1. Introduction
Pharmaceutical drug discovery is facing an overwhelming challenge in many areas of human medicine. Most chronic pathologies still form a huge burden to global healthcare and are tediously waiting for scientific research to figure out efficient remedies to reverse pathophysiological processes and/or cure diseases after their onset.
Cancer, for example, continues to be one of the most challenging health burdens worldwide. In 2022, there were close to 20 million new diagnosed cases along with 9.7 million deaths from cancer [
1]. Neurodegenerative diseases (NDD) are another example of global challenges where disease modifying therapies are still desperately lacking. This kind of disorders are currently the leading cause of disability and the second foremost cause of death worldwide [
2]. Dementia, the obvious manifestation of neurodegeneration, is developing at a frightening pace in the absence of any concrete disease modifying treatments. While the percentage of aged persons (>60 years) will exceed 21% in 2050 compared to 10% in 2010 [
3], World Health Organization estimates that there are 50 million dementia cases currently in the world with an annual increase rate of 10 million new cases and one new case every 3 seconds [
4]. Cardiovascular diseases are also spreading at an alarming rate in recent years [
5], causing at least 18 million deaths worldwide annually [
6]. Metabolic diseases are another well-known global epidemic. Diabetes mellitus affect more than 536 million people worldwide i.e., 10.5% of the world adult population, with an increase rate of more than 36 million persons annually [
7]. Obesity is affecting around 14% of world population (650 million) with 2 billion people being overweight according to the most recent available statistics [
8]. In addition, there are at least more than 1000 viruses known to infect humans, and some have killed hundred millions of people just in the 20th century [
9]. The last pandemic of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) infection is just a new example.
This global health conundrum, while needing urgent measures, results from an interplay of diverse circumstances. First, the understanding of diseases remains largely deficient despite the enormous advances in diagnosis technologies and many promising insights especially from new scientific fields such as biotechnologies, omics, and genetics. Pathophysiological mechanisms and environmental factors interplay as well as social, behavioral, and genetic factors’ implication in disease mechanisms are still so far from being totally understood. Second, the conception of therapeutic procedures and tools remains widely debated and controversial in many areas and medical specialties. The relatively “mechanical” approach to the human body has largely been shown to impair successful outcomes of therapeutic management and caregiving. Most of chronic conditions are managed in a symptomatic manner and medications are widely prescribed according to the patient’s complaint about its straight symptomatic feelings. Nevertheless, the human body reacts as a whole and complex entity to physiological alterations and pathological triggers and, thus, must be considered as such either in fundamental and translational research, or in clinical practice. The recently renewed interest in integrative approaches in medical practice and in public behaviors related to health maintenance is clear-cut evidence of such exigency. Third, the lack of quality and the low profitability of experimental studies are great impediments to clinically relevant advancements. An enormous number of research works are continuously published in all fields related to disease management but very frequently lead to conflicting results. Scientific reviews of hopefully new treatments in most chronic diseases frequently fail to draw clear and evidence-based conclusions and recommendations. This problem is broadly present and well known among the scientific community, especially for natural product prospection in major global health burdens, such as cancers and neurodegeneration.
Optimistically speaking, the current situation of pharmaceutical research, especially in experimental and preclinical phases, borrows undeniable advantages that give us confidence in a more propitious future to overcome the most challenging global health problems. The amount of deployed material resources and human effort in natural product research is huge and remains unfortunately not traduced by a similar amount of success, neither in translational research nor in clinical relevance. The current easiness of collaboration and coordinated transnational works is unprecedented. Scientists can take this opportunity to bypass old hurdles and conduct collective works via a participatory approach that can save a lot of time and resources. In addition, modern technologies such as artificial intelligence, high throughput techniques, data analysis tools, and many new techniques for experimental and preclinical studies open outstanding perspectives and possibilities for bioprospecting and drug discovery.
Bioprospection is taking advantage of a more opened world and easy moving of persons, knowledge, and resources to dig in an endless reservoir of natural products that are particularly promising in many complicated pathophysiological processes by delivering multiple actions via multiple targets as we will see for BP. Plants and their endophytes, for example, are an endless source of phytochemicals that are extremely diverse in occurrence and in pharmacological interest. At global scale, about 374.000 plant species are known to humanity and only 6% of them had been investigated for their pharmacological interest, considering that phytochemicals constitute about 70% of all known natural products [
10]. Correspondingly, this provides an extremely huge stockpile of bee derivatives, including BP, that can originate from some of these plants and be used for nutritional, preventive and curative purposes in humans and animals. We will hereinafter see detailed examples concerning BP, but these observations are assuredly true for other bee products.
BP is an extremely variable product from biological, chemical, and pharmacological points of view. As we will detail throughout this work, recent studies have shown that BP is implicated in many biochemical and physiological processes related to common disorders including neurodegenerative, cancerous, inflammatory, cardiovascular, and metabolic ones. This product is becoming widely promoted and consumed as a nutritional supplement and functional food due to its richness in valuable nutrients and growing data about its role in ameliorating several physiological and pathological conditions. Its composition can largely vary depending on its botanical origins even at the subspecies levels, and generally reflects a mixture of these origins in the same multifloral BP sample [
11]. Moreover, such composition may vary depending on the bee colony, even in the same apiary, and also depending on the hour, the weekday or other timing periods [
12]. It also varies depending on environmental conditions and geographical origin [
13]. All these conditioning factors make BP a very rich cocktail and distinguish it from other bee products.
Phenolic profile of BP, one of the most impacting factors in its biological activities, may vary widely depending on floral origin and harvesting season, thus leading not only to marked variation of nutritional and pharmacological activities, but also to physiological changes in nurse bees as well as to possible effects on the whole bee colony [
14]. It is noteworthy to mention that such variations remain insufficiently studied and poorly understood, showing that chemical fingerprinting is more accurate and relevant to identify and study BP at pharmacological level [
14].
2. Phytochemical Overview of Bee Pollen
In addition to the vast array of nutrients that are present in BP and that we have already detailed in a recent publication [
15], this matrix contains a myriad of other phytochemicals manifesting a wide range of biological activities. BP is particularly rich in phenolic compounds, mainly flavonoids and phenolic acids, and carotenoids [
15,
16,
17], but also in widespread and recently identified compounds such as phenolamides, betaines, and others [
18,
19,
20]. Phenolic acids and flavonoids are most likely responsible for the major part of antioxidant properties of BP [
21]. Indeed, several neurodegeneration- and cancer-related bioactivities of BP were reported by studies from different regions and environmental conditions worldwide. It has been especially reported to exert exceptionally marked antioxidant and anti-inflammatory activities [
15,
22,
23], as well as anti-cancer (anticarcinogenic and anti-mutagenic) [
24,
25], immunomodulatory and immunostimulant activities [
26,
27]. BP have also been reported to possess preventive and ameliorative effects on neurodegeneration [
28,
29], overall aging process [
18,
25], and cell death [
30], as well as to promote recovery from chronic diseases and to have chemo-preventive properties [
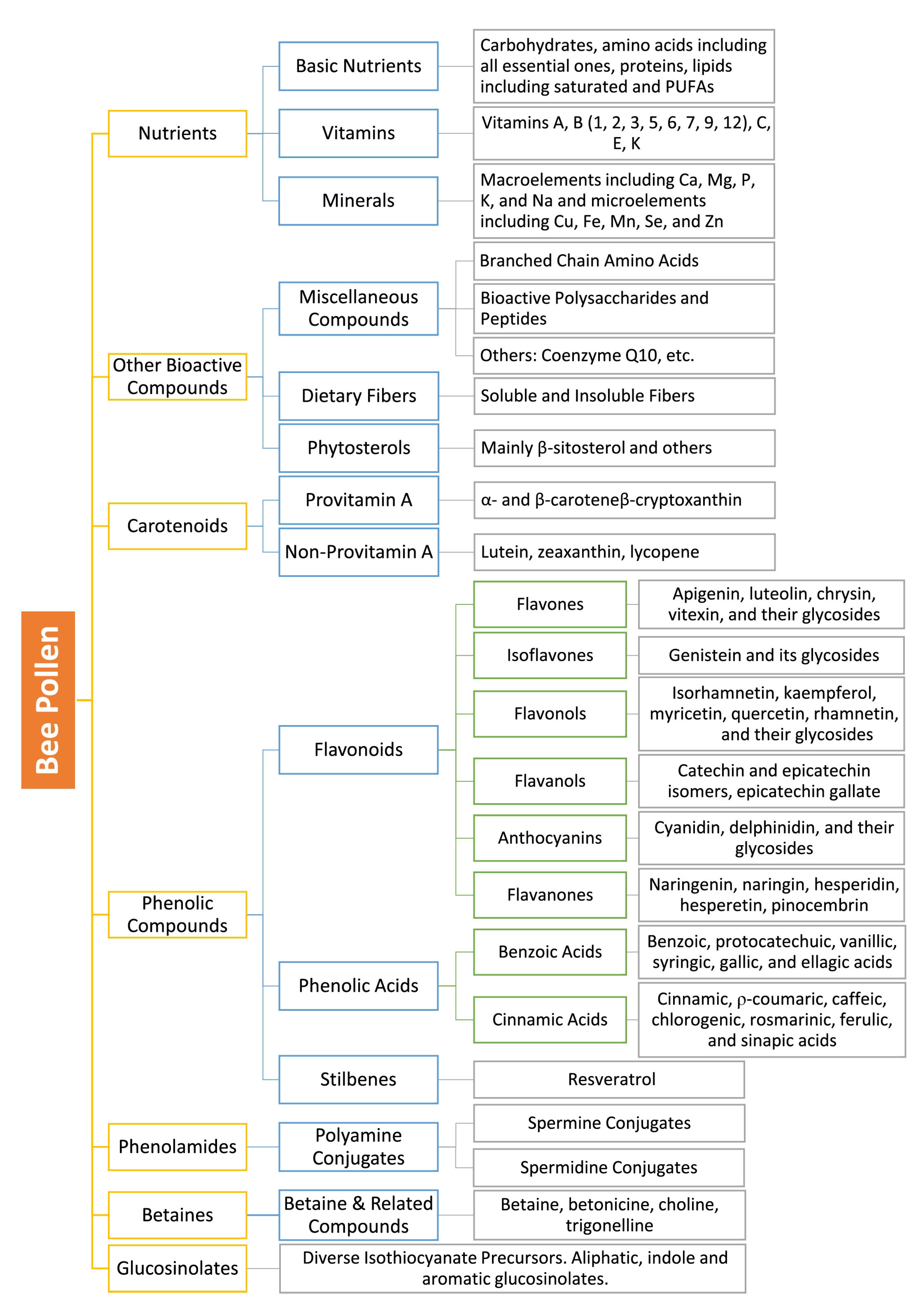
31]. These properties, added to its very high nutritional value, endow BP with many interesting and complementary bioactivities that may be of great usefulness in complex and multifactorial diseases such as cancers and NDD. During our current review, we will discuss many key molecules and chemical families in BP that are highly relevant to cancer and neurodegeneration. A summary of these families and their amounts that have been reported in BP so far are presented in
Table 1. In addition, numerous vital micronutrients have been highlighted for their potential interest in cancer and neurodegeneration in our current review (see our previous publication for details about their presence in BP [
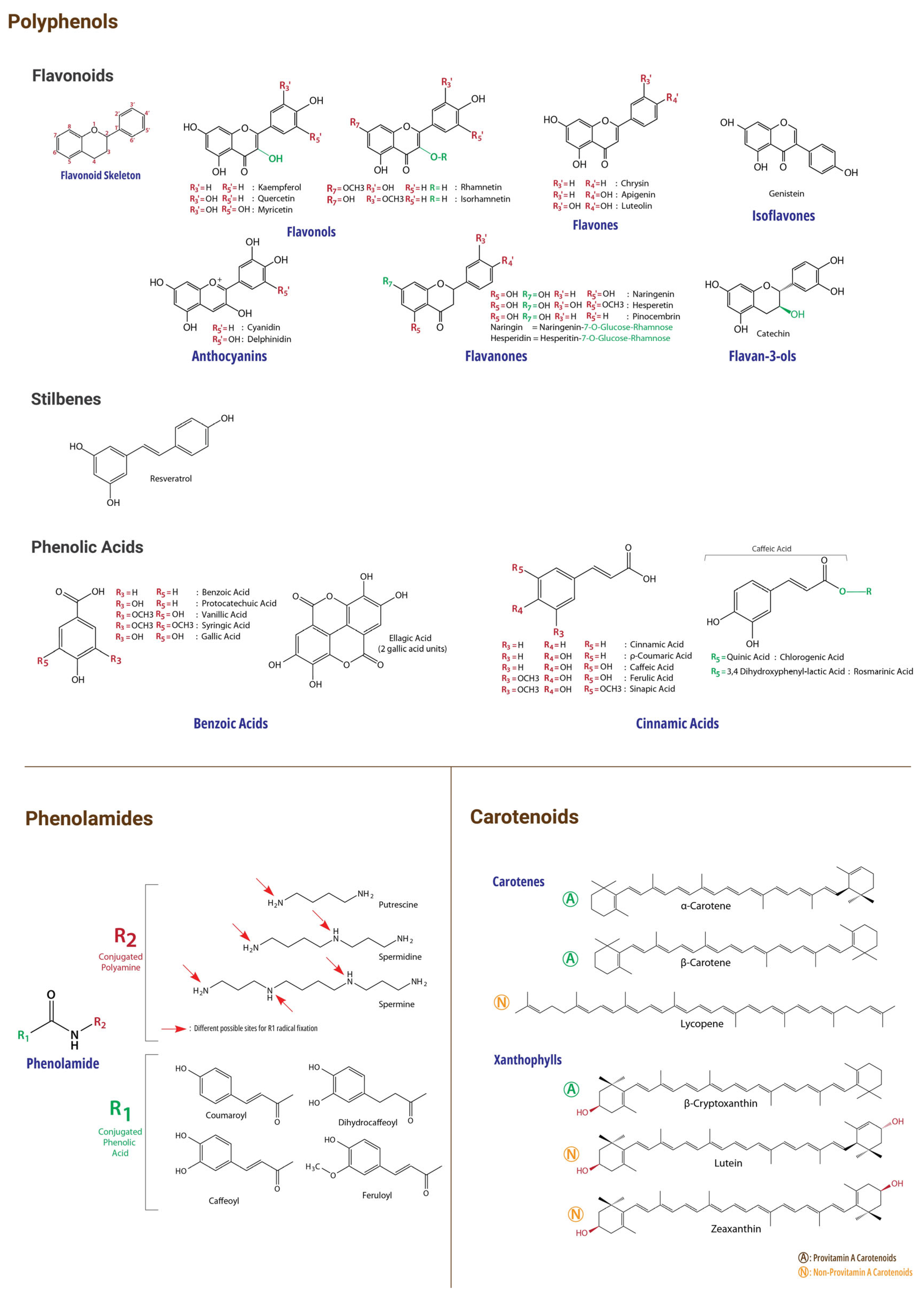
15]). For illustrative purposes, chemical structures and chemical classification of the main bioactive BP compounds are presented in
Figure 1 and
Figure 2.
We will first briefly underline some BP compounds that we will frequently invoke during this work for their relevant bioactivities, and which are not righteously underlined in many BP-related studies. Carotenoids, for example, are ubiquitous BP compounds (see
Table 1 and Table 2 [
32,
33,
34]). Some members of this family act as vitamin A sources in human organism and are referred to as provitamin A carotenoids, with β-carotene being the main representative, and other precursors such as α-carotene and β-cryptoxanthin [
35] which are also found at high levels in some BPs. Non-provitamin A carotenoids include lycopene, lutein, and zeaxanthin [
35] and are also frequent in BP. Lycopene is not only a “tomato mark”. BP has also been reported to contain important amounts of this well-studied carotenoid. A recent study reported that lycopene content in fresh and dried tomato was 25.4-33.5 and 701-1181 mg/kg respectively depending on the harvest period [
36]. Lycopene was found to be present at substantial amounts in some BPs (31.82, 49.67 and 59.18 mg/kg in
Erica spp.
Castanea sativa and a multifloral BPs respectively [
37]).
Phenolamides are a new class of compounds reported in BPs. They belong to a family of phenolic derivatives formed by the combination of phenolic acids and primary amines (mono- or poly-amines), that are widely present in plants and more abundant in flowers and pollen grains [
38]. Due to their unveiled abundance in BPs, these phytochemicals will markedly increase the value of pollen as functional food and pharmacological pool. A study of 20 BP samples reported that phenolamides percentages exceeded 1% of the total weight in 11 BP samples, and even reached 2.8% in pear and 3.9% in rose BPs [
18]. Another recent study reported that content in phenolamides ranged from 23.1 to 25.6 mg/g in
Castanea sp. and
Rubus sp. monofloral BPs respectively [
39]. These amounts greatly exceeded those of flavonoid compounds in the studied samples. Phenolamides present a great diversity in their structures, but their bioactivities remain largely unknown in human organism, while antioxidant, anti-inflammatory, neuroprotective, antiallergic, anti-obesity, anti-atherosclerotic, antimicrobial and antiproliferative activities being the main reported properties from
in vitro and
in vivo studies [
38,
40].
Spermidine is a polyamine which exist in all living cells and is provided to the human organism by three sources viz. endogenous biosynthesis, food intake, and gut microbial activity [
41]. This molecule has been aberrantly marketed as a “revolutionary” lifespan prolonger and neurodegeneration “counteragent”, but “appearances are often deceiving” as Aesop said centuries ago. Spermidine was reported by many studies to extend lifespan in diverse living organisms, and verified to exert many marked activities which are tightly related to neurodegeneration animal models and human cell lines [
41,
42,
43,
44,
45]. In clinical trials and other studies in humans, spermidine has also been frequently reported to exert similar effects [
46,
47,
48]. Conversely, a recent clinical trial in 100 human participants with subjective cognitive decline reported no difference of 12 month supplementation of spermidine with placebo on memory performance [
49]. Other more appealing results about spermidine safety and dosage have been reported by experimental and clinical studies. As an example, high doses of spermidine were shown to induce oxidative stress and toxic aldehyde production, leading possibly to cell death in retinal cell cultures, and to damage retina structure and function in animals [
50]. Another recent population-based study reported that high spermidine plasma levels were associated with pronounced brain aging and served as markers of the onset of Alzheimer’s disease following a mild cognitive impairment [
46]. These examples must drive a cautious attitude toward any translation of experimental data, especially in complex and ununderstood disorders.
Polyamines, especially spermidine, have also been widely flaunted for their possible anticancer potential but special attention should be drawn to these compounds and further studies are undeniably needed. As we have seen for spermidine in neurodegeneration, excessive levels of polyamines have been reported to exert some prooncogenic roles as these molecules are necessary for the viability of both normal and malignant cells, considering however that their interplay in tumor microenvironment and progression are just beginning to be understood and that the targeting of their metabolic pathways are showing promising outcomes in some cancers [
51,
52,
53,
54,
55].
Betaines have also been recently isolated in large quantities from BPs. A study of BPs from
Brassica napus, Cytisus sp.,
Papaver sp.,
Quercus sp.,
Reseda sp.,
Retama sp.,
Rosa sp.,
Rubus sp.,
Teucrium sp., and
Vicia sativa reported that betaines were present in all of them and that the concentration of some of the most known betaine in these BPs ranged were present in notable amounts 7–4910, 264–52,834, 12–3628, and 13–723 mg/kg BP dry weight for betaine, betonicine, trigonelline, and choline respectively [
20]. As we have seen for phenolamides, these amounts may also exceed those of many flavonoids and other phenolics that were reported in numerous BP samples (see
Table 1, Table 2 and Table 3 for other comparisons). In addition to their anti-inflammatory and antioxidant effects in diverse anatomical locations, betaines, especially betaine and/or choline, were reported to drive a wide range of metabolic and cardiovascular benefits [
56,
57]. Betaine may also exert some neuroprotective, neurodevelopmental, anti-neurodegenerative (e.g., ameliorate brain redox homeostasis, excitatory/inhibitory balance, and dendritic and other transmission alterations, and preserve neuronal structure) [
56,
58,
59] and anticancer (reduces experimentally-induced tumorigenesis and malignant cell proliferation and possibly linked to some cancer risk reduction in humans) effects [
57,
60,
61,
62,
63,
64].
Glucosinolates have also been recently reported to be present in some BPs at substantial amounts and have even been proposed as a reliable differentiating biomarker of BP origin [
65,
66]. These compounds and some of their derivatives are known to encompass an important anticancer potential [
67,
68] in addition to, perhaps less studied but encouraging, potential against NDD [
69,
70]. However, due to the rarity of the studies that cover their amounts in BP, we will not succinctly review them in our current work.
Anthocyanidins and their glycosides (anthocyanins) are also widely reported flavonoids in BP. Cyanidin, delphinidin, and their 3-glycosides are among the main representants of these two subgroups that are frequently reported in BP [
16,
71]. Notably, these flavonoids subgroups are widely known for their very diverse bioactivities against cancer [
72,
73,
74] and neurodegeneration [
75,
76,
77].
Some uncommon but interesting compounds were also reported in BP. Genistein, the soybean “mark”, is also the major isoflavone present in BP [
78]. Genistein is widely known to exert a pleiotropic series of biological activities that are tightly linked to neurodegeneration and cancer as we will see throughout this work. Resveratrol is also a pleiotropic phenolic compound that is abundant in many vegetables and is widely studied and marketed as a valuable ingredient to tackle aging and age-related pathophysiological mechanisms. Resveratrol was reported at widely different concentrations in BP samples from distant geographical locations and diverse botanical origins [
79,
80,
81,
82,
83].
BP is also rich in dietary fibers although widely variable percentages have been reported in the literature. Recent studies reported percentages from 13 to 22% in most cases, and more rarely 3-10% or 23-31% ranges [
71,
84]. Old studies reported that dietary fibers were present in BP at percentages ranging from 0.3 to 20% [
85].Some rare studies reported low percentages of dietary fibers in some BPs [
71], but this may also be due to methodological issues. In fact, BP-covering layers are mostly composed by polysaccharides including sporopollenin in the exine and cellulose and pectin in the intine [
15,
86], and a considerable amount of fibers will thus be surely present at least due to these polysaccharides. Different extraction media may also obviously result in different dietary fiber yield from the same BP [
87].
A mineral that is of a particular relevance to carcinogenesis and neurodegeneration pathophysiology is selenium as we will see. This vital microelement is present in high quantities in many BPs. Surprisingly, ratios up to 3 mg/kg have been reported by a study in Jordanian BP [
88] and up to 5 mg/kg in Turkish BP samples [
89]. These samples, if the reported results are valid and reproducible, will make these BPs extremely rich sources of selenium. The recommended dietary intake of this vital element will then be largely covered with small quantities of BP. In fact, an expert panel requested by the European Commission have just published an opinion and stated that 255 µg/day should be considered as the tolerable upper intake level of Selenium [
90].
Studies evaluating phenolic content of BP generally report their results with reference to a simple and well-known phenolic compound. The most frequently used measure to assess phenolic function-related potential is the milligram of gallic acid equivalent which is generally expressed in a gram of BP sample (mg GAE/g). However, a standardization problem arises frequently in experimental studies as some of them report the “mg GAE/g” value by reference to a gram of the used extract. Some other studies also report this concentration value by reference to the gram of fresh BP. Although most of studies that we have reviewed reported the “mg GAE/g” value by reference to a gram of raw dry BP, these discrepancies alter the reported results and harden analytical and comparative reviews. The same observation is also correct for total flavonoid content. The latter is generally evaluated in mg of quercetin equivalent (mg QE) in a gram or kilogram of the studied sample. Some rare studies also report their results in mg rutin equivalent. Total phenolic acids are generally not evaluated due to the lack of standard evaluating method. These assessment methods do not give a clear idea about quantitative composition in the weight of phenolic compounds but permit to have a good general estimation about the possible potential in bioactivities that are related to phenolic compounds. As a rough overview, the total phenolic content in a gram of raw dry BP ranged habitually around 10-40 mg GAE/g in the studies that have been published in the last five years [
29,
32,
39,
71,
91,
92,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102,
103,
104,
105,
106,
107]. A minimal number of samples were reported to bear phenolic amounts significantly outside this range. The phenolic content in extracts was obviously higher. Amounts up to 173.52 mg GAE/g have been reported in an extract of a Turkish multifloral BP collected by
Apis mellifera [
102]. Chinese monofloral BP from
Schisandra chinensis was also reported to have a phenolic content of 101.83 mg GAE/g [
103]. Monofloral
Nelumbo nucifera BP from China was among the poorest BPs in phenolic compounds (0.37 mg GAE/g) [
104]. All these examples are from studies that have been published in the last five years. In older studies, total phenolic content of BP from around the world was reported in recent reviews [
32] and [
71], and ranged between 0.69 and 213.20 mg GAE/g in BPs from different botanical and bee species. In these two reviews, total flavonoid content ranged between 0.9 and 77.88 mg QE/g in [
32] and between 0.1-107.00 mg QE/g in [
71]. These studies did not screen findings based on the matrix in which results were expressed, i.e. BP extract, dry BP, and fresh BP. This is a frequent problem in experimental investigations and may explain the large variation intervals in reported results. Some of the reviewed studies also expressed results in rutin or catechin equivalent.
We have chosen to investigate a series of the main reported compounds in BP as examples for diverse bioactivities that are tightly associated to cancer and neurodegeneration pathogenesis and therapeutic targeting. As examples of the most reported flavonoids in BP [
18,
29,
32,
71,
93,
96,
102,
106,
108,
109,
110,
111], we chose apigenin, catechin, chrysin, cyanidin, delphinidin, epicatechin, genistein, hesperidin, hesperetin, isorhamnetin, kaempferol, luteolin, myricetin, naringenin, naringin, pinocembrin, quercetin and and respective glycosides. The main reported phenolic acids in BP [
29,
32,
71,
96,
102,
106,
108,
111] and which we chose as examples to study were benzoic, caffeic, chlorogenic, cinnamic, coumaric, dihydroxybenzoic, ellagic, ferulic, gallic, hydroxycinnamic, protocatechuic, rosmarinic, syringic and vanillic acids. Although less frequently reported, we chose resveratrol as a relevant example of stilbene derivatives. The most frequent BP carotenoids [
16,
17,
32,
112,
113] that we chose as study examples were α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene. Other BP ubiquitous compounds that we studied as examples were spermidine and its glycosides among phenolamides, betaine and choline as relevant betaines, and glucosinolates which we always reviewed as a chemical family without specific examples. Coenzyme Q10 as a BP compound with very relevant importance to the studied diseases was also reviewed. Among BP nutrients, we mainly focused on all vitamins, and on copper, iron, selenium and zinc as very relevant elements to neurodegeneration and cancer, in addition to phytosterols (mainly β-sitosterol as a major representative in BP [
27,
71,
114]).
Table 1.
Main Chemical Families and Their Reported Abundance in BP (1).
Table 1.
