; Bennet, F.; Ciornii, D.; Nymark, P.; Grafström, R.; Hodoroaba, V.D. Reliable Surface Analysis Data of Nanomaterials in Support of Risk Assessment Based on Minimum Information Requirements. Nanomater. 2021, 11, 639. [Google Scholar] [CrossRef]
- Wang, Z.; Charly, C.; Antoine, S.; Sandrine, Z.; Dimitri, M.; Philippe, M. XPS and ToF-SIMS Investigation of Native Oxides and Passive Films Formed on Nickel Alloys Containing Chromium and Molybdenum. J. Electrochem. Soc. 2021, 168, 041503. [Google Scholar] [CrossRef]
- Jovičević-Klug, P.; Lipovšek, N.; Jovičević-Klug, M.; Mrak, M.; Ekar, J.; Ambrožič, B.; Dražić, G.; Kovač, J. Podgornik. Assessment of deep cryogenic heat-treatment impact on the microstructure and surface chemistry of austenitic stainless steel. Surfaces and Interfaces 2022, 35, 102456. [Google Scholar] [CrossRef]
- Omerzu, A.; Peter, R.; Jardas, D.; Turel, I.; Salamon, K.; Podlogar, M.; Vengust, D.; Badovinac, I.J.; Piltaver, I.K.; Petravic, M. Large enhancement of photocatalytic activity in ZnO thin films grown by plasma-enhanced atomic layer deposition. Surfaces and Interfaces 2021, 23, 100984. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H.; Jung, Y.; Shin, J.C.; Lee, Y.; Kim, K.; Kim, N.; van der Zande, A.M.; Son, J.; Lee, G.H. Evolution of defect formation during atomically precise desulfurization of monolayer MoS2. Commun. Mater. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Kim, B.; Kim, M.; Yoo, S.; Nam, S.K. Atomistic insights on hydrogen plasma treatment for stabilizing High-k/Si interface. Appl. Surf. Sci. 2022, 593, 153297. [Google Scholar] [CrossRef]
- Marinov, D.; de Marneffe, J.F.; Smets, Q.; Arutchelvan, G.; Bal, K.M.; Voronina, E.; Rakhimova, T.; Mankelevich, Y.; El Kazzi, S.; Mehta, A.N.; Wyndaele, P.J.; Heyne, M.H.; Zhang, J.; With, P.C.; Banerjee, S.; Neyts, E.C.; Asselberghs, I.; Lin, D.; De Gendt, S. Reactive plasma cleaning and restoration of transition metal dichalcogenide monolayers. Npj 2D Mater. Appl. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, H.; Kim, Y.; Song, H.; Lee, H.; Choi, J.; Kim, K.T.; Song, Y.H. Plasma-assisted hydrogen generation: A mechanistic review. Fuel Process. Technol. 2023, 247, 107761. [Google Scholar] [CrossRef]
- Tschersich, K.G.; Fleischhauer, J.P.; Schuler, H. Design and characterization of a thermal hydrogen atom source. J. Appl. Phys. 2008, 104, 34908. [Google Scholar] [CrossRef]
- Tschersich, K.G. Intensity of a source of atomic hydrogen based on a hot capillary. J. Appl. Phys. 2000, 87, 2565–2573. [Google Scholar] [CrossRef]
- Tschersich, K.G.; Von Bonin, V. Formation of an atomic hydrogen beam by a hot capillary. J. Appl. Phys. 1998, 84, 4065–4070. [Google Scholar] [CrossRef]
- Mozetič, M.; Drobnič, M.; Zalar, A. Recombination of neutral hydrogen atoms on AISI 304 stainless steel surface. Appl. Surf. Sci. 1999, 144–145, 399–403. [Google Scholar] [CrossRef]
- Cong, Z.; Fu, X.; Liu, S.; Wang, W.; Liu, H.; Lei, G.; Zhao, B.; Wu, H.; Gao, C. Enhancing the organic solar cells performances by elevating cesium carboxylate content of graphene oxide based cathode interface layer. Surfaces and Interfaces 2022, 31, 102068. [Google Scholar] [CrossRef]
