Submitted:
25 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Keywords:
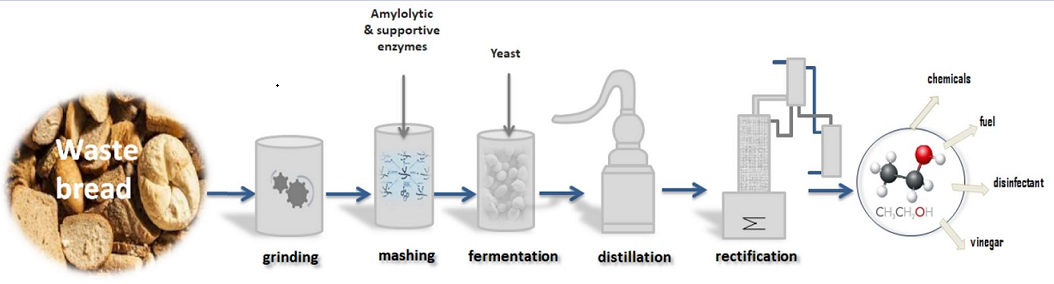
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Enzymatic Preparations
- Termamyl SC (α-amylase from Bacillus stearothermophilus, EC 3.2.1.1),
- SAN Extra (glucan 1,4-α-glucosidase from Aspergillus niger, EC 3.2.1.3),
- Viscoferm® (a multienzyme complex containing non-starch-degrading enzymes: cellulase, EC 3.2.1.4; xylanase (endo-1,4-), EC 3.2.1.8; β-glucanase (endo-1,3(4)-), EC 3.2.1.6),
- Neutrase® (neutral protease from Bacillus amyloliquefaciens, EC.3.4.24.28).
- GC 626 (acid α-amylase from Trichoderma reesei, EC 3.2.1.1),
- Stargen 002® (a blend of α-amylase from Aspergillus kawachi expressed in Trichoderma reesei, EC 3.2.1.1, and glucoamylase from Trichoderma reesei, EC 3.2.1.3).
- as supportive preparations, the aforementioned Viscoferm® and Neutrase® were applied.
2.3. Yeast
2.4. Sweet Mashes Preparation
- -
- without starch ‘activation’ (NSH-N/A) - milled bread was combined with tap water in a ratio of 3.5 L water per 1 kg of bread. This mixture was placed in a vessel within a water bath, equipped with a laboratory stirrer and thermometer. The whole mixture was heated to 50 ± 1 °C and digested with a GC 626 preparation (0.3 mL per 1 kg of raw material), and a viscosity-reducing Viscoferm® preparation (0.15 mL/kg raw material). The mixture was immediately cooled to 35 °C, and digested with the saccharifying Stargen 002® preparation (1.2 mL per 1 kg of raw material), along with the supportive Neutrase® preparation (0.1 mL/kg raw material). Next, the mash was cooled to a temperature of 30 °C, supplemented with an aqueous solution of (NH4)2HPO4 (0.2 g/L mash), and inoculated with yeast.
- -
- with starch ‘activation’ (NSH-A) - milled bread was combined with tap water in a ratio of 3.5 L water per 1 kg of bread in a vessel placed in a water bath and equipped with a laboratory stirrer and thermometer. The mixture was heated to 50 ± 1 °C, and digested with a GC 626 preparation (0.3 mL per 1 kg of raw material) along with a viscosity-reducing Viscoferm® preparation (0.15 mL/kg raw material). This was kept at the aforementioned temperature for 30 min in order to facilitate the activation of the starch. Further procedure was the same as in the case of the variant without starch ‘activation’.