Main Chemical Families and Their Reported Abundance in BP (1).
| Chemical (Sub)Family |
Evaluated Matrix |
Concentration Value or Range |
BP Botanical Origin |
Country of Origin |
References |
| Polyphenols (2)
|
Raw Dry BP |
20-30 mg GAE/g |
Numerous monofloral and multifloral BPs |
Many from Asia & Europe |
[91,92,93,105,106,107] |
| 30-40 mg GAE/g |
Two multifloral BPs |
Türkiye |
[105] |
| ND |
Poland |
[96] |
| BP from Brassica campestris
|
China |
[97] |
| 40-50 mg GAE/g |
Multifloral or monofloral from Castanea sativa
|
Türkiye |
[105] |
| Fresh BP |
20-30 mg GAE/g |
Monofloral from Castanea sativa or Hedera helix
|
Italy |
[98] |
| 30-40 mg GAE/g |
BP from Castanea sp. (100%) [39] |
Portugal |
[39] |
| Flavonoids (2)
|
Raw Dry BPs |
16.13–35.04 mg QE/g |
Multifloral pot pollen from stingless bees (Tetragonula biroi) |
Philippines |
[93] |
| 31.59 mg/g |
BP from Ranunculus spp. |
Finland |
[99] |
| 11.03-18.81 mg QE/g |
Monofloral and Multifloral BPs. |
Romania |
[29] |
| 12.28-15.61 mg QE/g |
Monofloral and Multifloral BPs. |
India |
[106] |
| 3.26-11.89 mg QE/g |
Diverse multifloral BPs |
Türkiye |
[91] |
| 11.77 mg QE/g |
Multifloral sample |
Poland |
[96] |
| 11.09 mg QE/g |
BP from Clematis vitalba
|
Hungary |
[92] |
| BP Extracts |
79.21 mg QE/g |
Multifloral sample |
Türkiye |
[102] |
| 6.74-104.13 mg QE/g |
Dichloromethane partitions from diverse Monofloral BPs |
Thailand |
[101] |
| Anthocyanins (monomeric) |
BP Extract |
0.19-0.74 mg CGE/g |
Multifloral pot pollen from stingless bees (Tetragonula biroi) |
Philippines |
[93] |
| Fresh BP |
58.16 mg CGE/l (3)
|
Monofloral BP from Castanea sativa
|
Italy |
[25] |
| Dry BP |
5.16-11.57 mg CE/g (4)
|
Diverse Monofloral BPs |
Italy |
[95] |
| Raw BP |
450-800 mg/kg |
Blue BPs |
Spain |
[16] |
| Phenolic Acids |
Dry BP |
1.83-6.97mg FAE/g |
Many monofloral BPs |
Italy |
[95] |
| 15.75-41.95 mg/g (5)
|
BP from Brassica, Filipendula, Trifolium and Vicia spp. |
Finland |
[99] |
| Stilbenes |
Dry BP |
0.156 & 0.193 mg GAE/g |
BP from Trifolium spp. and Coriandrum spp. respectively |
Italy |
[95] |
| 2.49 and 3.32mg/g |
BP from Taraxacum spp. and Rubus spp. respectively |
Finland |
[99] |
| Carotenoids |
Fresh BP |
11.78 mg/kg |
Monofloral BP from Castanea sativa sp. (88.8%) |
Italy |
[25] |
| 12.78-98.62 mg/kg |
ND, from diverse geographical locations |
Türkiye |
[115] |
| Dried BP |
261.33 mg/kg |
Monofloral BP from Helianthus annuus
|
Slovakia |
[116] |
| Frozen BP |
235.17 mg/kg |
Monofloral BP from Helianthus annuus
|
| Phenolamides |
Fresh BP |
Less than 6 mg/g |
Monofloral BPs from Carduus sp., Cistaceae (100%), and Echium sp. |
Portugal |
[39] |
| 11.5-25.6 mg/g |
BPs from Rubus sp. (100%) and Castanea sp. (100%) |
| 39.02 mg/g |
Rosa chinensis BP |
China |
[18] |
| 27.58 mg/g |
Pyrus bretschneideri BP |
| 22.24 mg/g |
Prunus armeniaca BP |
| 19.66 mg/g |
Crataegus bretschneideri BP |
| More than 15 mg/g |
Monofloral BPs from Brassica rapa, Helianthus annuus, Actinidia arguta, and Prunus salicina. |
| Dry BP |
38.7 mg/g |
Bi-floral BP: Crepis capillaris (59.84%) and Plantago sp. (20.14%) |
Portugal |
[110] |
| 16.09 mg/g |
Monofloral BPs from Ononis spinosa and Astralagus sp. (˃90%) |
Morocco |
[117] |
| Glucosinolates |
Dried BP |
Up to 1065 mg/kg (6)
|
Brassica spp. BPs were the richest ones |
Spain |
[66] |
| Phytosterols |
Dried BP |
3.153–6.863 mg/g (7) |
Multifloral pot pollen from stingless bees (Tetragonula biroi) |
Philippines |
[93] |
| Lyophilized BP |
9.46 mg/g |
Sorbus aucuparia |
All BP samples were collected by the bumblebee Bombus terrestris
|
|
[24] |
| 7.36 mg/g |
Calluna vulgaris |
| 5.33 mg/g |
Salix caprea |
| 2.47 mg/g |
Cistus sp. |
| 2.46 mg/g |
Cytisus scoparius |
| Betaines |
Raw BP |
5432-10104 mg/kg |
71 mono- and multi-floral BP samples |
Spain |
[20] |
Figure 1.
Phytochemical Classification of BP Bioactive Compounds.
Figure 1.
Phytochemical Classification of BP Bioactive Compounds.
Notes:
Figure 2.
Chemical Structures of Reviewed BP Bioactive Compounds.
Figure 2.
Chemical Structures of Reviewed BP Bioactive Compounds.
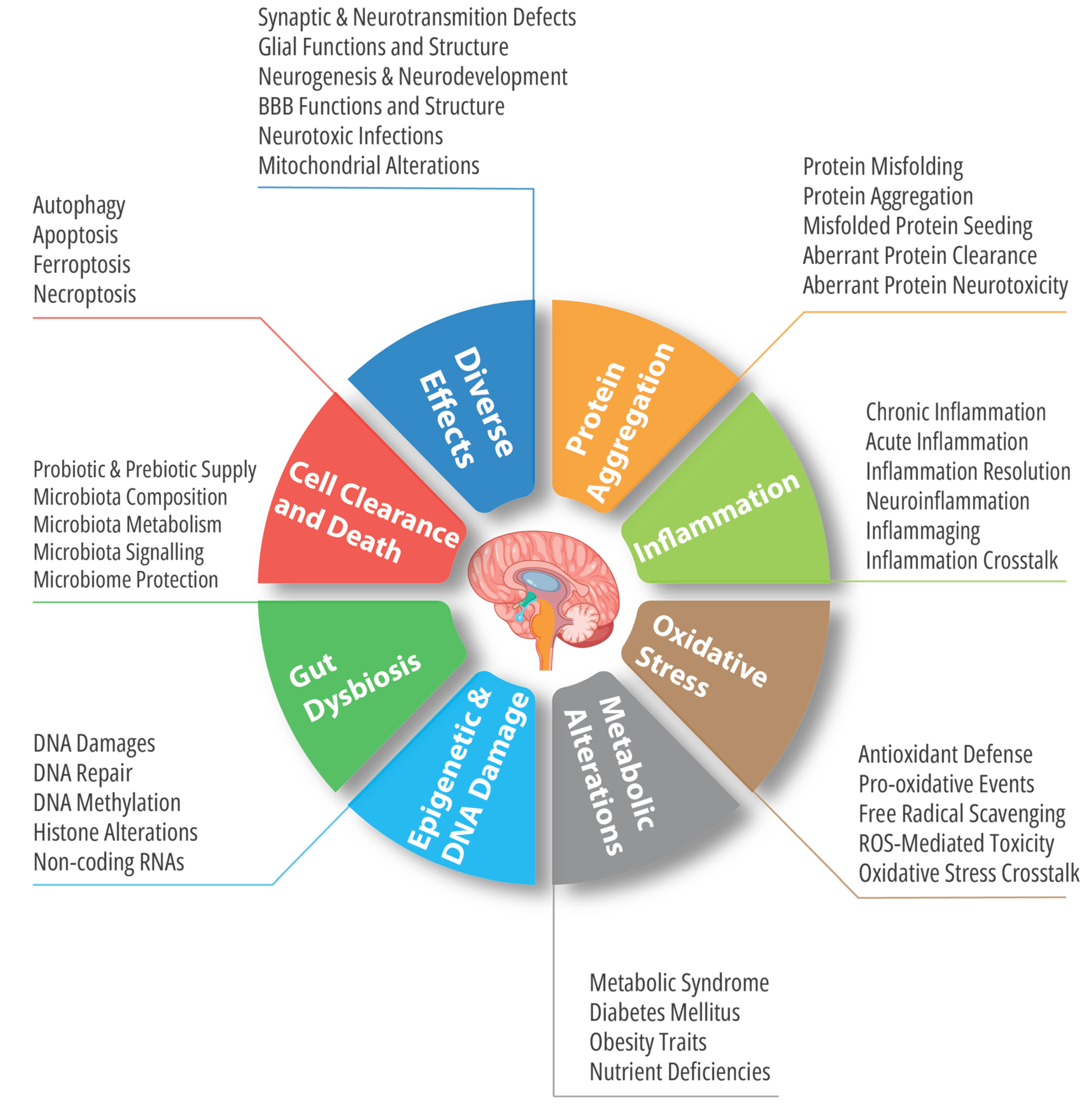
3. Shared Alterations in Neurodegeneration and Cancer
Many pathophysiological traits are ubiquitously marking both neurodegeneration and cancer. Some of them are also widely known to be present in other diseases such as cardiovascular and metabolic diseases. Among these shared mechanisms, we will highlight the most importantly investigated ones, i.e., oxidative stress, chronic inflammation, imbalances of cell death and clearance mechanisms, functional and compositional alterations of microbiota, and aberrant epigenetic signaling.
3.1. Oxidative Stress
Antioxidant activity is one of the most studied properties of BP [
110]. The latter has been proven by a great number of studies [
15,
120] to be a powerful antioxidant with a synergy of numerous antioxidant compounds that it gathers, and which are represented mainly by phenolic compounds, but include also diverse other phytochemicals. Antioxidative properties that are exerted by BP are evidently present in parallel and complementary ways with its marked anti-inflammatory effect, but also participate in the latter [
121]. Obviously, antioxidant activities, although generally present regardless of how and where studies were conducted, vary widely depending on the bee and plant species, and geographical, soil, timing and environmental conditions [
120,
122]. Many experimental and preclinical studies have been published in this context. A review of BP antioxidant studies and unveiled mechanisms can be found in our recent publication [
15].
Miscellaneous animal studies have reported that BP dietary supplementation resulted in a significantly higher activation of antioxidant and prophylactic mechanisms in different organs [
123,
124,
125], as well as in ameliorating different pathogenic biochemical, hematological, toxicological, and inflammatory parameters at systemic level in animals [
126,
127,
128]. Hence, BP has a universal and marked antioxidant potential by acting on major known redox pathways via a spectrum of pleiotropic mechanisms including radical scavenging, activity modulation of redox enzymes, metal reduction reactions, and cell protection and functional improvement [
15]. Although variating depending on their types, BP extracts encompassed antioxidant activities regardless of the used solvent.
Facing such variability, extraction methods will evidently be crucial in determining the antioxidant activity of BP. While ethanolic extracts was repeatedly reported to be more active than other extracts after comparing numerous samples of multifloral BP [
122,
129], methanolic extract was also reported to be more active than water extract of
Tilia BP for example [
120]. A recent comprehensive review concluded that 70% aqueous ethanol extraction using the agitation extraction without pulverizing the BP extracts provided the optimal condition to maximize antioxidant principles in these extracts [
129].
Antioxidant activity of BP has been shown by many studies to depend not only on botanical origin, but also on the geographical origin for the similar botanical composition elucidated by palynological studies [
94]. A comparative evaluation review has recently concluded that multifloral pollen showed a higher antioxidant activity, evaluated by radical scavenging and metal reducing potentials, and a richer phenolic composition [
91]. The authors of this study also recommended that prior chemical analysis and bioactivity tests would be a better parameter than palynological analysis to assess the value of BP.
3.2. Inflammation
Chronic inflammation is a key factor in aging and is sustained and triggered in an auto-maintained loop in aging persons, especially in immunosenescent ones and those hosting pathophysiological events linked to neurodegeneration and cancer [
130,
131]. In particular, chronic low-grade inflammation has been acknowledged as a major pathophysiological event and bidirectional crosstalk effector in neurodegeneration [
132,
133] and cancers including carcinogenesis and metastasis phases [
134,
135].
A wide range of basic and preclinical studies have reported diverse and interesting activities of BP against acute and chronic inflammation. BP is particularly promising in tackling chronic low-grade inflammation due to its richness in anti-inflammatory compounds, its high safety and long history of human use and acceptance [
15]. Indeed, BP has been reported to act on main pathophysiological mechanisms of chronic inflammation. This has been experimentally illustrated by the downregulation of pro-inflammatory cytokines, diverse inflammatory enzymes, inflammation-mediating protein complexes (e.g., nucleotide oligomerization domain-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome), inflammatory cell flux and activation, and major inflammatory signaling pathways, i.e., nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK) and nuclear factor-erythroid 2-related factor 2 (Nrf2) pathways. A comprehensive review of the most recent of these reports was done in our recent publication [
15].
Inhibiting inflammatory process initiation and execution may not be the sole necessary intervention to limit inflammation’s deleterious effects. Inflammation resolution is also a vital phase to abolish these effects in the body and restore tissular and inflammatory response homeostasis, and is thus recently becoming a novel therapeutic target in chronic inflammatory diseases [
136,
137]. Its mechanisms, although remaining not well understood, appear to rely on specialized lipidic pro-resolving mediators (SPMs, which are all derived from omega-3 and omega-6 fatty acids), and other molecules and immune cells, and culminate in regenerating intact endothelial, vascular and tissular states [
136,
137]. The failure of these critical processes is likely a key driver behind the settlement of chronic inflammation and resulting chronic diseases, including cancer [
138] and NDD [
139].
Many natural compounds were reported to accelerate inflammation resolution including some BP compounds. The main SPMs source molecules, viz. omega-3 fatty acids, are present in BP as we have seen. In addition, anthocyanins, some of the most ubiquitous polyphenolic components of BP from different bee species [
16,
25,
93,
140], were reported to be important promoters of inflammation resolution [
141,
142]. Note that anthocyanins are also well studied for their antioxidant potential and interesting inhibitory effects on chronic low-grade inflammation mechanisms [
143].
Polarizing macrophages to the M2 phenotype is known to exert an anti-inflammatory action and enhance inflammation resolution [
144]. Inducing such polarization was evidenced for diverse BP compounds such as kaempferol [
145], luteolin [
146], resveratrol [
147], and other phenolics [
148]. Another important observation to keep in mind, but to verify by further studies, is the fact that BP appears to possess more marked effects against chronic inflammation than other bee products, which may be another advantageous argument deeming its possible use. A recent study compared the effects of BP, bee bread, honey, propolis and royal jelly on chronic inflammation rodent models and found that BP and bee bread were more potent than other bee products [
149]. From these preliminary results and considering the substantial evidence on the potential of BP against oxidative stress and inflammation, BP ability to promote inflammation resolution and suppress chronic inflammation settlement appears to be promising research avenue in preventing and managing pathophysiological events in neurodegeneration and cancers.
3.3. Imbalances in Cell Death and Clearance
3.3.1. Autophagy Modulation
Autophagy is a cellular “quality-control” process encompassing metabolic and innate-immune pathways that culminate in the degradation of hazardous and dysfunctional material present in the cytoplasm [
150]. Autophagy should not be easily appraised as it is very frequently described in aging and neurodegeneration studies, where a simplistic conception stipulates that impaired autophagy is detrimental and enhanced autophagy is beneficial for aging and neuro-performances. A complex relation between autophagy, aging and neurodegeneration pathophysiology is revealed by research and the exact relations and interplays remain unclear [
151]. Similarly, in cancers, numerous studies roughly revealed that autophagy prevents neoplastic transformation of cells and preserve their genomic integrity, but growing data reveal that the impact of autophagy in cancer pathophysiology depend on a large plethora of factors, and that autophagy may also increase neoplastic cell performances, tumor relapse and resistance to treatments [
152].
The correcting potential of BP on autophagy processes remains very poorly studied and only a few experimental studies investigated it. A study in lipopolysaccharide-intoxicated rats recently reported that a one-month feeding with BP resulted in a dramatical decrease of C/EBP homologous protein (CHOP) gene expression [
153]. CHOP is known to be a key transcription factor in inducing stress-related autophagy with a particular impact in neurodegeneration process [
154]. Unfortunately, the authors used a commercial BP and did not mention its botanical origin. Accordingly, a pectic polysaccharide fraction isolated from
Rosa rugosa BP was tested in mice submitted to a high fat diet and was reported to promote autophagy by enhancing adenosine-monophosphate activated-protein kinase (AMPK) activity and suppressing Mammalian target of rapamycin (mTOR) activity in liver tissues [
155]. AMPK and mTOR were classically considered as master regulators of metabolism and autophagy [
156], but recent data appear to “subvert” the classical conception that ruled during the last decade. An intricate and even possibly an inhibitory role of AMPK in autophagy were reported by many recent studies [
157]. In contrast, deprivation of nurse bees from pollen leads to an increased expression of the autophagy-related (ATG) genes [
158] which is a series of genes mainly orchestrating macro-autophagy but also appearing to be involved in other known forms of autophagy, namely directly in some types of micro-autophagy [
159]. Many ATG genes have recently been identified as causative or risk factors of neurodegenerative, cardiovascular and metabolic diseases, a relationship which is still by far less clear in cancer [
152,
159].
To sum up, the limited number of available studies propose that BP has an intricate role in autophagy. Possible relations of numerous action mechanisms of its known components and the resulting effect of the whole product remain to be elucidated. The exact regulating mechanisms and sensors in autophagy regulation also remain not perfectly clear, a fact that unfortunately further complicates the design and conduction of preliminary experimental studies. When they were studied as pure molecules, many BP components were reported to modulate autophagy and to result in great benefits in some hurdling diseases such as NDD and cancers. However, how these molecules will behave when ingested in BP or in BP-based preparations, and how they will interact with each other when present in the same BP remain unfortunately unknown.
Many BP compounds modulate autophagy in an intricate manner, comprising neuroprotective modulation in healthy nerve and glial cells and cell death-promoting modulation in malignant cells. Among BP compounds that have been reported to exert this duality in autophagic regulation, we cite naringenin [
160], apigenin [
161], luteolin [
162], hesperetin [
163], kaempferol [
164], quercetin [
165], cyanidin-3-glycoside [
166], chrysin [
167], pinocembrin [
168], protocatechuic acid [
169], ellagic acid [
170], and spermidine [
171]. Myriad other studies have been published on this duality and could not be reviewed here.
3.3.2. Apoptosis Modulation
Contrariwise to what have been long thought in biomedical research, understanding and appraisal of cell death is subjected to a deep revolution in the very recent years. It has particularly been demonstrated that a clear-cut distinction cannot be made between cell death mechanisms because of their frequent overlap and mutual regulation, and the emergence of formerly unknown or poorly understood mechanisms [
172,
173]. Accordingly, we will see that BP acts on diverse pathways involved in this overlapping. The understanding of this action should therefore be with a novel holistic approach which will certainly be advantageous in bioprospection, translational research, and disease management, either in neurodegeneration where cell death is the main disease fatal outcome which unfortunately remains neither understood nor stoppable, or in cancer where resistance to cell death remains the biggest hurdle in targeting malignant cells.
B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) is the key initiator of intrinsic (mitochondrial) pathway of apoptosis by being translocated from the cytosol to the mitochondrial outer membrane, and then “spark” a quasi-non-returning point toward cell death by creating mitochondrial permeabilization pores and oligomerizing [
174,
175]. Targeting Bax and other key apoptosis effectors from Bcl-2 family was proposed as a feasible approach to considerably overcome cancer cell resistance to apoptosis [
176]. Bax is also activated, jointly with caspase cascade, by the accumulation of neurotoxic misfolded proteins such as Aβ and phosphorylated Tau [
177] and was generally found to be overexpressed in many major NDD [
178]. On the other hand, tumor protein 53 (p53) is a central effector in many stress-related molecular cascades by mainly acting as tumor suppressor [
179]. When muted, p53 mediates the generation of malignant cells that excessively proliferate despite their muted genome [
180]. Moreover, Cytochrome C generates the active form of caspase-9 which induce the effectors Caspase-3 and caspase-7 that, besides cleaving hundreds of proteins, undergo a series of cell demolishing processes to mediate both intrinsic and extrinsic pathways of apoptosis [
181,
182].
In programmed cell death, BP has been reported to intricately modulate apoptosis in cell lines and experimental models of neurodegeneration, cancers, and other diseases. Interestingly, this modulation manifested by inhibiting apoptosis in healthy cells and promoting it in malignant cells as we will see throughout some examples.
A methanolic extract of
Filipendula ulmaria BP was found to potently induce apoptosis in a dose- and exposition time-dependent ways in a murine colorectal cancer cell line [
183]. A peptide fraction of a hydrolysate from commercial BP originating mainly from
Mimosaceae was reported to promote apoptosis in a human lung cancer cell line in a timely-dependent manner (apoptosis rate passed 80% 72h after cell treatment with BP extract) [
184]. Involved mechanisms were not investigated by these two studies as the assessment of apoptotic effect was done only optically through cell counting. In a human lung cancer cell line, a polyphenolic extract of BP was reported to promote apoptosis by upregulating Bax and caspase-3 and downregulating Bcl-2 [
185].
Regarding the protective effects on healthy cells, a hydroethanolic extract of
Schisandra chinensis BP was found to inhibit cisplatin-induced apoptosis in rodent liver and kidney tissues by downregulating gene expression of apoptosis mediators Bax, p53, caspase-3, caspase-9 and cytochrome C [
186]. Accordingly, in rat models of propionic acid-induced autism, BP feeding resulted in significantly reducing caspase-3 levels in animal brains [
121]. The authors used a commercial BP and did not report its botanical origin or composition. Likewise, BP feeding was found to markedly protect rat pups against prenatal methylmercury-induced neurotoxicity. Caspase-3 inhibition was among many mechanisms that this study found to be involved in this protection [
187].
3.3.3. Ferroptosis Modulation
Ferroptosis is a newly discovered form of programmed cell death(first described in 2012), mainly triggered by an intracellular overload of iron and a deficiency in glutathione peroxidase 4 (GPX4); the former resulting in an increased reactive species production through Fenton reactions and the latter resulting from glutathione depletion [
188]. These two factors then result in an accumulation of lipid peroxides and subsequent cell death ultimately driven by membrane lipid peroxidation [
189]. Other glutathione-independent inhibitors of lipid peroxidation have been discovered and may therefore act as regulators of ferroptosis [
188,
190]. Ferroptosis has been implicated in many aging-related alterations such as cellular senescence [
191] and inflammation to which ferroptosis is bidirectionally related [
192]. It has been especially found to crucially participate in neurodegeneration pathogenesis and to inhibit malignant cell proliferation, hence being coveted as therapeutic target both through its induction in cancer cells and its inhibition in neurodegeneration [
193,
194].
Some mechanisms directly related to ferroptosis, such as reducing the levels of lipid peroxidation byproduct MDA (e.g.,
Fagopyrum esculentum,
Schisandra chinensis and other multifloral BP) [
195,
196], and increasing glutathione levels (e.g.,
Schisandra chinensis), were reported for some BPs in animal models and cell cultures [
15]; but direct assessments of anti-ferroptosis effect of BP as a whole product or an extract are still lacking.
However, A wide range of polyphenols, including many ubiquitous BP compounds, have especially been shown to exert potent ferroptosis modulation. Quercetin, apigenin, and gallic acid for example were reported to markedly reduce the viability of many cancer cell lines through ferroptosis induction, while significantly preventing this cell death form in different non-malignant neuronal lines [
197,
198,
199,
200,
201,
202]. Numerous phenolics including those present in BP have been reported to exert similar effects but could not be detailed here (see good recent reviews in [
203,
204]).
Diverse other BP compounds have been verified to exert important ferroptosis modulatory effects. Uronic acid was reported to act as ferroptosis promoter in ovarian cancer cells [
205]. This polysaccharide compound has been found at high concentrations in a
Lycium barbarum BP extract and drove antioxidant potential of the polysaccharide fraction of this extract [
206]. Carotenoids and retinol metabolites have also been reported as ferroptosis inhibitors. Lycopene inhibited chemically-induced ferroptosis in the mice hippocampus [
207]. Lutein’s metabolites in the human body, viz. 3'-epilutein and 3'-oxolutein, inhibited glutamate-induced ferroptosis in a neuronal cell line [
208]. β-carotene and vitamin A have been reported to inhibit ferroptosis and are importantly both endowed with a high potential to cross the blood-brain barrier (BBB [
209,
210]. Vitamin A and its metabolites inhibited experimentally-induced ferroptosis in neuronal and non-neuronal cells [
211].
Vitamin C was also shown to intricately intervene in ferroptosis regulation through multifaceted ways. In murine normal cells it prevented chemically-induced ferroptosis [
212], while it manifested a selective ferroptosis-mediated killing of pancreatic cancerous cells [
212]. Vitamin E is also an acknowledged endogenous ferroptosis inhibitor due to its effective lipid peroxidation-inhibitory potential [
209,
213] and this has been shown to mediate advantageous effects in some clinical and preclinical studies of NDD [
214,
215]. Selenium is another key element in ferroptosis process and regulation and is abundantly present in some BPs as we have already seen. It is a crucial component and activity determinant of selenoproteins such as GPX4 [
216]. Its supplementation upregulated GPX4 expression and increased resistance to ferroptosis in cell cultures and animal models [
216]. Multiple studies reported that selenium protects against ferroptosis in NDD [
217]. Coenzyme Q10 was recently found to play crucial roles in preventing ferroptosis mainly through regulating lipid peroxidation in plasma membrane [
218]. Other nutrients that are known as universal BP compounds are directly involved in regulating ferroptosis but are not discussed in this paper due to their very expanded and complex roles and interactions e.g.: unsaturated fatty acids and other lipidic compounds, amino acids such as cysteine, glucose and its sources, and minerals such as iron). These nutrients have been well reviewed in recent studies [
216,
218].
Zinc is perhaps the last unveiled element in ferroptosis. It has very recently been identified to be essential for ferroptosis by discovering that a zinc chelator suppressed ferroptosis, and zinc addition promoted ferroptosis, even with iron chelation [
219]. Treatment of different cancer cell lines with high levels of zinc induced ferroptosis [
220]. Zinc is also known to be deeply involved in central nervous system (CNS) functions and neurodegeneration. It was found to suppress ferroptosis in animal models of spinal cord injury [
221]. Zinc-mediated ferroptosis modulation, either through inhibiting it in neurodegeneration or selectively inducing it in cancer cells, appears therefore to be a novel therapeutic targeting avenue.
3.4. Metabolic and Nutritional Disorders
Metabolic imbalances are widely viewed to instigate or accelerate carcinogenesis and neurodegeneration, especially when chronically settled in the human body. Metabolic dysfunction, roughly defined as some or all metabolic syndrome criteria combined in one person, has been implicated in the pathogenesis of at least thirteen types of cancers and are considered responsible for at least 6% of acquired cancers worldwide [
222].
The importance of BP in preventing and managing diverse aspects of the deleterious interplays between nutrition, metabolic disorders and cancer appears to be well founded in our point of view due to the substantial amount of preclinical data that highlight BP importance in overall nutrition- and age-related diseases, as well as in their major accompanying triggers such as oxidative stress and chronic inflammation. Due to its high and relatively easy to adapt variability, low to moderate caloric value, richness in all necessary nutrients for humans, and potential safety, we can hypothesize that BP is a notable candidate to drive dietary interventions in cancer prevention and clinical management. It is also noteworthy to mention that numerous known bioactive components of BP (e.g., polyphenols), are known to intricately and advantageously modulate autophagy depending on healthy or abnormal state of cells, and to tackle oxidative stress and inflammation as we have detailed in this work and in our recent publication [
15]. Moreover, since modulating autophagy, oxidative stress and inflammation are the main highlighted mechanisms to explain benefits of caloric restriction in cancer and neurodegeneration [
223,
224,
225], the importance of BP in such context can be further deemed. Some calorie restriction mimetics have been actively studied recently and are being reported to produce caloric restriction effects without the need to cut caloric supply. These candidates include, for example, apigenin, quercetin, resveratrol and spermidine [
226,
227,
228]. Interestingly, these examples are reported to be present in many BPs and are endowed with fast-growing experimental and clinical evidence, which opens new research horizons for novel BP-based candidates. Likewise, a large body of evidence connects metabolic dysfunctions to neurodegeneration initiation and exacerbation. Caloric restrictions have been extensively reported to decelerate aging process and to tackle neurodegeneration mechanisms in animal models, with autophagy, oxidative stress and inflammation modulations being reported among major involved mechanisms [
223,
228,
229]. Given these considerations, BP appears to be a distinct candidate in dietary management of neurodegeneration, either in the early risky stages or during the disease course.
On the other hand, the possible potential of BP in preventing and holistically managing metabolic diseases was investigated by a considerable number of studies. A review of the most recent ones was done in our recent publication which can be consulted for more details [
15]. Briefly, BP either from monofloral or multifloral sources and from different geographical origins, was found to enhance digestive health and function and repair digestive tract injuries, exert numerous hepatoprotective activities and enhance liver functions, lower glycemia and possess many other antidiabetic properties, correct dyslipidemia by reducing cholesterol and/or triglyceride levels, reduce body weight, and tackle numerous obesity mechanisms [
15]. BP potential to enhance digestive microbiota and correct its dysbiosis will be discussed in the next subsection. Moreover, it is well known that at least some metabolic diseases are tightly linked to the development of neurodegeneration and cancers. Diabetes mellitus for example has been recently widely linked to AD and the latter becomes frequently called type 3 diabetes. Obesity is also directly linked to some cancer types as we have already seen. BP may therefore also be of great interest in preventing metabolic disease comorbidities, especially those that may culminate in NDD or cancers. Owing to the amount of evidence that we expose in this publication, the use of BP in patient suffering metabolic diseases may be advocated under specialized supervision and according to personalized data as a part of clinical programs to prevent secondary complications towards more deleterious diseases especially in persons being at increased risk to develop neurodegeneration and cancer. However, more solid and reliable preclinical and clinical data on this specific context are needed.
