Submitted:
24 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fusarium poae Isolates Selection.
2.2. Brachypodium Accessions
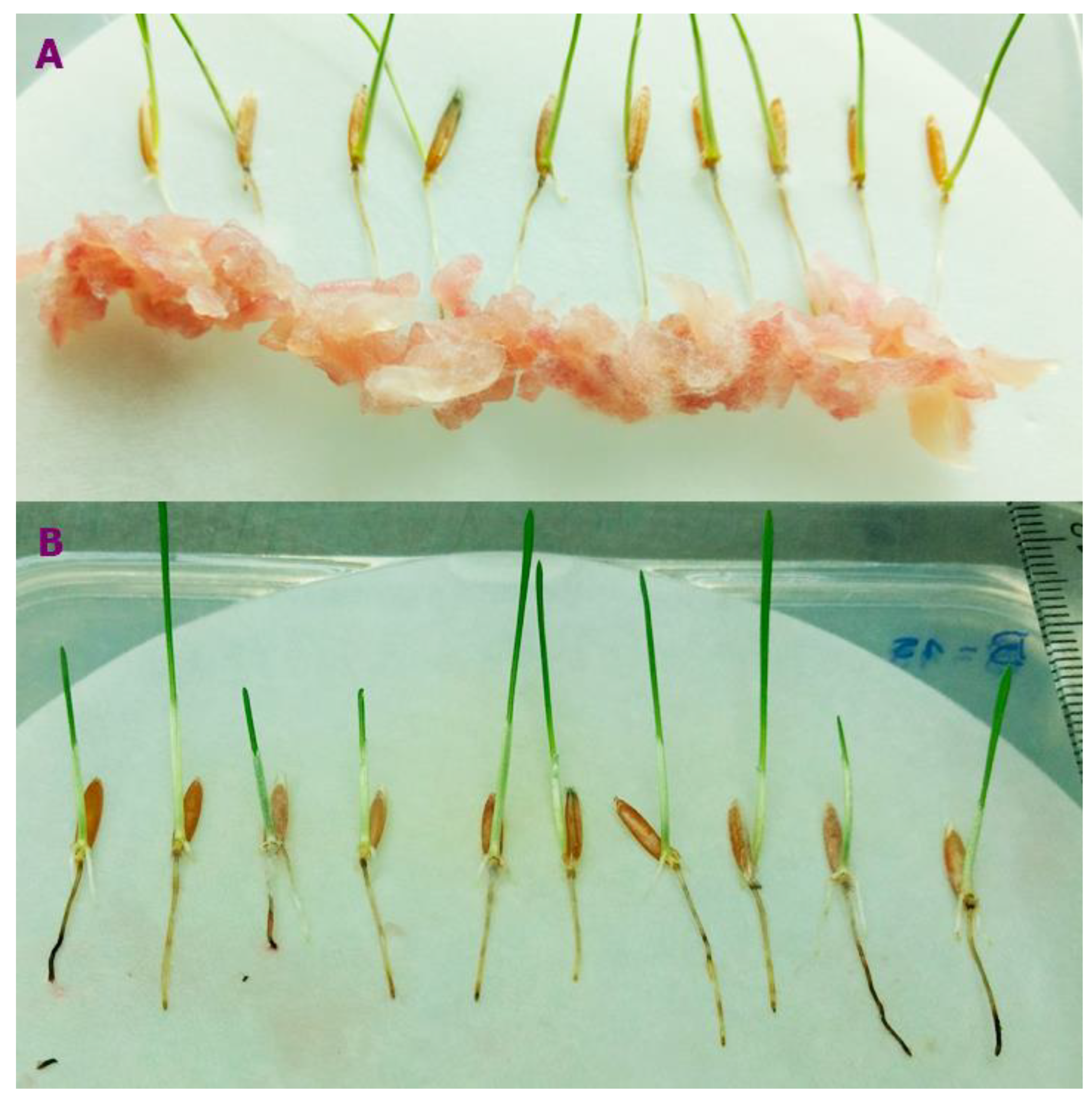
2.3. Pathogenicity Assay and F. poae Inoculation
2.4. Disease Severity and Quantification of Fungal Sporulation
2.5. Fungal Genomic DNA Quantification
2.6. NIV Quantification
3. Results
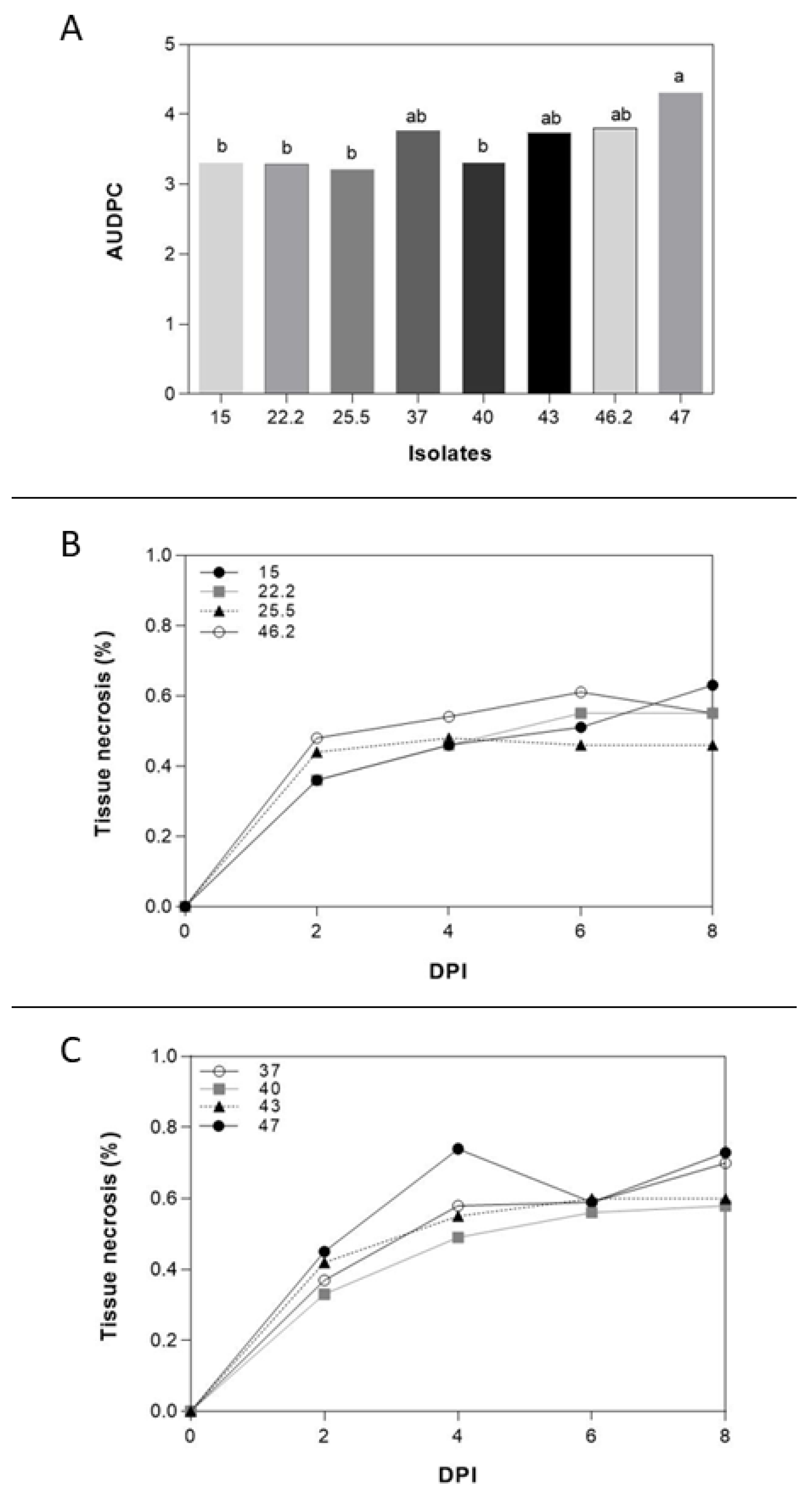
3.1. Fusarium poae Selection
3.2. Conidial Quantification and Disease Severity
3.3. Fusarium poae DNA and NIV Quantification
4. Discussion
5. Conclusion
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catalan, P.; Chalhoub, B.; Chochois, V.; Garvin, D.F.; Hasterok, R.; Manzaneda, A.J.; et al. Update on the genomics and basic biology of Brachypodium: International Brachypodium Initiative (IBI). Trends Plant Sci. 2014, 19, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Alberione, E.J. Sanitary update on wheat in Argentina. J. Plant Sci. Phytopathol. 2022, 6, 087–090. [Google Scholar]
- Dinolfo, M.I.; Martínez, M.; Nogueira, M.S.; Nicholson, P.; Stenglein, S.A. Evaluation of interaction between Brachypodium distachyon roots and Fusarium species. Eur. J. Plant Pathol. 2021, 159, 269–278. [Google Scholar] [CrossRef]
- Ng, C.A.; Poštulková, M.; Matoulková, D.; Psota, V.; Hartman, I.; Branyik, T. Methods for suppressing Fusarium infection during malting and their effect on malt quality. Czech J. Food Sci. 2021, 39, 340–359. [Google Scholar] [CrossRef]
- Pereyra, S.; Dill-Macky, R. Fusarium species recovered from wheat and barley grains in Uruguay, pathogenicity and deoxynivalenol content. Agrociencia Uruguay 2010, 14, 33–44. [Google Scholar] [CrossRef]
- Imathiu, S.M.; Edwards, S.G.; Ray, R.V.; Back, M.A. Fusarium langsethiae–a HT-2 and T-2 toxins producer that needs more attention. J. Phytopathol. 2013, 161, 1–10. [Google Scholar] [CrossRef]
- Ma, Z.; Xie, Q.; Li, G.; Jia, H.; Zhou, J.; Kong, Z.; et al. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020, 133, 1541–1568. