Submitted:
27 September 2024
Posted:
27 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
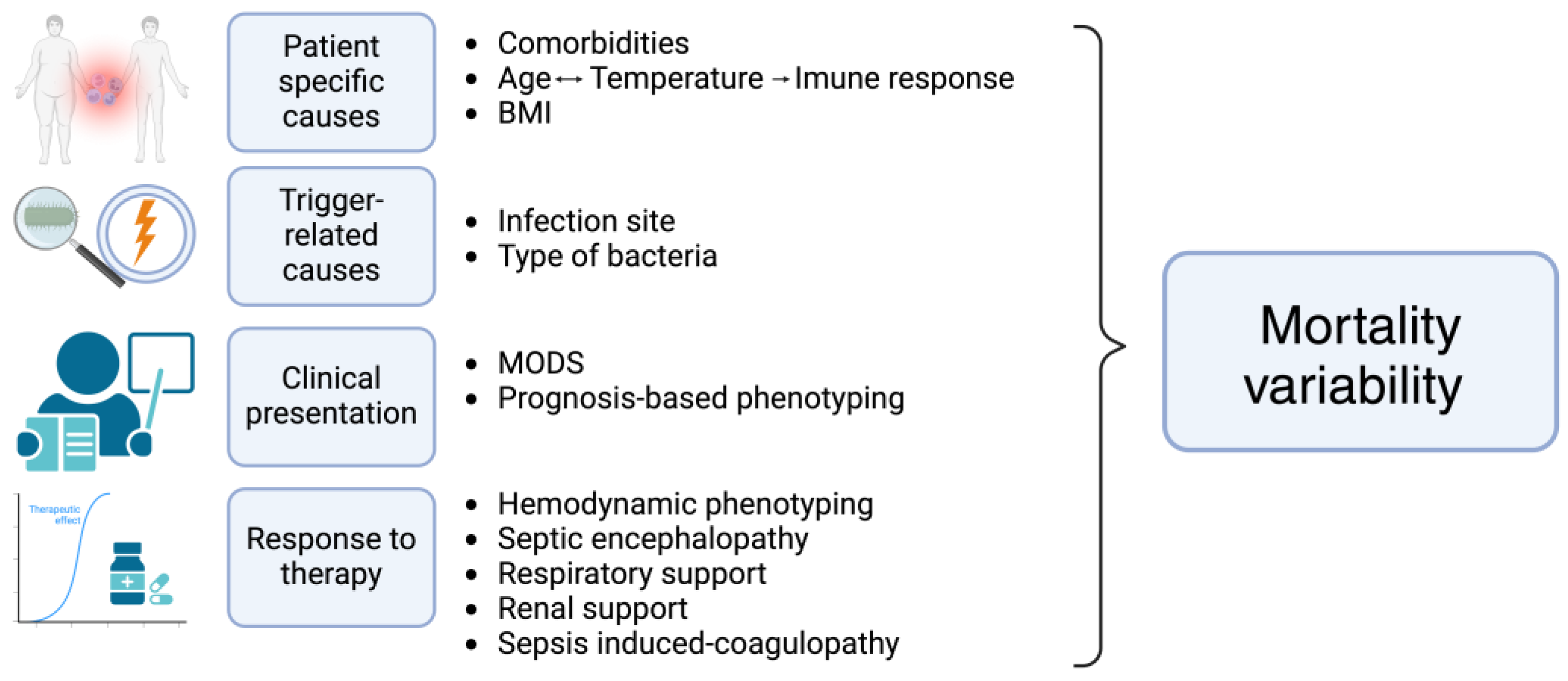
2. Patient Specific Causes of Heterogeneity
2.1. Comorbidities
2.2. Age, Temperature and Immune Response Relationship
2.3. Body-Mass Index
3. Trigger-Related Causes of Heterogeneity
3.1. Infection Site
3.2. Type of Bacteria
4. Clinical Manifestations and Prognosis-Based Phenotyping
4.1. Multiorgan Dysfunction Phenotyping
4.2. Prognosis-Based Phenotyping
5. Organ Dysfunction Trajectory and Response to the Therapeutic Approach in Clinical Sepsis Phenotyping
5.1. Hemodynamic Phenotyping
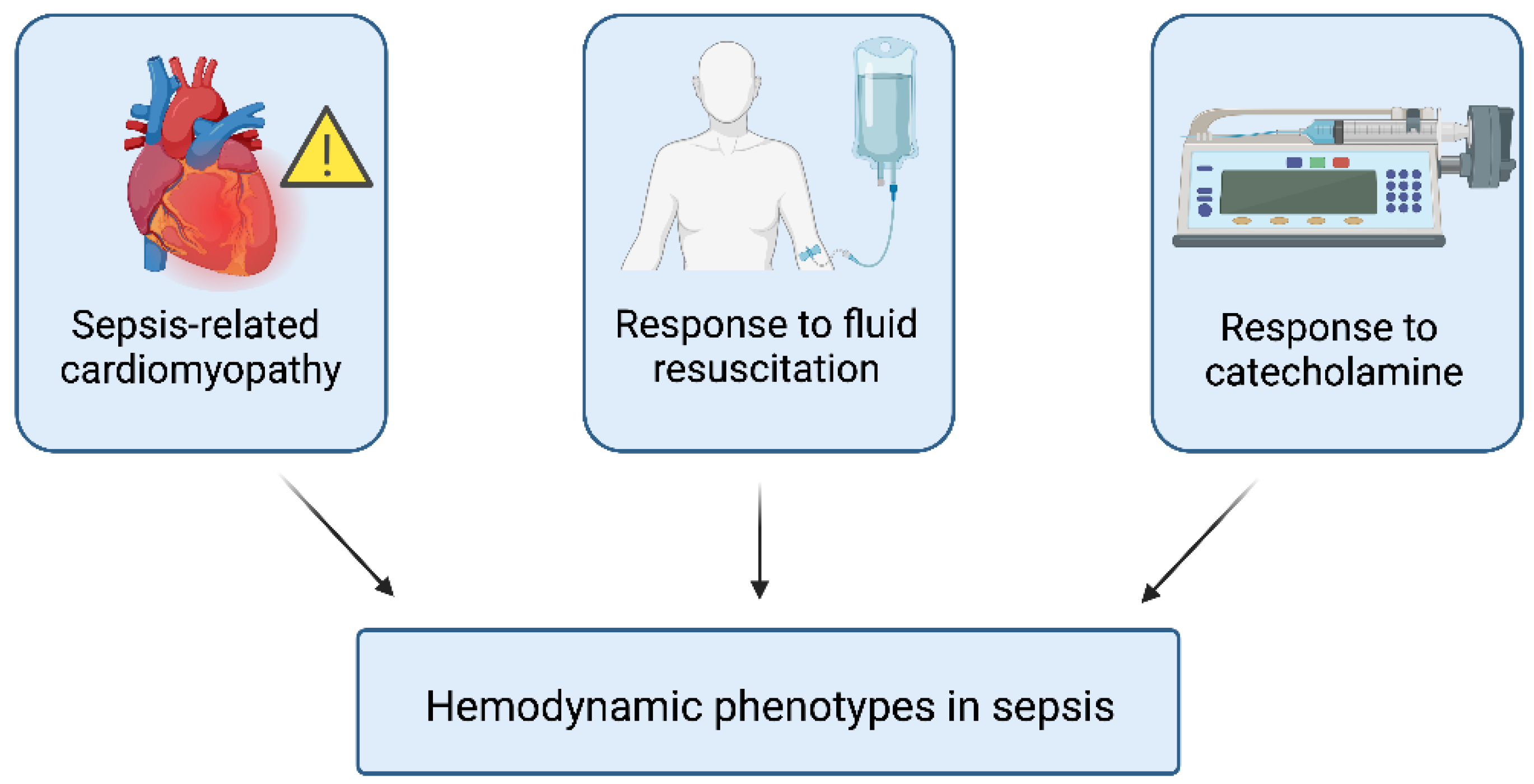
5.2. Sepsis-Associated Encephalopathy (SAE)
5.3. Respiratory Trajectory
5.4. Renal Injury and Renal Replacement Therapy (RRT)
5.5. Sepsis-Induced Coagulopathy (SIC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Isac, S.; Isac, T.; Tanasescu, M.D.; Pavel, B.; Andreescu, C.V.; Badea, A.-G.; Ojog, D.; Teodorescu, G.-D.; Laceanu, A.; Trifan, C.-B.; et al. The Omics Complexity in Sepsis: The Limits of the Personalized Medicine Approach. J. Pers. Med. 2024, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Cotorogea-Simion, M.; Pavel, B.; Isac, S.; Telecan, T.; Matache, I.-M.; Bobirca, A.; Bobirca, F.-T.; Rababoc, R.; Droc, G. What Is Different in Acute Hematologic Malignancy-Associated ARDS? An Overview of the Literature. Medicina (Mex.) 2022, 58, 1215. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003. [Google Scholar] [CrossRef]
- Ding, M.; Luo, Y. Unsupervised Phenotyping of Sepsis Using Nonnegative Matrix Factorization of Temporal Trends from a Multivariate Panel of Physiological Measurements. BMC Med. Inform. Decis. Mak. 2021, 21, 95. [Google Scholar] [CrossRef]
- Taylor, S.P.; Bray, B.C.; Chou, S.-H.; Burns, R.; Kowalkowski, M.A. Clinical Subtypes of Sepsis Survivors Predict Readmission and Mortality after Hospital Discharge. Ann. Am. Thorac. Soc. 2022, 19, 1355–1363. [Google Scholar] [CrossRef]
- Kudo, D.; Goto, T.; Uchimido, R.; Hayakawa, M.; Yamakawa, K.; Abe, T.; Shiraishi, A.; Kushimoto, S. Coagulation Phenotypes in Sepsis and Effects of Recombinant Human Thrombomodulin: An Analysis of Three Multicentre Observational Studies. Crit. Care 2021, 25, 114. [Google Scholar] [CrossRef]
