1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are formed as a result of incomplete combustion of organic materials such as coal, oil, gas, wood, tobacco, charbroiled meats, garbage, or other organic materials [
1,
2,
3]. They are released from natural and mainly anthropogenic sources [
4].
PAHs can mean health concerns due to their probable carcinogenic, mutagenic and teratogenic effects in humans [
5,
6]. The degree of toxicity, carcinogenicity and mutagenicity depends on the type of PAH. The heavy PAHs (containing 4-6 rings) are more toxic and stable compared to light ones (2-3 ring) [
7] (
Table 1). The U.S. Environmental Protection Agency (US EPA 1993, Risk and International Cancer Agency for Research of Cancer [
6,
8] classified PAHs according to their carcinogenicity shown in
Table 1. In addition, some PAHs classified as no-carcinogenic compounds have shown effects on the immune system and endocrine regulation [
9].
Human exposure of PAHs may occur via inhalation, dermal contact, or by the ingestion of food contaminated with these compounds [
11,
12]. Benzo(a)pyrene is generally considered the most toxic and carcinogenic PAH. The Contam panel [
8] considers PAH4 (sum of benzo(
a))anthracene (BaA), chrysene (CHr), benzo(
b)fluoranthene (BbF), and Benzo(
a)pyrene (BaP)) best suitable indicator for occurrence of PAHs in food. The European Commission recognizes the maximum permitted levels (EU limits) of PAHs in oils and fats as 2 μg/kg for BaP and 10 μg/kg for PAH4 [
13]. The human body metabolizes and eliminates PAHs [
14,
15] however, the continuous exposure to PAHs in the diet and atmospheric contamination may provide a pseudo persistence [
16,
17].
These compounds can accumulate in lipophilic regions such as adipose tissue, liver and kidney. PAHs may be excreted in breast milk because of their apolar character [
18]. Breast milk is further susceptible for accumulating PAHs as it contains lipophilic tissue [
19]. Contamination depends on environmental factors, source of exposition, stage of lactation, medical state, rearing system, and lifestyle [
20,
21].
There is evidence that chronic exposure in motherhood and in early childhood can have long-term consequences including birth defects, premature birth, low birth weight,malformations [
22], reduced growth and development [
23], asthma [
24], and lower intelligence quotient (IQ) [
22].
PAHs-contaminated breastmilk has been reported in numerous studies all over the world including Europe, Asia, America, and Africa: Europe: Italy [
14,
20,
25] Portugal [
26] Czech republic [
27], Asia: Turkey [
28], China [
29], Hong Kong [
30] India [
31], America: [
16,
32,
33] and Africa: Egypt [
19], Ghana [
34], Uganda [
35].
The aim of this study is to assess the levels of PAHs in the breast milk of healthy Hungarian women and to perform a carcinogenic risk assessment to determine whether levels of PAHs in breast milk pose any risk to breastfed infants. Our aim is to further reveal factors which can have effect on the PAH concentrations in breast milk. To the best of our knowledge, this is the first study about monitoring the concentration of PAHs in breast milk of Hungarian mothers.
2. Results
2.1. PAH Profile of Breast Milk Samples and Exposure Assessment
The concentration of 12 PAHs were determined in the breast milk of 50 Hungarian mothers. The sum of all individually measured components and 4 PAHs were also calculated in each sample and indicated as 4 PAHs and total PAHs, respectively.
Out of the 50 samples analyzed, 48 showed the presence of at least 2 PAHs, however in the other two samples no PAHs were detected. Phenanthrene was appeared most frequently (found in 92% of the samples). Dibenzo (a.h) anthracene was not detected in any of the analyzed samples. Fluorene was the most abundant PAH (5.3 ng/mL) followed by phenanthrene (3.2 ng/mL) and pyrene (2.5 ng/mL). 10 components were found in the sample, which contained the most different kind of PAHs. The concentration of 4 PAHs ranging from ND to 13.6 ng/ml with mean concentration of 4.4 ng/mL.
The most frequently detected PAH (Phenantrene) and the most abundant PAH (fluorene) was also a low molecular PAH compound, which may be transferred in human breast milk most rapidly and in greater amounts, because the mammary transport is inversely related to molecular weight [
38].
The total PAHs in human breast milk ranged from 0 to 77.95 ng/mL with a mean level 17.87 ng/mL. Mean concentrations of individual PAHs in the breast milk samples of Hungarian women fell within the range of 0-6 ng/mL.
The analysis of PAH levels in the present study reveals that the detected concentrations are within a comparable range to those reported in previous research conducted in European countries, specifically Italy and the Czech Republic. This finding is consistent with previous publications [
20,
27] indicating a similar exposure pattern across these regions. The comparable PAH levels suggest that common sources and exposure pathways may exist, potentially related to similar environmental pollution, dietary habits, or lifestyle factors prevalent in these areas.
There is a significant trend observed consisting of higher mean levels of low molecular weight (LMW) PAHs compared to heavy PAHs. This trend aligns with findings of previous studies, where LMW PAHs-such as naphthalene and phenanthrene- were more prevalent in breast milk than heavier compounds like benzo(a)pyrene. The higher concentration of LMW PAHs may be attributed to their greater volatility and higher abundance in the environment, which facilitates their absorption and transfer into breast milk. Additionally, these compounds are often found in higher concentrations in urban areas due to traffic emissions and residential heating, which are common sources of LMW PAHs.
The consistency of these results across different geographic locations underscores the importance of monitoring PAH levels in human breast milk as a marker of environmental exposure. It also highlights the need for public health interventions aimed at reducing exposure to these hazardous compounds, particularly for breastfeeding infants who are among the most vulnerable populations. Future research should focus on identifying specific sources of PAHs in these regions, as well as examining the potential long-term health effects on infants exposed to these contaminants through breastfeeding. Such efforts are crucial for developing effective strategies to minimize exposure and protect public health.
2.2. Risk Assessment
ILCR values were calculated to estimate carcinogenic risk for infants. Out of the 50 samples, 5 had ILCR values less than 10-6, which is considered safe. Only one of the samples had an ILCR value higher than 10-4, indicating a potential carcinogenic risk. It is understandable that this milk sample showed a high ILCR value because it came from one of three mothers who smoke tobacco.
This sample was additionally characterized by the following factors: frequent consumption of meat, lack of organic products in the diet, no higher education qualifications, and living in an urban setting. All these factors may collectively indicate a higher risk level.
The remaining 44 samples had ILCR values within the range of 10-6 and 10-4, which is generally considered an acceptable for breast feeding infants.
3.3. Questionnaires
Several factors that can influence the levels of PAHs in breast milk. These factors include the geographical location, residence, diet, smoking habits, and lifestyle of the individuals.
