Submitted:
25 September 2024
Posted:
29 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cancer Immunotherapy Landscape: Advances and Hurdles
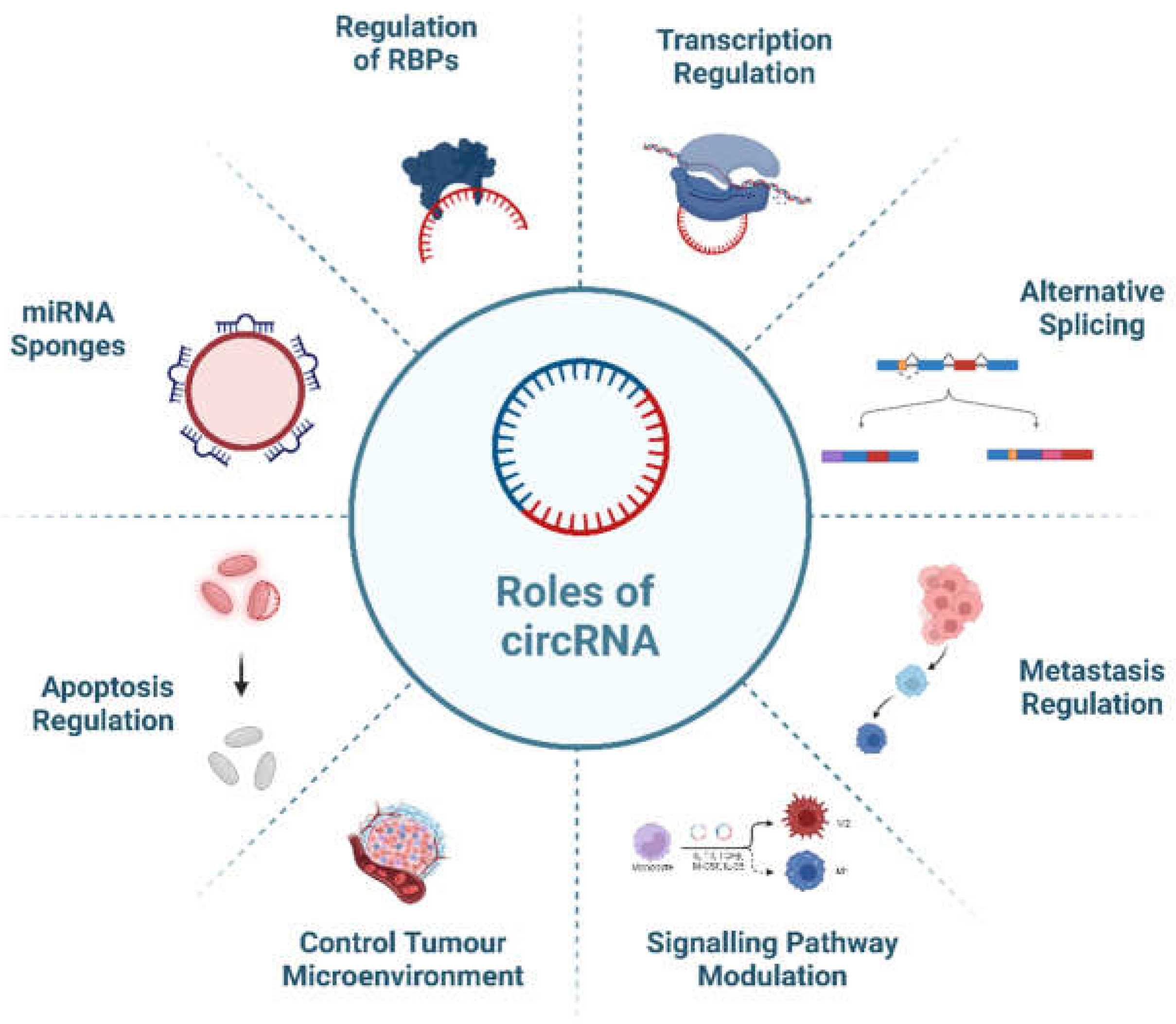
3. Role of Circ-RNAS in Cancer Progression and Therapeutic Resistance
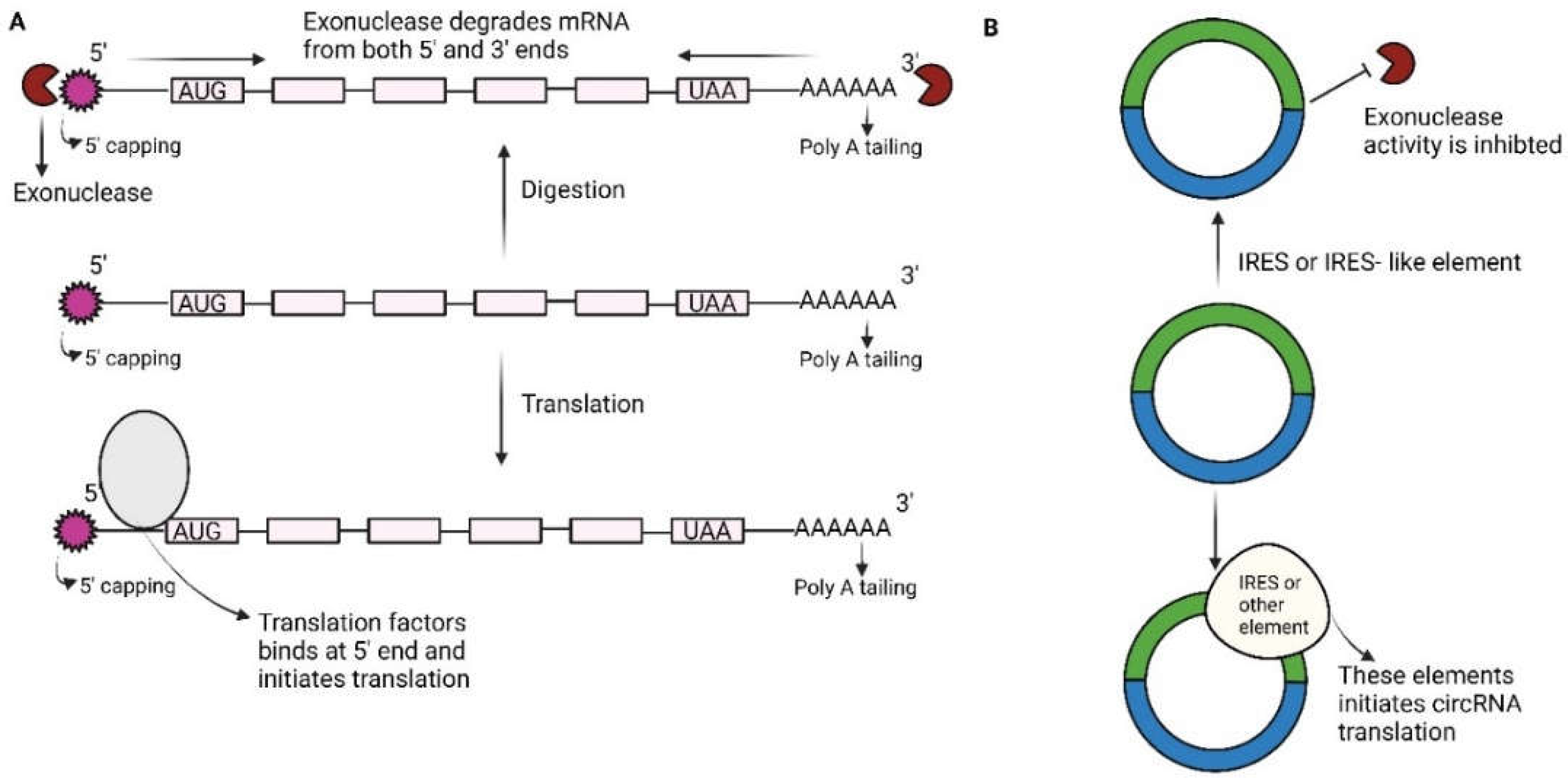
4. Unlocking the Power of Circular RNAS over Mrnas: Advantages in Cancer Vaccines
| Vaccines | Advantages | Disadvantages | References |
|---|---|---|---|
| SLP (Synthetic long peptide) Vaccine | Stable The long peptide can stimulate the response of CD4+ and CD8+ T cells |
Complex synthesis in vitro, high cost Weak anti-tumor immune response Potential adverse reactions. |
Chen et al., 2021b; Guo et al., 2018; Jou et al., 2021; Supabphol et al., 2021; Wei et al., 2021 |
| DNA Vaccine (Plasmid DNA encoding a new tumor antigen) |
Production process is simple, rapid, and low-cost Virus-like infection mechanism Allows the modification of coding antigens |
Risk of genomic integration | Chen et al., 2019b; Li et al., 2021; Supabphol et al., 2021; X. Yang et al., 2021b |
| mRNA Vaccine (mRNA transcribed in vitro encoding neoantigens) |
Production process is simple and rapid, and low-cost Translation without entering the nucleus No risk of genome integration Self-adjuvant effect. |
Poor stability Lack of effective transfection method for nuclease susceptibility. |
Barbier et al., 2022; Beck et al., 2021; Cafri et al., 2020; Chakraborty et al., 2021; Guo et al., 2018; Huang et al., 2021; Huff et al., 2022; Jain et al., 2021; Miao et al., 2021a; Qu et al., 2022b; Xu et al., 2020 |
| circRNA Vaccine (CircRNA encoding new tumor antigen constructed in vitro) | Good stability Simple and rapid production process with low-cost Translating without entering the nucleus High antigen expression efficiency No risk of genomic integration Self-adjuvant effect. |
Lack of large standardized production and purification methods Lack of effective transfection methods. |
Liu et al., 2019a; Prats et al., 2020; Wei et al., 2021; Zhao et al., 2022 |