- Honore, P.M.; Barreto Gutierrez, L.; Kugener, L.; Redant, S.; Attou, R.; Gallerani, A.; De Bels, D. Mortality in Non-Elderly Septic Patients Was Increased with Hypothermia and Decreased with Fever While Mortality in Elderly Patients Was Not Associated with Body Temperature: Beware of Some Confounders! Crit. Care 2020, 24, 606. [Google Scholar] [CrossRef]
- Shimazui, T.; Nakada, T.; Walley, K.R.; Oshima, T.; Abe, T.; Ogura, H.; Shiraishi, A.; Kushimoto, S.; Saitoh, D.; Fujishima, S.; et al. Significance of Body Temperature in Elderly Patients with Sepsis. Crit. Care 2020, 24, 387. [Google Scholar] [CrossRef]
- Ito, Y.; Kudo, D.; Kushimoto, S. Association between Low Body Temperature on Admission and In-Hospital Mortality According to Body Mass Index Categories of Patients with Sepsis. Medicine (Baltimore) 2022, 101, e31657. [Google Scholar] [CrossRef]
- Bhavani, S.V.; Carey, K.A.; Gilbert, E.R.; Afshar, M.; Verhoef, P.A.; Churpek, M.M. Identifying Novel Sepsis Subphenotypes Using Temperature Trajectories. Am. J. Respir. Crit. Care Med. 2019, 200, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.S.; Kim, J.H.; Kwon, Y.S. Cluster Analysis Integrating Age and Body Temperature for Mortality in Patients with Sepsis: A Multicenter Retrospective Study. Sci. Rep. 2022, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gan, Q.; Zhou, D.; Xia, X.; Xiang, W.; Xiao, R.; Tang, J.; Li, J. Evaluating the Risk of Sepsis Attributing to Obesity: A Two-Sample Mendelian Randomization Study. Postgrad. Med. J. 2023, 99, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huang, J.; Wang, D.; Zhu, D.; Zhao, Q.; Li, T.; Zhou, X.; Xu, Y. Association of Body Mass Index with Mortality of Sepsis or Septic Shock: An Updated Meta-Analysis. J. Intensive Care 2023, 11, 27. [Google Scholar] [CrossRef]
- Sato, T.; Kudo, D.; Kushimoto, S.; Hasegawa, M.; Ito, F.; Yamanouchi, S.; Honda, H.; Andoh, K.; Furukawa, H.; Yamada, Y.; et al. Associations between Low Body Mass Index and Mortality in Patients with Sepsis: A Retrospective Analysis of a Cohort Study in Japan. PLOS ONE 2021, 16, e0252955. [Google Scholar] [CrossRef]
- Jeganathan, N.; Yau, S.; Ahuja, N.; Otu, D.; Stein, B.; Fogg, L.; Balk, R. The Characteristics and Impact of Source of Infection on Sepsis-Related ICU Outcomes. J. Crit. Care 2017, 41, 170–176. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Liao, T.-Y.; Hsu, T.-C.; Hsu, W.-T.; Lee, M.-T.G.; Lee, C.-C. Temporal Trend and Survival Impact of Infection Source among Patients with Sepsis: A Nationwide Study. Crit. Care Resusc. 2020, 22, 126–132. [Google Scholar] [CrossRef]