3.5. Microbiota Alterations
Human microbiota studies are emerging at an incredible rate in the very recent years due to the discovery of very complex and diverse interferences of this micro-world with human physiology, health, and diseases. The keyword “microbiota” strikingly returns more than 85.000 scientific works published during the last five years in PubMed database (consulted on December 12
th, 2023). The gut microbiome manifests a great variability and is now very well known to determine or modulate a large array of health- and disease-related aspects. This variability is linked in a great part to modulable factors of which nutritional and lifestyle ones are the most determinant [
230]. A healthy microbiota has been found to potentiate cancerous process prevention and suppression, while some infectious or pro-inflammatory strains in dysbiosis may exert many tumorigenesis- and metastasis-enhancing effects, and colonize local and distant tumors [
231,
232]. On the other hand, gut microbiota has been shown to play pivotal roles in neurogenesis, neurodevelopment, microglial homeostasis and activation, cognitive and behavioral functions, and a large plethora of other neurological processes [
233,
234]. Gut dysbiosis is tightly and bidirectionally linked to a wide range of neurodegeneration triggers such as oxidative stress, neuroinflammation, aberrant protein deposition, and other structural, biochemical and functional alterations inside the CNS [
235,
236].
BP is rich in a large plethora of nutrients and other bioactive compounds that have been widely reported to exert manifold enhancing effects on gut microbiota. An extensive number of studies unveiled important microbiota modulating effects of polyphenols, especially flavonoids, with numerous ones frequently reported in BP (see recent dedicated reviews [
237,
238]. Carotenoids are also endowed with substantial evidence supporting their benefits on gut microbiota via different mechanisms such as promoting beneficial strains and reducing harmful ones, suppressing pro-inflammatory signaling, mitigating gut alterations, and ameliorating intestinal wall structure and functions [
239,
240]. In addition, other natural compounds that are present in BP, viz. phytosterols, glucosinolates, and betaines were also repeatedly described for their ameliorating effects on microbiota in animals and humans [
56,
67,
241,
242].
Vitamins and minerals, which are present in considerable amounts in BP [
15], have a great and complex impact on microbiota. Vitamin A indirectly enhances microbiota diversity and functions by promoting mucins production [
243]. B vitamins are essential for gut microbiota growth and metabolism [
244]. They exert diverse modulatory effects on this microbiota and its surrounding environment, while microbiota also exerts a very significant influence on vitamin B metabolism and functions [
244]. Vitamin E can regulate gut microbiota directly and indirectly, while gut microbiota may have a great influence on vitamin E metabolism and fate [
245,
246]. Studies in humans reported that vitamin C enhanced microbiota diversity, evenness, and beneficial strains [
245,
247]. Microbiota is involved in the liberation from food matrix and digestive absorption of vital minerals, while many of these elements are known to enhance microbiota diversity, prevent gut dysbiosis and/or regulate microbiota metabolism [
248,
249].
Carbohydrates, the most abundant BP compounds, exert diverse effects on microbiota depending on their structural differences (for recent comprehensive reviews of these effects, see [
250,
251]). BP may also be a rich source of dietary fibers as we have explained. With a good fiber supply, BP can modulate gut microbiota via diverse mechanisms, that have been verified by experimental studies [
87,
108], such as enhancing SCFA production, promoting some advantageous strains, and ameliorating microbiota diversity and
Firmicutes/
Proteobacteria ratio. The latter have been considered as an indicator of gut microbiota health and a biomarker of proneness to develop many diseases such as metabolic disorders, cancers and some NDD including dementia [
252,
253]. Clinical studies that assessed the effects of prebiotic dietary fiber-based microbiota modulation on some cancers (e.g., melanoma) and NDD (e.g., autism) reported generally that observed effects were rapid and reversible, which suggests that long course of these interventions may be more helpful [
254,
255]. Due to its composition (own microbiome, prebiotic fibers, micronutrients, etc.) and safety, BP endows a great potential to mimic such interventions.
In addition, BP bears a valuable microbiome comprising mainly
Lactobacillus strains and others such as
Pseudomonas genus bacteria and
Saccharomyces genus yeasts, and remaining poorly exploited despite its potential [
15]. More specific roles such as vitamin synthesis, immune system development and performance mediation, microglia enhancement and angiogenesis modulation, are attributed to gut microbiota [
256]. The multifaceted and positive effects of BP on gut microbiota may thus potentiate these roles and thus result in beneficial impact in cancer and neurodegeneration pathophysiology.
3.6. Infections
Some infectious diseases or long-lasting minor or localized infections have been shown to trigger neurodegenerative and oncogenic mechanisms. On the other hand, the anti-infective potential of numerous BP compounds is largely known. However, studies of BP as a whole product for anti-infective potential are more recent. The anti-infectious potential of BP was largely reported against many bacterial strains, but also against some viral and fungal pathogens. Many of these pathogens are involved in cancer and neurodegeneration as we will see.
3.6.1. Viral Infections
Several viral infections, including coronavirus, influenza, pneumonia, herpes, hepatitis C, and papillomavirus infections have been linked to diverse NDD [
257,
258] and cancers [
259,
260], as well as to their related pathophysiological events.
Many recent studies have shown the potential of BP and many of its ubiquitous compounds which are frequently present together at substantial levels, mainly phenolics, against the coronavirus SARS-CoV-2 [
261,
262,
263]. Inflammation is a central mechanism by which this virus mediates its deleterious effects including those linked to neurodegeneration and cancer [
264,
265]. Thus, in addition to its direct inhibitory effect on the SARS-CoV-2, BP as an important anti-inflammatory and antioxidant cocktail, is expected to present an important potential in tackling this mediation. BP and some compounds that were isolated from it have also been found to exhibit inhibitory activity against many influenza strains such as H1N1, H3N2, and H5N1 [
266,
267]. Comparable activities were also reported for a wide range of ubiquitous BP compounds against herpes simplex virus, human papillomavirus, and hepatitis C viruses [
268,
269,
270].
3.6.2. Bacterial Infections
Bacteria involved in periodontal diseases, particularly
Porphyromonas gingivalis, have been implicated in AD pathogenesis by numerous studies [
271]. A one-month feeding of BP to mice reported a strong inhibition of
P. gingivalis in oral cavities of animals [
272]. Many phenolics that are frequent in BP separately showed marked inhibitory effects on this pathogens [
273]. The very ubiquitous commensal bacteria
Chlamydia pneumoniae is also linked to NDD and other disorders including lung cancer [
274,
275]. Catechin, epicatechin, myricetin, quercetin and rhamnetin were reported to effectively inhibit
Chlamydia pneumoniae [
276,
277].
Helicobacter pylori has also been implicated in many human diseases, including gastric cancer and ND [
278,
279], via mechanisms involving mainly its high potential to alter and circumvent host’s immune response, thus culminating in prolonged inflammation, redox imbalance and epigenetic alterations [
278,
280]. Based on the already elucidated unequaled potential of BP in fighting chronic low-grade inflammation and the fact that some BP compounds have been reported [
281,
282] for their marked inhibitory effects against this germ, we can firmly advocate preclinical and clinical research works which unfortunately remain absent on BP as an apart product in this topic.
3.6.3. Fungal Infections
The commensal fungi
Candida albicans has also been linked to cancer [
283] and neurodegeneration [
284]. Many BP samples showed a potent activity against
C. albicans and other bacterial strains with the activity extent not always correlating with total phenolic contents [
91,
108]. Studies of other multifloral or botanically-unidentified BP samples reported that the sensitivity of
C. albicans and other
Candida species to these samples ranged from very high to absent depending on the study [
108,
285,
286].
3.7. Genetic and Epigenetic Alterations
Genetic and epigenetic alterations are among the most noticeable other mechanisms that are implicated in the pathophysiology of both neurodegeneration and oncogenesis. A wide range of intra- and extra-cellular factors can induce deoxyribonucleic acid (DNA) damages and ribonucleic acid (RNA) defects, which are known to have critical roles in numerous NDD [
287] and cancers [
288].
3.7.1. DNA Damage
DNA damage response (DDR) is a vital process that declines with age, but other factors can compromise it and therefore result in unrepaired or mistakenly repaired DNA damage [
289]. The latter is a well-established contributor to ageing by inducing cell death and senescence, but has also been recently verified to induce inflammation, implicating a newly unveiled role in inflammaging which is a major culprit in aging and age-related disease [
290]. Insufficiency and chronic activation of DDR may result in sustained neuronal dysfunction and consequent death [
291]. DNA damages interact also with neuronal plasticity in a mutual triggering manner resulting in a vicious cycle of altered neurotransmission and impaired DDR, and culminating in cognitive decline [
289]. Defects in DDR are also a pivotal phenomenon in triggering carcinogenic process. They fuel tumorigenesis through excessive genomic instability, but also make malignant cells more prone to further alterations of DNA and in host immune response against oncogenic process [
292].
An ethanolic extract of
Castanea sativa BP drastically reduced DNA damage byproducts by 34%
in vitro [
293]. Protective roles against experimental DNA damages were also evidenced for aqueous and ethanolic extracts of
Actinidia arguta BP[
294] and an ethanolic extract of a multifloral BP [
295]. Polyphenols in general enjoy a strong preclinical evidence as agents that lower DNA and other cellular damages, and thus manifest an important potential in fighting related diseases, such as cancer and NDD [
296]. Carotenoids are also ubiquitous compounds in BP that may encompass preventive and corrective effects against DNA damage [
297,
298]. Carotenoid effects against DNA damage may obviously not emanate only from their known antioxidant potential, but further studies are needed to elucidate this eventuality. Many vitamins and minerals have also been shown to prevent DNA damage in different contexts but could not be detailed here.
3.7.1. Epigenetic Regulation
Epigenetic regulation, which is mediated by three major types of mechanisms, i.e., DNA methylation, histone modification and non-coding RNA, is a major genome modulator that may shape human phenotype; and thus deeply contribute to define health and disease factors and critically mediate numerous pathological events including those implicated cancer and neurodegeneration [
299,
300]. Although playing a key role in genetic expression and being inheritable and transmissible during cell division, epigenetic modifications can be reversed and are fortunately ”reprogrammable” or “erasable” due to pharmacological and nutritional interventions [
299,
300]. In this context, BP, as an unequaled nutritive resource and a rich pool in bioactive compounds, may represent a potential tool to carry out such interventions.
Many BP ubiquitous phytochemicals have been found to modulate major epigenetic mechanisms. Some of them potently suppressed oncogenic epigenetic signaling and promoted epigenetic induction of tumor suppressor gene expression in experimental studies. Polyphenols are widely reported for their countless effects resulting from epigenetic modulatory mechanisms in neurodegeneration and cancer pathophysiology (good recent reviews can be found in [
301,
302]). All these effects are not limited to differentiated fully functional cells. The three major mechanisms of epigenetic modulations have also been verified for numerous polyphenols in cancer stem cells which play a crucial role in cancer renewal and resistance (reviewed in [
303]).
4. BP & Neurodegeneration
Despite the great impediments in understanding the pathophysiology of NDD, many common mechanisms are widely recognized and known to be always involved in disease course. Oxidative stress [
304], neuro- [
305] and systemic [
306] inflammation, and metabolic disorders, especially metabolic syndrome and diabetes [
307], are today among well-known interfering triggers in the long course of neurodegeneration settlement. Other pathophysiological mechanisms which remain less understood, and for which targeting did not greatly succeed in clinical applications are more specific to each disease. This includes for example acetylcholinesterase activity of which inhibition is clinically used in AD pharmacotherapy with a limited efficacy rate, and monoamine oxidases circulating levels which are also used as targets in Parkinson disease (PD) treatments with a limited clinical success rate. All these examples of pathophysiological mechanisms have been targeted by BP and encouraging results were reported, but studies remain very scarce and all in the very early stages of screening and prospection as we will see.
In such context and given that multitargeting is becoming a universally coveted approach in preclinical and translational research regarding NDD, BP evidently arises as a “perfect” candidate owing its importance to its very diversified and balanced composition. In the very early triggering of neurodegenerative processes, BP may virtually be of great importance in fighting oxidative stress, malnutrition, metabolic disorders, and chronic low-grade inflammation. Its widely recognized potential for these indications is clearly valuable, especially in such initial stages. Other pathophysiological mechanisms are widely verified to be linked to neurodegeneration but remain less studied than the previous one. They include some that have been recently deeply investigated such as ferroptosis and epigenetic mechanisms and others that are less understood and more controversially implicated in NDD such as autophagy- and microbiota-related effects. All these mechanisms are modulated by BP and its compounds in complex and promising ways as we have long discussed. In addition to these mechanisms which are, at certain level, shared between neurodegeneration and cancer, BP showed interesting potential in other neurodegeneration-specific processes such as misfolded protein aggregation and glial cell-mediated neuroinflammation.
Some major translational issues remain to be solved with BP in the case of any prospective use in NDD tackling. The first one is the insufficient understanding of BP metabolism in the digestive tract and especially the modifications mediated by gut microbiota which may even imply the microbiota-driven de novo synthesis of some compounds that are already present in BP as we have seen for some examples. In other cases, these modifications may consist in very complex interactions involving gut microbiota but also other localizations and physiological microenvironments as it is the case in the metabolism of BP phenolic compounds. The second challenge is the insufficient understanding of pharmacodynamic and pharmacokinetic behavior and fate of all BP compounds at the systemic levels as well as inside the CNS. Third, the ability of BP compounds to pass the blood-brain and blood-cerebrospinal fluid barriers and the effects that these compounds may exert in these barriers remain largely unknown. Fourth, many translational challenges originate from the persistent shortfalls in standardizing BP research and the difficulties in ensuring the reproductivity of its investigated compositions. The fifth major translational challenge emanates obviously from the poor understanding of neurodegeneration pathophysiology which will impact any preventive or therapeutic intervention.
4.1. BP in Neuroinflammation
Anti-inflammatory properties of BP have been described in the CNS by experimental studies. Feeding of rodent models of autism spectrum disorder with BP remarkably decreased pro-inflammatory cytokines and mediators such as IFN-γ, IL-1α, IL-6, TNF-α, and VEGF and increased the anti-inflammatory cytokine IL-10 in animal brains [
121,
308]. This effect was accompanied by a significant decrease in caspase-3 and oxidative stress markers, in addition to the correction of neurotransmitter defects. Unfortunately, neither geographical nor botanical origins of used BP were reported by the authors.
Stress, especially in its chronic conditions, is known to be a potential risk factor for neurodegeneration through causing a series of neurobiological alterations including the induction and maintenance of neuroinflammation [
309]. In rats with chronic immobilization stress, BP was found to suppress neuroinflammation (mainly evaluated by TNF-α and IL-1β levels in the hippocampus) and concomitant oxidative events and to enhance corrective mechanisms such as brain-derived neurotrophic factor (BDNF) levels and antioxidant defense, in addition to the reduction of the anxiety-like behavior [
310].
In the same context, another study in rodents reported that BP presented marked protective properties against neuroinflammation (evaluated mainly by IFN-γ levels) and related mechanisms (e.g., neural cell apoptosis and neurotransmitter defects) in neonates delivered by mothers which were treated with methylmercury before [
187]. Another newer study reported comparable results [
311] viz. neuroinflammation and oxidative stress inhibition, excitotoxicity reduction, and neurotransmission disturbance correction in rat pups, but unfortunately used a mixture of BP and probiotics and did not evaluate the effects of BP alone to draw comparative inferences.
These preliminary data, added to those available from numerous studies which evaluated the anti-inflammatory effect of BP in different anatomical locations and at systemic levels (see our previous publication [
15] for a comprehensive review), as well as the multiple enhancing effects of BP on gut microbiota that we have detailed previously in the current work, unveil a very interesting potential of BP to mitigate neuroinflammation etiologies and mechanisms. The essence of focus and effort should for now go to the roles of different components and the very likely presence of synergistic mechanisms, as well as to solve pharmacokinetic and bioavailability issues which remain among the most difficult challenges in exploiting the great nutritional and pharmacological potential of BP.
4.2. BP Enzyme Inhibitory Potential
Concerning the enzyme inhibitory potential, a limited number of studies have been conducted until now on BP, mainly against known enzymes that are implicated in AD pathogenesis. Despite the encouraging results from preliminary studies, as we will see hereinafter, investigations are still in embryonic stages and many aspects remain to be studied even in in vitro phases, such as the role of each of BP compounds and the possible synergies which appear most likely to exist between many ubiquitous BP components.
An
in vitro study of 18 different BP samples recently reported that their hydroethanolic extracts downregulated betasecretase-1 (BACE1, the cleaving enzyme of amyloid precursor protein (APP) that generates amyloid beta (Aβ) protein) activity with multifloral pollens being more potent than mono-floral ones [
104]. This study, which is to our knowledge the first published one about this bioactivity, reported also that the 18 pollens did not show a strong inhibition of acetylcholinesterase (synaptic acetylcholine cleaver).
A recent
in vitro study of
Sabal blackburniana reported that the methanolic extract of pollen grains from this plant manifested an important inhibitory activity of the acetylcholinesterase [
312]. Interestingly, this study noted that the IC
50 of the anti-acetylcholinesterase activity of pollen grain extract (0.5 µg/ml) was dramatically reduced by the encapsulation of these extracts in zinc oxide nanoparticles (became 0.064 µg/ml). Moreover, the IC
50 of BP extract was even slightly lower than that of donepezil which is a reference in the acetylcholinesterase inhibitory treatment for AD. Unfortunately, this study did not investigate the BP of the same plant to figure out a possible difference between the pollen grains collected manually from the plant and those crafted by honeybees. An older study of diverse BP methanolic extracts [
313] also reported that a multifloral pollen originating mainly from
Myrcia spp. (60%) and
Cocos nucifera (33.3%) presented a very potent inhibitory activity on acetylcholinesterase (IC
50 of 3.93 ± 0.64 µg/ml), followed by samples from
Eucalyptus spp. monofloral BP which were also very potent (IC
50 of 26.85 ± 1.61 and 38.38 ± 2.47 µg/ml for two different samples). Other samples from
Spondias spp. and
Miconia spp. also presented a potent activity (IC
50 of 71.52 ± 3.58 89.66 ± 8.28 µg/ml respectively).
A very recent study investigated, for the first time to our knowledge, the effect of BP on catechol-
O-methyltransferase, a key enzyme in PD pathogenesis. This study found that hydroxycinnamoyl acid amides were the major drivers of the inhibitory effect on this enzyme that was strongly exerted by some of the studied extracts [
314]. Some of these molecules were largely more active than some known phenolics such as caffeic acid and p-coumaric acid. Note that some widely studied flavonoids which are repeatedly isolated in BP were reported as more potent inhibitors of catechol-
O-methyltransferase (e.g., IC
50 was 0.96μM for myricetin and 1.38μM for quercetin [
315]).
4.3. BP Compounds and Protein Aggregation
A growing number of preclinical and clinical studies are recently focusing on tackling or delaying aging-related processes. Indeed, altered proteostasis is acknowledged as a major hallmark of NDD and of overall aging process[
316]. Diverse widespread BP compounds have been reported to inhibit misfolded protein aggregation in the CNS. We will only review some BP bioactive compounds that were reported to impact the aggregation of most pathogenic proteins involved in the major NDDs, i.e., Aβ and tau in AD and α-synuclein in PD. Studies of BP as a whole product or extract are still absent.
4.3.1. BP Compounds and Amyloid-β
Under physiological conditions in humans, Aβ isoforms consist mainly of Aβ
1-40 (about 90% of total Aβ) and Aβ
1-42 (about 5 to 10% of total Aβ) [
317]. Aβ
1-42 is more neurotoxic so as a higher ratio of Aβ
1-42/Aβ
1-40 correlates with faster aggregation and pattern alteration of spontaneously formed oligomeric species [
318]. The transition from monomeric Aβ to a sufficient ratio of Aβ
1-42/Aβ
1-40 for oligomers and β-sheets forming is thought to happen usually 10 to 15 years before clinically confirmed pathological state of AD [
319]. The importance of natural products with known safety profiles is therefore of vital importance in early long-term mitigation of Aβ aggregation.
Numerous BP ubiquitous flavonoids (e.g., naringenin, naringin, apigenin, luteolin, hesperidin, hesperetin, kaempferol, quercetin, and myricetin) and phenolic acids (e.g., gallic, ellagic, ferulic, and rosmarinic acids) are widely recognized for their multifaceted potential to counter Aβ genesis, aggregation, and toxicity [
320,
321,
322]. Many non-phenolic BP compounds have also been reported to mitigate aberrant Aβ genesis, deposition, and/or toxicity, e.g.: β-carotene [
323], lycopene [
324], spermidine [
45], and betaine [
325]. Diverse polysaccharides from plants were shown to exert anti-amyloidogenic, Aβ disaggregating, and cognition promoting effects [
326,
327]. However, studies of such potential in BP polysaccharides are still lacking and highly recommended.
4.3.2. BP Compounds and Tau Protein
The microtubule-associated protein tau is mainly known for its role in stabilizing and protecting neuronal cytoskeleton microtubules, as well as in regulating microtubule dynamics and axonal transport [
328]. A basal level of phosphorylation, which is intricately regulated by the interplay of kinases and phosphatases, is necessary for tau physiological functions, but an alteration of this equilibrium may result in an hyperphosphorylation of tau leading to its excessive dissociation from microtubules and aggregation in form of neurofibrillary tangles, and ultimately to neuronal death [
328,
329]. Tau phosphorylation is mediated by tau kinases such as glycogen synthase kinase 3β (GSK3β), cyclin-dependent kinase 5 (CDK5), and others, while its hyperphosphorylation may result from these kinases’ overexpression or from an inactivation of tau phosphatases [
328,
329].
Numerous BP compounds have been shown to inhibit tau aggregation. A wide range of micronutrients that are present in BP, especially many vitamins and minerals, have been linked to preserving tau homeostasis and preventing its pathology [
330,
331]. BP flavonoids (e.g., apigenin, quercetin, myricetin, genistein, naringenin, luteolin) and other phenolics (e.g., rosmarinic, caffeic and ferulic acids, resveratrol) have been reported to tackle tau aggregation and pathology through diverse mechanisms including GSK3β and CDK5 modulation, and tau-mediated cytotoxicity mitigation.
4.3.3. BP Compounds and α-Synuclein
Alpha-synuclein (αS) is highly expressed in diverse organelles of CNS neurons including dopaminergic neurons of the substantia nigra pars compacta, and other excitatory and inhibitory neurons [
332]. αS is present in neuronal presynaptic terminals possibly traducing a role in vesicular trafficking and synaptic transmission and plasticity, in addition to a wide range of functions that are still not fully understood [
333,
334]. Diverse factors, such as mutations and aberrances in PTM, proteostasis defects and other factors, may intervene, to trigger and promote αS misfolding and aggregation.
Many polyphenols including some flavonoids and phenolic acids that are present in BP have been shown to prevent or reduce αS aggregation. Among unveiled mechanisms, the formation of non-toxic irregular aggregates, rearrangement of preformed toxic intermediates, or disassembly of mature fibrils, in addition to microbiota-mediated modulation, were mainly reported [
335,
336]. Being more recent and still less coveted than in other protein aggregation pathologies such as Aβ, this bioprospection is still mainly focusing on phenolic compounds with many ones being widely present in BP.
Apart from phenolics, other BP compounds remain poorly understood in αS-related pathology. A recent study found that retinoic acid, one of the main metabolites of BP carotenoids as we have seen, prevented the formation of intracellular αS inclusions, prevented αS-induced toxicity and dopaminergic neuronal loss in αS-injured neuronal cell lines and mice models [
337]. Other studies have reported a certain interest of vitamins such as A and E, and other micronutrients such as selenium in mitigating αS aggregation and pathology [
338,
339].
Figure 3.
General Aspects of the Potential of BP and Its Major Compounds in Neurodegeneration.
Figure 3.
General Aspects of the Potential of BP and Its Major Compounds in Neurodegeneration.
5. BP & Cancer
Carcinogenesis is a normal process occurring in human organism, and immune system should normally eradicate produced cancerous cells; but an overly complex interplay of different intrinsic, extrinsic, and behavioral factors can sustain carcinogenic process and promote tumor formation and metastasis. Among these factors, bad dietary habits, long lasting oxidative and inflammatory conditions, obesity, dysbiosis, epigenetic changes, some infectious diseases, and sedentary lifestyle are widely known [
340,
341]. Apparently, these examples of factors are all modifiable early before disease onset.
Bee products, especially BP, may be of foremost importance in such conditions. It is repeatedly reported to encompass anticancer properties by scientific experiments and reviews, and furthermore to interestingly potentiate cancer pharmacotherapies, and alleviate their side effects. Main known mechanisms of BP anticancer effects include apoptosis stimulation in malignant cells, antiproliferative bioactivities, and reduction of tumor growth [
342], evidently in addition to the anti-inflammatory and antioxidant effects, the possible modulation of some pre-carcinogenic troubles such as autophagic and epigenetic aberrances, and the tackling of healthy cell death mechanisms as we have discussed.
Due to its safety and rich composition, BP could be used in the long term as a prophylactic product especially in at-risk persons. Its verified pharmacological properties could be of a great help in early stages of tumorigenesis especially if innovative formulations assembling well targeted galenic forms and standardized compositions at efficient levels of desired compounds. The fact that other bee products have been repeatedly reported to have higher cytotoxic activities than BP, which however remains less investigated in cancers, should not be taken as a rule. Some studies have challenged such allegations by showing that these activities depend on the extraction process and yield, bee species and tested cell lines. In addition, some BP extracts were more potent than other bee products [
343] or than chemotherapeutic drugs
in vitro on selected cell lines [
344].
In addition to the extraction process, pollen preliminary preparation, treatments and formulation will evidently also be key determinants of its activity. A proteases-mediated pre-treatment of BP resulted in potently active peptide fractions against a lung cancer cell line [
184]. Hydrostatic treatment of BP also resulted in markedly ameliorated its active compound release, and permitted a marked increase of both its antiproliferative and anti-apoptotic activities as well as a potentiation of cancerous cell cycle destruction [
345]. Fermentation was also frequently reported to enhance BP compound content, bioavailability, and bioactivities [
15].
Furthermore, anticancer properties seem to be present in BP regardless of the bee species that forage it. We will see many examples, especially with BP collected by different genera and species of stingless bees, in the subsection hereinafter. Due to the scarcity of studies on whole BP, we will also briefly discuss some selected ubiquitous BP compounds that we are giving as examples throughout this work.
5.1. Effects on Carcinogenesis and Cancerous Cells
Nelumbo nucifera BP was reported to exert a multifaceted effect against prostate cancer, including antiproliferative, apoptosis promoting, and cell cycle disturbing effects on the human PC-3 cell line [
345]. Interestingly, this study reported that all treated pollen extracts (methanolic which was the most potent, ethanolic, and aqueous) increased glutathione depletion (despite its antioxidant role, glutathione is key element for tumor growth). The observed effect in all extracts were dose- and time-dependent.
A study of methanolic extracts of six mono-floral BP samples, collected by
Apis mellifera, on three cell lines of human breast cancer showed that some of these samples exerted an anticancer effect [
346].
Bidens pilosa pollen had a very potent antiproliferative effect (cell viability was drastically 0% on BT-20 and Hs 578T cells and 43.5% on MCF-7 cells), while
Camellia sinensis pollen extract had a lower but also marked effect on only two cell lines (BT-20 and Hs 578T). These two BP were the richest in flavonoids and carotenoids.
Brassica napus and
Nelumbo nucifera pollens did not show marked effects on the studied cell lines.
Another study of a BP originating predominantly from
Filipendula ulmaria reported that a methanolic extract of this BP exhibited marked dose- and time-dependent antiproliferative effect and a weak apoptosis-inducing effect on a colon carcinoma cell line [
183]. Two samples were used in this study; the first contained 70% and the second 80% of
F. ulmaria pollen. Other old studies have reported that BP had an inhibitory effect on bone marrow cancer and leukemia cell lines [
347].
An ethanolic extract of BP manifested an antiproliferative activity on lung adenocarcinoma (A549) and breast cancer (MCF-7) cell lines [
185]. Analysis of this extract found that it was composed of flavonoids and phenolic acids that are frequently reported in BP (naringenin, quercetin-diglycoside, catechin, and caffeic, chlorogenic, cinnamic, coumaric, ferulic, gallic, and syringic acids). Another BP ethanolic extract that was particularly rich in an apigenin and benzoic acid showed an antiproliferative effect in a mouse myeloma cell line [
348]. Although a tested propolis extract in this study had a higher phenolic content and antioxidant potential, BP was more active against myeloma cells than propolis.
