Submitted:
27 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
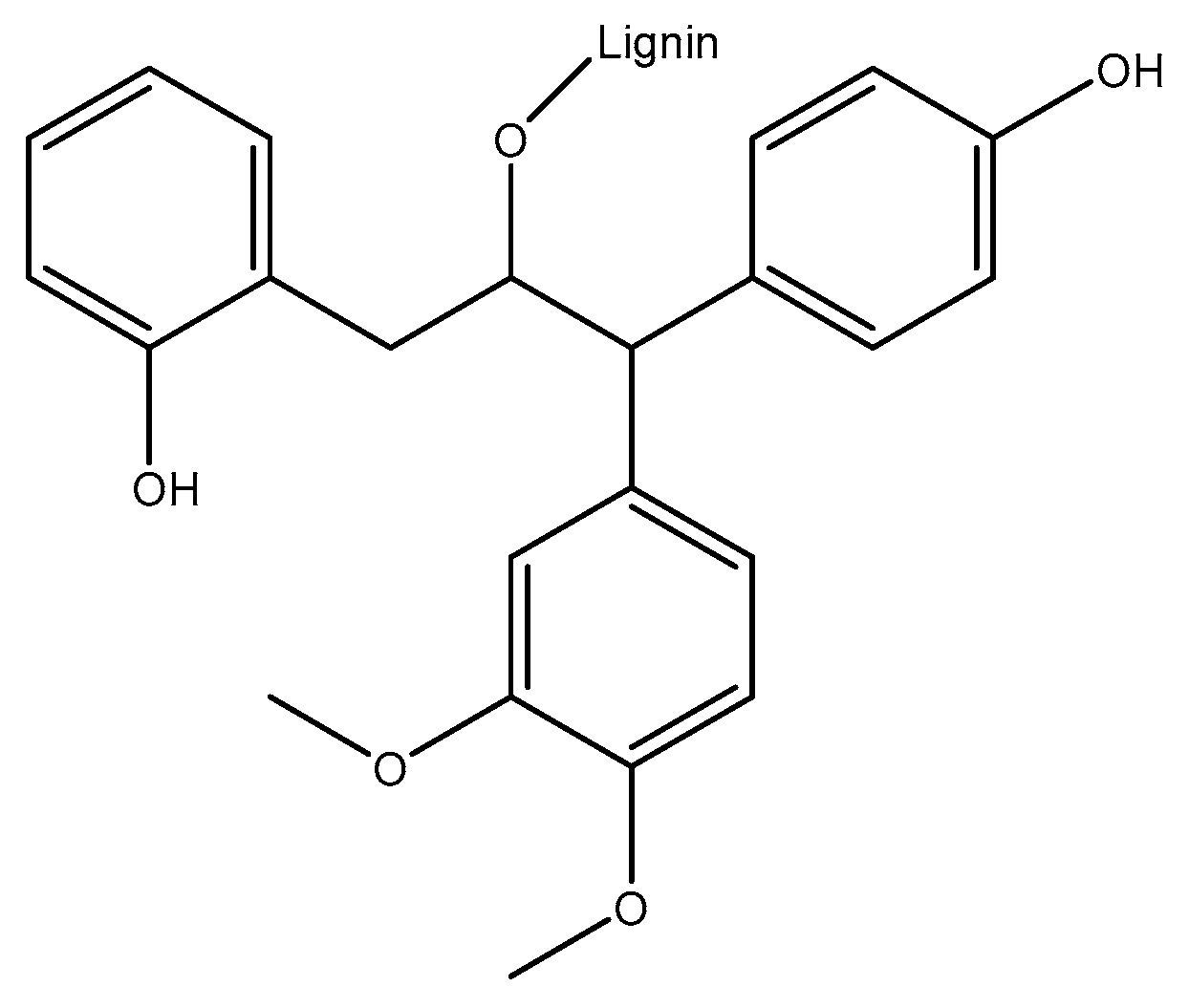
1.1. Lignin Isolation
1.2. Lignin Synthesis
2. Production of Bioactive Substances
3. Lignin Bioactivity Antimicrobial and Antifungal Properties
4. Research and Application
4.1. Lignin as a Binder
4.2. Structural Adaptability in Nature; Role in Plants
4.3. Biodegradation Resistance
4.4. Industrial Versatility: Material Science
4.5. Energy Production
4.6. Chemical Production
4.7. Environmental and Agricultural Applications Soil Enhancement
4.8. Biodegradable Mulches
4.9. Pharmaceutical and Cosmetic Uses Drug Delivery Systems
4.10. Cosmetics
4.11. Pharmaceutical Formulation
4.12. Role in Sustainable Development Circular Economy
4.13. Renewable Raw Material
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annual Review of Plant Biology 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiology 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; Boerjan, W. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochemistry Reviews 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Kang, K.Y.; Chapple, C. Designed for Deconstruction—Poplar Trees Altered in Lignification Improve the Outcome of Biomass Conversion. The Plant Journal 2012, 72, 569–582. [Google Scholar]
- Hatfield, R.; Vermerris, W. Lignin Formation in Plants. The Dilemma of Linkage Specificity, Plant Physiology 2001, 126, 1351–1357. [Google Scholar]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustainable Chemistry & Engineering 2014, 2, 1072–1092. [Google Scholar]
- Dee, S.J.; Bell, A.T. A Study of the Reactivity of Lignin during Pyrolysis Using Pyrolysis Gas Chromatography Combined with Density Functional Theory Calculations. Energy & Fuels 2011, 25, 4086–4095. [Google Scholar]
- Norgren, M.; Edlund, H. Lignin: Recent Advances and Emerging Applications. Current Opinion in Colloid & Interface Science 2014, 19, 409–416. [Google Scholar]
- Zhao, X.; Zhang, L.; Liu, D. Biomass Recalcitrance. Part I: The Chemical Compositions and Physical Structures Affecting the Enzymatic Hydrolysis of Lignocellulose. Biofuels, Bioproducts and Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Progress in Polymer Science 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Wyman, C.E. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid Fuels, Hydrogen and Chemicals from Lignin: A Critical Review. Renewable and Sustainable Energy Reviews 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Gosselink, R.J.; de Jong, E.; Guran, B.; Abächerli, A. Co-Production of Dyes and Biofuels from Lignin. Biomacromolecules 2004, 5, 1240–1246. [Google Scholar]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Wyman, C.E. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Xu, C.; Arancon RA, D.; Labidi, J.; Luque, R. Lignin Depolymerisation Strategies: Towards Valuable Chemicals and Fuels. Chemical Society Reviews 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent Advances and Emerging Applications. Current Opinion in Colloid & Interface Science 2014, 19, 409–416. [Google Scholar]
- Gosselink RJ, A.; de Jong, E.; Guran, B.; Abächerli, A. Co-Production of Dyes and Biofuels from Lignin. Biomacromolecules 2004, 5, 1240–1246. [Google Scholar]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy & Fuels 2006, 20, 848–889. [Google Scholar]
- Kögel-Knabner, I. The Macromolecular Organic Composition of Plant and Microbial Residues as Inputs to Soil Organic Matter. Soil Biology and Biochemistry 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, A.J.; Simpson, M.J. Chemical and Mineralogical Controls on Humic Acid Sorption to Clay Mineral Surfaces. Organic Geochemistry 2005, 36, 1553–1566. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Ma, P.L. Whole Crop Utilization of Cereal Grains. Lignins and Hemicelluloses in Straw. In Cereal Chemistry and Technology (pp. 377-396). Springer: Boston, MA, USA, 2000.
