Submitted:
25 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
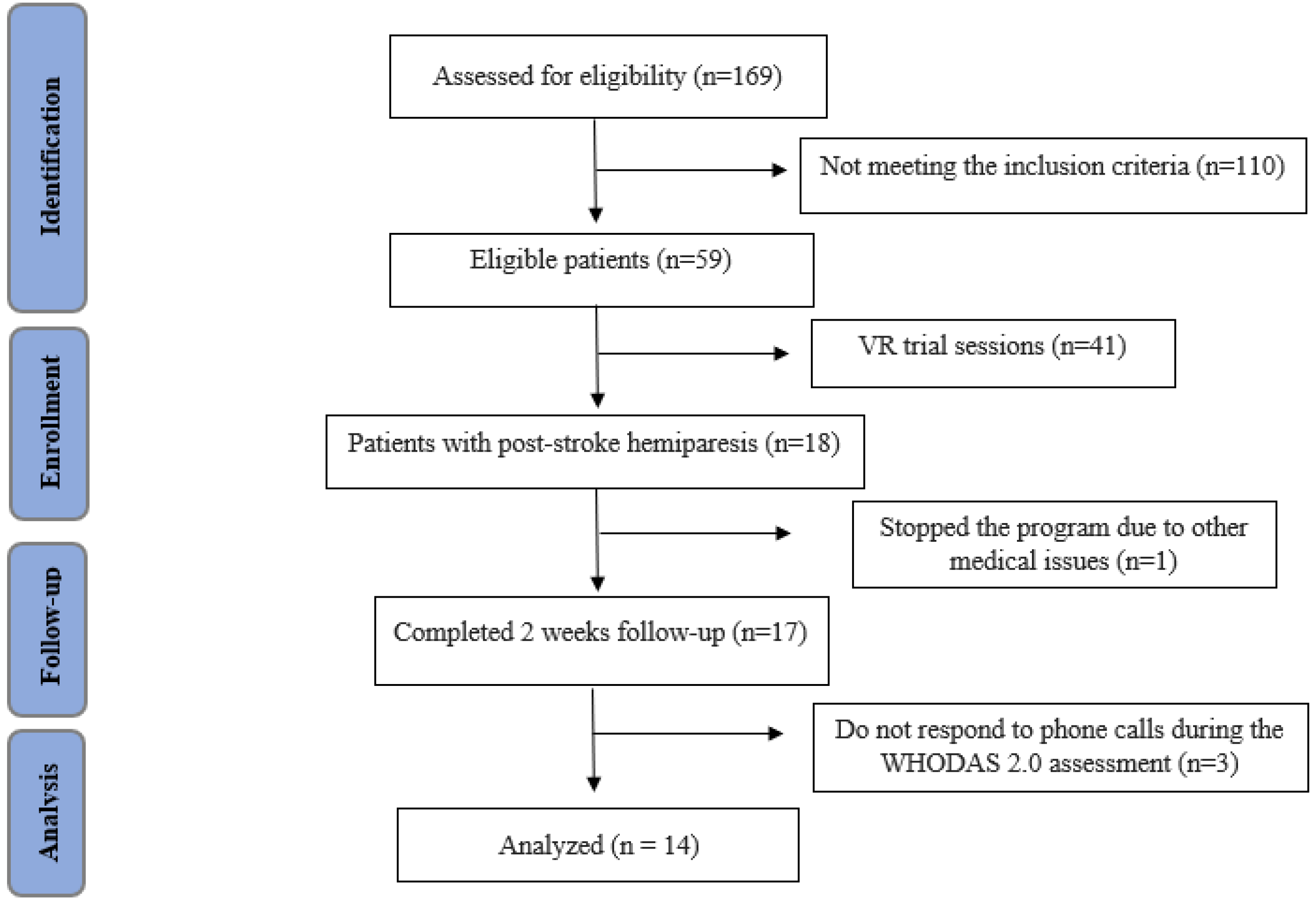
2.1. Study Design, Protocol and Patients
2.2. CnvT and VR Rehabilitation Program
2.3. Muscle Tone Assessment
2.4. Range of Motion Joint Assessment
2.5. Health and Disability Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. International Journal of Stroke 2022, 17, 18–29. [CrossRef]
- GBD 2019 Stroke Collaborators Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. The Lancet. Neurology 2021, 20, 795. [CrossRef]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circulation research 2017, 120, 439–448.