A BP extract prepared with a hexane and methanol solvent mixture showed important anticancer properties in experimentally-induced mice breast cancer [
349]. In these animals, BP significantly reduced tumor size and body weight loss, and increased survival rate. Interestingly BP extract at 200 mg/kg was able to ameliorate survival rate significantly more than doxorubicin alone, and to correct body weight loss while doxorubicin alone induced a greater weight loss than the one observed in untreated cancerous mice. In addition, BP ameliorated oxidative and inflammatory markers and diverse hormonal level alterations in fed animals. This study, which used a commercial botanically- and chemically-unidentified BP, reported a synergistic effect that we will discuss later.
An
in vitro study used BP hydrolysates obtained by commercial proteases on a human bronchogenic carcinoma cell line, and showed that some of these hydrolysates manifested a very marked apoptosis-inducing activity (up to 80% after 72h by an alcalase-resulting fraction) [
184]. Moreover, this activity was selectively exerted by BP on apoptosis process either in its early and late stages and no significant effect on cell necrosis was noted. The authors used commercial pollen and did not describe its botanical origin.
Evidently, not all studies of BP anticancer potential reported similar results. A study of the effect of nineteen ethanolic extracts samples, including mono- and multi-floral BPs on five human cell lines of prostate (PC-3), breast (MCF-7), stomach (AGS), and lung (A549 and NCI-H727) carcinomas, found that all extracts presented, at variable levels, an antiproliferative activity which was however much weaker than cisplatin (assessed by the inhibitory concentration IC
50) [
104]. The only exceptions were that two monofloral BP samples (
Actinidia arguta and
Quercus palustris) did not show a significant antiproliferative potential (IC
50 >25 mg/ml) in A549 cell line.
Inhibitory activity of multiple BP samples was also tested
in vitro on cell growth of gastric, colorectal, cervical, breast, and non-small-cell lung cancer cell lines [
117]. In this study most of the studied BP samples failed to show antiproliferative activity on the studied cell lines. Only one sample (originating at 100% of
Coriandrum and
Daucus sp.) showed significant inhibitory activity on breast cancer cells and another sample (composed of
Olea europaea at ˃85%) showed significant inhibitory activity on cervical cancer cells.
One study which went “against the wave” in anticancer activity of BP was recently published. This study reported that BP feeding to zebrafish models of melanoma not only didn’t result in a protective effect against tumor cell proliferation, but promoted tumor growth in fed animals, and, although it significantly modified animal intestinal microbiota, gene transcript levels of proinflammatory mediators in the intestine didn’t differ between pollen-fed and control animals [
350]. This bring conflictual results with some previous experimental results [
342] that reported a BP activity against melanoma. We have already seen numerous examples where BP presents antineoplastic potential. In addition, many studies reported that BP may have an important tyrosinase inhibitor potential [
19,
83]. Studies on BP in melanoma remain extremely scarce and such result discrepancies are perfectly normal in the absence of sufficient study sampling and diversity.
BP from bee species other than
Apis mellifera have also been shown to be endowed with interesting anticancer effects. A recent study reported that ethanolic extracts from BPs collected by seven stingless bees showed, at different extents with few exceptions, inhibitory effects against colorectal (Caco-2), cervical (HeLa), and breast (MCF-7) cancer cell lines [
344]. BP collected by
Homotrigona fimbriata showed a potent antiproliferative effect, which largely exceeded that of 5-fluorouracil at the same mass dosage (500 µg/ml), against all tested cancer cell lines. Accordingly, a methanolic extract of BP collected by the stingless bee
Lepidotrigona terminate showed a significant dose-dependent antiproliferative effect on a breast cancer cell line (the IC
50 was 15 mg/ml on MCF-7 cells) [
351]. The authors however did not mention the botanical origin nor the approximate composition of BP, but they reported that it presented a marked antioxidant activity.
A recent study that used a pollen, collected by the stingless bees
Trigona spp. without indicating its botanical origin, reported that water extract of this pollen had a certain antiproliferative activity on a human breast cancer cell line (the IC
50 value was 18.6 mg/ml on MCF-7 cells), and that activity was similar to that of water extract of propolis [
352]. Interestingly, this study reported that measured antioxidant potential was greater in BP water extract than in propolis or honey water extract (honey extract did not show significant effect on the studied cell line). Another interesting observation reported by this study was the fact that pollen water extract was less toxic than propolis water extract on a normal human keratinocyte cell line. This study, being done only
in vitro, evidently needs further investigations. The reported observations about selective toxicity on cancerous cells and the higher potential and safety of pollen, as we have already seen also in a prostate cancer example, commend a potential use of BP in anticancer bioprospection. All given examples, and generally all available data until nowadays, provide an encouraging and considerable amount of experimental evidence, but which remains still in “embryonic” stage clinically.
A study of BP collected by three stingless bee species reported that all the three types of pollen manifested antiproliferative activity on human breast adenocarcinoma cell lines, although obviously at different extents [
353]. However, BP collected by
Geniotrigona thoracica presented markedly higher potential than that collected by
Heterotrigona itama and
Tetrigona apicalis. Similar selective effect on cancerous cells as the one reported by the previous example [
352] was also reported by this study.
Geniotrigona thoracica BP exerted marked antiproliferative activity and presented a much higher therapeutic index when assessed by reference to a human mammary epithelial cell lines taken as control normal cells [
353], which indicate that this pollen exerted selective antiproliferative effect on cancerous cells with higher safety regarding normal cells.
5.2. Effects on Anticancer Therapies
In addition to its direct anticancer potential, BP has been reported to exert multiple advantageous effects on other anticancer therapies. Specifically, BP was able to reduce toxicity of chemotherapeutic agents, and enhance the effectiveness of anticancer chemo-, radio-, and immuno-therapies in a multitude of in vitro and animal models of cancers. A multitude of anticancer mechanisms was unveiled by these studies and included the suppression of multidrug resistance effectors, the promotion of apoptosis and cell cycle arrest induction, the stimulation of mitochondrial altering effects, and diverse other molecular mechanisms. We will briefly see the studies of BP as a whole product or extract, and give some examples of the most abundant bioactive compounds of BP.
5.2.1. Reducing the Toxicity of Anticancer Therapies
A water extract of BP markedly reduced cisplatin-induced histological alteration in hepatic, renal and testicular tissues of mice models [
354]. Moreover, this extract prevented the genotoxic and cytotoxic effects of cisplatin in bone marrow cells, in addition to correcting the aberrances in multiple oxidative stress indicators in intoxicated animals. It is noteworthy that the preventive effect of BP was more pronounced than that of propolis extracted with similar protocol [
354]. BP botanical origin and chemical composition were not investigated in this study. Accordingly, BP from
Schisandra chinensis prevented many cisplatin-induced toxic alterations in liver and kidneys of rat models [
186]. The observed preventive effects consisted mainly in reducing tissular degenerations and correcting the levels of diverse inflammatory, oxidative stress, and apoptosis mediators and regulators, in addition to markedly reducing the alterations in hepatic transaminases, blood urea nitrogen, and creatinine levels. Water extract of another commercial BP was reported to counteract the doxorubicin-induced suppression of hematopoiesis in rat models [
355]. BP feeding reduced bone marrow and spleen histological alterations and increased spleen and body weight and survival rate, which were all altered by doxorubicin, in addition to preventing the decline in the abundance of diverse blood cells including red and white blood cells and platelets. At molecular level, BP corrected many alterations in hematopoiesis inducers, inflammatory mediators, and oxidative stress and apoptosis markers. Used BP showed a high safety profile in the studied animals. Another
in vivo study reported that BP feeding protected rats against methotrexate-induced testicular damage [
356]. Water extract of BP completely corrected the decrease in testosterone and the increase in luteinizing hormone, and significantly suppressed the alterations in oxidative stress markers, testicular and epididymal weights, and sperm structure and composition, that were all induced by methotrexate injection. The authors used a commercial BP and did not indicate its geographical and botanical origins or chemical composition.
Some major BP compounds have also been reported repeatedly to prevent chemotherapeutics-induced toxicity. All the examples that we will report hereinafter have been realized in murine models and the underlying mechanisms appeared to rely mainly on the antioxidant, anti-inflammatory, anti-genotoxic and antiapoptotic potential of the studied compounds. Evidently, most reported studies concerned flavonoids. Investigated organs where such protective effects were observed were mainly liver, kidney, spleen, heart, lung, and nervous system. Polyphenols, mainly flavonoids and phenolic acids, including many of those ubiquitously present in BP, have been extensively studied for their chemoprotective potential
in vitro and
in vivo. Some examples that showed those protective effects
in vivo were naringenin [
357], luteolin [
358], hesperidin [
359], quercetin [
360], apigenin [
361], kaempferol [
362], isorhamnetin [
363], myricetin [
364], pinocembrin [
365], and protocatechuic [
366], ellagic [
367], chlorogenic [
368], rosmarinic [
369], and ferulic [
370] acids. Other BP compounds, such as lutein [
371], lycopene [
372], choline [
373], coenzyme Q10 [
374], have also been reported to mitigate the toxicity of anticancer therapies.
It is also important to note that many of the studies that investigated the combination of these natural compounds with chemotherapeutic agents reported a synergistic potential and an achievement of the desired anticancer effect with a lower drug dosage. This may evidently imply that reducing necessary dose will also be a potential way to reduce chemotherapeutic toxicity to non-malignant cells and to preserve diverse physiological functions. Moreover, combinations of these BP compounds together generally resulted in a greater chemoprotective effect. This was also the same for combining these natural bioactive compounds with known protective drugs that are already used in clinical practice. Indeed, a combination of naringenin with metformin resulted in a synergistic potential to markedly reduce the necessary dose of liposomal doxorubicin to achieve similar therapeutic effect in xenograft models of breast cancer [
375]. Quercetin and resveratrol combination markedly reduced doxorubicin cardiotoxicity which is the major impediment of this important antineoplastic agent [
376]. Myricetin and quercetin had also a synergistic preventive effect against cisplatin-induced nephrotoxicity in mice [
377]. Histopathological alterations were not completely prevented by either of the two flavonols, but their combination almost normalized the histological aspect of the microscopic slices. Other studies with similar results are available in literature. These synergisms roughly suggest an important potential of more combinations of chemoprotective bioactive compounds in cancer management. Such combinations are unequally abundant in BP from all botanical and geographical sources, but the real extent of eventual synergisms remain to be unveiled by further studies.
In addition to chemoprotection, some BP compounds have also begun to be recently reported to reduce the toxicity of cancer immunotherapies. In murine models, chrysin markedly prevented trastuzumab-induced cardiotoxicity [
378], while resveratrol prevented pembrolizumab-induced hepatotoxicity and neurotoxicity [
379]. Coenzyme Q10 mitigated trastuzumab toxicity in human cardiomyocyte and hepatocyte cultures [
380]. This kind of protective effects are of great importance if sufficiently verified with other BP compounds and studied for their possible synergisms. Immunotherapies have revolutionized cancer management and their side effects appear to emanate mainly from their ability to modulate immune processes, while their combination with chemotherapies, which may hold greater promises in some cancers, is hindered by cumulative toxicities [
381].
Additionally, many BP compounds have shown important radioprotective effects in experimental studies. Evidently, phenolic compounds were the most studied, but other BP bioactive compounds and micronutrients have also been shown to encompass important protective potential against radiotherapy injuries. This kind of protective effects were especially reported for flavonoids (e.g.: anthocyanins, apigenin, genistein, quercetin, and naringin) and phenolic acids (e.g.: caffeic, chlorogenic, cinnamic, ferulic, gallic, and rosmarinic acids) [
382,
383,
384]. Radioprotective effects were also evidenced for carotenoids including β-carotene, lutein, zeaxanthin, and lycopene, coenzyme Q10, vitamins A, B, C and E, zinc, and selenium [
384,
385,
386].
5.2.2. Enhancing the Effects of Anticancer Therapies
An ethanolic extract of
Trifolium alexandrinum BP was found to synergistically enhance bevacizumab activity against A549 lung cancer cell lines and reduced its IC
50 [
387]. Similar synergistic potential has also been reported on MCF-7 breast cancer cells where BP combination minimized the required dose of bevacizumab to achieve similar anticancer effect [
185]. Ethanolic extract of
Trifolium alexandrinum BP was additionally verified to synergistically potentiate bevacizumab anticancer effects, including the reduction of tumoral nodule incidence and size and the activation of diverse proapoptotic and other molecular anticancer mechanisms in murine models of non-small lung cancer [
388]. Methanolic extract of a BP collected by the stingless bee
Lepidotrigona terminate potently enhanced the antiproliferative effect of cisplatin even if when the latter was used at low doses on a breast cancer (MCF-7) cell line. A dose of 15 mg/ml of BP quasi-doubled the antiproliferative effect of cisplatin even at its lowest used dosage (2.5 µmol/l). BP was also reported to synergistically promote the cytotoxicity of 5-fluorouracil and bee products on the HTC-116 human colon cancer cell line [
389]. In this study the IC50 of 5-fluorouracil was reduced from 6.94 to 1.90 µM when combined with BP. Interestingly, the potentiating effect of BP was more potent than that of honey or royal jelly.
BP also acted synergistically with immunotherapies. A nano-encapsulated ethanolic extract of BP synergistically exerted an antiproliferative effect on non-small lung cancer cell line in combination with Bevacizumab [
185]. Another BP extract was found to significantly enhance apoptosis induction and tumor volume and growth reduction that were exerted by Bevacizumab in mouse models of chemically-induced lung cancer [
388]. Finally, a small clinical trial reported that BP ameliorated menopausal symptoms in breast cancer patients receiving antihormonal treatment [
390].
Many BP ubiquitous compounds were reported to enhance chemotherapeutic effects on cancer cells. In aim to overcome the two major challenges of cancer management, viz. toxicity and resistance, the combination of small molecules, especially polyphenols, with traditional anticancer therapies has been extensively investigated recently. Numerous studies showed that phenolics (including many of those found in BP) suppress multidrug resistance and synergistically enhance anticancer effects of conventional chemotherapeutics. They may encompass many pharmacokinetic amelioration capabilities toward chemotherapeutic drugs such as reducing drug efflux, which is the main cause of multi-drug resistance in cancer cells, enhancing drug cellular uptake, and modulating drug metabolism. All these results were thoroughly reviewed in recent studies [
391,
392,
393].
In addition to enhancing the effect of chemotherapeutic agents on cancer cells, many bioactive compounds that are present in BP have been shown to sensitize malignant cells to radiotherapy. This was for example evidenced for many phenolics such as quercetin, kaempferol, genistein, ellagic acid, and resveratrol [
394].
5.3. Anticancer Potential of BP Compounds
A wide range of BP compounds have been highlighted for their important cytotoxic, antiproliferative, antimetastatic, antiangiogenic and other destructive effects activities on malignant cells
in vitro and
in vivo. Due to the complexity of these data and the great diversity of BP compounds, we will only overview some compounds that we have already highlighted during this work. Chemopreventive, cell death-inducing (mainly pro-apoptotic), antiproliferative, antimetastatic, and/or antiangiogenic effects were reported for example for the majority of BP flavonoids and phenolic acids that we enumerated in this publication (for review examples, see [
395,
396,
397]). Moreover, many of these molecules have been reported to suppress cancer cell stemness and reduce malignant stem cell pools in experimental models (e.g., kaempferol [
398], quercetin [
399], genistein [
400], and protocatechuic [
401], ellagic [
402], and caffeic [
403] acids), while others have been shown to enhance antitumor immunity (e.g., apigenin [
404], cyanidin-3-
O-glucoside [
405], delphinidin-3-
O-glucoside [
405], and chlorogenic acid [
406]).
Carotenoids are other abundant BP compounds that are widely studied for their anticancer potential. Except some limited controversies, mainly in lung cancer, carotenoids (including those that are present in BP, i.e., α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin) have been endowed with chemopreventive, proapoptotic, and antimetastatic potential against different cancers in numerous studies in animal models and observational studies in humans [
407,
408,
409].
In addition to polyphenols and carotenoids, many other BP compounds are known for their anticancer potential. Steroid compounds from
Brassica napus and
Brassica campestris showed important anticancer effects on prostate cancer [
345]. Another study of BP of the famous plant
Lycium barbarum, reported that lipopolysaccharides from this pollen induced,
in vitro and
in vivo, a significant dose-dependent increase in cancerous cell apoptosis and a tumor growth suppression by different mechanisms including a particularly marked inhibition of the PI3K/Akt [
410]. The latter is known to be one of the major signaling pathways that promote carcinogenesis, tumor growth and dissemination, and resistance to chemotherapeutic agents [
411].
Phytosterols are also endowed with multifaceted preventive and pharmacological potentials against many cancers according to a considerable body of preclinical evidence. Mehanistically, they were reported to induce cancer cell death, exert anti-proliferative, antimetastatic, and antiangiogenic effects, stimulate antitumor immunity, and to alter tumor microenvironment [
412,
413,
414]. Spermidine and its analogues have been shown to induce apoptosis and cell cycle arrest in diverse cancer cells [
415]. In addition, some BP micronutrients are also widely known, at least from nutritional point of view, to be related to cancer risk and progression. Anticancer properties were for example widely reported for vitamin E [
416,
417], polyunsaturated fatty acids [
418,
419], and selenium [
420,
421]. Regulatory authorities and scientific communities remain skeptical about the use of micronutrients for cancer prevention or treatment due to insufficient or unclear evidence, but studies of these nutrients in a holistic approach considering their natural existence milieus and association with other nutrients and phytochemicals, either from BP or other natural sources, are still lacking.
Figure 4.
General Anticancer Mechanism of BP and Its Major Compounds.
Figure 4.
General Anticancer Mechanism of BP and Its Major Compounds.
6. Materials and Methods
Human observational and interventional studies about BP in neurodegeneration and cancer are both still lacking. Likewise, studies investigating these topics in experimental animal models are also still very scarce. However, an immense amount of experimental evidence on different pathophysiological mechanisms that are involved in neurodegeneration and/or carcinogenesis is available on selected BP compounds and less abundantly on BP as a whole product or on its extracts. We have therefore estimated that a literature review is very needed. Furthermore, and given that available experimental evidence is still heterogenous and insufficiently clear despite its abundance, we have decided to adopt a scoping review methodology in our investigation. Scoping review is more adapted to the topic that we discuss in this work than other review methods. On one hand, a scoping review permits to expound a large extent of literature in a more flexible way that may include diversified and heterogenous data. On the other hand, it ensures a systematic and comprehensive selection of available scientific literature while maintaining a greater openness to receive scientific community criticism and draw diversified conclusions [
15]. In this context, scoping reviews are perfectly adapted to map available evidence on a complex and diversified product such as BP, especially in complex and insufficiently understood pathophysiological contexts such as cancers and neurodegeneration. In the current work, we adopt the same reference (PRISMA checklist and guidelines) that we adopted and explained in our recent review which was, at our current level of knowledge, the first one that focused BP [
15]. Our review protocol is resumed in Figure 7 and the procedural checklist is presented in
Table 5.
Our current work was undertaken in a biphasic procedure. In addition to the scoping review to gather and map available evidence, we undertook an analytical approach to figure out possible research avenues to develop future investigational works in the tackled topic. Since the data about BP in neurodegeneration and cancer are roughly known to be encouraging, we adopted this approach concomitantly with reviewing the recent achievements in understanding the pathophysiological mechanisms for which we searched a possible interest of BP and its known compounds. Such an approach consequently obligated us to analyze and report a great number of scientific publications, a number that is normally very uncommon in biomedical and pharmaceutical research regardless of the review type and topic.
The main international databases dealing with the medical and pharmaceutical fields, viz. PubMed, ScienceDirect, Scopus, Cochrane Library, and Google Scholar were consulted. Two preliminary searches separately used the keywords “bee pollen cancer” and “bee pollen neurodegener*” to screen potentially relevant publications. In parallel, the mechanisms that are well studied and present in both neurodegeneration and cancer were identified. We selected oxidative stress, inflammation, cellular clearance, cell death, microbiota alterations, infections, and genetic and epigenetic alterations. For specific mechanisms, we selected neuroinflammation, enzyme modulation and misfolded protein aggregation for neurodegenerative diseases. We focused only on the major neurotoxic proteins that are well documented in the most prevalent NDD, viz. amyloid-β, tau and α-synuclein. In cancer we selected the two main issues in cancer therapies, viz. tumor suppression and therapy toxicity. In the tumor suppression, we searched major anticancer mechanisms, viz. cell death induction, inhibition of proliferation, metastasis and neovascularization, anticancer immunity, and carcinogenesis prevention, in addition to the synergism with anticancer therapeutics. To evaluate the role of BP on the toxicity of anticancer therapeutics, we searched for studies that covered the modulation of this toxicity by BP. In anticancer therapeutics, we considered all kinds, i.e., chemotherapy, radiotherapy, and immunotherapy. More affined terms were subsequently used including “bee AND pollen” and one of the following words: “oxidation”, “inflammation”, “autophagy”, “apoptosis”, “ferroptosis”, “microbiota”, “bee DNA damage”, and “epigenetic”. The search term “bee pollen neurodegener*” was replaced by the “bee pollen neurodegenerative” in Science Direct because this database does not support searches with wildcard characters (“*” in our case). In this database, the “article type” filter was applied and limited to “review articles” and “research articles”.
After this first screening, we undertook a general phytochemical overview of BP studies and chose a list of the most frequent bioactive compounds and nutrients. We selected apigenin, catechin, chrysin, cyanidin, delphinidin, epicatechin, genistein, hesperidin, hesperetin, isorhamnetin, kaempferol, luteolin, myricetin, naringenin, naringin, pinocembrin, and quercetin from flavonoids, resveratrol from stilbenes, and benzoic, caffeic, chlorogenic, cinnamic, coumaric, dihydroxybenzoic, ellagic, ferulic, gallic, hydroxycinnamic, protocatechuic, rosmarinic, syringic, and vanillic acids from phenolic acids. From carotenoids, we selected α-carotene, β-carotene, β-cryptoxanthin, lutein, lycopene, and zeaxanthin. We selected also other less studied compounds from BP which were betaines, glucosinolates, polyamines (especially spermidine), and micronutrients including vitamins A, B, C, and E and two microelements viz. selenium and zinc. The names of these compounds were therefore included to replace “bee pollen” in all the keywords searches that we have just enumerated. Other database consultations were continuously done while advancing in our work to elucidate pathophysiological mechanisms or some properties of the studied BP compounds.
Due to the huge amount of data, we also decided to limit the time interval of includible literature. In the general anticancer and anti-neurodegenerative mechanisms of BP, we extended the search on the last ten-year interval, while in the case of BP specific compounds, we limited the search to the last five years. The search on BP effect on specific mechanisms, (e.g., oxidative, inflammation, autophagy, etc.) were also limited to the last five years. However, when data were unclear or insufficient, we extended the search on the concerned issue to larger time intervals. When studying disease pathophysiological parameters and related mechanistic issues, the search was limited to the last three years.
For the keyword “bee pollen” associated to “cancer” or “neurodegeneration”, a total of 42, 1228, 67, 2, and 20280 articles were retrieved from PubMed, ScienceDirect, Scopus, Cochrane Library, and Google Scholar, respectively from the last 10 years. For the keyword “bee pollen” associated to the keywords “oxidative”, “inflammation”, “autophagy”, “apoptosis”, “ferroptosis, “microbiota”, “DNA”, and “epigenetic”, the number of retrieved research works in PubMed were 116, 29, 4, 21, 0, 112, and 94 respectively. The number of results was large with singular BP compounds and a reference sampling was adapted to the investigated mechanism, or the biological or pathophysiological process.
The selection of research publications was then limited to those published in English, French, and Spanish languages. was used in Google Scholar to refine the search. From all initial search results, we have eliminated the following types from consideration: duplication across databases, books and conference proceedings, irrelevant publications for our research topic, and works that were published without a digital object identifier (DOI). The final number of articles that were included in the present review was 421.
Figure 5.
Flow Diagram of Literature Resource Identification and Screening (The protocol conception relies on PRISMA 2020 Standards).
Figure 5.
Flow Diagram of Literature Resource Identification and Screening (The protocol conception relies on PRISMA 2020 Standards).
7. Inferring Remarks and Perspectives
Following all what we have seen throughout this work, we could ascertain that BP is an unequaled stockpile of functional bioactive compounds and complementary nutraceuticals that could be perfectly joined to a healthy lifestyle for healthy and ill persons. This is especially of great relevance to carcinogenesis and neurodegeneration contexts where the major pathophysiological mechanisms that are linked to these pathologies are among the most important ones that are mitigated by BP. The spectrum of hallmarks of both aging and age-related diseases that are widely accepted among the scientific community mainly includes genomic and epigenetic alterations, loss of protein homeostasis, impaired autophagy, mitochondrial dysfunction, cellular senescence, compromised neuronal function and plasticity, chronic inflammation, immuno-senescence, dysbiosis, and deregulated nutrient-sensing. We have thoroughly elucidated in this work and in our recent publication [
15] that BP and its known compounds act through a multitude of mechanisms in most of these hallmarks.
In all discussed mechanisms, chronicity of resulting modifications is a shared attribute before the settlement of both neurodegenerative and malignant disorders. Effective mitigation of such deleterious results imposes the presence of a safe and complementary rich tool to suppress chronic triggers. BP then rises as a candidate encompassing a large plethora of verified bioactivities that could be a valuable contribution either in cancer prophylaxis or in certain disease stages. The number of preclinical and clinical studies remains very limited regarding the extensive diversity of BP and the great diversity, ambiguity, and entanglement of carcinogenesis-related processes.
In our current work, we have evoked, for the first time to our knowledge, a substantial and clear evidence coming from individual studies of a wide range of BP compounds to support the existence of other very relevant bioactivities of BP that remain completely untapped. These bioactivities are mainly the modulation of ferroptosis and the regulation of epigenetic mechanisms. Most studies investigating natural, especially phenolic, compounds in ferroptosis as it relates to neurodegeneration and cancer were published in the last three-five years depending on the molecules. Data is still raw and, despite the promising results that emanate from preclinical evidence, our understanding of these effects remains elementary and further research works are needed to end up in beneficial clinical outcomes. BP is packed with diverse ferroptosis modulators that appear to act in an intricate manner depending on the cellular context. Although a great part of the studied compounds exerts this bimodal regulation, many others have been reported to only mitigate ferroptosis. These intriguing qualities undeniably warrant further and substantial research efforts. The ferroptosis inhibitory effect of its compounds may endow BP with a great interest in mitigating neurodegeneration process which is installed through decades of changes and culminate in cell death that appear to be driven by ferroptosis in many NDD. This does not alter its potential in managing cell death also in carcinogenesis due to numerous examples that we have seen. In fact, aggressive, metastatic and treatment-refractory cancer cells are known to be highly sensitive to ferroptosis. Figuring out safe and chronically usable compounds is thus of foremost interest. On the other side, the plethora of epigenetic regulatory mechanisms that we have seen for many major BP compounds is clearly relevant. Epigenetic regulation is a very promising research area in BP and a highly complex and multifaceted potential is a priori verified for a wide spectrum of BP compounds. Since epigenetic processes are among the most studied and influential etiological factors in neurodegeneration, cancers and age-related diseases in general, more focused studies are strongly recommended to decipher possible interventions, especially in long-term preventive interventions, but also in managing confirmed pathological states and/or in conducing prenatal interventions.
The other promising research avenue is the modulation of microbiota. The latter has been investigated by a few experimental studies of BPs, but focused investigations on cancer and neurodegeneration are still lacking. The fourth research avenue which may encompass a great importance for BP is the mitigation of infections and other disorders that accompany them in the context of increasing risks to develop oncological and neurodegenerative disorders or to exacerbate them through promoting comorbidities. The fifth research topic that remains untapped by BP research is the potential of this bee-fashioned cocktail to mitigate aberrant protein aggregation and the neurotoxicity that is mediated by these misfolded proteins. We have also clearly exposed the abundance of experimental evidence emanating from individual studies of BP compounds in this topic. Other specific topics were also evoked during our discussions. Among appealing examples, we underline the possible effects of BP in neurotoxic and carcinogenic infections, as well as the possible bimodal epigenetic regulation by BP, the possible potential role of BP polysaccharides in ferroptosis, and the calorie restriction mimetics that was unveiled for some BP compounds.
