Submitted:
26 September 2024
Posted:
27 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Synthesis of Triallyl Derivative of Curcumin 2
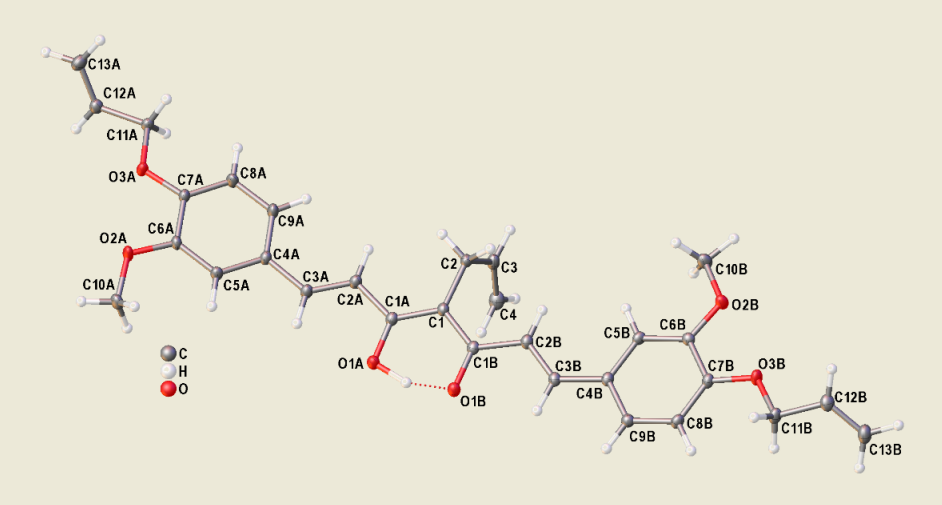
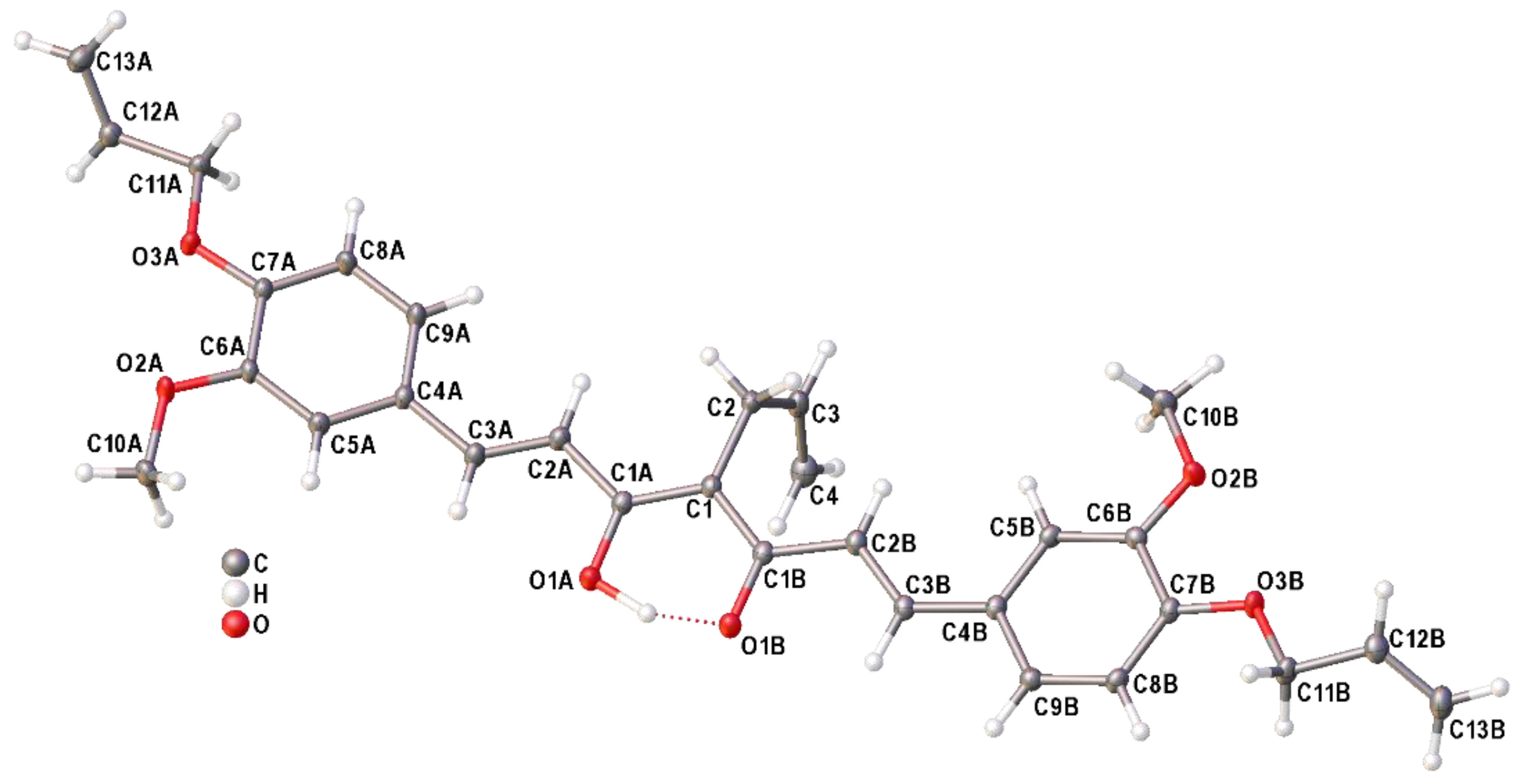
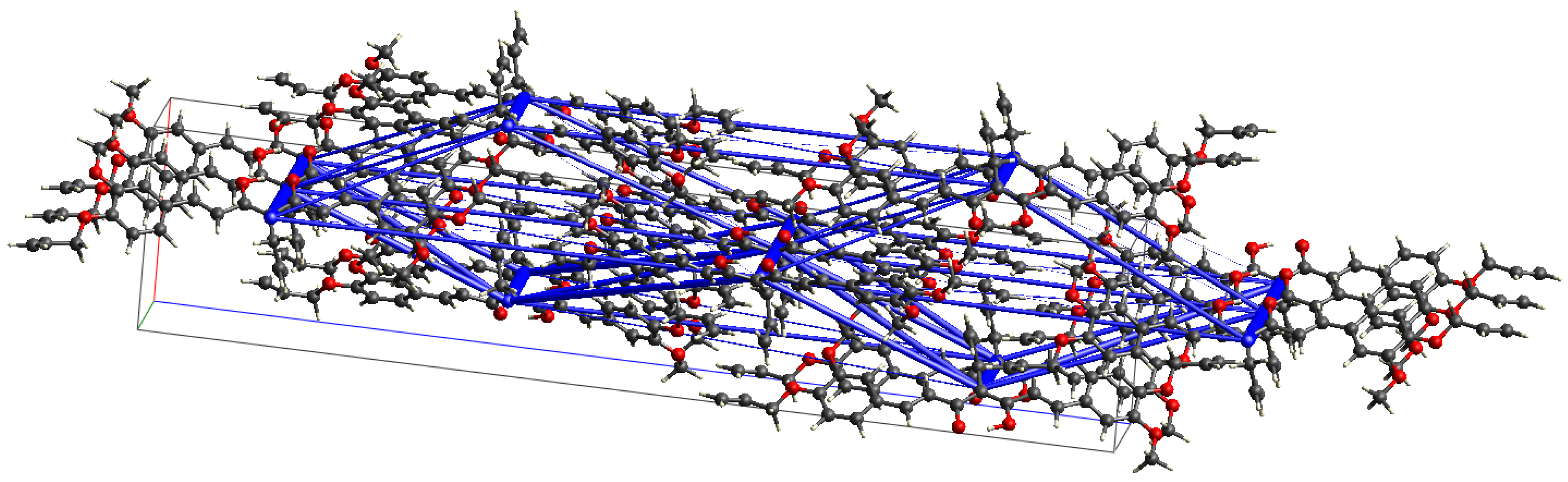
2.2. Single-Crystal X-ray Diffraction Studies of Allyl Derivative of Curcumin 2
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of (1E,6E)-4-allyl-1,7-bis(4’-allyloxy-3’-methoxyphenyl)hepta-1,6-diene-3,5-dione 2
3.3. Single Crystal X-ray Diffraction Study
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites. 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.C.; Faustino, M.A.F.; Braga, S.S. Curcumin innovative delivery forms: paving the ‘yellow brick road’ of antitumoral phytotherapy. Appl. Sci. 2020, 10, 8990–9019. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: biological activities and modern pharmaceutical forms. Antibiotics. 2022, 11, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radical Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, T.; Zou, Y.; Zhang, J.H.; Feng, H. Curcumin inhibits microglia inflammation and confers neuroprotection in intracerebral hemorrhage. Immunol. Lett. 2014, 160, 89–95. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zu, J.N.; Li, J.; Chen, C.; Xi, C.Y.; Yan, J.L. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci. Lett. 2014, 560, 51–56. [Google Scholar] [CrossRef]
- Oelkrug, C. , Lange, C.M.; Wenzel, E.; Fricke, S.; Hartke, M.; Simasi, J.; Schubert, A. Analysis of the tumoricidal and anti-cachectic potential of curcumin. Anticancer Res. 2014, 34, 4781–4788. [Google Scholar] [PubMed]
- Ohtsu, H.; X. Zhiyan; Ishida, J.; Nagai, M.; Wang, H.K.; Itokawa, H.; Su, C.Y.; Shih, C.; Chiang, T.; Chang, E.; Lee, Y.; et al. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J. Med. Chem., 2002, 45, 5037–5042. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, C.; Liu, D.; Yan, J.; Liang, H. The clinical applications of curcumin: current state and the future. Curr. Pharm. Des. 2013, 19, 2011–2031. [Google Scholar] [PubMed]
