Submitted:
24 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Several medical graduates, avoid surgical specialties especially those with complex and delicate anatomy due to their doubt in their visual spatial abilities. Many patients are deemed inoperable, particularly in the field of pediatric cardiology, due to the complexity of the anatomy. On a similar note, stratification of risk of cardiac interventions, and decision making, is a room of significant person-based and center-based bias. Two inter-related technologies, namely immersive virtual reality (VR) and artificial intelligence have made impossible things possible and can help to standardize decision making strategies. This review aimed at reviewing the main anatomical and lesion-based scopes of these advancements in the field of cardiac care, with a special focus on pediatric cardiology.
Keywords:
Background
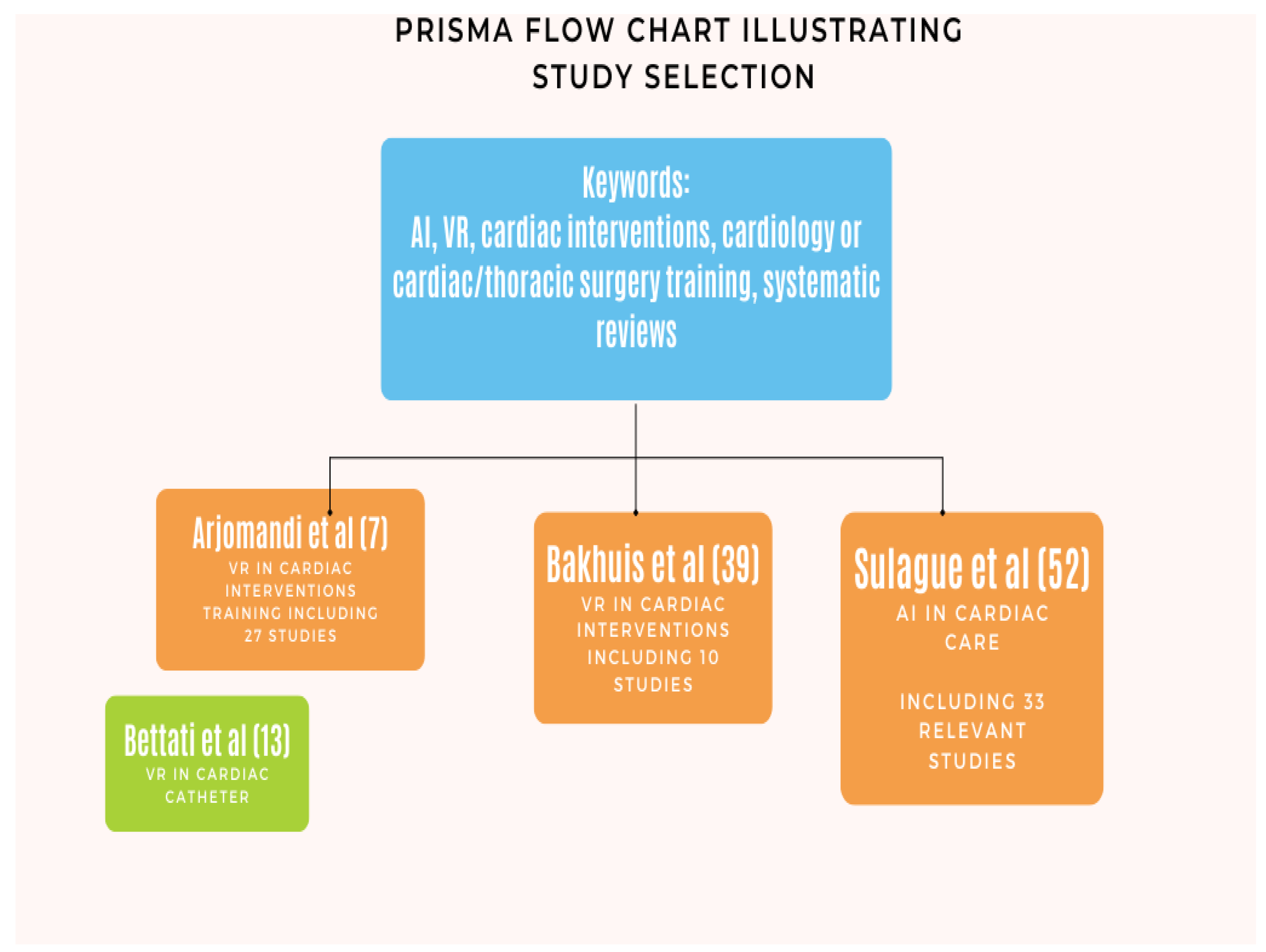
Main Body
Virtual Reality in Cardiology/Cardiac Surgery Training
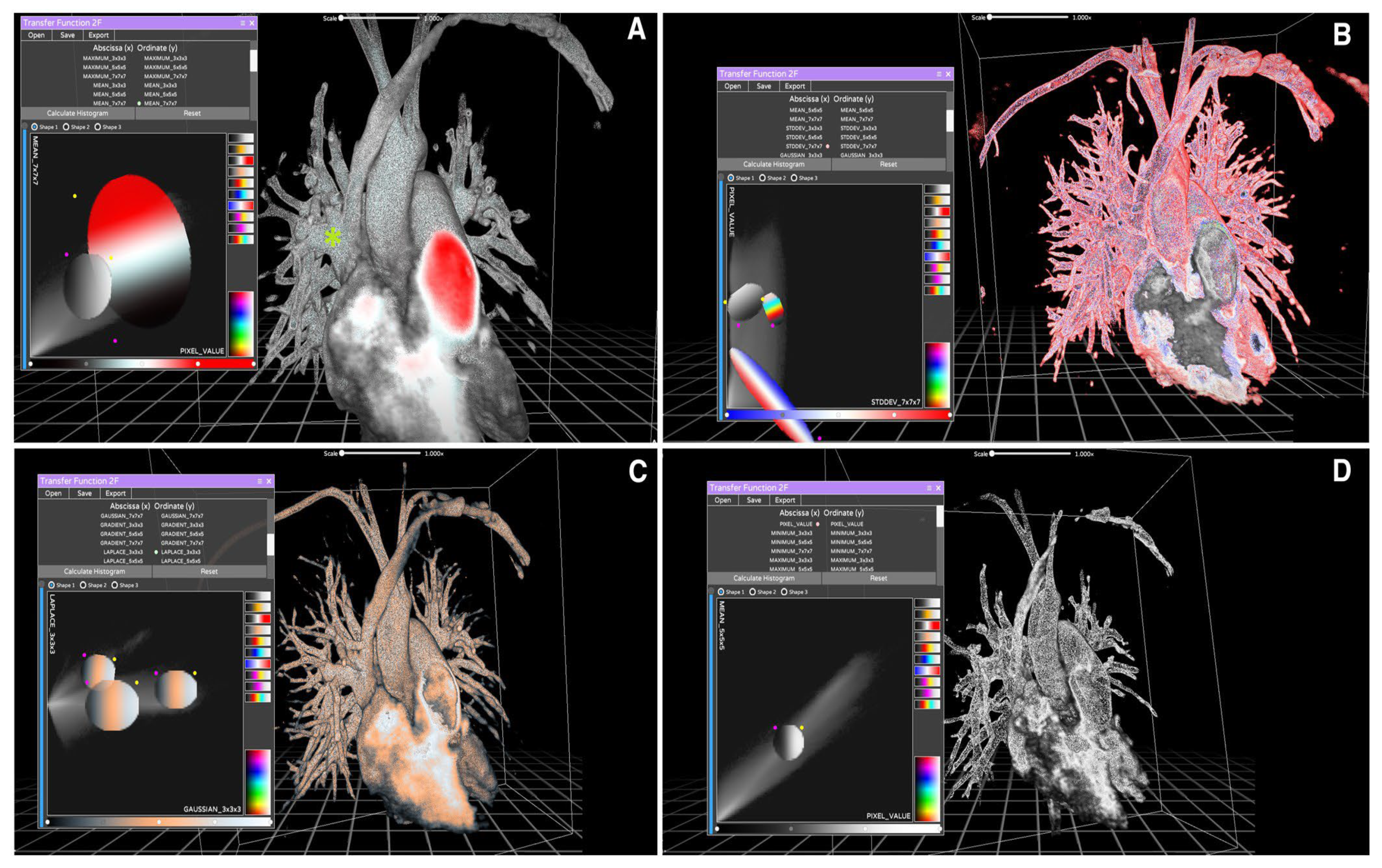
Virtual Reality in Planning Cardiac Interventions
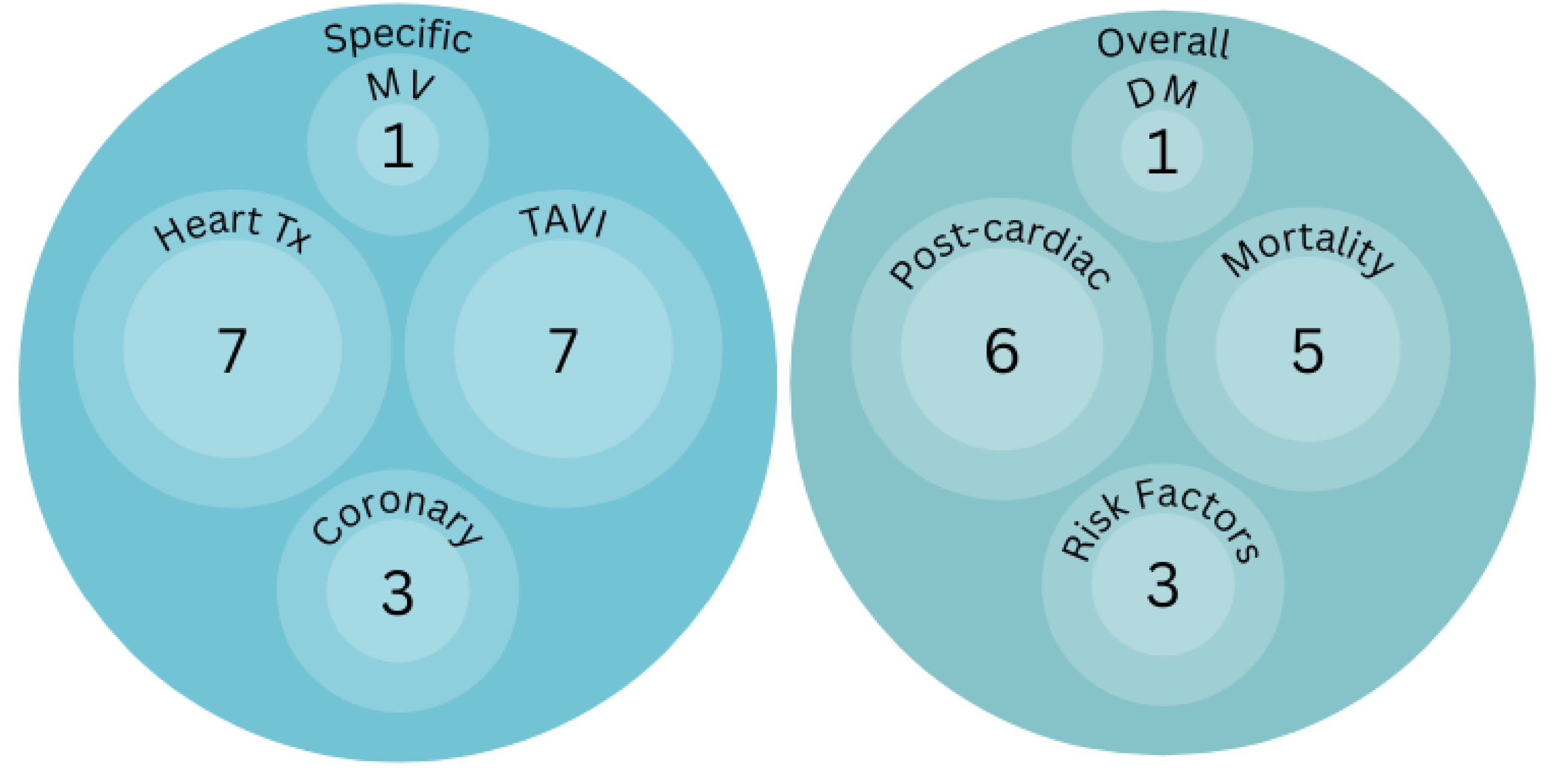
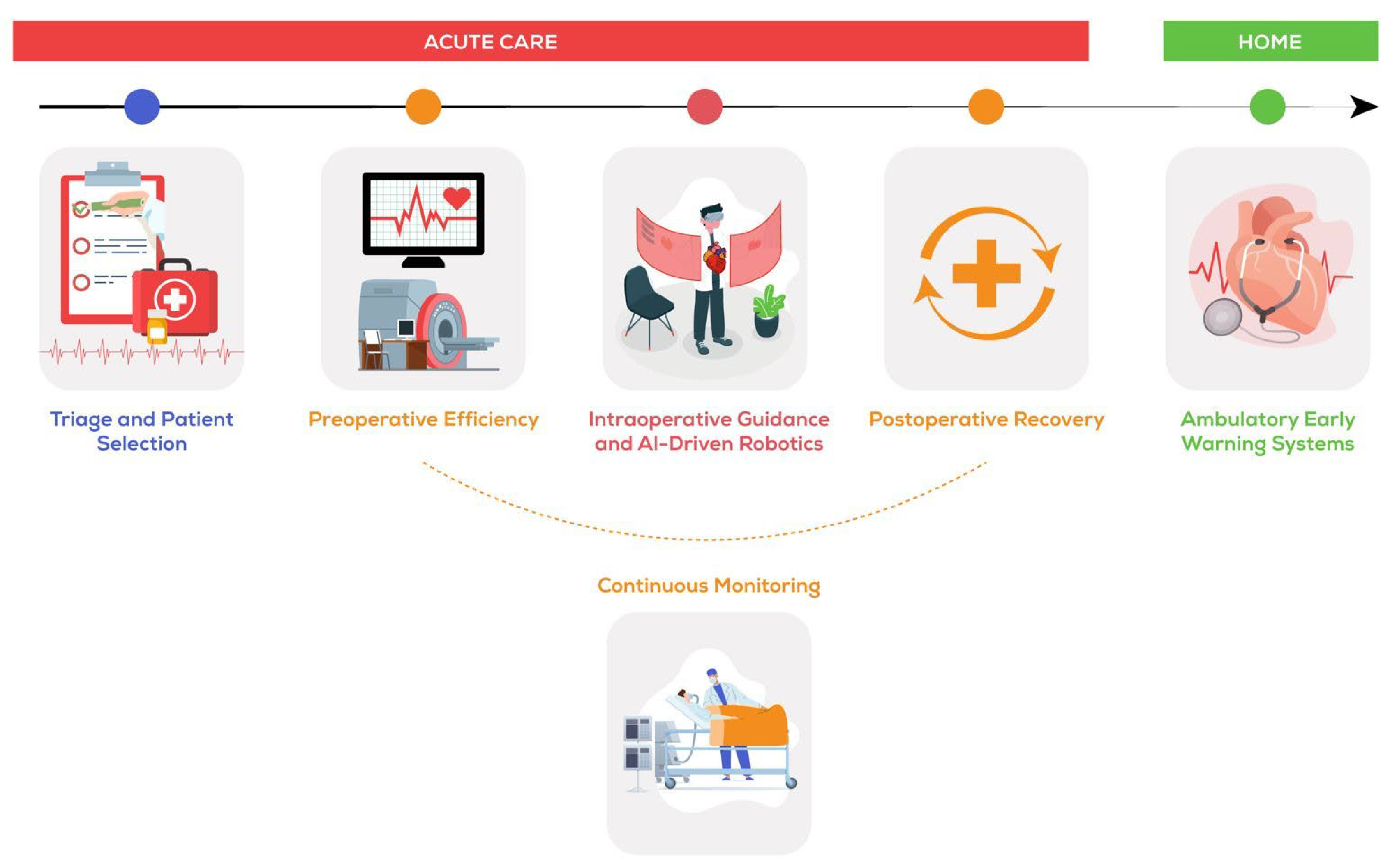
Big Data, Is Artificial Intelligence, Substituting the Multidisciplinary Approach in Cardiac Surgery?