2.5. Fermentation of Mashes
2.6. Distillation
2.7. Analytical Methods
2.8. Calculations
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Chemical Composition of Raw Materials
3.2. Chemical Composition of Mashes before and after Fermentation
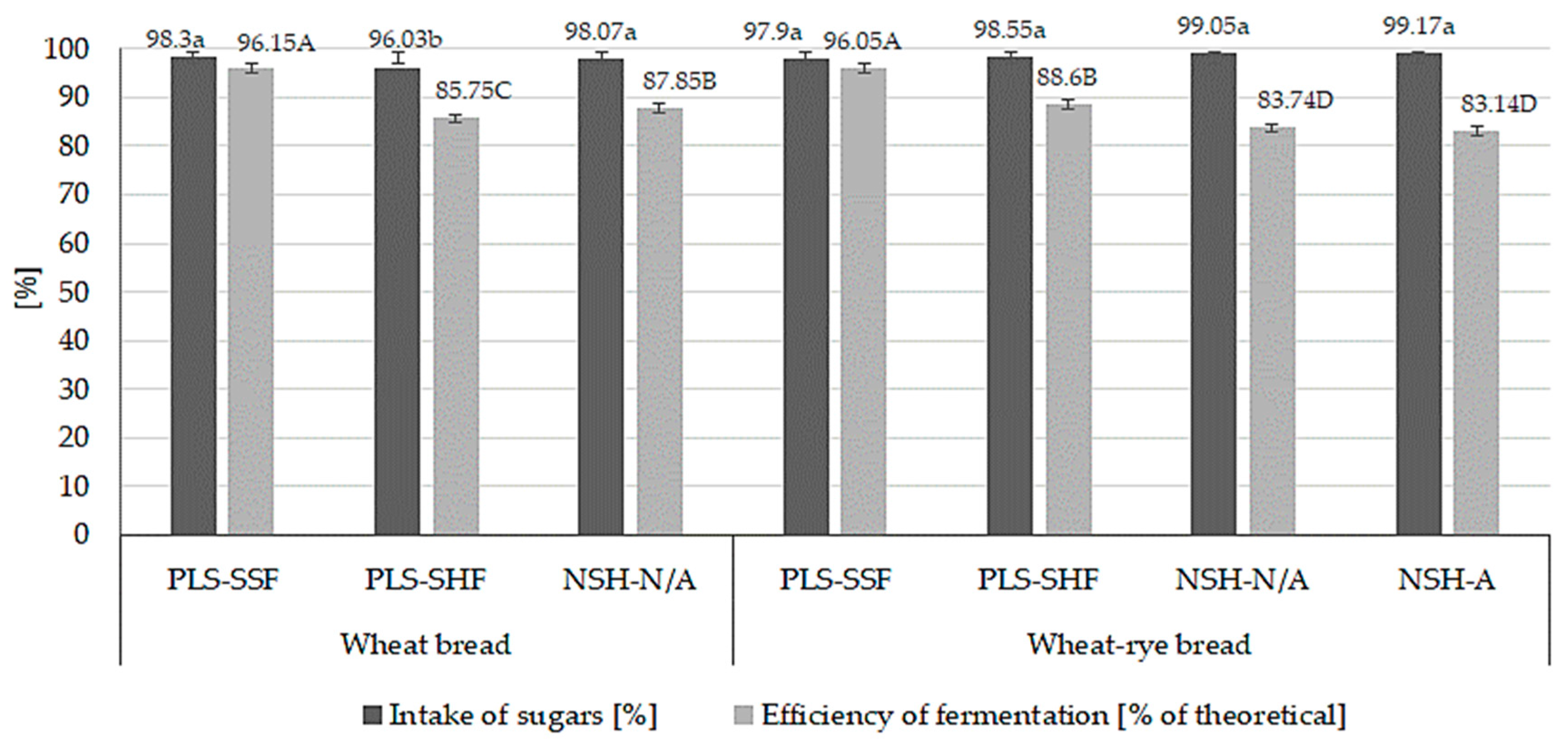
3.3. Fermentation Indicators
3.4. Chemical Composition of the Obtained Distillates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachs, J.D.; Kroll, C.; Lafortune, G.; Fuller, G.; Woelm, F. Sustainable Development Report 2022. Cambridge University Press; 2022. [CrossRef]
- Jung, J.M.; Kim, J.Y.; Kim, J.H.; Kim, S.M.; Jung, S.; Song H., Kwon E., Choi Y.E. Zero-waste strategy by means of valorization of bread waste. J. Clean. Prod. 2022, 6(1), 365-370.