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of Lignin from residue of corn stover to ethanol production. Industrial Crops and Products 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, V. A new approach to biopolymer composite synthesis using Lignin and bacterial biopolyester. Journal of Materials Science 2013, 48, 7848–7855. [Google Scholar]
- Qiu, X.; Kong, Q.; Rong, L.; Tan, C. Antimicrobial and antifungal properties of lignin-based polyols with high phenolic hydroxyl content. International Biodeterioration & Biodegradation 2016, 111, 8–14. [Google Scholar]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.; Kostiainen, M.A.; Santos, H.A.; Yli-Kauhaluoma, J. Properties and chemical modifications of Lignin: Towards lignin-based nanomaterials for biomedical applications. Progress in Materials Science 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Qiu, X.; Kong, Q.; Rong, L.; Tan, C. Antimicrobial and antifungal properties of lignin-based polyols with high phenolic hydroxyl content. International Biodeterioration & Biodegradation 2016, 111, 8–14. [Google Scholar]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.; Kostiainen, M.A.; Santos, H.A.; Yli-Kauhaluoma, J. Properties and chemical modifications of Lignin: Towards lignin-based nanomaterials for biomedical applications. Progress in Materials Science 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of Lignin from residue of corn stover to ethanol production. Industrial Crops and Products 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Liang, Z. Lignin-based antimicrobial materials: Preparation and applications. Green Chemistry 2015, 17, 3926–3930. [Google Scholar]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Viksna, A.; Dobele, G.; Telysheva, G. Characterization of softwood and hardwood LignoBoost kraft lignins with emphasis on their antioxidant activity. BioResources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Prasetyo, E.N.; Kudanga, T.; Ostergaard, L.H.; Rencoret, J.; Gutierrez, A.; del Rio, J.C.; Murkovic, M. Polymerization of lignosulfonates by the laccase-HBT (1-hydroxybenzotriazole) system improves dispersant properties and reduces antimicrobial activity. Bioresource Technology 2010, 101, 5054–5062. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of Lignin to high-value polymeric materials: Review and perspective. Chemical Reviews 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chemistry 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Pang, B.; Fu, K.; Liu, H.; Yang, Z.; Lu, X. Antibacterial mechanism of lignin-derived carbon dots and their application in immunoassays. Journal of Materials Chemistry B 2018, 6, 3656–3664. [Google Scholar]
- Constant, S.; Wienk HL, J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel RJ, H.; Weckhuysen, B.M.; Huijgen WJ, J.; Gosselink RJ, A.; Bruijnincx PC, A. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chemistry 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Riva, R.; Pantos, A.; Fina, A. Lignin-based materials: Present, future, and perspectives. Chemical Reviews 2019, 119, 1204–1261. [Google Scholar]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Progress in Polymer Science 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Spokas, K.A.; Reicosky, D.C. Impacts of Sixteen Different Biochars on Soil Greenhouse Gas Production. Annals of Environmental Science 2009, 3, 179–193. [Google Scholar]
- Bilbao-Sainz, C.; Avena-Bustillos, R.J.; Wood, D.F.; Williams, T.G.; McHugh, T.H.; Krochta, J.M. Composite Edible Films Based on Hydroxypropyl Methylcellulose, Lignin, and Polyphenol. Journal of Agricultural and Food Chemistry 2010, 58, 3774–3780. [Google Scholar] [CrossRef]
- Cheng, S.; D’cruz, I.; Wang, M.; Leitch, M.; Xu, C. Highly Efficient Liquefaction of Woody Biomass in Hot-Compressed Alcohol–Water Co-Solvents for the Production of Biopolyols. Bioresource Technology 2009, 100, 3541–3547. [Google Scholar]
- Hayes, D.G.; Anunciado MB, G.; DeBruyn, J.M.; Bandopadhyay, S.; Schaeffer, S.; English, M.E.; Wadsworth, L.C. Biodegradable Plastic Mulch Films for Sustainable Specialty Crop Production. Horticulture Research 2019, 6, 1–9. [Google Scholar]
- Zhiwen Wang, Peter J. The isolation of Lignin with native-like structure. Biotechnol Adv. 2023, 68, 108230.