Various parameters provided by mothers in the questionnaires were analysed. We hypothesized that polycyclic aromatic hydrocarbon (PAH) compounds would occur in higher quantities in women with a higher BMI due to their lipophilic properties. However, we failed to confirm this outcome. Maternal BMI before and after pregnancy had no correlation with the total PAH concentration (pbefore=0.262, pafter=0.054).
Environmental pollution may vary in different geographical areas, contributing to higher concentrations of PAHs in the air, water, soil, and food sources. Fortunately, only three mothers smoked during breastfeeding, which made statistical comparisons and conclusions unfeasible. However, the average PAH concentration was still higher in smokers compared to non-smokers, based on the data from these three mothers (smoker: 18.00±17.84 and non-smoker: 17.10±11.65).
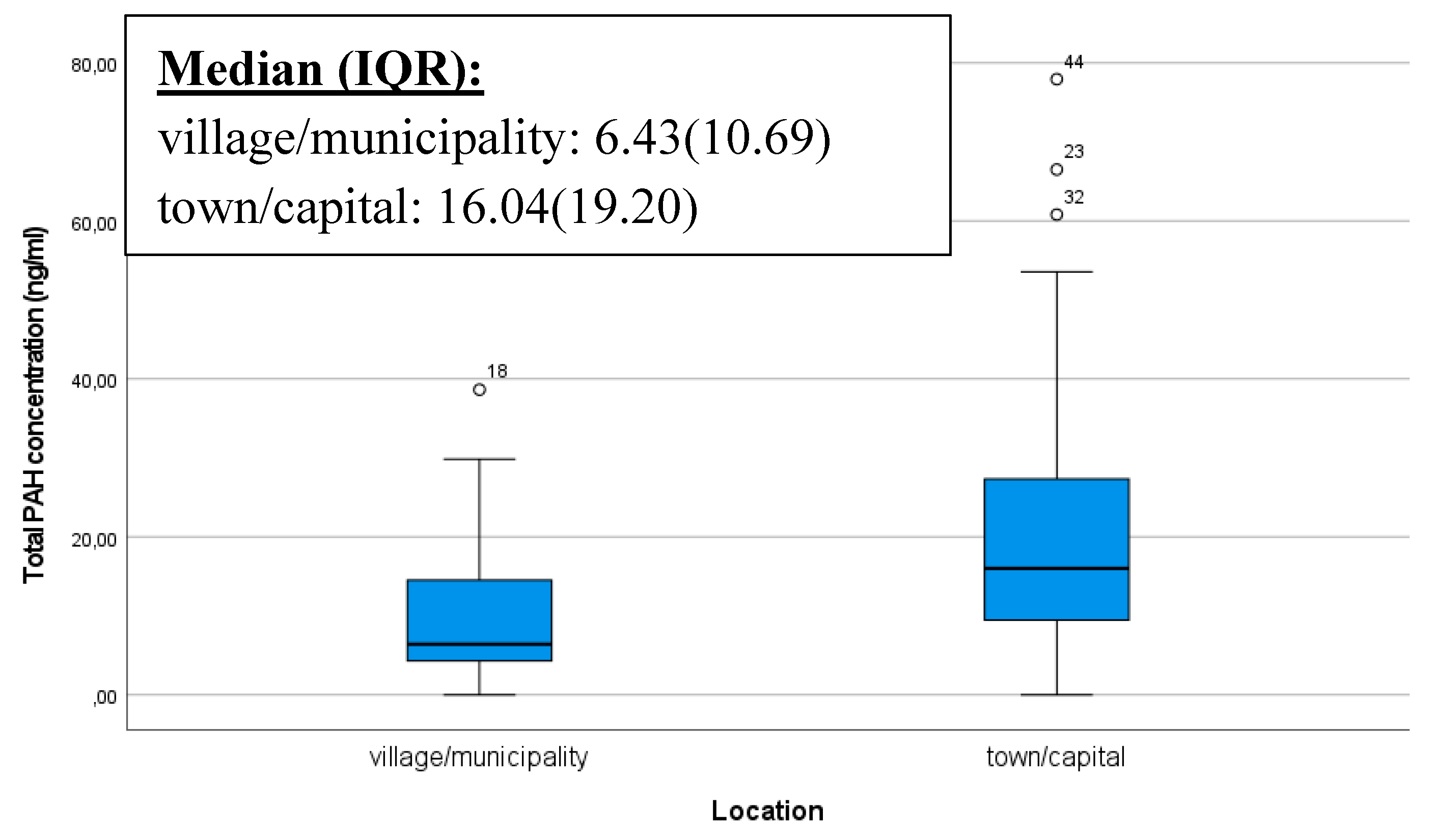
Industrial activities, vehicle emissions, and the proximity of contaminated areas can also increase the presence of PAHs in the living environment. Individuals living in areas with higher pollution levels (such as in the capital or major cities, near busy roads or factories) may face an increased risk of exposure to PAHs, which can later affect the PAH levels in breast milk. Thus, in our analysis, we examined whether there was a significant difference in the total PAHs quantity in the breast milk of mothers living in two different areas categorized as village/municipality and town/capital. Our results confirmed that samples from mothers, living in urban environments, contained significantly more PAHs. The box plot illustrates that outstandingly high levels of PAHs are also present in mothers living in urban environments (p<0,001) (
Figure 1).