- World Health Organization International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY.; World Health Organization, 2007; ISBN 92-4-154732-4.
- Miller, E.L.; Murray, L.; Richards, L.; Zorowitz, R.D.; Bakas, T.; Clark, P.; Billinger, S.A. Comprehensive Overview of Nursing and Interdisciplinary Rehabilitation Care of the Stroke Patient: A Scientific Statement from the American Heart Association. Stroke 2010, 41, 2402–2448. [CrossRef]
- Warburton, E.; Alawneh, J.A.; Clatworthy, P.L.; Morris, R.S. Stroke Management. BMJ Clinical Evidence 2010, 2010.
- Wade, D.T. Measurement in Neurological Rehabilitation. Current Opinion in Neurology 1992, 5, 682–686.
- Langhorne, P.; Coupar, F.; Pollock, A. Motor Recovery after Stroke: A Systematic Review. The Lancet Neurology 2009, 8, 741–754. [CrossRef]
- Dobkin, B.; Carmichael, T. Principles of Recovery after Stroke. Recovery after stroke 2005, 47–66.
- Levin, M.F.; Kleim, J.A.; Wolf, S.L. What Do Motor “Recovery” and “Compensation” Mean in Patients Following Stroke? Neurorehabilitation and neural repair 2009, 23, 313–319. [CrossRef]
- Dancause, N.; Nudo, R.J. Shaping Plasticity to Enhance Recovery after Injury. Progress in brain research 2011, 192, 273–295. [CrossRef]
- Kwakkel, G.; Kollen, B.; Lindeman, E. Understanding the Pattern of Functional Recovery after Stroke: Facts and Theories. Restorative neurology and neuroscience 2004, 22, 281–299.
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of Virtual Reality as a Tool for Upper Limb Rehabilitation: Incorporation of Motor Control and Motor Learning Principles. Physical therapy 2015, 95, 415–425. [CrossRef]
- Viau, A.; Feldman, A.G.; McFadyen, B.J.; Levin, M.F. Reaching in Reality and Virtual Reality: A Comparison of Movement Kinematics in Healthy Subjects and in Adults with Hemiparesis. Journal of neuroengineering and rehabilitation 2004, 1, 1–7. [CrossRef]
- Thornton, M.; Marshall, S.; McComas, J.; Finestone, H.; McCormick, A.; Sveistrup, H. Benefits of Activity and Virtual Reality Based Balance Exercise Programmes for Adults with Traumatic Brain Injury: Perceptions of Participants and Their Caregivers. Brain injury 2005, 19, 989–1000. [CrossRef]
- Ferche, O.; Moldoveanu, A.; Cinteza, D.; Toader, C.; Moldoveanu, F.; Voinea, A.; Taslitchi, C. From Neuromotor Command to Feedback: A Survey of Techniques for Rehabilitation through Altered Perception.; IEEE, 2015; pp. 1–4.