- Sindiswa, L. Dube, Foluso O. Osunsanmi, Bongekile P. Ngcobo, Londiwe B. Mkhwanazi, Zanele Z. Jobe, Raphael T. Aruleba, Rebamang A. Mosa, and Andrew R. Opoku. Isolation and Characterization of Potential Lignin Peroxidase-Producing Bacteria from Compost Samples at Richards Bay (South Africa).Pol J Microbiol. 2023, 72, 117–124. [Google Scholar]
- Kpalo, S.Y.; Zainuddin, M.F.; Manaf, L.A. A Review of Technical and Economic Aspects of Biomass Briquetting. Sustainability. 2020, 12, 4609. [Google Scholar] [CrossRef]
- Deshannavar, U.B.; Hegde, P.G.; Dhalayat, Z.; Patil, V.; Gavas, S. Production and characterization of agro-based briquettes and estimation of calorific value by regression analysis: An energy application. Mater. Sci. Energy Technol. 2018, 1, 175–181. [Google Scholar] [CrossRef]
- Berghel, J.; Frodeson, S.; Granström, K.; Renström, R.; Ståhl, M.; Nordgren, D.; Tomani, P. The effects of kraft lignin additives on wood fuel pellet quality, energy use and shelf life. Fuel Process. Technol. 2013, 112, 64–69. [Google Scholar] [CrossRef]
- Davies, R.M.; Davies, O.A. Physical and combustion characteristics of briquettes made from water hyacinth and phytoplankton scum as binder. J. Combust. 2013, 2013, 549894. [Google Scholar] [CrossRef]
- Miao, L. , Li, Q. , Diao, A., Zhang, X., Ma, Y., Construction of a novel phenol synthetic pathway in Escherichia coli through 4-hydroxybenzoate decarboxylation. Appl. Microbiol. Biotechnol. 2015, 99, 5163–5173. [Google Scholar]
- Ni, J. , Gao, Y. Y., Tao, F., Liu, H. Y., Xu, P., Temperature-directed biocatalysis for the sustainable production of aromatic aldehydes or alcohols. Angew. Chem. Int. Ed. Engl. 2018, 57, 1214–1217. [Google Scholar]
- Hansen, E. H. , Moller, B. L., Kock, G. R., Bunner, C. M. et al., De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker's yeast (Saccharomyces cerevisiae). Appl. Environ. Microbiol. 2009, 75, 2765–2774. [Google Scholar]
- Adeboye, P. T. , Bettiga, M. , Aldaeus, F., Larsson, P. T., Olsson, L., Catabolism of coniferyl aldehyde, ferulic acid and p-coumaric acid by Saccharomyces cerevisiae yields less toxic products. Microbial. Cell. Fact. 2015, 14, 149. [Google Scholar]
- Ludmila Martínková, Michal Grulich, Miroslav Pátek, Barbora Křístková, Margit Winkler. Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review. Biomolecules. 2023, 13, 717.
- Jian Yang, Xingye An, Bin Lu. Lignin: A multi-faceted role/function in 3D printing inks. Int Biol Macromol 2024, 267 Pt 2, 131364. [Google Scholar] [CrossRef]
- Thenapakiam Sathasivam, Jing Kai, Sigit Sugiarto, Yong Yu, Debbie Xiang Yun Soo, Qiang Zhu, Jasmeen Merzaban, Dan Kai. Nano-Strategies for Lignin Biomaterials toward Cancer Therapy. Adv Health Mater. 2023, 12, e2300024.
- Qing-Hu, Ma. Lignin Biosynthesis and Its Diversified Roles in Disease Resistance. Genes. 2024, 15, 295. [Google Scholar]
- Manzhao Yao, Xiaoyun Bi, Zuhao Wang. Recent advances in lignin-based carbon materials and their applications: A review. Int J Biol Macromol. 2022, 223 Pt A, 980–1014. [Google Scholar]
- Xiaolan Rao, Jaime Barros. Modeling lignin biosynthesis: A pathway to renewable chemicals.Trends Pland Sci. 2024, 29, 546–559.
- Maurice N Collins. Lignin and its blends.Int J Biol macromol. 2019, 135, 560.
- Dominik Rais, Susanne Zibek. Biotechnological and Biochemical Utilization of Lignin. Adv Biochem Eng Biotechnol. 2019, 166, 469–518.
- Jeong Gu Lee, Ho Young Yoon, Joon-Yung Cha, Woe-Yeon Kim, Pil Joo Kim, Jong-Rok Jeon. Artificial humification of lignin architecture: Top-down and bottom-up approaches. Biotechnol Adv. 2019, 37, 107416.
- Sie Shing Wong, Riyang Shu, Jiaguang Zhang, Haichao Liu, Ning Yan. Downstream processing of lignin derived feedstock into end products. Chem Soc. Rev. 2020, 49, 5510–5560.
- Christopher C Azubuike, Marco N Allemann, Joshua K Michener. Microbial assimilation of lignin-derived aromatic compounds and conversion to value-added products. Curr Opin Microbiol. 2022, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Adam Ekielski, Pawan Kumar Mishra. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int J Mol Sci. 2020, 22, 63.
- Arren Liu, Dylan Ellis, Apurv Mhatre, Sumant Brahmankar, Jong Seto, David R Nielsen, Arul M Varman. Biomanufacturing of value-added chemicals from lignin. Curr Opinj Biotechnol. 2024, 89, 103178.
- Thomas S Lankiewicz, Hemant Choudhary, Yu Gao, Bashar Amer, Stephen P Lillington, Patrick A Leggieri, Jennifer L Brown, Candice L Swift, Anna Lipzen, Hyunsoo Na, Mojgan Amirebrahimi, Michael K Theodorou, Edward E K Baidoo, Kerrie Barry, Igor V Grigoriev, Vitaliy I Timokhin, John Gladden, Seema Singh, Jenny C Mortimer, John Ralph, Blake A Simmons, Steven W Singer, Michelle A O'Malley. Lignin deconstruction by anaerobic fungi. Nat Miccrobiol. 2023, 8, 596–610.
- Vinoth Kumar Ponnusamy, Dinh Duc Nguyen, Jeyaprakash Dharmaraja, Sutha Shobana, J Rajesh Banu, Rijuta Ganesh Saratale, Soon Woong Chang, Gopalakrishnan Kumar. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour Technol. 2019, 271, 462–472.