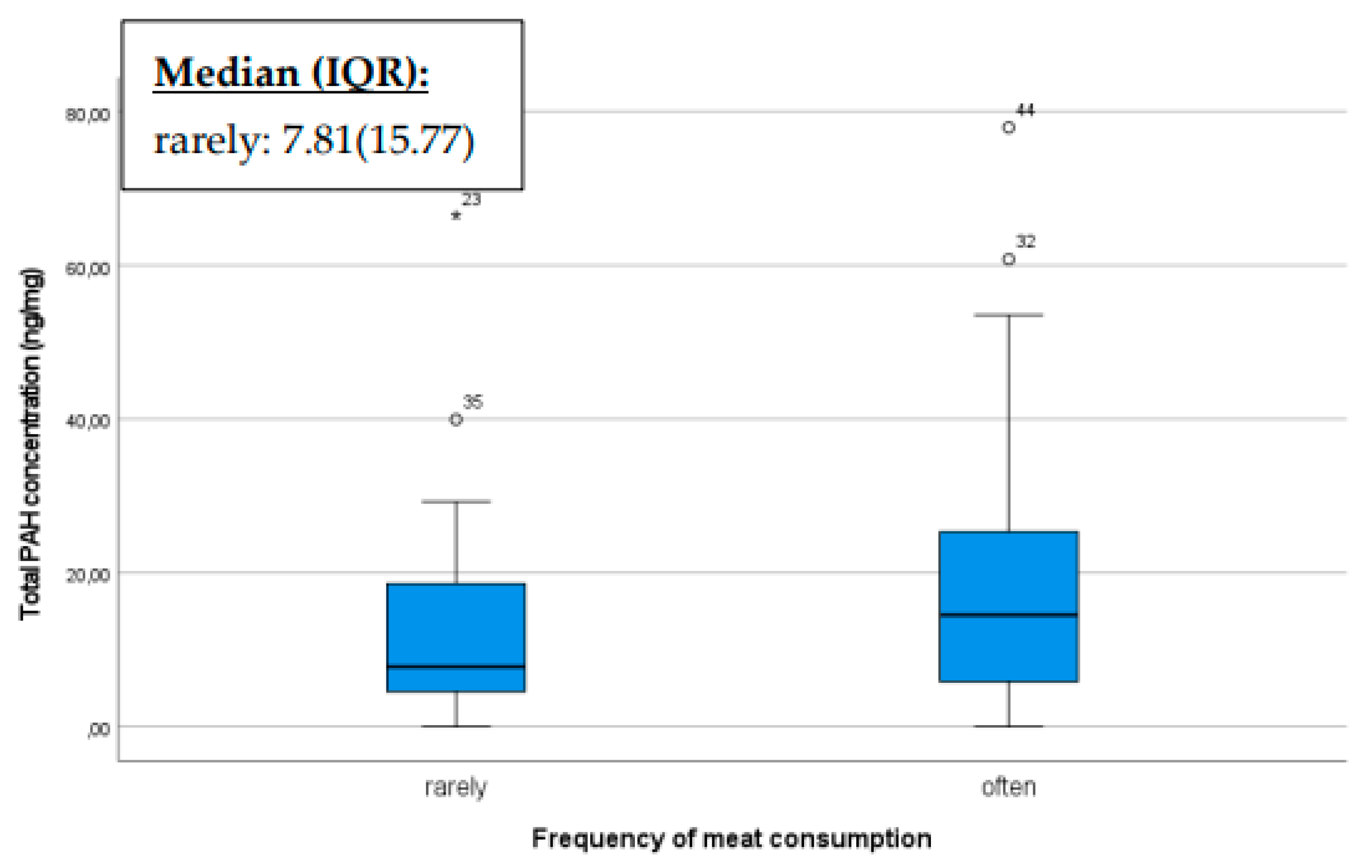
Our results also confirmed that diet can influence the levels of polycyclic aromatic hydrocarbons (PAHs) in breast milk, including meat, milk, and organic products that potentially affecting the overall PAH concentration. If someone consumes meat or milk contaminated with PAHs, these compounds can enter the bloodstream and subsequently be excreted into various body fluids, including breast milk [
35]. Consequently, the PAH concentration in breast milk may increase with higher meat or milk consumption, especially if the meat is cooked at high temperatures or exposed to significant levels of smoke or charring. Based on our sample, mothers who consumed meat more frequently had higher PAH concentrations in their breast milk compared to those who consumed it less (p=0.044) (
Figure 2).
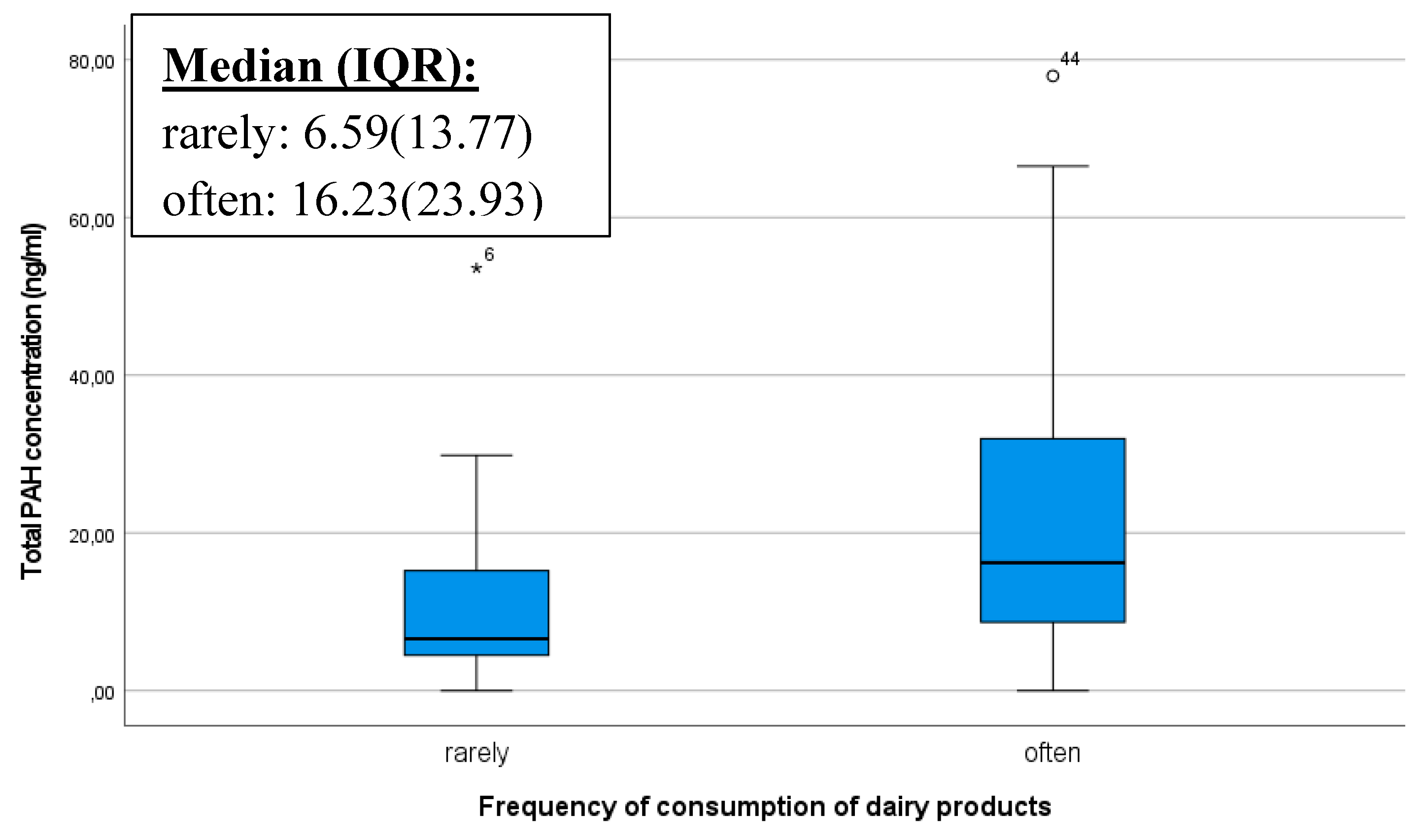
The frequency of maternal consumption of dairy products and its influence on the PAH content of breast milk was investigated with curiosity. We obtained the result that individuals who frequently consume dairy products had a significantly higher PAH concentration (p=0.027) (
Figure 3). PAHs are typically lipophilic (fat-soluble) compounds, and as such, they can easily accumulate in fatty tissues and animal fats. If foods such as cow milk, contain PAHs, they can be stored in the body when dissolved in fats [
39].
