1. Introduction
Coronavirus disease 2019 (COVID-19) is a serious lung disease, that may be complicated by severe acute respiratory syndrome (SARS-CoV-2) needing mechanical ventilation and intensive care unit treatment. Among the clinical features of COVID-19, thrombotic events in the venous and arterial are frequent complications, that are associated with poor clinical outcomes [
1]. Warning signs of thrombosis include essentially elevated markers of D-dimer, that is expression of systemic fibrinolysis consequent to thrombin generation [
2]; elevated markers of D-dimer have been consistently reported in COVID-19 patients and closely associated with thrombotic events and poor survival [
3,
4]. The mechanism accounting for hypercoagulation in COVID-19 has not been clarified but may include direct interaction of virus or its components such as Spike protein with cells devoted to activating coagulation systems such as endothelial cells, platelets and leucocytes or abnormal response to virus injury with production for example of cytokines [
1,
5] or oxidative stress that may in turn activate clotting system. On the basis that patients with community-acquired pneumonia (CAP) display enhanced levels of NOX2, one the most important cellular producers of reactive oxidant species (ROS), and lipopolysaccharides (LPS) coincidentally with an ongoing prothrombotic state, suggesting that low-grade endotoxemia may be implicated in the thrombotic complications occurring in CAP [
6,
7,
8], we speculated that oxidative stress and low-grade endotoxemia may be implicated also in COVID-19. Analysis of NOX2 and LPS in COVID-19 showed, in fact, that they are elevated compared to controls and associated with thrombosis suggesting that LPS could promote a hypercoagulation state [
9]. The biological plausibility of this hypothesis relies on an experimental model of thrombosis where low-grade endotoxemia enhanced thrombus growth via the LPS-specific receptor, i.e. Toll-like receptor 4 (TLR4) [
10]. Previous studies [
9,
11,
12] have linked higher intestinal translocation with severe cases of COVID-19, describing a higher abundance of bacterial components in the bloodstream compared to milder cases.
However, so far, the relationship between NOX2, LPS and clotting activation has not been investigated in COVID-19, nor it has clarified the reason for low-grade endotoxemia.
Albumin is the most relevant protein in human blood, which possesses anti-inflammatory, antioxidant, and anticoagulant properties [
13,
14]; also, albumin can neutralize LPS preventing its toxic effects [
15,
16]. Previous studies showed that in COVID-19 serum albumin is seriously reduced during the acute phase of the disease and correlated with mortality and thrombotic events [
14,
17]. Therefore, the aim of the study was to explore the interplay between NOX2, LPS and albumin and to assess if such interplay may be implicated in the hypercoagulation state of COVID-19. In addition, we would aim to verify if NOX2 and LPS could predict disease severity especially ARDS.
2. Materials and Methods
2.1. Study Design and Population
This is an observational retrospective cohort multi-center study performed in Italian Hospitals devoted to COVID-19 care. This study was performed in non-intensive care unit (ICU) medical wards. We enrolled consecutive patients, according to the inclusion/exclusion criteria form cohort from Rome Hospitals and Latina Hospital.
We included in the study adult (>18 years) patients with laboratory-confirmed COVID-19 or SARS-CoV-2–related pneumonia, requiring or not mechanical ventilation, consecutively hospitalized from March 2020 to May 2020. COVID-19 was diagnosed based on the World Health Organization interim guidance, as previously described [
18]. Patients were classified as having or not having acute respiratory distress syndrome (ARDS), that was defined as the acute onset of respiratory failure, bilateral infiltrates on chest radiograph, hypoxemia as defined by a PaO
2/FiO
2 ratio ≤200 mmHg, and no evidence of left atrial hypertension or a pulmonary capillary pressure <18 mmHg (if measured) to rule out cardiogenic edema [
19]. Healthy subjects matched for demographic characteristics and without acute infections were used as control; they were recruited as routine screening for cardiovascular disease from the Division of I Clinica Medica, Atherothrombosis center, Policlinico Umberto I, Rome.
The routine analysis included P/F ratio and hs-CRP executed within 48 h from the admission at the hospital. Ethical approval for this study was obtained from Ethics Committee of Azienda Ospedaliera Universitaria Policlinico Umberto I (ID Prot. 6192) and was conducted in accordance with the Declaration of Helsinki.
2.2. sNOX2-dp Assay
sNOX2-dp concentration was measured with an ELISA method. Briefly, the assay is based on coating standards and serum samples into ELISA 96 wells plate overnight at 4 °C. After incubation an anti-sNOX2dp-horseradish peroxidase (HRP) monoclonal antibody was added. The immobilized antibody-enzyme conjugates were quantified by adding tetramethylbenzidine (TMB) and a stop solution. The enzyme activity was measured spectrophotometrically at 450 nm, values were expressed as pg/mL. Intra-assay and inter-assay coefficients of variation were <10%.
2.3. Hydrogen Peroxide (H2O2) Production
Hydrogen peroxide (H2O2) concentrations were determined by a colorimetric assay according to the manufacturer’s instructions (Abcam, Cambridge, UK). Values were expressed as μM and the intra- and inter-assay CV were both <10%.
2.4. LPS Assay
LPS levels were measured using a commercial ELISA kit (Cusabio, Wuhan, China) as previously described [
10]. Values were expressed as picograms per milliliter; intra-assay and inter-assay coefficients of variation were <10%.
2.5. Zonulin Assay
Serum zonulin levels were measured using a commercial ELISA kit (Elabscience, Houston, TX). The amount of zonulin was measured with a microplate autoreader at 450 nm. Values were expressed as nanograms per milliliter; both intra-assay and interassay coefficients of variation were <10%.
2.6. Plasma D-Dimer Assay
D-dimer levels in human plasma were measured using a commercial ELISA kit (Abcam, Cambridge, UK). Values were expressed as micrograms per milliliter; intra-assay and inter-assay coefficients of variation were <10%.
2.7. Albumin Assay
Albumin levels in human serum were measured using a commercial colorimetric assay kit (Abcam, Cambridge, UK). Values were expressed as grams per deciliter; intra-assay and inter-assay coefficients of variation were <10%.
2.8. Assessment of Intrahospital Ischemic and Embolic Events
The clinical course of the disease and its evolution were monitored during hospitalization. The appearance of new ischemic/embolic events was diagnosed as follows: (i) pulmonary thromboembolism by lung computed tomography scan [
20]; (ii) myocardial infarction by electrocardiogram (ECG) changes associated with enhanced markers of cell necrosis [
21] ; (iii) acute brain ischemia by onset of new focal neurological signs and symptoms and confirmed, whenever possible, by nuclear magnetic resonance or computed tomography imaging [
22]; and (iv) acute limb ischemia diagnosed according to American Heart Association guidelines [
23].
2.9. In Vitro Study
HUVEC (Human Umbilical Vein Endothelial Cells) were purchased from Lonza (Amboise, France) and cultured in EGM-2 complete medium as previously described [
24,
25,
26]. Sub-confluent cultures (2500/cm
2) were expanded between passages 3–5. Cell morphology and growth were monitored by light microscopy and assessed by Trypan Blue. To stimulate the cultures, cells were starved overnight (EBM Endothelial basal medium, Lonza supplemented with 0.2% FBS). The following day, starvation media was withdrawn and HUVEC were treated with or without LPS (160 pg/mL) in the presence or not of albumin (3-5 g/dL) [
27]. As a negative control (NC), the same percentage of albumin-diluent (1% PBS) was used.