- Brancoli, P.; Lundin, M.; Bolton, K.; Eriksson, M. Bread loss rates at the supplier-retailer interface – Analysis of risk factors to support waste prevention measures. Resour. Conserv. Recycl. 2019, 147, 128–136. [CrossRef]
- Samray, M.N.; Masatcioglu, T.M.; Koksel, H. Bread crumbs extrudates: A new approach for reducing bread waste. J. Cereal Sci. 2019, 85, 130–136. [CrossRef]
- Zhang, A.Y.-Z.; Sun, Z.; Leung, C.C.J.; Han, W.; Lau, K.Y.; Li, M.; Lin, C.S.K. Valorisation of bakery waste for succinic acid production. Green Chem. 2013, 15, 690–695. [CrossRef]
- Verni, M.; Minisci, A.; Convertino, S.; Nionelli, L.; Rizzello, C.G. Wasted Bread as Substrate for the Cultivation of Starters for the Food Industry. Front. Microbiol. 2020, 11, 293. [CrossRef]
- Korzeniowska-Ginter, R.; Dereszewska, A. THE SCALE OF WASTE OF BAKERY PRODUCTS IN HOUSEHOLDS. Ann. Pol. Assoc. Agric. Agribus. Econ. 2018, XX, 91–97. [CrossRef]
- Narisetty, V.; Cox, R.; Willoughby, N.; Aktas, E.; Tiwari, B.; Matharu, A.S.; Salonitis, K.; Kumar, V. Recycling bread waste into chemical building blocks using a circular biorefining approach. Sustain. Energy Fuels 2021, 5, 4842–4849. [CrossRef]
- Kiran, E.U.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [CrossRef]
- Muñoz, I.; Flury, K.; Jungbluth, N.; Rigarlsford, G.; i Canals, L.M.; King, H. Life cycle assessment of bio-based ethanol produced from different agricultural feedstocks. Int. J. Life Cycle Assess. 2014, 19, 109–119. [CrossRef]
- Kawa-Rygielska, J.; Czubaszek, A.; Pietrzak, W. Some aspects of baking industry wastes utilization in bioethanol production. Zesz. Probl. Post. Nauk Roln. 2013, 575, 71–77 (in Polish).
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric Acid Pretreatment of Jerusalem Artichoke Stalks for Enzymatic Saccharification and Bioethanol Production. Energies 2018, 11, 2153. [CrossRef]
- Lane, R.H. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists, Inc.: Arlington, WA, USA, 1995; Vol. 2, pp. 777–796.
- Polish Standard PN-A-79528-6. Spirit (Ethyl Alcohol). Test Methods. Determination of methyl alcohol content. Polish Committee for Standardization: Warsaw, Poland, 2000.
- Polish Standard PN-A-79528-4. Spirit (Ethyl Alcohol). Test Methods. Determination of aldehydes content. Polish Committee for Standardization: Warsaw, Poland, 2000.
- Polish Standard PN-A-79528-7. Spirit (Ethyl Alcohol). Test Methods. Determination of acidity. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Pomeranz, Y. Biochemical, Functional and Nutritive Changes during Storage. In Storage of Cereal Grains and Their Products; Christensen, C.M., Ed.; Monograph Series; American Association of Cereal Chemists: St. Paul, MN, USA, 1974; pp. 56–114.
- Wilkin, D.R.; Stenning, B.C. Moisture content of cereal grains. Available online: https://cereals.ahdb.org.uk/publications/1989/september/01/moisture-content-of-cereal-grains.aspx (accessed on 15 May 2024).
- Rhazi, L.; Méléard, B.; Daaloul, O.; Grignon, G.; Branlard, G.; Aussenac, T. Genetic and Environmental Variation in Starch Content, Starch Granule Distribution and Starch Polymer Molecular Characteristics of French Bread Wheat. Foods 2021, 10, 205. [CrossRef]
- Mesta-Corral, M.; Gómez-García, R.; Balagurusamy, N.; Torres-León, C.; Hernández-Almanza, A.Y. Technological and Nutritional Aspects of Bread Production: An Overview of Current Status and Future Challenges. Foods 2024, 13, 2062. [CrossRef]
- Paterson, A.; Swanston, J.S.; Piggott, J.R. Production of fermentable extracts from cereals and fruits. In Fermented Beverage Production; Lea, A.G.H., Piggott, J., Eds.; Springer Science + Business: New York, NY, USA, 1995; pp. 1–24.
- Ben Rejeb, I.; Charfi, I.; Baraketi, S.; Hached, H.; Gargouri, M. Bread Surplus: A Cumulative Waste or a Staple Material for High-Value Products? Molecules 2022, 27, 8410.
- Lineback, D.R.; Rasper, V.F. Wheat carbohydrates. In Wheat: Chemistry and Technology, 3rd ed.; Pomeranz, Y., Ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1998; Volume 1, pp. 277–372.