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, D.R.; Patriarca, A.; Pinto, V.F. Can discrepancies between Fusarium graminearum trichothecene genotype and chemotype be explained by the influence of temperature in the relative production of 3-ADON and 15-ADON. Fungal Biol. 2021, 125, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Castañares, E.; Albuquerque, D.R.; Dinolfo, M.I.; Pinto, V.F.; Patriarca, A.; Stenglein, S.A. Trichothecene genotypes and production profiles of Fusarium graminearum isolates obtained from barley cultivated in Argentina. Int. J. Food Microbiol. 2014, 179, 57–63. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; et al. Fusarium: more than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100–116. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Transcriptomics of cereal–Fusarium graminearum interactions: what we have learned so far. Mol. Plant Pathol. 2018, 19, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Kikot, G.E.; Moschini, R.; Consolo, V.F.; Rojo, R.; Salerno, G.; Hours, R.A.; et al. Occurrence of different species of Fusarium from wheat in relation to disease levels predicted by a weather-based model in Argentina pampas region. Mycopathologia 2011, 171, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.C.; Chaouch, S.; Macadré, C.; Balzergue, S.; Huguet, S.; Martin-Magniette, M.L.; Bellvert, F.; Deguercy, X.; Thareau, V.; Heintz, D.; Saindrenan, P.; et al. Differential gene expression and metabolomics analyses of Brachypodium distachyon infected by deoxynivalenol producing and non-producing strains of Fusarium graminearum. BMC Genom. 2014, 15, 629. [Google Scholar] [CrossRef] [PubMed]
- Peraldi, A.; Beccari, G.; Steed, A.; Nicholson, P. Brachypodium distachyon: A new pathosystem to study Fusarium head blight and other Fusarium diseases on wheat. BMC Plant Biol. 2011, 11, 100. [Google Scholar] [CrossRef]
- Snijders, C.H.A. Resistance in wheat to Fusarium infection and trichothecene formation. Toxicology Letters 2004, 153(1), 37–46. [Google Scholar] [CrossRef]
- Boughalleb, N.; Souli, M.; Ouled Sghaier, H. Occurrence and geographic distribution of wheat Fusarium head blight and Fusarium root rot in Jendouba’s areas of Tunisia. African J. Plant Sci. Biotechnol. 2008, 2, 23–26. [Google Scholar]
- Stenglein, S.A. Fusarium poae a pathogen that needs more attention. J. Plant Pathol. 2009, 91, 25–36. [Google Scholar]