After stimulation, HUVEC-derived supernatants were stored at −80 °C until use. Each experiment was replicated five times. Supernatants were analyzed for sNOX2dp and H2O2 concentration as previously described. Factor VIII was also measured by ELISA used according to the manufacturer’s guidelines (Lifespan Bioscience, Seattle, Washington, USA). The values of the release of factor VIII were expressed as U/dL. Inter- and intra-assay coefficients of variations were < 10%.
2.10. Statistical Analysis
All continuous variables were tested for normality with the Shapiro–Wilk test. Continuous variables with normal distribution were reported as mean ± standard deviation (SD), non-parametric variables as median and interquartile range (IQR). Between-groups comparisons were performed using an unpaired T test for normally distributed variables and using an appropriate non-parametric test for non-normally distributed variables (Mann–Whitney U test). Correlations were performed by the Spearman’s Rank Correlation Coefficient and described as Rs.
The prediction role of serum LPS, zonulin, hs-CRP and D-dimer were evaluated by means of area under the curve (AUC) for the receiver operating characteristic curve for predicting incident ARDS.
The bivariate and multivariate effects of prognostic factors on ARDS occurrence in COVID-19 patients were also assessed by means of logistic regression models. Wald confidence intervals and tests for odds-ratios and adjusted odds-ratios were computed based on the estimated standard errors. The stochastic level of entry into the multivariable model was set at 0.10.
Only p values <0.05 were considered statistically significant. All tests were 2-tailed, and analyses were performed using computer software packages (IBM SPSS Statistics, ver. 27).
3. Results
Clinical characteristics of patients with COVID-19 and controls are reported in
Table 1.
No significant differences were present in age, sex, body mass index and smoking habit between patients and controls. Conversely, significant differences were present for prevalence of arterial hypertension, diabetes, atrial fibrillation, and chronic obstructive pulmonary disease (COPD) between patients and controls. As expected, COVID-19 patients with ARDS showed an increased prevalence of COPD, atrial fibrillation and routinary markers of inflammation and hypercoagulation.
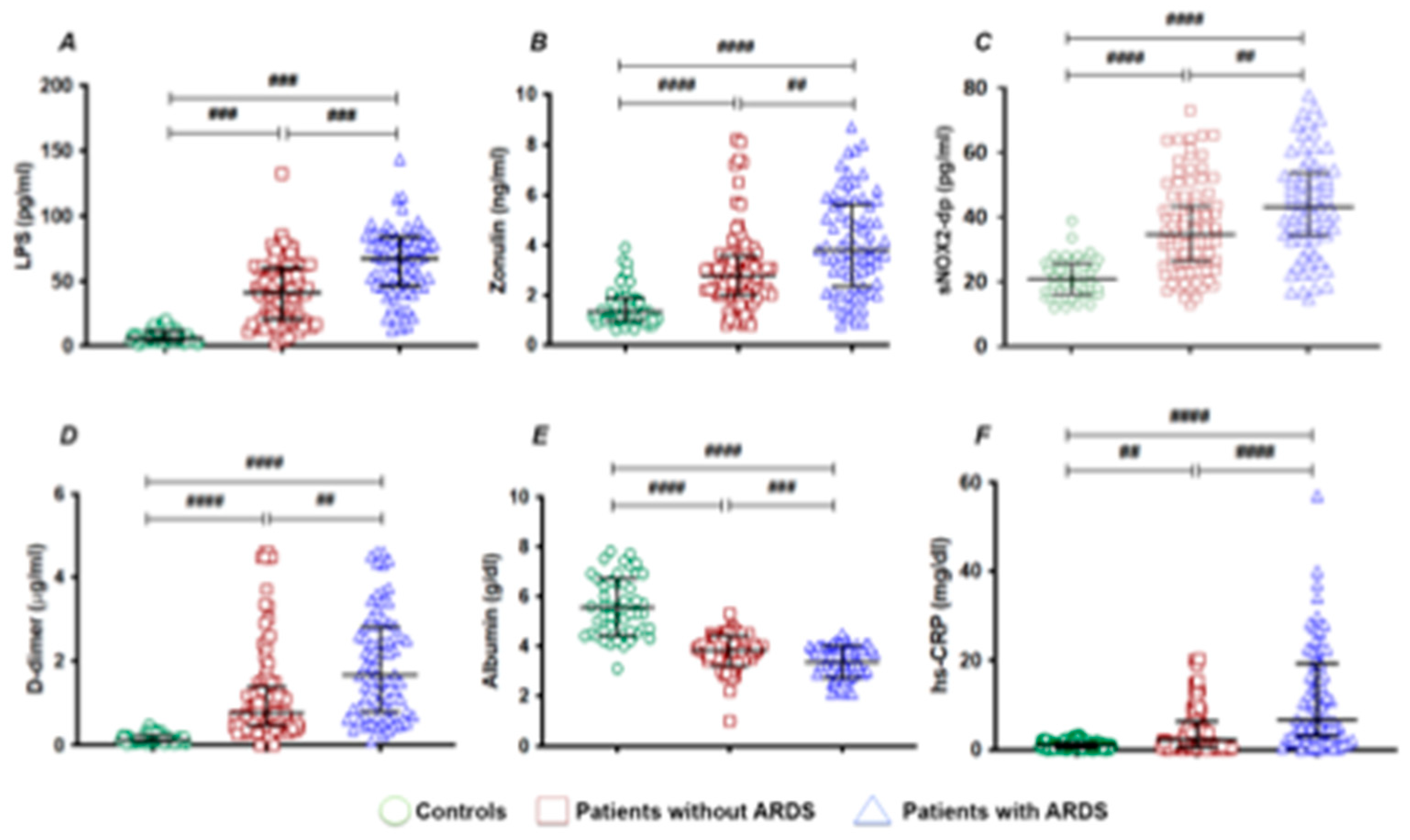
To assess the importance of gut permeability in the developed of COVID-19 severity, we analyzed two markers of intestinal barrier dysfunction such as LPS and zonulin. Serum LPS and zonulin were higher in patients with COVID-19 than in control subjects (
Table 1); in COVID-19 patients with ARDS, LPS and zonulin were significantly higher compared to COVID-19 patients without ARDS (
Table 1 and
Figure 1 A and B).
To analyze the interplay between oxidative stress and COVID-19 severity, we analyzed NOX2 levels in patients with and without ARDS. Significant differences were found in baseline levels of sNOX2-dp between controls and COVID-19 patients (
Table 1 and
Figure 1 C). The levels of sNOX2-dp were higher in patients with compared with those without ARDS (
Table 1 and
Figure 1 C).
In addition, to analyze the interplay between coagulation and inflammation and COVID-19 severity, we analyzed levels of D-dimer, albumin and hs-CRP in patients with and without ARDS. Significant differences were found in baseline levels of D-dimer, albumin, and hs-CRP between controls and COVID-19 patients (
Table 1 and
Figure 1 D-F). Among COVID-19, serum concentrations of D-dimer and hs-CRP were higher in patients with ARDS (
Table 1 and
Figure 1 D and F). Conversely, the serum concentration of albumin was lower in patients with ARDS compared with those without ARDS (
Table 1 and
Figure 1 E).
