Submitted:
20 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
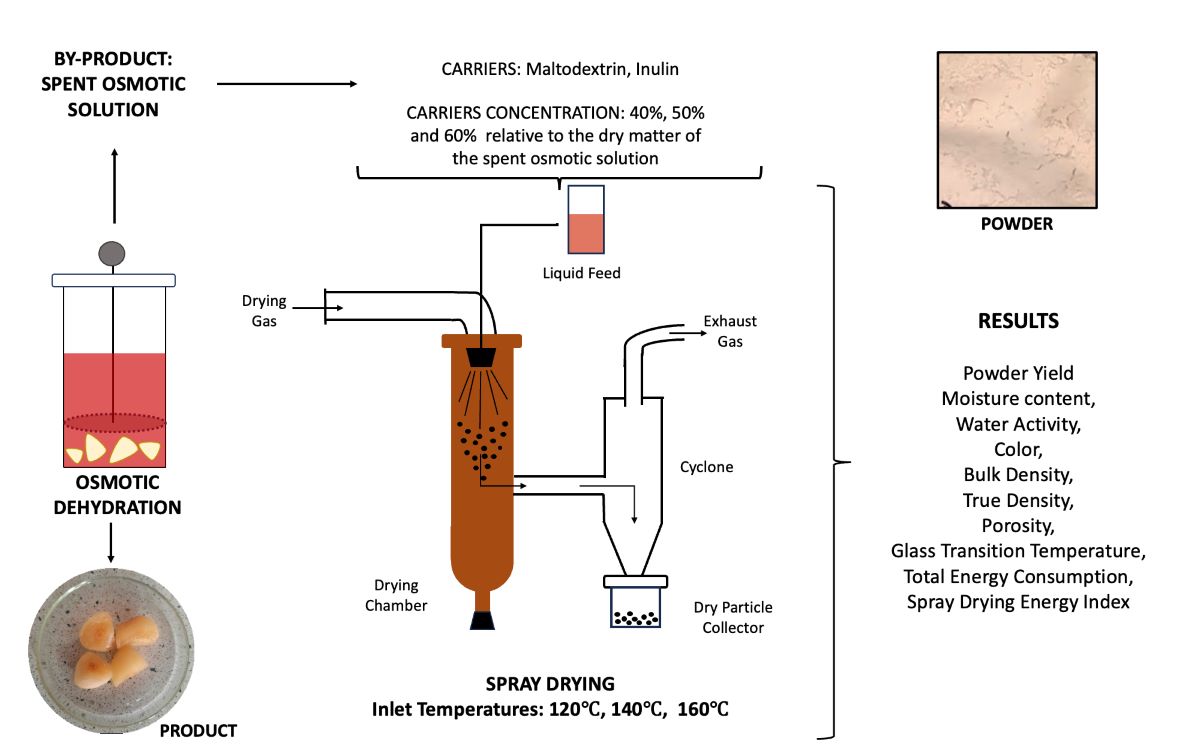
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions for Spray Drying
2.3. pH of the Solutions
2.4. Viscosity
2.5. Total Soluble Solids
2.6. Spray Drying
2.7. Yield
2.8. Water Activity
2.9. Color of the Solutions and Obtained Powders
2.10. Moisture Content of Obtained Powders
2.11. Bulk Density
2.12. True Density
2.13. Porosity
2.14. Glass Transition Temperature
2.15. Total Energy Consumption and Spray Drying Energy Index
2.16. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Solutions
3.2. Spray Drying Process Parameters
3.3. Properties of Obtained Powders
5. Conclusions
- The type of carrier used had the greatest impact on the efficiency of the drying process. The use of maltodextrin resulted in over 50% higher powder yield compared to inulin.
- The maximum yield of 32% was achieved for the sample containing 60% maltodextrin at the highest inlet temperature (M60-160).
- The highest energy consumption was observed at the highest inlet temperatures, although the type and concentration of the carrier did not have a significant effect on energy consumption.
- To assess the energy efficiency of the process, the Spray Drying Energy Index (SDEI) was proposed, which relates total energy consumption to powder yield. Despite higher energy consumption at higher inlet temperatures, the powder yields were greater, indicating higher energy efficiency for these processes. The highest energy efficiency was observed for the M60-160 sample, where high energy consumption was offset by a large powder yield.
- The moisture content of the powders was primarily influenced by the concentration of the carrier, while water activity was significantly affected by the inlet temperature. The lowest moisture content and water activity were observed for the M60-160 and M50-160 samples.