- Li, K.; Zhong, W.; Li, P.; Ren, J.; Jiang, K.; Wu, W. Antibacterial mechanism of lignin and lignin-based antimicrobial materials in different fields. Int. J. Biol. Macromol. 2023, 252, 126281. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Chen, Z.; Xie, Y.; Khan, S.S.; Singh, S.; Yu, C.; Cheng, G. Recent advances in biological activities of lignin and emerging biomedical applications: A short review. Int. J. Biol. Macromol. 2022, 208, 819–832. [Google Scholar] [CrossRef]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for white natural sunscreens. Int. J. Biol. Macromol. 2018, 122, 549–554. [Google Scholar] [CrossRef]
- Cesarino, I. Structural features and regulation of lignin deposited upon biotic and abiotic stresses. Curr. Opin. Biotechnol. 2019, 56, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Guebitz, G.M.; Pellis, A.; Nyanhongo, G.S. Harnessing the Power of Enzymes for Tailoring and Valorizing Lignin. Trends Biotechnol. 2020, 38, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- Ayub, R.; Raheel, A. High-Value Chemicals from Electrocatalytic Depolymerization of Lignin: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 3767. [Google Scholar] [CrossRef] [PubMed]
- Bausch, F.; Owusu, D.D.; Jusner, P.; Rosado, M.J.; Rencoret, J.; Rosner, S.; del Río, J.C.; Rosenau, T.; Potthast, A. Lignin Quantification of Papyri by TGA—Not a Good Idea. Molecules 2021, 26, 4384. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- A Tuskan, G.; Muchero, W.; Tschaplinski, T.J.; Ragauskas, A.J. Population-level approaches reveal novel aspects of lignin biosynthesis, content, composition and structure. Curr. Opin. Biotechnol. 2019, 56, 250–257. [Google Scholar] [CrossRef]
- Xuewen Xu, Penghui Li, Yidan Zhong, Jiangdong Yu, Chen Miao, Guolin Tong. Int J Biol Macromol. 2023, 243, 125203.
- Wang, J.; Wang, J.; Chen, W.; Chen, W.; Yang, D.; Yang, D.; Fang, Z.; Fang, Z.; Liu, W.; Liu, W.; et al. Monodispersed Lignin Colloidal Spheres with Tailorable Sizes for Bio-Photonic Materials. Small 2022, 18, e2203561. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Pu, Y.; Meng, X.; Xu, J.; Yuan, J.S.; Ragauskas, A.J. Emerging Strategies for Modifying Lignin Chemistry to Enhance Biological Lignin Valorization. ChemSusChem 2020, 13, 5423–5432. [Google Scholar] [CrossRef]
- Renders, T.; Bossche, G.V.D.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Ohtani, M.; Demura, T. The quest for transcriptional hubs of lignin biosynthesis: Beyond the NAC-MYB-gene regulatory network model. Curr. Opin. Biotechnol. 2018, 56, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Ottesen, V.; Opedal, M.T.; Moe, S.T. Morphology of lignin structures on fiber surfaces after organosolv pretreatment. Biopolymers 2022, 113, e23520. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.-W.; Tsang, D.C. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef]
- Joseph J Bozell. Approaches to the selective catalytic conversion of lignin: A grand challenge for biorefinery development.Top Curr Chem. 2014, 353, 229–55.
- Radhika, N.; Sachdeva, S.; Kumar, M. Microbe assisted depolymerization of lignin rich waste and its conversion to gaseous biofuel. J. Environ. Manag. 2021, 300, 113684. [Google Scholar] [CrossRef]
- Liu, S.-C.; Shi, T.; He, Z.-J.; Chen, K.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Ethylenediamine pretreatment simultaneously improved carbohydrate hydrolysis and lignin valorization. Bioresour. Technol. 2023, 386, 129552. [Google Scholar] [CrossRef]
- Hutterer, C.; Schild, G.; Kliba, G.; Potthast, A. Lignin profiling in extracted xylans by size-exclusion chromatography. Carbohydr. Polym. 2016, 151, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Hararak, B.; Wanmolee, W.; Wijaranakul, P.; Prakymoramas, N.; Winotapun, C.; Kraithong, W.; Nakason, K. Physicochemical properties of lignin nanoparticles from softwood and their potential application in sustainable pre-harvest bagging as transparent UV-shielding films. Int. J. Biol. Macromol. 2022, 229, 575–588. [Google Scholar] [CrossRef]
- Ibarra, D.; García-Fuentevilla, L.; Domínguez, G.; Martín-Sampedro, R.; Hernández, M.; Arias, M.E.; Santos, J.I.; Eugenio, M.E. NMR Study on Laccase Polymerization of Kraft Lignin Using Different Enzymes Source. Int. J. Mol. Sci. 2023, 24, 2359. [Google Scholar] [CrossRef]
- Sun, S.-F.; Yang, H.-Y.; Yang, J.; Shi, Z.-J.; Deng, J. Revealing the structural characteristics of lignin macromolecules from perennial ryegrass during different integrated treatments. Int. J. Biol. Macromol. 2021, 178, 373–380. [Google Scholar] [CrossRef]
- Rencoret, J.; Gutiérrez, A.; Nieto, L.; Jiménez-Barbero, J.; Faulds, C.B.; Kim, H.; Ralph, J.; Martínez. T.; del Río, J.C. Lignin Composition and Structure in Young versus AdultEucalyptus globulusPlants. Plant Physiol. 2010, 155, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi Sakagami 1, Ken Hashimoto, Fumika Suzuki, Takako Ogiwara, Kazue Satoh, Hideyuki Ito, Tsutomu Hatano, Yoshida Takashi, Sei-ichiro Fujisawa. Molecular requirements of lignin-carbohydrate complexes for expression of unique biological activities.Phytochemistry 2005, 66, 2108–2.
- Nilsson, T.Y.; Wagner, M.; Inganäs, O. Lignin Modification for Biopolymer/Conjugated Polymer Hybrids as Renewable Energy Storage Materials. ChemSusChem 2015, 8, 4081–4085. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.; Liebentritt, S.; Vicente, A.A.; Teixeira, J.A. Lignin from an integrated process consisting of liquid hot water and ethanol organosolv: Physicochemical and antioxidant properties. Int. J. Biol. Macromol. 2018, 120, 159–169. [Google Scholar] [CrossRef]
- Xin Zhang, Jinxu Zhang, Hua Yang, Chunyong He, Yubin Ke, Seema Singh, Gang Cheng. Determination of the Structures of Lignin Subunits and Nanoparticles in Solution by Small-Angle Neutron Scattering: Towards Improving Lignin Valorizatio. ChemSusChem 2022, 15, e202201230. [CrossRef]
- Qin, Z.; Zhao, T.-P.; He, M.-K.; Yuan, J.-Y.; Li, B.-B.; Qin, Z.; Liu, H.-M.; Wang, X.-D. Chemical and structural transformations of lignin in sesame seed hull during roasting. Int. J. Biol. Macromol. 2024, 277, 134121. [Google Scholar] [CrossRef]
- Weiland, F.; Kohlstedt, M.; Wittmann, C. Guiding stars to the field of dreams: Metabolically engineered pathways and microbial platforms for a sustainable lignin-based industry. Metab. Eng. 2021, 71, 13–41. [Google Scholar] [CrossRef]
- C Lapierre 1, B Pollet, J J MacKay, R R Sederoff. Lignin structure in a mutant pine deficient in cinnamyl alcohol dehydrogenase.J Agric Food Chem. 2000, 48, 2326–31.
