1. Introduction
Prostate cancer (PC) is the second most commonly diagnosed cancer in men with the incidence varying worldwide [
1]. Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein (glutamate carboxypeptidase II or N-acetyl-L-aspartyl-L-glutamate peptidase I). Physiological PSMA expression is detected in salivary and lacrimal glands, normal prostate epithelium, sympathetic ganglia, duodenum and colon, and the proximal tubules of the kidneys [
2].
PSMA-PET/CT is an advanced imaging modality that targets prostate cancer cells. The antigen, highly expressed in primary and metastatic prostate cancer, is also detected in various other tumors and their metastases due to neovascularization. Interestingly, uptake can occur in several benign granulomatous and inflammatory diseases as well [
3]. The expression levels in prostate cancer are elevated by approximately 100–1000 times compared to normal tissue, though the precise mechanism behind this overexpression is not fully understood [
4].
To plan the most effective treatment, it is essential to accurately assess both the local and systemic staging of newly diagnosed prostate cancer (PCa). Risk stratification is based on several pre-treatment factors, including findings from a digital rectal examination (DRE), prostate-specific antigen (PSA) levels, and the Gleason Score (GS) from prostate biopsy [
5]. Current guidelines recommend using MRI, computed tomography (CT), and bone scintigraphy for PCa staging to inform treatment decisions. Conventional imaging modalities, such as BS and CT, exhibit limited accuracy in identifying metastases to lymph nodes and bones, particularly among patients with low PSA levels. Consequently, the limitations of these imaging tools can lead to inaccurate assessment, potentially resulting in under-staging and under-treatment [6,7].
In the last 5 years, Ga-68 PSMA-PET/CT has become a revolutionary imaging technique for detecting PC relapse. Numerous studies have consistently illustrated that PSMA exhibits superior sensitivity and specificity compared to traditional approaches or choline PET, particularly in identifying tumor recurrence, especially in patients with low PSA levels (<1.0 ng/mL) [
8]. While promising results suggest a significant clinical impact in altering the therapeutic approach, based on PSMA PET/CT evaluation of BCR, demonstrating improvement in long-term outcomes is crucial to validate the clinical utility of this transformative molecular imaging technique [
9].
The aim of the present study was to assess the intensity of 68Ga-PSMA uptake in the primary tumor in patients with biopsy-proven treatment-naive PC, and to determine whether a correlation exists between 68Ga-PSMA accumulation in the primary tumor, and the Gleason score and/or PSA concentration. Moreover, we compared preoperative histopathology report with definitive pathological report after robot-assisted radical prostatectomy (RARP).
2. Material and Methods
2.1. Patients
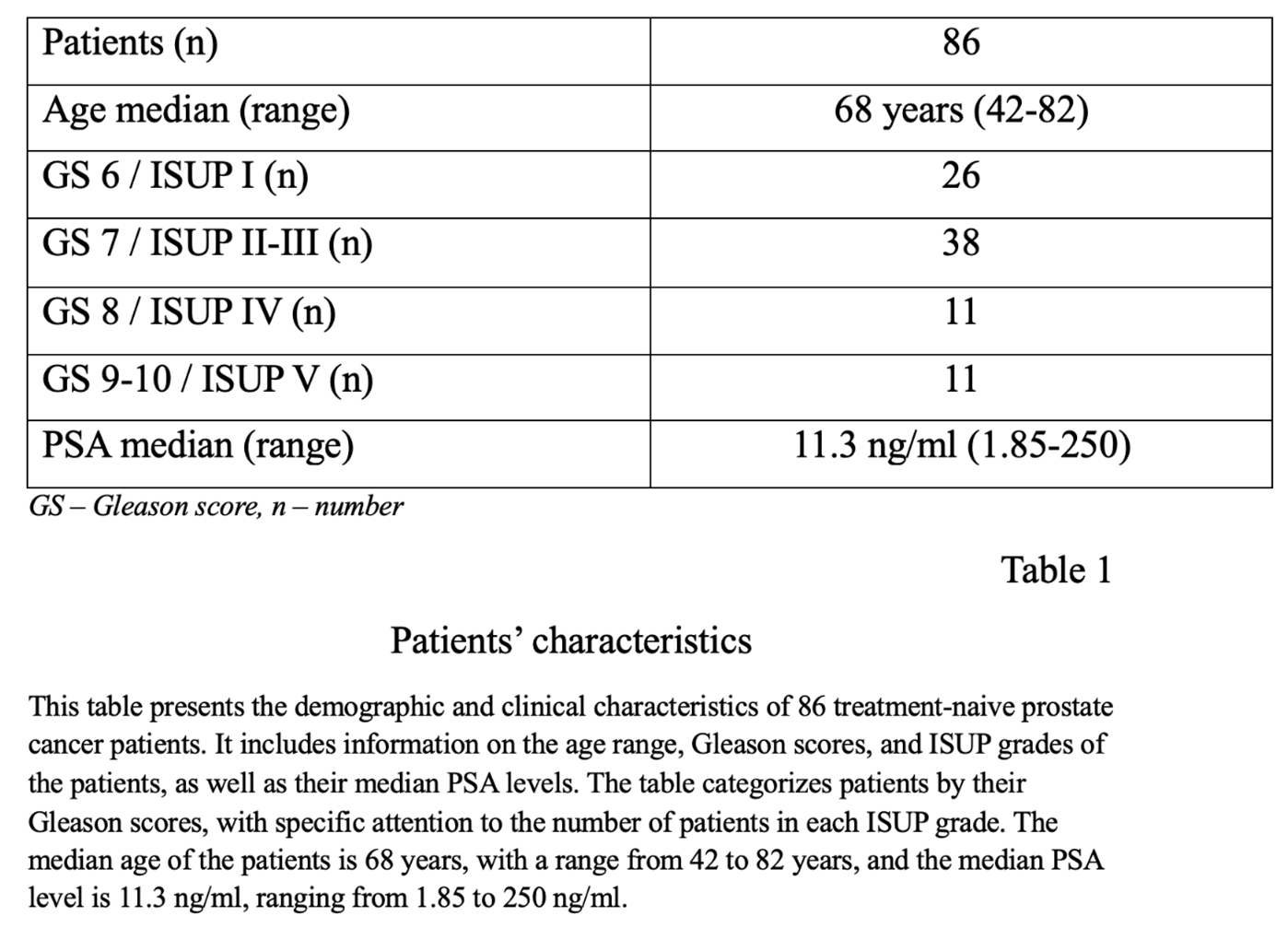
We retrospectively evaluated the files of 86 patient who had the 68Ga-PSMA PET/CT imaging for the purpose of PCa staging and 40 patient who underwent RARP. All patients had PCa diagnosed based on transrectal ultrasound-guided biopsy. Furthermore, all patients were subjected to initial biopsy and final postoperative specimen analysis. In addition to the Gleason scoring system, grade groups were reported according to the 2014 International Society of Urological Pathology 19 Consensus, which was later on adopted by the WHO for the 2016 edition of Pathology and Genetics: grade group 1 (GS ≤6), grade group 2 (GS 3+4=7), grade group 3 (GS 4+3=7), grade group 4 (GS 4+4=8, 3+5=8, 5+3=8), and grade group 5 (GS 9–10). For analytic purpose, grade groups are used to underline the clinical significance of differentiating between GS 3+4 and 4+3. All patients had a PSA concentration assessed within maximum of 28 days before a 68Ga-PSMA PET/CT scan. The study was carried out in accordance with the Helsinki Declaration and its later amendments. Written consent was obtained from all patients in accordance with our institution rules.