- Van Coller, G.J.; Rose, L.J.; Boutigny, A.L.; Ward, T.J.; Lamprecht, S.C.; Viljoen, A. The distribution and type B trichothecene chemotype of Fusarium species associated with head blight of wheat in South Africa during 2008 and 2009. PLoS ONE 2022, 17, e0275084. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.A.; Klink, H. Composition and predominance of Fusarium species causing Fusarium head blight in winter wheat grain depending on cultivar susceptibility and meteorological factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Lázaro, L.; Stenglein, S.A.; Dinolfo, M.I. Effects of waterlogging stress on plant-pathogen interaction between Fusarium poae and wheat/barley. Acta Sci.-Agron. 2019, 41, e42629. [Google Scholar] [CrossRef]
- Martínez, M.; Ramírez Albuquerque, L.D.; Arata, A.F.; Biganzoli, F.; Fernández Pinto, V.; Stenglein, S.A. Effects of Fusarium graminearum and Fusarium poae on disease parameters, grain quality and mycotoxins contamination in bread wheat (part I). J. Sci. Food Agric. 2020, 100, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, S.A.; Dinolfo, M.I.; Barros, G.; Bongiorno, F.; Chulze, S.; Moreno, M.V. Fusarium poae pathogenicity and mycotoxin accumulation on selected wheat and barley genotypes at a single location in Argentina. Plant Dis. 2014, 98, 1733–1738. [Google Scholar] [CrossRef]
- Witte, T.E.; Hicks, C.; Hermans, A.; Shields, S.; Overy, D.P. Debunking the myth of Fusarium poae T-2/HT-2 toxin production. J. Agric. Food Chem. 2024, 72, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Barreto, D.; Carmona, M.; Ferrazini, M.; Zanelli, M.; Perez, B.A. Occurrence and pathogenicity of Fusarium poae in barley in Argentina. Cereal Res. Commun. 2004, 32, 53–60. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Decundo, J.; Martínez, M.; Dieguez, S.N.; Moreyra, F.; Moreno, M.V.; et al. Natural contamination with mycotoxins produced by Fusarium graminearum and Fusarium poae in malting barley in Argentina. Toxins 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Dinolfo, M.I.; Castañares, E.; Stenglein, S.A. Characterization of a Fusarium poae world-wide collection by using molecular markers. Eur. J. Plant Pathol. 2014, 140, 119–132. [Google Scholar] [CrossRef]