- Moilanen, U.; Kellock, M.; Galkin, S.; Viikari, L. The laccase-catalyzed modification of lignin for enzymatic hydrolysis. Enzym. Microb. Technol. 2011, 49, 492–498. [Google Scholar] [CrossRef]
- Asikkala, J.; Tamminen, T.; Argyropoulos, D.S. Accurate and Reproducible Determination of Lignin Molar Mass by Acetobromination. J. Agric. Food Chem. 2012, 60, 8968–8973. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Ralph, J.; Marques, G.; Gutiérrez, A.; Martínez. T.; del Río, J.C. Structural Characterization of Lignin Isolated from Coconut (Cocos nucifera) Coir Fibers. J. Agric. Food Chem. 2013, 61, 2434–2445. [Google Scholar] [CrossRef]
- Raquel Martin-Sampedro 1, Ewellyn A Capanema, Ingrid Hoeger, Juan C Villar, Orlando J Rojas. Lignin changes after steam explosion and laccase-mediator treatment of eucalyptus wood chips.J Agric Food Chem. 2011, 59, 8761–8769.
- van Parijs, F.R.; Morreel, K.; Ralph, J.; Boerjan, W.; Merks, R.M. Modeling Lignin Polymerization. I. Simulation Model of Dehydrogenation Polymers. Plant Physiol. 2010, 153, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gwak, K.; Treasure, T.; Jameel, H.; Chang, H.; Park, S. Effect of Lignin Chemistry on the Enzymatic Hydrolysis of Woody Biomass. ChemSusChem 2014, 7, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Zaitoon, A.; Sharma, S.; Manickavasagan, A.; Lim, L.-T. Enhanced hydrophobic paper-sheet derived from Miscanthus × giganteus cellulose fibers coated with esterified lignin and cellulose acetate blend. Int. J. Biol. Macromol. 2022, 223, 1243–1256. [Google Scholar] [CrossRef]
- Pereira, A.A.; Martins, G.F.; Antunes, P.A.; Conrrado, R.; Pasquini, D.; Job, A.E.; Curvelo, A.A.S.; Ferreira, M.; Riul, A.; Constantino, C.J.L. Lignin from Sugar Cane Bagasse: Extraction, Fabrication of Nanostructured Films, and Application. Langmuir 2007, 23, 6652–6659. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Catalytic Lignin Valorization Process for the Production of Aromatic Chemicals and Hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef]
- Chazal, R.; Robert, P.; Durand, S.; Devaux, M.-F.; Saulnier, L.; Lapierre, C.; Guillon, F. Investigating Lignin Key Features in Maize Lignocelluloses Using Infrared Spectroscopy. Appl. Spectrosc. 2014, 68, 1342–1347. [Google Scholar] [CrossRef]
- Espiñeira, J.M.; Uzal, E.N.; Ros, L.V.G.; Carrión, J.S.; Merino, F.; Barceló, A.R.; Pomar, F. Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biol. 2010, 13, 59–68. [Google Scholar] [CrossRef]
- Donohoe, B.S.; Decker, S.R.; Tucker, M.P.; Himmel, M.E.; Vinzant, T.B. Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 2008, 101, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Cauley, A.N.; Wilson, J.N. Functionalized lignin biomaterials for enhancing optical properties and cellular interactions of dyes. Biomater. Sci. 2017, 5, 2114–2121. [Google Scholar] [CrossRef]
- Zinovyev, G.; Sumerskii, I.; Rosenau, T.; Balakshin, M.; Potthast, A. Ball Milling’s Effect on Pine Milled Wood Lignin’s Structure and Molar Mass. Molecules 2018, 23, 2223. [Google Scholar] [CrossRef]
- Bécsy-Jakab, V.E.; Savoy, A.; Saulnier, B.K.; Singh, S.K.; Hodge, D.B. Extraction, recovery, and characterization of lignin from industrial corn stover lignin cake. Bioresour. Technol. 2024, 399, 130610. [Google Scholar] [CrossRef] [PubMed]
- Tonin, F.; Vignali, E.; Pollegioni, L.; D’arrigo, P.; Rosini, E. A novel, simple screening method for investigating the properties of lignin oxidative activity. Enzym. Microb. Technol. 2017, 96, 143–150. [Google Scholar] [CrossRef]
- Mazar, A.; Paleologou, M. Comparison of the effects of three drying methods on lignin properties. Int. J. Biol. Macromol. 2023, 258, 128974. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.-Y.; Youn, H.J.; Choi, J.W. Fractionation of lignin macromolecules by sequential organic solvents systems and their characterization for further valuable applications. Int. J. Biol. Macromol. 2018, 106, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Li, J.; Ek, M.; Ligero, P.; de Vega, A. Native Lignin Structure of Miscanthus x giganteus and Its Changes during Acetic and Formic Acid Fractionation. J. Agric. Food Chem. 2009, 57, 6262–6270. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Pan, T.-F.; Guo, Z.-X.; Pan, D.-M. Specific Lignin Accumulation in Granulated Juice Sacs ofCitrus maxima. J. Agric. Food Chem. 2014, 62, 12082–12089. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Jin, X.; Xia, C.; Ma, S.; Hong, S.; Aladejana, J.T.; Dong, A.; Luo, Y.; Li, J.; et al. High-Strength, High-Swelling-Resistant, High-Sensitivity Hydrogel Sensor Prepared with Wood That Retains Lignin. Biomacromolecules 2024, 25, 1696–1708. [Google Scholar] [CrossRef]