When mothers consume dairy products, the fat content of milk provides an opportunity for the accumulation of PAHs in the body, which can then be detectable in breast milk. This is particularly noteworthy when mothers frequently consume dairy products, as the processing methods of these products, such as pasteurization or heat treatment, may potentially contribute to the formation of PAHs [
31].
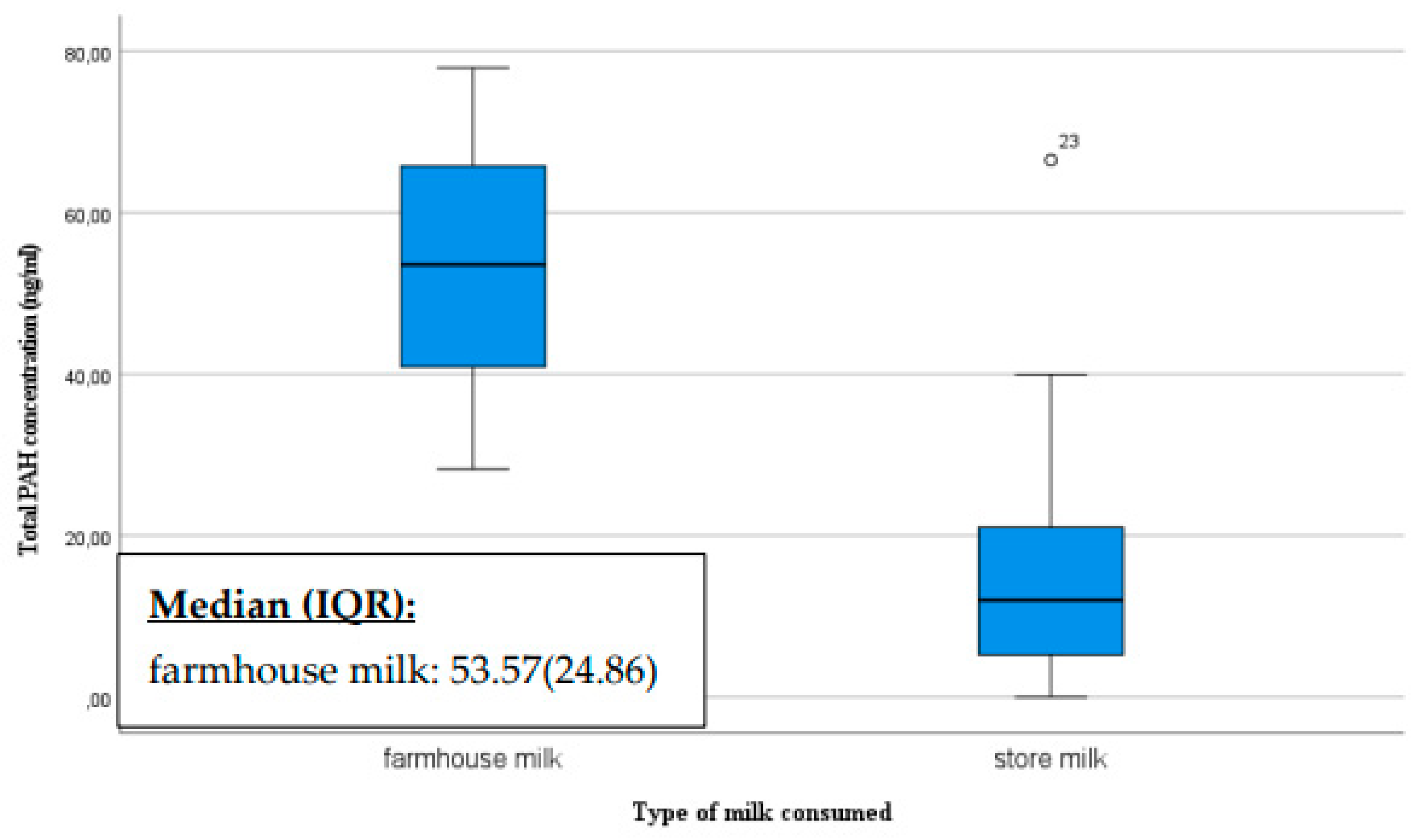
We also asked mothers about their milk consumption habits, specifically whether they consume store-bought milk or home-produced milk. The ratio of mothers consuming home-produced milk versus store-bought milk varied significantly, with a total of ten consuming home-produced milk and 33 consuming store-bought milk. The breast milk of mothers who consume home-produced milk had a significantly higher PAH concentration than those consuming store-bought milk (p=0.007) (
Figure 4).
The presence of PAHs in dairy products may therefore depend on the processing and preparation methods of the milk. The farmhouse milk samples may have contained PAHs levels that were higher than that detected in pasteurized milk as raw milk contained more triglycerides which may explain the higher level of PAHs [
40].
Processing methods, the source of the milk, and storage conditions can vary for home-produced milk, all of which may influence the quantity of PAHs. Factors such as the diet and living conditions of the animals may also play a role. Store-bought dairy products are also processed and produced in various ways, and their PAH levels are regularly monitored to comply with standards.
