1. Introduction
Enterovirus infections have been linked to various clinical syndromes in newborns, ranging from mildly febrile illnesses with or without rash, herpangina, hand, foot, and mouth disease, conjunctivitis, respiratory and gastrointestinal tract diseases, to more severe, sometimes life-threatening conditions such as aseptic meningitis, encephalitis, polio-like paralysis, myopericarditis, and chronic enterovirus infections in immunocompromised individuals[
1].
Echoviruses (Enteric Cytopathgenic Human Orphan viruses), 30μm in diameter, are non-enveloped viruses with a positive -sense single-stranded RNA genome with 30 serotypes, and are members of the genus Enterovirus from the family Picornaviridae[
2]., and are classified in the species of human Enterovirus B[
3]. Since its initial isolation and description in the 1950s[
4], Echovirus are known to be a common cause of human diseases, including an acute nonspecific febrile illness with or without rash, and aseptic meningitis[
5].Echovirus infections, especially in neonates, are usually mild and self-limited, however, there have been several outbreaks of severe illness with occasional fatal outcomes[
6,
7]. Echovirus 11, (EV-11), is the most commonly identified serotype that is typically associated with mild gastrointestinal and respiratory infections, hand foot mouth disease, and uveitis[8-10], however, it can escalate to more serious conditions, such as myocarditis, meningitis, encephalitis, and sepsis, in vulnerable populations, including neonates[
3,
11,
12]. Immature immune systems and increased vulnerability to viral infections and inadequate inflammatory reactions in neonates may be two of the causes[
13,
14] .
During a 2018 outbreak in Taiwan, 181 cases were confirmed nationwide via viral culture surveillance by Taiwan Centers for Disease Control (CDC), with neonates comprising 19% (35 cases), and the mortality rate is 3.8% (7 cases) of the cases; nursery epidemics of EV-11 are common, with frequent reports of outbreaks [
15]. A local study retrospectively analyzed 60 patients with EV-11 infection and found that 35% of infants with younger than 3 months old had positive virus culture from CSF specimens and 13% of severe hepatitis, and only one case died due to myocarditis and severe sepsis[
13]. Another retrospective analysis targeted 10 infants with confirmed EV-11 infection found that 50% of infants (5 cases) with aseptic meningitis, and one case with fulminant hepatitis [
16]. All highlight the vulnerability of newborns to EV-11 and underscores the importance of effective outbreak management.
In April 2019, a teaching hospital in Taichung, Taiwan experienced a significant EV-11 outbreak in the sick neonate room (SN), which is a nursery that provides nursing care to those infants convalescing or those sick infants not requiring intensive care. This outbreak presented a severe challenge to the hospital’s infection control practices, as the SN housed a particularly vulnerable group of neonates, many of whom were already in a fragile state due to their underlying conditions. The emergence of EV-11 in this setting prompted an urgent investigation to understand the source of the outbreak and the mechanisms of viral transmission.
This investigation aimed to identify the root causes of the outbreak and to implement effective control measures to prevent further spread of EV-11. Given the high risk of severe illness due to EV-11 in neonates, it is crucial to respond swiftly and efficiently to mitigate the impact of outbreaks. This study provides insights into the successful control of EV-11 outbreaks in neonatal settings and highlights the importance of robust infection control practices and timely interventions.
3. Results
3.1. Descriptive Epidemiology
The demographic and epidemiological characteristics of the five neonates diagnosed with EV-11 infection are summarized in
Table 1. The cohort consisted of three males and two females, with birth weights ranging from 2472 grams to 4224 grams. Two infants were premature. The age at symptom onset varied, ranging from 6th to 27th day. The age at the laboratory confirmation of EV-11 infection ranged from 20th day to 47th day of life, indicating variability in the time to diagnosis. All infants developed a fever as the primary clinical manifestation, highlighting it as a consistent symptom of EV-11 infection in this cohort. Despite variations in the timing of symptom onset and diagnosis, the presence of fever was a clinical feature common to all cases, suggesting that early fever in neonates may be a critical indicator of EV-11 infection.
Table 1.
Demographic and Clinical Data of Neonatal Patients with EV-11 meningitis.
Table 1.