- The color change (ΔE) was most affected by the type of carrier used, but the concentration of the carrier and the inlet temperature also had a significant impact. The largest color change was observed for the M60-120 sample, while the smallest was noted for the I40-160 sample.
- No statistically significant differences were observed in the true density of the various powder samples. The bulk density and porosity were most affected by the type of carrier used. Samples containing maltodextrin generally exhibited higher values for these parameters. The lowest bulk density and porosity were observed for the M60-160 sample, with values of 1.431±0.019% and 58.3±0.7% respectively.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, B.; Ramaswamy, H.S. Evaluation of Mass Transfer Kinetics and Quality of Microwave-osmotic Dehydrated Mango Cubes under Continuous Flow Medium Spray (MWODS) Conditions in Sucrose Syrup as Moderated by Dextrose and Maltodextrin Supplements. Drying Technology 2020, 38, 1036–1050. [CrossRef]
- Yadav, A.K.; Singh, S.V. Osmotic Dehydration of Fruits and Vegetables: A Review. J Food Sci Technol 2014, 51, 1654–1673. [CrossRef]
- Salehi, F. Recent Advances in the Ultrasound-Assisted Osmotic Dehydration of Agricultural Products: A Review. Food Bioscience 2023, 51, 102307. [CrossRef]
- Sareban, M.; Abbasi Souraki, B. Anisotropic Diffusion during Osmotic Dehydration of Celery Stalks in Salt Solution. Food and Bioproducts Processing 2016, 98, 161–172. [CrossRef]
- de Oliveira, L.F.; Corrêa, J.L.G.; Botrel, D.A.; Vilela, M.B.; Batista, L.R.; Freire, L. Reuse of Sorbitol Solution in Pulsed Vacuum Osmotic Dehydration of Yacon (Smallanthus Sonchifolius). J Food Process Preserv 2017, 41, e13306. [CrossRef]
- Bourdoux, S.; Li, D.; Rajkovic, A.; Devlieghere, F.; Uyttendaele, M. Performance of Drying Technologies to Ensure Microbial Safety of Dried Fruits and Vegetables. Comp Rev Food Sci Food Safe 2016, 15, 1056–1066. [CrossRef]
- Abrahão, F.R.; Corrêa, J.L.G. Osmotic Dehydration: More than Water Loss and Solid Gain. Critical Reviews in Food Science and Nutrition 2023, 63, 2970–2989. [CrossRef]
- Fernández, P.R.; Lovera, N.; Ramallo, L.A. Sucrose Syrup Reuse during One- and Multi-stage Osmotic Dehydration of Pineapple. J Food Process Engineering 2020, 43, e13399. [CrossRef]
- Singh, P.; Ban, Y.G.; Kashyap, L.; Siraree, A.; Singh, J. Sugar and Sugar Substitutes: Recent Developments and Future Prospects. In Sugar and Sugar Derivatives: Changing Consumer Preferences; Mohan, N., Singh, P., Eds.; Springer Singapore: Singapore, 2020; pp. 39–75 ISBN 9789811566622.
- Md Salim, N.S. Potential Utilization of Fruit and Vegetable Wastes for Food through Drying or Extraction Techniques. NTNF 2017, 1. [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems Associated with Spray Drying of Sugar-Rich Foods. Drying Technology 1997, 15, 671–684. [CrossRef]