- Fanuel, M.; Ropartz, D.; Guillon, F.; Saulnier, L.; Rogniaux, H. Distribution of cell wall hemicelluloses in the wheat grain endosperm: a 3D perspective. Planta 2018, 248, 1505–1513. [CrossRef]
- Schweizer, T.F.; Würsch, P. Analysis of dietary fiber. In The Analysis of Dietary Fiber in Food; James, W.P.T., Theander, O., Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 203–216.
- Diowksz, A. Taste as a key element of bread quality. In Bread - taste, health, economy. SITSpoż Publishing House, Warsaw 2019, pp. 21-39.
- Liu, X.; Jia, B.; Sun, X.; Ai, J.; Wang, L.; Wang, C.; Zhao, F.; Zhan, J.; Huang, W. Effect of Initial PH on Growth Characteristics and Fermentation Properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, M800–8. [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K. Effect of simultaneous saccharification and fermentation conditions of native triticale starch on the dynamics and efficiency of process and composition of the distillates obtained. J. Chem. Technol. Biotechnol. 2013, 88, 615–622. [CrossRef]
- Strąk-Graczyk, E.; Balcerek, M. Effect of Pre-hydrolysis on Simultaneous Saccharification and Fermentation of Native Rye Starch. Food Bioprocess Technol. 2020, 13, 923–936. [CrossRef]
- Russell, I. Understanding yeast fundamentals. In The Alcohol Textbook, 4th ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Alltech Inc.: Nicholasville, KY, USA, 2003; pp. 85–120.
- Kotarska, K.; Czupryński, B.; Kłosowski, G. Effect of various activators on the course of alcoholic fermentation. J. Food Eng. 2006, 77, 965–971. [CrossRef]
- Lagos, M.A.P.; Caviativa, J.A.C.; Pinzón, D.C.T.; Roa, D.H.R.; Basso, T.O.; Lozano, M.E.V. Xylose Metabolization by a Saccharomyces cerevisiae Strain Isolated in Colombia. Indian J. Microbiol. 2023, 63, 84–90. [CrossRef]
- Graves, T.; Narendranath, N.V.; Dawson, K.; Power, R. Interaction effects of lactic acid and acetic acid at different temperatures on ethanol production by Saccharomyces cerevisiae in corn mash. Appl. Microbiol. Biotechnol. 2007, 73, 1190–1196. [CrossRef]
- Baroň, M.; Fiala, J. Chasing after minerality, relationship to yeast nutritional stress and succinic acid production. Czech J. Food Sci. 2012, 30, 188–193.
- Yalcin, S.K.; Yesim Ozbas, Z. Effects of pH and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from Turkey. Brazilian J. Microbiol. 2008, 39(2), 325-332.
- Kawa-Rygielska, J.; Pietrzak, W. UTILIZATION OF WASTE BREAD FOR BIOETHANOL PRODUCTION. Zywnosc.Nauka.Technologia.Jakosc/Food.Science.Technology.Quality 2011. [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Nowak, A.; Wojtczak, M.; Czyżowska, A.; Dziekońska-Kubczak, U.; Patelski, P. The Effect of Different Starch Liberation and Saccharification Methods on the Microbial Contaminations of Distillery Mashes, Fermentation Efficiency, and Spirits Quality. Molecules 2017, 22, 1647. [CrossRef]
- Regulation (EU) 2019/787 of the European Parliament and of the Council. Off. J. Eur. Union 2019, 130, 1–54.
- Plutowska, B.; Biernacka, P.; Wardencki, W. Identification of Volatile Compounds in Raw Spirits of Different Organoleptic Quality. J. Inst. Brew. 2010, 116, 433–439. [CrossRef]
- Polish Standard, PN-A-79523:2002 Agricultural Distillate; Polish Committee for Standardization: Warsaw, Poland, 2002.