Demographic and Clinical Data of Neonatal Patients with EV-11 meningitis.
| Case |
Clinical manifestations |
Outborn |
Birth weight (g)/ gender |
Gestational age(weeks) |
Age at onset (d) |
Age at Lab confirm (d) |
| Case 1 |
fever, poor activity, acrocyanosis, apnea, bradycardia |
- |
4224/F |
36 2/7 |
7 |
23 |
| Case 2 |
fever, tachypnea, poor activity |
- |
2472/M |
34 3/7 |
6 |
20 |
| Case 3 |
fever, poor feeding, poor activity, skin rash |
+ |
3670/F |
39 |
9 |
23 |
| Case 4 |
fever, skin rash |
+ |
3035/M |
38 3/7 |
27 |
47 |
| Case 5 |
fever, poor feeding, skin rash |
+ |
3040/M |
39 |
9 |
39 |
This table provides detailed demographic and clinical characteristics of the five neonates diagnosed with Echovirus 11 (EV-11) infection during the outbreak. The variables include primary symptoms, outborn or inborn, gestational age, gender, birth weight, age at symptom onset, and age at EV-11 isolated from CSF.
3.2. Laboratory Investigations
The laboratory investigations provided comprehensive data on the hematological, biochemical, and CSF parameters of the infected infants (
Table 2). White blood cell (WBC) counts varied significantly among the infants and ranged from 5000 cells/µL to 26,400 cells/µL. Differential counts showed a predominance of segmented neutrophils, which ranged from 53.3% to 88%, with corresponding lymphocyte percentages varying between 7.6% and 38%. Platelet counts varied significantly among the infants and ranged from 60,000 platelets/µL to 11,300 platelets/µL. The variation in these parameters indicates differing immune response levels among the infants, possibly reflecting the severity of the infection.
Liver enzyme levels, including glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT), were elevated in Case 2, who had significantly high GOT and GPT levels of 8879 IU/L and 549 IU/L, respectively. These elevated liver enzymes suggest hepatic involvement, a common feature in viral infections, particularly those caused by EV-11[4, 14–15].
C-reactive protein (CRP), a marker of inflammation, was notably elevated in several cases, particularly in Case 1 at 7.79 mg/dL. This increase is indicative of a strong inflammatory response, which is consistent with the acute phase of EV-11 infection. The troponin I level is a marker of cardiac injury[
17]. B type natriuretic peptide (brain natriuretic peptide; BNP) a hormone is produced by the ventricular muscle in human hearts [
18]. Both showed a significant elevation in case 2 (troponin I: 0.292ng/mL; BNP: 717 pg/mL), and the ejection fraction calculated with a cardioechogram was 82%, which was not compatible with the criteria of myocarditis. CSF analysis yielded significant findings: glucose levels ranging from 51 mg/dL to 73 mg/dL and protein levels between 43 mg/dL and 229 mg/dL. WBC counts in the CSF varied, with Case 3 showing a particularly high count of 56 cells/µL, and the presence of RBCs (56,000 cells/µL ) in the CSF suggested a robust inflammatory response in the central nervous system, or a traumatic tapping. The presence of RBCs in the CSF was also noted, although in low quantities, indicating mild hemorrhagic involvement in some cases.
Overall, the laboratory investigations highlighted the systemic nature of EV-11 infection, which affected multiple organ systems including the liver, blood cells, and central nervous system. The variability in laboratory parameters among the infants underscores the diverse clinical presentations and the need for comprehensive monitoring and management of neonates with suspected or confirmed EV-11 infection.
Table 2.
Laboratory Investigations of Neonatal Patients with EV-11 meningitis.
Table 2.