2.2. Image Acquisition
After the preparation and quality control of the radiotracer, all patients received an activity dose of 2 MBq per kilogram of body weight of 68Ga-PSMA based on the radiolabeling yield. Following the radiotracer injection, patients were administered 20 mg of Furosemide.
Whole-body imaging was performed 50 minutes post-injection of the radiotracer using one of two integrated PET/CT scanners: Discovery PET-CT 680 (Gorzów Wielkopolski, Poland). Patients were positioned supine on the scanner table, and a CT transmission scan was conducted without intravenous contrast enhancement. Following this, PET emission scanning was performed with a duration of 3 minutes per bed position, covering the same transverse field of view in the caudocranial direction. CT transmission images were used for attenuation correction, and image reconstruction was done using an iterative method. SUV values are related to lean body mass.
2.3. Image Analysis
PET images were reviewed using Advantage Workstation Server ver. 3.2 ext 1.2. Region of interests were drawn manually around the prostate gland, avoiding the bladder activity, to calculate the maximum SUV (SUVmax) values. A segmental analysis of the prostate gland was not carried out. The SUVmax value from the whole prostate gland was assumed to be the highest site of PSMA expression and recorded for further evaluation.
2.4. Statistical Analysis
All results were expressed as median and mean ± standard deviation. Pearson’s correlation test was used to assess the correlation between different parameters. Grade group comparisons were conducted using the chi-square test. Sensitivity and specificity were calculated through receiver operating characteristic (ROC) curve analysis. All statistical significance tests were two-tailed, with a P value of less than 0.05 considered significant.
3. Results
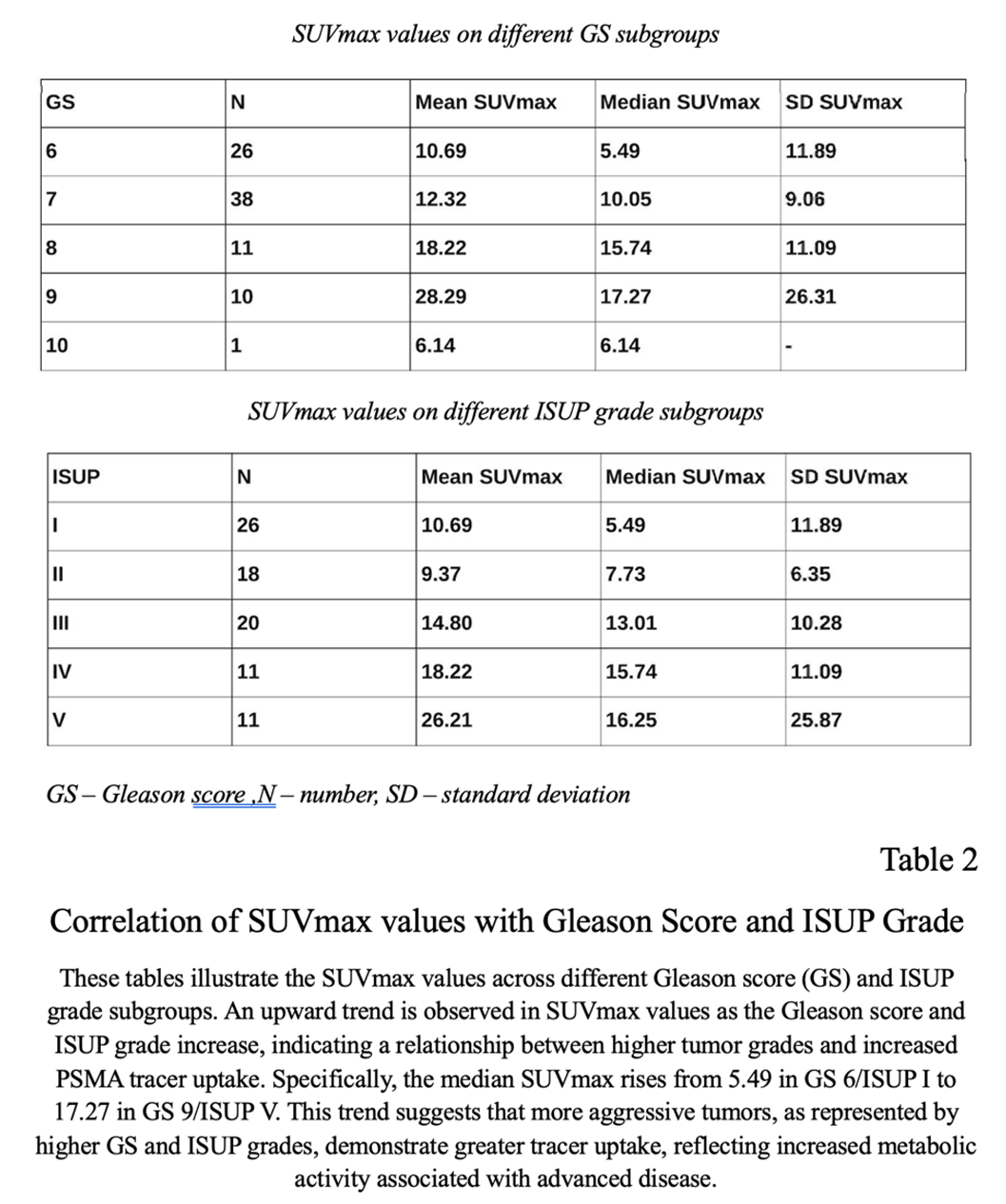
A total of 86 male patients were included, with median age of 68 years (range 42 to 82 years) (Table 1). Representative 68Ga-PSMA PET/CT fusion images for each grade group are presented in Figure 1. PSA median concentration was 11.3 ng/ml (range 2 to 250 ng/ml). No correlation was found between PSA values and tumor grade groups (Pearson's ρ = 0.24, p = 0.027). However, there was a moderately positive correlation between SUVmax and tumor grade group (Pearson's ρ = 0.34, p < 0.001) (Figure 1).