- Tontul, I.; Topuz, A.; Ozkan, C.; Karacan, M. Effect of Vegetable Proteins on Physical Characteristics of Spray-Dried Tomato Powders. Food sci. technol. int. 2016, 22, 516–524. [CrossRef]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Microencapsulation by Spray Drying of a Lycopene-Rich Tomato Concentrate: Characterization and Stability. LWT 2018, 91, 286–292. [CrossRef]
- Sidhu, G.K.; Singh, M.; Kaur, P. Effect of Operational Parameters on Physicochemical Quality and Recovery of Spray-dried Tomato Powder. J Food Process Preserv 2019, 43. [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, S.C.; Duarte, D.F.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Tomato (Solanum Lycopersicum L.) Pomace Ethanolic Extract by Spray Drying: Optimization of Process Conditions. Applied Sciences 2019, 9, 612. [CrossRef]
- Zimmer, A.; Masztalerz, K.; Lech, K. Effect of Osmotic Dehydration in Tomato Juice on Microstructure of Garlic and on Drying Using Different Methods. Agriculture 2024, 14, 1164. [CrossRef]
- Zimmer, A. A.; Masztalerz, K.; Lech, K. The Influence of the Pretreatment of Garlic (Allium sativum L.) and Tomato (Solanum lycopersicum L.) Osmotic Solution on Physical Properties of the Material. J. Food Process Eng. 2023, 46, e14314. [CrossRef]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A Review of Encapsulation of Carotenoids Using Spray Drying and Freeze Drying. Critical Reviews in Food Science and Nutrition 2020, 60, 3547–3572. [CrossRef]
- Shahidi, F.; Han, X. Encapsulation of Food Ingredients. Critical Reviews in Food Science and Nutrition 1993, 33, 501–547. [CrossRef]
- Khwanpruk, K.; Akkaraphenphan, C.; Wattananukit, P.; Kaewket, W.; Chusai, S. Effect of Drying Air Condition and Feed Composition on the Properties of Orange Juice Spray Dried Powder. MATEC Web Conf. 2018, 192, 03013. [CrossRef]
- Abadio, F.D.B.; Domingues, A.M.; Borges, S.V.; Oliveira, V.M. Physical Properties of Powdered Pineapple (Ananas Comosus) Juice––Effect of Malt Dextrin Concentration and Atomization Speed. Journal of Food Engineering 2004, 64, 285–287. [CrossRef]
- Perinelli, D.R.; Santanatoglia, A.; Caprioli, G.; Bonacucina, G.; Vittori, S.; Maggi, F.; Sagratini, G. Inulin Functionalized “Giuncata” Cheese as a Source of Prebiotic Fibers. Foods 2023, 12, 3499. [CrossRef]
- Liu, S.; Ellars, C.E.; Edwards, D.S. Ascorbic Acid: Useful as a Buffer Agent and Radiolytic Stabilizer for Metalloradiopharmaceuticals. Bioconjugate Chem. 2003, 14, 1052–1056. [CrossRef]
- Goula, A.M.; Adamopoulos, K.G.; Kazakis, N.A. Influence of Spray Drying Conditions on Tomato Powder Properties. Drying Technology 2004, 22, 1129–1151. [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of Drying Methods on the Physical Properties and Microstructures of Mango (Philippine ‘Carabao’ Var.) Powder. Journal of Food Engineering 2012, 111, 135–148. [CrossRef]
- Anisuzzaman, S.M.; G. Joseph, C.; Ismail, F.N. Influence of Carrier Agents Concentrations and Inlet Temperature on the Physical Quality of Tomato Powder Produced by Spray Drying. JST 2023, 31, 1379–1411. [CrossRef]
- Roccia, P.; Martínez, M.L.; Llabot, J.M.; Ribotta, P.D. Influence of Spray-Drying Operating Conditions on Sunflower Oil Powder Qualities. Powder Technology 2014, 254, 307–313. [CrossRef]