- Sode, M.; Schwarz-Selinger, T.; Jacob, W.; Kersten, H. Surface loss probability of atomic hydrogen for different electrode cover materials investigated in H2-Ar low-pressure plasmas. J. Appl. Phys. 2014, 116, 013302. [Google Scholar] [CrossRef]
- Zyryanov, S.M.; Kovalev, A.S.; Lopaev, D.V.; Malykhin, E.M.; Rakhimov, A.T.; Rakhimova, T.V.; Koshelev, K.N.; Krivtsun, V.M. Loss of hydrogen atoms in H2 plasma on the surfaces of materials used in EUV lithography. Plasma Phys. Reports. 2011, 37, 881–889. [Google Scholar] [CrossRef]
- Rutigliano, M.; Santoro, D.; Balat-Pichelin, M. Hydrogen atom recombination on tungsten at high temperature: Experiment and Molecular Dynamics Simulation. Surf. Sci. 2014, 628, 66–75. [Google Scholar] [CrossRef]
- Wood, B.J.; Wise, H. Kinetics of hydrogen atom recombination on surfaces. J. Phys. Chem. 1961, 65, 1976–1983. [Google Scholar] [CrossRef]
- Paul, D.; Mozetic, M.; Zaplotnik, R.; Primc, G.; Đonlagić, D.; Vesel, A. A Review of Recombination Coefficients of Neutral Oxygen Atoms for Various Materials. Mater. 2023, 16, 1774. [Google Scholar] [CrossRef]
- Vlekken, J.; D’Olieslaeger, M.; Knuyt, G.; Vandervorst, W.; De Schepper, L. Investigation of the formation process of MCs+-molecular ions during sputtering. J. Am. Soc. Mass Spectrom. 2000, 11, 650–658. [Google Scholar] [CrossRef]
- Honda, F.; Lancaster, G.M.; Fukuda, Y.; Rabalais, J.W. SIMS study of the mechanism of cluster formation during ion bombardment of alkali halides. J. Chem. Phys. 1978, 69, 4931–4937. [Google Scholar] [CrossRef]
- Lancaster, G.M.; Honda, F.; Fukuda, Y.; J. W. Rabalais. Secondary Ion Mass Spectrometry of Molecular Solids. Cluster Formation During Ion Bombardment of Frozen Water, Benzene, and Cyclohexane. J. Am. Chem. Soc. 1979, 101, 1951–1958. [Google Scholar] [CrossRef]
- Saha, B.; Chakraborty, P. Secondary ion mass spectrometry of MCsn+ molecular ion complexes. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms. 2007, 258, 218–225. [Google Scholar] [CrossRef]
- Vlekken, J.; Wu, T.D.; D’Olieslaeger, M.; Knuyt, G.; De Schepper, L.; Stals, L.M. Monte Carlo simulation of the formation of MCs+ molecular ions. Int. J. Mass Spectrom. Ion Process. 1996, 156, 61–66. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering – An Introduction, Publisher: John Wiley & Sons, Hoboken, New Jersey, USA, 2018; pp. III.
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Fundamentals of Gas Permeation Through Membranes. In Gas Separation Membranes: Polymeric and Inorganic, Publisher: Springer, Berlin, Germany, 2015; pp. 14.
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A. 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
- Markelj, S.; Schwarz-Selinger, T.; Založnik, A.; Kelemen, M.; Vavpetič, P.; Pelicon, P.; Hodille, E.; Grisolia, C. Deuterium retention in tungsten simultaneously damaged by high energy W ions and loaded by D atoms. Nucl. Mater. Energy. 2017, 12, 169–174. [Google Scholar] [CrossRef]
- Marquardt, N. Introduction to the principles of vacuum physics. Cern Accel. Sch. Vac. Technol. 1999, 1–24. [Google Scholar] [CrossRef]
- Sameshima, J.; Numao, S. Behavior and process of background signal formation of hydrogen, carbon, nitrogen, and oxygen in silicon wafers during depth profiling using dual-beam TOF-SIMS. Surf. Interface Anal. 2022, 54, 165–173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