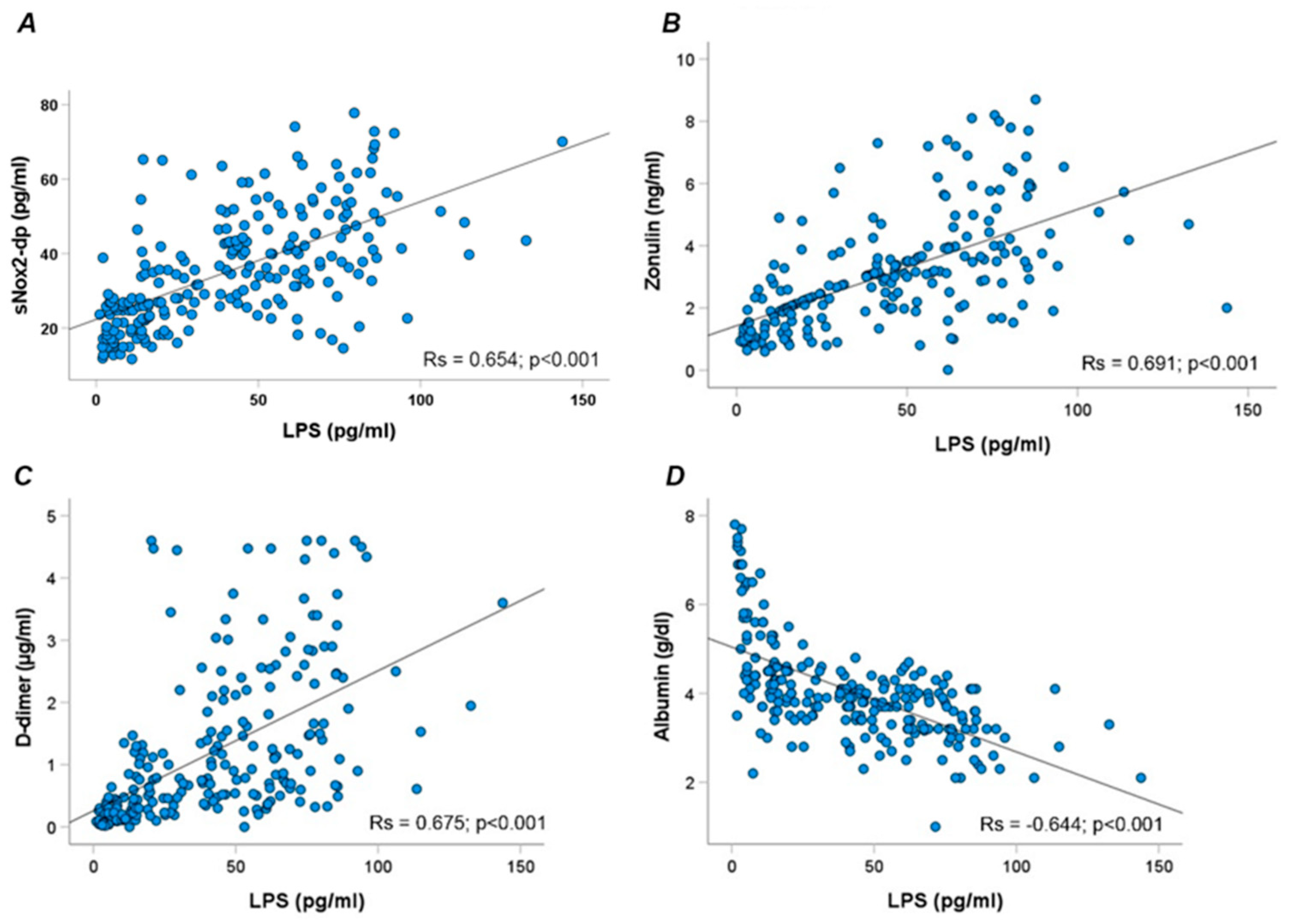
Overall, LPS correlated directly with sNOX2dp (Rs=0.654; p<0.001), zonulin (Rs = 0.691; p<0.001), D-dimer (Rs = 0.675; p<0.001) and inversely with albumin (Rs = -0.644; p<0.001) (
Figure 2 A-D).
Moreover, NOX2 correlated directly with D-dimer (Rs=0.525; p<0.001) and, inversely, with albumin (Rs=-0.443; p<0.001).
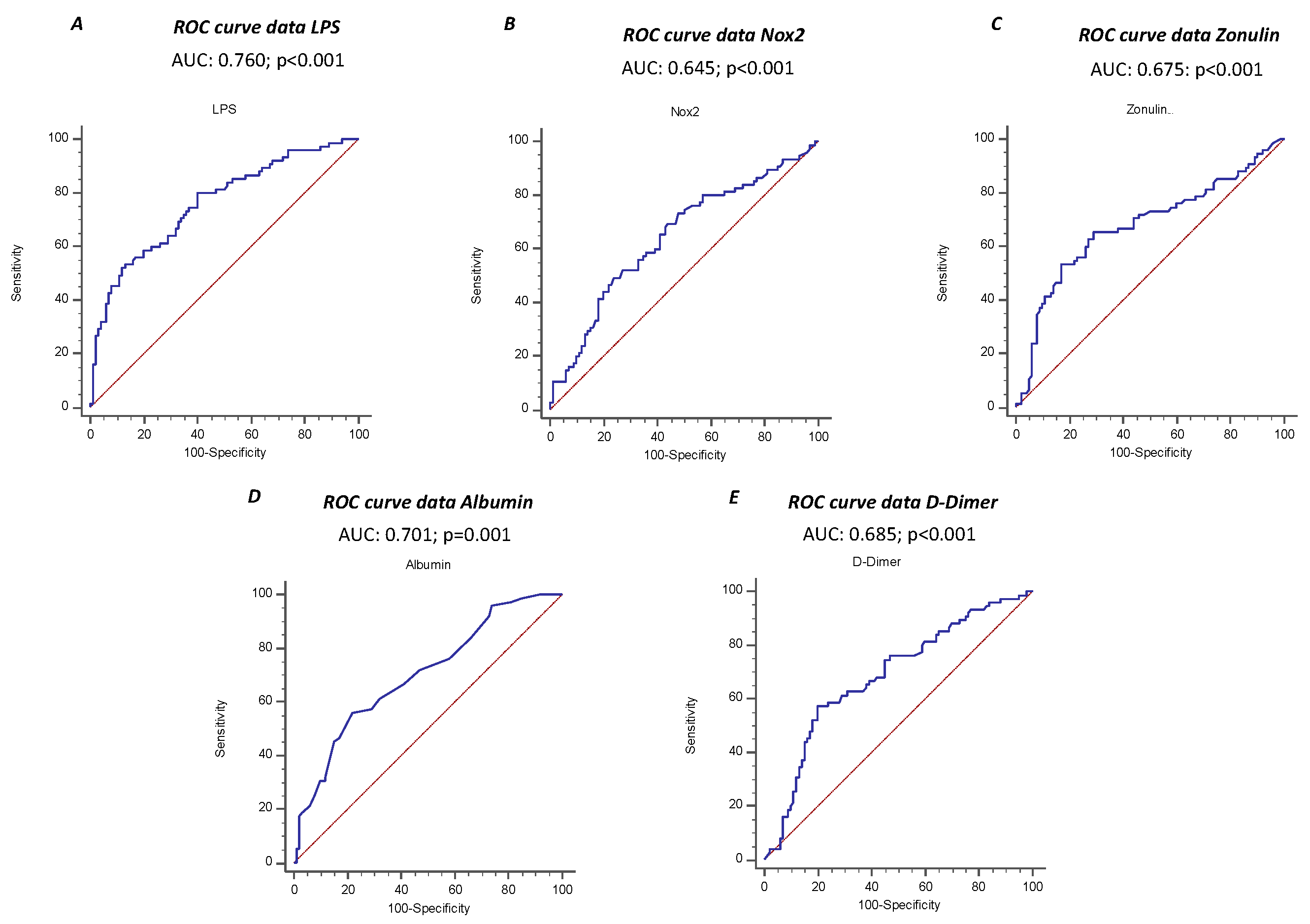
The ROC curve analyses showed that sNOX2-dp, LPS, zonulin, albumin and D-dimer predicted ARDS, with LPS showing the highest AUC (AUC for LPS: 0.760; 95% CI: 0.690-0.821; AUC for NOX2: 0.645; 95% CI: 0.562-0.728; AUC for zonulin: 0.675; 95% CI: 0.600-0.744; AUC for albumin 0.701; 95% CI: 0.627-0.767; AUC for D-Dimer 0.685; 95% CI: 0.610-0.753) (
Figure 3 A-E).