Conclusion
List of Abbreviations
| AI | Artificial intelligence |
| AV | Atrioventricular |
| CMR | Cardiac magnetic resonance |
| CT | computed tomography |
| DM | Decision making |
| DORV | Double outlet right ventricle |
| KD | Kawasaki disease |
| LVAD | Left ventricular assist device |
| MAPCAs | Main aorto-pulmonary connecting arteries |
| MV | Mitral valve |
| TAVI | Transcatheter aortic valve implantation |
| TEE | Transesophageal echocardiography |
| Tx | Transplantation |
| VR | Virtual reality |
| VSA | Visual spatial ability |
| VSD | Ventricular septal defect |
| VSD | Ventricular septal defect |
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgement
Conflicts of Interest
References
- Sonnadara R, Kalun P, Dunn K, Wagner N, Pulakunta T (2020) Canadian Medical Education Journal Recent evidence on visual-spatial ability in surgical education: A scoping review Des preuves récentes sur les habiletés visuo-spatiales pour la formation en chirurgie : revue exploratoire. Can Med Educ J 2020:111–127.
- Ntakakis G, Plomariti C, Frantzidis C, Antoniou PE, Bamidis PD, Tsoulfas G (2023) Exploring the use of virtual reality in surgical education. World J Transplant 13:36–43. [CrossRef]
- Laspro M, Groysman L, Verzella AN, Kimberly LL, Flores RL (2023) The Use of Virtual Reality in Surgical Training: Implications for Education, Patient Safety, and Global Health Equity. Surgeries 4:635–646. [CrossRef]
- Khor WS, Baker B, Amin K, Chan A, Patel K, Wong J (2016) Augmented and virtual reality in surgery—the digital surgical environment: applications, limitations and legal pitfalls. Ann Transl Med 4:454–454. [CrossRef]
- Giordano C, Brennan M, Mohamed B, Rashidi P, Modave F, Tighe P (2021) Accessing Artificial Intelligence for Clinical Decision-Making. Front Digit Heal 3:. [CrossRef]
- Khine MS (2016) Visual-spatial ability in STEM education: Transforming research into practice. Vis Abil STEM Educ Transform Res into Pract 1–263. [CrossRef]
- Arjomandi Rad A, Hajzamani D, Sardari Nia P (2023) Simulation-based training in cardiac surgery: A systematic review. Interdiscip Cardiovasc Thorac Surg 37:. [CrossRef]
- Brandão CM de A, Dallan LRP, Dinato FJ, Monteiro R, Fiorelli AI, Jatene FB (2021) Evaluation method of training simulation on biological models for cardiovascular surgery residents. J Card Surg 36:2247–2252. [CrossRef]
- Sharma VJ, Barton C, Page S, Ganesh JS, Patel N, Pirone F, Lin Z, Kejriwal NK, El Gamel A, McCormack DJ, Meikle F (2021) Cardiac surgery simulation: a low-cost feasible option in an Australasian setting. ANZ J Surg 91:2042–2046. [CrossRef]
- Brown M, Krishnananthan N, Paul V (2022) Right heart catherisation – a virtual reality. Eur Heart J 43:2787. [CrossRef]
- Hermsen JL, Yang R, Burke TM, Dardas T, Jacobs LM, Verrier ED, Mokadam NA (2018) Development of a 3-D printing-based cardiac surgical simulation curriculum to teach septal myectomy. J Thorac Cardiovasc Surg 156:1139-1148.e3. [CrossRef]
- Kenny L, Booth K, Freystaetter K, Wood G, Reynolds G, Rathinam S, Moorjani N (2018) Training cardiothoracic surgeons of the future: The UK experience. J Thorac Cardiovasc Surg 155:2526-2538.e2. [CrossRef]
- Bettati P, Dormer JD, Young J, Shahedi M, Fei B (2021) Virtual reality assisted cardiac catheterization. 82. [CrossRef]
- Spooner AJ, Faulkner CM, Novick RJ, Kent WDT (2019) Optimizing Surgical Skills in Cardiac Surgery Residents with Cardiac Transplant in the High-Fidelity Porcine Model. Innov Technol Tech Cardiothorac Vasc Surg 14:37–42. [CrossRef]
- Joyce DL, Lahr BD, Maltais S, Said SM, Stulak JM, Nuttall GA, Joyce LD (2018) Integration of simulation components enhances team training in cardiac surgery. J Thorac Cardiovasc Surg 155:2518-2524.e5. [CrossRef]
- Hermsen JL, Mohamadipanah H, Yang S, Wise B, Fiedler A, DiMusto P, Pugh C (2021) Multimodal Cardiopulmonary Bypass Skills Assessment Within a High-Fidelity Simulation Environment. Ann Thorac Surg 112:652–660. [CrossRef]
- Hicks GL, Gangemi J, Angona RE, Ramphal PS, Feins RH, Fann JI (2011) Cardiopulmonary bypass simulation at the Boot Camp. J Thorac Cardiovasc Surg 141:284–292. [CrossRef]