- Davídek, T.; Devaud, S.; Robert, F.; Blank, I. Sugar fragmentation in the Maillard reaction cascade: Isotope labeling studies on the formation of acetic acid by a hydrolytic α-dicarbonyl cleavage mechanism. J. Agric. Food Chem. 2006, 54, 6667–6676.
| Parameters | Wheat bread | Wheat-rye bread | ||
|---|---|---|---|---|
| Content [g/100 g] | ||||
| Mean | SD | Mean | SD | |
| Moisture | 24.35 b | 0.15 | 19.80 a | 0.37 |
| Total protein (Nx6,25) | 7.08 b | 0.01 | 8.18 a | 0.05 |
| Total reducing sugars expressed as glucose, incl.: | 2.29 a | 0.15 | 1.62 b | 0.25 |
| Maltotriose | 0.15 a | 0.01 | 0.17 a | 0.01 |
| Maltose | 0.17 a | 0.01 | 0.10 b | 0.01 |
| Glucose | 1.95 a | 0.18 | 1.33 b | 0.16 |
| Starch | 54.24 b | 1.29 | 61.88 a | 3.99 |
| Xylose | 0.12 b | 0.02 | 0.39 a | 0.02 |
| Arabinose | 0.08 b | 0.03 | 0.22 a | 0.00 |
| Succinic acid | 0.03 a | 0.01 | 0.03 a | 0.01 |
| Lactic acid | 0.04 b | 0.01 | 0.08 a | 0.01 |
| Acetic acid | 0.03 b | 0.01 | 0.06 a | 0.01 |
| Glycerol | 0.03 b | 0.01 | 0.11 a | 0.01 |
| Parameter | Wheat bread-based mashes | Wheat-rye bread-based mashes | ||||||||||||
| PLS-SSF | PLS-SHF | NSH-N/A | PLS-SSF | PLS-SHF | NSH-N/A | NSH-A | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| pH | 5.10 a | 0.10 | 5.10 a | 0.10 | 5.10 a | 0.20 | 4.50 b | 0.10 | 4.50 b | 0.10 | 4.50 b | 0.10 | 4.50 b | 0.10 |
| Extract [% w/w] | 17.60 c | 0.28 | 18.93 b | 0.17 | 20.00 a | 0.10 | 16.40 d | 0.21 | 18.00 c | 0.10 | 20.00 a | 0.10 | 20.00 a | 0.10 |
| Total reducing sugars expressed as glucose [g/L], incl.: |
120.44 d | 1.58 | 135.25 c | 1.22 | 145.92 a | 0.12 | 113.43 e | 1.67 | 102.49 f | 1.20 | 140.34 b | 0.17 | 148.24 a | 0.13 |
| Maltotriose | 0.95 b | 0.17 | 5.85 a | 0.05 | 0.13 de | 0.02 | 0.60 c | 0.06 | 0.32 d | 0.02 | 0.11 e | 0.00 | 0.12 e | 0.00 |
| Maltose | 35.10 a | 3.74 | 8.12 b | 0.11 | 0.29 c | 0.02 | 33.79 a | 1.70 | 0.88 c | 0.04 | 1.15 c | 0.01 | 0.29 c | 0.01 |
| Glucose | 82.57 e | 5.91 | 120.45 c | 3.67 | 145.48 ab | 1.85 | 77.29 e | 1.62 | 101.23 d | 2.34 | 139.01 b | 1.13 | 147.81 a | 0.51 |
| Dextrins [g/L] | 22.71 f | 0.21 | 23.97 f | 0.25 | 68.50 a | 0.09 | 30.84 e | 1.10 | 51.53 b | 0.46 | 45.11 c | 0.57 | 38.02 d | 0.34 |
| Parameter | Wheat bread-based mashes | Wheat-rye bread-based mashes | |||||||||||||
| PLS-SSF | PLS-SHF | NSH-N/A | PLS-SSF | PLS-SHF | NSH-N/A | NSH-A | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| pH | 4.40 a | 0.02 | 4.30 ab | 0.00 | 4.30 ab | 0.10 | 4.10 b | 0.10 | 4.40 a | 0.00 | 4.20 ab | 0.10 | 4.20 ab | 0.10 | |
| Apparent extract [% w/w] |
0.71 bc | 0.07 | 1.11 a | 0.06 | 0.70 bc | 0.01 | 0.63 c | 0.02 | 0.78 b | 0.01 | 0.760 b | 0.02 | 0.75 b | 0.02 | |