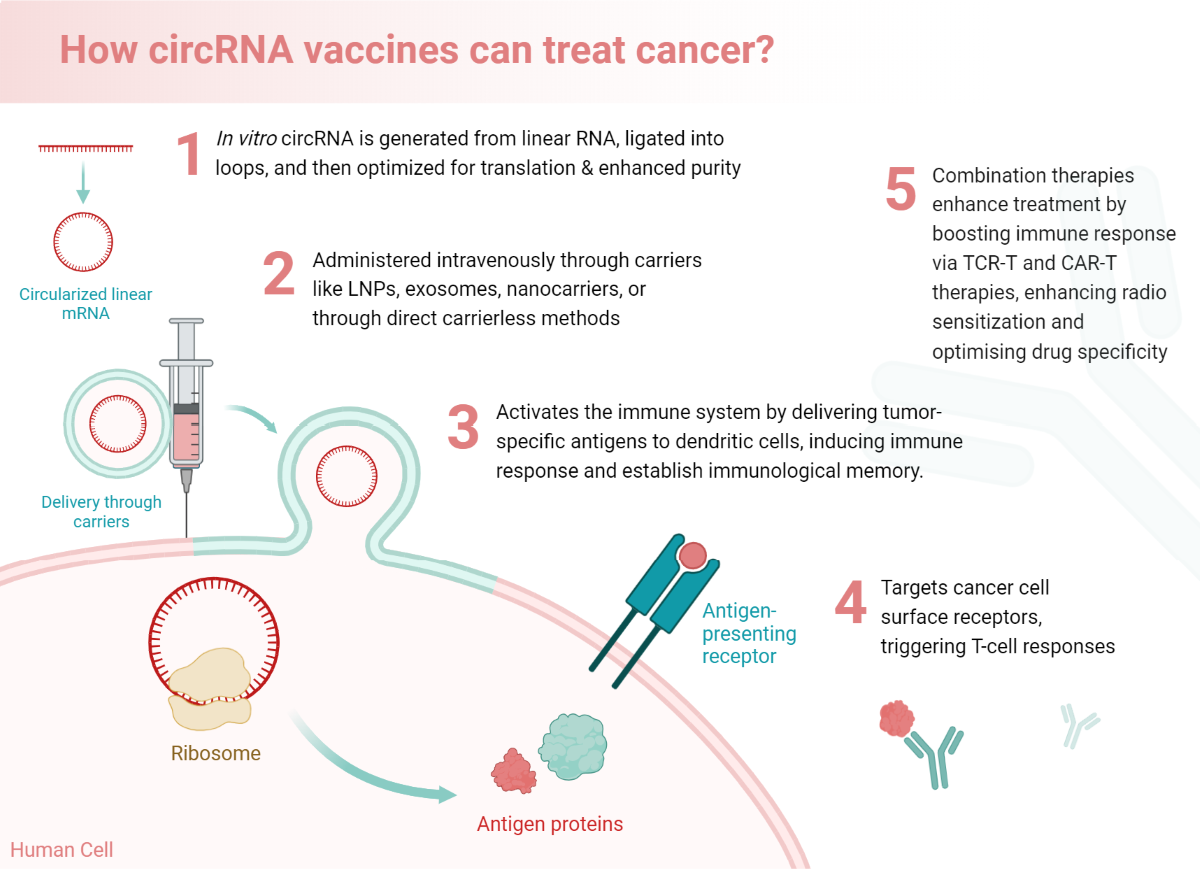
4. Exploring circRNA Vaccines in Cancer: Immunological Perspective
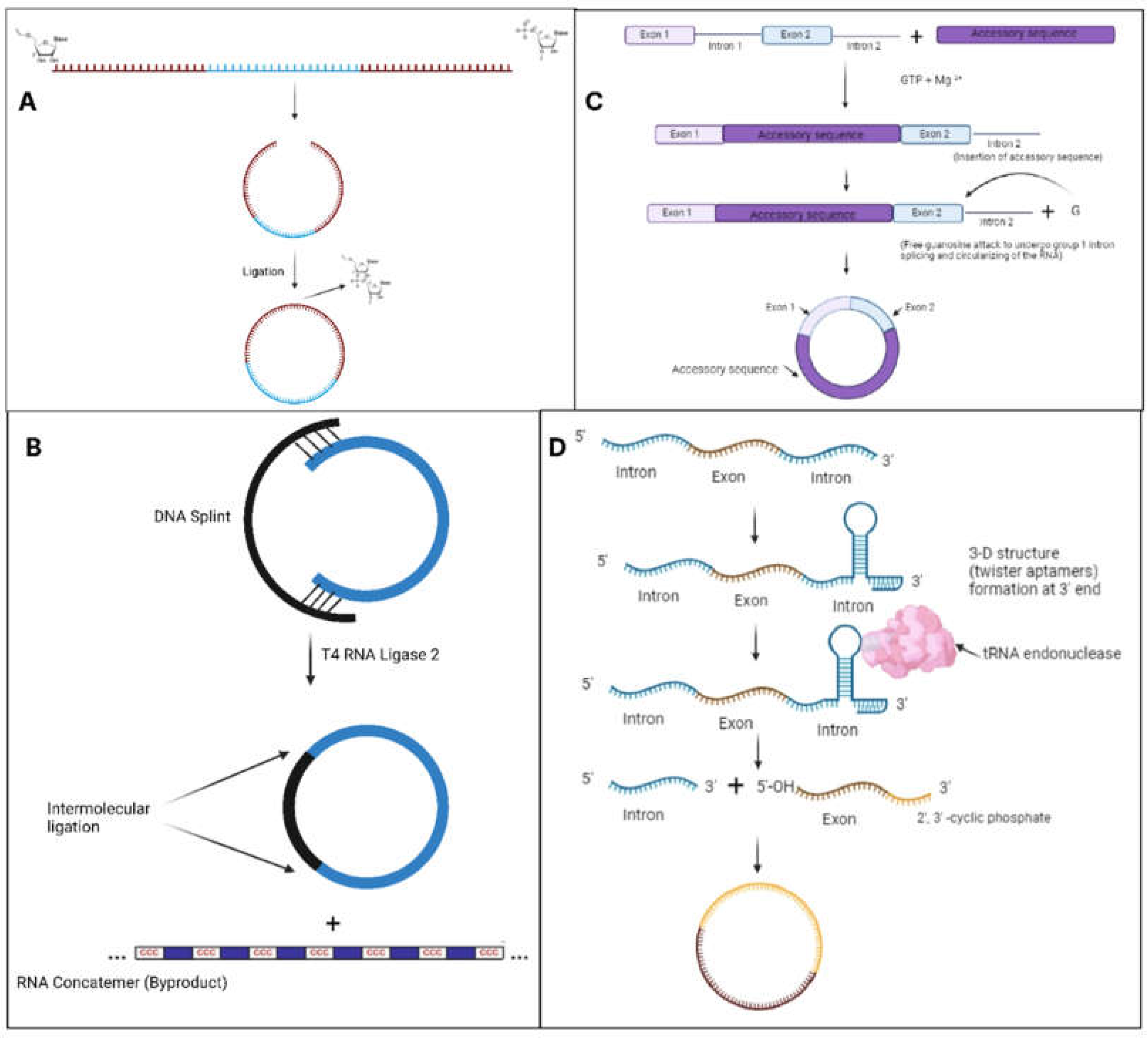
5. Innovations in Designing circRNA-Based Cancer Vaccines
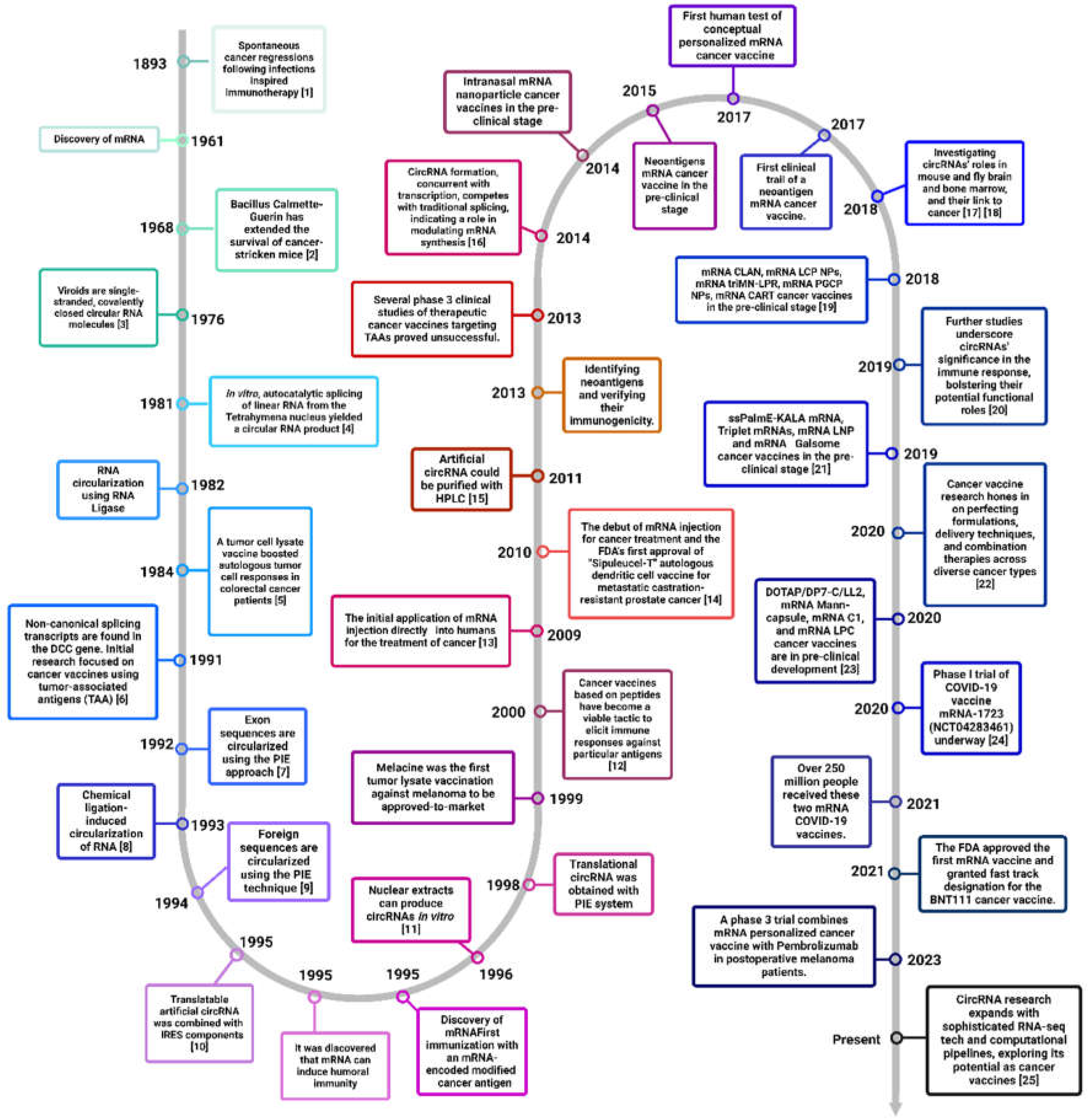
6. Translational Milestones: from Bench to Bedside with circRNA Vaccines
7. Emerging Trends and Challenges
7.1. Personalized Treatment
7.2. Challenges
| Challenges | Description |
| Detection and Quantification | The circular nature makes circRNAs elusive to accurate detection and quantification as this can be omitted by traditional RNA sequencing approaches or underestimated in terms of abundance (Dong et al., 2023; Kristensen et al., 2018; Nguyen et al., 2022; Szabo & Salzman, 2016). |
| Functional Annotation | It becomes difficult to understand the roles of circRNA because they may act as miRNA sponges, bind to RBPs, regulate gene transcription, and their mechanisms of action are diverse (Lee et al., 2022). |
| Design and Engineering | This is challenging since there will be a need to design circRNA constructs for experiments such as overexpression or knockdown which require inherent circularity necessitating specialized techniques such as antisense oligonucleotides or CRISPR-based strategies (Feng et al., 2023; Gao et al., 2022; Li et al., 2018; Obi & Chen, 2021; Szabo & Salzman, 2016). |
| Stability | CircRNAs possess varying stability across several different subcellular localizations (He et al., 2021; Kristensen et al., 2018; Ren et al., 2022; Wu et al., 2022). |
| Delivery systems | CircRNA-based constructs from specific cells or tissues require efficient delivery systems that include viral vectors or nanoparticles (Feng et al., 2023; He et al., 2021; Loan Young et al., 2023; Zhao et al., 2022). |
| Bioinformatics tools | Bioinformatics tools require circRNA sequencing data to differentiate between circular and linear RNA reads to determine back-splicing regions (Cheng et al., 2021; Feng et al., 2023; Miao et al., 2021; Niu et al., 2024). |
8. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldous, A. R., & Dong, J. Z. (2018). Personalized neoantigen vaccines: A new approach to cancer immunotherapy. Bioorganic & Medicinal Chemistry, 26(10), 2842–2849. [CrossRef]
- Amaya, L., Grigoryan, L., Li, Z., Lee, A., Wender, P. A., Pulendran, B., & Chang, H. Y. (2023). Circular RNA vaccine induces potent T-cell responses. Proceedings of the National Academy of Sciences, 120(20). [CrossRef]
- Ashwal-Fluss, R., Meyer, M., Pamudurti, N. R., Ivanov, A., Bartok, O., Hanan, M., Evantal, N., Memczak, S., Rajewsky, N., & Kadener, S. (2014). CircRNA biogenesis competes with pre-mRNA splicing. Molecular Cell, 56(1), 55–66. [CrossRef]
- Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R., & Anderson, D. G. (2022, May 9). The clinical progress of mRNA vaccines and immunotherapies. Nature Biotechnology, 40(6), 840–854. [CrossRef]
- Baxevanis, C. N., Fortis, S. P., Ardavanis, A., & Perez, S. A. (2020). Exploring essential issues for improving therapeutic cancer vaccine trial design. Cancers, 12(10), 2908. [CrossRef]
- Beck, J. D., Reidenbach, D., Salomon, N., & et al. (2021). mRNA therapeutics in cancer immunotherapy. Molecular Cancer, 20(69). [CrossRef]
- Cafri, G., Gartner, J. J., Zaks, T., Hopson, K., Levin, N., Paria, B. C., Parkhurst, M. R., Yossef, R., Lowery, F. J., Jafferji, M. S., Prickett, T. D., Goff, S. L., McGowan, C. T., Seitter, S., Shindorf, M. L., Parikh, A., Chatani, P. D., Robbins, P. F., & Rosenberg, S. A. (2020, October 5). mRNA vaccine–induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. Journal of Clinical Investigation, 130(11), 5976–5988. [CrossRef]
- Cardillo, F., Bonfim, M., Da Silva Vasconcelos Sousa, P., Mengel, J., Ribeiro Castello-Branco, L. R., & Pinho, R. T. (2021). Bacillus Calmette–Guérin Immunotherapy for Cancer. Vaccines, 9(5), 439. [CrossRef]