Laboratory Investigations of Neonatal Patients with EV-11 meningitis.
| Case |
Blood |
CSF |
WBC
(X1000) |
N/L |
PLT
(X1000) |
Hb |
GOT
/GPT |
BUN
/Cr |
CPK |
CRP |
TPNI |
BNP |
Glu |
Glu |
Protein |
WBC /RBC |
| Case 1 |
19.6 |
88.2/7.6 |
225 |
17 |
56
/8 |
11
/0.52 |
308 |
7.79 |
0.017 |
198 |
59 |
57 |
71 |
2/1 |
| Case 2 |
26.4 |
74/10 |
60 |
11.7 |
8879
/549 |
19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This table highlights the hematological, biochemical, and cerebrospinal fluid (CSF) parameters for the neonates infected with EV-11. The variables include white blood cell count (WBC), platelet count, hemoglobin (Hb), liver enzyme levels (GOT/GPT: glutamic-oxaloacetic transaminase/ glutamic-pyruvic transaminase), kidney function levels (BUN/Cr: blood urea nitrogen/ creatinine), muscle enzyme levels (CPK: Creatine phosphokinase); CRP (C-reactive protein), TPNI ( Troponin I), BNP (B-type natriuretic peptide), Glu (Glucose), and cerebrospinal fluid analysis results.
3.3. Further Interventions
During the outbreak, new patient admissions were suspended until designated quarantine areas were established. To prevent community infections, additional beds were allocated in general wards for non-critical outborn neonates. Critical patients were admitted and quarantined within an isolation zone in the SN. Throat and rectal swabs from all new cases underwent viral isolation surveillance; negative results permitted release from quarantine, while cases exhibiting EV-11-related symptoms required further viral isolation and testing and remained quarantined until their symptoms were free.
3.4. Hospital Information System and Control Measures
During the outbreak, confirmed cases and exposed patients from April to June 2019 were recorded as EV-11 contacts in the hospital information system for tracking by the emergency and outpatient departments. Follow-up was conducted one week post-discharge, and healthcare workers were promptly notified of new EV-11 infection results via text messages and the hospital information system. To prevent the vertical transmission of EV-11 from mothers, infection control measures were implemented in the delivery room and nursery, allowing only one designated visitor during hospitalization. Disinfection procedures mirrored those of isolation zones, breastfeeding and rooming-in were prohibited, and electronic and paper-posters were displayed to raise awareness. Symptomatic neonates were promptly transferred to quarantined areas.
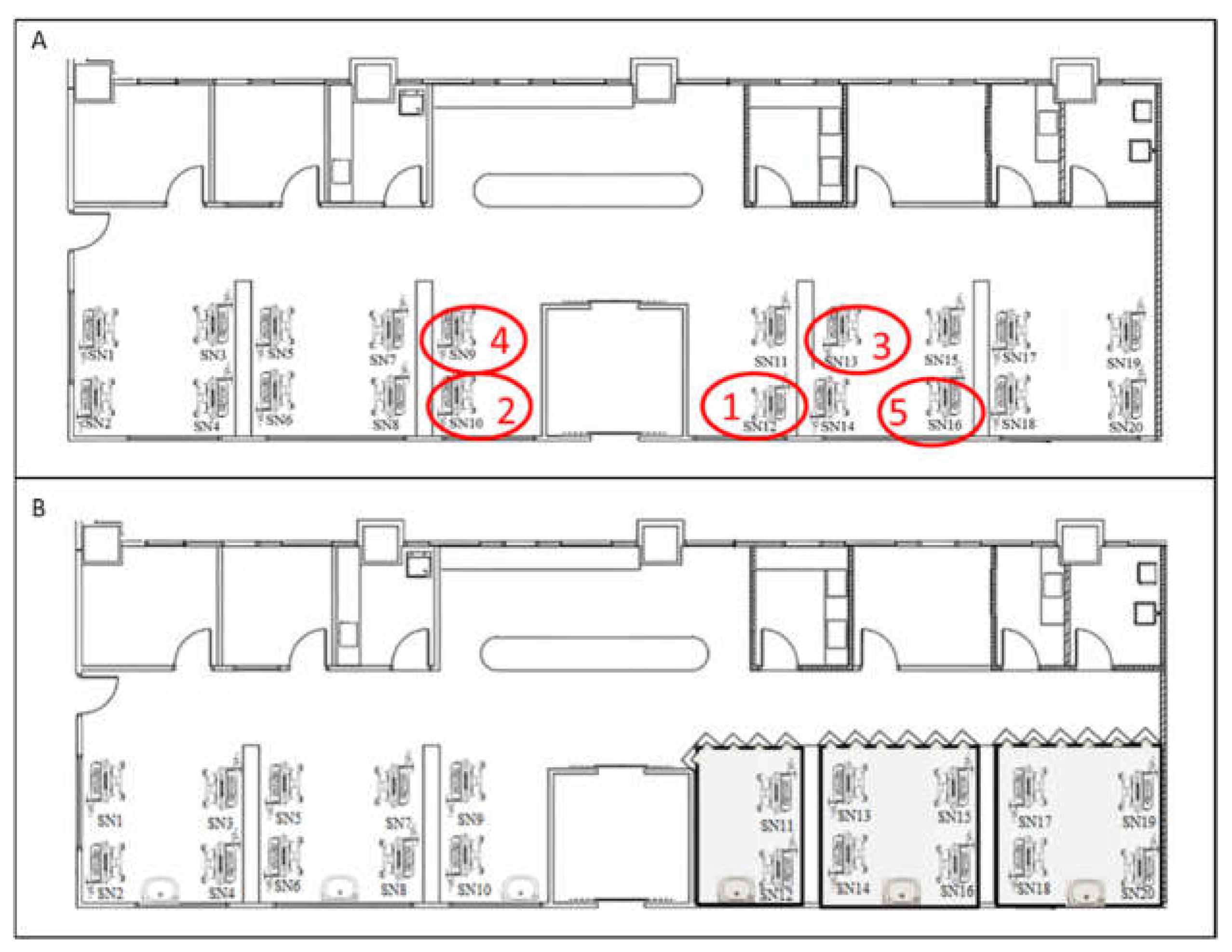
Figure 1.
Layout of the Sick Neonatal Room and Isolation Zones.
Figure 1.
Layout of the Sick Neonatal Room and Isolation Zones.