- Fouilloux V, Gsell T, Lebel S, Kreitmann B, Berdah S (2014) Assessment of team training in management of adverse acute events occurring during cardiopulmonary bypass procedure: a pilot study based on an animal simulation model (Fouilloux, Team training in cardiac surgery). Perfusion 29:44–52. [CrossRef]
- Premyodhin N, Mandair D, Ferng AS, Leach TS, Palsma RP, Albanna MZ, Khalpey ZI (2018) 3D printed mitral valve models: affordable simulation for robotic mitral valve repair. Interact Cardiovasc Thorac Surg 26:71–76. [CrossRef]
- Smelt J, Corredor C, Edsell M, Fletcher N, Jahangiri M, Sharma V (2015) Simulation-based learning of transesophageal echocardiography in cardiothoracic surgical trainees: A prospective, randomized study. J Thorac Cardiovasc Surg 150:22–25. [CrossRef]
- Valdis M, Chu MWA, Schlachta C, Kiaii B (2016) Evaluation of robotic cardiac surgery simulation training: A randomized controlled trial. J Thorac Cardiovasc Surg 151:1498-1505.e2. [CrossRef]
- Valdis M, Chu MWA, Schlachta CM, Kiaii B (2015) Validation of a Novel Virtual Reality Training Curriculum for Robotic Cardiac Surgery a Randomized Trial. Innov Technol Tech Cardiothorac Vasc Surg 10:383–388. [CrossRef]
- Sardari Nia P, Heuts S, Daemen J, Luyten P, Vainer J, Hoorntje J, Cheriex E, Maessen J (2016) Preoperative planning with three-dimensional reconstruction of patient’s anatomy, rapid prototyping and simulation for endoscopic mitral valve repair. Interact Cardiovasc Thorac Surg ivw308. [CrossRef]
- Duffy MC, Ibrahim M, Lachapelle K (2019) Development of a saphenous vein harvest model for simulation-based assessment. J Thorac Cardiovasc Surg 157:1082–1089. [CrossRef]
- Sardari Nia P, Daemen JHT, Maessen JG (2019) Development of a high-fidelity minimally invasive mitral valve surgery simulator. J Thorac Cardiovasc Surg 157:1567–1574. [CrossRef]
- Russo M, Koenigshofer M, Stoiber M, Werner P, Gross C, Kocher A, Laufer G, Moscato F, Andreas M (2020) Advanced three-dimensionally engineered simulation model for aortic valve and proximal aorta procedures. Interact Cardiovasc Thorac Surg 30:887–895. [CrossRef]
- Yasuda S, Van den Eynde J, Vandendriessche K, Masuda M, Meyns B, Oosterlinck W (2021) Implementation of a beating heart system for training in off-pump and minimally invasive coronary artery bypass. BMC Surg 21:26. [CrossRef]
- Jebran A-F, Saha S, Waezi N, Al-Ahmad A, Niehaus H, Danner BC, Baraki H, Kutschka I (2019) Design and training effects of a physical reality simulator for minimally invasive mitral valve surgery. Interact Cardiovasc Thorac Surg 29:409–415. [CrossRef]
- Feins RH, Burkhart HM, Conte J V., Coore DN, Fann JI, Hicks GL, Nesbitt JC, Ramphal PS, Schiro SE, Shen KR, Sridhar A, Stewart PW, Walker JD, Mokadam NA (2017) Simulation-Based Training in Cardiac Surgery. Ann Thorac Surg 103:312–321. [CrossRef]
- Nesbitt JC, St Julien J, Absi TS, Ahmad RM, Grogan EL, Balaguer JM, Lambright ES, Deppen SA, Wu H, Putnam JB (2013) Tissue-based coronary surgery simulation: Medical student deliberate practice can achieve equivalency to senior surgery residents. J Thorac Cardiovasc Surg 145:1453–1459. [CrossRef]
- Joyce DL, Dhillon TS, Caffarelli AD, Joyce DD, Tsirigotis DN, Burdon TA, Fann JI (2011) Simulation and skills training in mitral valve surgery. J Thorac Cardiovasc Surg 141:107–112. [CrossRef]
- Tavlasoglu M, Durukan AB, Arslan Z, Kurkluoglu M, Amrahov A, Jahollari A (2013) Evaluation of Skill-Acquisition Process in Mitral Valve Repair Techniques: A Simulation-Based Study. J Surg Educ 70:318–325. [CrossRef]
- Zhang L-F, Feng H-B, Yu Z-G, Jing S, Wan F (2018) Surgical Training Improves Performance in Minimally Invasive Left Ventricular Assist Device Implantation Without Cardiopulmonary Bypass. J Surg Educ 75:195–199. [CrossRef]
- Arango S, Gorbaty B, Tomhave N, Shervheim D, Buyck D, Porter ST, Iaizzo PA, Perry TE (2023) A High-Resolution Virtual Reality-Based Simulator to Enhance Perioperative Echocardiography Training. J Cardiothorac Vasc Anesth 37:299–305. [CrossRef]
- Luo X, Luo F, Li B, Li B, Tang Y, Sun H (2020) A tissue-based simulation model for cardiopulmonary bypass cannulation/decannulation training. Perfusion 35:680–686. [CrossRef]