- Adams, B.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G. , Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef]
- Conteas, C.N.; Panossian, A.M.; Tran, T.T.; Singh, H.M. Treatment of HIV-associated diarrhea with curcumin. Dig. Dis. Sci. 2009, 2009. 54, 2188–2191. [Google Scholar] [CrossRef]
- Kesharwani, R.K.; Srivastava, V.; Singh, P.; Rizvi, S.I.; Adeppa, K.; Misra, K. A Novel approach for overcoming drug resistance in breast cancer chemotherapy by targeting new synthetic curcumin analogues against aldehyde dehydrogenase 1 (ALDH1A1) and glycogen synthase kinase-3 β (GSK-3β). Appl. Biochem. Biotechnol. 2015, 54, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar. P.; Lall, R. Metal-curcumin complexes in therapeutics: An approach to enhance pharmacological effects of curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, U.; Rasmussen, P.B.; Lawesson, S.-O. Synthesis of naturally occurring curcuminoids and related compounds. Liebigs Ann. Chem. 1985, 1985, 1557–1569. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, W.; Kang, J.; Chong, Y. Significant enhancement in radical-scavenging activity of curcuminoids conferred by acetoxy substituent at the central methylene carbon. Bioorg. Med. Chem. 2011, 19, 3793–3800. [Google Scholar] [CrossRef]
- Hsieh, M.-T.; Chang, L.-C.; Hung, H.-Y.; Lin, H.-Y.; Shih, M.-H.; Tsai, C.-H.; Kuo, S.C.; Lee, K.-H. New bis(hydroxymethyl) alkanoate curcuminoid derivatives exhibit activity against triple-negative breast cancer in vitro and in vivo. Eur. J. Med. Chem. 2017, 131, 141–151. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Hou, Y.-C.; Yang, J.-S.; Lin, H.-Y.; Chang, T.-Y.; Lee, K.-H.; Kuo, S.-C.; Hsieh, M.-T. Synthesis, anticancer activity, and preliminary pharmacokinetic evaluation of 4,4-disubstituted curcuminoid 2,2-bis(hydroxymethyl)propionate derivatives. Molecules 2020, 25, 479. [Google Scholar] [CrossRef]