This figure illustrates the floor plan of the SN before (A) and after (B) the implementation of infection control measures. The positions of case 1 to case 5 were demonstrated in red circles. The case 1, case 2 and case 4 shared . The implementation of of infection control measures including setting up isolation zones (grey color) ;

: basin;
: insulation board; and

:curtains. Soap, 75% alcohol, and 0.5% Chlorhexidine gluconate gluconate-based hand rubs were all available nearby any basins and incubators.
Figure 2.
Epidemiological Curve of the EV-11 Outbreak.
Figure 2.
Epidemiological Curve of the EV-11 Outbreak.
The epidemiological curve shows the timeline of symptom onset, hospital admissions, and infection control interventions during the EV-11 outbreak. It maps the dates on which patients exhibited symptoms, received diagnoses, and when containment measures were put in place. Blue line: The period of admission to the SN.

: Date at symptom onset.

: Date at IVIG administered.

: date at infection control interventions.
4. Discussion
EV-11 can cause severe illnesses in neonates and infants, often leading to high morbidity and mortality rates. During the 2018 outbreak in Taiwan, 7 out of 8 severe cases resulted in death[
13]. In May 2019, a nosocomial EV-11 infection in Guangzhou City, Guangdong Province, caused the deaths of five infants suffering from neonatal pneumonia and other underlying conditions [
19]. Between July 2022 and April 2023, seven out of nine infants with severe EV-11 infection died in France [
6]. Even though no mortality was recorded, the EV-11 outbreak discussed in this study presented a significant challenge, particularly given the vulnerability of neonates to severe complications from enterovirus infections. This study assesses the management of the outbreak, compares findings with previous research, and highlights the critical importance of stringent infection control measures in neonatal care settings.
The outbreak was initiated by Case 1, and Case 2 admitted to the SN, both of whom were neonates born inside the hospital presenting fever and EV-11 was isolated from their CSF. A further investigation found that Case 3, Case4, and Case 5 were born outside the hospital, and presented fever and later transferred without adequate isolation measures. Case 1 and Case 2 were highly possible to be infected by the Case 4, because hospitalization periods of those three cases showed overlapped. Additionally, Case1, Case 2 and Case 4 were cared by the same caregivers during the same shifts. Some caregivers may underestimate the danger of outbreak and not follow stricter isolation and control measures. This led to rapid intrahospital transmission, highlighting a significant lapse in infection control. The response of the hospital involved immediate establishment of a designated isolation zone, suspension of new patient admissions, and strict quarantine measures in controlling further spread.