- Kiraly L (2018) Three-dimensional modelling and three-dimensional printing in pediatric and congenital cardiac surgery. Transl Pediatr 7:129–138. [CrossRef]
- Yoo SJ, Hussein N, Peel B, Coles J, Arsdell GS va., Honjo O, Haller C, Lam CZ, Seed M, Barron D (2021) 3D Modeling and Printing in Congenital Heart Surgery: Entering the Stage of Maturation. Front Pediatr 9:1–11. [CrossRef]
- Batteux C, Haidar MA, Bonnet D (2019) 3D-printed models for surgical planning in complex congenital heart diseases: A systematic review. Front Pediatr 7:1–8. [CrossRef]
- Bakhuis W, Max SA, Maat APWM, Bogers AJJC, Mahtab EAF, Sadeghi AH (2023) Preparing for the future of cardiothoracic surgery with virtual reality simulation and surgical planning: a narrative review. Shanghai Chest 7:0–3. [CrossRef]
- Nanchahal S, Arjomandi Rad A, Naruka V, Chacko J, Liu G, Afoke J, Miller G, Malawana J, Punjabi P (2024) Mitral valve surgery assisted by virtual and augmented reality: Cardiac surgery at the front of innovation. Perfusion 39:244–255. [CrossRef]
- Pushparajah K, Chu KYK, Deng S, Wheeler G, Gomez A, Kabir S, Schnabel JA, Simpson JM (2021) Virtual reality three-dimensional echocardiographic imaging for planning surgical atrioventricular valve repair. JTCVS Tech 7:269–277. [CrossRef]
- Pelizzo G, Costanzo S, Roveri M, Lanfranchi G, Vertemati M, Milani P, Zuccotti G, Cassin S, Panfili S, Rizzetto F, Campari A, Camporesi A, Calcaterra V (2022) Developing Virtual Reality Head Mounted Display (HMD) Set-Up for Thoracoscopic Surgery of Complex Congenital Lung MalFormations in Children. Children 9:50. [CrossRef]
- Ong CS, Krishnan A, Huang CY, Spevak P, Vricella L, Hibino N, Garcia JR, Gaur L (2018) Role of virtual reality in congenital heart disease. Congenit Heart Dis 13:357–361. [CrossRef]
- Mendez A, Hussain T, Hosseinpour A-R, Valverde I (2019) Virtual reality for preoperative planning in large ventricular septal defects. Eur Heart J 40:1092–1092. [CrossRef]
- Ghosh RM, Mascio CE, Rome JJ, Jolley MA, Whitehead KK (2021) Use of Virtual Reality for Hybrid Closure of Multiple Ventricular Septal Defects. JACC Case Reports 3:1579–1583. [CrossRef]
- Ramaswamy R, Marimuthu S, Ramarathnam K, Vijayasekharan S, Rao KS, Balakrishnan K (2021) Virtual reality-guided left ventricular assist device implantation in pediatric patient: Valuable presurgical tool. Ann Pediatr Cardiol 14:388. [CrossRef]
- Sadeghi AH, Taverne YJHJ, Bogers AJJC, Mahtab EAF (2020) Immersive virtual reality surgical planning of minimally invasive coronary artery bypass for Kawasaki disease. Eur Heart J 41:3279–3279. [CrossRef]
- Ayerbe VMC, Morales MLV, Rojas CJL, Cortés MLA (2020) Visualization of 3D Models Through Virtual Reality in the Planning of Congenital Cardiothoracic Anomalies Correction: An Initial Experience. World J Pediatr Congenit Hear Surg 11:627–629. [CrossRef]
- van de Woestijne PC, Bakhuis W, Sadeghi AH, Peek JJ, Taverne YJHJ, Bogers AJJC (2021) 3D Virtual Reality Imaging of Major Aortopulmonary Collateral Arteries: A Novel Diagnostic Modality. World J Pediatr Congenit Hear Surg 12:765–772. [CrossRef]
- Abjigitova D, Sadeghi AH, Peek JJ, Bekkers JA, Bogers AJJC, Mahtab EAF (2022) Virtual Reality in the Preoperative Planning of Adult Aortic Surgery: A Feasibility Study. J Cardiovasc Dev Dis 9:31. [CrossRef]
- Bertelli F, Raimondi F, Godard C, Bergonzoni E, Cattapan C, Gastino E, Galliotto F, Boddaert N, El Beheiry M, Masson J-B, Guariento A, Vida VL (2023) Fast-track virtual reality software to facilitate 3-dimensional reconstruction in congenital heart disease. Interdiscip Cardiovasc Thorac Surg 36:. [CrossRef]
- Sulague RM, Beloy FJ, Medina JR, Mortalla ED, Cartojano TD, Macapagal S, Kpodonu J (2024) Artificial intelligence in cardiac surgery: A systematic review. World J Surg 48:2073–2089. [CrossRef]
- Agasthi P, Ashraf H, Pujari SH, Girardo ME, Tseng A, Mookadam F, Venepally NR, Buras M, Khetarpal BK, Allam M, Eleid MF, Greason KL, Beohar N, Siegel RJ, Sweeney J, Fortuin FD, Holmes DR, Arsanjani R (2021) Artificial Intelligence Trumps TAVI2-SCORE and CoreValve Score in Predicting 1-Year Mortality Post-Transcatheter Aortic Valve Replacement. Cardiovasc Revascularization Med 24:33–41. [CrossRef]