- He, X.-L.; Liao, X.-L.; Xie, Z.-C.; Han, L.; Yang, X.-L.; Kang, Y. Pulmonary Infection Is an Independent Risk Factor for Long-Term Mortality and Quality of Life for Sepsis Patients. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Jeganathan, N. Burden of Sepsis in India. Chest 2022, 161, 1438–1439. [Google Scholar] [CrossRef]
- Chou, E.H.; Mann, S.; Hsu, T.-C.; Hsu, W.-T.; Liu, C.C.-Y.; Bhakta, T.; Hassani, D.M.; Lee, C.-C. Incidence, Trends, and Outcomes of Infection Sites among Hospitalizations of Sepsis: A Nationwide Study. PLOS ONE 2020, 15, e0227752. [Google Scholar] [CrossRef]
- Papin, G.; Bailly, S.; Dupuis, C.; Ruckly, S.; Gainnier, M.; Argaud, L.; Azoulay, E.; Adrie, C.; Souweine, B.; Goldgran-Toledano, D.; et al. Clinical and Biological Clusters of Sepsis Patients Using Hierarchical Clustering. PLOS ONE 2021, 16, e0252793. [Google Scholar] [CrossRef] [PubMed]
- Schertz, A.R.; Eisner, A.E.; Smith, S.A.; Lenoir, K.M.; Thomas, K.W. Clinical Phenotypes of Sepsis in a Cohort of Hospitalized Patients According to Infection Site. Crit. Care Explor. 2023, 5, e0955. [Google Scholar] [CrossRef] [PubMed]
- IOANA MARINA GRINȚESCU, L.M. EFFICACY OF TIGECYCLINE TREATMENT IN SEVERE AND COMPLICATED CLOSTRIDIUM DIFFICILE INFECTION. 2017, 65,4, 600–604. [Google Scholar]
- Falcone, M.; Tiseo, G.; Carbonara, S.; Marino, A.; Di Caprio, G.; Carretta, A.; Mularoni, A.; Mariani, M.F.; Maraolo, A.E.; Scotto, R.; et al. Mortality Attributable to Bloodstream Infections Caused by Different Carbapenem-Resistant Gram-Negative Bacilli: Results From a Nationwide Study in Italy (ALARICO Network). Clin. Infect. Dis. 2023, 76, 2059–2069. [Google Scholar] [CrossRef]
- Maraolo, A.E.; Corcione, S.; Grossi, A.; Signori, A.; Alicino, C.; Hussein, K.; Trecarichi, E.M.; Viale, P.; Timsit, J.-F.; Veeraraghavan, B.; et al. The Impact of Carbapenem Resistance on Mortality in Patients With Klebsiella Pneumoniae Bloodstream Infection: An Individual Patient Data Meta-Analysis of 1952 Patients. Infect. Dis. Ther. 2021, 10, 541–558. [Google Scholar] [CrossRef]