- Araujo-Díaz, S.B.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the Physical Properties and Conservation of the Antioxidants Content, Employing Inulin and Maltodextrin in the Spray Drying of Blueberry Juice. Carbohydrate Polymers 2017, 167, 317–325. [CrossRef]
- Adhikari, B.; Howes, T.; Bhandari, B.R.; Troung, V. Effect of Addition of Maltodextrin on Drying Kinetics and Stickiness of Sugar and Acid-Rich Foods during Convective Drying: Experiments and Modelling. Journal of Food Engineering 2004, 62, 53–68. [CrossRef]
- Adhikari, B.; Howes, T.; Bhandari, B.R.; Truong, V. Characterization of the Surface Stickiness of Fructose–Maltodextrin Solutions During Drying. Drying Technology 2003, 21, 17–34. [CrossRef]
- Largo Ávila, E.; Cortés Rodríguez, M.; Ciro Velásquez, H.J. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. Rev. Fac. Nac. Agron. Medellín 2015, 68, 7509–7520. [CrossRef]
- Aghbashlo, M.; Mobli, H.; Rafiee, S.; Madadlou, A. Energy and Exergy Analyses of the Spray Drying Process of Fish Oil Microencapsulation. Biosystems Engineering 2012, 111, 229–241. [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The Physicochemical Properties of Spray-Dried Watermelon Powders. Chemical Engineering and Processing: Process Intensification 2007, 46, 386–392. [CrossRef]
- Selvamuthukumaran, M.; Khanum, F. Optimization of Spray Drying Process for Developing Seabuckthorn Fruit Juice Powder Using Response Surface Methodology. J Food Sci Technol 2014, 51, 3731–3739. [CrossRef]
- Wang, B.; Duke, S.R.; Wang, Y. Microencapsulation of Lipid Materials by Spray Drying and Properties of Products. J Food Process Engineering 2017, 40, e12477. [CrossRef]
- Mishra, P.; Mishra, S.; Mahanta, C.L. Effect of Maltodextrin Concentration and Inlet Temperature during Spray Drying on Physicochemical and Antioxidant Properties of Amla (Emblica Officinalis) Juice Powder. Food and Bioproducts Processing 2014, 92, 252–258. [CrossRef]
- Chegini, G.R.; Ghobadian, B. Effect of Spray-Drying Conditions on Physical Properties of Orange Juice Powder. Drying Tech. 2005, 23, 657–668. [CrossRef]
- Tkacz, K.; Wojdyło, A.; Michalska-Ciechanowska, A.; Turkiewicz, I.P.; Lech, K.; Nowicka, P. Influence Carrier Agents, Drying Methods, Storage Time on Physico-Chemical Properties and Bioactive Potential of Encapsulated Sea Buckthorn Juice Powders. Molecules 2020, 25, 3801. [CrossRef]
- Lacerda, E.C.Q.; Calado, V.M. de A.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, Inulin and Maltodextrin as Encapsulating Agents Affect the Quality and Stability of Jussara Pulp Microparticles. Carbohydrate Polymers 2016, 151, 500–510. [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Stability of Lycopene during Spray Drying of Tomato Pulp. LWT - Food Science and Technology 2005, 38, 479–487. [CrossRef]
- Li, J.; Pettinato, M.; Casazza, A.A.; Perego, P. A Comprehensive Optimization of Ultrasound-Assisted Extraction for Lycopene Recovery from Tomato Waste and Encapsulation by Spray Drying. Processes 2022, 10, 308. [CrossRef]