- Lo Muzio FP, Rozzi G, Rossi S, Luciani GB, Foresti R, Cabassi A, Fassina L, Miragoli M (2021) Artificial Intelligence Supports Decision Making during Open-Chest Surgery of Rare Congenital Heart Defects. J Clin Med 10:5330. [CrossRef]
- Hernandez-Suarez DF, Kim Y, Villablanca P, Gupta T, Wiley J, Nieves-Rodriguez BG, Rodriguez-Maldonado J, Feliu Maldonado R, da Luz Sant’Ana I, Sanina C, Cox-Alomar P, Ramakrishna H, Lopez-Candales A, O’Neill WW, Pinto DS, Latib A, Roche-Lima A (2019) Machine Learning Prediction Models for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 12:1328–1338. [CrossRef]
- Aranda-Michel E, Sultan I, Kilic A, Bianco V, Brown JA, Serna-Gallegos D (2022) A machine learning approach to model for end-stage liver disease score in cardiac surgery. J Card Surg 37:29–38. [CrossRef]
- Ayers B, Sandholm T, Gosev I, Prasad S, Kilic A (2021) Using machine learning to improve survival prediction after heart transplantation. J Card Surg 36:4113–4120. [CrossRef]
- Fan Y, Dong J, Wu Y, Shen M, Zhu S, He X, Jiang S, Shao J, Song C (2022) Development of machine learning models for mortality risk prediction after cardiac surgery. Cardiovasc Diagn Ther 12:12–23. [CrossRef]
- Hu L-H, Betancur J, Sharir T, Einstein AJ, Bokhari S, Fish MB, Ruddy TD, Kaufmann PA, Sinusas AJ, Miller EJ, Bateman TM, Dorbala S, Di Carli M, Germano G, Commandeur F, Liang JX, Otaki Y, Tamarappoo BK, Dey D, Berman DS, Slomka PJ (2020) Machine learning predicts per-vessel early coronary revascularization after fast myocardial perfusion SPECT: results from multicentre REFINE SPECT registry. Eur Hear J - Cardiovasc Imaging 21:549–559. [CrossRef]
- Luo L, Huang SQ, Liu C, Liu Q, Dong S, Yue Y, Liu KZ, Huang L, Wang SJ, Li HY, Zheng S, Wu ZK (2022) Machine Learning–Based Risk Model for Predicting Early Mortality After Surgery for Infective Endocarditis. J Am Heart Assoc 11:. [CrossRef]
- Molina RS, Molina-Rodríguez MA, Rincón FM, Maldonado JD (2022) Cardiac Operative Risk in Latin America: A Comparison of Machine Learning Models vs EuroSCORE-II. Ann Thorac Surg 113:92–99. [CrossRef]
- Kampaktsis PN, Siouras A, Doulamis IP, Moustakidis S, Emfietzoglou M, Van den Eynde J, Avgerinos D V., Giannakoulas G, Alvarez P, Briasoulis A (2023) Machine learning-based prediction of mortality after heart transplantation in adults with congenital heart disease: A UNOS database analysis. Clin Transplant 37:. [CrossRef]
- Kampaktsis PN, Tzani A, Doulamis IP, Moustakidis S, Drosou A, Diakos N, Drakos SG, Briasoulis A (2021) State-of-the-art machine learning algorithms for the prediction of outcomes after contemporary heart transplantation: Results from the UNOS database. Clin Transplant 35:. [CrossRef]
- Agasthi P, Buras MR, Smith SD, Golafshar MA, Mookadam F, Anand S, Rosenthal JL, Hardaway BW, DeValeria P, Arsanjani R (2020) Machine learning helps predict long-term mortality and graft failure in patients undergoing heart transplant. Gen Thorac Cardiovasc Surg 68:1369–1376. [CrossRef]
- Hasimbegovic E, Papp L, Grahovac M, Krajnc D, Poschner T, Hasan W, Andreas M, Gross C, Strouhal A, Delle-Karth G, Grabenwöger M, Adlbrecht C, Mach M (2021) A Sneak-Peek into the Physician’s Brain: A Retrospective Machine Learning-Driven Investigation of Decision-Making in TAVR versus SAVR for Young High-Risk Patients with Severe Symptomatic Aortic Stenosis. J Pers Med 11:1062. [CrossRef]
- Zhou Y, Chen S, Rao Z, Yang D, Liu X, Dong N, Li F (2021) Prediction of 1-year mortality after heart transplantation using machine learning approaches: A single-center study from China. Int J Cardiol 339:21–27. [CrossRef]
- Shou BL, Chatterjee D, Russel JW, Zhou AL, Florissi IS, Lewis T, Verma A, Benharash P, Choi CW (2022) Pre-operative Machine Learning for Heart Transplant Patients Bridged with Temporary Mechanical Circulatory Support. J Cardiovasc Dev Dis 9:311. [CrossRef]
- Li Y, Xu J, Wang Y, Zhang Y, Jiang W, Shen B, Ding X (2020) A novel machine learning algorithm, Bayesian networks model, to predict the high-risk patients with cardiac surgery-associated acute kidney injury. Clin Cardiol 43:752–761. [CrossRef]