- Dinolfo, M.I.; Stenglein, S.A.; Moreno, M.V.; Nicholson, P.; Jennings, P.; Salerno, G.L. ISSR markers detect high genetic variation among Fusarium poae isolates from Argentina and England. Eur. J. Plant Pathol. 2010, 127, 483–491. [Google Scholar] [CrossRef]
- Stenglein, S.A.; Dinolfo, M.I.; Bongiorno, F.; Moreno, M.V. Response of wheat (Triticum spp.) and barley (Hordeum vulgare) to Fusarium poae. Agrociencia 2012, 46, 46–299. [Google Scholar]
- Chhabra, B.; Tiwari, V.; Gill, B.S.; Dong, Y.; Rawat, N. Discovery of a promising susceptibility factor for Fusarium head blight in wheat. bioRxiv 2020. [Google Scholar]
- Campos, M.D.; Patanita, M.; Varanda, C.; Materatski, P.; Felix, M.D.R. Plant-pathogen interaction. Biology 2021, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ali, S.; Leng, Y.; Wang, R.; Garvin, D.F. Brachypodium distachyon-Cochliobolus sativus pathosystem is a new model for studying plant-pathogen interactions in cereal crops. Phytopathology 2015, 105, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Donn, S.; Ryan, P.R.; Mathesius, U.; Devilla, R.; Jones, A.; et al. Microbiome and exudates of the root and rhizosphere of Brachypodium distachyon, a model for wheat. PLoS ONE 2016, 11, e0164533. [Google Scholar] [CrossRef] [PubMed]
- Peraldi, A.; Griffe, L.L.; Burt, C.; McGrann, G.R.D.; Nicholson, P. Brachypodium distachyon exhibits compatible interactions with Oculimacula spp. and Ramularia collo-cygni, providing the first pathosystem model to study eyespot and ramularia leaf spot diseases. Plant Pathol. 2014, 63, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Verelst, W.; Bertolini, E.; De Bodt, S.; Vandepoele, K.; Demeulenaere, M.; Pè, M.E.; et al. Molecular and physiological analysis of growth-limiting drought stress in Brachypodium distachyon leaves. Mol. Plant 2013, 6, 311–322. [Google Scholar] [CrossRef]
- Luo, N.; Liu, J.; Yu, X.; Jiang, Y. Natural variation of drought response in Brachypodium distachyon. Physiol. Plant. 2011, 141, 19–29. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Powell, J.J.; Schneebeli, K.; Hsia, M.M.; Gardiner, D.M.; Bragg, J.N.; et al. Brachypodium as an emerging model for cereal–pathogen interactions. Ann. Bot. 2015, 115, 717–731. [Google Scholar] [CrossRef]