- Cech, T. R., Zaug, A. J., & Grabowski, P. J. (1981). In vitro splicing of the ribosomal RNA precursor of tetrahymena: Involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell, 27(3), 487–496. [CrossRef]
- Chakraborty, C., Sharma, A. R., Bhattacharya, M., & Lee, S. S. (2021, July 7). From COVID-19 to Cancer mRNA Vaccines: Moving from Bench to Clinic in the Vaccine Landscape. Frontiers in Immunology, 12. [CrossRef]
- Chen, C., & Sarnow, P. (1995). Initiation of Protein Synthesis by the Eukaryotic Translational Apparatus on Circular RNAs. Science, 268(5209), 415–417. [CrossRef]
- Chen, C., Chen, X., Tang, H., Jiang, Y., Wang, S., Zhang, X., Huang, T., Yuan, X., Wang, J., & Peng, L. (2022). Circular RNAs involve in immunity of digestive cancers From bench to bedside: a review. Frontiers in Immunology, 13. [CrossRef]
- Chen, F., Huang, C., Wu, Q., Jiang, L., Chen, S., & Chen, L. (2019a). Circular RNA expression profiles in plasma exosomes from early-stage lung adenocarcinoma and the potential biomarkers. Journal of Cellular Biochemistry, 121(3), 2525–2533. [CrossRef]
- Chen, F., Zou, Z., Du, J., Su, S., Shao, J., Meng, F., Yang, J., Xu, Q., Ding, N., Yang, Y., Liu, Q., Wang, Q., Sun, Z., Zhou, S., Du, S., Wei, J., & Liu, B. (2019b, April 8). Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. Journal of Clinical Investigation, 129(5), 2056–2070. [CrossRef]
- Chen, L. L. (2020a, May 4). The expanding regulatory mechanisms and cellular functions of circular RNAs. Nature Reviews Molecular Cell Biology, 21(8), 475–490. [CrossRef]
- Chen, Z., Zhang, S., Han, N., Jiang, J., Xu, Y., Ma, D., Lu, L., Guo, X., Qiu, M., Huang, Q., Wang, H., Mo, F., Chen, S., & Yang, L. (2021b, August 13). A Neoantigen-Based Peptide Vaccine for Patients with Advanced Pancreatic Cancer Refractory to Standard Treatment. Frontiers in Immunology, 12. [CrossRef]
- ClinicalTrials.gov. (n.d.). Phase 1, open-label, dose-ranging study of the safety and immunogenicity of 2019-nCoV vaccine (mRNA-1273) in healthy adults. Retrieved May 23, 2024, from https://clinicaltrials.gov/ct2/show/NCT04283461.