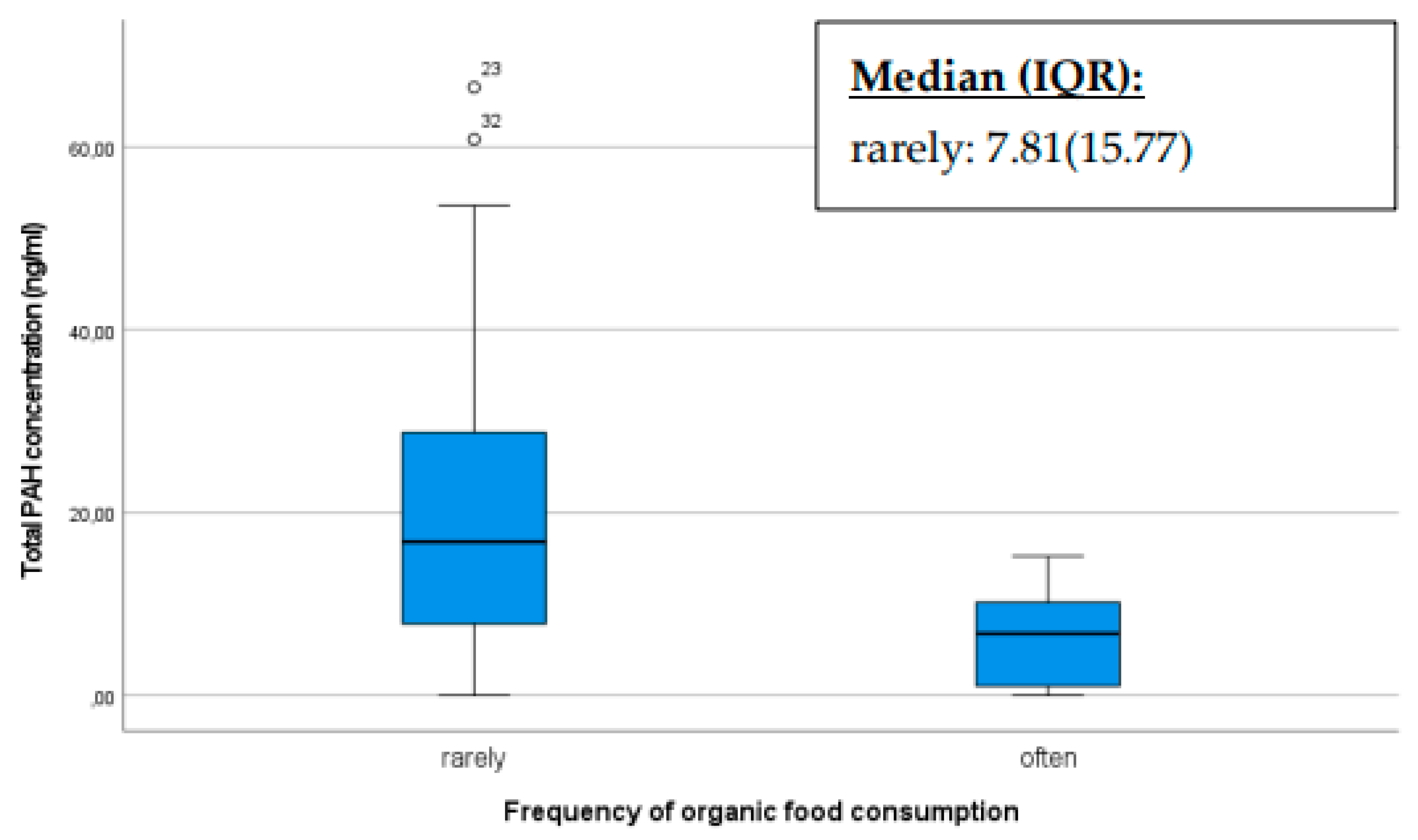
Organic farming methods prioritize soil and air purity, thereby minimizing the input of chemical substances such as synthetic pesticides that may contain polycyclic aromatic hydrocarbons (PAHs) or contribute to their formation. Individuals can reduce their exposure to PAHs originating from pesticide residues by consuming organic products. The production of organic food may involve different processing methods compared to traditional foods. Producers of organic food may employ alternative processing techniques that could result in potentially lower PAH levels. In our study, we assessed how the total PAH concentration in the breast milk of mothers would differ between mothers who frequently consumed organic products compared to those that do not [
41].
Our results indicate that mothers who preferentially consumed organic products had a significantly lower PAH concentration as shown in the box plot below (p<0.001). Mothers who infrequently consumed organic products exhibited more pronounced outliers with higher PAH levels (
Figure 5).
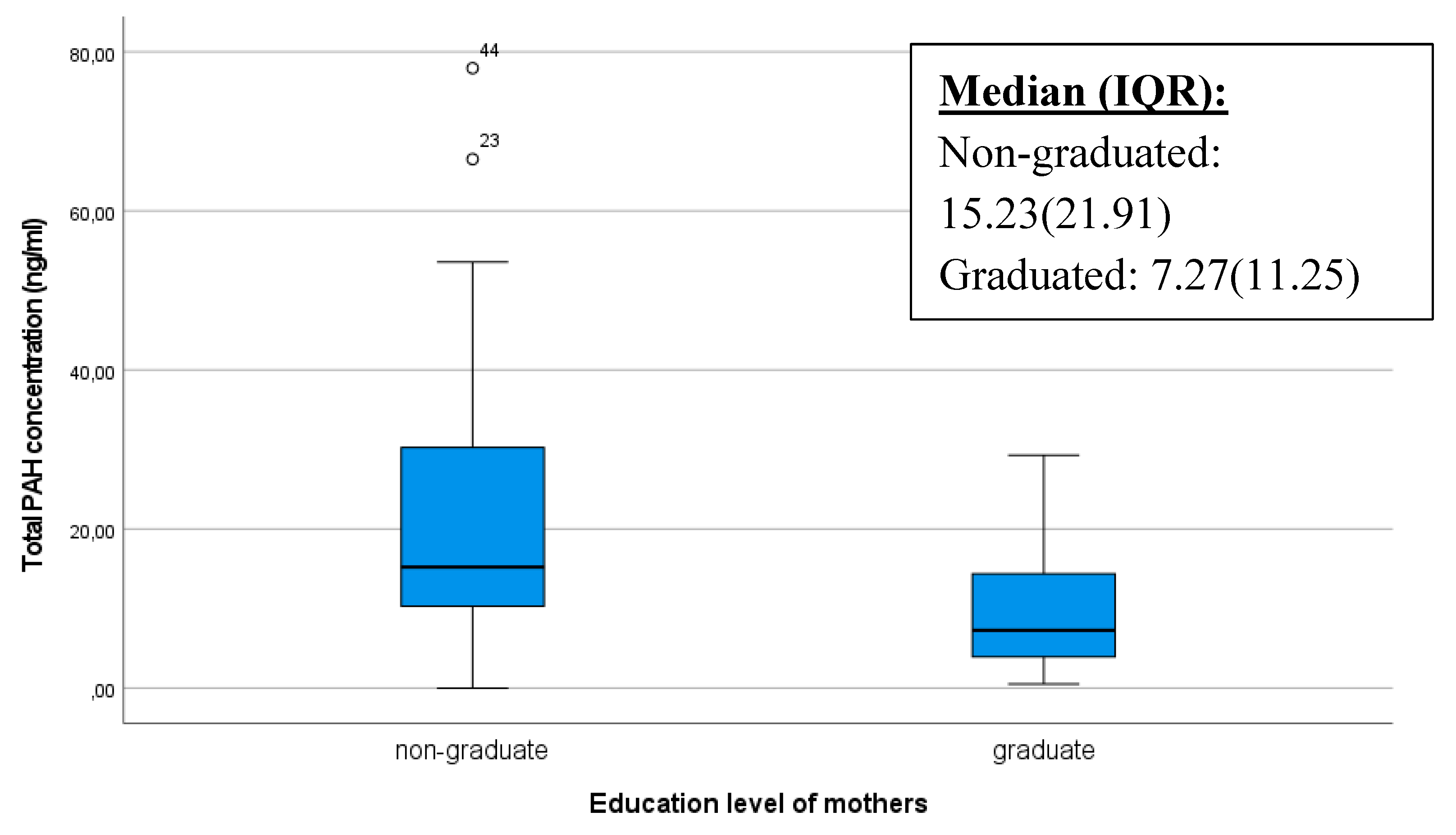
It is conceivable that educated or better informed mothers possess more knowledge about potential risks affecting infants, including exposure to pollutants such as polycyclic aromatic hydrocarbons (PAHs). Consequently, they are more likely to adopt healthier lifestyles and make decisions that minimize exposure to harmful substances. The box plot below also illustrates that extremely high PAH values occurred among mothers without a diploma, and their median value was significantly higher (p=0.005) (
Figure 5.).