- Bicudo, M.O.P.; Jó, J.; Oliveira, G.A. de; Chaimsohn, F.P.; Sierakowski, M.R.; Freitas, R.A. de; Ribani, R.H. Microencapsulation of Juçara (Euterpe Edulis M.) Pulp by Spray Drying Using Different Carriers and Drying Temperatures. Drying Technology 2015, 33, 153–161. [CrossRef]
- Syamaladevi, R.M.; Insan, S.K.; Dhawan, S.; Andrews, P.; Sablani, S.S. Physicochemical Properties of Encapsulated Red Raspberry (Rubus Idaeus) Powder: Influence of High-Pressure Homogenization. Drying Technology 2012, 30, 484–493. [CrossRef]
- Averardi, A.; Cola, C.; Zeltmann, S.E.; Gupta, N. Effect of Particle Size Distribution on the Packing of Powder Beds: A Critical Discussion Relevant to Additive Manufacturing. Materials Today Communications 2020, 24, 100964. [CrossRef]
- Zouari, A.; Mtibaa, I.; Triki, M.; Jridi, M.; Zidi, D.; Attia, H.; Ayadi, M.A. Effect of Spray-drying Parameters on the Solubility and the Bulk Density of Camel Milk Powder: A Response Surface Methodology Approach. Int J of Dairy Tech 2020, 73, 616–624. [CrossRef]
- Harnkarnsujarit, N.; Charoenrein, S.; Roos, Y.H. Microstructure Formation of Maltodextrin and Sugar Matrices in Freeze-Dried Systems. Carbohydrate Polymers 2012, 88, 734–742. [CrossRef]
- Farías-Cervantes, V.S.; Salinas-Moreno, Y.; Chávez-Rodríguez, A.; Luna-Solano, G.; Medrano-Roldan, H.; Andrade-González, I. Stickiness and Agglomeration of Blackberry and Raspberry Spray Dried Juices Using Agave Fructans and Maltodextrin as Carrier Agents. Czech J. Food Sci. 2020, 38, 229–236. [CrossRef]
- Jumah, R.Y.; Tashtoush, B.; Shaker, R.R.; Zraiy, A.F. MANUFACTURING PARAMETERS AND QUALITY CHARACTERISTICS OF SPRAY DRIED JAMEED. Drying Technology 2000, 18, 967–984. [CrossRef]
- Nguyen, D.Q.; Nguyen, T.H.; Mounir, S.; Allaf, K. Effect of Feed Concentration and Inlet Air Temperature on the Properties of Soymilk Powder Obtained by Spray Drying. Drying Technology 2018, 36, 817–829. [CrossRef]
- Baenas, N.; Bravo, S.; García-Alonso, F.J.; Gil, J.V.; Periago, M.J. Changes in Volatile Compounds, Flavour-Related Enzymes and Lycopene in a Refrigerated Tomato Juice during Processing and Storage. Eur Food Res Technol 2021, 247, 975–984. [CrossRef]
- Lee, S.M.; Cho, A.R.; Yoo, S.; Kim, Y. Effects of Maltodextrins with Different Dextrose-equivalent Values. Flavour & Fragrance J 2018, 33, 153–159. [CrossRef]
- Yu, A.B.; Feng, C.L.; Zou, R.P.; Yang, R.Y. On the Relationship between Porosity and Interparticle Forces. Powder Technology 2003, 130, 70–76. [CrossRef]
- Michalska-Ciechanowska, A.; Majerska, J.; Brzezowska, J.; Wojdyło, A.; Figiel, A. The Influence of Maltodextrin and Inulin on the Physico-Chemical Properties of Cranberry Juice Powders. ChemEngineering 2020, 4, 12. [CrossRef]
- Sousa, A.S. de; Borges, S.V.; Magalhães, N.F.; Ricardo, H.V.; Azevedo, A.D. Spray-Dried Tomato Powder: Reconstitution Properties and Colour. Braz. arch. biol. technol. 2008, 51, 607–614. [CrossRef]
| Solutions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Dry Mass (%) |
Water Activity (-) |
pH (-) | Viscosity (mPa*s) |
Color Parameters | |||
| L* | a* | b* | ∆E | |||||