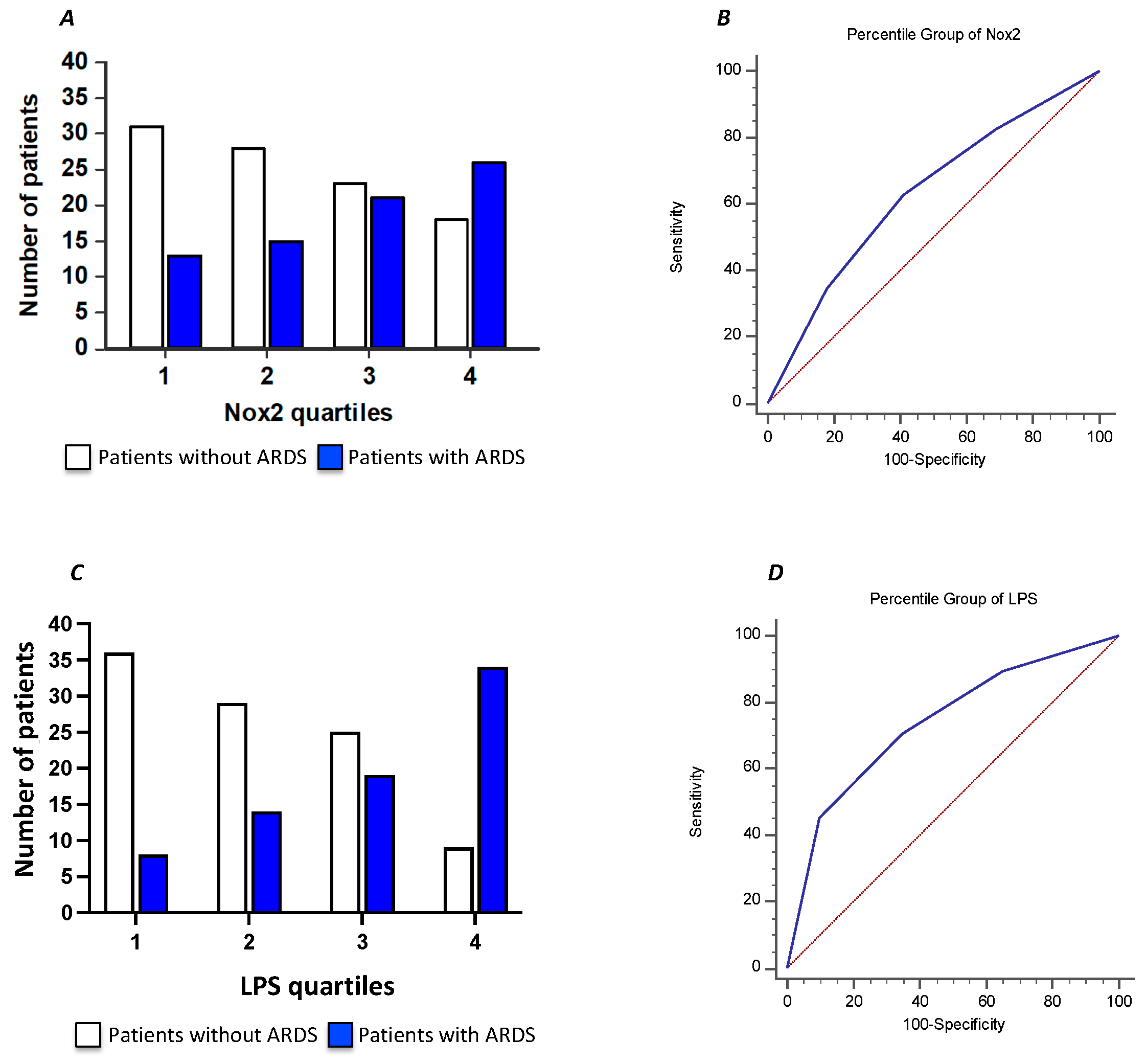
To further characterize the relationship between serum sNOX2-dp and ARDS in COVID-19 patients, we divided the COVID-19 cohort according to sNOX2-dp quartiles (I quartile, n=44; sNOX2-dp ≤ 28 pg/mL; II quartile, n=43; sNOX2-dp > 28 and ≤ 38.9 pg/mL; III quartile, n=44; sNOX2-dp > 38.9 and ≤ 50.2 pg/mL; IV quartile, n=44; sNOX2-dp > 50.2 pg/mL). The number of patients with ARDS progressively increased between the first to the fourth quartile (29%, 35%, 48% and 59%, respectively; p=0.024) (
Figure 4 A). The AUC of NOX2 quartiles was 0.630 (95% CI: 0.554-0.701), with values ≥ III quartiles predicting ARDS with a sensitivity of 63% and a specificity of 59% (
Figure 4 B).
Moreover, we characterized the relationship between serum LPS and ARDS in COVID-19 patients dividing the COVID-19 cohort according to LPS quartiles (I quartile, n=43; LPS ≤ 29.4 pg/mL; II quartile, n=44, LPS > 29.4 and ≤50.3 pg/mL; III quartile, n=44; LPS > 50.3 and ≤ 71.7 pg/mL; IV quartile, n=44 LPS > 71.7 pg/mL). The number of patients with ARDS progressively increased between the first to the fourth quartile (19%, 32%,43% and 77% respectively; p<0.001) (
Figure 4 C). The AUC of LPS quartiles was 0.739 (95% CI: 0.667-0.802), with values ≥ III quartiles predicting ARDS with a sensitivity of 70.7% and a specificity of 65% (
Figure 4 D).
Finally, a multivariable logistic regression analysis showed that the IV quartile of LPS was independently associated with an increased risk of ARDS, together with low-albumin serum levels and an increased hs-CRP, after adjusting for NOX2, D-dimer, age, sex, and comorbidities (
Table 2).
During a median follow-up of 18 days 21 patients experienced thrombotic events, 15 with ARDS and 6 without ARDS. Among the 75 patients with ARDS, 15 patients (20%) experienced thrombotic events in the arterial (n=7) and venous circulation (n=8) (interquartile range 11–27 days). Patients without ARDS (n= 100) had 6 (6%) (n= 6) thrombotic events (in the arterial n=2 and venous circulation n=4).