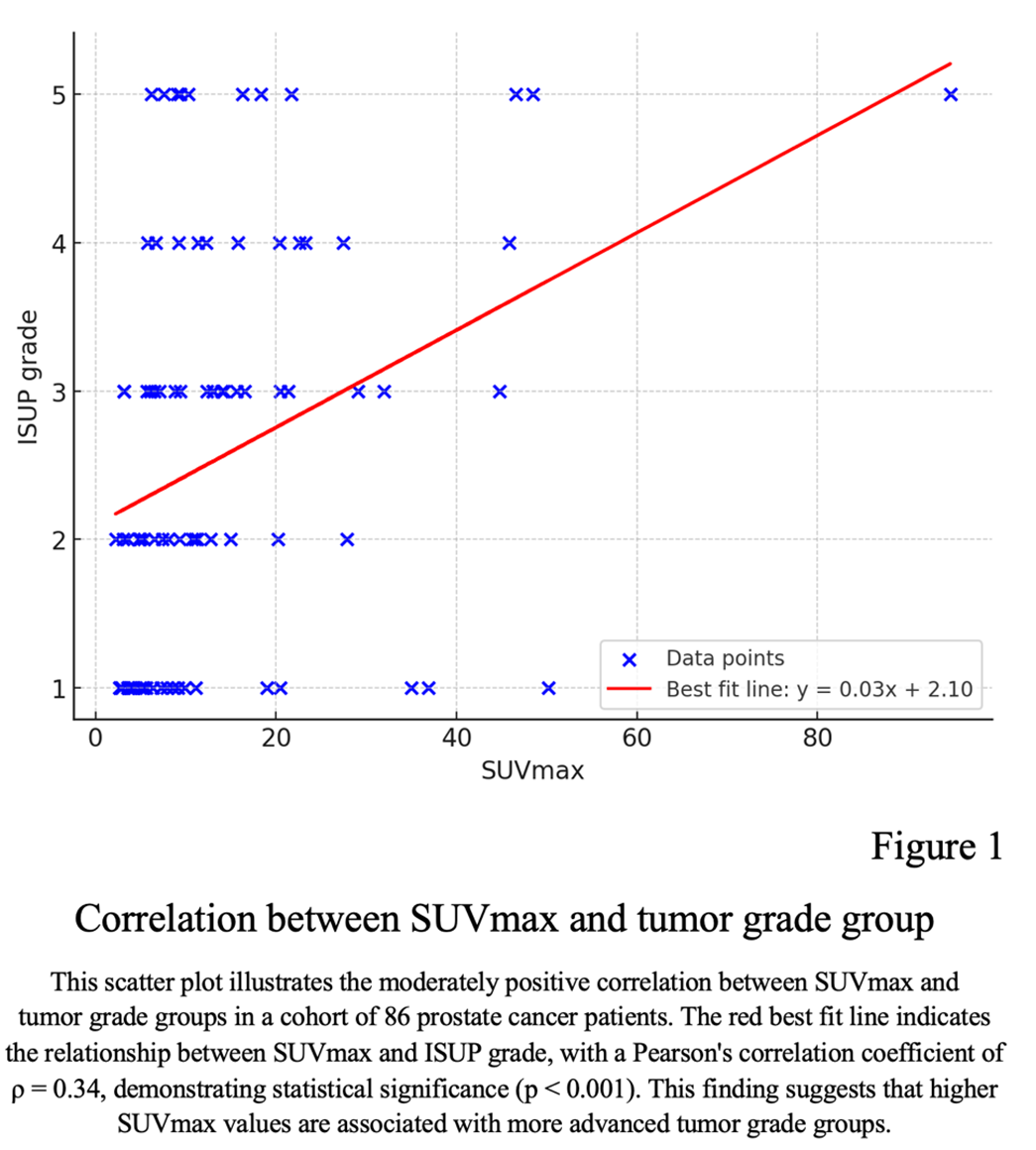
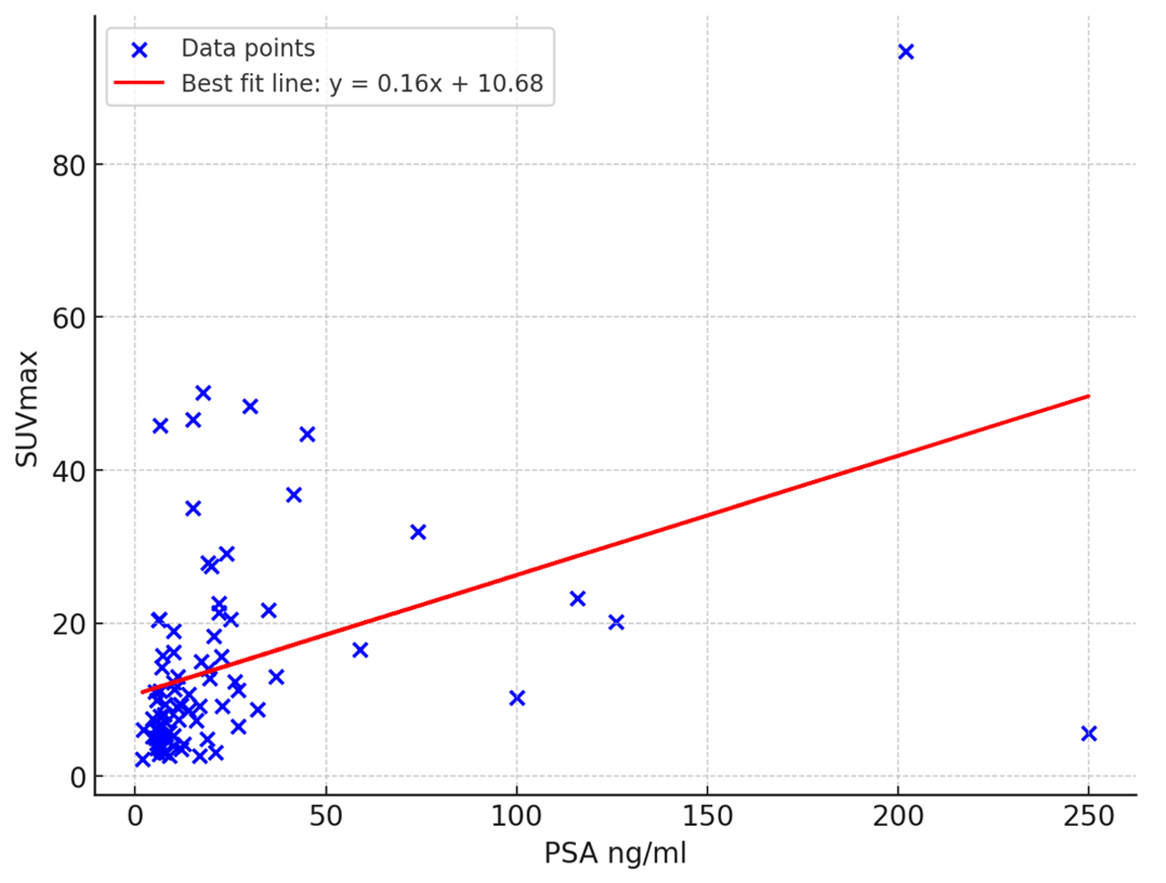
The Gleason score among patients ranged from 6-10. Patients presented with a median PSA concentration of 11,3 ng/ml. The median SUVmax among all tumors was 9,33 (range: 2.20 – 94.72).
There are moderately positive correlation (Pearson’s ρ=0.42 , p <0.001) between PSA concentration and SUVmax. (Figure 2)
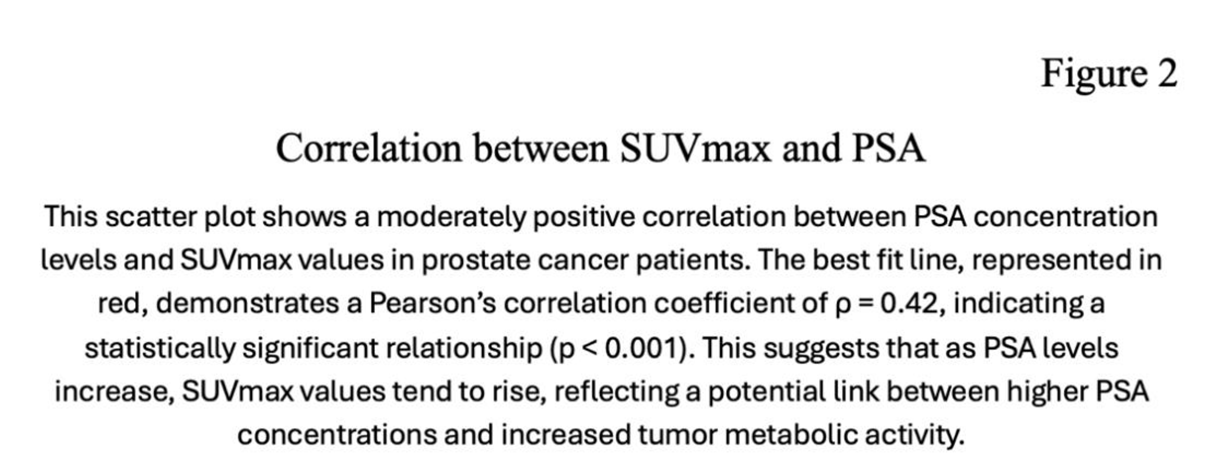
68Ga PSMA uptake in the primary tumors was higher in patients with PSA levels >10 ng/ml than those with PSA values <10 ng/ml, exhibiting a median SUVmax of 12.98 vs. 6.09 respectively, and resulting in a statistically difference (p<0.001).
Combining GS and tumor-related tracer (PSMA) uptake revealed an upward trend of elevation the SUVmax in higher GS/ISUP grade: GS 6/ISUP I (SUVmax = 5.49), GS 7a/ISUP II(SUVmax = 7.73), GS 7b /ISUP III(SUVmax = 13.01), GS 8/ISUP IV(SUVmax = 15.74), GS 9/ISUP 5 (SUVmax = 17.27) (Table 2). The same trend was found between the SUVmax and ISUP grade.