| OS† | 16.86±0.18 b* | 0.9754±0.0008 a | 3.81±0.00 d | 1.91±0.01a | 23.19±0.50 a | 13.05±0.26 e | 9.15±0.27 e | - |
| OS-M40 | 18.64±0.04 a | 0.9896±0.0012 c | 3.72±0.01 a | 2.39±0.03 b, c | 23.65±0.50 a | 5.44±0.19 a | 5.20±0.13 a | 8.59±0.26 b |
| OS-M50 | 20.46±0.01 a | 0.9873±0.0003 b, c | 3.74±0.01b, c | 3.09±0.04 d | 25.43±0.19 b | 7.91±0.07 c | 6.40±0.08 c | 6.25±0.16 a |
| OS-M60 | 19.69±0.16 a | 0.9872±0.0026 c | 3.77±0.01c | 2.99±0.11d | 26.39±0.49 b | 6.52±0.15 b | 6.71±0.19 a, b | 8.05±0.40 b |
| OS-I40 | 20.47±0.53 a | 0.9853±0.0025 b, c | 3.72±0.00 a | 2.20±0.05 b | 28.05±0.34 c | 8.86±0.48 d | 7.91±0.39 d | 6.55±0.51a |
| OS-I50 | 20.59±0.13 a | 0.9809±0.0033 a, b | 3.73±0.00 a, b | 2.51±0.08 c | 27.71±0.26 c | 7.07±0.27 b | 6.25±0.15 b, c | 8.04±0.23 b |
| OS-I60 | 22.30±0.18 c | 0.9866±0.0009 b, c | 3.63±0.01a, b | 3.34±0.03 e | 37.52±0.44 d | 13.29±0.14 e | 13.69±0.24 f | 15.05±0.34 c |
| Total Soluble Solids, % | Water Activity, - | pH, - | Viscosity, mPa⋅s | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| M/I† | 60.68 | 0.0002 | 16 | 0.0073 | 25 | 0.0024 | 15.61 | 0.0075 |
| CC | 12.48 | 0.0073 | 5 | 0.0014 | 24 | 0.0013 | 202.56 | 0.0001 |
| M/I x CC | 23.33 | 0.0015 | 1 | 0.3483 | 15 | 0.0044 | 55.63 | 0.0001 |
| L* | a* | b* | ∆E | |||||
| F | p | F | p | F | p | F | p | |
| M/I | 1052.52 | 0.0001 | 679.33 | 0.0001 | 1126.18 | 0.0001 | 200.79 | 0.0001 |
| CC | 443.35 | 0.0001 | 210.34 | 0.0001 | 432.55 | 0.0001 | 312.12 | 0.0001 |
| M/I x CC | 213.04 | 0.0001 | 338.79 | 0.0001 | 518.91 | 0.0001 | 271.95 | 0.0001 |
| Sample | Outlet Temperature, °C |
Feed Flow Rate, g⋅min-1 | Yield, % | Energy Consumption, kJ |
Spray Drying Energy Index, kJ | |
|---|---|---|---|---|---|---|
| M40-120† | 84±1.4 a* | 3.62±0.16 a | 15.43±1.34 d, e | 1791±81 a | 11672±1535 a | |
| M40-140 | 95±0.1 b | 3.66±0.08 a | 13.51±0.71c-e | 2097±44 b, c | 15552±1155 a | |
| M40-160 | 109±1.4 c | 3.58±0.08 a | 23.17±0.61f-h | 2295±51 d | 9912±479 a | |
| M50-120 | 85±2.1a | 3.74±0.02 a, b | 15.83±0.28 d, e | 1731±8 a | 10936±246 a | |
| M50-140 | 95±0.0 b | 3.82±0.06 a, b | 25.58±1.91g, h | 2012±33 b | 7891±718 a | |
| M50-160 | 108±1.4 c | 3.73±0.01 a, b | 28.83±2.73 h, i | 2201±3 c, d | 7670±738 a | |
| M60-120 | 83±2.1a | 3.70±0.06 a, b | 18.91±1.36 e, f | 1752±27 a | 9297±813 a | |
| M60-140 | 96±0.7 b | 3.72±0.02 a, b | 26.52±0.36 f, g | 2064±13 b, c | 9165±85 a | |
| M60-160 | 109±0.1c | 3.75±0.07 a, b | 32.13±0.64 i | 2195±41c, d | 6831±8 a | |
| I40-120 | 85±1.4 a | 3.73±0.07 a, b | 2.75±0.48 a | 1736±33 a | 64249±12315 b | |
| I40-140 | 96±1.4 b | 3.81±0.01 a, b | 9.27±1.21b, c | 2017±1 b | 21943±2842 a | |
| I40-160 | 109±1.4 c | 3.71±0.07 a, b | 5.15±1.55 a, b | 2218±40 c, d | 45271±14444 b | |
| I50-120 | 83±1.4 a | 3.67±0.08 a | 12.57±0.43 c, d | 1767±38 a | 14067±782 a | |
| I50-140 | 96±0.7 b | 3.68±0.07 a, b | 11.54±0.98 c, d | 2087±37 b, c | 118140±1223 a | |
| I50-160 | 108±0.7 c | 4.00±0.21b | 8.98±1.83 b, c | 2058±109 b, c | 23526±5998 a | |
| I60-120 | 85±2.1a | 3.76±0.05 a, b | 14.12±2.13 c-e | 1724±24 a | 12338±1689 a | |