We must underline that our current work has some limitations. The first and most important one is the insufficient understanding of the pathophysiological mechanisms, especially in the case of neurodegeneration which may have influenced the definition of the pathophysiological processes to focus on in the current study. The second limitation was the quasi-absence of the coordination and officially agreed standardization of experimental studies of BP, resulting frequently in scattered results and insufficient approaches of the main studied bioactivities. The third limitation was the great scarcity in some major pathophysiological processes in neurodegeneration for BP as a whole product, especially in the areas that we have proposed to investigate for the first time such as epigenetic regulation and ferroptosis modulation. The fourth major limitation was the absence of the studies evaluating the interaction between different BP compounds, either in synergistic or antagonistic manners, in the different bioactivities that we have studied in this review. Other minor limitations, such as the impossibility to access to some publications and some clearly inconsistent results reported by some experimental studies, were encountered but did not significantly influence the essence of our review, analysis, and inferences.
We could index all the novel research avenues that we have just proposed as the first major result of our investigation. The second part is the well identified challenges that may still hinder the application of the encouraging evidence that results from experimental research in translational research and in clinical practice. One of the main challenges to resolve is the problem of bioavailability and bioaccessibility of BP bioactive compounds. In fact, and as it is well known in natural product pharmacology, some very promising compounds of BP still could not be efficiently delivered in situ to the desired targets in different parts of the body. Many aspects, or more righteously most aspects, of gastrointestinal and systemic metabolism as well as overall pharmacokinetic and pharmacodynamic behaviors of these compounds remain poorly understood. In addition, some drawbacks that characterize some phenolics and other compounds such as great instability and short half-life remain still unsolved. The second most important challenge is the standardization of BP composition. Unfortunately, the establishment of agricultural, apicultural, and experimental guidelines to conceive reproducible BP composition, as well as reliable analytical procedures and efficient formulations remain still far from our reach. Another great deficiency that persists in understanding BP is the rarity of comparative studies between the phytochemical composition of plant pollens in flowers and the composition of BPs. Comparative analysis of pharmacokinetic and pharmacodynamic traits of both pollens is also still lacking. A final important parameter to consider is the fact that multitargeting is a highly coveted tendency in pharmacological prospection regarding complex and poorly understood diseases such as NDD and cancers. In the case of BP, this can be either an advantage or a limitation, depending on the investigational and clinical contexts. The synergistic and eventual antagonistic behaviors among its compounds in bioactivities that are relevant for neurodegeneration and cancer remain almost absent.
This work is the first comprehensive review of BP potential in neurodegenerative and malignant disorders. Accordingly, we hope that this work will give motivating insights to deeply tackle the research issue that we evoke in our current work and constitute a valuable starting point to future more fruitful scientific works.
Finally, the multiple, encouraging and very promising bioactivities and insights that we have detailed in this work let us to firmly bolster, not only the preventive, but also the possible curative potential of BP in neurodegeneration and cancer.
Author Contributions
Conceptualization, Campos M.G. and Kacemi R.; methodology, Kacemi R.; validation, Campos M.G. and Kacemi R.; writing—original draft preparation, Kacemi R.; writing—review and editing, Campos M.G.; funding acquisition, Campos M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Center of Chemistry from Faculty of Sciences and Technology of University of Coimbra, Portugal by Fundação para a Ciência e a Tecnologia I.P. (FCT), Portugal (UI0204): UIDB/00313/2020.
References
- Bray et al., “Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries185 Countries.,” CA Cancer J Clin. 2024 May-Jun;74(3):229-263. [CrossRef]
- V. L. Feigin et al., “The global burden of neurological disorders: translating evidence into policy,” Lancet Neurol., vol. 19, no. 3, pp. 255–265, 2020. [CrossRef]
- M. Duggan, B. M. Duggan, B. Torkzaban, T. M. Ahooyi, K. Khalili, and J. Gordon, “Age-related neurodegenerative diseases,” J. Cell. Physiol., vol. 235, no. 4, pp. 3131–3141, 2020. [CrossRef]
- World Health Organisation, “Dementia: Key Facts,” Key Facts. Accessed: Feb. 10, 2023. [Online]. Available: https://www.who.
- O. Gaidai, Y. O. Gaidai, Y. Cao, and S. Loginov, “Global cardiovascular diseases death rate prediction,” Curr. Probl. Cardiol., p. 101622, Jan. 2023. [CrossRef]
- Z. Wang, A. Z. Wang, A. Du, H. Liu, Z. Wang, and J. Hu, “Systematic Analysis of the Global, Regional and National Burden of Cardiovascular Diseases from 1990 to 2017,” J. Epidemiol. Glob. Health, vol. 12, no. 1, pp. 92–103, 2022. [CrossRef]
- M. G. Savelieff, K. S. M. G. Savelieff, K. S. Chen, S. E. Elzinga, and E. L. Feldman, “Diabetes and dementia: Clinical perspective, innovation, knowledge gaps,” J. Diabetes Complications, vol. 36, no. 11, p. 108333, 2022. [CrossRef]
- C. Boutari and C. S. Mantzoros, “A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on,” Metabolism., vol. 133, 2022. [CrossRef]
- Harper, A.; et al. , “Viral Infections, the Microbiome, and Probiotics,” Front. Cell. Infect. Microbiol., vol. 10, no. February, pp. 1–21, 2021. [CrossRef]
- S. Theodoridis, E. G. S. Theodoridis, E. G. Drakou, T. Hickler, M. Thines, and D. Nogues-Bravo, “Evaluating natural medicinal resources and their exposure to global change,” Lancet Planet. Heal., vol. 7, no. 2, pp. e155–e163, 2023. [CrossRef]
- R. Mărgăoan, A. R. Mărgăoan, A. Özkök, Ş. Keskin, N. Mayda, A. C. Urcan, and M. Cornea-Cipcigan, “Bee collected pollen as a value-added product rich in bioactive compounds and unsaturated fatty acids: A comparative study from Turkey and Romania,” Lwt, vol. 149, no. June, p. 111925, Sep. 2021. [CrossRef]
- I. K. Karabagias, V. K. I. K. Karabagias, V. K. Karabagias, I. Gatzias, and K. A. Riganakos, “Bio-functional properties of bee pollen: The case of ‘bee pollen yoghurt,’” Coatings, vol. 8, no. 12, 2018. [CrossRef]
- V. Adaškevičiūtė, V. V. Adaškevičiūtė, V. Kaškonienė, P. Kaškonas, K. Barčauskaitė, and A. Maruška, “Comparison of physicochemical properties of bee pollen with other bee products,” Biomolecules, vol. 9, no. 12, pp. 1–22, 2019. [CrossRef]
- T. de S. Bovi et al., “Seasonal variation of flavonoid content in bee bread: Potential impact on hypopharyngeal gland development in Apis mellifera honey bees,” J. Apic. Res., vol. 59, no. 2, pp. 170–177, 2020. [CrossRef]
- R. Kacemi and M. G. Campos, “Translational Research on Bee Pollen as a Source of Nutrients: A Scoping Review from Bench to Real World.,” Nutrients, vol. 15, no. 10, p. 2413, 23. 20 May. [CrossRef]
- M. H. Baky, M. B. M. H. Baky, M. B. Abouelela, K. Wang, and M. A. Farag, “Bee Pollen and Bread as a Super-Food: A Comparative Review of Their Metabolome Composition and Quality Assessment in the Context of Best Recovery Conditions,” Molecules, vol. 28, no. 2, 2023. [CrossRef]
- P. Velásquez, E. P. Velásquez, E. Muñoz-Carvajal, L. Grimau, D. Bustos, G. Montenegro, and A. Giordano, “Floral Pollen Bioactive Properties and Their Synergy in Honeybee Pollen.,” Chem. Biodivers., vol. 20, no. 4, p. e202201138, Apr. 2023. [CrossRef]
- J. Qiao et al., “Phenolamide and flavonoid glycoside profiles of 20 types of monofloral bee pollen,” Food Chem., vol. 405, no. PA, p. 134800, Mar. 2023. [CrossRef]
- X. Zhang, M. X. Zhang, M. Yu, X. Zhu, R. Liu, and Q. Lu, “Metabolomics reveals that phenolamides are the main chemical components contributing to the anti-tyrosinase activity of bee pollen,” Food Chem., vol. 389, no. March, p. 133071, 2022. [CrossRef]
- Ares, A.M.; et al. , “Differentiation of bee pollen samples according to the betaines and other quaternary ammonium related compounds content by using a canonical discriminant analysis,” Food Res. Int., vol. 160, no. July, 2022. [CrossRef]
- S. Rojo, O. S. Rojo, O. Escuredo, M. S. Rodríguez-Flores, and M. C. Seijo, “Botanical Origin of Galician Bee Pollen (Northwest Spain) for the Characterization of Phenolic Content and Antioxidant Activity,” Foods, vol. 12, no. 2, p. 294, 2023. [CrossRef]
- H. R. El-Seedi et al., “Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties,” Front. Nutr., vol. 8, no. January, 2022. [CrossRef]
- P. Filannino et al., “Nutrients Bioaccessibility and Anti-inflammatory Features of Fermented Bee Pollen: A Comprehensive Investigation,” Front. Microbiol., vol. 12, no. February, Feb. 2021. [CrossRef]
- R. Mărgăoan et al., “Bee collected pollen and bee bread: Bioactive constituents and health benefits,” Antioxidants, vol. 8, no. 12, pp. 1–33, Nov. 2019. [CrossRef]
- E. Chelucci, C. E. Chelucci, C. Chiellini, A. Cavallero, and M. Gabriele, “Bio-Functional Activities of Tuscan Bee Pollen,” Antioxidants, vol. 12, no. 1, p. 115, Jan. 2023. [CrossRef]
- W. A. Weis et al., “An overview about apitherapy and its clinical applications,” Phytomedicine Plus, vol. 2, no. 2, 2022. [CrossRef]
- S. A. M. Khalifa et al., “Bee Pollen: Current Status and Therapeutic Potential,” Nutrients, vol. 13, no. 6, p. 1876, May 2021. [CrossRef]
- F. Xue and C. Li, “Effects of ultrasound assisted cell wall disruption on physicochemical properties of camellia bee pollen protein isolates,” Ultrason. Sonochem., vol. 92, no. November 2022, p. 106249, 2023. [CrossRef]
- M. Oroian, F. M. Oroian, F. Dranca, and F. Ursachi, “Characterization of Romanian Bee Pollen—An Important Nutritional Source,” Foods, vol. 11, no. 17, p. 2633, Aug. 2022. [CrossRef]
- S. Han et al., “Lotus Bee Pollen Extract Inhibits Isoproterenol-Induced Hypertrophy via JAK2/STAT3 Signaling Pathway in Rat H9c2 Cells,” Antioxidants, vol. 12, no. 1, p. 88, 2023. [CrossRef]
- K. Alshallash et al., “Bee Pollen as a Functional Product – Chemical Constituents and Nutritional Properties,” J. Ecol. Eng., vol. 24, no. 2, pp. 173–183, Feb. 2023. [CrossRef]
- V. Aylanc, S. I. V. Aylanc, S. I. Falcão, S. Ertosun, and M. Vilas-Boas, “From the hive to the table: Nutrition value, digestibility and bioavailability of the dietary phytochemicals present in the bee pollen and bee bread,” Trends Food Sci. Technol., vol. 109, no. October 2020, pp. 464–481, Mar. 2021. [CrossRef]
- C. Salazar-González, C. A. C. Salazar-González, C. A. Fuenmayor, C. Díaz-Moreno, C. M. Stinco, and F. J. Heredia, “Stability and shelf life modeling of natural colorant from bee pollen,” Food Packag. Shelf Life, vol. 40, no. September, p. 101169, Dec. 2023. [CrossRef]
- C. Y. Salazar-González et al., “Characterization of carotenoid profile and α-tocopherol content in Andean bee pollen influenced by harvest time and particle size,” Lwt, vol. 170, no. July, p. 114065, Dec. 2022. [CrossRef]
- M. A. González-Peña, A. E. M. A. González-Peña, A. E. Ortega-Regules, C. Anaya de Parrodi, and J. D. Lozada-Ramírez, “Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids-A Review.,” Plants (Basel, Switzerland), vol. 12, no. 2, pp. 1–22, Jan. 2023. [CrossRef]
- D. Górecka et al., “Lycopene in tomatoes and tomato products,” Open Chem., vol. 18, no. 1, pp. 752–756, Jun. 2020. [CrossRef]
- L. M. Estevinho, T. L. M. Estevinho, T. Dias, and O. Anjos, “Influence of the Storage Conditions (Frozen vs. Dried) in Health-Related Lipid Indexes and Antioxidants of Bee Pollen,” Eur. J. Lipid Sci. Technol., vol. 121, no. 2, pp. 1–9, Feb. 2019. [CrossRef]
- M. Roumani, R. E. M. Roumani, R. E. Duval, A. Ropars, A. Risler, C. Robin, and R. Larbat, “Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests,” Biomed. Pharmacother., vol. 131, no. July, 2020. [CrossRef]
- S. Larbi et al., “Differentiating between Monofloral Portuguese Bee Pollens Using Phenolic and Volatile Profiles and Their Impact on Bioactive Properties,” Molecules, vol. 28, no. 22, p. 7601, Nov. 2023. [CrossRef]
- W. Wang, H. D. W. Wang, H. D. Snooks, and S. Sang, “The Chemistry and Health Benefits of Dietary Phenolamides,” J. Agric. Food Chem., vol. 68, no. 23, pp. 6248–6267, 2020. [CrossRef]
- N. Varghese, S. N. Varghese, S. Werner, A. Grimm, and A. Eckert, “Dietary mitophagy enhancer: A strategy for healthy brain aging?,” Antioxidants, vol. 9, no. 10, pp. 1–28, 2020. [CrossRef]
- A. Stacchiotti and G. Corsetti, “Natural Compounds and Autophagy: Allies Against Neurodegeneration,” Front. Cell Dev. Biol., vol. 8, no. September, pp. 1–16, 2020. [CrossRef]
- X. Yang et al., “Spermidine inhibits neurodegeneration and delays aging via the PINK1-PDR1-dependent mitophagy pathway in C. elegans,” Aging (Albany. NY)., vol. 12, no. 17, pp. 16852–16866, Sep. 2020. [CrossRef]
- G. Lou, K. G. Lou, K. Palikaras, S. Lautrup, M. Scheibye-Knudsen, N. Tavernarakis, and E. F. Fang, “Mitophagy and Neuroprotection,” Trends Mol. Med., vol. 26, no. 1, pp. 8–20, 2020. [CrossRef]
- K. Freitag et al., “Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer’s disease mouse model,” J. Neuroinflammation, vol. 19, no. 1, pp. 1–19, 2022. [CrossRef]
- S. M. Wortha et al., “Association of spermidine plasma levels with brain aging in a population-based study,” Alzheimer’s Dement., no. September, pp. 1–9, 2022. [CrossRef]
- F. Madeo, M. A. F. Madeo, M. A. Bauer, D. Carmona-Gutierrez, and G. Kroemer, “Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans?,” Autophagy, vol. 15, no. 1, pp. 165–168, 2019. [CrossRef]
- C. Schwarz et al., “Spermidine intake is associated with cortical thickness and hippocampal volume in older adults,” Neuroimage, vol. 221, no. July, p. 117132, 2020. [CrossRef]
- C. Schwarz et al., “Effects of Spermidine Supplementation on Cognition and Biomarkers in Older Adults with Subjective Cognitive Decline: A Randomized Clinical Trial,” JAMA Netw. Open, vol. 5, no. 5, p. E2213875, 2022. [CrossRef]
- W. Han, H. W. Han, H. Li, and B. Chen, “Research Progress and Potential Applications of Spermidine in Ocular Diseases,” Pharmaceutics, vol. 14, no. 7, pp. 1–17, 2022. [CrossRef]
- C. E. Holbert, M. T. C. E. Holbert, M. T. Cullen, R. A. Casero, and T. M. Stewart, “Polyamines in cancer: integrating organismal metabolism and antitumour immunity.,” Nat. Rev. Cancer, vol. 22, no. 8, pp. 467–480, Aug. 2022. [CrossRef]
- V. Tulluri and V. V. Nemmara, “Role of Antizyme Inhibitor Proteins in Cancers and Beyond.,” Onco. Targets. Ther., vol. 14, pp. 667–682, 2021. [CrossRef]
- I. Novita Sari, T. I. Novita Sari, T. Setiawan, K. Seock Kim, Y. Toni Wijaya, K. Won Cho, and H. Young Kwon, “Metabolism and function of polyamines in cancer progression,” Cancer Lett., vol. 519, no. June, pp. 91–104, 2021. [CrossRef]
- Q.-Z. Li, Z.-W. Q.-Z. Li, Z.-W. Zuo, Z.-R. Zhou, and Y. Ji, “Polyamine homeostasis-based strategies for cancer: The role of combination regimens.,” Eur. J. Pharmacol., vol. 910, no. 28, p. 174456, Nov. 2021. [CrossRef]
- J. Fan, Z. J. Fan, Z. Feng, and N. Chen, “Spermidine as a target for cancer therapy.,” Pharmacol. Res., vol. 159, no. March, p. 104943, Sep. 2020. [CrossRef]
- M. K. Arumugam, M. C. M. K. Arumugam, M. C. Paal, T. M. Donohue, M. Ganesan, N. A. Osna, and K. K. Kharbanda, “Beneficial Effects of Betaine: A Comprehensive Review.,” Biology (Basel)., vol. 10, no. 6, pp. 1–24, May 2021. [CrossRef]
- D. Dobrijević et al., “Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods,” Molecules, vol. 28, no. 12, p. 4824, Jun. 2023. [CrossRef]
- H. Ahmed et al., “Microbiota-derived metabolites as drivers of gut–brain communication,” Gut Microbes, vol. 14, no. 1, pp. 1–33, 2022. [CrossRef]
- M. Bhatt, A. M. Bhatt, A. Di Iacovo, T. Romanazzi, C. Roseti, and E. Bossi, “Betaine-The dark knight of the brain.,” Basic Clin. Pharmacol. Toxicol., no. November 2022, pp. 1–11, Feb. 2023. [CrossRef]
- M. S. Seyyedsalehi et al., “Dietary Choline and Betaine Intake and Risk of Colorectal Cancer in an Iranian Population.,” Cancers (Basel)., vol. 15, no. 9, pp. 1–12, Apr. 2023. [CrossRef]
- J. Y. Huang et al., “A prospective evaluation of serum methionine-related metabolites in relation to pancreatic cancer risk in two prospective cohort studies,” Int. J. Cancer, vol. 147, no. 7, pp. 1917–1927, 2020. [CrossRef]
- T. Ghazi, T. T. Ghazi, T. Arumugam, A. Foolchand, and A. A. Chuturgoon, The Impact of Natural Dietary Compounds and Food-Borne Mycotoxins on DNA Methylation and Cancer, vol. 9, no. 9. 2020. [CrossRef]
- N. D’onofrio et al., “Synergistic effect of dietary betaines on sirt1-mediated apoptosis in human oral squamous cell carcinoma cal 27,” Cancers (Basel)., vol. 12, no. 9, pp. 1–19, 2020. [CrossRef]
- S. Martín-Fernández-de-Labastida, I. S. Martín-Fernández-de-Labastida, I. Alegria-Lertxundi, M. M. de Pancorbo, and M. Arroyo-Izaga, “Association between nutrient intake related to the one-carbon metabolism and colorectal cancer risk: a case–control study in the Basque Country,” Eur. J. Nutr., vol. 62, no. 8, pp. 3181–3191, 2023. [CrossRef]
- A. M. Ares, J. A. A. M. Ares, J. A. Tapia, A. V. González-Porto, M. Higes, R. Martín-Hernández, and J. Bernal, “Glucosinolates as Markers of the Origin and Harvesting Period for Discrimination of Bee Pollen by UPLC-MS/MS,” Foods, vol. 11, no. 10, pp. 1–14, 2022. [CrossRef]
- M. Ares et al., “Differentiation of bee pollen samples according to their intact-glucosinolate content using canonical discriminant analysis,” Lwt, vol. 129, no. April, p. 109559, Jul. 2020. [CrossRef]
- G. Na, C. G. Na, C. He, S. Zhang, S. Tian, Y. Bao, and Y. Shan, “Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy,” Int. J. Mol. Sci., vol. 24, no. 3, 2023. [CrossRef]
- N. Orouji, S. K. N. Orouji, S. K. Asl, Z. Taghipour, S. Habtemariam, S. M. Nabavi, and R. Rahimi, “Glucosinolates in cancer prevention and treatment: experimental and clinical evidence.,” Med. Oncol., vol. 40, no. 12, p. 344, Nov. 2023. [CrossRef]
- R. M. Kamal et al., “Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases.,” Molecules, vol. 27, no. 3, pp. 1–49, Jan. 2022. [CrossRef]
- M. S. Jaafaru, N. A. M. S. Jaafaru, N. A. Abd Karim, M. E. Enas, P. Rollin, E. Mazzon, and A. F. Abdull Razis, “Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs).,” Nutrients, vol. 10, no. 5, pp. 1–15, May 2018. [CrossRef]
- M. Thakur and V. Nanda, “Composition and functionality of bee pollen: A review,” Trends Food Sci. Technol., vol. 98, no. November 2019, pp. 82–106, 2020. [CrossRef]
- A. M. Posadino et al., “An updated overview of cyanidins for chemoprevention and cancer therapy.,” Biomed. Pharmacother., vol. 163, p. 114783, Jul. 2023. [CrossRef]
- W. Li, C. W. Li, C. Peng, L. Zhaojie, and W. Wei, “Chemopreventive and therapeutic properties of anthocyanins in breast cancer: A comprehensive review,” Nutr. Res., vol. 107, no. 700, pp. 48–64, 2022. [CrossRef]
- R. de P. do Nascimento and A. P. da F. Machado, “The preventive and therapeutic effects of anthocyanins on colorectal cancer: A comprehensive review based on up-to-date experimental studies,” Food Res. Int., vol. 170, no. January, p. 113028, Aug. 2023. [CrossRef]
- H. Zhong et al., “Protective Effect of Anthocyanins against Neurodegenerative Diseases through the Microbial-Intestinal-Brain Axis: A Critical Review.,” Nutrients, vol. 15, no. 3, Jan. 2023. [CrossRef]
- A. Zaa, Á. J. Marcelo, Z. An, J. L. Medina-Franco, and M. A. Velasco-Velázquez, “Anthocyanins: Molecular Aspects on Their Neuroprotective Activity.,” Biomolecules, vol. 13, no. 11, p. 1598, Oct. 2023. [CrossRef]
- S. K. Panchal and L. Brown, “Potential Benefits of Anthocyanins in Chronic Disorders of the Central Nervous System.,” Molecules, vol. 28, no. 1, pp. 1–14, Dec. 2022. [CrossRef]
- A. El Ghouizi et al., “Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties,” Antioxidants, vol. 12, no. 3, pp. 1–31, 2023. [CrossRef]
- J. Zhang et al., “Protective Mechanism of Fagopyrum esculentum Moench. Bee Pollen EtOH Extract Against Type II Diabetes in a High-Fat Diet/Streptozocin-Induced C57BL/6J Mice,” Front. Nutr., vol. 9, no. June, pp. 1–12, 2022. [CrossRef]
- M. F. Santa Bárbara et al., “Storage methods, phenolic composition, and bioactive properties of Apis mellifera and Trigona spinipes pollen,” J. Apic. Res., vol. 60, no. 1, pp. 99–107, Jan. 2021. [CrossRef]
- M. Bakour et al., “Exploring the Palynological, Chemical, and Bioactive Properties of Non-Studied Bee Pollen and Honey from Morocco,” Molecules, vol. 27, no. 18, 2022. [CrossRef]
- H. Laaroussi et al., “Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats,” Food Res. Int., vol. 138, no. May, 2020. [CrossRef]
- F. Li et al., “Bioactive constituents of f. Esculentum bee pollen and quantitative analysis of samples collected from seven areas by HPLC,” Molecules, vol. 24, no. 15, 2019. [CrossRef]
- J. Bertoncelj, T. J. Bertoncelj, T. Polak, T. Pucihar, N. Lilek, A. Kandolf Borovšak, and M. Korošec, “Carbohydrate composition of Slovenian bee pollens,” Int. J. Food Sci. Technol., vol. 53, no. 8, pp. 1880–1888, 2018. [CrossRef]
- M. G. R. Campos et al., “Pollen composition and standardisation of analytical methods,” J. Apic. Res., vol. 47, no. 2, pp. 154–161, 2008. [CrossRef]
- V. Aylanc, S. I. V. Aylanc, S. I. Falcão, and M. Vilas-Boas, “Bee pollen and bee bread nutritional potential: Chemical composition and macronutrient digestibility under in vitro gastrointestinal system,” Food Chem., vol. 413, no. January, p. 135597, Jul. 2023. [CrossRef]
- H. Zheng et al., “Effects of Four Extraction Methods on Structure and In Vitro Fermentation Characteristics of Soluble Dietary Fiber from Rape Bee Pollen,” Molecules, vol. 28, no. 12, p. 4800, 2023. [CrossRef]
- H. M. M. Aldgini, A. H. M. M. Aldgini, A. Abdullah Al-Abbadi, E. S. M. Abu-Nameh, and R. O. Alghazeer, “Determination of metals as bio indicators in some selected bee pollen samples from Jordan,” Saudi J. Biol. Sci., vol. 26, no. 7, pp. 1418–1422, 2019. [CrossRef]
- S. S. Altunatmaz, D. S. S. Altunatmaz, D. Tarhan, F. Aksu, U. B. Barutçu, and M. E. Or, “Mineral element and heavy metal (Cadmium, lead and arsenic) levels of bee pollen in Turkey,” Food Sci. Technol., vol. 37, pp. 136–141, 2017. [CrossRef]
- D. Turck et al., “Scientific opinion on the tolerable upper intake level for selenium,” EFSA J., vol. 21, no. 1, 2023. [CrossRef]
- G. Alimoglu, E. G. Alimoglu, E. Guzelmeric, P. I. Yuksel, C. Celik, I. Deniz, and E. Yesilada, “Monofloral and polyfloral bee pollens: Comparative evaluation of their phenolics and bioactivity profiles,” LWT, vol. 142, no. August 2020, p. 110973, May 2021. [CrossRef]
- R. Végh, G. R. Végh, G. Sipiczki, and M. Csóka, “Investigating the Antioxidant and Color Properties of Bee Pollens of Various Plant Sources,” Chem. Biodivers., 2023. [CrossRef]
- M. D. Belina-Aldemita, M. M. D. Belina-Aldemita, M. Schreiner, and S. D’Amico, “Characterization of phenolic compounds and antioxidative potential of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines,” J. Food Biochem., vol. 44, no. 1, pp. 1–14, 2020. [CrossRef]