- Verstraete, E.; Boelens, J.; Coen, K.D.; Claeys, G.; Vogelaers, D.; Vanhaesebrouck, P.; Blot, S. Healthcare-Associated Bloodstream Infections in a Neonatal Intensive Care Unit over a 20-Year Period (1992–2011): Trends in Incidence, Pathogens, and Mortality. Infect. Control Hosp. Epidemiol. 2014, 35, 511–518. [Google Scholar] [CrossRef]
- Arvaniti, K.; Dimopoulos, G.; Antonelli, M.; Blot, K.; Creagh-Brown, B.; Deschepper, M.; De Lange, D.; De Waele, J.; Dikmen, Y.; Eckmann, C.; et al. Epidemiology and Age-Related Mortality in Critically Ill Patients with Intra-Abdominal Infection or Sepsis: An International Cohort Study. Int. J. Antimicrob. Agents 2022, 60, 106591. [Google Scholar] [CrossRef]
- Knox, D.B.; Lanspa, M.J.; Kuttler, K.G.; Brewer, S.C.; Brown, S.M. Phenotypic Clusters within Sepsis-Associated Multiple Organ Dysfunction Syndrome. Intensive Care Med. 2015, 41, 814–822. [Google Scholar] [CrossRef]
- Ibrahim, Z.M.; Wu, H.; Hamoud, A.; Stappen, L.; Dobson, R.J.B.; Agarossi, A. On Classifying Sepsis Heterogeneity in the ICU: Insight Using Machine Learning. J. Am. Med. Inform. Assoc. 2020, 27, 437–443. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Goyal, H.; Mo, L.; Hong, Y. Identification of Subclasses of Sepsis That Showed Different Clinical Outcomes and Responses to Amount of Fluid Resuscitation: A Latent Profile Analysis. Crit. Care 2018, 22, 347. [Google Scholar] [CrossRef]
- Xu, Z.; Mao, C.; Su, C.; Zhang, H.; Siempos, I.; Torres, L.K.; Pan, D.; Luo, Y.; Schenck, E.J.; Wang, F. Sepsis Subphenotyping Based on Organ Dysfunction Trajectory. Crit. Care 2022, 26, 197. [Google Scholar] [CrossRef] [PubMed]
- Aldewereld, Z.T.; Zhang, L.A.; Urbano, A.; Parker, R.S.; Swigon, D.; Banerjee, I.; Gómez, H.; Clermont, G. Identification of Clinical Phenotypes in Septic Patients Presenting With Hypotension or Elevated Lactate. Front. Med. 2022, 9, 794423. [Google Scholar] [CrossRef] [PubMed]
- Sharafoddini, A.; Dubin, J.A.; Lee, J. Identifying Subpopulations of Septic Patients: A Temporal Data-Driven Approach. Comput. Biol. Med. 2021, 130, 104182. [Google Scholar] [CrossRef] [PubMed]
- McLymont, N.; Glover, G.W. Scoring Systems for the Characterization of Sepsis and Associated Outcomes. Ann. Transl. Med. 2016, 4, 527. [Google Scholar] [CrossRef]
- Zhang, Z.; Ho, K.M.; Gu, H.; Hong, Y.; Yu, Y. Defining Persistent Critical Illness Based on Growth Trajectories in Patients with Sepsis. Crit. Care 2020, 24, 57. [Google Scholar] [CrossRef]