- Karri R, Kawai A, Thong YJ, Ramson DM, Perry LA, Segal R, Smith JA, Penny-Dimri JC (2021) Machine Learning Outperforms Existing Clinical Scoring Tools in the Prediction of Postoperative Atrial Fibrillation During Intensive Care Unit Admission After Cardiac Surgery. Hear Lung Circ 30:1929–1937. [CrossRef]
- Park J, Bonde PN (2022) Machine Learning in Cardiac Surgery: Predicting Mortality and Readmission. ASAIO J 68:1490–1500. [CrossRef]
- Li T, Yang Y, Huang J, Chen R, Wu Y, Li Z, Lin G, Liu H, Wu M (2022) Machine learning to predict post-operative acute kidney injury stage 3 after heart transplantation. BMC Cardiovasc Disord 22:1–9. [CrossRef]
- Kim RB, Alge OP, Liu G, Biesterveld BE, Wakam G, Williams AM, Mathis MR, Najarian K, Gryak J (2022) Prediction of postoperative cardiac events in multiple surgical cohorts using a multimodal and integrative decision support system. Sci Rep 12:11347. [CrossRef]
- Kilic A, Goyal A, Miller JK, Gleason TG, Dubrawksi A (2021) Performance of a Machine Learning Algorithm in Predicting Outcomes of Aortic Valve Replacement. Ann Thorac Surg 111:503–510. [CrossRef]
- Fernandes MPB, Armengol de la Hoz M, Rangasamy V, Subramaniam B (2021) Machine Learning Models with Preoperative Risk Factors and Intraoperative Hypotension Parameters Predict Mortality After Cardiac Surgery. J Cardiothorac Vasc Anesth 35:857–865. [CrossRef]
- Truong VT, Beyerbach D, Mazur W, Wigle M, Bateman E, Pallerla A, Ngo TNM, Shreenivas S, Tretter JT, Palmer C, Kereiakes DJ, Chung ES (2021) Machine learning method for predicting pacemaker implantation following transcatheter aortic valve replacement. Pacing Clin Electrophysiol 44:334–340. [CrossRef]
- Thalappillil R, Datta P, Datta S, Zhan Y, Wells S, Mahmood F, Cobey FC (2020) Artificial Intelligence for the Measurement of the Aortic Valve Annulus. J Cardiothorac Vasc Anesth 34:65–71. [CrossRef]
- Xue X, Chen W, Chen X (2022) A Novel Radiomics-Based Machine Learning Framework for Prediction of Acute Kidney Injury-Related Delirium in Patients Who Underwent Cardiovascular Surgery. Comput Math Methods Med 2022:1–16. [CrossRef]
- Chang Junior J, Binuesa F, Caneo LF, Turquetto ALR, Arita ECTC, Barbosa AC, Fernandes AM da S, Trindade EM, Jatene FB, Dossou P-E, Jatene MB (2020) Improving preoperative risk-of-death prediction in surgery congenital heart defects using artificial intelligence model: A pilot study. PLoS One 15:e0238199. [CrossRef]
- Zea-Vera R, Ryan CT, Havelka J, Corr SJ, Nguyen TC, Chatterjee S, Wall MJ, Coselli JS, Rosengart TK, Ghanta RK (2022) Machine Learning to Predict Outcomes and Cost by Phase of Care After Coronary Artery Bypass Grafting. Ann Thorac Surg 114:711–719. [CrossRef]
- Allyn J, Allou N, Augustin P, Philip I, Martinet O, Belghiti M, Provenchere S, Montravers P, Ferdynus C (2017) A Comparison of a Machine Learning Model with EuroSCORE II in Predicting Mortality after Elective Cardiac Surgery: A Decision Curve Analysis. PLoS One 12:e0169772. [CrossRef]
- Bodenhofer U, Haslinger-Eisterer B, Minichmayer A, Hermanutz G, Meier J (2021) Machine learning-based risk profile classification of patients undergoing elective heart valve surgery. Eur J Cardio-Thoracic Surg 60:1378–1385. [CrossRef]
- Gao Y, Liu X, Wang L, Wang S, Yu Y, Ding Y, Wang J, Ao H (2022) Machine learning algorithms to predict major bleeding after isolated coronary artery bypass grafting. Front Cardiovasc Med 9:. [CrossRef]
- Jiang H, Liu L, Wang Y, Ji H, Ma X, Wu J, Huang Y, Wang X, Gui R, Zhao Q, Chen B (2021) Machine Learning for the Prediction of Complications in Patients After Mitral Valve Surgery. Front Cardiovasc Med 8:. [CrossRef]
- Evertz R, Lange T, Backhaus SJ, Schulz A, Beuthner BE, Topci R, Toischer K, Puls M, Kowallick JT, Hasenfuß G, Schuster A (2022) Artificial Intelligence Enabled Fully Automated CMR Function Quantification for Optimized Risk Stratification in Patients Undergoing Transcatheter Aortic Valve Replacement. J Interv Cardiol 2022:1–9. [CrossRef]
- He K, Liang W, Liu S, Bian L, Xu Y, Luo C, Li Y, Yue H, Yang C, Wu Z (2022) Long-term single-lead electrocardiogram monitoring to detect new-onset postoperative atrial fibrillation in patients after cardiac surgery. Front Cardiovasc Med 9:. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