Previous studies have underscored the high transmissibility of EV-11 in neonatal units[
13,
16,
20,
21]. For instance, Ho et al. documented a similar EV-11 outbreak in a NICU, where inadequate isolation practices contributed to widespread transmission. They reported that, of the 10 confirmed cases, three were likely community-acquired infections, while seven were healthcare-associated. The initial cases were not properly isolated, leading to the spread of EV-11 to seven others, likely transmitted by healthcare workers during the same shift[
16]. The prompt identification and containment measures taken during the outbreak discussed in this study reflect lessons learned from previous such incidents, in which early intervention was the key to controlling viral spread.
Ho et al. highlighted the broad clinical spectrum of neonatal echovirus infections, ranging from mild febrile illness to severe multi-organ involvement [
16]. Fang et al. also emphasized the role of multi-organ involvement in neonates, which contributes to the severity of enteroviral infections and the need for vigilant monitoring[
22]. In this study, EV-11 symptoms included fever (5 cases); poor activity (3 cases); skin rash (3 cases); poor feeing (2 cases); hepatitis (1 case); thrombocytopenia (1 case). The clinical manifestations of EV-11 in infants primarily included fever, consistent with previous reports on neonatal enteroviral infections[
16,
19,
23]. Skin rash associated with EV-11 was reported to be unique as vesicular skin lesions[
24]. However, due to the limitation of this retrospective study, we could not make this conclusion. Moreover, skin rash was only found four out of sixty patients with EV-11 [
13].
Non-specific symptoms such as poor feeding, and poor activity were also noted in this study, as reported in the literature[
13,
16,
25]. A report analyzing genotypes of EV-11 in China from 1994 to 2017 collected specimens from patients with acute flaccid paralysis, and hand-foot-mouth disease. Interestingly, EV-11 could be detected in healthy individuals[
26], and this could explain subjects with EV-11 show no symptoms.
Laboratory findings showed significantly elevated WBC counts and CRP, indicating systemic inflammation related to EV-11[
13]. While echovirus types 6, 9, 14, 19, 21, 30, and 11 have been associated with hepatitis, particularly severe and fatal cases involving EV-11[
13,
16,
27], only one case (Case 2) in this study showed elevated GOT and GPT levels. This suggests that the link between hepatitis and EV-11 infections may not be as consistent as previously thought. Although enterovirus infections, especially EV-9[
28] and EV-30[
29], are known to cause rhabdomyolysis with high CPK levels [
30,
31], CPK levels in these five EV-11 cases were normal, indicating that muscle tissue may not be involved in EV-11 infections in infants.
Myocarditis is a serious and potentially fatal complication of EV-11 infection, though its incidence was low in the studies by Ho et al.[
16] (1/10) and Chen et al.[
13] (1/37). Although Case 2 had elevated troponin I and BNP levels, his ejection fraction of 82% did not meet the criteria for myocarditis, and none of the infants in this study showed signs of myocarditis, suggesting that the incidence of myocarditis in EV-11-infected infants is uncommon.
Meningitis is a common and serious complication of EV-11 infection, and the incidence in the studies by Ho et al.[
16] (5/10), Chen et al.[
13] (10/37), Bina Rai et al. [
32](2/11), and Grapin et al.[
6] (5/9). All five cases were found EV-11 positive in the CSF specimens in this study, suggesting that EV-11 is prone to spread and enter the CNS in this local area.
The pathophysiology of echovirus 11 (EV-11) is not yet fully understood. As EV-11 initiates infection by targeting host cells using the cell surface protein decay-accelerating factor (DAF or CD55) [
33] and β2-microglobulin[
34]. Once attached, the virus enters the host cells through various endocytic pathways, including clathrin-mediated endocytosis, caveolin-mediated endocytosis, and macropinocytosis[35-37]. Upon entry, EV-11-containing vesicles are transported through the cytoplasm along microtubules or actin filaments to the endosome, where the virus undergoes uncoating. After uncoating, the viral genome is released into the cytoplasm and viral genome replication occurs within double-membrane vesicles in the cytoplasm, and new virions are ultimately released from the host cell through lysis[
38]. Through those steps, EV-11 can disseminate from the gastrointestinal tract to the bloodstream, leading to systemic infections[
39]. EV-11 causing severe complications such as myocarditis, encephalitis, and sepsis in neonates may attribute to their immature immune systems and increased vulnerability[
13]. Recent research suggests that EV-11 can efficiently infect liver cells and macrophages through triggering inflammatory responses and pyroptosis, a form of programmed cell death associated with inflammation[
14]. Future research should focus on evaluating multi-organ involvement in a larger cohort, which was not fully explored in this study. Understanding the full spectrum of the clinical impact of EV-11 is essential for improving neonatal care and outcomes.