- Yang, R.; Han, D.; Zhang, L.; Huang, T.; Xu, F.; Zheng, S.; Yin, H.; Lyu, J. Analysis of the Correlation between the Longitudinal Trajectory of SOFA Scores and Prognosis in Patients with Sepsis at 72 Hour after Admission Based on Group Trajectory Modeling. J. Intensive Med. 2022, 2, 39–49. [Google Scholar] [CrossRef]
- Geri, G.; Vignon, P.; Aubry, A.; Fedou, A.-L.; Charron, C.; Silva, S.; Repessé, X.; Vieillard-Baron, A. Cardiovascular Clusters in Septic Shock Combining Clinical and Echocardiographic Parameters: A Post Hoc Analysis. Intensive Care Med. 2019, 45, 657–667. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Yin, W.; Zhang, H.; Liu, L.; Pan, P.; Zhu, Y.; Huang, W.; Xing, Z.; Yao, B.; et al. A Multicenter Prospective Cohort Study of Cardiac Ultrasound Phenotypes in Patients with Sepsis: Study Protocol for a Multicenter Prospective Cohort Trial. Front. Med. 2022, 9, 938536. [Google Scholar] [CrossRef]
- Kattan, E.; Bakker, J.; Estenssoro, E.; Ospina-Tascón, G.A.; Cavalcanti, A.B.; Backer, D.D.; Vieillard-Baron, A.; Teboul, J.-L.; Castro, R.; Hernández, G. Hemodynamic Phenotype-Based, Capillary Refill Time-Targeted Resuscitation in Early Septic Shock: The ANDROMEDA-SHOCK-2 Randomized Clinical Trial Study Protocol. Rev. Bras. Ter. Intensiva 2022, 34. [Google Scholar] [CrossRef]
- Zhu, J.-L.; Yuan, S.-Q.; Huang, T.; Zhang, L.-M.; Xu, X.-M.; Yin, H.-Y.; Wei, J.-R.; Lyu, J. Influence of Systolic Blood Pressure Trajectory on In-Hospital Mortality in Patients with Sepsis. BMC Infect. Dis. 2023, 23, 90. [Google Scholar] [CrossRef]
- Kenny, J.-E.S. Assessing Fluid Intolerance with Doppler Ultrasonography: A Physiological Framework. Med. Sci. 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Shald, E.A.; Erdman, M.J.; Ferreira, J.A. Impact of Clinical Sepsis Phenotypes on Mortality and Fluid Status in Critically Ill Patients. Shock 2022, 57, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Liu, J.; Shen, F.; Liao, X.; Xiu, M.; Zhao, H.; Zhao, M.; Xie, J.; Wang, P.; Huang, M.; et al. Individualized Resuscitation Strategy for Septic Shock Formalized by Finite Mixture Modeling and Dynamic Treatment Regimen. Crit. Care 2021, 25, 243. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, S.V.; Semler, M.; Qian, E.T.; Verhoef, P.A.; Robichaux, C.; Churpek, M.M.; Coopersmith, C.M. Development and Validation of Novel Sepsis Subphenotypes Using Trajectories of Vital Signs. Intensive Care Med. 2022, 48, 1582–1592. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509. [Google Scholar] [CrossRef]