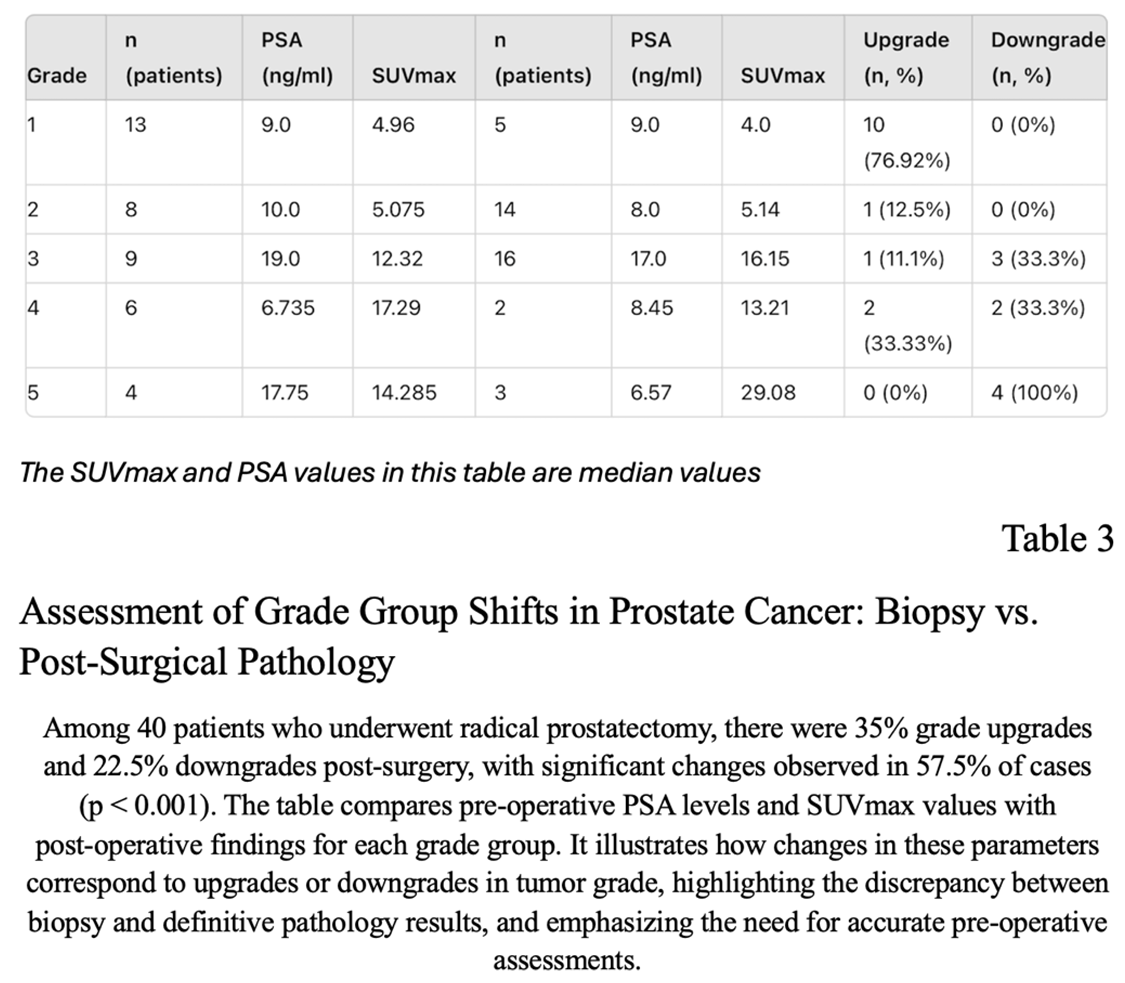
In the group of 86 patients we had 40 who underwent RARP.There were 13 (32,5%) patients in grade group 1, 8 (20%) patients in grade group 2, 9 (22,5%) patients in grade group 3, 6 (15%) patients in grade group 4 and 4 (10%) patients in grade group 5 according to pre-operative biopsy reports. In terms of ISUP grade, there were 35% upgrade and 22,5% downgrade following radical prostatectomy. When we compared the pathology reports of prostatectomy specimens with the biopsy results, we observed 57,5% changes in grade groups, which was significant (P<0.001). There were 1 level upgrade of grade groups in 7 patients, 2 level upgrade in 6 patients, 3 level upgrade in one patient,. We also observed 1 level downgrade in 5 patients and 2 level downgrade in 4 patients(Table 3)
Following radical prostatectomy, 27,5% (n=11) of patients moved from a low-risk level (grade groups 1+2) to a high-risk level (grade groups 3+4+5) and 7,5% (n=3) of patients moved from a high-risk level to a low-risk level. An SUVmax cut-off of 5.64 was seen in 4 out of 11 patients (36.36%) who transitioned to higher-risk groups, indicating a potential pattern rather than a predictive threshold (Figure 3).

In addition, the SUVmax values for patients in whom radical prostatectomy was performed were compared to definitive postoperative histology results (n = 40), which didn’t reveal a statistically significant correlation (p<0.289) between the median SUVmax and GS: a median SUVmax of 6.51 in the group with GS of 6 and 7 (range 2.2-50.15) and a median SUVmax of 14.19 in GS 8-9 tumors (range 6.69-45.84).