| I60-140 | 96±0.7 b | 3.73±0.03 a, b | 13.05±1.80 c-e | 2061±15 b, c | 16951±2312 a | |
| I60-160 | 107±2.8 c | 3.70±0.02 a, b | 14.12±2.65 c-e | 2224±13 c, d | 12044±3103 a |
| Outlet Temperature, °C |
Feed Flow Rate, g⋅min-1 | Yield, % | Total Energy Consumption, kJ |
Spray Drying Energy Index, kJ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| M/I† | 0 | 0.9093 | 3.35 | 0.084 | 550 | 0.0001 | 3.66 | 0.0719 | 94.5897 | 0.0001 |
| CC | 1 | 0.3969 | 3.49 | 0.05237 | 85.041 | 0.0001 | 4.02 | 0.0359 | 40.5008 | 0.0001 |
| Tin | 854 | 0.0001 | 0.82 | 0.4581 | 40.674 | 0.0001 | 341.88 | 0.0001 | 4.0753 | 0.0347 |
| M/I x CC | 0.5 | 0.6186 | 1.99 | 0.1652 | 0.906 | 0.4220 | 2.33 | 0.1263 | 23.278 | 0.0001 |
| M/I x Tin | 0.9 | 0.4425 | 1.42 | 0.2672 | 52.404 | 0.0001 | 1.67 | 0.2169 | 6.0926 | 0.0095 |
| CC x Tin | 0.2 | 0.9276 | 2.31 | 0.0974 | 2.946 | 0.0491 | 2.67 | 0.0654 | 6.0007 | 0.003 |
| M/I x CC x Tin |
0.9 | 0.5102 | 3.04 | 0.0445 | 11.082 | 0.0001 | 3.12 | 0.0411 | 9.4102 | 0.0003 |
| Powders | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Mc, % | Water Activity, - | Color Parameters | Bulk Density, g⋅cm-3 | True Density, g⋅cm-3 | Porosity, % | Glass Transition Temperature, °C | |||
| L* | a* | b* | ∆E | |||||||
| M40-120† | 3.81±0.58 a-d* | 0.218±0.017 b-e | 83.91±0.26 f, g | 9.58±1.55 b-d | 13.70±0.92 b-d | 26.17±2.47 e-g | 0.558±0.019 b-f | 1.590±0.009 a | 64.9±1.0 a, b | 38.1±0.3 d |
| M40-140 | 5.23±1.65 d-f | 0.215±0.010 b-e | 81.78±0.01d-f | 11.53±0.01d-f | 14.96±0.10 b-f | 23.33±0.20 d-f | 0.538±0.001b-f | 1.514±0.029 a | 64.4±0.6 a, b | 38.3±0.6 e |
| M40-160 | 3.21±0.13 a-c | 0.222±0.011 b-e | 78.295±0.04 b, c | 12.39±2.08 f, g | 17.49±0.44 h | 19.84±1.76 a-c | 0.591±0.018 r, f | 1.549±0.034 a | 61.8±0.3 a, b | 38.4±0.1f |
| M50-120 | 3.80±0.39 a-d | 0.228±0.007 c-e | 84.58±0.72 g | 9.55±0.71 b-d | 13.16±0.51a, b | 26.84±0.81g | 0.540±0.003 b-f | 1.511±0.056 a | 64.2±1.1 a, b | 38.4±0.3 e |
| M50-140 | 2.87±0.13 a-c | 0.214±0.011 b-e | 83.95±0.63 f, g | 9.36±0.52 b, c | 13.08±0.07 a, b | 26.36±0.55 f, g | 0.562±0.008 c-f | 1.686±0.069 a | 66.6±1.9 a, b | 38.6±0.2 i |
| M50-160 | 2.27±0.03 a | 0.198±0.009 a, b | 82.22±0.10 d-g | 9.73±0.58 b-e | 14.19±0.48 b-f | 24.54±0.44 d-g | 0.548±0.001b-f | 1.463±0.071 a | 62.5±1.7 a, b | 38.0±0.3 j |
| M60-120 | 3.56±0.39 a-d | 0.214±0.005 b-e | 87.80±0.34 h | 7.32±1.16 a | 11.30±0.52 a | 30.91±1.50 h | 0.594±0.012 f | 1.486±0.016 a | 60.0±1.3 a, b | 37.2±0.3 g |
| M60-140 | 2.98±0.13 a-c | 0.233±0.004 e | 84.22±0.08 g | 8.20±0.17 a, b | 13.60±1.75 b, c | 27.10±1.44 g | 0.576±0.007 d-f | 1.481±0.029 a | 61.1±1.3 a, b | 36.8±0.3h, i |
| M60-160 | 2.28±0.10 a | 0.177±0.006 a | 82.83±0.01e-g | 9.62±1.34 b-d | 13.60±0.77 b, c | 25.18±2.08 e-g | 0.597±0.002 f | 1.431±0.019 a | 58.3±0.7 a | 36.1±0.1i |
| I40-120 | 6.12±0.21 e, f | 0.231±0.021c-e | 79.98±0.13 c, d | 11.48±0.90 d-f | 15.97±0.28 f-h | 21.69±1.49 c, d | 0.486±0.016 a-e | 1.379±0.390 a | 63.4±9.2 a, b | 35.0±0.2 c |