- Cameirao, M.S.; Badia, S.B. i; Duarte, E.; Frisoli, A.; Verschure, P.F. The Combined Impact of Virtual Reality Neurorehabilitation and Its Interfaces on Upper Extremity Functional Recovery in Patients with Chronic Stroke. Stroke 2012, 43, 2720–2728. [CrossRef]
- Moldoveanu, A.; Ferche, O.-M.; Moldoveanu, F.; Lupu, R.G.; Cinteză, D.; Constantin Irimia, D.; Toader, C. The TRAVEE System for a Multimodal Neuromotor Rehabilitation. IEEE Access 2019, 7, 8151–8171. [CrossRef]
- Gibson, L.M.; Brazzelli, M.; Thomas, B.M.; Sandercock, P.A. A Systematic Review of Clinical Trials of Pharmacological Interventions for Acute Ischaemic Stroke (1955-2008) That Were Completed, but Not Published in Full. Trials 2010, 11, 1–12. [CrossRef]
- ZAMPOLINI, M.; SELB, M.; BOLDRINI, P.; BRANCO, C.A.; GOLYK, V.; HU, X.; KIEKENS, C.; NEGRINI, S.; NULLE, A.; ORAL, A.; et al. The Individual Rehabilitation Project as the Core of Person-Centered Rehabilitation: The Physical and Rehabilitation Medicine Section and Board of the European Union of Medical Specialists Framework for Rehabilitation in Europe. Eur J Phys Rehabil Med 2022, 58, 503–510. [CrossRef]
- Ustun, T.B.; Kostanjesek, N.; Chatterji, S.; Rehm, J.; World Health Organization Measuring Health and Disability : Manual for WHO Disability Assessment Schedule (WHODAS 2.0) / Edited by T.B. Üstün, N. Kostanjsek, S. Chatterji, J.Rehm. 2010.
- Potcovaru, C.-G.; Salmen, T.; Bîgu, D.; Săndulescu, M.I.; Filip, P.V.; Diaconu, L.S.; Pop, C.; Ciobanu, I.; Cinteză, D.; Berteanu, M. Assessing the Effectiveness of Rehabilitation Interventions through the World Health Organization Disability Assessment Schedule 2.0 on Disability—A Systematic Review. Journal of Clinical Medicine 2024, 13, 1252. [CrossRef]
- Unit Calculator Available online: https://alcoholchange.org.uk/alcohol-facts/interactive-tools/unit-calculator (accessed on 15 August 2024).
- White, I.R.; Altmann, D.R.; Nanchahal, K. Alcohol Consumption and Mortality: Modelling Risks for Men and Women at Different Ages. Bmj 2002, 325, 191. [CrossRef]
- Caraiman, S.; Stan, A.; Botezatu, N.; Herghelegiu, P.; Lupu, R.G.; Moldoveanu, A. Architectural Design of a Real-Time Augmented Feedback System for Neuromotor Rehabilitation.; IEEE, 2015; pp. 850–855.
- Harb, A.; Kishner, S. Modified Ashworth Scale. In StatPearls [Internet]; StatPearls Publishing, 2023.
- Norkin, C.C.; White, D.J. Measurement of Joint Motion: A Guide to Goniometry; FA Davis, 2016; ISBN 0-8036-5847-8.
- WHO Disability Assessment Schedule (WHODAS 2.0) Available online: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health/who-disability-assessment-schedule (accessed on 30 June 2024).
- Faria, A.L.; Cameirão, M.S.; Couras, J.F.; Aguiar, J.R.O.; Costa, G.M.; Bermúdez i Badia, S. Combined Cognitive-Motor Rehabilitation in Virtual Reality Improves Motor Outcomes in Chronic Stroke – A Pilot Study. Front. Psychol. 2018, 9. [CrossRef]
- Henderson, A.; Korner-Bitensky, N.; Levin, M. Virtual Reality in Stroke Rehabilitation: A Systematic Review of Its Effectiveness for Upper Limb Motor Recovery. Topics in stroke rehabilitation 2007, 14, 52–61. [CrossRef]
- Dąbrowská, M.; Pastucha, D.; Janura, M.; Tomášková, H.; Honzíková, L.; Baníková, Š.; Filip, M.; Fiedorová, I. Effect of Virtual Reality Therapy on Quality of Life and Self-Sufficiency in Post-Stroke Patients. Medicina 2023, 59, 1669. [CrossRef]