4. Materials and Methods
4.1. Human Subjects
A total of 50 volunteering lactating mothers, randomly selected from Hungarian population, were included in our study. The recruitment of potential donors was done with the help of the Hungarian Nursing Association (Magyar Védőnői Szolgálat). All volunteers were above the age of 18. Participating mothers signed an informed consent form and filled a questionnaire to provide information on their education, residence, dietary habits, Body Mass Index (BMI), smoking, and other relevant information which can influence the levels of PAHs in their breast milk. More details about the donors are summarized in
Table 2. Permission to collect breastmilk samples in the Hungarian University of Agriculture and Life Sciences (MATE) (formerly Szent István University) was granted by the Ministry of Human Resources (decision number: 14069-6/2018/EÜIG) and the University of Pécs (Regional Research Ethics Committee, approval number: 5579) and was performed in line with the principles of the Declaration of Helsinki.
Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
The sampling of breast milk took place between 2014 and 2019. The breast milk samples were collected manually by the mothers. Approximately 10-20 ml from each participating mother were expressed and placed into clean 50 mL FALCON tubes, were frozen immediately and stored at -20 °C until the analysis. PAHs measurement were performed at the Institute of Bioanalysis at the University of Pécs.
4.2. Chemicals and Reagents
The EPA 525 PAH standard mix (500 μg/mL in dichloromethane) contained 13 PAHs acenaphthylene (Ace), anthracene (Ant), benzo(a)anthracene (BaA), benzo(a)pyrene (BaP), benzo(b)fluoranthene (BbF), benzo(g,h,i)perylene (BP), benzo(k)fluoranthene (BkF), chrysene (Chry), dibenzo(a,h)anthracene (DB), fluorene (Flu), indeno(1,2,3-c,d)pyrene (IndP) phenanthrene (Phen), and pyrene (Pyr) was purchased from Sigma Adrich, (St. Louis, MO, USA).
4.3. Sample Preparation and Chemical Analysis
The sample preparation included liquid-liquid extraction of PAHs from breast milk, followed by silica gel purification and concentration steps. Just prior to the analysis, the breast milk samples were thawed in the dark in a water bath at 37 °C for 10 minutes. An amount of 5 ml breast milk was taken to a polypropylene centrifuge tube and was mixed with 3 ml of hexane. The mixture was vortexed for 5 minutes, incubated for 30 minutes in a water bath at 37 °C and then centrifuged at 3000 rpm for 10 minutes at 4 °C. The supernatant was removed and transferred for further purification. Pasteur pipette was filled up with 2 g silica gel and was conditioned with 3 ml of 3:1 n-hexane: dichloromethane (3:1, v/v) and then washed with 3 ml of hexane, whole volume of the supernatant was put onto the silica column. The column was washed again with 3 ml of hexane and then the PAHs eluted with 3 ml of n-hexane: dichloromethane (3:1, v/v). The extract was evaporated to dryness by a gentle stream of nitrogen, then redissolved in 1 ml of acetonitrile and transferred into dark glass HPLC vials for HPLC-FLD analysis. PAHs was extracted from breast milk in duplicates. The distilled water was used as method blank and subjected to the entire analytical procedures to determine possible background interference.
All organic solvents such as hexane, cyclohexane, dichloromethane, and acetonitrile that were used for the preparation and analysis of samples were LC-MS grade (VWR International, USA).
4.4. Instrumental Analysis
HPLC-FLD separation was carried on a HPLC apparatus Shimadzu LC 20 AD model which was equipped with a binary solvent delivery system, an autosampler, a column heater, an RF-20A/RF-20Axs Fluorescence Detector (FLD), and a UV diode array detector (DAD). The chromatographic separation of PAHs was achieved on a PAH kinetex column (4.6mm x 100 mm, 3.5 μm) by Phenomenex (California, United States). The injected sample volume was 10 μl. PAHs were separated using a gradient elution program with a flow rate of 1 ml/minute. The initial mobile phase was 50% acetonitrile and 50% water for 7 min which was then gradiently changed to 100% acetonitrile over 7 min, slightly 100/0 % (8 min) and then decreased to initial phase (50-0%) over 12 min. The temperature of the column was maintained at 20°C during the analysis. The PAHs were monitored using the FLD detector. Due to the lack of fluorescent property of acenaphthylene no quantitative determination was made in case of this compound. The excitation and emission wavelengths were changed during the run to obtain the highest sensitivity for all compounds. PAHs were identified based on retention time and fluorescence spectrum, and compound quantification was performed by using external calibration method. The HPLC analysis of the samples were repeated in triplicate.
4.5. Method Validation
Different concentrations of standard calibration solutions were prepared at 9 concentration levels in acetonitrile. The calibration levels were: 0.0625, 0.125, 0.25, 0.5, 1, 5, 10, 20 and 50 ng/mL PAHs. For each level, three independent replicates were prepared in a random order. Spiked samples (final concentrations: 5, 10 and 20 ng/mL) were prepared for recovery studies. Limit of detection (LOD) and limit of quantitation (LOQ) calculation was based on the signal to noise ratio 3:1 and 10:1 respectively. All solutions were stored in amber vials at 4 °C.
All 12 PAHs have a good linearity at 9 concentrations (0.0625, 0.125, 0.25, 0.5, 1, 5, 10, 20 and 50 ng/mL) with a regression coefficient greater than 0.9870. The LOD and LOQ of the method was ranging from 0.07-0.25 ng/mL and 0.21-0.76 ng/mL, respectively. The method performed well in the recovery of PAHs in mother milk both at low (5 ng/mL) medium (10 ng/mL) and high (20 ng/mL) concentrations. The mean recovery rates were ranging from 72.81 and 135.08 %.
4.6. Risk Assessment Analysis
4.6.1. Exposure Assessment
Toxicity Equivalent Factors (TEF) [
8] have been defined as carcinogenic or probable carcinogenic PAHs. TEF values represent the relative toxicity of individual PAH compounds compared to a reference compound, which is generally applied to the most toxic, carcinogenic, and mutagenic PAH specifically BaP. The TEF value of BaP is 1. TEF values of other PAHs are shown in
Table 1. PEQBaP is BaP equivalent toxicity calculated by the sum of individual PAH concentrations (Ci) multiplies with its corresponding TEF (TEFi) as follows:
The chronic daily intake (CDI) of PAHs was calculated using the BaP equivalent concentration according to the following equation:
where Ci is the PAH12 (PAH 13 standard mix without acenaphthylene calculated BaP equivalent concentration (ng PEQBaP ng/g). IR is the ingestion rate of breast milk in grams per day (700 g milk/day); EF is the exposure frequency to carcinogenic or mutagenic PAHs in days per year (350 day/year); ED is exposure duration in years (1 year); BW is the average body weight of a baby in kg (5kg) and AT is the average exposure time in days (2 years). The default values were made in consistent US EPA guidelines)
4.6.2. Risk Characterization
Estimating the infant risk for cancer related to PAH content in breast milk can involve the use of various models. We used the Incremental Lifetime Cancer Risk model (ILCR) to calculate carcinogenic risk for infants. The ILCR method is widely used in the risk assessment of PAH [
36] as follows.