The transmission of EV-11 is respiratory and fecal–oral spread. Although the source of the outbreak had not been identified, the infected inborn infants (Case 1 and 2) could have acquired the virus from infected asymptomatic caregivers through hand contacts, or through baby-to-baby transmission. The infection control measures taken during the EV-11 outbreak were comprehensive and proved effective in stopping viral transmission. Key actions included the establishment of a designated isolation zone, enforcement of strict hand hygiene protocols, and use of protective equipment. As EV-11 was reported to widespread circulation and increasing prevalence in local sewage[
19], environmental cleaning protocols, particularly the use of sodium hypochlorite for surface disinfection, were implemented based on evidence from previous outbreaks. The efficacy of ethanol against echoviruses is rather good. Study shows that ethanol at 92.4% are effective against EV-11[
40,
41]. Although studies indicate that enteroviruses are resistant to most commercial alcohol-based disinfectants and require either a minimum of 95% ethanol to be effectively inactivated[42-44], however Chang et al. admitted that 95% ethanol still cannot fully inactivate EV71 and may be impractical for use in many instances[
42]. Our findings align with those of previous studies that underscored the importance of hand-washing with soap and water, the use of gloves, 75% alcohol, and 0.5% Chlorhexidine gluconate gluconate-based hand rubs enhances compliance and effectiveness of infection control.
Ho et al. and Chen et al. highlighted that rigorous infection control practices, including the use of isolation rooms and strict hand hygiene, are crucial for controlling EV-11 outbreaks in NICUs[
13,
16] . The success of the outbreak management described in this study can be credited to the hospital’s swift implementation of these measures. In addition, the hospital’s decision to prohibit breastfeeding and rooming-in during the outbreak was essential for minimizing the risk of vertical transmission. This approach, although challenging, was supported by Chang et al.’s and Maus et al.’s , which showed that enteroviruses could be transmitted from mother to infant during delivery or through breastfeeding[
45,
46]. By enforcing these restrictions, the hospital significantly reduced the risk of mother-to-infant transmission, a critical factor in managing neonatal outbreaks.
Despite the successful containment of the outbreak, several limitations are noted. The study was conducted in a single hospital setting, which may limit the generalizability of the findings to other healthcare institutions. The small sample size of infected neonates also constrains the ability to draw broader conclusions about the epidemiology and clinical outcomes of EV-11 infections. In addition, the emotional and psychological impact on families due to the restrictions on breastfeeding and rooming-in was not assessed, which is an important consideration in managing such outbreaks. Lastly, while the infection control measures were effective, the long-term outcomes of the infected infants were not monitored, leaving gaps in the understanding of the full impact of the outbreak.
The successful control of this EV-11 outbreak provides several important lessons for managing future outbreaks in neonatal and pediatric settings. First, the importance of early detection cannot be overstated. The rapid identification of the index cases and the prompt implementation of isolation and quarantine measures were critical in preventing widespread transmission. The initial lapse in isolating the index case (Case 4) upon admission allowed the virus to spread, illustrating the need for stringent infection control protocols to be in place at all times. This includes the use of protective equipment, rigorous hand hygiene, and environmental cleaning, as well as clear protocols for managing suspected infectious cases.
Finally, this study underscores the value of public health education for managing infectious diseases. By educating healthcare workers and the public about the risks of enteroviral infections and the necessary precautions, the hospital was able to enhance compliance with infection control measures and prevent further transmission. This approach is consistent with the recommendations of the World Health Organization, which emphasize the role of public health education in controlling infectious disease outbreaks
 : basin;
: basin;  : insulation board; and
: insulation board; and  :curtains. Soap, 75% alcohol, and 0.5% Chlorhexidine gluconate gluconate-based hand rubs were all available nearby any basins and incubators.