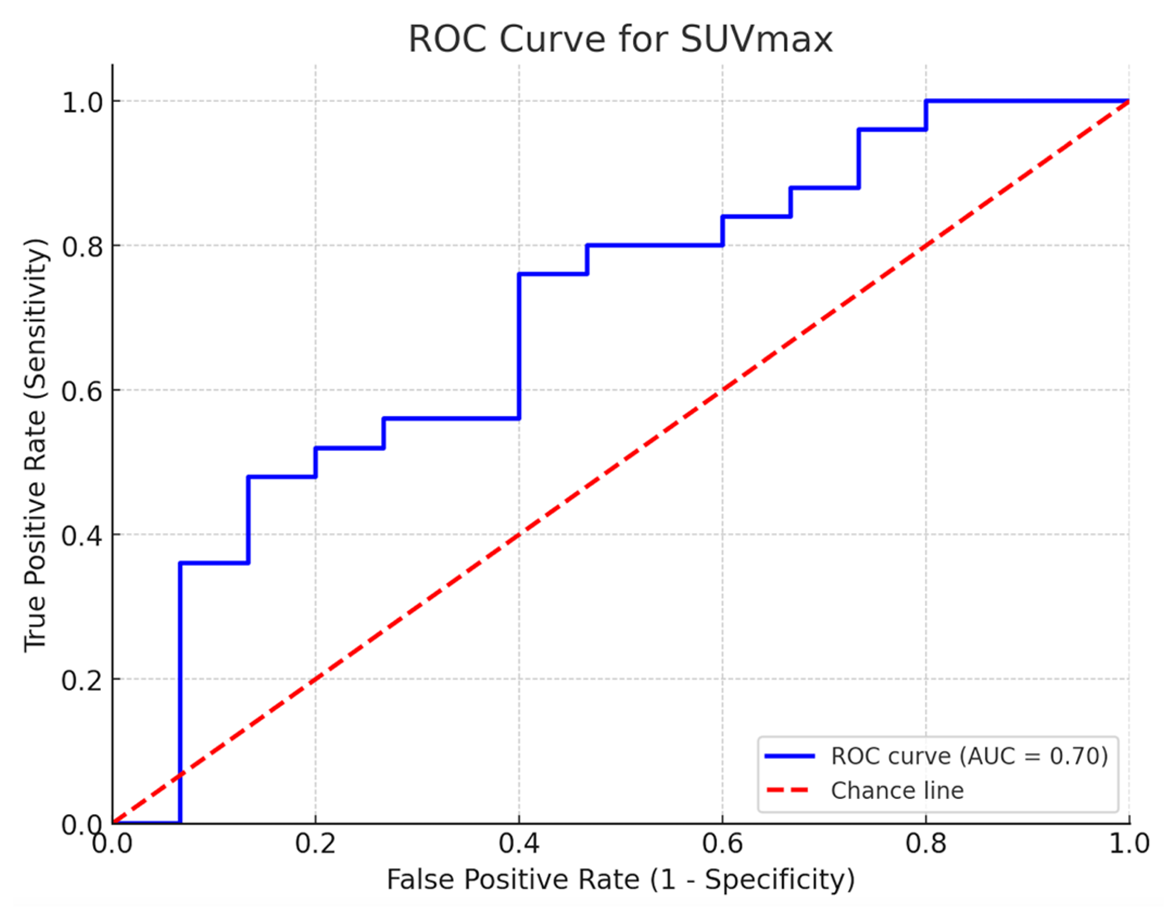
Receiver operation characteristic curve analysis of SUVmax (area under curve=0.82, 95% CI: 0.70–0.94) showed a cut-off value of 5.64, yielding a sensitivity and a specificity of 76% and 60%, respectively.
4. Discussion
The primary objective of our retrospective study was to assess whether a correlation exists between the intensity of 68Ga PSMA uptake in the primary prostate cancer patients and their Gleason score (GS) and/or ISUP grade. Additionally, we compared the histopathology findings from preoperative biopsies with definitive pathology results in patients who underwent radical prostatectomy (RP), aiming to determine if the SUVmax value can predict a higher risk in definitive pathology than initially indicated by the biopsy.
Prostate cancer cells commonly exhibit elevated PSMA expression, allowing for targeted PET imaging using PSMA ligands. Among these, Ga68 PSMA has shown a strong affinity for PSMA [
10]. Benign prostatic epithelium also shows elevated PSMA expression, as demonstrated by immunohistochemical studies, though the expression is less intense compared to prostate cancer cells [11,12]
In general, adequate tracer uptake in the primary tumor is essential for the visualization and detection of metastases through PET imaging. The use of 68Ga PSMA PET/CT for staging should be reserved for prostate cancer patients with a high probability of significant PET positivity in the primary tumor. It would be valuable to have predictive parameters at the initial diagnosis of prostate cancer to determine whether the primary tumor is suitable for imaging with 68Ga PSMA PET/CT.
This issue was addressed in part in a recently published studies by Sachpekidis et al. and Uprimny et al. [13,14]. In his research, Uprimny demonstrated that the intensity of tumor-related tracer uptake on 68Ga PSMA PET/CT correlates with PSA concentration and GS in newly diagnosed PC. The same upward trend in SUVmax was observed when combining Gleason scores and tumor-related tracer uptake, with higher values corresponding to increased GS/ISUP grades was found in our work.
Based on our findings, Ga68 PSMA PET/CT for primary staging of prostate cancer may be useful in tumors with a Gleason score greater than 7 or in patients with PSA levels ≥10 ng/ml, that is intermediate or high risk patients. Staging in cases with a Gleason score of 6 or 7 and PSA levels <10 ng/ml may be futile, given the significantly lower detection rates on 68Ga PSMA PET/CT [
15]. Additionally, our results showed a similar correlation between the median SUVmax values and Gleason score/ISUP grades.