- da Mata, A.K.A.; Felipe, V.T.d.A.; Mazzetto, S.E.; Lomonaco, D.; Avelino, F. Development of an eco-friendly acetosolv protocol for tuning the acetylation of coconut shell lignin: Structural, antioxidant, solubility and UV-blocking properties. Int. J. Biol. Macromol. 2022, 211, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, C.; Baumberger, S.; Cathala, B.; Dole, P. Lignin–polymer blends: Evaluation of compatibility by image analysis. Comptes Rendus Biol. 2004, 327, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, B.; Zhou, W.; Liu, X.; Chen, F. High-value utilization of eucalyptus kraft lignin: Preparation and characterization as efficient dye dispersant. Int. J. Biol. Macromol. 2018, 109, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.; Radovic-Hrapovic, Z.; Ahvazi, B.; Thiboutot, S.; Ampleman, G.; Hawari, J. Sorption of 2,4-dinitroanisole (DNAN) on lignin. J. Environ. Sci. 2012, 24, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Majeke, B.; García-Aparicio, M.; Biko, O.; Viljoen-Bloom, M.; van Zyl, W.; Görgens, J. Synergistic codon optimization and bioreactor cultivation toward enhanced secretion of fungal lignin peroxidase in Pichia pastoris: Enzymatic valorization of technical (industrial) lignins. Enzym. Microb. Technol. 2020, 139, 109593. [Google Scholar] [CrossRef] [PubMed]
- Voxeur, A.; Wang, Y.; Sibout, R. Lignification: Different mechanisms for a versatile polymer. Curr. Opin. Plant Biol. 2015, 23, 83–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Wan, S.; Wu, W.; Wu, S.; Jin, Y. Adsorption behavior of two glucanases on three lignins and the effect by adding sulfonated lignin. J. Biotechnol. 2020, 323, 1–8. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.J.; Cho, E.J.; Song, Y.; Bae, H.-J. Isolation and characterization of lignin from the oak wood bioethanol production residue for adhesives. Int. J. Biol. Macromol. 2015, 72, 1056–1062. [Google Scholar] [CrossRef]
- S Lepifre 1, M Froment, F Cazaux, S Houot, D Lourdin, X Coqueret, C Lapierre, S Baumberger. Lignin incorporation combined with electron-beam irradiation improves the surface water resistance of starch films.Biomacromolecules. 2004, 5, 1678–86.
- Moon, S.-J.; Eom, I.-Y.; Kim, J.-Y.; Kim, T.-S.; Lee, S.M.; Choi, I.-G.; Choi, J.W. Characterization of lignin-rich residues remaining after continuous super-critical water hydrolysis of poplar wood (Populus albaglandulosa) for conversion to fermentable sugars. Bioresour. Technol. 2011, 102, 5912–5916. [Google Scholar] [CrossRef]
- Kontur, W.S.; Olmsted, C.N.; Yusko, L.M.; Niles, A.V.; Walters, K.A.; Beebe, E.T.; Meulen, K.A.V.; Karlen, S.D.; Gall, D.L.; Noguera, D.R.; et al. A heterodimeric glutathione S-transferase that stereospecifically breaks lignin's β(R)-aryl ether bond reveals the diversity of bacterial β-etherases. J. Biol. Chem. 2019, 294, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Lu, F.; Ralph, S.; Ralph, J. Identification of 4–O–5-Units in Softwood Lignins via Definitive Lignin Models and NMR. Biomacromolecules 2016, 17, 1909–1920. [Google Scholar] [CrossRef]
- B M Majeke, F-X Collard 2, L Tyhoda, J F Görgens. The synergistic application of quinone reductase and lignin peroxidase for the deconstruction of industrial (technical) lignins and analysis of the degraded lignin products. Bioresour Technol. 2021, 319, 124152.
- Bai, Y.-Y.; Xiao, L.-P.; Shi, Z.-J.; Sun, R.-C. Structural Variation of Bamboo Lignin before and after Ethanol Organosolv Pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. [Google Scholar] [CrossRef]
- Haiwei Guo, Bo Zhang, Zaojuan Qi, Changzhi Li, Jianwei Ji, Tao Dai, Aiqin Wang, Tao Zhang. Valorization of Lignin to Simple Phenolic Compounds over Tungsten Carbide: Impact of Lignin Structure.Chem SusChem. 2017, 10, 523–532.
- Bai, J.; Wang, S.; Li, Y.; Wang, Z.; Tang, J. Effect of chemical structure and molecular weight on the properties of lignin-based ultrafine carbon fibers. Int. J. Biol. Macromol. 2021, 187, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Pepijn Prinsen, Anand Narani, Gadi Rothenberg. Lignin Depolymerisation and Lignocellulose Fractionation by Solvated Electrons in Liquid AmmoniaChemSus Chem. 2017, 10, 1022–1032.
- Martín-Sampedro, R.; Santos, J.I.; Eugenio, M.E.; Wicklein, B.; Jiménez-López, L.; Ibarra, D. Chemical and thermal analysis of lignin streams from Robinia pseudoacacia L. generated during organosolv and acid hydrolysis pre-treatments and subsequent enzymatic hydrolysis. Int. J. Biol. Macromol. 2019, 140, 311–322. [Google Scholar] [CrossRef]
- Ran Bi, Martin Lawoko, Gunnar Henriksson. Phoma herbarum, a soil fungus able to grow on natural lignin and synthetic lignin (DHP) as sole carbon source and cause lignin degradation. J Ind Microbiol Biotechnol. 2016, 43, 1175–1182.
- Maria Pilar Vinardell, Montserrat Mitjans. Lignins and Their Derivatives with Beneficial Effects on Human HealthInt J Mol Sci 2017, 18, 1219.
- Wang, Z.; Jönsson, L.J. Comparison of catalytically non-productive adsorption of fungal proteins to lignins and pseudo-lignin using isobaric mass tagging. Bioresour. Technol. 2018, 268, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Romualdo S Fukushima 1, Monty S Kerley. Use of lignin extracted from different plant sources as standards in the spectrophotometric acetyl bromide lignin methodJ Agric Food Chem 2011, 59, 3505–3509.
- Omid Hosseinaei, David P Harper, Joseph J Bozell, Timothy G Rials. Improving Processing and Performance of Pure Lignin Carbon Fibers through Hardwood and Herbaceous Lignin BlendsInt J Mol Sci. 2017, 18, 1410.