SFBaP is the carcinogenic slope factor of BaP (7.3 mg/kg), CF is the unit transformation 10-6 mg/ng
According to the USEPA guidelines, ILCR lower than 10
-6 is a safe value, IRCL in the range 10
-6 and 10
-4 is acceptable, and IRCL higher than 10
-4 indicate risk for infants [
37].
4.7. Statistical Analysis
Statistical analysis was carried out using IBM SPSS Statistics 29 Software (IBM Corporation, Armonk, NY, USA) to find correlations between maternal dietary habits, lifestyle, and the PAH concentrations in their breast milk. A descriptive statistical analysis was performed. Data (including maternal demographic characteristics and PAH concentrations) are presented as mean ± standard deviation, or as median with interquartile range (IQR), that depended on whether the data followed a normal or non-normal distribution. The sample distribution of PAH concentrations was examined before statistical analysis using Kolgomorov-Smirnov test. The datasets followed a normal distribution in case of BaA, BbF and BkF, while in case of other PAHs, and total PAHs concentration did not follow normal distribution. Hypotheses concerning the equality of total PAHs concentration were tested using non-parametric procedures based on the Mann-Whitney U-test for comparing two different groups and Kruskal-Wallis test in case of more than two groups. Statistical significance was established as a p-value of <0.05.
5. Conclusions
The results of the study confirm the presence of PAHs in breast milk samples from Hungarian volunteering mothers. The average level of PAH concentration in breast milk is comparable to average concentrations measured in other European studies. According to the calculations made with the help of the IRCL model, most breast milk samples can be considered safe or show acceptable carcinogenic risk for infants (between 10% and 88%). Monitoring of PAHs level during lactation is recommended to follow potential changes and challenges of infant health risks. The results of this study suggest that lifestyle and dietary habits can play a significant role in regulating the concentration of PAHs in breast milk. Informing mothers about possibilities to avoid PAH affected activities (eg. smoking) and food types (eg. smoked meat) could also be important. Based on the findings, adopting a healthy lifestyle and a mindful choosing of food may contribute to a minimal quantity of PAHs in the diet, which is crucial for proper maternal and infant health. Further research should thoroughly investigate the impact of individual foods, processing methods, and other lifestyle factors on PAH concentration. Healthcare advice should emphasize the role of healthy nutrition and lifestyle in minimizing the presence of PAHs in breast milk.
Author Contributions
Conceptualization, Viktória Poór. and Timea Dergez.; methodology, Anditi Bernard Collins, Mátyás Wahr, Dénes Szerencsés.; validation, Viktória Poór, Anditi Bernard Collins; formal analysis, Timea Dergez, Anikó Kőnig-Péter, Kinga Sándor-Bajusz, István Szabó; resources, István Szabó, Tímea Dergez; data curation, Anditi Bernard Collins, Viktória Poór, Timea Dergez; writing—original draft preparation, Anditi Bernard Collins, Timea Dergez, Viktória Poór; writing—review and editing, Timea Dergez, Viktória Poór, István Szabó, Kinga Sándor-Bajusz; visualization, Timea Dergez, Viktória Poór; supervision, Vitkória Poór, Timea Dergez, István Szabó; project administration, Timea Dergez; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This Research was supported by Grants from the Stipendium Hungaricum and the National Research and Development Fund, NVKP_16-1-2016-0009 project.
Institutional Review Board Statement
Permission to collect breastmilk samples in the Hungarian University of Agriculture and Life Sciences (MATE) (formerly Szent István University) was granted by the Ministry of Human Resources (decision number: 14069-6/2018/EÜIG) and the University of Pécs (Regional Research Ethics Committee, approval number: 5579) and was performed in line with the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author, in order to protect the personal information of the mothers participating in the study.
Acknowledgments
We are grateful to the women who participated in this volunteering study. Authors especially thank to the Hungarian Nursing Association (Magyar Védőnői Szolgálat) for their excellent assistance in sample collecting. We would like to thank Kinga Amália Sándor-Bajusz for reviewing our manuscript for language accuracy.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Polynuclear aromatic compounds, Chemical, Environmental and Experimental Data. ed.; Part 1; IARC: Lyon, France, 1983, Volume 32. 94-448.
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Polynuclear aromatic compounds, Industrial exposures in aluminium production, coal gasification, coke production, and iron and steel founding. ed.; Part 3; IARC: Lyon, France, 1984, Volume 34. 34-181.
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Polynuclear aromatic compounds, Bitumens, coal-tars and derived products, shale-oils and soots. ed.; Part 4; IARC: Lyon, France, 1985, Volume 35. 38-245.
- Kim SR, Halden RU, Buckley TJ. Polycyclic aromatic hydrocarbons in human milk of nonsmoking US women. Environ. Sci. Technol. 2008, 42: 2663-2667.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. ed. 2002:82:1-556.
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. ed.; IARC: Lyon, France, 2010, 35-110.
- Bandow N, Altenburger R, Lubcke-von Varel U, Paschke A, Streck G, Brack W. Partitioning-Based Dosing: An Approach To Include Bioavailability in the Effect-Directed Analysis of Contaminated Sediment Samples. Environ. Sci. Technol. 2009, 43: 3891-3896.
- Polycyclic Aromatic Hydrocarbons in Food - Scientific Opinion of the Panel on Contaminants in the Food Chain. ed. Wiley Online Library: EFSA Journal 2008, 724:1-114.
- Hylland, K. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. J. Toxicol. Environ.-Part a-Current Issues 2006, 69, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Fisher TT, Law RJ, Rumney HS, Kirby MF, Kelly C. Towards a scheme of toxic equivalency factors (TEFs) for the acute toxicity of PAHs in sediment. Ecotoxicol. Environ. Saf. 2011: 2245–2251.
- Ma YN, Harrad S. Spatiotemporal analysis and human exposure assessment on polycyclic aromatic hydrocarbons in indoor air, settled house dust, and diet: A review. Environ. Int. 2015, 84, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ruby MV, Lowney YW, Bunge AL, Roberts SM, Gomez-Eyles JL, Ghosh U, Kissel JC, et al. Oral Bioavailability, Bioaccessibility, and Dermal Absorption of PAHs from Soil-State of the Science. Environ. Sci. Technol. 2016, 50: 2151-2164.