Of note, patients who experienced a thrombotic event showed higher levels of LPS, than patients without thrombotic events (75.5 [52.2-85.1] vs. 46.4 [26.3-67.6] pg/mL; p<0.001). In particular, the number of thrombotic events progressively increased from the I to the IV quartile of serum LPS (2% in the I, 9% in the II, 9% in the III and 27% in the IV quartile, p<0.001). Univariate logistic regression analyses showed that increased levels of LPS (OR: 1.037; 95% CI: 1.017-1.057; p<0.001) and D-dimer (OR: 2.239; 95% CI: 1.1569-3.194; p<0.001) and decreased levels of albumin (OR: 0.350; 95% CI: 0.178-0.688; p=0.002) were associated with increased risk of thrombotic events.
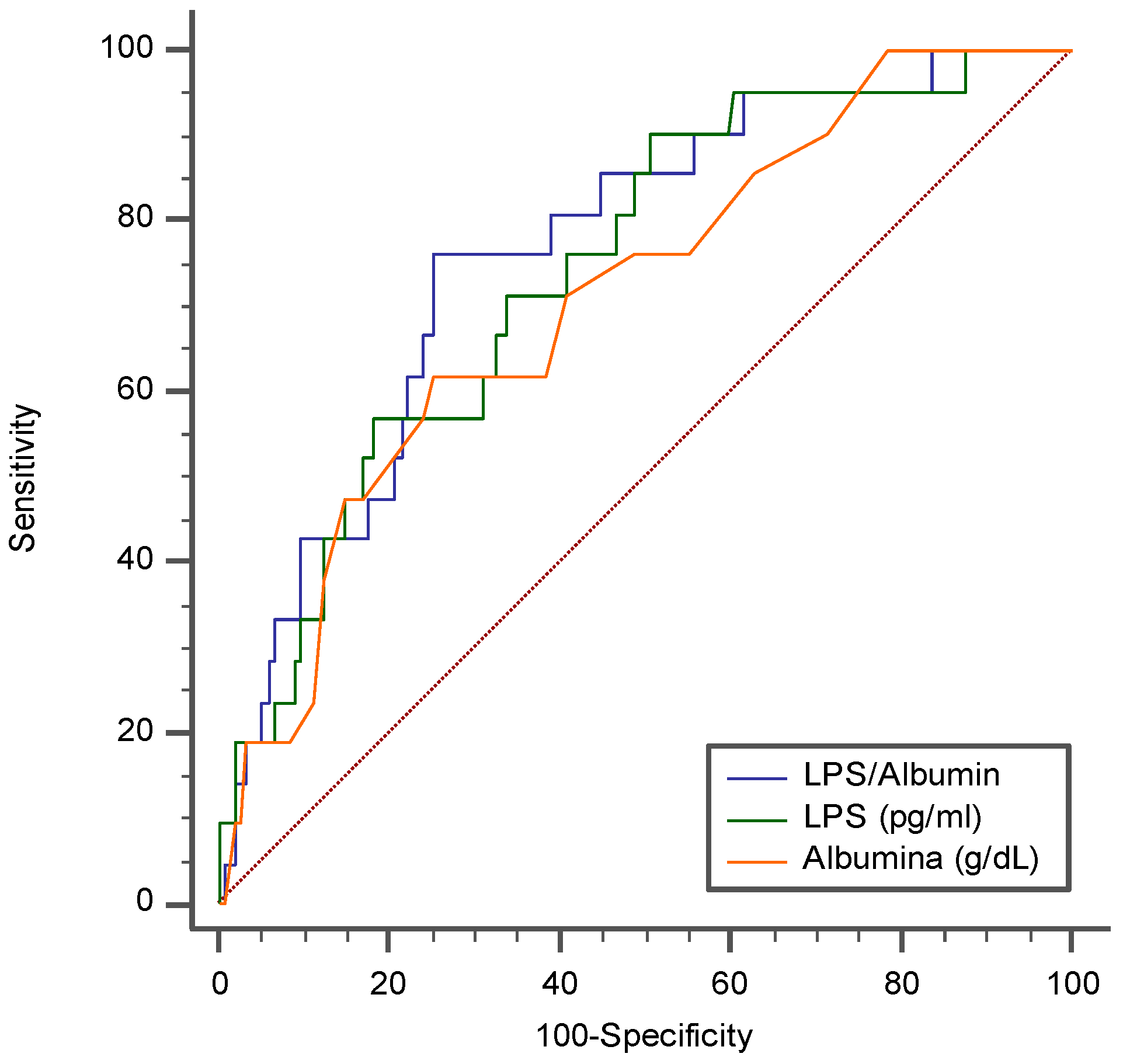
Due to the functional interplay between LPS and albumin, we evaluated the role of the LPS/albumin ratio as a predictor of thrombotic events. In a multivariable logistic regression model, both LPS/Albumin levels (OR: 1.045; 95% CI: 1.005-1.087; p=0.029 and D-dimer (OR: 2.002; 95% CI: 1.369-2.926; p<0.001) predicted thrombotic events. The ROC curve analyses showed that the LPS/albumin ratio showed a higher AUC than LPS albumin alone (LPS/Albumin AUC: 0.768; 95% CI: 0.699 to 0.829; p<0.0001; LPS AUC: 0.745; 95% CI: 0. 0.673 to 0.808; p<0.0001; Albumin AUC 0.712; 95% CI: 0. 0.638 to 0.778; p=0.0003) (
Figure 5).
In particular, the LPS/Albumin ratio > 18.5 showed a sensitivity of 76.2% and a specificity of 74.7% to detect a thrombotic event. Interestingly, overall, the correlation between the LPS/Albumin ratio and D-dimer was stronger (Rs=0.687; p<0.001), than the correlation between the correlation between LPS and D-dimer (Rs=0.675; p<0.001) and Albumin and D-dimer (-0.596; p<0.001).