- S. Castiglioni, M. S. Castiglioni, M. Stefano, P. Astolfi, M. Pisani, and P. Carloni, “Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour, Using a Chemometric Approach,” Molecules, vol. 27, no. 22, p. 7996, Nov. 2022. [CrossRef]
- G. Rocchetti, S. G. Rocchetti, S. Castiglioni, G. Maldarizzi, P. Carloni, and L. Lucini, “UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin,” Int. J. Food Sci. Technol., vol. 54, no. 2, pp. 335–346, 2019. [CrossRef]
- T. Sawicki, M. T. Sawicki, M. Starowicz, L. Kłębukowska, and P. Hanus, “The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products,” Molecules, vol. 27, no. 4, p. 1301, Feb. 2022. [CrossRef]
- S. Yan et al., “Effect of fermented bee pollen on metabolic syndrome in high-fat diet-induced mice,” Food Sci. Hum. Wellness, vol. 10, no. 3, pp. 345–355, 2021. [CrossRef]
- A. Castagna et al., “Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen?,” Molecules, vol. 25, no. 21, pp. 1–15, 2020. [CrossRef]
- Salonen, A. Lavola, V. Virjamo, and R. Julkunen-Tiitto, “Protein and phenolic content and antioxidant capacity of honey bee-collected unifloral pollen pellets from Finland,” J. Apic. Res., vol. 60, no. 5, pp. 744–750, Oct. 2021. [CrossRef]
- J. E. Oyarzún et al., “Honeybee Pollen Extracts Reduce Oxidative Stress and Steatosis in Hepatic Cells,” Molecules, vol. 26, no. 1, Dec. 2021. [CrossRef]
- P. Khongkarat, P. P. Khongkarat, P. Phuwapraisirisan, and C. Chanchao, “Phytochemical content, especially spermidine derivatives, presenting antioxidant and antilipoxygenase activities in Thai bee pollens.,” PeerJ, vol. 10, p. e13506, 2022. [CrossRef]
- Y. C. Gercek, S. Y. C. Gercek, S. Celik, and S. Bayram, “Screening of Plant Pollen Sources, Polyphenolic Compounds, Fatty Acids and Antioxidant/Antimicrobial Activity from Bee Pollen.,” Molecules, vol. 27, no. 1, pp. 1–13, Dec. 2021. [CrossRef]
- N. Cheng, S. N. Cheng, S. Chen, X. Liu, H. Zhao, and W. Cao, “Impact of schisandrachinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in highfat diet induced obese mice,” Nutrients, vol. 11, no. 2, 2019. [CrossRef]
- Y. Zou et al., “The botanical origin and antioxidant, anti-BACE1 and antiproliferative properties of bee pollen from different regions of South Korea.,” BMC Complement. Med. Ther., vol. 20, no. 1, p. 236, Jul. 2020. [CrossRef]
- N. Mayda, A. N. Mayda, A. Özkök, N. Ecem Bayram, Y. C. Gerçek, and K. Sorkun, “Bee bread and bee pollen of different plant sources: determination of phenolic content, antioxidant activity, fatty acid and element profiles,” J. Food Meas. Charact., vol. 14, no. 4, pp. 1795–1809, 2020. [CrossRef]
- M. Thakur and V. Nanda, “Screening of Indian bee pollen based on antioxidant properties and polyphenolic composition using UHPLC-DAD-MS/MS: A multivariate analysis and ANN based approach,” Food Res. Int., vol. 140, no. December 2020, p. 110041, 2021. [CrossRef]
- S. Çelik, N. S. Çelik, N. Kutlu, Y. C. Gerçek, S. Bayram, R. Pandiselvam, and N. E. Bayram, “Optimization of Ultrasonic Extraction of Nutraceutical and Pharmaceutical Compounds from Bee Pollen with Deep Eutectic Solvents Using Response Surface Methodology,” Foods, vol. 11, no. 22, pp. 1–19, 2022. [CrossRef]
- I. Ilie et al., “Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria,” Antioxidants, vol. 11, no. 5, 2022. [CrossRef]
- M. S. Rodríguez-Flores, O. M. S. Rodríguez-Flores, O. Escuredo, M. C. Seijo, S. Rojo, M. Vilas-Boas, and S. I. Falcão, “Phenolic Profile of Castanea Bee Pollen from the Northwest of the Iberian Peninsula,” Separations, vol. 10, no. 4, pp. 1–13, Apr. 2023. [CrossRef]
- V. Aylanc, A. V. Aylanc, A. Tomás, P. Russo-Almeida, S. I. Falcão, and M. Vilas-Boas, “Assessment of bioactive compounds under simulated gastrointestinal digestion of bee pollen and bee bread: Bioaccessibility and antioxidant activity,” Antioxidants, vol. 10, no. 5, 2021. [CrossRef]
- V. Adaškevičiūtė, V. V. Adaškevičiūtė, V. Kaškonienė, K. Barčauskaitė, P. Kaškonas, and A. Maruška, “The Impact of Fermentation on Bee Pollen Polyphenolic Compounds Composition.,” Antioxidants (Basel, Switzerland), vol. 11, no. 4, pp. 1–16, Mar. 2022. [CrossRef]
- Y. Salazar-González et al., “Carotenoid profile determination of bee pollen by advanced digital image analysis,” Comput. Electron. Agric., vol. 175, no. May, p. 105601, Aug. 2020. [CrossRef]
- Y. X. Bi et al., “Effects of hot-air drying temperature on drying characteristics and color deterioration of rape bee pollen,” Food Chem. X, vol. 16, no. July, p. 100464, 2022. [CrossRef]
- M. Vanderplanck, P.-L. M. Vanderplanck, P.-L. Zerck, G. Lognay, and D. Michez, “Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies?,” Apidologie, vol. 51, no. 5, pp. 826–843, Oct. 2020. [CrossRef]
- M. M. Özcan et al., “Determination of Antioxidant Activity, Phenolic Compound, Mineral Contents and Fatty Acid Compositions of Bee Pollen Grains Collected from Different Locations,” J. Apic. Sci., vol. 63, no. 1, pp. 69–79, Jun. 2019. [CrossRef]
- K. Fatrcová-Šramková, J. K. Fatrcová-Šramková, J. Nôžková, M. Máriássyová, and M. Kačániová, “Biologically active antimicrobial and antioxidant substances in the Helianthus annuus L. bee pollen.,” J. Environ. Sci. Health. B., vol. 51, no. 3, pp. 176–81, 2016. [CrossRef]
- V. Aylanc et al., “Evaluation of Antioxidant and Anticancer Activity of Mono- and Polyfloral Moroccan Bee Pollen by Characterizing Phenolic and Volatile Compounds.,” Molecules, vol. 28, no. 2, pp. 1–20, Jan. 2023. [CrossRef]
- H. Zhang, Q. H. Zhang, Q. Lu, and R. Liu, “Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen,” Food Chem., vol. 375, no. July 2021, p. 131908, May 2022. [CrossRef]
- N. Rzetecka, E. N. Rzetecka, E. Matuszewska, S. Plewa, J. Matysiak, and A. Klupczynska-Gabryszak, “Bee products as valuable nutritional ingredients: Determination of broad free amino acid profiles in bee pollen, royal jelly, and propolis,” J. Food Compos. Anal., vol. 126, no. November 2023, p. 105860, 2024. [CrossRef]
- M. Martinello and F. Mutinelli, “Antioxidant Activity in Bee Products: A Review.,” Antioxidants (Basel, Switzerland), vol. 10, no. 1, pp. 1–42, Jan. 2021. [CrossRef]
- H. A. Alfawaz, A. H. A. Alfawaz, A. El-Ansary, L. Al-Ayadhi, R. S. Bhat, and W. M. Hassan, “Protective Effects of Bee Pollen on Multiple Propionic Acid-Induced Biochemical Autistic Features in a Rat Model.,” Metabolites, vol. 12, no. 7, p. 571, Jun. 2022. [CrossRef]
- M. Węglińska, R. M. Węglińska, R. Szostak, A. Kita, A. Nemś, and S. Mazurek, “Determination of nutritional parameters of bee pollen by Raman and infrared spectroscopy,” Talanta, vol. 212, no. November 2019, p. 120790, 2020. [CrossRef]
- S. N. Al-Kahtani, A. A. S. N. Al-Kahtani, A. A. Alaqil, and A. O. Abbas, “Modulation of Antioxidant Defense, Immune Response, and Growth Performance by Inclusion of Propolis and Bee Pollen into Broiler Diets,” Animals, vol. 12, no. 13, pp. 1–13, 2022. [CrossRef]
- Y. Xu et al., “Impact of camellia japonica bee pollen polyphenols on hyperuricemia and gut microbiota in potassium oxonate-induced mice,” Nutrients, vol. 13, no. 8, p. 2665, Jul. 2021. [CrossRef]
- J. B. Suleiman, A. B. A. J. B. Suleiman, A. B. A. Bakar, and M. Mohamed, “Review on Bee Products as Potential Protective and Therapeutic Agents in Male Reproductive Impairment,” Molecules, vol. 26, no. 11, p. 3421, Jun. 2021. [CrossRef]
- W. Kędzierski et al., “Bee Pollen Supplementation to Aged Horses Influences Several Blood Parameters,” J. Equine Vet. Sci., vol. 90, p. 103024, Jul. 2020. [CrossRef]
- A. M. K. K. Nassar et al., “Ameliorative effects of honey, propolis, pollen, and royal jelly mixture against chronic toxicity of sumithion insecticide in white albino rats,” Molecules, vol. 25, no. 11, pp. 1–15, Jun. 2020. [CrossRef]
- J. O. Lopes et al., “Anti-Inflammatory and antinociceptive activity of pollen extract collected by stingless bee Melipona fasciculata,” Int. J. Mol. Sci., vol. 20, no. 18, pp. 1–20, Sep. 2019. [CrossRef]
- L. Lawag, O. L. Lawag, O. Yoo, L. Y. Lim, K. Hammer, and C. Locher, “Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents,” Antioxidants, vol. 10, no. 7, p. 1113, Jul. 2021. [CrossRef]
- K. A. Walker, N. K. A. Walker, N. Basisty, D. M. Wilson, and L. Ferrucci, “Connecting aging biology and inflammation in the omics era,” J. Clin. Invest., vol. 132, no. 14, 2022. [CrossRef]
- X. Li, C. X. Li, C. Li, W. Zhang, Y. Wang, P. Qian, and H. Huang, “Inflammation and aging: signaling pathways and intervention therapies,” Signal Transduct. Target. Ther., vol. 8, no. 1, p. 239, Jun. 2023. [CrossRef]
- T. Behl et al., “Current trends in neurodegeneration: Cross talks between oxidative stress, cell death, and inflammation,” Int. J. Mol. Sci., vol. 22, no. 14, 2021. [CrossRef]
- A. Merelli, M. A. Merelli, M. Repetto, A. Lazarowski, and J. Auzmendi, “Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases,” J. Alzheimer’s Dis., vol. 82, no. s1, pp. S109–S126, Dec. 2021. [CrossRef]
- S. M. Afify, G. S. M. Afify, G. Hassan, A. Seno, and M. Seno, “Cancer-inducing niche: the force of chronic inflammation,” Br. J. Cancer, vol. 127, no. 2, pp. 193–201, 2022. [CrossRef]
- S. S. Sohrab et al., “Chronic Inflammation’s Transformation to Cancer: A Nanotherapeutic Paradigm,” Molecules, vol. 28, no. 11, pp. 1–19, 2023. [CrossRef]
- S. Alfaro, V. S. Alfaro, V. Acuña, R. Ceriani, M. F. Cavieres, C. R. Weinstein-Oppenheimer, and C. Campos-Estrada, “Involvement of Inflammation and Its Resolution in Disease and Therapeutics,” Int. J. Mol. Sci., vol. 23, no. 18, 2022. [CrossRef]
- J. Park, C. J. J. Park, C. J. Langmead, and D. M. Riddy, “New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery,” ACS Pharmacol. Transl. Sci., vol. 3, no. 1, pp. 88–106, 2020. [CrossRef]
- A. Fishbein, B. D. A. Fishbein, B. D. Hammock, C. N. Serhan, and D. Panigrahy, “Carcinogenesis: Failure of resolution of inflammation?,” Pharmacol. Ther., vol. 218, 2021. [CrossRef]
- S. Chamani et al., “Resolution of Inflammation in Neurodegenerative Diseases: The Role of Resolvins,” Mediators Inflamm., vol. 2020, 2020. [CrossRef]
- Habryka, R. Socha, and L. Juszczak, “Effect of Bee Pollen Addition on the Polyphenol Content, Antioxidant Activity, and Quality Parameters of Honey,” Antioxidants, vol. 10, no. 5, p. 810, May 2021. [CrossRef]
- Sears and A., K. Saha, “Dietary Control of Inflammation and Resolution,” Front. Nutr., vol. 8, no. August, pp. 1–19, 2021. [CrossRef]
- B. Sears, M. B. Sears, M. Perry, and A. K. Saha, “Dietary Technologies to Optimize Healing from Injury-Induced Inflammation,” Antiinflamm. Antiallergy. Agents Med. Chem., vol. 20, no. 2, pp. 123–131, 2020. [CrossRef]
- A. Kozłowska and T. Dzierżanowski, “Targeting inflammation by anthocyanins as the novel therapeutic potential for chronic diseases: An update,” Molecules, vol. 26, no. 14, 2021. [CrossRef]
- A. Merecz-Sadowska, P. A. Merecz-Sadowska, P. Sitarek, T. Śliwiński, and R. Zajdel, “Anti-inflammatory activity of extracts and pure compounds derived from plants via modulation of signaling pathways, especially pi3k/akt in macrophages,” Int. J. Mol. Sci., vol. 21, no. 24, pp. 1–32, 2020. [CrossRef]
- Y. Sun et al., “Kaempferol has potential anti-coronavirus disease 2019 (COVID-19) targets based on bioinformatics analyses and pharmacological effects on endotoxin-induced cytokine storm.,” Phytother. Res., vol. 37, no. 6, pp. 2290–2304, Jun. 2023. [CrossRef]
- Y. Wang, W. Y. Wang, W. Smith, D. Hao, B. He, and L. Kong, “M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds,” Int. Immunopharmacol., vol. 70, no. January, pp. 459–466, 2019. [CrossRef]
- K. D. Moudgil and S. H. Venkatesha, “The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation,” Int. J. Mol. Sci., vol. 24, no. 1, 2023. [CrossRef]
- Z. Chang, Y. Z. Chang, Y. Wang, C. Liu, W. Smith, and L. Kong, “Natural Products for Regulating Macrophages M2 Polarization.,” Curr. Stem Cell Res. Ther., vol. 15, no. 7, pp. 559–569, 2020. [CrossRef]
- M. Kosedag and M. Gulaboglu, “Pollen and bee bread expressed highest anti-inflammatory activities among bee products in chronic inflammation: an experimental study with cotton pellet granuloma in rats,” Inflammopharmacology, no. 0123456789, 2023. [CrossRef]
- V. Deretic, “Autophagy in inflammation, infection, and immunometabolism,” Immunity, vol. 54, no. 3, pp. 437–453, 2021. [CrossRef]
- Y. Aman et al., “Autophagy in healthy aging and disease,” Nat. Aging, vol. 1, no. 8, pp. 634–650, Aug. 2021. [CrossRef]
- J. Klionsky et al., “Autophagy in major human diseases.,” EMBO J., vol. 40, no. 19, p. e108863, Oct. 2021. [CrossRef]
- R. M. Abdel-Megeed and M. O. Kadry, “Amelioration of autophagy and inflammatory signaling pathways via α-lipoic acid, burdock and bee pollen versus lipopolysaccharide-induced insulin resistance in murine model,” Heliyon, vol. 9, no. 5, p. e15692, 2023. [CrossRef]
- Y. Yang, L. Y. Yang, L. Liu, I. Naik, Z. Braunstein, J. Zhong, and B. Ren, “Transcription factor C/EBP homologous protein in health and diseases,” Front. Immunol., vol. 8, no. NOV, 2017. [CrossRef]
- X. Li, H. X. Li, H. Gong, S. Yang, L. Yang, Y. Fan, and Y. Zhou, “Pectic Bee Pollen Polysaccharide from Rosa rugosa Alleviates Diet-Induced Hepatic Steatosis and Insulin Resistance via Induction of AMPK/mTOR-Mediated Autophagy,” Molecules, vol. 22, no. 5, 2017. [CrossRef]
- A.M. Ali and H. Kunugi, Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen, vol. 9, no. 10. 2020. [CrossRef]
- J. M. Park, D. H. J. M. Park, D. H. Lee, and D. H. Kim, “Redefining the role of AMPK in autophagy and the energy stress response,” Nat. Commun., vol. 14, no. 1, 2023. [CrossRef]
- V. Corby-Harris et al., “Diet and pheromones interact to shape honey bee (Apis mellifera) worker physiology,” J. Insect Physiol., vol. 143, no. August, p. 104442, 2022. [CrossRef]
- H. Yamamoto, S. H. Yamamoto, S. Zhang, and N. Mizushima, “Autophagy genes in biology and disease,” Nat. Rev. Genet., vol. 24, no. 6, pp. 382–400, 2023. [CrossRef]
- A. U. Ahsan, V. L. A. U. Ahsan, V. L. Sharma, A. Wani, and M. Chopra, “Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid (1-42) Evoked Neurotoxicity.,” Mol. Neurobiol., vol. 57, no. 8, pp. 3589–3602, Aug. 2020. [CrossRef]
- A. Yammine et al., “Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases.,” Cells, vol. 9, no. 11, Oct. 2020. [CrossRef]
- L. Li, R. L. Li, R. Zhou, H. Lv, L. Song, X. Xue, and L. Wu, “Inhibitive Effect of Luteolin on Sevoflurane-Induced Neurotoxicity through Activation of the Autophagy Pathway by HMOX1.,” ACS Chem. Neurosci., vol. 12, no. 18, pp. 3314–3322, Sep. 2021. [CrossRef]
- H. Moon and J.-M. Yun, “Neuroprotective effects of hesperetin on H2O2-induced damage in neuroblastoma SH-SY5Y cells.,” Nutr. Res. Pract., vol. 17, no. 5, pp. 899–916, Oct. 2023. [CrossRef]
- Z. Liu et al., “Kaemperfol Protects Dopaminergic Neurons by Promoting mTOR-Mediated Autophagy in Parkinson’s Disease Models,” Neurochem. Res., vol. 48, no. 5, pp. 1395–1411, 2023. [CrossRef]
- Z. Cui et al., “Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism,” Front. Immunol., vol. 13, no. July, pp. 1–26, 2022. [CrossRef]
- H. Baek, Sanjay, M. Park, and H.-J. Lee, “Cyanidin-3-O-glucoside protects the brain and improves cognitive function in APPswe/PS1ΔE9 transgenic mice model.,” J. Neuroinflammation, vol. 20, no. 1, p. 268, Nov. 2023. [CrossRef]
- L. Zhou et al., “Chrysin induces autophagy-dependent ferroptosis to increase chemosensitivity to gemcitabine by targeting CBR1 in pancreatic cancer cells.,” Biochem. Pharmacol., vol. 193, no. September, p. 114813, Nov. 2021. [CrossRef]
- H. Gong, “Pinocembrin suppresses proliferation and enhances apoptosis in lung cancer cells in vitro by restraining autophagy.,” Bioengineered, vol. 12, no. 1, pp. 6035–6044, Dec. 2021. [CrossRef]
- L. Huang, X. L. Huang, X. Zhong, S. Qin, and M. Deng, “Protocatechuic acid attenuates β-secretase activity and okadaic acid-induced autophagy via the Akt/GSK-3β/MEF2D pathway in PC12 cells,” Mol. Med. Rep., vol. 21, no. 3, pp. 1328–1335, 2020. [CrossRef]
- M. T. Ardah, N. M. T. Ardah, N. Eid, T. Kitada, and M. E. Haque, “Ellagic Acid Prevents α-Synuclein Aggregation and Protects SH-SY5Y Cells from Aggregated α-Synuclein-Induced Toxicity via Suppression of Apoptosis and Activation of Autophagy.,” Int. J. Mol. Sci., vol. 22, no. 24, Dec. 2021. [CrossRef]
- B. Coeli-Lacchini et al., “Spermidine Suppresses Oral Carcinogenesis through Autophagy Induction, DNA Damage Repair, and Oxidative Stress Reduction,” Am. J. Pathol., vol. 193, no. 12, pp. 2172–2181, 2023. [CrossRef]
- L. Leak and S. J. Dixon, “Surveying the landscape of emerging and understudied cell death mechanisms,” Biochim. Biophys. Acta - Mol. Cell Res., vol. 1870, no. 3, p. 119432, 2023. [CrossRef]
- M. E. Mercau, S. M. E. Mercau, S. Patwa, K. P. L. Bhat, S. Ghosh, and C. V. Rothlin, “Cell death in development, maintenance, and diseases of the nervous system,” Semin. Immunopathol., vol. 44, no. 5, pp. 725–738, 2022. [CrossRef]
- M. Bonora et al., “Physiopathology of the permeability transition pore: Molecular mechanisms in human pathology,” Biomolecules, vol. 10, no. 7, pp. 1–25, 2020. [CrossRef]
- A. Z. Spitz and E. Gavathiotis, “Physiological and pharmacological modulation of BAX,” Trends Pharmacol. Sci., vol. 43, no. 3, pp. 206–220, 2022. [CrossRef]
- A. Lopez et al., “Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer,” Nat. Commun., vol. 13, no. 1, 2022. [CrossRef]
- S. Kumari, R. S. Kumari, R. Dhapola, and D. H. K. Reddy, “Apoptosis in Alzheimer’s disease: insight into the signaling pathways and therapeutic avenues,” Apoptosis, vol. 28, no. 7, pp. 943–957, 2023. [CrossRef]
- N. S. Erekat, “Apoptosis and its therapeutic implications in neurodegenerative diseases,” Clin. Anat., vol. 35, no. 1, pp. 65–78, Jan. 2022. [CrossRef]
- A. Lahalle, M. A. Lahalle, M. Lacroix, C. de Blasio, M. Y. Cissé, L. K. Linares, and L. le Cam, “The p53 pathway and metabolism: The tree that hides the forest,” Cancers (Basel)., vol. 13, no. 1, pp. 1–17, Jan. 2021. [CrossRef]
- P. L. Vaddavalli and B. Schumacher, “The p53 network: cellular and systemic DNA damage responses in cancer and aging,” Trends Genet., vol. 38, no. 6, pp. 598–612, 2022. [CrossRef]
- S. Bedoui, M. J. S. Bedoui, M. J. Herold, and A. Strasser, “Emerging connectivity of programmed cell death pathways and its physiological implications,” Nat. Rev. Mol. Cell Biol., vol. 21, no. 11, pp. 678–695, 2020. [CrossRef]
- L. E. Araya, I. V. L. E. Araya, I. V. Soni, J. A. Hardy, and O. Julien, “Deorphanizing Caspase-3 and Caspase-9 Substrates in and out of Apoptosis with Deep Substrate Profiling,” ACS Chem. Biol., vol. 16, no. 11, pp. 2280–2296, 2021. [CrossRef]
- R. Mărgăoan, M. R. Mărgăoan, M. Zăhan, L. A. Mărghitaş, D. S. Dezmirean, S. Erler, and O. Bobiş, “Antiproliferative activity and apoptotic effects of Filipendula ulmaria pollen against C26 mice colon tumour cells,” J. Apic. Sci., vol. 60, no. 1, pp. 135–144, 2016. [CrossRef]
- T. Saisavoey, P. T. Saisavoey, P. Sangtanoo, P. Srimongkol, O. Reamtong, and A. Karnchanatat, “Hydrolysates from bee pollen could induced apoptosis in human bronchogenic carcinoma cells (ChaGo-K-1),” J. Food Sci. Technol., vol. 58, no. 2, pp. 752–763, 2021. [CrossRef]
- N. A. N. Hanafy, E. A. B. N. A. N. Hanafy, E. A. B. Eltonouby, E. I. Salim, M. E. Mahfouz, S. Leporatti, and E. H. Hafez, “Simultaneous Administration of Bevacizumab with Bee-Pollen Extract-Loaded Hybrid Protein Hydrogel NPs Is a Promising Targeted Strategy against Cancer Cells,” Int. J. Mol. Sci., vol. 24, no. 4, p. 3548, Feb. 2023. [CrossRef]
- H. Huang, Z. H. Huang, Z. Shen, Q. Geng, Z. Wu, P. Shi, and X. Miao, “Protective effect of Schisandra chinensis bee pollen extract on liver and kidney injury induced by cisplatin in rats,” Biomed. Pharmacother., vol. 95, no. September, pp. 1765–1776, 2017. [CrossRef]
- A. Ben Bacha, A. O. A. Ben Bacha, A. O. Norah, M. Al-Osaimi, A. H. Harrath, L. Mansour, and A. El-Ansary, “The therapeutic and protective effects of bee pollen against prenatal methylmercury induced neurotoxicity in rat pups,” Metab. Brain Dis., vol. 35, no. 1, pp. 215–224, 2020. [CrossRef]
- I. Costa et al., “Molecular mechanisms of ferroptosis and their involvement in brain diseases,” Pharmacol. Ther., vol. 244, p. 108373, Apr. 2023. [CrossRef]
- X. Wang, Y. X. Wang, Y. Zhou, J. Min, and F. Wang, “Zooming in and out of ferroptosis in human disease.,” Front. Med., vol. 17, no. 2, pp. 173–206, Apr. 2023. [CrossRef]
- Y. Du and Z. Guo, “Recent progress in ferroptosis: inducers and inhibitors,” Cell Death Discov., vol. 8, no. 1, pp. 1–10, Dec. 2022. [CrossRef]
- Coradduzza, A. Congiargiu, Z. Chen, A. Zinellu, C. Carru, and S. Medici, “Ferroptosis and Senescence: A Systematic Review.,” Int. J. Mol. Sci., vol. 24, no. 4, Feb. 2023. [CrossRef]
- Y. Chen, Z.-M. M. Y. Chen, Z.-M. M. Fang, X. Yi, X. Wei, and D.-S. S. Jiang, “The interaction between ferroptosis and inflammatory signaling pathways,” Cell Death Dis., vol. 14, no. 3, pp. 1–13, Mar. 2023. [CrossRef]
- S. Sun, J. S. Sun, J. Shen, J. Jiang, F. Wang, and J. Min, “Targeting ferroptosis opens new avenues for the development of novel therapeutics.,” Signal Transduct. Target. Ther., vol. 8, no. 1, p. 372, Sep. 2023. [CrossRef]
- Y. Gu, Y. Y. Gu, Y. Li, J. Wang, L. Zhang, J. Zhang, and Y. Wang, “Targeting ferroptosis: Paving new roads for drug design and discovery,” Eur. J. Med. Chem., vol. 247, no. November 2022, p. 115015, Feb. 2023. [CrossRef]
- X. Zhao, X. X. Zhao, X. Wang, and Y. Pang, “Phytochemicals Targeting Ferroptosis: Therapeutic Opportunities and Prospects for Treating Breast Cancer.,” Pharmaceuticals (Basel)., vol. 15, no. 11, Nov. 2022. [CrossRef]
- K. Khorsandi, Z. K. Khorsandi, Z. Kianmehr, Z. Hosseinmardi, and R. Hosseinzadeh, “Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis.,” Cancer Cell Int., vol. 20, no. 1, p. 18, 2020. [CrossRef]
- Z.-X. Wang et al., “Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis.,” Br. J. Pharmacol., vol. 178, no. 5, pp. 1133–1148, Mar. 2021. [CrossRef]
- Z.-H. Lin et al., “Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson’s Disease by Inhibiting Ferroptosis.,” Oxid. Med. Cell. Longev., vol. 2022, p. 7769355, 2022. [CrossRef]
- C. Shao et al., “Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis.,” Proc. Natl. Acad. Sci. U. S. A., vol. 117, no. 19, pp. 10155–10164, May 2020. [CrossRef]
- P. Amini et al., “Induction of Cancer Cell Death by Apigenin: A Review on Different Cell Death Pathways.,” Mini Rev. Med. Chem., vol. 23, no. 14, pp. 1461–1478, 2023. [CrossRef]
- O. A. Ojo, D. E. O. A. Ojo, D. E. Rotimi, A. B. Ojo, A. D. Ogunlakin, and B. O. Ajiboye, “Gallic acid abates cadmium chloride toxicity via alteration of neurotransmitters and modulation of inflammatory markers in Wistar rats.,” Sci. Rep., vol. 13, no. 1, p. 1577, Jan. 2023. [CrossRef]
- Z. Hong et al., “Ferroptosis-related Genes for Overall Survival Prediction in Patients with Colorectal Cancer can be Inhibited by Gallic acid.,” Int. J. Biol. Sci., vol. 17, no. 4, pp. 942–956, 2021. [CrossRef]
- M. Lesjak, N. M. Lesjak, N. Simin, and S. K. S. Srai, “Can Polyphenols Inhibit Ferroptosis?,” Antioxidants (Basel, Switzerland), vol. 11, no. 1, pp. 1–28, Jan. 2022. [CrossRef]
- K. Zheng, Y. K. Zheng, Y. Dong, R. Yang, Y. Liang, H. Wu, and Z. He, “Regulation of ferroptosis by bioactive phytochemicals: Implications for medical nutritional therapy.,” Pharmacol. Res., vol. 168, no. March, p. 105580, Jun. 2021. [CrossRef]