- EUR-Lex. EC/835/2011 COMMISSION REGULATION (EU) No 835/2011 of 19 August 2011. Amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. ed.: OJEU 2011: L 215/214.
- Del Bubba M, Zanieri L, Galvan P, Donzelli GP, Checchini L, Lepri L. Determination of polycyclic aromatic hydrocarbons (PAHs) and total fats in human milk. Annali Di Chimica 2005, 95: 629-641.
- Moon HB, Lee DH, Lee YS, Kannan K. Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere 2012, 86, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Santos PM, Sanchez MD, Pavon JLP, Cordero BM. Determination of polycyclic aromatic hydrocarbons in human biological samples: A critical review. TrAC. 2019, 113, 194–209. [Google Scholar]
- Torres-Moreno C, Puente-DelaCruz L, Codling G, Villa AL, Cobo M, Klanova J, Johnson-Restrepo B. Polycyclic aromatic hydrocarbons (PAHs) in human breast milk from Colombia: Spatial occurrence, sources and probabilistic risk assessment. Environ. Res. 2022, 204:111981.
- Wallace MAG, Pleil JD, Whitaker DA, Oliver KD. Recovery and reactivity of polycyclic aromatic hydrocarbons collected on selected sorbent tubes and analyzed by thermal desorption-gas chromatography/mass spectrometry. Chromatogr. A. 2019, 1602, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hegazy AM, Fakhreldin AR, Nasr SM. Monitoring of carcinogenic environmental pollutants in women's breast milk. BPJ. 2020, 13, 119–125. [Google Scholar]
- Zanieri L, Galvan P, Checchini L, Cincinelli A, Lepri L, Donzelli GP, Del Bubba M. Polycyclic aromatic hydrocarbons (PAHs) in human milk from Italian women: Influence of cigarette smoking and residential area. Chemosphere 2007, 67, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano R, Santonicola S, De Felice A, Anastasio A, Murru N, Ferrante MC, Cortesi ML. Occurrence and distribution of polycyclic aromatic hydrocarbons in mussels from the gulf of Naples, Tyrrhenian Sea, Italy. Mar. Pollut. Bull. 2016, 104, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Perera FP, Tang DL, Wang S, Vishnevetsky J, Zhang BZ, Diaz D, Camann D, et al. Prenatal Polycyclic Aromatic Hydrocarbon (PAH) Exposure and Child Behavior at Age 6-7 Years. EHP. 2012, 120: 921-926.
- Xu YZ, Che T, Li YJ, Fang C, Dai ZW, Li HX, Xu L, et al. Remediation of polycyclic aromatic hydrocarbons by sulfate radical advanced oxidation: Evaluation of efficiency and ecological impact. Ecotoxicol. Environ. Saf. 2021, 223:112594.
- Karimi P, Peters KO, Bidad K, Strickland PT. Polycyclic aromatic hydrocarbons and childhood asthma. Eur. J. Epidemiol. 2015, 30: 91-101.
- Santonicola S, De Felice A, Cobellis L, Passariello N, Murru N, Ferrante MC, Mercogliano R. Comparative study on the occurrence of polycyclic aromatic hydrocarbons in breast milk and infant formula and risk assessment. Chemosphere 2017, 175: 383-390.
- Oliveira M, Duarte S, Delerue-Matos C, Pena A, Morais S. Exposure of nursing mothers to polycyclic aromatic hydrocarbons: Levels of un-metabolized and metabolized compounds in breast milk, major sources of exposure and infants' health risks. Environ. Pollut. 2020, 266: 115243.
- Pulkrabova J, Stupak M, Svarcova A, Rossner P, Rossnerova A, Ambroz A, Sram R, et al. Relationship between atmospheric pollution in the residential area and concentrations of polycyclic aromatic hydrocarbons (PAHs) in human breast milk. Sci. Total Environ. 2016, 562: 640-647.
- Cok I, Mazmanci B, Mazmanci MA, Turgut C, Henkelmann B, Schramm KW. Analysis of human milk to assess exposure to PAHs, PCBs and organochlorine pesticides in the vicinity Mediterranean city Mersin, Turkey. Environ. Int. 2012, 40: 63-69.
- Wang L, Liu AP, Zhao Y, Mu X, Huang T, Gao H, Ma JM. The levels of polycyclic aromatic hydrocarbons (PAHs) in human milk and exposure risk to breastfed infants in petrochemical industrialized Lanzhou Valley, Northwest China. ESPR. 2018, 25: 16754-16766.
- Tsang HL, Wu SC, Leung CKM, Tao S, Wong MH. Body burden of POPs of Hong Kong residents, based on human milk, maternal and cord serum. Environ. Int. 2011, 37: 142-151.
- Khanverdiluo S, Talebi-Ghane E, Heshmati A, Mehri F. The concentration of polycyclic aromatic hydrocarbons (PAHs) in mother milk: A global systematic review, meta-analysis and health risk assessment of infants. Saudi J. Biol. Sci. 2021, 28: 6869-6875.
- Kim KH, Jahan SA, Kabir E, Brown RJC. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60: 71-80.
- Acharya N, Gautam B, Subbiah S, Rogge MM, Anderson TA, Gao WM. Polycyclic aromatic hydrocarbons in breast milk of obese vs normal women: Infant exposure and risk assessment. Sci. Total Environ. 2019, 668: 658-667.
- Asamoah A, Fini MN, Essumang DK, Muff J, Sogaard EG. PAHs contamination levels in the breast milk of Ghanaian women from an e-waste recycling site and a residential area. Sci. Total Environ. 2019, 666: 347-354.
- Ssepuya F, Odongo S, Bandowe BAM, Abayi JJM, Olisah C, Matovu H, Mubiru E, et al. Polycyclic aromatic hydrocarbons in breast milk of nursing mothers: Correlates with household fuel and cooking methods used in Uganda, East Africa. Sci. Total Environ. 2022, 842.
- Xia ZH, Duan XL, Qiu WX, Liu D, Wang B, Tao S, Jiang QJ, et al. Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci. Total Environ. 2010, 408: 5331-5337.
- Heshmati A, Mehri F, Karami-Momtaz J, Khaneghah AM. The concentration and health risk of potentially toxic elements in black and green tea-both bagged and loose-leaf. QAS 2020, 12: 140-150.
- Cavret S, Feidt C, Le Roux Y, Laurent F. Short communication: Study of mammary epithelial role in polycyclic aromatic hydrocarbons transfer to milk. JDS. 2005, 88: 67-70.
- Kipopoulou A, Manoli E, Samara C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ. Pollut. 1999, 106: 369-380.
- Raza N, Kim K-H. Quantification techniques for important environmental contaminants in milk and dairy products. TrAC 2018, 98: 79-94.
- Seralini GE, Douzelet J, Jungers G. Dataset of pollutants in organic and non-organic food. Data in Brief 2022, 42: 108295.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).