- Cong, J., Wang, X., Zheng, X., Wang, D., Fu, B., Sun, R., Tian, Z., & Wei, H. (2018, August). Dysfunction of Natural Killer Cells by FBP1-Induced Inhibition of Glycolysis during Lung Cancer Progression. Cell Metabolism, 28(2), 243-255.e5. [CrossRef]
- Derderian, C., Orunmuyi, A. T., Olapade-Olaopa, E. O., & Ogunwobi, O. O. (2019). PVT1 signaling is a mediator of cancer progression. Frontiers in Oncology, 9. [CrossRef]
- Dong, Y., Gao, Q., Chen, Y., Zhang, Z., Du, Y., Liu, Y., Zhang, G., Li, S., Wang, G., Chen, X., Liu, H., Han, L., & Ye, Y. (2023b). Identification of CircRNA signature associated with tumor immune infiltration to predict the therapeutic efficacy of immunotherapy. Nature communications, 14(1), 2540. [CrossRef]
- El Sharkawi, F. Z., Awad, M. S., Elagawy, W., Al Sawaf, H., & Taha, H. (2021). Circular RNAs 0064286 and 0000475: Potential Diagnostic Biomarkers in Hepatocellular Carcinoma. Asian Pacific Journal of Cancer Prevention, 22(9), 3039–3044. [CrossRef]
- Fan, Y.-N., Li, M., Luo, Y.-L., Chen, Q., Wang, L., Zhang, H.-B., Shen, S., Gu, Z., & Wang, J. (2018). Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomaterials Science, 6(11), 3009–3018. [CrossRef]
- Feng, X.-Y., Zhu, S.-X., Pu, K.-J., Huang, H.-J., Chen, Y.-Q., & Wang, W.-T. (2023). New insight into circRNAs: Characterization, strategies, and biomedical applications. Experimental Hematology & Oncology, 12(1), 91. [CrossRef]
- Garrido-Martin, E. M., Mellows, T. W. P., Clarke, J., Ganesan, A. P., Wood, O., Cazaly, A., Seumois, G., Chee, S. J., Alzetani, A., King, E. V., Hedrick, C. C., Thomas, G., Friedmann, P. S., Ottensmeier, C. H., Vijayanand, P., & Sanchez-Elsner, T. (2020, July). M1hottumor-associated macrophages boost tissue-resident memory T cells infiltration and survival in human lung cancer. Journal for ImmunoTherapy of Cancer, 8(2), e000778. [CrossRef]
- Ge, J., Wang, J., Xiong, F., Jiang, X., Zhu, K., Wang, Y., Mo, Y., Gong, Z., Zhang, S., He, Y., Li, X., Shi, L., Guo, C., Wang, F., Zhou, M., Xiang, B., Li, Y., Li, G., Xiong, W., & Zeng, Z. (2021, July 28). Epstein-–Barr Virus–Encoded Circular RNA CircBART2.2 Promotes Immune Escape of Nasopharyngeal Carcinoma by Regulating PD-L1. Cancer Research, 81(19), 5074–5088. [CrossRef]
- Guo, Y., Lei, K., & Tang, L. (2018, July 2). Neoantigen Vaccine Delivery for Personalized Anticancer Immunotherapy. Frontiers in Immunology, 9. [CrossRef]
- Haabeth, O. A. W., Blake, T. R., McKinlay, C. J., Waymouth, R. M., Wender, P. A., & Levy, R. (2018). mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proceedings of the National Academy of Sciences of the United States of America, 115(39). [CrossRef]
- Hassett, K. J., Benenato, K. E., Jacquinet, E., Lee, A., Woods, A., Yuzhakov, O., Himansu, S., Deterling, J., Geilich, B. M., Ketova, T., Mihai, C., Lynn, A., McFadyen, I., Moore, M. J., Senn, J. J., Stanton, M. G., Almarsson, Ö., Ciaramella, G., & Brito, L. A. (2019). Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Molecular Therapy. Nucleic Acids, 15, 1–11. [CrossRef]
- He, T., Xu, Y., Li, X., Wang, X., Li, J., Ou-Yang, D., Cheng, H., Li, H., Qin, J., Huang, Y., & Wang, H. (2023). A linear and circular dual-conformation noncoding RNA involved in oxidative stress tolerance in Bacillus altitudinis. Nature Communications, 14(1). [CrossRef]
- He, W., Zhang, X., Zou, Y., Li, J., Chang, L., He, Y., Jin, Q., & Ye, J. (2024). Effective synthesis of circRNA via a thermostable T7 RNA polymerase variant as the catalyst. Frontiers in Bioengineering and Biotechnology, 12. [CrossRef]
- Hewitt, S. L., Bai, A., Bailey, D., Ichikawa, K., Zielinski, J., Karp, R., Apte, A., Arnold, K., Zacharek, S. J., Iliou, M. S., Bhatt, K., Garnaas, M., Musenge, F., Davis, A., Khatwani, N., Su, S. V., MacLean, G., Farlow, S. J., Burke, K., & Frederick, J. P. (2019). Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Science Translational Medicine, 11(477). [CrossRef]
- Hiam-Galvez, K. J., Allen, B. M., & Spitzer, M. H. (2021). Systemic immunity in cancer. Nature Reviews. Cancer, 21(6), 345–359. [CrossRef]
- Hoover, H. C., Jr, Brandhorst, J. S., Peters, L. C., Surdyke, M. G., Takeshita, Y., Madariaga, J., Muenz, L. R., & Hanna, M. G., Jr. (1993). Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 11(3), 390–399. [CrossRef]
- Huang, Q., Zeng, J., & Yan, J. (2021, February). COVID-19 mRNA vaccines. Journal of Genetics and Genomics, 48(2), 107–114. [CrossRef]
- Huff, A. L., Jaffee, E. M., & Zaidi, N. (2022, March 15). Messenger RNA vaccines for cancer immunotherapy: progress promotes promise. Journal of Clinical Investigation, 132(6). [CrossRef]
- Islam, M. A., Xu, Y., Tao, W., Ubellacker, J. M., Lim, M., Aum, D., Lee, G. Y., Zhou, K., Zope, H., Yu, M., Cao, W., Oswald, J. T., Dinarvand, M., Mahmoudi, M., Langer, R., Kantoff, P. W., Farokhzad, O. C., Zetter, B. R., & Shi, J. (2018). Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nature Biomedical Engineering, 2(11), 850–864. [CrossRef]
- Jain, S., Venkataraman, A., Wechsler, M. E., & Peppas, N. A. (2021, December). Messenger RNA-based vaccines: Past, present, and future directions in the context of the COVID-19 pandemic. Advanced Drug Delivery Reviews, 179, 114000. [CrossRef]
- Ji, W., Qiu, C., Wang, M., Mao, N., Wu, S., & Dai, Y. (2018). Hsa_circ_0001649: A circular RNA and potential novel biomarker for colorectal cancer. Biochemical and Biophysical Research Communications, 497(1), 122–126. [CrossRef]
- Jin, H., Jin, X., Zhang, H., & Wang, W. (2017, March 10). Circular RNA hsa-circ-0016347 promotes proliferation, invasion, and metastasis of osteosarcoma cells. Oncotarget, 8(15), 25571–25581. [CrossRef]
- Jou, J., Harrington, K. J., Zocca, M. B., Ehrnrooth, E., & Cohen, E. E. (2021, February 1). The Changing Landscape of Therapeutic Cancer Vaccines—Novel Platforms and Neoantigen Identification. Clinical Cancer Research, 27(3), 689–703. [CrossRef]
- Kantoff, P. W., Higano, C. S., Shore, N. D., Berger, E. R., Small, E. J., Penson, D. F., Redfern, C. H., Ferrari, A. C., Dreicer, R., Sims, R. B., Xu, Y., Frohlich, M. W., & Schellhammer, P. F. (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England Journal of Medicine, 363(5), 411–422. [CrossRef]