- Pasquet, J.C.; Changenet, V.; Macadré, C.; Boex-Fontvieille, E.; Soulhat, C.; Bouchabké-Coussa, O.; et al. A Brachypodium UDP-glycosyltransferase confers root tolerance to deoxynivalenol and resistance to Fusarium infection. Plant Physiol. 2016, 172, 559–574. [Google Scholar] [CrossRef]
- Des Marais, D.L.; Razzaque, S.; Hernandez, K.M.; Garvin, D.F.; Juenger, T.E. Quantitative trait loci associated with natural diversity in water-use efficiency and response to soil drying in Brachypodium distachyon. Plant Sci. 2016, 251, 2–11. [Google Scholar] [CrossRef]
- Rancour, D.M.; Marita, J.M.; Hatfield, R.D. Cell wall composition throughout development for the model grass Brachypodium distachyon. Front. Plant Sci. 2012, 3, 266. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. (Eds.) The Fusarium Laboratory Manual; Blackwell Publishing, Oxford, UK, 2006.
- Parry, D.W.; Nicholson, P. Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 1996, 45, 383–391. [Google Scholar] [CrossRef]
- Goddard, R.; Peraldi, A.; Ridout, C.; Nicholson, P. Enhanced disease resistance caused by BRI1 mutation is conserved between Brachypodium distachyon and barley (Hordeum vulgare). Mol. Plant-Microbe Interact. 2014, 27, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Introduction to Plant Disease Epidemiology; Jhon Willey & Sons. New York, USA, 1990. Campbell, C.L.; Madden, L.V. (Eds.).
- Brennan, J.M.; Leonard, G.; Fagan, B.; Cooke, B.M.; Ritieni, A.; Ferracane, R.; Nicholson, P.; Simpson, D.; Thomsett, M.; et al. Comparison of commercial European wheat cultivars to Fusarium infection of head and seedling tissue. Plant Pathol. 2007, 56, 55–64. [Google Scholar] [CrossRef]
- Chen, J.; Griffey, C.A.; Saghai Maroof, M.A.; Stromberg, E.L.; Biyashev, R.M.; Zhao, W.; et al. Validation of two major quantitative trait loci for Fusarium head blight resistance in Chinese wheat line W14. Plant Breed. 2006, 125, 99–101. [Google Scholar] [CrossRef]
- Di Rienzo, J.; Balzarini, M.; Gonzalez, L.; Casanoves, F.; Tablada, M.; Robledo, C. W. Infostat: software para análisis estadístico; Universidad Nacional de Córdoba, Argentina, 2012.