- Yang, L.-C.; Wang, C.-C.; Lee,D. -Y.; Lin, W.-C.; Kuo, S.-C.; Juang, S.-H.; Hsieh, M.-T. 4,4-Diallyl curcumin bis(2,2-hydroxymethyl)propanoate ameliorates nonalcoholic steatohepatitis in methionine-choline-deficient diet and Western diet mouse models. Chem. Biol. Drug Design 2024, 193, e14532. [Google Scholar] [CrossRef]
- Kesharwani, R.K.; Srivastava, V.; Singh, P.; Rizvi, S.I.; Adeppa, K.; Misra, K. A novel approach for overcoming drug resistance in breast cancer chemotherapy by targeting new synthetic curcumin analogues against aldehyde dehydrogenase 1 (ALDH1A1) and glycogen synthase kinase-3 β (GSK-3β). Appl. Biochem. Biotechnol. 2015, 176, 1996–2017. [Google Scholar] [CrossRef]
- Barthelemy, S.; Vergnes, L.; Moynier, M.; Guyot, D.; Labidalle, S.; Bahraoui, E. Curcumin and curcumin derivatives inhibit Tat-mediated transactivation of type 1 human immunodeficiency virus long terminal repeat. Res. Virol. 1998, 149, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Somsakeesit, L.; Senawong, T.; Kumboonm, P.; Saenglee, S.; Samankul, A.; Senawong, G.; Yenjai, C.; Phaosiri, C. Influence of side-chain changes on histone deacetylase inhibitory and cytotoxicity activities of curcuminoid derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 127171. [Google Scholar] [CrossRef] [PubMed]
- Changtam, C. , Koning, H.P.; Ibrahim, H.; Sajid, M.S.; Gould, M.K.; Suksamrarn, A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur. J. Med. Chem. 2010, 45, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Arkles, B.C.; Curcumin and its derivatives for use as silicone colorants. US 2007/0204412, 6 September 2007. Available online: https://patents.google.com/patent/US20070204412A1/en (accessed on 22 September 2024).
- Galer, P.; Golobič, A.; Koller, J.; Košmrlj, B.; Šket, B. Structures in solid state and solution of dimethoxy curcuminoids: Regioselective bromination and chlorination. Chem. Centr. J. 2013, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J.; Mostad, A. Structural studies of curcuminoids. I. The crystal structure of curcumin. Acta Chem. Scand. 1982, 36, 475–479. [Google Scholar] [CrossRef]