- Karikó, K., Muramatsu, H., Ludwig, J., & Weissman, D. (2011). Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Research, 39(21), e142–e142. [CrossRef]
- Kazakov, S. A., Balatskaya, S. V., & Johnston, B. H. (2006). Ligation of the hairpin ribozyme in cis induced by freezing and dehydration. RNA, 12(3), 446–456. [CrossRef]
- Khansari, N. (2019). Neoantigen: A New Hope for Effective Cancer Immunotherapy. Vaccination Research – Open Journal, 4(1), 19–20. [CrossRef]
- Kleaveland, B., Shi, C. Y., Stefano, J., & Bartel, D. P. (2018). A network of noncoding regulatory RNAs acts in the mammalian brain. Cell, 174(2), 350-362.e17. [CrossRef]
- Kristensen, L. S., Hansen, T. B., Venø, M. T., & Kjems, J. (2018). Circular RNAs in cancer: Opportunities and challenges in the field. Oncogene, 37(5), 555–565. [CrossRef]
- Kumar, A., Weller, K. P., & Vilgelm, A. E. (2022). Personalized cancer immunotherapy. Engineering Technologies and Clinical Translation, 399–426. [CrossRef]
- Le Moignic, A., Malard, V., Benvegnu, T., Lemiègre, L., Berchel, M., Jaffrès, P.-A., Baillou, C., Delost, M., Macedo, R., Rochefort, J., Lescaille, G., Pichon, C., Lemoine, F. M., Midoux, P., & Mateo, V. (2018). Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. Journal of Controlled Release: Official Journal of the Controlled Release Society, 278, 110–121. [CrossRef]
- Lee, K. H., Kim, S., & Lee, S. (2022). Pros and cons of in vitro methods for circular RNA preparation. International Journal of Molecular Sciences, 23(21), 13247. [CrossRef]
- Legnini, I., Di Timoteo, G., Rossi, F., Morlando, M., Briganti, F., Sthandier, O., Fatica, A., Santini, T., Andronache, A., Wade, M., Laneve, P., Rajewsky, N., & Bozzoni, I. (2017). Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Molecular Cell, 66(1), 22-37. e9. [CrossRef]
- Lei, Z., Tian, Q., Teng, Q., Wurpel, J. N., Zeng, L., Pan, Y., & Chen, Z. (2023). Understanding and targeting resistance mechanisms in cancer. MedComm, 4(3). [CrossRef]
- Li, H., Peng, K., Yang, K., Ma, W., Qi, S., Yu, X., He, J., Lin, X., & Yu, G. (2022). Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics, 12(14), 6422–6436. [CrossRef]
- Li, J., Xu, Q., Huang, Z., Mao, N., Lin, Z., Cheng, L., Sun, B., & Wang, G. (2021a). CircRNAs: A new target for the diagnosis and treatment of digestive system neoplasms. Cell Death & Disease, 12(2), 1–13. [CrossRef]
- Li, W., Liu, J., Chen, M., Xu, J., & Zhu, D. (2020). Circular RNA in cancer development and immune regulation. Journal of Cellular and Molecular Medicine, 26(6), 1785–1798. [CrossRef]
- Lisiecka, U., & Kostro, K. (2016). Mechanisms of tumor escape from immune surveillance. Journal of Veterinary Research, 60(4), 453–460. [CrossRef]
- Liu, J., Song, S., Lin, S., Zhang, M., Du, Y., Zhang, D., Xu, W., & Wang, H. (2019a, June 14). Circ-SERPINE2 promotes the development of gastric carcinoma by sponging miR-375 and modulating YWHAZ. Cell Proliferation, 52(4). [CrossRef]
- Liu, L., Wang, Y., Miao, L., Liu, Q., Musetti, S., Li, J., & Huang, L. (2018). Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Molecular Therapy: The Journal of the American Society of Gene Therapy, 26(1), 45–55. [CrossRef]
- Liu, T., Ye, P., Ye, Y., Lu, S., & Han, B. (2020, January 19). Circular RNA hsa_circRNA_002178 silencing retards breast cancer progression via microRNA-328-3p-mediated inhibition of COL1A1. Journal of Cellular and Molecular Medicine, 24(3), 2189–2201. [CrossRef]
- Liu, X., Zhang, Y., Zhou, S., Dain, L., Mei, L., & Zhu, G. (2022, August). Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. Journal of Controlled Release, 348, 84–94. [CrossRef]
- Ma, Y., Wang, T., Zhang, X., Wang, P., & Long, F. (2024). The role of circular RNAs in regulating resistance to cancer immunotherapy: mechanisms and implications. Cell Death and Disease, 15(5). [CrossRef]
- Mai, Y., Guo, J., Zhao, Y., Ma, S., Hou, Y., & Yang, J. (2020). Intranasal delivery of cationicT liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cellular Immunology, 354(104143), 104143. [CrossRef]
- Mendes, B. B., Conniot, J., Avital, A., Yao, D., Jiang, X., Zhou, X., Sharf-Pauker, N., Xiao, Y., Adir, O., Liang, H., Shi, J., Schroeder, A., & Conde, J. (2022). Nanodelivery of nucleic acids. Nature Reviews Methods Primers, 2(1). [CrossRef]
- Miao, L., Zhang, Y., & Huang, L. (2021a, February 25). mRNA vaccine for cancer immunotherapy. Molecular Cancer, 20(1). [CrossRef]
- Mo, Y., Wang, Y., Wang, Y., Deng, X., Yan, Q., Fan, C., Zhang, S., Zhang, S., Gong, Z., Shi, L., Liao, Q., Guo, C., Li, Y., Li, G., Zeng, Z., Jiang, W., Xiong, W., & Xiang, B. (2022, October 5). Circular RNA circPVT1 promotes nasopharyngeal carcinoma metastasis via the β-TrCP/c-Myc/SRSF1 positive feedback loop. Molecular Cancer, 21(1). [CrossRef]
- Morse, M. A., Gwin, W. R., & Mitchell, D. A. (2021, January 29). Vaccine Therapies for Cancer: Then and Now. Targeted Oncology, 16(2), 121–152. [CrossRef]
- Nandakumar, J., & Shuman, S. (2004). How an RNA Ligase Discriminates RNA versus DNA Damage. Molecular Cell, 16(2), 211–221. [CrossRef]
- Nigro, J. M., Cho, K. R., Fearon, E. R., Kern, S. E., Ruppert, J. M., Oliner, J. D., Kinzler, K. W., & Vogelstein, B. (1991). Scrambled exons. Cell, 64(3), 607–613. [CrossRef]
- Niu, D., Wu, Y., & Lian, J. (2023). Circular RNA vaccine in disease prevention and treatment. Signal Transduction and Targeted Therapy, 8(1). [CrossRef]
- Obi, P., & Chen, Y. G. (2021). The design and synthesis of circular RNAs. Methods, 196, 85–103. [CrossRef]
- Pamudurti, N. R., Bartok, O., Jens, M., Ashwal-Fluss, R., Stottmeister, C., Ruhe, L., Hanan, M., Wyler, E., Perez-Hernandez, D., Ramberger, E., Shenzis, S., Samson, M., Dittmar, G., Landthaler, M., Chekulaeva, M., Rajewsky, N., & Kadener, S. (2017). Translation of CircRNAs. Molecular Cell, 66(1), 9-21. e7. [CrossRef]
- Pasman Z, Been MD & Garcia-Blanco MA (1996). Exon circularization in mammalian nuclear extracts. RNA (New York, N.Y.);2(6):603-10.