- Atoui, A.; Khoury, A.E.; Kallassy, M.; Lebrihi, A. Quantification of Fusarium graminearum and Fusarium culmorum by real-time PCR system and zearalenone assessment in maize. Int. J. Food Microbiol. 2012, 154, 59–65. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Meth. 2009, 76, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Park, J.H.; Cho, S.H.; Yang, M.S.; Park, C.M. Phenological growth stages of Brachypodium distachyon: codification and description. Weed Res. 2011, 51, 612–620. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A. M. Simple and efficient methodology to determine mycotoxins in cereal syrups. Food Chem. 2015, 177, 274–279. [Google Scholar] [CrossRef]
- Draper, J.; Mur, L.A.; Jenkins, G.; Ghosh-Biswas, G.C.; Bablak, P.; Hasterok, R.; et al. Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol. 2001, 127, 1539–1555. [Google Scholar] [CrossRef]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Kulik, T.; Jestoi, M. Quantification of Fusarium poae DNA and associated mycotoxins in asymptomatically contaminated wheat. Int. J. Food Microbiol. 2009, 130, 233–237. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Tini, F.; Covarelli, L.; Cowger, C.; Sulyok, M.; Benincasa, P.; Beccari, G. Infection timing affects Fusarium poae colonization of bread wheat spikes and mycotoxin accumulation in the grain. J. Sci. Food Agric. 2022, 102, 6358–6372. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.; Liu, C.; Van der Fels-Klerx, H.J. Fusarium infection and trichothecenes in barley and its comparison with wheat. World Mycotoxin J. 2018, 11, 33–46. [Google Scholar] [CrossRef]
- Malbrán, I.; Mourelos, C.A.; Girotti, J.R.; Aulicino, M.B.; Balatti, P.A.; Lori, G.A. Aggressiveness variation of Fusarium graminearum isolates from Argentina following point inoculation of field grown wheat spikes. Crop Protec. 2012, 42, 234–243. [Google Scholar] [CrossRef]
- Schneebeli, K.; Mathesius, U.; Matt, M. , Brachypodium distachyon is a pathosystem model for the study of the wheat disease rhizoctonia root rot. Plant Pathol. 2015, 64, 91–100. [Google Scholar] [CrossRef]
- Figueroa, M.; Alderman, S.; Garvin, D.F.; Pfender, W.F. Infection of Brachypodium distachyon by formae speciales of Puccinia graminis: early infection events and host-pathogen incompatibility. PLoS ONE 2013, 8, e56857. [Google Scholar] [CrossRef]
- Routledge, A.P.; Shelley, G.; Smith, J.V.; Talbot, N.J.; Draper, J.; Mur, L.A. Magnaporthe grisea interactions with the model grass Brachypodium distachyon closely resemble those with rice (Oryza sativa). Mol. Plant Pathol. 2004, 5, 253–265. [Google Scholar] [CrossRef]
- Sandoya, G.V.; de Oliveira Buanafina, M.M. Differential responses of Brachypodium distachyon genotypes to insect and fungal pathogens. Physiol. Mol. Plant Pathol. 2014, 85, 53–64. [Google Scholar] [CrossRef]
- Nazari, L.; Pattori, E.; Manstretta, V.; Terzi, V.; Morcia, C.; Somma, S.; et al. Effect of temperature on growth, wheat head infection, and nivalenol production by Fusarium poae. Food Microbiol. 2018, 76, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N. Mycotoxins are a component of Fusarium graminearum stress-response system. Front. Microbiol. 2015, 6, 133574. [Google Scholar] [CrossRef] [PubMed]
- Maier, F.J.; Miedaner, T.; Hadeler, B.; Felk, A.; Salomon, S.; Lemmens, M.; et al. Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol. 2006, 7, 449–461. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