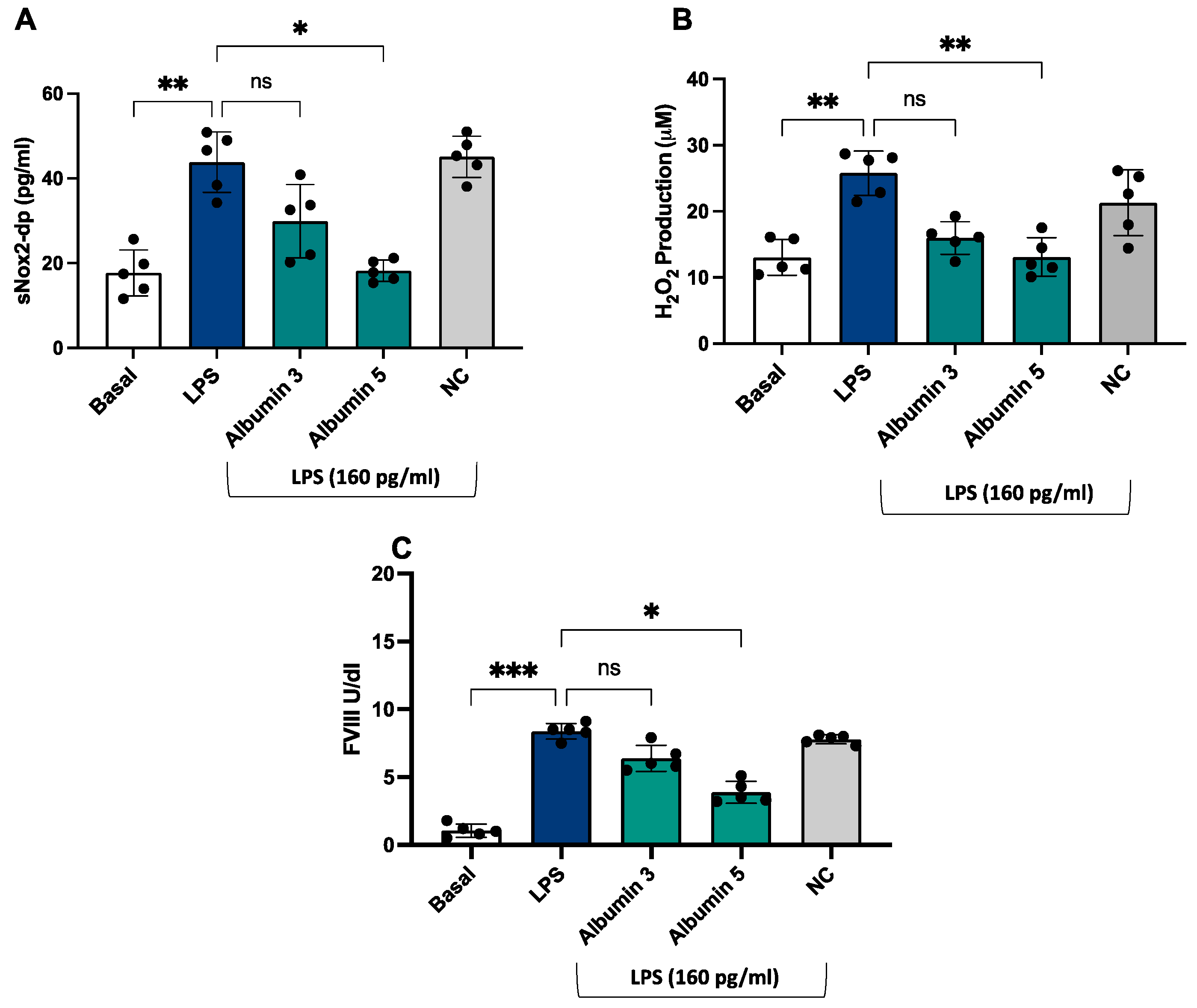
In vitro study
After stimulation with LPS (160 pg/mL), we observed an increase of sNOX2dp, H
2O
2 production and FVIII (
Figure 6 A-C) compared to unstimulated cells.
Albumin-treated endothelial cells before the stimulation with LPS showed a significant decrease of NOX2 activation (
Figure 6 A), H
2O
2 production (
Figure 6 B) and FVIII (
Figure 6 C) compared to LPS-stimulated cells; this effect was only evident at concentrations of 5 g/dL.
4. Discussion
The main findings of the present study show that 1) LPS and oxidative stress are significantly associated with a hypercoagulation state; 2) LPS and NOX2 are inversely associated with serum albumin; 3) LPS and NOX2 are significantly associated with disease severity and 4) LPS/albumin ration significantly predicts thrombotic events.
LPS is a glycolipid component of the outer membrane of Gram-negative gut bacteria and is composed of carbohydrates and a portion of lipid A. LPS may increase into systemic circulation depending upon the diet typology; after a high-fat-rich diet, its elevation is associated with enhanced intestinal biosynthesis of apolipoprotein B48, that serves to transport chylomicrons in the peripheral circulation [
28,
29]. Gut dysbiosis and/or impaired gut barrier dysfunction are key factors in determining LPS translocation into systemic circulation with ensuing low-grade endotoxemia and systemic inflammation [
28]. In addition to metabolic diseases, systemic infections may contribute to gut dysbiosis and low-grade endotoxemia as shown in patients with community-acquired pneumonia and more recently by COVID-19 [
28]
. Thus, the present study supports and extends previous study from our group showing that endotoxemia is detectable in the early phase of COVID-19 disease with a significant association with D-dimer, suggesting that LPS contributes to the hypercoagulation state of COVID-19 patients. As above outlined, the increase of LPS in patients with pneumonia may depend on several mechanisms including gut dysbiosis and/or impaired gut barrier dysfunction secondary to systemic infection or inflammation. Our previous data, which are confirmed by the present study, suggest a role for gut barrier dysfunction as a mechanism accounting for LPS translocation into systemic circulation as indicated by the significant increase of zonulin, an indirect marker of gut barrier dysfunction [
28]
, and its correlation with LPS. However, impaired gut permeability is not the only mechanism accounting for endotoxemia. Thus, the reduction of albumin may also play a role as albumin serves to blunt LPS hindering its toxic effects. Among the functions of albumin, its activity as an acute reactant protein largely explains its serum reduction during the acute phase of the disease [
30]. This change may have deleterious clinical effects in several ways, “in primis” by favoring LPS toxicity contributing to the systemic inflammation via LPS-induced overproduction of cytokines such as interleukin 6 or TNF-alpha [
31]
.
Previous reports showed that LPS may elicit a hypercoagulation state acting at the level of several cell lines such as platelets, leucocytes, and endothelial cells, that upon activation are involved in thrombin generation via NOX2-related platelet activation, macrophage Tissue Factor overexpression and factor VIII and von Willebrand secretion by endothelial cells [
28,
32]. Therefore, oxidative stress leads to the dysregulation of coagulation and fibrinolysis processes increasing the risk of thrombus formation [
33]. According to our previous studies, we show significant differences between controls and COVID-19 patients in NOX2 levels, that were higher according to COVID-19 severity. Moreover, we found a significant association between sNOX2-dp and D-dimer suggesting a role of NOX2 in eliciting the hypercoagulation state in these patients.
The clinical consequence of this phenomenon is worsening of systemic inflammation with a negative impact on infection progression; in accordance with this hypothesis is the significant association between LPS and COVID-19 severity, i.e., higher was LPS and more frequent was the occurrence of ARDS. The reduction of serum albumin is also important in the context of hypercoagulation of COVID-19. Thus, albumin possesses anticoagulant and antiplatelet effects by inhibiting the liver biosynthesis of coagulation factors or reducing the platelet biosynthesis of eicosanoids [
34,
35]; experimental studies in humans demonstrated that albumin supplementation exerts antiplatelet and anticoagulant effects [
36,
37]. Thus, the concomitant reduction of albumin along with the increase of LPS is a negative combination of factors that strongly contribute to enhanced thrombin generation and hypercoagulation and eventually higher thrombotic risk. To investigate this issue, we analyzed the impact of both variables on the thrombotic risk of our patients and found that the LPS/albumin ratio was more predictive of thrombotic risk than the single variable the combined changes of LPS and albumin could seriously influence thrombotic outcomes in COVID-19
.
Finally, we conducted an in vitro study to confirm the role of LPS/NOX2 axis in hypercoagulation and the effect of albumin. LPS increases endothelial oxidative stress and coagulation biomarkers, that were reduced in the presence of albumin 5 g/dl.