- Patop, I. L., & Kadener, S. (2018). CircRNAs in cancer. Current Opinion in Genetics & Development, 48, 121–127. [CrossRef]
- Petkovic, S., & Müller, S. (2015). RNA circularization strategies in vivo and in vitro. Nucleic Acids Research, 43(4), 2454–2465. [CrossRef]
- Piątkiewicz, P., Miłek, T., Bernat-Karpińska, M., Ohams, M., Czech, A., & Ciostek, P. (2013, March 2). The Dysfunction of NK Cells in Patients with Type 2 Diabetes and Colon Cancer. Archivum Immunologiae Et Therapiae Experimentalis, 61(3), 245–253. [CrossRef]
- Piwecka, M., Glažar, P., Hernandez-Miranda, L. R., Memczak, S., Wolf, S. A., Rybak-Wolf, A., Filipchyk, A., Klironomos, F., Cerda Jara, C. A., Fenske, P., Trimbuch, T., Zywitza, V., Plass, M., Schreyer, L., Ayoub, S., Kocks, C., Kühn, R., Rosenmund, C., Birchmeier, C., & Rajewsky, N. (2017). Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science (New York, N.Y.), 357(6357). [CrossRef]
- Poboży, K., Domańska, J., & Domański, P. (2023). Neoantigen therapeutic cancer vaccines: a promising approach to personalized immunotherapy. OncoReview. [CrossRef]
- Prats, A. C., David, F., Diallo, L. H., Roussel, E., Tatin, F., Garmy-Susini, B., & Lacazette, E. (2020, November 14). Circular RNA, the Key for Translation. International Journal of Molecular Sciences, 21(22), 8591. [CrossRef]
- Propper, D. J., & Balkwill, F. R. (2022). Harnessing cytokines and chemokines for cancer therapy. Nature Reviews. Clinical Oncology, 19(4), 237–253. [CrossRef]
- Puttaraju, M., & Been, M. (1992). Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Research, 20(20), 5357–5364. [CrossRef]
- Qu, H., Chen, M., Ge, J., Zhang, X., He, S., Xiong, F., Yan, Q., Zhang, S., Gong, Z., Guo, C., Wang, F., Zeng, Z., Li, X., Li, G., Xiong, W., & Wu, X. (2022a, January). A fluorescence strategy for circRNA quantification in tumor cells based on T7 nuclease-assisted cycling enzymatic amplification. Analytica Chimica Acta, 1189, 339210. [CrossRef]
- Qu, L., Yi, Z., Shen, Y., Lin, L., Chen, F., Xu, Y., Wu, Z., Tang, H., Zhang, X., Tian, F., Wang, C., Xiao, X., Dong, X., Guo, L., Lu, S., Yang, C., Tang, C., Yang, Y., Yu, W., . . . Wei, W. (2022b, May). Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell, 185(10), 1728-1744.e16. [CrossRef]
- Raskov, H., Orhan, A., Gaggar, S., & Gögenur, I. (2021). Cancer-Associated fibroblasts and Tumor-Associated macrophages in cancer and cancer immunotherapy. Frontiers in Oncology, 11. [CrossRef]
- Ren, D., Hua, Y., Yu, B., Ye, X., He, Z., Li, C., Wang, J., Mo, Y., Wei, X., Chen, Y., Zhou, Y., Liao, Q., Wang, H., Xiang, B., Zhou, M., Li, X., Li, G., Li, Y., Zeng, Z., & Xiong, W. (2020). Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Molecular Cancer, 19(1). [CrossRef]
- Romero, P., & Coukos, G. (2014). Successful engineering cancer immunotherapy. European Journal of Immunology, 44(2), 318–320. [CrossRef]
- Rubio, D. M., Schoenbaum, E. E., Lee, L. S., Schteingart, D. E., Marantz, P. R., Anderson, K. E., Platt, L. D., Baez, A., & Esposito, K. (2010). Defining Translational Research: Implications for Training. Academic Medicine, 85(3), 470–475. [CrossRef]
- Sahin, U., & Türeci, Z. (2018). Personalized vaccines for cancer immunotherapy. Science, 359(6382), 1355–1360. [CrossRef]
- Sanger, H. L., Klotz, G., Riesner, D., Gross, H. J., & Kleinschmidt, A. K. (1976). Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proceedings of the National Academy of Sciences of the United States of America, 73(11), 3852–3856. [CrossRef]
- Schindewolf, C., Braun, S., & Domdey, H. (1996). In vitro Generation of a Circular Exon from a Linear Pre-mRNA Transcript. Nucleic Acids Research, 24(7), 1260–1266. [CrossRef]
- Sebastian, M., Schröder, A., Scheel, B. et al. (2019). A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol Immunother, 68, 799–812. [CrossRef]
- Shen, T., Cheng, X., Liu, X., Xia, C., Zhang, H., Pan, D., Zhang, X., & Li, Y. (2019, October 14). Circ_0026344 restrains metastasis of human colorectal cancer cells via miR-183. Artificial Cells, Nanomedicine, and Biotechnology, 47(1), 4038–4045. [CrossRef]
- Son, S., Nam, J., Zenkov, I., Ochyl, L. J., Xu, Y., Scheetz, L., Shi, J., Farokhzad, O. C., & Moon, J. J. (2020). Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Letters, 20(3), 1499–1509. [CrossRef]
- Španielová, H., & Brdička, R. (2023). Oncolytic viruses and cancer treatment. Klinická Onkologie, 36(1). [CrossRef]
- Su, M., Xiao, Y., Ma, J., Tang, Y., Tian, B., Zhang, Y., Xu, L., Wu, Z., Yang, D., Zhou, Y., Wang, H., Liao, Q., & Wang, W. (2019). Circular RNAs in Cancer: emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Molecular Cancer, 18(1). [CrossRef]
- Sun, C., Sun, H. Y., Xiao, W. H., Zhang, C., & Tian, Z. G. (2015, June 15). Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacologica Sinica, 36(10), 1191–1199. [CrossRef]
- Supabphol, S., Li, L., Goedegebuure, S. P., & Gillanders, W. E. (2021, March 31). Neoantigen vaccine platforms in clinical development: understanding the future of personalized immunotherapy. Expert Opinion on Investigational Drugs, 30(5), 529–541. [CrossRef]