| Ethanol [g/L] | 71.42 b | 1.32 | 70.95 b | 1.02 | 85.68 a | 0.02 | 72.50 b | 2.53 | 72.54 b | 1.64 | 81.52 a | 2.25 | 80.95 a | 2.11 | |
| Total reducing sugars expressed as glucose [g/L], incl.: |
0.32 c | 0.08 | 4.34 a | 0.35 | 0.90 b | 0.05 | 0.30 c | 0.01 | 0.23 c | 0.01 | 0.98 b | 0.01 | 1.09 b | 0.03 | |
| Maltotriose | 0.16 ab | 0.06 | 0.21 a | 0.07 | 0.07 bc | 0.00 | 0.09 bc | 0.00 | 0.02 c | 0.00 | 0.07 bc | 0.00 | 0.07 bc | 0.00 | |
| Maltose | 0.14 d | 0.01 | 0.31 c | 0.05 | 0.72 b | 0.09 | 0.18 cd | 0.03 | 0.14 d | 0.02 | 0.80 ab | 0.07 | 0.90 a | 0.01 | |
| Glucose | 0.01 c | 0.00 | 3.79 a | 0.42 | 2.07 b | 0.15 | 0.02 c | 0.01 | 0.05 c | 0.01 | 0.08 c | 0.02 | 0.07 c | 0.02 | |
| Dextrins [g/L] | 1.94 a | 0.09 | 1.87 a | 0.56 | 0.72 b | 0.12 | 2.52 a | 0.58 | 1.89 a | 0.13 | 0.75 b | 0.02 | 0.44 b | 0.08 | |
| Other compounds [g/L]: | |||||||||||||||
| Xylose | 0.16 c | 0.01 | 0.10 cd | 0.02 | 0.16 c | 0.01 | 0.37 a | 0.02 | 0.03 d | 0.01 | 0.27 b | 0.05 | 0.36 a | 0.05 | |
| Arabinose | 0.02 c | 0.00 | 0.03 c | 0.00 | 0.01 c | 0.00 | 0.01 c | 0.00 | 0.10 a | 0.02 | 0.06 b | 0.01 | 0.030c | 0.00 | |
| Citric acid | 0.25 a | 0.01 | 0.22 a | 0.01 | 0.21 a | 0.02 | 0.01 c | 0.00 | 0.09 b | 0.01 | 0.07 bc | 0.05 | 0.12 b | 0.03 | |
| Succinic acid | 0.92 c | 0.11 | 1.01 bc | 0.03 | 1.09 bc | 0.08 | 1.24 ab | 0.02 | 1.05 bc | 0.02 | 1.47 a | 0.15 | 1.53 a | 0.22 | |
| Lactic acid | 0.62 cd | 0.08 | 2.61 a | 0.14 | 0.50 d | 0.05 | 0.81 c | 0.21 | 1.14 b | 0.05 | 0.46 d | 0.05 | 0.39 d | 0.05 | |
| Formic acid | 0.23 a | 0.01 | 0.05 b | 0.01 | 0.06 b | 0.02 | 0.09 b | 0.01 | 0.07 b | 0.03 | 0.06 b | 0.01 | 0.05 b | 0.01 | |
| Acetic acid | 0.09 cd | 0.02 | 0.24 ab | 0.05 | 0.31 a | 0.08 | 0.07 d | 0.02 | 0.28 ab | 0.04 | 0.22 abc | 0.06 | 0.15 bcd | 0.03 | |
| Glycerol | 7.23 b | 0.35 | 7.01 b | 0.16 | 7.97 a | 0.13 | 7.10 b | 0.09 | 6.71 b | 0.55 | 8.25 a | 0.03 | 8.36 a | 0.02 | |
| Parameter [g/L alcohol 100% v/v] |
Wheat bread-based mashes | Wheat-rye bread-based mashes | ||||||||||||
| PLS-SSF | PLS-SHF | NSH-N/A | PLS-SSF | PLS-SHF | NSH-N/A | NSH-A | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Methanol | 0.200 b | 0.04 | 0.240 b | 0.03 | 0.200 b | 0.03 | 0.150 b | 0.02 | 0.400 a | 0.08 | 0.210 b | 0.02 | 0.190 b | 0.03 |
| Aldehydes as acetaldehyde |
0.170 c | 0.01 | 0.120 d | 0.01 | 0.160 c | 0.02 | 0.220 a | 0.02 | 0.180 bc | 0.01 | 0.233 a | 0.02 | 0.210 ab | 0.01 |
| Acidity as acetic acid |
0.620 d | 0.01 | 0.940 a | 0.04 | 0.723 c | 0.05 | 0.583 d | 0.04 | 0.620 d | 0.01 | 0.710 c | 0.01 | 0.850 b | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