- Kuerbis, A.; Mulliken, A.; Muench, F.; Moore, A.; Gardner, D. Older Adults and Mobile Technology: Factors That Enhance and Inhibit Utilization in the Context of Behavioral Health. Publications and Research 2017. [CrossRef]
- Hodgson, J.C.; Benattayallah, A.; Hodgson, T.L. The Role of the Dominant versus the Non-Dominant Hemisphere: An fMRI Study of Aphasia Recovery Following Stroke. Aphasiology 2014, 28, 1426–1447. [CrossRef]
- Salmen, T.; Serbanoiu, L.-I.; Bica, I.-C.; Serafinceanu, C.; Muzurović, E.; Janez, A.; Busnatu, S.; Banach, M.; Rizvi, A.A.; Rizzo, M. A Critical View over the Newest Antidiabetic Molecules in Light of Efficacy—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences 2023, 24, 9760. [CrossRef]
- Oprea, E.; Berteanu, M.; Cintezã, D.; Manolescu, B.N. The Effect of the ALAnerv Nutritional Supplement on Some Oxidative Stress Markers in Postacute Stroke Patients Undergoing Rehabilitation. Applied Physiology, Nutrition, and Metabolism 2013, 38, 613–620. [CrossRef]
- Weintraub, M.S.; Rosen, Y.; Otto, R.; Eisenberg, S.; Breslow, J.L. Physical Exercise Conditioning in the Absence of Weight Loss Reduces Fasting and Postprandial Triglyceride-Rich Lipoprotein Levels. Circulation 1989, 79, 1007–1014. [CrossRef]
- Carraro, E.; Schilirò, T.; Biorci, F.; Romanazzi, V.; Degan, R.; Buonocore, D.; Verri, M.; Dossena, M.; Bonetta, S.; Gilli, G. Physical Activity, Lifestyle Factors and Oxidative Stress in Middle Age Healthy Subjects. International journal of environmental research and public health 2018, 15, 1152.
- Doussoulin, A.; Rivas, C.; Bacco, J.; Sepúlveda, P.; Carvallo, G.; Gajardo, C.; Soto, A.; Rivas, R. Prevalence of Spasticity and Postural Patterns in the Upper Extremity Post Stroke. Journal of Stroke and Cerebrovascular Diseases 2020, 29, 105253. [CrossRef]
- Seok Ryu, J.; Woo Lee, J.; Il Lee, S.; Ho Chun, M. Factors Predictive of Spasticity and Their Effects on Motor Recovery and Functional Outcomes in Stroke Patients. Topics in stroke rehabilitation 2010, 17, 380–388. [CrossRef]
- Lundström, E.; Terént, A.; Borg, J. Prevalence of Disabling Spasticity 1 Year after First-ever Stroke. European journal of neurology 2008, 15, 533–539. [CrossRef]
- Chheang, V.; Lokesh, R.; Chaudhari, A.; Wang, Q.; Baron, L.; Kiafar, B.; Doshi, S.; Thostenson, E.; Cashaback, J.; Barmaki, R.L. Immersive Virtual Reality and Robotics for Upper Extremity Rehabilitation. arXiv preprint arXiv:2304.11110 2023.
- Hosseini, Z.-S.; Peyrovi, H.; Gohari, M. The Effect of Early Passive Range of Motion Exercise on Motor Function of People with Stroke: A Randomized Controlled Trial. Journal of caring sciences 2019, 8, 39. [CrossRef]
- Indrawati, I.; Sudiana, K.; Sajidin, M. Active Passive and Active-Assistive Range of Motion (ROM) Exercise to Improve Muscle Strength in Post Stroke Clients: A Systematic Review.; 2019; pp. 329–337.
- Lindgren, I.; Jönsson, A.-C.; Norrving, B.; Lindgren, A. Shoulder Pain After Stroke: A Prospective Population-Based Study. Stroke 2007, 38, 343–348. [CrossRef]
- Funao, H.; Tsujikawa, M.; Momosaki, R.; Shimaoka, M. Virtual Reality Applied to Home-Visit Rehabilitation for Hemiplegic Shoulder Pain in a Stroke Patient: A Case Report. Journal of Rural Medicine 2021, 16, 174–178. [CrossRef]
- Shahrbanian, S.; Simmonds, M.J. Effects of Different Virtual Reality Environments on Experimental Pain Rating in Post-Stroke Individuals with and without Pain in Comparison to Pain Free Healthy Individuals. Annual Review Of Cybertherapy And Telemedicine 2008, 653, 659.