- Pereira, A.; Hoeger, I.C.; Ferrer, A.; Rencoret, J.; del Rio, J.C.; Kruus, K.; Rahikainen, J.; Kellock, M.; Gutiérrez, A.; Rojas, O.J. Lignin Films from Spruce, Eucalyptus, and Wheat Straw Studied with Electroacoustic and Optical Sensors: Effect of Composition and Electrostatic Screening on Enzyme Binding. Biomacromolecules 2017, 18, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Sarkanen, S. Macromolecular replication during lignin biosynthesis. Phytochemistry 2010, 71, 453–462. [Google Scholar] [CrossRef]
- Jaramillo-Carmona, S.; Fuentes-Alventosa, J.; Rodríguez-Gutiérrez, G.; Waldron, K.; Smith, A.; Guillén-Bejarano, R.; Fernández-Bolaños, J.; Jiménez-Araujo, A.; Rodríguez-Arcos, R. Characterization of Asparagus Lignin by HPLC. J. Food Sci. 2008, 73, C526–C532. [Google Scholar] [CrossRef]
- Singh, S.S.; Zaitoon, A.; Sharma, S.; Manickavasagan, A.; Lim, L.-T. Enhanced hydrophobic paper-sheet derived from Miscanthus × giganteus cellulose fibers coated with esterified lignin and cellulose acetate blend. Int. J. Biol. Macromol. 2022, 223, 1243–1256. [Google Scholar] [CrossRef]
- Pereira, A.A.; Martins, G.F.; Antunes, P.A.; Conrrado, R.; Pasquini, D.; Job, A.E.; Curvelo, A.A.S.; Ferreira, M.; Riul, A.; Constantino, C.J.L. Lignin from Sugar Cane Bagasse: Extraction, Fabrication of Nanostructured Films, and Application. Langmuir 2007, 23, 6652–6659. [Google Scholar] [CrossRef]
- Chazal, R.; Robert, P.; Durand, S.; Devaux, M.-F.; Saulnier, L.; Lapierre, C.; Guillon, F. Investigating Lignin Key Features in Maize Lignocelluloses Using Infrared Spectroscopy. Appl. Spectrosc. 2014, 68, 1342–1347. [Google Scholar] [CrossRef]
- Bécsy-Jakab, V.E.; Savoy, A.; Saulnier, B.K.; Singh, S.K.; Hodge, D.B. Extraction, recovery, and characterization of lignin from industrial corn stover lignin cake. Bioresour. Technol. 2024, 399, 130610. [Google Scholar] [CrossRef]
- de Menezes, F.F.; Martim, D.B.; Ling, L.Y.; Mulato, A.T.N.; Crespim, E.; Oliveira, J.V.d.C.; Driemeier, C.E.; de Giuseppe, P.O.; Rocha, G.J.d.M. Exploring the compatibility between hydrothermal depolymerization of alkaline lignin from sugarcane bagasse and metabolization of the aromatics by bacteria. Int. J. Biol. Macromol. 2022, 223, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.; Vermaas, J.V.; Liang, L.; Ragauskas, A.J.; Smith, J.C.; Petridis, L. Atomistic Simulations of Polydisperse Lignin Melts Using Simple Polydisperse Residue Input Generator. Biomacromolecules 2023, 25, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Andriani, F.; Lawoko, M. Oxidative Carboxylation of Lignin: Exploring Reactivity of Different Lignin Types. Biomacromolecules 2024, 25, 4246–4254. [Google Scholar] [CrossRef]
- Kimura, C.; Oh, S.-W.; Fujita, T.; Watanabe, T. Adsorptive Inhibition of Enveloped Viruses and Nonenveloped Cardioviruses by Antiviral Lignin Produced from Sugarcane Bagasse via Microwave Glycerolysis. Biomacromolecules 2022, 23, 789–797. [Google Scholar] [CrossRef]
- Burger, R.; Lindner, S.; Rumpf, J.; Do, X.T.; Diehl, B.W.; Rehahn, M.; Monakhova, Y.B.; Schulze, M. Benchtop versus high field NMR: Comparable performance found for the molecular weight determination of lignin. J. Pharm. Biomed. Anal. 2022, 212, 114649. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Chang, C.; Kulsreshtha, M.; Gupta, P. Bio-separation of value-added products from Kraft lignin: A promising two-stage lignin biorefinery via microbial electrochemical technology. Int. J. Biol. Macromol. 2022, 227, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Xiaochen Ma, Shujun Liu, Hongliang Wang, Yulu Wang, Zhen Li, Tianyi Gu, Yulong Li, Fengjiao Xin, Boting Wen. In Vitro Fermentation of Beechwood Lignin-Carbohydrate Complexes Provides Evidence for Utilization by Gut BacteriaNutrients 2023, 15, 220.
- Chen, M.; Ralph, J.; Luterbacher, J.S.; Shi, Q.-S.; Xie, X. Selecting Suitable Near-Native Lignins for Research. J. Agric. Food Chem. 2023, 71, 20751–20761. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Zhao, J.; Oyom, W.; Long, H.; Yang, R.; Pu, L.; Bi, Y.; Prusky, D. Meyerozyma guilliermondii promoted the deposition of GSH type lignin by activating the biosynthesis and polymerization of monolignols at the wounds of potato tubers. Food Chem. 2023, 416, 135688. [Google Scholar] [CrossRef]
- Diment, D.; Tkachenko, O.; Schlee, P.; Kohlhuber, N.; Potthast, A.; Budnyak, T.M.; Rigo, D.; Balakshin, M. Study toward a More Reliable Approach to Elucidate the Lignin Structure–Property–Performance Correlation. Biomacromolecules 2023, 25, 200–212. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Wang, X.; Shi, H.; Niu, M.; Zhang, Y.; Wang, Z.; Guo, Y. Effect of Lignin Structure Characteristics on the Performance of Lignin Based Phenol Formaldehyde Adhesives. Macromol. Rapid Commun. 2024, 45, e2300663. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; He, M.; Li, Y.; Lu, W.; Li, M.; Sun, B.; Zheng, Y. A Joint Transcriptomic and Metabolomic Analysis Reveals the Regulation of Shading on Lignin Biosynthesis in Asparagus. Int. J. Mol. Sci. 2023, 24, 1539. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxia Duan, Xueke Wang, Ao Huang, Guijiang Liu, Yun Liu. Molecules. 2022, 27, 2905.