The study has implications and limitations. We did not provide experimental evidence that the combination of high LPS and low albumin enhances the activation of intrinsic and/or extrinsic coagulation pathways more than a single variable, thereby further study is necessary to explore this issue. Reduction of LPS or increase of albumin could contribute to lower systemic inflammation and hypercoagulation state, thereby further study should be addressed to modulate these two variables in COVID-19. A preliminary study with albumin supplementation showed a significant reduction of D-dimer but the sample size as well as study methodology did not allow definite conclusion [
37]. Even if the increase of zonulin suggests a dysfunctionality of the gut barrier further studies are necessary to investigate if factors intrinsic and/or extrinsic to gut microbiota are involved in low-grade endotoxemia in COVID-19.
5. Conclusions
Patients with COVID-19 show a simultaneous increase of LPS and NOX2-activity associated with a reduction of albumin that may contribute to a hypercoagulation state and eventually increase the thrombotic risk. Interventional studies to lower LPS, NOX2 activity or increase albumin are warranted to assess if this approach may reduce the thrombotic risk.
Author Contributions
R.C.: Conceptualization, writing – original draft, visualization, formal analysis, data curation; C.N.: writing – original draft, formal analysis; R.M., P.Z., A.C., Ri.C., A.O.: Patients’ recruitment; V.P., S.P.: methodology, investigation; R.Ca.: formal analysis; M.C.M., M.D., F.C., G.C.: investigation, formal analysis; L.C.: writing – original draft; P.P., G.F., M.V., F.P., C.M., D.A.: project administration, writing - original draft; F.V.: supervision, writing – original draft; L.R., C.D., E.V.: Investigation; M.L.: Data Curation, Supervision; V.C. Conceptualization, Supervision, Writing – original draft.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Azienda Ospedaliera Universitaria Policlinico Umberto I (ID Prot. 6192).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data analyzed in this article will be shared upon reasonable request from the corresponding author.
Acknowledgments
The authors highly acknowledge the contribution of the entire staff at S.M Goretti Hospital, Latina and COVID-19 Latina Study Group: Valeria Belvisi, Tiziana Tieghi, Margherita De Masi, Paola Zuccalà, Paolo Fabietti, Angelo Vetica, Vito Sante Mercurio, Anna Carraro, Laura Fondaco, Blerta Kertusha, Alberico Parente, Giulia Mancarella, Silvia Garattini, Andrea Gasperin, Davide Caianiello, Marco Perla, Jessica Luchetti, Giulia Passariello, Ginevra Gargiulo, Gaetano Brignone, Gianluca Gabrielli, Daniela Di Trento, Emanuela Del Giudice, Riccardo Lubrano, Melania Garante, Maria Gioconda Zotti, Antonella Puorto, Marcello Ciuffreda, Antonella Sarni, Gabriella Monteforte, Rita Dal Piaz, Emanuela Viola, Carla Damiani, Antonietta Barone, Barbara Mantovani, Daniela Di Sanzo, Vincenzo Gentili, Massimo Carletti, Massimo Aiuti, Andrea Gallo, Piero Giuseppe Meliante, Salvatore Martellucci, Oliviero Riggio, Maria Consiglia Bragazzi, Stefania Gioia, Silvia Nardelli, Rosanna Venere, Emiliano Valenzi, Camilla Graziosi, Niccolò Bina, Martina Fasolo, Silvano Ricci, Maria Teresa Gioacchini, Antonella Lucci, Luisella Corso, Daniela Tornese, Francesco Equitani, Carmine Cosentino, Antonella Melucci, Iavarone Carlo, Desirè Mancini, Frida Leonetti, Gaetano Leto, Camillo Gnessi, Giuseppe Pelle, Iannarelli Angelo, Mario Iozzino, Adriano Ascarelli, Cesare Ambrogi, Iacopo Carbone, Giuseppe Campagna, Roberto Cesareo, Giuseppe Straface, Clelia Di Pippo, Valentina Isgrò, Gabriele Bagaglini, Gabriella Bonanni, Alessandra Mecozzi, Sergio Parrocchia, Giuseppe Visconti, Giorgio Casati, Laide Romagnoli, Silvia Cavalli. This work was developed with support from the COVID-19 Infectious Diseases Study Group from the Policlinico Umberto I Hospital, Sapienza, University of Rome, Italy whose members are: Ciardi Maria Rosa, Ajassa Camilla, d’Ettorre Gabriella, D’Agostino Claudia, Russo Gianluca, Trinchieri Vito, Guariglia Paola, Antonelli Laura, Cuomo Maria Rosaria, Carnevalini Martina, Andreoni Carolina, Mastropietro Cristina, Iaiani Giancarlo, Mezzaroma Ivano, Falciano Mario, Ceccarelli Giancarlo, Zingaropoli MariaAntonella, Perri Valentina, Massimiliano De Angelis, Santinelli Letizia, Pinacchio Claudia, Nijhawan Parni, Miele Maria Claudia, Innocenti Giuseppe Pietro, Mengoni Fabio, Pasculli Patrizia, Cogliati Dezza Francesco, Vassalini Paolo, De Girolamo Gabriella, Savelloni Giulia, Valeri Serena, Siccardi Guido, Alessi Federica, Recchia Gregorio, Ridolfi Marco, Cavallari Eugenio Nelson, Celani Luigi, Romani Francesco Eugenio, Aronica Raissa, Filippi Valeria, Mauro Vera, Volpicelli Lorenzo, Candy Matteo, Alban Rugova, Di Bari Silvia, Gavaruzzi Francesca, Casali Elena and Carli Serena Maria.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Violi, F.; Pignatelli, P.; Cammisotto, V.; Carnevale, R.; Nocella, C. COVID-19 and thrombosis: Clinical features, mechanism of disease, and therapeutic implications. Kardiol Pol 2021, 79, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.D.; Schell, J.C.; Rodgers, G.M. The D-dimer assay. Am J Hematol 2019, 94, 833–839. [Google Scholar] [CrossRef]
- Berger, J.S.; Kunichoff, D.; Adhikari, S.; Ahuja, T.; Amoroso, N.; Aphinyanaphongs, Y.; Cao, M.; Goldenberg, R.; Hindenburg, A.; Horowitz, J.; et al. Prevalence and Outcomes of D-Dimer Elevation in Hospitalized Patients With COVID-19. Arterioscler Thromb Vasc Biol 2020, 40, 2539–2547. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Nijhawan, P.; Carraro, A.; Pasculli, P.; Zuccala, P.; Perri, V.; Marocco, R.; Kertusha, B.; Siccardi, G.; Del Borgo, C.; et al. Increased sCD163 and sCD14 Plasmatic Levels and Depletion of Peripheral Blood Pro-Inflammatory Monocytes, Myeloid and Plasmacytoid Dendritic Cells in Patients With Severe COVID-19 Pneumonia. Front Immunol 2021, 12, 627548. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Della Valle, P.; Calvieri, C.; Taliani, G.; Ferroni, P.; Falcone, M.; Carnevale, R.; Bartimoccia, S.; D’Angelo, A.; Violi, F.; et al. Low-grade endotoxemia and clotting activation in the early phase of pneumonia. Respirology 2016, 21, 1465–1471. [Google Scholar] [CrossRef]
- Cangemi, R.; Casciaro, M.; Rossi, E.; Calvieri, C.; Bucci, T.; Calabrese, C.M.; Taliani, G.; Falcone, M.; Palange, P.; Bertazzoni, G.; et al. Platelet activation is associated with myocardial infarction in patients with pneumonia. J Am Coll Cardiol 2014, 64, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Calvieri, C.; Bucci, T.; Carnevale, R.; Casciaro, M.; Rossi, E.; Calabrese, C.M.; Taliani, G.; Grieco, S.; Falcone, M.; et al. Is NOX2 upregulation implicated in myocardial injury in patients with pneumonia? Antioxid Redox Signal 2014, 20, 2949–2954. [Google Scholar] [CrossRef]