- Poenaru, D.; Sandulescu, M.I.; Cinteza, D. Pain Modulation in Chronic Musculoskeletal Disorders: Botulinum Toxin, a Descriptive Analysis. Biomedicines 2023, 11, 1888. [CrossRef]
- Trofin, D.; Salmen, B.-M.; Salmen, T.; Trofin, D.M.; Reurean-Pintilei, D. Advancing the Diagnosis of Diabetic Neuropathies: Electrodiagnostic and Skin Autofluorescence Methods. Journal of Personalized Medicine 2024, 14, 884. [CrossRef]
- Küçükdeveci, A.A.; Kutlay, Ş.; Yıldızlar, D.; Öztuna, D.; Elhan, A.H.; Tennant, A. The Reliability and Validity of the World Health Organization Disability Assessment Schedule (WHODAS-II) in Stroke. Disability and rehabilitation 2013, 35, 214–220. [CrossRef]
- Cheung, K.L.; Tunik, E.; Adamovich, S.V.; Boyd, L.A. Neuroplasticity and Virtual Reality. Virtual reality for physical and motor rehabilitation 2014, 5–24.
- Hao, J.; Xie, H.; Harp, K.; Chen, Z.; Siu, K.-C. Effects of Virtual Reality Intervention on Neural Plasticity in Stroke Rehabilitation: A Systematic Review. Archives of Physical Medicine and Rehabilitation 2022, 103, 523–541. [CrossRef]
- Nielsen, J.B.; Willerslev-Olsen, M.; Christiansen, L.; Lundbye-Jensen, J.; Lorentzen, J. Science-Based Neurorehabilitation: Recommendations for Neurorehabilitation from Basic Science. Journal of motor behavior 2015, 47, 7–17. [CrossRef]

| Game | Description | Gameplay screenshot | Impairments/ movements targeted |
|---|---|---|---|
| Balls | The patients are asked to touch a set of balls coming toward them in the virtual environment. |  |
Extension of the shoulder Flexion and extension of the wrist |
| Fruits | The patients are asked to "pick" a set of fruits floating in the air and place them in a basket in front of them. |  |
Extension and supination |
| An alternative to the game mentioned above is one where the patients are asked to "pick" the fruits and bring them to their mouths. | Flexion and pronation | ||
| Road | The patients are asked to touch the entire perimeter of a curve with their hands. This curve is shaped like a branch and fills in with leaves, as shown in the adjacent figure. |  |
Supination and pronation |
| Rocket | The patients are asked to raise their hands to a certain height to launch a rocket aimed at a little monster. At regular intervals, a little monster appears in front of the patients and moves toward them. The patients have a few seconds to raise their hands to the required height to launch the rocket that stops the monster. |  |
Abduction and flexion of the shoulder |
| Fires | The patients are asked to control a stream of water coming from their hands to extinguish flames at the windows of a building. There are several types of games. |  |
|
| Cooking | The patients are asked to prepare burgers on a tray in front of them. Ingredients for the burgers appear on the left and right sides of the tray, and the patients must move them to a specific position in the center of the tray to layer the burger. The first and last ingredients are always bun slices. |  |
Internal and external rotation of the shoulder |
| Piano | The patients are asked to touch the keys of a keyboard to play the piano. Markers indicating which key to press come toward the patients. |  |