- Ishigami, Y.; Goto, M.; Masuda, T.; Takizawa, Y.; Suzum, S. The crystal structure and the fluorescent properties of curcumin. J. Jpn. Soc. Colour Mater. 1999, 72, 71–77. [Google Scholar] [CrossRef]
- Lozada, M.C.; Lobato, C.E.; Enriquez, R.G.; Ortiz, B.; Gnecco, D.; Reynolds, W.F.; Soriano-Garsia, M. Crystal structures and synthesis of 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl) hept-1,4,6-trien-3-one. Anal. Sci.: X-Ray Struct. Anal. Online 2005, 21, x59–x60. [Google Scholar] [CrossRef]
- Parimita, S.P.; Ramshankar, Y.V.; Suresh, S.; Row, T.N.G. Redetermination of curcumin: (1E,4Z,6E)-5-hydroxy-1,7-bis(4-hydroxy-3-methoxy-phenyl)hepta-1,4,6-trien-3-one. Acta Cryst. E 2007, 63, o860–o862. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013–5015. [Google Scholar] [CrossRef]
- Parameswari, A.R.; Devipriya, B.; Jenniefer, S.J.; Muthiah, P.T.; Kumaradhas, P. Low temperature crystal structure of 5-hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-dien-3-one. J. Chem. Cryst. 2012, 42, 227–231. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Solid-state phase transformations and storage stability of curcumin polymorphs. Cryst. Growth Des. 2015, 15, 1757–1770. [Google Scholar] [CrossRef]
- Matlinska, M.A.; Wasylishen, R.E.; Bernard, G.M.; Terskikh, V.V.; Brinkmann, A.; Michaelis, V.K. Capturing elusive polymorphs of curcumin: A structural characterization and computational study. Cryst. Growth Des. 2018, 18, 5556–5563. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G. Curcumin, a biological wonder molecule: A crystal engineering point of view. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03; Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- APEX2 and SAINT; Bruker AXS: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