:curtains. Soap, 75% alcohol, and 0.5% Chlorhexidine gluconate gluconate-based hand rubs were all available nearby any basins and incubators.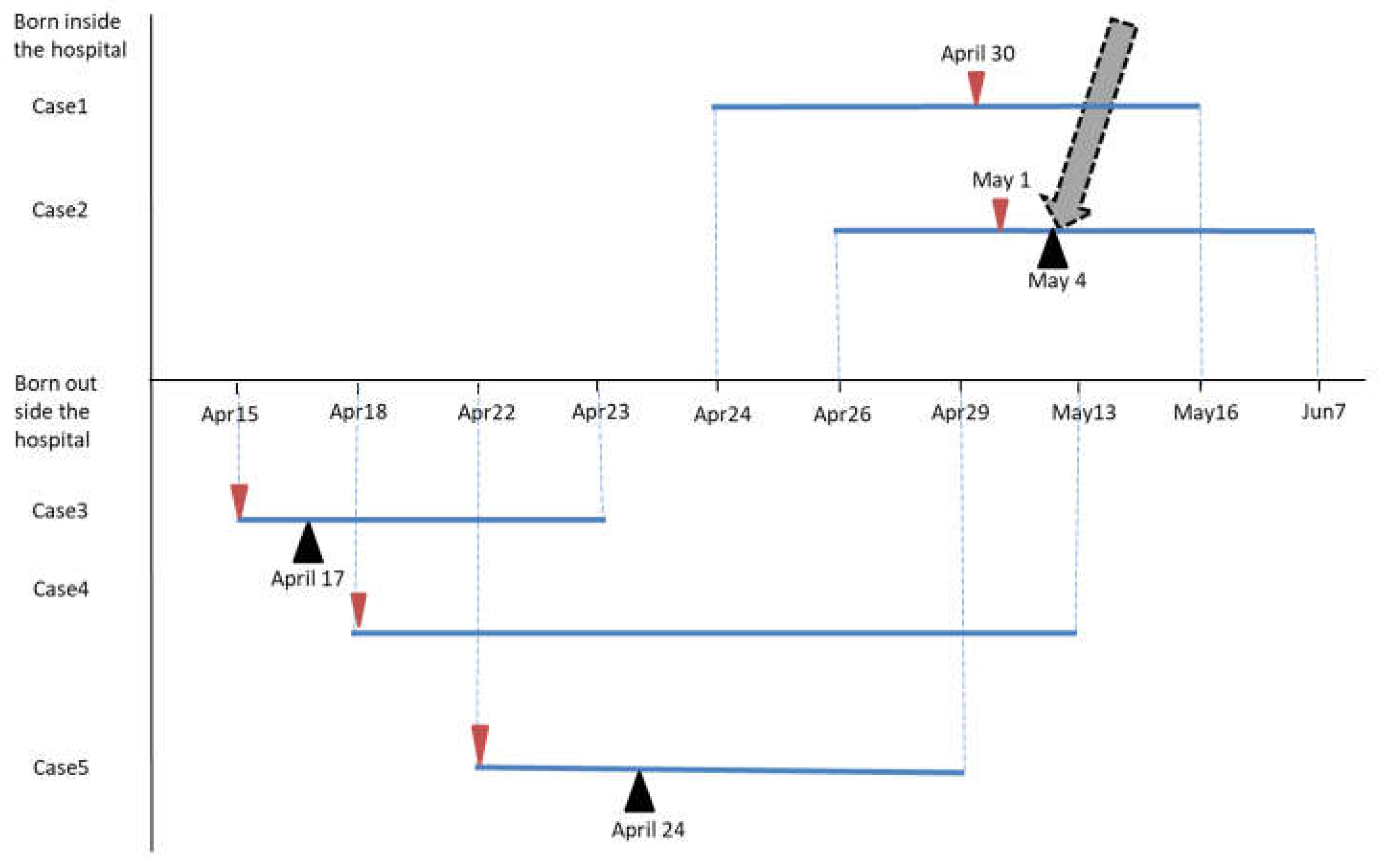
 : Date at symptom onset.
: Date at symptom onset.  : Date at IVIG administered.
: Date at IVIG administered.  : date at infection control interventions.
: date at infection control interventions.