Flexion and extension of the wrist |
| An alternative version of the game described above is where the patients are only asked to touch the markers coming toward them. | |||
| Diorama | The patients are asked to place 14 pieces of a diorama. The pieces appear on the left or right side of the patients and must be placed in a fixed position on a model. |  |
Flexion and extension of the wrist |
| Characteristic | n=14 |
|---|---|
| Age (years) ± mean (SD) | 55.29±8.6 |
| Females Males |
14.27% 85.71% |
| Urban Rural |
71,42% 28,57% |
| Education (years) ± mean (SD) | 13.36±2.72 |
| Marital status (married) | 70.4% |
| Employment status (retirees) | 78.57% |
| Stroke Ischemic Haemorrhagic |
78.57% 21.42% |
| Left side affected Right side affected |
85.71% 14.27% |
| Months since the onset of stroke, median (IQR) | 26.25 (5.25 to 31.5) |
| Smoker | 50% |
| Alcohol consumer | 21.42 |
| MMSE, median (IQR) | 27.50 (25 to 30) |
| Number of patients injected with Botulinum toxin | 8 |
| Average time since last injection (months) | 1.81 |
| Characteristic | n=14 |
|---|---|
| HBP (%) | 92.86% |
| Type 2 Diabetes Mellitus (%) | 21.43% |
| Dyslipidemia | 85.71% |
| Atrial Fibrillation | 14.29% |
| Clinical endpoints | T0 Median (IQR) |
T1 Median (IQR) |
p |
|---|---|---|---|
| MAS shoulders adductors | 1.5 (1.0 to 2.0) | 1.0 (1.0 to 1.5) | 0.02 |
| MAS elbow flexors | 1.5 (1.0 to 2.0) | 1.0 (1.0 to 1.5) | 0,008 |
| MAS pronators | 1 (1 to 1.5) | 1 (1 to 1.5) | 0.083 |
| MAS wrist flexors | 1.5 (0.75 to 2.0) | 1 (0.75 to 1.5) | 0,023 |
| MAS fingers flexors |
1.25 (0.0 to 1.62) | 1 (0.0 to 1.5) | 0.034 |
| Clinical endpoints | T0 Media (SD) |
T1 Media (SD) |
p |
|---|---|---|---|
| AROM abduction (°) | 98.57 (18.95) | 102,28 (18.28) | ˂0.001 |
| AROM shoulder flexion (°) | 101,07 (31.90) | 104.42 (30.85) | ˂0.001 |
| AROM internal rotation (°) | 53 (5.96) | 54 (5.96) | ˂0.001 |
| AROM external rotation (°) | 41.07 (28.07) | 45.28 (26.28) | ˂0.001 |
| AROM elbow flexion (°) | 135.28 (16.16) | 136.14 (14.26) | ˂0.001 |
| AROM pronation (°) | 80.07 (3.51) | 80.28 (3.60) | ˂0.001 |
| AROM supination (°) | 61.28 (28.26) | 64.28 (26.01) | ˂0.001 |
| AROM wrist flexion (°) | 65.28 (14.77) | 65.71 (14.67) | ˂0.001 |
| AROM wrist extension (°) | 32.92 (22.27) | 35.21 (21.41) | ˂0.001 |
| AROM metacarpophalangeal flexion (°) | 83.00 (9.68) | 83.71 (9.44) | ˂0.001 |
| AROM metacarpophalangeal extension (°) | 7.64 (8.68) | 10.42 (8.13) | ˂0.001 |
| NRS shoulder | 2.28 (1.85) | 1.68 (1.27) | ˂0.001 |
| Disability categories | T0 Disability percentage | 30 days Disability percentage | P value | |
|---|---|---|---|---|
| Cognition | 23.21% | 14.88% | 0.011 | |
| Mobility | 39.29% | 37.50% | 0.714 | |
| Self-care | 41.07% | 36.16% | 0.366 | |
| Getting along with people | 27.86% | 21.07% | 0.142 | |
| Life activities | Household | 65.63% | 0.363 | 0.363 |
| Work or school activities | 54.69% | 0.391 | 0.392 | |
| Participation in society | 34.82% | 31.55% | 0.340 | |
| Overall disability | 40.94% | 36.30% | 0.127 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