| I40-140 | 4.48±0.20 c-e | 0.230±0.069 d-e | 79.82±0.001c, d | 11.71±0.03 e, f | 15.79±0.01e-h | 21.44±0.29 b-d | 0.480±0.049 a-d | 1.594±0.011 a | 69.9±3.3 a, b | 35.6±0.1b |
| I40-160 | 5.33±0.03 d-f | 0.232±0.011e | 75.59±0.01b | 14.3±0.11g | 17.00±0.09 g, h | 16.55±0.40 a | 0.511±0.010 a-f | 1.440±0.081 a | 64.5±2.7 a, b | 36.6±0.3 a |
| I50-120 | 3.95±0.34 a-d | 0.208±0.007 b-e | 83.11±0.03 e-g | 9.27±1.48 a-c | 13.85±1.18 b-e | 25.57±1.81e, g | 0.466±0.034 a-c | 1.517±0.221 a | 68.8±6.8 a, b | 38.3±0.2 f |
| I50-140 | 3.32±0.10 a-c | 0.196±0.006 a, b | 80.96±0.13 c-e | 10.77±0.09 c-f | 15.22±0.06 c-g | 22.89±0.44 c-e | 0.484±0.025 a-d | 1.601±0.026 a | 69.8±0.6 a, b | 38.2±0.3 e |
| I50-160 | 4.23±0.23 b-d | 0.232±0.004 e | 76.64±0.10 b | 12.77±0.73 f, g | 14.47±1.38 h | 18.22±0.54 a, b | 0.495±0.032 a-f | 1.520±0.037 a | 67.4±2.9 a, b | 38.6±0.2 c |
| I60-120 | 3.56±0.35 a-d | 0.205±0.010 b-d | 83.69±0.21e- g | 8.66±0.59 a, b | 14.33±2.61b-f | 26.38±1.40 f, g | 0.530±0.029 b-f | 1.476±0.024 a | 64.1±2.6 a, b | 41.4±0.2 h |
| I60-140 | 3.31±0.06 a-c | 0.208±0.005 b-e | 82.00±0.03 d-g | 10.19±0.20 b-e | 14.69±0.04 b-f | 24.10±0.11 d-g | 0.455±0.004 a, b | 1.507±0.045 a | 69.8±0.6 a, b | 40.3±0.3 f |
| I60-160 | 2.64±0.17 a, b | 0.199±0.010 a, b | 79.95±0.09 c, d | 11.29±1.16 c-f | 15.63±0.17 d-h | 21.74±1.65 c, d | 0.596±0.063 f | 1.429±0.023 a | 58.3±3.7 a, b | 41.3±0.4 g |
| FD | 6.49±0.12 f | 0.196±0.001 a-c | 60.38±0.20 a | 20.75±0.33 h | 16.08±0.32 f-h | - | 0.413±0.045 a | 1.491±0.069 a | 72.4±1.7 b | - |
| Moisture Content, % | Water Activity, - |
Bulk Density, g⋅cm-3 | True Density, g⋅cm-3 |
Porosity, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| M/I† | 24.839 | 0.0001 | 0.81 | 0.3716 | 63.67 | 0.0001 | 0.54 | 0.4718 | 31.53 | 0.0001 |
| CC | 41.486 | 0.0001 | 3.07 | 0.5479 | 8.94 | 0.0020 | 1.564 | 0.2365 | 18.35 | 0.0001 |
| Tin | 9.105 | 0.0018 | 18.82 | 0.0001 | 8.16 | 0.0030 | 2.172 | 0.1429 | 19.25 | 0.0001 |
| M/I x CC | 3.528 | 0.051 | 17.53 | 0.0001 | 0.08 | 0.9220 | 0.49 | 0.6208 | 1.24 | 0.2978 |
| M/I x Tin | 7.507 | 0.0043 | 3.7 | 0.0312 | 1.97 | 0.1679 | 0.384 | 0.6869 | 3.00 | 0.0583 |
| CC x Tin | 1.177 | 0.3541 | 10.67 | 0.0001 | 3.02 | 0.0456 | 0.386 | 0.8285 | 1.36 | 0.2586 |
| M/I x CC x Tin | 5.324 | 0.0052 | 5.77 | 0.0001 | 2.03 | 0.1336 | 0.878 | 0.4967 | 2.96 | 0.0279 |
| L* | a* | b* | ∆E | Glass Transition Temperature, °C | ||||||
| F | p | F | p | F | p | F | p | F | p | |
| M/I | 145.4 | 0.0001 | 62.09 | 0.0001 | 81.4 | 1E-04 | 134.2 | 0.0001 | 4372.6 | 0.0001 |
| CC | 62.9 | 0.0001 | 67.68 | 0.0001 | 39.79 | 1E-04 | 70.46 | 0.0001 | 3739 | 0.0001 |
| Tin | 108.8 | 0.0001 | 55.28 | 0.0001 | 47.93 | 1E-04 | 100.92 | 0.0001 | 63.6 | 0.0001 |
| M/I x CC | 0.3 | 0.743 | 0.330 | 0.7221 | 4.56 | 0.015 | 0.25 | 0.7822 | 443.3 | 0.0001 |
| M/I x Tin | 2.2 | 0.116 | 4.130 | 0.0214 | 1.05 | 0.357 | 2.24 | 0.1158 | 1283.8 | 0.0001 |
| CC x Tin | 2.1 | 0.087 | 0.900 | 0.4693 | 1.93 | 0.119 | 1.6 | 0.1868 | 59.4 | 0.0001 |
| M/I x CC x Tin | 4.1 | 0.006 | 4.160 | 0.0052 | 6.96 | 1E-04 | 4.13 | 0.0054 | 210.8 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