- Russell, J.A.; Wellman, H.; Walley, K.R. Vasopressin versus Norepinephrine in Septic Shock: A Propensity Score Matched Efficiency Retrospective Cohort Study in the VASST Coordinating Center Hospital. J. Intensive Care 2018, 6, 73. [Google Scholar] [CrossRef]
- Mazeraud, A.; Righy, C.; Bouchereau, E.; Benghanem, S.; Bozza, F.A.; Sharshar, T. Septic-Associated Encephalopathy: A Comprehensive Review. Neurotherapeutics 2020, 17, 392–403. [Google Scholar] [CrossRef]
- Isac, S.; Panaitescu, A.M.; Iesanu, M.I.; Zeca, V.; Cucu, N.; Zagrean, L.; Peltecu, G.; Zagrean, A.-M. Maternal Citicoline-Supplemented Diet Improves the Response of the Immature Hippocampus to Perinatal Asphyxia in Rats. Neonatology 2020, 117, 729–735. [Google Scholar] [CrossRef]
- Isac, S.; Pavel, B.; Dobre, M.; Milanesi, E.; Matache, I.-M.; Paun, R.-M.; Klimko, A.; Iesanu, M.I.; Droc, G.; Zagrean, A.-M. Does a Single Exposure to General Anesthesia Have a Cumulative Effect on the Developing Brain after Mild Perinatal Asphyxia? Life 2022, 12, 1568. [Google Scholar] [CrossRef]
- Sonneville, R.; Benghanem, S.; Jeantin, L.; De Montmollin, E.; Doman, M.; Gaudemer, A.; Thy, M.; Timsit, J.-F. The Spectrum of Sepsis-Associated Encephalopathy: A Clinical Perspective. Crit. Care 2023, 27, 386. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Qin, M.; Walline, J.H.; Gao, Y.; Yu, S.; Ge, Z.; Gong, C.; Zhu, H.; Annane, D.; Li, Y. CLINICAL PHENOTYPES OF SEPSIS-ASSOCIATED ENCEPHALOPATHY: A RETROSPECTIVE COHORT STUDY. Shock 2023, 59, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Sanders, R.D.; Girard, T.D.; McGrane, S.; Thompson, J.L.; Shintani, A.K.; Herr, D.L.; Maze, M.; Ely, E.W. MENDS investigators Effect of Dexmedetomidine versus Lorazepam on Outcome in Patients with Sepsis: An a Priori-Designed Analysis of the MENDS Randomized Controlled Trial. Crit. Care Lond. Engl. 2010, 14, R38. [Google Scholar] [CrossRef] [PubMed]
- Shehabi, Y.; Howe, B.D.; Bellomo, R.; Arabi, Y.M.; Bailey, M.; Bass, F.E.; Bin Kadiman, S.; McArthur, C.J.; Murray, L.; Reade, M.C.; et al. Early Sedation with Dexmedetomidine in Critically Ill Patients. N. Engl. J. Med. 2019, 380, 2506–2517. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Santhakumaran, S.; Prevost, A.T.; Ward, J.K.; Marshall, T.; Bradley, C.; Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; et al. Defining Phenotypes and Treatment Effect Heterogeneity to Inform Acute Respiratory Distress Syndrome and Sepsis Trials: Secondary Analyses of Three RCTs. Effic. Mech. Eval. 2021, 8, 1–104. [Google Scholar] [CrossRef]