- Oliva, A.; Cammisotto, V.; Cangemi, R.; Ferro, D.; Miele, M.C.; De Angelis, M.; Cancelli, F.; Pignatelli, P.; Venditti, M.; Pugliese, F.; et al. Low-Grade Endotoxemia and Thrombosis in COVID-19. Clin Transl Gastroenterol 2021, 12, e00348. [Google Scholar] [CrossRef]
- Carnevale, R.; Sciarretta, S.; Valenti, V.; di Nonno, F.; Calvieri, C.; Nocella, C.; Frati, G.; Forte, M.; d’Amati, G.; Pignataro, M.G.; et al. Low-grade endotoxaemia enhances artery thrombus growth via Toll-like receptor 4: implication for myocardial infarction. Eur Heart J 2020, 41, 3156–3165. [Google Scholar] [CrossRef]
- Giron, L.B.; Dweep, H.; Yin, X.; Wang, H.; Damra, M.; Goldman, A.R.; Gorman, N.; Palmer, C.S.; Tang, H.Y.; Shaikh, M.W.; et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front Immunol 2021, 12, 686240. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Gilboa, T.; Ogata, A.F.; Senussi, Y.; Lazarovits, R.; Boribong, B.P.; Bartsch, Y.C.; Loiselle, M.; Rivas, M.N.; Porritt, R.A.; et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pignatelli, P.; Castellani, V.; Carnevale, R.; Cammisotto, V. Gut dysbiosis, endotoxemia and clotting activation: A dangerous trio for portal vein thrombosis in cirrhosis. Blood Rev 2023, 57, 100998. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Ceccarelli, G.; Cangemi, R.; Alessandri, F.; D’Ettorre, G.; Oliva, A.; Pastori, D.; Loffredo, L.; Pignatelli, P.; Ruberto, F.; et al. Hypoalbuminemia, Coagulopathy, and Vascular Disease in COVID-19. Circ Res 2020, 127, 400–401. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum Albumin: A Multifaced Enzyme. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Chen, T.A.; Tsao, Y.C.; Chen, A.; Lo, G.H.; Lin, C.K.; Yu, H.C.; Cheng, L.C.; Hsu, P.I.; Tsai, W.L. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol 2009, 44, 619–625. [Google Scholar] [CrossRef]
- Violi, F.; Cangemi, R.; Romiti, G.F.; Ceccarelli, G.; Oliva, A.; Alessandri, F.; Pirro, M.; Pignatelli, P.; Lichtner, M.; Carraro, A.; et al. Is Albumin Predictor of Mortality in COVID-19? Antioxid Redox Signal 2021, 35, 139–142. [Google Scholar] [CrossRef]
- Bartimoccia, S.; Cammisotto, V.; Nocella, C.; Del Ben, M.; D’Amico, A.; Castellani, V.; Baratta, F.; Pignatelli, P.; Loffredo, L.; Violi, F.; et al. Extra Virgin Olive Oil Reduces Gut Permeability and Metabolic Endotoxemia in Diabetic Patients. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Matuschak, G.M.; Lechner, A.J. Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. Mo Med 2010, 107, 252–258. [Google Scholar]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019, 54. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Gutierrez, J.A.; Creager, M.A.; Scirica, B.M.; Olin, J.; Murphy, S.A.; Braunwald, E.; Morrow, D.A. Acute Limb Ischemia and Outcomes With Vorapaxar in Patients With Peripheral Artery Disease: Results From the Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-Thrombolysis in Myocardial Infarction 50 (TRA2 degrees P-TIMI 50). Circulation 2016, 133, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- De Falco, E.; Carnevale, R.; Pagano, F.; Chimenti, I.; Fianchini, L.; Bordin, A.; Siciliano, C.; Monticolo, R.; Equitani, F.; Carrizzo, A.; et al. Role of NOX2 in mediating doxorubicin-induced senescence in human endothelial progenitor cells. Mech Ageing Dev 2016, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Catasca, E.; Pignatelli, P.; Brancorsini, M.; Nocella, C.; De Falco, E.; Bartimoccia, S.; Frati, G.; Carnevale, R.; et al. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J Am Heart Assoc 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Menna, C.; De Falco, E.; Pacini, L.; Scafetta, G.; Ruggieri, P.; Puca, R.; Petrozza, V.; Ciccone, A.M.; Rendina, E.A.; Calogero, A.; et al. Axitinib affects cell viability and migration of a primary foetal lung adenocarcinoma culture. Cancer Invest 2014, 32, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Osband, A.J.; Deitch, E.A.; Hauser, C.J.; Lu, Q.; Zaets, S.; Berezina, T.; Machiedo, G.W.; Rajwani, K.K.; Xu, D.Z. Albumin protects against gut-induced lung injury in vitro and in vivo. Ann Surg 2004, 240, 331–339. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat Rev Cardiol 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Carnevale, R.; Pastori, D.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Novo, M.; Del Ben, M.; Farcomeni, A.; Angelico, F.; Violi, F. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: effect of extra-virgin olive oil. Eur J Nutr 2019, 58, 843–851. [Google Scholar] [CrossRef]
- Pignatelli, P.; Farcomeni, A.; Menichelli, D.; Pastori, D.; Violi, F. Serum albumin and risk of cardiovascular events in primary and secondary prevention: a systematic review of observational studies and Bayesian meta-regression analysis. Intern Emerg Med 2020, 15, 135–143. [Google Scholar] [CrossRef]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med 1997, 185, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Raparelli, V.; Nocella, C.; Bartimoccia, S.; Novo, M.; Severino, A.; De Falco, E.; Cammisotto, V.; Pasquale, C.; Crescioli, C.; et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J Hepatol 2017, 67, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, X.; Huang, G. Understanding thrombosis: the critical role of oxidative stress. Hematology 2024, 29, 2301633. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, K.A.; Stoffersen, E. On the inhibitory effect of albumin on platelet aggregation. Thromb Res 1980, 17, 13–18. [Google Scholar] [CrossRef]
- Maclouf, J.; Kindahl, H.; Granstrom, E.; Samuelsson, B. Interactions of prostaglandin H2 and thromboxane A2 with human serum albumin. Eur J Biochem 1980, 109, 561–566. [Google Scholar] [CrossRef]
- Basili, S.; Carnevale, R.; Nocella, C.; Bartimoccia, S.; Raparelli, V.; Talerico, G.; Stefanini, L.; Romiti, G.F.; Perticone, F.; Corazza, G.R.; et al. Serum Albumin Is Inversely Associated With Portal Vein Thrombosis in Cirrhosis. Hepatol Commun 2019, 3, 504–512. [Google Scholar] [CrossRef]
- Violi, F.; Ceccarelli, G.; Loffredo, L.; Alessandri, F.; Cipollone, F.; D’Ardes, D.; D’Ettorre, G.; Pignatelli, P.; Venditti, M.; Mastroianni, C.M.; et al. Albumin Supplementation Dampens Hypercoagulability in COVID-19: A Preliminary Report. Thromb Haemost 2021, 121, 102–105. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).