- Tateshita, N., Miura, N., Tanaka, H., Masuda, T., Ohtsuki, S., Tange, K., Nakai, Y., Yoshioka, H., & Akita, H. (2019). Development of a lipoplex-type mRNA carrier composed of an ionizable lipid with a vitamin E scaffold and the KALA peptide for use as an ex vivo dendritic cell-based cancer vaccine. Journal of Controlled Release: Official Journal of the Controlled Release Society, 310, 36–46. [CrossRef]
- Verbeke, R., Lentacker, I., Breckpot, K., Janssens, J., Van Calenbergh, S., De Smedt, S. C., & Dewitte, H. (2019). Broadening the message: A nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano. [CrossRef]
- Viollet, S., Fuchs, R. T., Munafo, D. B., Zhuang, F., & Robb, G. B. (2011). T4 RNA Ligase 2 truncated active site mutants: improved tools for RNA analysis. BMC Biotechnology, 11(1). [CrossRef]
- Wang, B., Yin, H., Zhang, Y., Zhao, Q., Wang, H., Wei, J., Meng, L., Xin, Y., & Jiang, X. (2023). Overcoming acquired resistance to cancer immune checkpoint therapy: potential strategies based on molecular mechanisms. Cell & Bioscience, 13(1). [CrossRef]
- Wang, W. (2015a). NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Frontiers in Immunology, 6. [CrossRef]
- Wei, G., Zhu, J., Hu, H. B., & Liu, J. Q. (2021, March). Circular RNAs: Promising biomarkers for cancer diagnosis and prognosis. Gene, 771, 145365. [CrossRef]
- Wesselhoeft, R. A., Kowalski, P. S., & Anderson, D. G. (2018). Engineering circular RNA for potent and stable translation in eukaryotic cells. Nature Communications, 9(1). [CrossRef]
- Wesselhoeft, R. A., Kowalski, P. S., Parker-Hale, F. C., Huang, Y., Bisaria, N., & Anderson, D. G. (2019, May). RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In vivo. Molecular Cell, 74(3), 508-520.e4. [CrossRef]
- Wu, C. J. (2018). Abstract IA11: Developing and improving personalized neoantigen-targeting cancer vaccines. Cancer Immunology Research, 6(9_Supplement), IA11. [CrossRef]
- Xie, J., Ye, F., Deng, X., Tang, Y., Liang, J.-Y., Huang, X., Sun, Y., Tang, H., Lei, J., Zheng, S., & Zou, Y. (2023). Circular RNA: A promising new star of vaccine. Journal of Translational Internal Medicine, 11(4), 372–381. [CrossRef]
- Xin, Y., Huang, M., Guo, W., Huang, Q., Zhang, L. Z., & Jiang, G. (2017). Nano-based delivery of RNAi in cancer therapy. Molecular Cancer, 16(1). [CrossRef]
- Xu, S., Yang, K., Li, R., & Zhang, L. (2020, September 9). mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection. International Journal of Molecular Sciences, 21(18), 6582. [CrossRef]
- Yang, H., Wang, W., Romano, K. A., Gu, M., Sanidad, K. Z., Kim, D., Yang, J., Schmidt, B., Panigrahy, D., Pei, R., Martin, D. A., Ozay, E. I., Wang, Y., Song, M., Bolling, B. W., Xiao, H., Minter, L. M., Yang, G. Y., Liu, Z., . . . Zhang, G. (2018). A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Science Translational Medicine, 10(443). [CrossRef]
- Yang, Q., Li, F., He, A. T., & Yang, B. B. (2021a, May). Circular RNAs: Expression, localization, and therapeutic potentials. Molecular Therapy, 29(5), 1683–1702. [CrossRef]
- Yang, X., Fan, J., Wu, Y., Ma, Z., Huang, J., Zhang, Y., Zhou, Z., Mo, F., Liu, X., Yuan, H., Xu, Y., Pan, L., & Chen, S. (2021b, October). Synthetic multiepitope neoantigen DNA vaccine for personalized cancer immunotherapy. Nanomedicine: Nanotechnology, Biology and Medicine, 37, 102443. [CrossRef]
- Yao, T., Chen, Q., Shao, Z., Song, Z., Fu, L., & Xiao, B. (2018). Circular RNA 0068669 as a new biomarker for hepatocellular carcinoma metastasis. Journal of Clinical Laboratory Analysis, 32(8). [CrossRef]
- Yuan, X., Diao, J., Du, A., Wen, S., Zhou, L., & Pan, Y. (2020). Circular RNA expression profiles and features in NAFLD mice: a study using RNA-seq data. Journal of Translational Medicine, 18(1). [CrossRef]
- Yue, S., Li, Y., Qiao, Z., Song, W., & Bi, S. (2021, November). Rolling Circle Replication for Biosensing, Bioimaging, and Biomedicine. Trends in Biotechnology, 39(11), 1160–1172. [CrossRef]
- Zhang, P., Gao, C., Huang, X., Lu, J., Guo, X., Shi, G., Cai, J., & Ke, A. (2020a). Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Molecular Cancer, 19(1). [CrossRef]
- Zhang, Q., Wang, W., Zhou, Q., Chen, C., Yuan, W., Liu, J., Li, X., & Sun, Z. (2020b, January 23). Roles of circRNAs in the tumour microenvironment. Molecular Cancer, 19(1). [CrossRef]
- Zhang, Z. G., Buller, B., & Chopp, M. (2019). Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nature Reviews Neurology, 15(4), 193–203. [CrossRef]
- Zhang, Z., Lu, M., Qin, Y., Gao, W., Tao, L., Su, W., & Zhong, J. (2021, April 16). Neoantigen: A New Breakthrough in Tumor Immunotherapy. Frontiers in Immunology, 12. [CrossRef]
- Zhao, X., Zhong, Y., Wang, X., Shen, J., & An, W. (2022). Advances in Circular RNA and Its Applications. International Journal of Medical Sciences, 19(6), 975–985. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