- Z. Tan, H. Z. Tan, H. Huang, W. Sun, Y. Li, and Y. Jia, “Current progress of ferroptosis study in ovarian cancer.,” Front. Mol. Biosci., vol. 9, no. August, p. 966007, 2022. [CrossRef]
- W. Zhou et al., “Antioxidant and immunomodulatory activities in vitro of polysaccharides from bee collected pollen of Chinese wolfberry,” Int. J. Biol. Macromol., vol. 163, pp. 190–199, 2020. [CrossRef]
- S. Y. Zhu, J. Z. S. Y. Zhu, J. Z. Jiang, J. Lin, L. Liu, J. Y. Guo, and J. L. Li, “Lycopene ameliorates atrazine-induced spatial learning and memory impairments by inhibiting ferroptosis in the hippocampus of mice,” Food Chem. Toxicol., vol. 174, no. January 2022, p. 113655, 2023. [CrossRef]
- R. Pap et al., “Protective Effects of 3’-Epilutein and 3’-Oxolutein against Glutamate-Induced Neuronal Damage.,” Int. J. Mol. Sci., vol. 24, no. 15, Jul. 2023. [CrossRef]
- Xu, H. Wang, X. Li, R. Huang, and L. Luo, “Recent progress on targeting ferroptosis for cancer therapy.,” Biochem. Pharmacol., vol. 190, no. April, p. 114584, Aug. 2021. [CrossRef]
- V. Stepanić and M. Kučerová-Chlupáčová, “Review and Chemoinformatic Analysis of Ferroptosis Modulators with a Focus on Natural Plant Products,” Molecules, vol. 28, no. 2, Jan. 2023. [CrossRef]
- M. Jakaria, A. A. M. Jakaria, A. A. Belaidi, A. I. Bush, and S. Ayton, “Vitamin A metabolites inhibit ferroptosis,” Biomed. Pharmacother., vol. 164, p. 114930, Aug. 2023. [CrossRef]
- Y. Liu et al., “Vitamin C Sensitizes Pancreatic Cancer Cells to Erastin-Induced Ferroptosis by Activating the AMPK/Nrf2/HMOX1 Pathway,” Oxid. Med. Cell. Longev., vol. 2022, 2022. [CrossRef]
- J. Li et al., “Ferroptosis: past, present and future,” Cell Death Dis., vol. 11, no. 2, 2020. [CrossRef]
- Y. Wang, S. Y. Wang, S. Wu, Q. Li, H. Sun, and H. Wang, “Pharmacological Inhibition of Ferroptosis as a Therapeutic Target for Neurodegenerative Diseases and Strokes.,” Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger., vol. 10, no. 24, p. e2300325, Aug. 2023. [CrossRef]
- T. Matsuo et al., “Involvement of ferroptosis in human motor neuron cell death,” Biochem. Biophys. Res. Commun., vol. 566, pp. 24–29, Aug. 2021. [CrossRef]
- Mishima and, M. Conrad, “Nutritional and Metabolic Control of Ferroptosis.,” Annu. Rev. Nutr., vol. 42, pp. 275–309, Aug. 2022. [CrossRef]
- X.-S. Ding et al., “Ferroptosis in Parkinson’s disease: Molecular mechanisms and therapeutic potential.,” Ageing Res. Rev., vol. 91, no. September, p. 102077, Sep. 2023. [CrossRef]
- Y. Qi et al., “Ferroptosis Regulation by Nutrient Signalling,” Nutr. Res. Rev., vol. 35, no. 2, pp. 282–294, 2022. [CrossRef]
- P. H. Chen et al., “Zinc transporter ZIP7 is a novel determinant of ferroptosis,” Cell Death Dis., vol. 12, no. 2, 2021. [CrossRef]
- L. Zhang et al., “Crosstalk between regulated necrosis and micronutrition, bridged by reactive oxygen species,” Front. Nutr., vol. 9, no. September, pp. 1–22, 2022. [CrossRef]
- M.-H. Ge et al., “Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway.,” CNS Neurosci. Ther., vol. 27, no. 9, pp. 1023–40, May 2021. [CrossRef]
- P. Karra et al., “Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome,” Obesity, vol. 30, no. 7, pp. 1323–1334, Jul. 2022. [CrossRef]
- Q. Wu, Z. J. Q. Wu, Z. J. Gao, X. Yu, and P. Wang, “Dietary regulation in health and disease,” Signal Transduct. Target. Ther., vol. 7, no. 1, 2022. [CrossRef]
- C. Vidoni et al., “Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy,” J. Cancer Prev., vol. 26, no. 4, pp. 224–236, 2021. [CrossRef]
- M. Alidadi, M. M. Alidadi, M. Banach, P. C. Guest, S. Bo, T. Jamialahmadi, and A. Sahebkar, “The effect of caloric restriction and fasting on cancer,” Semin. Cancer Biol., vol. 73, no. September 2020, pp. 30–44, 2021. [CrossRef]
- S. J. Hofer, S. S. J. Hofer, S. Davinelli, M. Bergmann, G. Scapagnini, and F. Madeo, “Caloric Restriction Mimetics in Nutrition and Clinical Trials,” Front. Nutr., vol. 8, no. September, pp. 4–6, 2021. [CrossRef]
- J. Martel et al., “Recent advances in the field of caloric restriction mimetics and anti-aging molecules,” Ageing Res. Rev., vol. 66, no. December 2020, p. 101240, 2021. [CrossRef]
- A. Sharma and A. K. Singh, “Molecular mechanism of caloric restriction mimetics-mediated neuroprotection of age-related neurodegenerative diseases: an emerging therapeutic approach,” Biogerontology, vol. 24, no. 5, pp. 679–708, 2023. [CrossRef]
- B. Erbaba, A. B. Erbaba, A. Arslan-Ergul, and M. M. Adams, “Effects of caloric restriction on the antagonistic and integrative hallmarks of aging,” Ageing Res. Rev., vol. 66, no. November 2020, p. 101228, 2021. [CrossRef]
- M. Parizadeh and M.-C. Arrieta, “The global human gut microbiome: genes, lifestyles, and diet.,” Trends Mol. Med., vol. 14, no. 2, pp. 135–159, Jul. 2023. [CrossRef]
- I. Benešová, Ľ. Křížová, and M. Kverka, “Microbiota as the unifying factor behind the hallmarks of cancer,” J. Cancer Res. Clin. Oncol., no. 0123456789, 2023. [CrossRef]
- J. Sun, F. J. Sun, F. Chen, and G. Wu, “Potential effects of gut microbiota on host cancers: focus on immunity, DNA damage, cellular pathways, and anticancer therapy,” ISME J., no. March, pp. 1–17, 2023. [CrossRef]
- H. Zhang et al., “Implications of Gut Microbiota in Neurodegenerative Diseases,” Front. Immunol., vol. 13, no. February, pp. 1–15, 2022. [CrossRef]
- S. G. Sorboni, H. S. S. G. Sorboni, H. S. Moghaddam, R. Jafarzadeh-Esfehani, and S. Soleimanpour, “A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders,” Clin. Microbiol. Rev., vol. 35, no. 1, 2022. [CrossRef]
- Gubert, J. Gasparotto, and L. H Morais, “Convergent pathways of the gut microbiota-brain axis and neurodegenerative disorders.,” Gastroenterol. Rep., vol. 10, p. goac017, 2022. [CrossRef]
- M. P. Singh et al., “Influence of the Gut Microbiota on the Development of Neurodegenerative Diseases,” Mediators Inflamm., vol. 2022, 2022. [CrossRef]
- H. Cheng et al., “Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review,” Phytomedicine, vol. 119, no. April, p. 154979, 2023. [CrossRef]
- P. L. de Freitas, J. P. N. P. L. de Freitas, J. P. N. Miranda, L. M. França, and A. M. de A. Paes, “Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota,” Nutrients, vol. 14, no. 17, pp. 1–15, 2022. [CrossRef]
- T. Bohn et al., “Carotenoids in Health as Studied by Omics-Related Endpoints.,” Adv. Nutr., no. August, p. 138824, Sep. 2023. [CrossRef]
- T. Silva Meneguelli, M. T. Silva Meneguelli, M. Duarte Villas Mishima, H. H. M. Hermsdorff, H. S. D. Martino, J. Bressan, and E. Tako, “Effect of carotenoids on gut health and inflammatory status: A systematic review of in vivo animal studies.,” Crit. Rev. Food Sci. Nutr., pp. 1–16, Jul. 2023. [CrossRef]
- K. Tsiantas, S. J. K. Tsiantas, S. J. Konteles, E. Kritsi, V. J. Sinanoglou, T. Tsiaka, and P. Zoumpoulakis, “Effects of Non-Polar Dietary and Endogenous Lipids on Gut Microbiota Alterations: The Role of Lipidomics,” Int. J. Mol. Sci., vol. 23, no. 8, 2022. [CrossRef]
- N. Zhao et al., “Betaine supplementation alleviates dextran sulfate sodium-induced colitis via regulating the inflammatory response, enhancing the intestinal barrier, and altering gut microbiota.,” Food Funct., vol. 13, no. 24, pp. 12814–12826, Dec. 2022. [CrossRef]
- P. Brecht et al., “How Do Minerals, Vitamins, and Intestinal Microbiota Affect the Development and Progression of Heart Disease in Adult and Pediatric Patients?,” Nutrients, vol. 15, no. 14, Jul. 2023. [CrossRef]
- K. S. Hossain, S. K. S. Hossain, S. Amarasena, and S. Mayengbam, “B Vitamins and Their Roles in Gut Health.,” Microorganisms, vol. 10, no. 6, Jun. 2022. [CrossRef]
- X. Y. Li, L. X. Y. Li, L. Meng, L. Shen, and H. F. Ji, “Regulation of gut microbiota by vitamin C, vitamin E and β-carotene,” Food Res. Int., vol. 169, no. March, p. 112749, 2023. [CrossRef]
- V. Stacchiotti, S. V. Stacchiotti, S. Rezzi, M. Eggersdorfer, and F. Galli, “Metabolic and functional interplay between gut microbiota and fat-soluble vitamins,” Crit. Rev. Food Sci. Nutr., vol. 61, no. 19, pp. 3211–3232, 2021. [CrossRef]
- Lin and D., M. Medeiros, “The microbiome as a major function of the gastrointestinal tract and its implication in micronutrient metabolism and chronic diseases,” Nutr. Res., vol. 112, pp. 30–45, 2023. [CrossRef]
- M. Barone, F. M. Barone, F. D’Amico, P. Brigidi, and S. Turroni, “Gut microbiome–micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins?,” BioFactors, vol. 48, no. 2, pp. 307–314, 2022. [CrossRef]
- V. Bielik and M. Kolisek, “Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome,” Int. J. Mol. Sci., vol. 22, no. 13, 2021. [CrossRef]
- L. Payling, K. L. Payling, K. Fraser, S. M. Loveday, I. Sims, N. Roy, and W. McNabb, “The effects of carbohydrate structure on the composition and functionality of the human gut microbiota,” Trends Food Sci. Technol., vol. 97, no. September 2019, pp. 233–248, 2020. [CrossRef]
- L. P. Mora-Flores, R. L. P. Mora-Flores, R. Moreno-Terrazas Casildo, J. Fuentes-Cabrera, H. A. Pérez-Vicente, G. de Anda-Jáuregui, and E. E. Neri-Torres, “The Role of Carbohydrate Intake on the Gut Microbiome: A Weight of Evidence Systematic Review,” Microorganisms, vol. 11, no. 7, pp. 1–22, 2023. [CrossRef]
- M. Cazzaniga, G. B. M. Cazzaniga, G. B. Zonzini, F. Di Pierro, S. Moricoli, and A. Bertuccioli, “Gut Microbiota, Metabolic Disorders and Breast Cancer: Could Berberine Turn Out to Be a Transversal Nutraceutical Tool? A Narrative Analysis,” Int. J. Mol. Sci., vol. 23, no. 20, 2022. [CrossRef]
- M. Hashim and S. Makpol, “A review of the preclinical and clinical studies on the role of the gut microbiome in aging and neurodegenerative diseases and its modulation,” Front. Cell. Neurosci., vol. 16, no. November, 2022. [CrossRef]
- M. Zhao et al., “Gut microbiota: a potential target for improved cancer therapy,” J. Cancer Res. Clin. Oncol., vol. 149, no. 1, pp. 541–552, 2023. [CrossRef]
- N. Spencer et al., “Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response,” Science (80-. )., vol. 374, no. 6575, pp. 1632–1640, 2021. [CrossRef]
- V. Nandwana et al., “The Role of Microbiome in Brain Development and Neurodegenerative Diseases,” Molecules, vol. 27, no. 11, 2022. [CrossRef]
- M. Blackhurst and K. E. Funk, “Viral pathogens increase risk of neurodegenerative disease.,” Nat. Rev. Neurol., vol. 19, no. 5, pp. 259–260, May 2023. [CrossRef]
- Faccioli, S. Nardelli, S. Gioia, O. Riggio, and L. Ridola, “Neurological and psychiatric effects of hepatitis C virus infection.,” World J. Gastroenterol., vol. 27, no. 29, pp. 4846–4861, Aug. 2021. [CrossRef]
- W. Nelson and L. Mirabello, “Human papillomavirus genomics: Understanding carcinogenicity.,” Tumour virus Res., vol. 15, no. February, p. 200258, Jun. 2023. [CrossRef]
- M. A. Luna-Cuadros, H.-W. M. A. Luna-Cuadros, H.-W. Chen, H. Hanif, M. J. Ali, M. M. Khan, and D. T.-Y. Lau, “Risk of hepatocellular carcinoma after hepatitis C virus cure.,” World J. Gastroenterol., vol. 28, no. 1, pp. 96–107, Jan. 2022. [CrossRef]
- A. Zarei, A. A. Zarei, A. Ramazani, A. Rezaei, and S. Moradi, “Screening of honey bee pollen constituents against COVID-19: an emerging hot spot in targeting SARS-CoV-2-ACE-2 interaction.,” Nat. Prod. Res., vol. 37, no. 6, pp. 974–980, Mar. 2023. [CrossRef]
- H. İ. Güler et al., “Targeting CoV-2 spike RBD and ACE-2 interaction with flavonoids of Anatolian propolis by in silico and in vitro studies in terms of possible COVID-19 therapeutics.,” Turkish J. Biol. = Turk Biyol. Derg., vol. 45, no. 4, pp. 530–548, 2021. [CrossRef]
- W. G. Lima, J. C. M. W. G. Lima, J. C. M. Brito, and W. S. da Cruz Nizer, “Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2),” Phyther. Res., vol. 35, no. 2, pp. 743–750, 2021. [CrossRef]
- A. Vanderheiden and R. S. Klein, “Neuroinflammation and COVID-19,” Curr. Opin. Neurobiol., vol. 76, p. 102608, 2022. [CrossRef]
- M. du Plessis, C. M. du Plessis, C. Fourie, J. Riedemann, W. J. S. de Villiers, and A. M. Engelbrecht, “Cancer and Covid-19: Collectively catastrophic,” Cytokine Growth Factor Rev., vol. 63, no. October 2021, pp. 78–89, 2022. [CrossRef]
- T. G. Dimitriou et al., “Antiviral Activity of Beebread, Bee-Collected Pollen and Artificially Fermented Follen against Influenza A Virus,” Foods, vol. 12, no. 10, pp. 1–16, 2023. [CrossRef]
- F. Nainu et al., “Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties,” Antibiotics, vol. 10, no. 7, 2021. [CrossRef]
- M. Šudomová, K. M. Šudomová, K. Berchová-Bímová, A. Mazurakova, D. Šamec, P. Kubatka, and S. T. S. S. Hassan, “Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights,” Viruses, vol. 14, no. 3, Mar. 2022. [CrossRef]
- M. Barchitta et al., “Dietary antioxidant intake and human papillomavirus infection: Evidence from a cross-sectional study in Italy,” Nutrients, vol. 12, no. 5, pp. 1–10, 2020. [CrossRef]
- W. H. El-Tantawy and A. Temraz, “Natural products for the management of the hepatitis C virus: a biochemical review,” Arch. Physiol. Biochem., vol. 126, no. 2, pp. 116–128, May 2020. [CrossRef]
- Visentin, I. Gobin, and Ž. Maglica, “Periodontal Pathogens and Their Links to Neuroinflammation and Neurodegeneration.,” Microorganisms, vol. 11, no. 7, pp. 1–26, Jul. 2023. [CrossRef]
- A. Khurelchuluun et al., “Bee Pollen Diet Alters the Bacterial Flora and Antimicrobial Peptides in the Oral Cavities of Mice,” Foods, vol. 10, no. 6, p. 1282, Jun. 2021. [CrossRef]
- B. Curuțiu, L. M. B. Curuțiu, L. M. Dițu, A. M. Grumezescu, and A. M. Holban, “Polyphenols of honeybee origin with applications in dental medicine,” Antibiotics, vol. 9, no. 12, pp. 1–19, Nov. 2020. [CrossRef]
- C. S. G. Catumbela, V. V. C. S. G. Catumbela, V. V. Giridharan, T. Barichello, and R. Morales, “Clinical evidence of human pathogens implicated in Alzheimer’s disease pathology and the therapeutic efficacy of antimicrobials: an overview.,” Transl. Neurodegener., vol. 12, no. 1, p. 37, Jul. 2023. [CrossRef]
- S. K. Lotz, B. M. S. K. Lotz, B. M. Blackhurst, K. L. Reagin, and K. E. Funk, “Microbial Infections Are a Risk Factor for Neurodegenerative Diseases,” Front. Cell. Neurosci., vol. 15, no. July, 2021. [CrossRef]
- U. Kumar et al., “Role of Dietary Flavonoids in Preventing COVID-19 Infection and Other Infectious Diseases: A Mini Review,” Eur. J. Gen. Dent., vol. 11, no. 3, pp. 158–165, 2022. [CrossRef]
- C. Hou et al., “Alternative strategies for Chlamydia treatment: Promising non-antibiotic approaches,” Front. Microbiol., vol. 13, 2022. [CrossRef]
- J. Kountouras et al., “Controlling the Impact of Helicobacter pylori-Related Hyperhomocysteinemia on Neurodegeneration,” Med., vol. 59, no. 3, pp. 1–17, 2023. [CrossRef]
- Alipour, “Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer.,” J. Gastrointest. Cancer, vol. 52, no. 1, pp. 23–30, Mar. 2021. [CrossRef]
- C. O. C. D. Mărginean, C. O. C. D. C. O. C. D. Mărginean, C. O. C. D. Mărginean, and L. E. Meliț, “Helicobacter pylori-Related Extraintestinal Manifestations-Myth or Reality.,” Child. (Basel, Switzerland), vol. 9, no. 9, pp. 1–28, Sep. 2022. [CrossRef]
- R. Ivyna de Araújo Rêgo et al., “Flavonoids-Rich Plant Extracts Against Helicobacter pylori Infection as Prevention to Gastric Cancer,” Front. Pharmacol., vol. 13, no. August, pp. 1–20, 2022. [CrossRef]
- S. Sathianarayanan, A. V. S. Sathianarayanan, A. V. Ammanath, R. Biswas, A. B, S. Sukumaran, and B. Venkidasamy, “A new approach against Helicobacter pylori using plants and its constituents: A review study,” Microb. Pathog., vol. 168, no. September 2021, p. 105594, Jul. 2022. [CrossRef]
- J. Talapko et al., “A Putative Role of Candida albicans in Promoting Cancer Development: A Current State of Evidence and Proposed Mechanisms,” Microorganisms, vol. 11, no. 6, p. 1476, 2023. [CrossRef]
- Z. X. Phuna and P. Madhavan, “A reappraisal on amyloid cascade hypothesis: the role of chronic infection in Alzheimer’s disease.,” Int. J. Neurosci., pp. 1–19, Mar. 2022. [CrossRef]
- A. Didaras, K. A. Didaras, K. Karatasou, T. G. Dimitriou, G. D. Amoutzias, and D. Mossialos, “Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives.,” Antibiot. (Basel, Switzerland), vol. 9, no. 11, p. 811, Nov. 2020. [CrossRef]
- I. Kizilpinar-Temizer, A. I. Kizilpinar-Temizer, A. Guder, E. D. Candan, and U. Yolcu, “Antioxidant properties, element contents and antimicrobial activities of bee pollen collected by Apis mellifera L. in Türkiye,” Spanish J. Agric. Res., vol. 20, no. 4, p. e0506, Nov. 2022. [CrossRef]
- D. M. Wilson, M. R. D. M. Wilson, M. R. Cookson, L. Van Den Bosch, H. Zetterberg, D. M. Holtzman, and I. Dewachter, “Hallmarks of neurodegenerative diseases,” Cell, vol. 186, no. 4, pp. 693–714, 2023. [CrossRef]
- K.-I. Yoshioka, R. K.-I. Yoshioka, R. Kusumoto-Matsuo, Y. Matsuno, and M. Ishiai, “Genomic Instability and Cancer Risk Associated with Erroneous DNA Repair.,” Int. J. Mol. Sci., vol. 22, no. 22, Nov. 2021. [CrossRef]
- A. Konopka and J. D. Atkin, “The Role of DNA Damage in Neural Plasticity in Physiology and Neurodegeneration,” Front. Cell. Neurosci., vol. 16, no. June, pp. 1–14, 2022. [CrossRef]
- Y. Zhao, M. Y. Zhao, M. Simon, A. Seluanov, and V. Gorbunova, “DNA damage and repair in age-related inflammation.,” Nat. Rev. Immunol., vol. 23, no. 2, pp. 75–89, Feb. 2023. [CrossRef]
- Z. X. Wang, Y. L. Z. X. Wang, Y. L. Li, J. L. Pu, and B. R. Zhang, “DNA Damage-Mediated Neurotoxicity in Parkinson’s Disease,” Int. J. Mol. Sci., vol. 24, no. 7, 2023. [CrossRef]
- J. L. Hopkins, L. J. L. Hopkins, L. Lan, and L. Zou, “DNA repair defects in cancer and therapeutic opportunities,” Genes Dev., vol. 34, no. 5–6, pp. 278–293, Mar. 2022. [CrossRef]
- S. Şahin and B. Karkar, “The antioxidant properties of the chestnut bee pollen extract and its preventive action against oxidatively induced damage in DNA bases,” J. Food Biochem., vol. 43, no. 7, pp. 1–8, Jul. 2019. [CrossRef]
- S. Chen, X. S. Chen, X. Wang, and N. Cheng, “Ultrasound-assisted ethanol extraction of Actinidia arguta pollen possesses antioxidant activity and protects DNA from oxidative damage,” J. Food Biochem., vol. 45, no. 4, pp. 1–12, 2021. [CrossRef]
- R. Bridi et al., “Honeybee Pollen From Southern Chile: Phenolic Profile, Antioxidant Capacity, Bioaccessibility, and Inhibition of DNA Damage,” Front. Pharmacol., vol. 13, no. March, pp. 1–12, 2022. [CrossRef]
- M. Rudrapal et al., “Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action,” Front. Pharmacol., vol. 13, no. February, pp. 1–15, 2022. [CrossRef]
- Crupi, M. F. Faienza, M. Y. Naeem, F. Corbo, M. L. Clodoveo, and M. Muraglia, “Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being.,” Antioxidants (Basel, Switzerland), vol. 12, no. 5, May 2023. [CrossRef]
- K. Starska-Kowarska, “Dietary Carotenoids in Head and Neck Cancer-Molecular and Clinical Implications.,” Nutrients, vol. 14, no. 3, Jan. 2022. [CrossRef]
- C. Dion, C. C. Dion, C. Laberthonnière, and F. Magdinier, “Épigénétique, principes et exemples d’applications,” La Rev. Médecine Interne, vol. 44, no. 11, pp. 594–601, Nov. 2023. [CrossRef]
- A. Farsetti, B. A. Farsetti, B. Illi, and C. Gaetano, “How epigenetics impacts on human diseases,” Eur. J. Intern. Med., vol. 114, no. May, pp. 15–22, 2023. [CrossRef]
- Arora, M. Sharma, L. Y. Sun, and T. O. Tollefsbol, “The epigenetic link between polyphenols, aging and age-related diseases,” Genes (Basel)., vol. 11, no. 9, pp. 1–21, Sep. 2020. [CrossRef]
- Rajendran, S. A. Abdelsalam, K. Renu, V. Veeraraghavan, R. Ben Ammar, and E. A. Ahmed, “Polyphenols as Potent Epigenetics Agents for Cancer,” Int. J. Mol. Sci., vol. 23, no. 19, p. 11712, Oct. 2022. [CrossRef]
- S. Ghasemi et al., “Epigenetic targeting of cancer stem cells by polyphenols (cancer stem cells targeting).,” Phytother. Res., vol. 35, no. 7, pp. 3649–3664, Jul. 2021. [CrossRef]
- S. Wakatsuki and T. Araki, “Novel insights into the mechanism of reactive oxygen species-mediated neurodegeneration,” Neural Regen. Res., vol. 18, no. 4, pp. 746–749, 2023. [CrossRef]
- M. Amanollahi, M. M. Amanollahi, M. Jameie, A. Heidari, and N. Rezaei, The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases, vol. 60, no. 2. Springer US, 2023. [CrossRef]
- Y. Mou et al., “Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging,” Front. Immunol., vol. 13, no. April, pp. 1–25, 2022. [CrossRef]
- A. P. de A Boleti et al., “Adipose tissue, systematic inflammation, and neurodegenerative diseases,” Neural Regen. Res., vol. 18, no. 1, pp. 38–46, Jan. 2023. [CrossRef]
- K. Aabed et al., “Bee pollen and propolis improve neuroinflammation and dysbiosis induced by propionic acid, a short chain fatty acid in a rodent model of autism.,” Lipids Health Dis., vol. 18, no. 1, p. 200, Nov. 2019. [CrossRef]
- C. Peña-Bautista, E. C. Peña-Bautista, E. Casas-Fernández, M. Vento, M. Baquero, and C. Cháfer-Pericás, “Stress and neurodegeneration,” Clin. Chim. Acta, vol. 503, no. November 2019, pp. 163–168, 2020. [CrossRef]
- Ö. Saral et al., “Bee pollen increases hippocampal brain-derived neurotrophic factor and suppresses neuroinflammation in adult rats with chronic immobilization stress,” Neurosci. Lett., vol. 766, no. November 2021, p. 136342, Jan. 2022. [CrossRef]
- A. Al Suhaibani, A. A. Al Suhaibani, A. Ben Bacha, M. Alonazi, R. S. Bhat, and A. El-Ansary, “Testing the combined effects of probiotics and prebiotics against neurotoxic effects of propionic acid orally administered to rat pups,” Food Sci. Nutr., vol. 9, no. 8, pp. 4440–4451, 2021. [CrossRef]
- S. S. El-Hawwary et al., “Green-synthesized zinc oxide nanoparticles, anti-Alzheimer potential and the metabolic profiling of: Sabal blackburniana grown in Egypt supported by molecular modelling,” RSC Adv., vol. 11, no. 29, pp. 18009–18025, 2021. [CrossRef]
- J. S. Araújo et al., “Chemical composition and biological activities of mono- and heterofloral bee pollen of different geographical origins,” Int. J. Mol. Sci., vol. 18, no. 5, p. 921, Apr. 2017. [CrossRef]
- Miyata, S. Hoshino, M. R. Ahn, and S. Kumazawa, “Chemical Profiles of Korean Bee Pollens and Their Catechol- O-methyltransferase Inhibitory Activities,” J. Agric. Food Chem., vol. 70, no. 4, pp. 1174–1181, 2022. [CrossRef]
- D. F. Zhao et al., “Discovery and characterization of flavonoids in vine tea as catechol-O-methyltransferase inhibitors,” Fitoterapia, vol. 152, no. April, p. 104913, 2021. [CrossRef]
- C. López-Otín, M. A. C. López-Otín, M. A. Blasco, L. Partridge, M. Serrano, and G. Kroemer, “Hallmarks of aging: An expanding universe.,” Cell, vol. 186, no. 2, pp. 243–278, Jan. 2023. [CrossRef]
- A. Kent, T. L. A. Kent, T. L. Spires-Jones, and C. S. Durrant, “The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics,” Acta Neuropathol., vol. 140, no. 4, pp. 417–447, Oct. 2020. [CrossRef]
- L. Cerofolini et al., “Mixing Aβ(1-40) and Aβ(1-42) peptides generates unique amyloid fibrils,” Chem. Commun., vol. 56, no. 62, pp. 8830–8833, 2020. [CrossRef]
- A. Nabers, H. A. Nabers, H. Hafermann, J. Wiltfang, and K. Gerwert, “Aβ and tau structure-based biomarkers for a blood- and CSF-based two-step recruitment strategy to identify patients with dementia due to Alzheimer’s disease,” Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit., vol. 11, pp. 257–263, 2019. [CrossRef]
- E. R. Kabir et al., “Unveiling the Potential of Polyphenols as Anti-Amyloid Molecules in Alzheimer’s Disease,” Curr. Neuropharmacol., vol. 21, no. 4, pp. 787–807, Apr. 2023. [CrossRef]