4.1. Both Poorly-Differentiated and Well-Differentiated Tumors May Not Be Detected in PSMA PET/CT
Several studies support the notion that PSMA PET/CT has reduced efficacy in detecting poorly-differentiated tumors as well as well-differentiated. For instance, research has shown that tumors with both low PSA concentration and Gleason scores,that is low-risk PCa, tend to have lower PSMA uptake, which makes their detection more challenging, using this imaging modality. This is particularly problematic in patients with Gleason scores of 3+3 and 3+4, where detection rates on PSMA PET/CT are lower compared to higher scores, such as Gleason 9 [16,17]. Aykanat, I.C. et al. found that 38 of 62 (61.2%) patients who what Gleason 8 in biopsy were downgraded after RP. Interestingly, in our work 6 of 10 (60%) patients with GS 9-10 also was downgraded after RP. Downgrading of Gleason scores after radical prostatectomy can occur due to several reasons. One key factor is sampling error during biopsy, where the most aggressive parts of the tumor may not be captured. This leads to an overestimation of the Gleason score in the biopsy. Additionally, heterogeneity within the tumor means that different regions may show varying Gleason patterns, which the biopsy might not fully represent. These issues underscore the challenges of accurately predicting the true tumor grade based solely on biopsy [18,19].
While PSMA PET/CT is highly sensitive for detecting prostate cancer, studies have noted that it may not detect all high-grade tumors, including those with Gleason scores of 9-10. Tumor heterogeneity can result in variable PSMA expression, leading to instances where aggressive cancers exhibit low or no PSMA uptake. For example, a study demonstrated that a subset of patients with advanced prostate cancer (Gleason 9-10) showed poor detection on PSMA PET/CT due to low PSMA expression, despite the aggressiveness of their disease. These cases may require additional imaging modalities, such as FDG-PET, which can help identify tumors that are PSMA-negative but FDG-positive [
20].
Additionally, smaller or poorly differentiated high-grade tumors may exhibit lower PSMA avidity, making them harder to detect. High-grade prostate cancer tumors may exhibit lower PSMA avidity due to the loss of differentiation as the tumor becomes more aggressive. This loss of differentiation can lead to reduced PSMA expression, making these tumors harder to detect on PSMA PET/CT scans. Additionally, tumor heterogeneity can result in varying levels of PSMA expression within different regions of the same tumor, further complicating detection [
21]. This underlines the importance of combining PSMA PET/CT with other diagnostic tools, especially in cases of high Gleason scores.
According to Rosenzweig et al., low Gleason scores or poorly differentiated tumors may not be detected on PSMA PET/CT [
22]. In our study, one patient with a Gleason score of 10 exhibited an SUVmax of 6.14, which is comparable to values typically associated with a Gleason score of 6. However, this trend was not observed in the findings of Uprimny et al. While PSMA PET/CT generally correlates with higher Gleason scores, poorly differentiated tumors might have altered PSMA expression due to changes in tumor biology or microenvironment. This could result in lower SUVmax values, as observed in our study. Differences in tumor vascularization, cellular density, or metabolic pathways could also impact PSMA uptake. These variations highlight the need for a multifactorial approach in interpreting PSMA PET/CT findings, especially in high-risk cases.
These findings indicate that while PSMA PET/CT is a powerful tool for staging prostate cancer, it is not infallible, especially in some number of both well-differentiated and poorly-differentiated PCa.
4.2. Changes of Histopathological Grade after RP
Following the introduction of PSA screening, there was a significant rise in the number of patients diagnosed with low-grade, indolent prostate cancer [23,24]. Many of these patients are deemed suitable for active surveillance rather than radical intervention, as such treatments can result in significant adverse effects, including urinary, sexual, and bowel dysfunction [25,26]. Unfortunately, the discordance between biopsy Gleason scores and definitive pathology Gleason scores is a well-documented and widely acknowledged challenge [27,28].
Due to the work of Demirci et al., for all patients following radical prostatectomy, 21% (n=16) of patients moved from a low-risk level (grade groups 1+2) to a high-risk level (grade groups 3+4+5) and 10% (n=6) of patients moved from a high-risk level to a low-risk level and based of PSMA PET images of subgroup of patients who were moved from low-risk to high risk group, the SUVmax cut-off of 9.1 would have predicted upstage in 10 out of those 16 (62.5%) patients [
29].
In agreement with the previous report, we observed a 27,5% upgrade to grade group 2 or higher after radical prostatectomy that was initially reported to be grade group 1 in biopsy reports. With a SUVmax cut-off value of 5.64, PSMA PET predicted the upgrading with a rate of 36,6% . Unfortunately, we did not have a large enough number of patients, which may have resulted in a lower sensitivity and specificity.
Assessing the rate of clinically significant upstaging may hold substantial value for initial treatment planning and outcome prediction. However, this study has limitations due to its retrospective design. Additionally, we did not perform a segmental analysis of pathology specimens or tracer accumulation sites in the 68Ga-PSMA PET/CT scan. Instead, we relied on the SUVmax value across the prostatic lesions and compared it with the grade group. However, SUVmax has limitations, as it only represents the single most intense point of uptake and may not reflect the entire tumor’s metabolic activity. An alternative, SUVmean (SUVavr), which measures the average uptake within the lesion, could provide a more comprehensive assessment of tumor burden and heterogeneity, potentially offering a better correlation with tumor grade and clinical outcomes. A prospective study is needed to further evaluate the role of tracer uptake in predicting clinically significant cancer.
This study underscores the potential of 68Ga-PSMA PET/CT in correlating tumor uptake with Gleason scores and ISUP grades in prostate cancer patients. Our results demonstrate a significant relationship between SUVmax values and tumor grade, suggesting that higher SUVmax is associated with more aggressive disease. The observed grade shifts between preoperative biopsy and post-radical prostatectomy pathology highlight the limitations of biopsy alone in accurate staging. These findings indicate that 68Ga-PSMA PET/CT can serve as a valuable tool in enhancing the accuracy of prostate cancer staging and guiding treatment decisions.
Author Contributions
O.P.: Conceptualization, Methodology, Writing – Original Draft. P.P.: Conceptualization, Methodology, Writing – Original Draft, Supervision P.Z.: Conceptualization, Methodology, Writing – Original Draft, Supervision M.G.: Conceptualization P.S.: Conceptualization K.K.: Conceptualization K.B.-J.: Conceptualization T.D.: Conceptualization K.K.: Conceptualization M.C.C.: Conceptualization, Writing – Review&Editing, Methodology, Visualisation, Supervision J.A.: Conceptualization, Methodology, Writing – Original Draft. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front Public Health. 2022;10.