- Sinha, P.; Meyer, N.J.; Calfee, C.S. Biological Phenotyping in Sepsis and Acute Respiratory Distress Syndrome. Annu. Rev. Med. 2023, 74, 457–471. [Google Scholar] [CrossRef]
- The Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group; Bagshaw, S.M.; Lapinsky, S.; Dial, S.; Arabi, Y.; Dodek, P.; Wood, G.; Ellis, P.; Guzman, J.; Marshall, J.; et al. Acute Kidney Injury in Septic Shock: Clinical Outcomes and Impact of Duration of Hypotension Prior to Initiation of Antimicrobial Therapy. Intensive Care Med. 2009, 35, 871–881. [Google Scholar] [CrossRef]
- Bouchard, J.; Acharya, A.; Cerda, J.; Maccariello, E.R.; Madarasu, R.C.; Tolwani, A.J.; Liang, X.; Fu, P.; Liu, Z.-H.; Mehta, R.L. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. 2015, 10, 1324–1331. [Google Scholar] [CrossRef]
- Wiersema, R.; Jukarainen, S.; Vaara, S.T.; Poukkanen, M.; Lakkisto, P.; Wong, H.; Linder, A.; Van Der Horst, I.C.C.; Pettilä, V. Two Subphenotypes of Septic Acute Kidney Injury Are Associated with Different 90-Day Mortality and Renal Recovery. Crit. Care 2020, 24, 150. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Priyanka, P.; Wang, S.; Smith, A.; Singbartl, K.; Palevsky, P.M.; Chawla, L.S.; Yealy, D.M.; Angus, D.C.; Kellum, J.A.; et al. Sepsis-Associated Acute Kidney Disease. Kidney Int. Rep. 2020, 5, 839–850. [Google Scholar] [CrossRef]
- Williams, B.; Zou, L.; Pittet, J.-F.; Chao, W. Sepsis-Induced Coagulopathy: A Comprehensive Narrative Review of Pathophysiology, Clinical Presentation, Diagnosis, and Management Strategies. Anesth. Analg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Vincent, J.-L.; Laterre, P.-F.; LaRosa, S.P.; Dhainaut, J.-F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Reinhart, K.; Opal, S.; Demeyer, I.; Doig, C.; Rodriguez, A.L.; Beale, R.; Svoboda, P.; Laterre, P.F.; Simon, S.; et al. Efficacy and Safety of Tifacogin (Recombinant Tissue Factor Pathway Inhibitor) in Severe Sepsis: A Randomized Controlled Trial. JAMA 2003, 290, 238. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