- Q. Zhang and Y. Yan, “The role of natural flavonoids on neuroinflammation as a therapeutic target for Alzheimer’s disease: a narrative review.,” Neural Regen. Res., vol. 18, no. 12, pp. 2582–2591, Dec. 2023. [CrossRef]
- R. Kaur et al., “Potential of flavonoids as anti-Alzheimer’s agents: bench to bedside,” Environ. Sci. Pollut. Res., vol. 29, no. 18, pp. 26063–26077, Apr. 2022. [CrossRef]
- Banerjee, D. Baghel, A. Pacheco de Oliveira, and A. Ghosh, “β-Carotene, a Potent Amyloid Aggregation Inhibitor, Promotes Disordered Aβ Fibrillar Structure,” Int. J. Mol. Sci., vol. 24, no. 6, 2023. [CrossRef]
- Z. Batool et al., “Natural Carotenoids as Neuroprotective Agents for Alzheimer’s Disease: An Evidence-Based Comprehensive Review,” J. Agric. Food Chem., vol. 70, no. 50, pp. 15631–15646, 2022. [CrossRef]
- F. Alipourfard, H. F. Alipourfard, H. Shajiee, F. Nazari-Serenjeh, V. Hojati, and M. Alirezaie, “Betaine attenuates oxidative stress and cognitive dysfunction in an amyloid β-induced rat model of Alzheimer’s disease.,” Res. Pharm. Sci., vol. 18, no. 3, pp. 270–278, 2023. [CrossRef]
- Z. Zhang et al., “Advances in polysaccharides of natural source of the anti-Alzheimer’s disease effect and mechanism,” Carbohydr. Polym., vol. 296, no. August, p. 119961, 2022. [CrossRef]
- O. Makshakova et al., “The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein.,” Pharmaceutics, vol. 15, no. 2, Feb. 2023. [CrossRef]
- S. Y. Liang, Z. T. S. Y. Liang, Z. T. Wang, L. Tan, and J. T. Yu, “Tau Toxicity in Neurodegeneration,” Mol. Neurobiol., vol. 59, no. 6, pp. 3617–3634, 2022. [CrossRef]
- P. Rawat, U. P. Rawat, U. Sehar, J. Bisht, A. Selman, J. Culberson, and P. H. Reddy, “Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies,” Int. J. Mol. Sci., vol. 23, no. 21, 2022. [CrossRef]
- H. Shah et al., “Revisiting the Role of Vitamins and Minerals in Alzheimer’s Disease.,” Antioxidants (Basel, Switzerland), vol. 12, no. 2, pp. 1–32, Feb. 2023. [CrossRef]
- P. Lei, S. P. Lei, S. Ayton, and A. I. Bush, “The essential elements of Alzheimer’s disease.,” J. Biol. Chem., vol. 296, p. 100105, 2021. [CrossRef]
- P. Gracia, J. D. P. Gracia, J. D. Camino, L. Volpicelli-Daley, and N. Cremades, “Multiplicity of α-synuclein aggregated species and their possible roles in disease,” Int. J. Mol. Sci., vol. 21, no. 21, pp. 1–27, 2020. [CrossRef]
- L. D. Bernal-Conde et al., “Alpha-Synuclein Physiology and Pathology: A Perspective on Cellular Structures and Organelles,” Front. Neurosci., vol. 13, no. January, pp. 1–22, 2020. [CrossRef]
- Grozdanov and K., M. Danzer, “Intracellular Alpha-Synuclein and Immune Cell Function,” Front. Cell Dev. Biol., vol. 8, no. October, pp. 1–6, 2020. [CrossRef]
- S. Peña-Díaz, J. S. Peña-Díaz, J. García-Pardo, and S. Ventura, “Development of Small Molecules Targeting α-Synuclein Aggregation: A Promising Strategy to Treat Parkinson’s Disease,” Pharmaceutics, vol. 15, no. 3, pp. 1–34, Mar. 2023. [CrossRef]
- Riegelman, K. S. Xue, J. Wang, and L. Tang, “Gut–Brain Axis in Focus: Polyphenols, Microbiota, and Their Influence on α-Synuclein in Parkinson’s Disease,” Nutrients, vol. 16, no. 13, p. 2041, Jun. 2024. [CrossRef]
- L. Zhou, Z. L. Zhou, Z. Ma, and X. Gao, “Retinoic Acid Prevents α-Synuclein Preformed Fibrils-Induced Toxicity via Inhibiting STAT1-PARP1 Signaling.,” Mol. Neurobiol., vol. 60, no. 8, pp. 4828–4841, Aug. 2023. [CrossRef]
- Sandeep, M. R. Sahu, L. Rani, A. S. Kharat, and A. C. Mondal, “Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease?,” Brain Sci., vol. 13, no. 2, 2023. [CrossRef]
- S. Salaramoli, H. R. S. Salaramoli, H. R. Joshaghani, M. Hosseini, and S. I. Hashemy, “Therapeutic Effects of Selenium on Alpha-Synuclein Accumulation in Substantia Nigra Pars Compacta in a Rat Model of Parkinson’s Disease: Behavioral and Biochemical Outcomes,” Biol. Trace Elem. Res., no. 0123456789, 2023. [CrossRef]
- D. E. O’Sullivan et al., “Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis,” Clin. Gastroenterol. Hepatol., vol. 20, no. 6, pp. 1229-1240.e5, 2022. [CrossRef]
- M. Cao et al., “Current cancer burden in China: epidemiology, etiology, and prevention,” Cancer Biol. Med., vol. 19, no. 8, pp. 1121–1138, 2022. [CrossRef]
- J. S. Algethami et al., “Bee Pollen: Clinical Trials and Patent Applications,” Nutrients, vol. 14, no. 14, p. 2858, Jul. 2022. [CrossRef]
- P. M. Kustiawan, S. P. M. Kustiawan, S. Puthong, E. T. Arung, and C. Chanchao, “In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines,” Asian Pac. J. Trop. Biomed., vol. 4, no. 7, pp. 549–556, Jul. 2014. [CrossRef]
- T. Arung et al., “Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines,” Saudi J. Biol. Sci., vol. 28, no. 12, pp. 7182–7189, 2021. [CrossRef]
- T. Tuoheti, H. A. T. Tuoheti, H. A. Rasheed, L. Meng, and M. sheng Dong, “High hydrostatic pressure enhances the anti-proliferative properties of lotus bee pollen on the human prostate cancer PC-3 cells via increased metabolites,” J. Ethnopharmacol., vol. 261, no. 1, 2020. [CrossRef]
- C. NGUYEN, L.-C. C. NGUYEN, L.-C. LIU, M.-C. WU, T.-P. LIN, C.-Y. YANG, and M.-Y. HUANG, “Chemical constituents, antioxidant, and anticancer activities of bee pollen from various floral sources in Taiwan,” Not. Bot. Horti Agrobot. Cluj-Napoca, vol. 50, no. 2, p. 12644, May 2022. [CrossRef]
- B. Denisow and M. Denisow-Pietrzyk, “Biological and therapeutic properties of bee pollen: a review,” J. Sci. Food Agric., vol. 96, no. 13, pp. 4303–4309, 2016. [CrossRef]
- A. Kahraman et al., “Ethanolic extract of Turkish bee pollen and propolis: phenolic composition, antiradical, antiproliferative and antibacterial activities,” Biotechnol. Biotechnol. Equip., vol. 36, no. 1, pp. 44–55, Dec. 2022. [CrossRef]
- Li, P. Khongkarat, R. Ramadhan, P. Phuwapraisirisan, and C. Chanchao, “Bee Pollen and Doxorubicin by Synergistic Effects Inhibit the Proliferation of Breast Tumors in 4T1 Tumor-bearing BALB/c Mice: A Biochemical, Immunohistochemical,and Molecular Approach,” Pharmacogn. Mag., vol. 65, no. 1, pp. 71–83, Jun. 2023. [CrossRef]
- M. Di Chiacchio et al., “Bee pollen in zebrafish diet affects intestinal microbiota composition and skin cutaneous melanoma development,” Sci. Rep., vol. 12, no. 1, pp. 1–18, 2022. [CrossRef]
- A. Wan Omar, N. A. A. Wan Omar, N. A. Azhar, N. Harif Fadzilah, N. N. S. Nik Mohamed Kamal, N. H. Fadzilah, and N. N. S. Nik Mohamed Kamal, “Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines,” Asian Pac. J. Trop. Biomed., vol. 6, no. 3, pp. 265–269, Mar. 2016. [CrossRef]
- Amalia, A. Diantini, and A. Subarnas, “Water-soluble propolis and bee pollen of Trigona spp. From South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line,” Oncol. Lett., vol. 20, no. 5, 2020. [CrossRef]
- N. H. Fadzilah, W. A. N. H. Fadzilah, W. A. Wan Omar, and W. A. W. Omar, “Therapeutic Evaluation of Ethanolic Bee Pollen Extract from Malaysian Stingless Bee in MCF-7 and MCF-10A Cell Lines,” Pertanika J. Trop. Agric. Sci., vol. 46, no. 1, pp. 37–48, Dec. 2023. [CrossRef]
- A. A. Tohamy, E. M. A. A. Tohamy, E. M. Abdella, R. R. Ahmed, and Y. K. Ahmed, “Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts.,” Cytotechnology, vol. 66, no. 2, pp. 283–97, Mar. 2014. [CrossRef]
- Shaldoum, A. F. El-kott, M. M. A. Ouda, and E. M. Abd-Ella, “Immunomodulatory effects of bee pollen on doxorubicin-induced bone marrow/spleen immunosuppression in rat,” J. Food Biochem., vol. 45, no. 6, pp. 1–14, Jun. 2021. [CrossRef]
- O. Saral et al., “The effect of bee pollen on reproductive and biochemical parameters in methotrexate-induced testicular damage in adult rats,” J. Basic Clin. Physiol. Pharmacol., vol. 32, no. 5, pp. 1001–1006, 2021. [CrossRef]
- S. S. Dhanisha, S. S. S. Dhanisha, S. Drishya, K. P. Gangaraj, M. K. Rajesh, and C. Guruvayoorappan, “Molecular Docking Studies of Naringenin and its Protective Efficacy against Methotrexate Induced Oxidative Tissue Injury.,” Anticancer. Agents Med. Chem., vol. 22, no. 1, pp. 169–180, 2022. [CrossRef]
- I. O. Imosemi, S. E. I. O. Imosemi, S. E. Owumi, and U. O. Arunsi, “Biochemical and histological alterations of doxorubicin-induced neurotoxicity in rats: Protective role of luteolin.,” J. Biochem. Mol. Toxicol., vol. 36, no. 3, p. e22962, Mar. 2022. [CrossRef]
- C. Gur, F. M. C. Gur, F. M. Kandemir, C. Caglayan, and E. Satıcı, “Chemopreventive effects of hesperidin against paclitaxel-induced hepatotoxicity and nephrotoxicity via amendment of Nrf2/HO-1 and caspase-3/Bax/Bcl-2 signaling pathways,” Chem. Biol. Interact., vol. 365, no. March, p. 110073, 2022. [CrossRef]
- M. R. Farag et al., “Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats.,” Antioxidants (Basel, Switzerland), vol. 10, no. 12, Nov. 2021. [CrossRef]
- D. Singh, M. A. D. Singh, M. A. Khan, K. Akhtar, F. Arjmand, and H. R. Siddique, “Apigenin alleviates cancer drug Sorafenib induced multiple toxic effects in Swiss albino mice via anti-oxidative stress,” Toxicol. Appl. Pharmacol., vol. 447, no. March, p. 116072, 2022. [CrossRef]
- S. Safarpour et al., “Protective Effect of Kaempferol and Its Nanoparticles on 5-Fluorouracil-Induced Cardiotoxicity in Rats,” Biomed Res. Int., vol. 2022, 2022. [CrossRef]
- M. D. Navarro-Hortal et al., “Role of flavonoids against adriamycin toxicity,” Food Chem. Toxicol., vol. 146, no. August, 2020. [CrossRef]
- M. U. Rehman and I. A. Rather, “Myricetin Abrogates Cisplatin-Induced Oxidative Stress, Inflammatory Response, and Goblet Cell Disintegration in Colon of Wistar Rats.,” Plants (Basel, Switzerland), vol. 9, no. 1, Dec. 2019. [CrossRef]
- J. Gu et al., “Pinocembrin inhibited cardiomyocyte pyroptosis against doxorubicin-induced cardiac dysfunction via regulating Nrf2/Sirt3 signaling pathway,” Int. Immunopharmacol., vol. 95, no. November 2020, p. 107533, 2021. [CrossRef]
- Shafiee, L. Safaeian, and F. Gorbani, “Protective effects of protocatechuic acid against doxorubicin- and arsenic trioxide-induced toxicity in cardiomyocytes.,” Res. Pharm. Sci., vol. 18, no. 2, pp. 149–158, Apr. 2023. [CrossRef]
- Shabani, D. Bayrami, A. A. Moghadam, Z. Jamali, and A. Salimi, “Pretreatment of ellagic acid protects ifosfamide-induced acute nephrotoxicity in rat kidneys: A mitochondrial, histopathological and oxidative stress approaches.,” Toxicol. reports, vol. 10, no. April, pp. 441–447, 2023. [CrossRef]
- S. E. Owumi et al., “Chlorogenic acid abates oxido-inflammatory and apoptotic responses in the liver and kidney of Tamoxifen-Treated rats,” Toxicol. Res. (Camb)., vol. 10, no. 2, pp. 345–353, Mar. 2021. [CrossRef]
- G. Rahbardar, F. G. Rahbardar, F. Eisvand, M. Rameshrad, B. M. Razavi, and H. Hosseinzadeh, “In Vivo and In Vitro Protective Effects of Rosmarinic Acid against Doxorubicin-Induced Cardiotoxicity,” Nutr. Cancer, vol. 74, no. 2, pp. 747–760, 2022. [CrossRef]
- S. A. Helmy, S. S. A. Helmy, S. El-Mofty, A. M. El Gayar, I. M. El-Sherbiny, and Y. M. El-Far, “Novel Doxorubicin / Folate-Targeted Trans-Ferulic Acid-Loaded doxorubicin/folate-targeted trans-ferulic acid-loaded PLGA nanoparticles combination: In-vivo superiority over standard chemotherapeutic regimen for breast cancer treatment,” Biomed. Pharmacother., August. [CrossRef]
- O. Oyovwi et al., “Lutein Attenuates Cyclosporin-induced Testicular Impairment in Male Rats through Modulation of Androgenic Hormones and Enzymes,” Pharmacol. Toxicol. Nat. Med. (ISSN 2756-6838), vol. 2, no. 1, pp. 12–24, Feb. 2022. [CrossRef]
- M. Aboubakr, A. M. Aboubakr, A. Farag, A. Elfadadny, M. Alkafafy, A. Soliman, and M. Elbadawy, “Antioxidant and anti-apoptotic potency of allicin and lycopene against methotrexate-induced cardiac injury in rats,” Environ. Sci. Pollut. Res., vol. 30, no. 38, pp. 88724–88733, 2023. [CrossRef]
- B. E. Johns, M. B. E. Johns, M. Ficken, M. E. Engberg, L. Wecker, and R. M. Philpot, “Increasing dietary choline attenuates spatial memory deficits resulting from exposure to the chemotherapeutic agents cyclophosphamide and doxorubicin,” J. Psychopharmacol., vol. 35, no. 10, pp. 1300–1309, 2021. [CrossRef]
- D. A. Shabaan, N. D. A. Shabaan, N. Mostafa, M. M. El-Desoky, and E. A. Arafat, “Coenzyme Q10 protects against doxorubicin-induced cardiomyopathy via antioxidant and anti-apoptotic pathway,” Tissue Barriers, vol. 11, no. 1, pp. 1–14, 2023. [CrossRef]
- B. Pateliya, V. B. Pateliya, V. Burade, and S. Goswami, “Combining naringenin and metformin with doxorubicin enhances anticancer activity against triple-negative breast cancer in vitro and in vivo,” Eur. J. Pharmacol., vol. 891, no. October 2020, p. 173725, 2021. [CrossRef]
- A. Al Fatease et al., “Chemosensitization and mitigation of Adriamycin-induced cardiotoxicity using combinational polymeric micelles for co-delivery of quercetin/resveratrol and resveratrol/curcumin in ovarian cancer.,” Nanomedicine, vol. 19, pp. 39–48, Jul. 2019. [CrossRef]
- S. M. Hassan, M. M. S. M. Hassan, M. M. Khalaf, S. A. Sadek, and A. M. Abo-Youssef, “Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice.,” Pharm. Biol., vol. 55, no. 1, pp. 766–774, Dec. 2017. [CrossRef]
- L. R. Shirmard, M. L. R. Shirmard, M. Shabani, A. A. Moghadam, N. Zamani, H. Ghanbari, and A. Salimi, “Protective Effect of Curcumin, Chrysin and Thymoquinone Injection on Trastuzumab-Induced Cardiotoxicity via Mitochondrial Protection,” Cardiovasc. Toxicol., vol. 22, no. 7, pp. 663–675, Jul. 2022. [CrossRef]
- B. Türkmen et al., “Protective effect of resveratrol against pembrolizumab-induced hepatotoxicity and neurotoxicity in male rats.,” J. Biochem. Mol. Toxicol., vol. 37, no. 3, p. e23263, Mar. 2023. [CrossRef]
- V. Quagliariello et al., “Nano-encapsulation of coenzyme q10 in secondary and tertiary nano-emulsions for enhanced cardioprotection and hepatoprotection in human cardiomyocytes and hepatocytes during exposure to anthracyclines and trastuzumab,” Int. J. Nanomedicine, vol. 15, pp. 4859–4876, 2020. [CrossRef]
- O. Gumusay, J. O. Gumusay, J. Callan, and H. S. Rugo, “Immunotherapy toxicity: identification and management.,” Breast Cancer Res. Treat., vol. 192, no. 1, pp. 1–17, Feb. 2022. [CrossRef]
- Zhang, Y. Huang, Z. Li, H. Wu, B. Zou, and Y. Xu, “Exploring Natural Products as Radioprotective Agents for Cancer Therapy: Mechanisms, Challenges, and Opportunities,” Cancers (Basel)., vol. 15, no. 14, Jul. 2023. [CrossRef]
- S. Faramarzi, S. S. Faramarzi, S. Piccolella, L. Manti, and S. Pacifico, “Could Polyphenols Really Be a Good Radioprotective Strategy?,” Molecules, vol. 26, no. 16, Aug. 2021. [CrossRef]
- A. Altomare et al., “Protective Role of Natural Compounds under Radiation-Induced Injury,” Nutrients, vol. 14, no. 24, pp. 1–21, Dec. 2022. [CrossRef]
- Y. Shimizu et al., “Amelioration of Radiation Enteropathy by Dietary Supplementation With Reduced Coenzyme Q10,” Adv. Radiat. Oncol., vol. 4, no. 2, pp. 237–245, 2019. [CrossRef]
- H. Bagheri et al., “Radioprotective effects of zinc and selenium on mice spermatogenesis,” J. Biomed. Phys. Eng., vol. 10, no. 6, pp. 707–712, Aug. 2020. [CrossRef]
- N. A. N. Hanafy, E. I. N. A. N. Hanafy, E. I. Salim, M. E. Mahfouz, E. A. Eltonouby, and I. H. Hamed, “Fabrication and characterization of bee pollen extract nanoparticles: Their potential in combination therapy against human A549 lung cancer cells,” Food Hydrocoll. Heal., vol. 3, no. December 2022, 2023. [CrossRef]
- I. Salim, M. E. I. Salim, M. E. Mahfouz, E. A. Eltonouby, N. A. N. Hanafy, and E. H. Hafez, “Based polymer nanoparticles from bee pollen attenuate non-small lung cancer through enhancement of apoptosis and cell cycle arrest in vivo,” Cancer Nanotechnol., vol. 14, no. 1, p. 77, Dec. 2023. [CrossRef]
- L. Kurdi and F. Alhusayni, “Cytotoxicity Effect of 5-fluorouracil and bee products on the HTC-116 Human colon Cancer Cell Line in vitro,” Int. J. Pharm. Phytopharm. Res., vol. 10, no. 2, pp. 19–26, 2020. [CrossRef]
- K. Münstedt, B. K. Münstedt, B. Voss, U. Kullmer, U. Schneider, and J. Hübner, “Bee pollen and honey for the alleviation of hot flushes and other menopausal symptoms in breast cancer patients.,” Mol. Clin. Oncol., vol. 3, no. 4, pp. 869–874, Jul. 2015. [CrossRef]
- E. Khatoon et al., “Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives,” Semin. Cancer Biol., vol. 80, no. 19, pp. 306–339, May 2022. 20 November. [CrossRef]
- Maleki Dana, F. Sadoughi, Z. Asemi, and B. Yousefi, “The role of polyphenols in overcoming cancer drug resistance: a comprehensive review.,” Cell. Mol. Biol. Lett., vol. 27, no. 1, p. 1, Jan. 2022. [CrossRef]
- C. Jakobušić Brala, A. C. Jakobušić Brala, A. Karković Marković, A. Kugić, J. Torić, and M. Barbarić, “Combination Chemotherapy with Selected Polyphenols in Preclinical and Clinical Studies-An Update Overview.,” Molecules, vol. 28, no. 9, Apr. 2023. [CrossRef]
- J. Yi et al., “Potential of natural products as radioprotectors and radiosensitizers: Opportunities and challenges,” Food Funct., vol. 12, no. 12, pp. 5204–5218, 2021. [CrossRef]
- I. R. S. Vieira, L. I. R. S. Vieira, L. Tessaro, A. K. O. Lima, I. P. S. Velloso, and C. A. Conte-Junior, “Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer.,” Nutrients, vol. 15, no. 14, pp. 1–42, Jul. 2023. [CrossRef]
- Y. Zhang et al., “Natural Polyphenols for Treatment of Colorectal Cancer.,” Molecules, vol. 27, no. 24, pp. 1–23, Dec. 2022. [CrossRef]
- A. Lyubitelev and V. Studitsky, “Inhibition of Cancer Development by Natural Plant Polyphenols: Molecular Mechanisms,” Int. J. Mol. Sci., vol. 24, no. 13, p. 10663, 2023. [CrossRef]
- A. Sharma, S. A. Sharma, S. Sinha, H. Keswani, and N. Shrivastava, “Kaempferol and Apigenin suppresses the stemness properties of TNBC cells by modulating Sirtuins.,” Mol. Divers., vol. 26, no. 6, pp. 3225–3240, Dec. 2022. [CrossRef]
- W. Liao et al., “Targeting cancer stem cells and signalling pathways through phytochemicals: A promising approach against colorectal cancer,” Phytomedicine, vol. 108, no. June 2022, p. 154524, 2023. [CrossRef]
- S. S. Bhat et al., “Genistein: A potent anti-breast cancer agent,” Curr. Issues Mol. Biol., vol. 43, no. 3, pp. 1502–1517, 2021. [CrossRef]
- Z. Li et al., “Protocatechuic Acid-Based Supramolecular Hydrogel Targets SerpinB9 to Achieve Local Chemotherapy for OSCC,” ACS Appl. Mater. Interfaces, vol. 14, no. 32, pp. 36379–36394, 2022. [CrossRef]
- W. S. Ramadan et al., “Modulation of the Tumor Microenvironment by Ellagic Acid in Rat Model for Hepatocellular Carcinoma: A Potential Target against Hepatic Cancer Stem Cells.,” Cancers (Basel)., vol. 15, no. 19, Oct. 2023. [CrossRef]
- A. R. Aikins et al., “Caffeic Acid Inhibits Proliferation, Migration, and Stemness of DU-145 Prostate Cancer Cells,” Nat. Prod. Commun., vol. 18, no. 8, pp. 1–8, Aug. 2023. [CrossRef]
- Y. Zhou et al., “Apigenin in cancer therapy: From mechanism of action to nano-therapeutic agent,” Food Chem. Toxicol., vol. 168, no. July, 2022. [CrossRef]
- C. Mazewski, M. S. C. Mazewski, M. S. Kim, and E. Gonzalez de Mejia, “Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico.,” Sci. Rep., vol. 9, no. 1, p. 11560, Aug. 2019. [CrossRef]
- A. Zeng et al., “Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway.,” Oncol. Rep., vol. 45, no. 2, pp. 717–727, Feb. 2021. [CrossRef]
- J. L. Rowles and J. W. Erdman, “Carotenoids and their role in cancer prevention,” Biochim. Biophys. Acta - Mol. Cell Biol. Lipids, vol. 1865, no. 11, p. 158613, Nov. 2020. [CrossRef]
- L. Koklesova et al., “Carotenoids in Cancer Apoptosis-The Road from Bench to Bedside and Back.,” Cancers (Basel)., vol. 12, no. 9, pp. 1–41, Aug. 2020. [CrossRef]
- L. Koklesova et al., “Carotenoids in Cancer Metastasis-Status Quo and Outlook.,” Biomolecules, vol. 10, no. 12, pp. 1–26, Dec. 2020. [CrossRef]
- L. Ran et al., “Antitumor effects of pollen polysaccharides from Chinese wolfberry on DU145 cells via the PI3K/AKT pathway in vitro and in vivo,” Int. J. Biol. Macromol., vol. 152, pp. 1164–1173, 2020. [CrossRef]
- F. Rascio et al., “The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: an updated review,” Cancers (Basel)., vol. 13, no. 16, 2021. [CrossRef]
- Cioccoloni, C. Soteriou, A. Websdale, L. Wallis, M. A. Zulyniak, and J. L. Thorne, “Phytosterols and phytostanols and the hallmarks of cancer in model organisms: A systematic review and meta-analysis,” Crit. Rev. Food Sci. Nutr., vol. 62, no. 5, pp. 1145–1165, 2022. [CrossRef]
- A. U. Khan et al., “The critical role of the phytosterols in modulating tumor microenvironment via multiple signaling: A comprehensive molecular approach.,” Phytother. Res., vol. 37, no. 4, pp. 1606–1623, Apr. 2023. [CrossRef]
- F. Blanco-Vaca, L. F. Blanco-Vaca, L. Cedó, and J. Julve, “Phytosterols in Cancer: From Molecular Mechanisms to Preventive and Therapeutic Potentials.,” Curr. Med. Chem., vol. 26, no. 37, pp. 6735–6749, 2019. [CrossRef]
- P. Prasher et al., “Spermidine as a promising anticancer agent: Recent advances and newer insights on its molecular mechanisms.,” Front. Chem., vol. 11, no. April, p. 1164477, 2023. [CrossRef]
- M. do P. S. de Sousa Coelho et al., “Chemopreventive and anti-tumor potential of vitamin E in preclinical breast cancer studies: A systematic review,” Clin. Nutr. ESPEN, vol. 53, no. 2023, pp. 60–73, 2023. [CrossRef]
- O. Ekeuku, E. P. O. Ekeuku, E. P. Etim, K.-L. Pang, K.-Y. Chin, and C.-W. Mai, “Vitamin E in the management of pancreatic cancer: A scoping review.,” World J. Gastrointest. Oncol., vol. 15, no. 6, pp. 943–958, Jun. 2023. [CrossRef]
- M. Montecillo-Aguado, B. M. Montecillo-Aguado, B. Tirado-Rodriguez, and S. Huerta-Yepez, “The Involvement of Polyunsaturated Fatty Acids in Apoptosis Mechanisms and Their Implications in Cancer.,” Int. J. Mol. Sci., vol. 24, no. 14, Jul. 2023. [CrossRef]
- K. P. Liput et al., “Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis.,” Int. J. Mol. Sci., vol. 22, no. 13, Jun. 2021. [CrossRef]
- K. Kalimuthu et al., “The emerging role of selenium metabolic pathways in cancer: New therapeutic targets for cancer,” J. Cell. Biochem., vol. 123, no. 3, pp. 532–542, Mar. 2022. [CrossRef]
- E. G. Varlamova et al., “Mechanisms of the Cytotoxic Effect of Selenium Nanoparticles in Different Human Cancer Cell Lines.,” Int. J. Mol. Sci., vol. 22, no. 15, Jul. 2021. [CrossRef]
- C. M. Mangione et al., “Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement,” Jama, vol. 327, no. 23, pp. 2326–2333, 2022. [CrossRef]
- Iqbal and, I. Ali, “Dietary Trace Element Intake and Risk of Breast Cancer: A Mini Review,” Biol. Trace Elem. Res., vol. 200, no. 12, pp. 4936–4948, 2022. [CrossRef]
- W. Alam et al., “Micronutrient food supplements in patients with gastro-intestinal and hepatic cancers,” Int. J. Mol. Sci., vol. 22, no. 15, p. 8014, Jul. 2021. [CrossRef]
- J. Baechle, N. J. Baechle, N. Chen, P. Makhijani, S. Winer, D. Furman, and D. A. Winer, “Chronic inflammation and the hallmarks of aging,” Mol. Metab., vol. 74, no. June, p. 101755, 2023. [CrossRef]
- Z. Li et al., “Aging and age-related diseases: from mechanisms to therapeutic strategies.,” Biogerontology, vol. 22, no. 2, pp. 165–187, Apr. 2021. [CrossRef]
- C. López-Otín, F. C. López-Otín, F. Pietrocola, D. Roiz-Valle, L. Galluzzi, and G. Kroemer, “Meta-hallmarks of aging and cancer.,” Cell Metab., vol. 35, no. 1, pp. 12–35, Jan. 2023. [CrossRef]
- M. V. Blagosklonny, “Hallmarks of cancer and hallmarks of aging.,” Aging (Albany. NY)., vol. 14, no. 9, pp. 4176–4187, May 2022. [CrossRef]
- C. Koeberle, A. P. C. Koeberle, A. P. Kipp, H. Stuppner, and A. Koeberle, “Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling.,” Med. Res. Rev., vol. 43, no. 3, pp. 614–682, May 2023. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).