- Georgakopoulos A, Bamias A, Chatziioannou S. Current role of PSMA-PET imaging in the clinical management of prostate cancer. Vol. 15, Therapeutic Advances in Medical Oncology. 2023.
- De Galiza Barbosa F, Queiroz MA, Nunes RF, Costa LB, Zaniboni EC, Marin JFG, et al. Nonprostatic diseases on PSMA PET imaging: A spectrum of benign and malignant findings. Vol. 20, Cancer Imaging. 2020.
- Testa U, Castelli G, Pelosi E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines. 2019;6(3).
- Rodrigues G, Warde P, Pickles T, Crook J, Brundage M, Souhami L, et al. Pre-treatment risk stratification of prostate cancer patients: A critical review. Vol. 6, Journal of the Canadian Urological Association. 2012.
- Ogu J, Jayasekera M, Villanueva-Meyer J, Bhargava P. Gradual normalization of superscan in prostate cancer: A case report and literature review. Radiol Case Rep. 2023;18(12).
- Caroli P, Sandler I, Matteucci F, De Giorgi U, Uccelli L, Celli M, et al. 68 Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: prospective results in 314 patients. Eur J Nucl Med Mol Imaging. 2018;45(12).
- van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119(2).
- Matushita CS, da Silva AMM, Schuck PN, Bardisserotto M, Piant DB, Pereira JL, et al. 68Ga-Prostate-specific membrane antigen (PSMA) positron emission tomography (pet) in prostate cancer: a systematic review and meta-analysis. International Braz J Urol. 2021;47(4).
- Puranik AD, Dev ID. Ga-68 Prostate-Specific Membrane Antigen PET/CT: Imaging and Clinical Perspective in Prostate Cancer: Imaging and Clinical Perspective in Prostate Cancer. Vol. 17, PET Clinics. 2022.
- Wright GL, Haley C, Beckett M Lou, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urologic Oncology: Seminars and Original Investigations. 1995;1(1).
- Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer. 1998;82(11).
- Sachpekidis C, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68Ga-PSMA-11 dynamic PET/CT imaging in primary prostate cancer. Clin Nucl Med. 2016;41(11).
- Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44(6).
- Zhou C, Tang Y, Deng Z, Yang J, Zhou M, Wang L, et al. Comparison of 68Ga-PSMA PET/CT and multiparametric MRI for the detection of low- and intermediate-risk prostate cancer. EJNMMI Res. 2022;12(1).
- Ibrahim Can Aykanat YKHSEKAOBEKTIKOFBGDEBAECMDBMOD& TE. The role of PSMA PET/CT in predicting downgrading in patients with Gleason score 4+4 prostate cancer in prostate biopsy. Springer Link. 2024 May 21;341 .
- Xu L, Chen R, Yu X, Liu J, Wang Y. 18F-FDG PET Is Not Inferior to 68Ga-PSMA PET for Detecting Biochemical Recurrent Prostate Cancer with a High Gleason Score: A Head-to-Head Comparison Study. Diagnostics. 2024;14(1).
- Tohi Y, Matsuda I, Fujiwara K, Harada S, Ito A, Yamasaki M, et al. The predictive factor for pathological downgrading after prostatectomy in patients with biopsy gleason score 4+3 or 4+4 prostate cancer. Mol Clin Oncol. 2021;14(3).
- Hu J, Hong Y, Wang M, Zeng J, Liu W. The Changes in Gleason Score Between the Diagnostic Biopsy and Radical Prostatectomy Pathology. Indian Journal of Surgery. 2024;86(2).
- Gelardi F, Briganti A, Pini C, Ninatti G, Gandaglia G, Montorsi F, et al. European guidelines update on PSMA PET/CT for prostate cancer staging—snap back to reality. Vol. 50, European Journal of Nuclear Medicine and Molecular Imaging. 2023.
- Eder M, Eisenhut M, Babich J, Haberkorn U. PSMA as a target for radiolabelled small molecules. Vol. 40, European Journal of Nuclear Medicine and Molecular Imaging. 2013.
- Rosenzweig B, Haramaty R, Davidson T, Lazarovich A, Shvero A, Haifler M, et al. Very Low Prostate PET/CT PSMA Uptake May Be Misleading in Staging Radical Prostatectomy Candidates. J Pers Med. 2022;12(3).
- Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: A review of current evidence. Vol. 311, JAMA. 2014.
- Tidd-Johnson A, Sebastian SA, Co EL, Afaq M, Kochhar H, Sheikh M, et al. Prostate cancer screening: Continued controversies and novel biomarker advancements. Vol. 16, Current Urology. 2022.
- de Vos II, Luiting HB, Roobol MJ. Active Surveillance for Prostate Cancer: Past, Current, and Future Trends. Vol. 13, Journal of Personalized Medicine. 2023.
- Loeb S, Bruinsma SM, Nicholson J, Briganti A, Pickles T, Kakehi Y, et al. Active surveillance for prostate cancer: A systematic review of clinicopathologic variables and biomarkers for risk stratification. Vol. 67, European Urology. 2015.
- Boorjian SA, Karnes RJ, Crispen PL, Rangel LJ, Bergstralh EJ, Sebo TJ, et al. The Impact of Discordance Between Biopsy and Pathological Gleason Scores on Survival After Radical Prostatectomy. Journal of Urology. 2009;181(1).
- Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. Pathological Upgrading and Up Staging With Immediate Repeat Biopsy in Patients Eligible for Active Surveillance. Journal of Urology. 2008;180(5).
- Demirci E, Kabasakal L, Şahin OE, Akgün E, Gültekin MH, Doǧanca T, et al. Can SUVmax values of Ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun. 2019;40(1).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).