- Zhong, L.; Xu, M.; Wang, C.; Shao, L.; Mao, J.; Jiang, W.; Ji, X.; Yang, G.; Chen, J.; Lyu, G.; et al. Pretreatment of willow using the alkaline-catalyzed sulfolane/water solution for high-purity and antioxidative lignin production. Int. J. Biol. Macromol. 2020, 159, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Xue, Y.; Zhang, Y.; Li, T.; Yang, T.; Li, S. Effect of microstructure-scale features on lignin fluorescence for preparation of high fluorescence efficiency lignin-based nanomaterials. Int. J. Biol. Macromol. 2022, 202, 520–528. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Valente, A.J.; Antunes, F.E.; Romano, A.; Norgren, M.; Medronho, B. Levulinic acid: A novel sustainable solvent for lignin dissolution. Int. J. Biol. Macromol. 2020, 164, 3454–3461. [Google Scholar] [CrossRef]
- Popova, Y.A.; Shestakov, S.L.; Belesov, A.V.; Pikovskoi, I.I.; Kozhevnikov, A.Y. Comprehensive analysis of the chemical structure of lignin from raspberry stalks (Rubus idaeus L.). Int. J. Biol. Macromol. 2020, 164, 3814–3822. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, Y.; Lei, B.; Zhong, H. Impact mechanisms of supercritical CO2–ethanol–water on extraction behavior and chemical structure of eucalyptus lignin. Int. J. Biol. Macromol. 2020, 161, 1506–1515. [Google Scholar] [CrossRef]
- Rodríguez, A.; Espinosa, E. Special Issue “Lignocellulosic Biomass”. Molecules 2021, 26, 1483. [Google Scholar] [CrossRef]
- Eliana Capecchi, Elisabetta Tomaino, Davide Piccinino, Peter Elias Kidibule, Maria Fernández-Lobato, Daniele Spinelli 3, Rebecca Pogni, Ana Garcia Cabado, Jorge Lago, Raffaele Saladino. Nanoparticles of Lignins and Saccharides from Fishery Wastes as Sustainable UV-Shielding, Antioxidant, and Antimicrobial Biofillers.Biomacromolecules. 2022, 23, 3154–3164.
- Li, J.; Hu, H.-C.; Chai, X.-S. Rapid method for determination of carbonyl groups in lignin compounds by headspace gas chromatography. J. Chromatogr. A 2015, 1404, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Lima, C.F.; Barbosa, L.C.; Colodette, J.L.; Gouveia, A.; Silvério, F.O. Determination of Eucalyptus spp lignin S/G ratio: A comparison between methods. Bioresour. Technol. 2010, 101, 4056–4061. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Lu, F.; Sun, R.; Ralph, J. Synthesis and Characterization of New 5-Linked Pinoresinol Lignin Models. Chem. – A Eur. J. 2012, 18, 16402–16410. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Gaspar, A.R.; Contreras, S.; Lucia, L.A.; Crestini, C.; Argyropoulos, D.S. On the propensity of lignin to associate: A size exclusion chromatography study with lignin derivatives isolated from different plant species. Phytochemistry 2007, 68, 2570–2583. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Rivera, J.L.; Herrera, R.; Flores, N.; Rutiaga, J.G.; López, P. Evaluation of the chemical reactivity in lignin precursors using the Fukui function. J. Mol. Model. 2007, 14, 77–81. [Google Scholar] [CrossRef]
- Claudia Crestini, Maria Chiara Caponi, Dimitris S Argyropoulos, Raffaele Saladino. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds.Bioorg Med Chekm. 2006, 14, 5292–302.
- Marangon, C.A.; Otoni, C.G.; Bertuso, P.C.; Rossi, P.F.; dos Santos, D.M.; Lourençon, T.V.; Martins, V.C.; Plepis, A.M.G.; Mattoso, L.H.; Nitschke, M. Side-stream lignins: Potential antioxidant and antimicrobial agents in milk. Food Res. Int. 2024, 180, 114091. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The isolation, characterization and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef]
- Eugenio, M.E.; Martín-Sampedro, R.; Santos, J.I.; Wicklein, B.; Martín, J.A.; Ibarra, D. Properties versus application requirements of solubilized lignins from an elm clone during different pre-treatments. Int. J. Biol. Macromol. 2021, 181, 99–111. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Structural elucidation of inhomogeneous lignins from bamboo. Int. J. Biol. Macromol. 2015, 77, 250–259. [Google Scholar] [CrossRef]
- Rüttimann, C.; Vicuña, R.; Mozuch, M.D.; Kirk, T.K. Limited bacterial mineralization of fungal degradation intermediates from synthetic lignin. Appl. Environ. Microbiol. 1991, 57, 3652–3655. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Luo, X.-T.; Xu, L.-H.; Sun, Q.; Wen, J.-L.; Liang, X.-F.; Liu, H.-Z.; Yuan, T.-Q. Structural elucidation and targeted valorization of untractable lignin from pre-hydrolysis liquor of xylose production via a simple and robust separation approach. Int. J. Biol. Macromol. 2023, 253, 127029. [Google Scholar] [CrossRef]
- Sekeri, S.H.; Ibrahim, M.N.M.; Umar, K.; Yaqoob, A.A.; Azmi, M.N.; Hussin, M.H.; Othman, M.B.H.; Malik, M.F.I.A. Preparation and characterization of nanosized lignin from oil palm (Elaeis guineensis) biomass as a novel emulsifying agent. Int. J. Biol. Macromol. 2020, 164, 3114–3124. [Google Scholar] [CrossRef]
- Murillo-Morales, G.; Sethupathy, S.; Zhang, M.; Xu, L.; Ghaznavi, A.; Xu, J.; Yang, B.; Sun, J.; Zhu, D. Characterization and 3D printing of a biodegradable polylactic acid/thermoplastic polyurethane blend with laccase-modified lignin as a nucleating agent. Int. J. Biol. Macromol. 2023, 236, 123881. [Google Scholar] [CrossRef]
- Hernández-Ramos, F.; Alriols, M.G.; Antxustegi, M.M.; Labidi, J.; Erdocia, X. Valorisation of crude glycerol in the production of liquefied lignin bio-polyols for polyurethane formulations. Int. J. Biol. Macromol. 2023, 247, 125855. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.-C.; Kim, J.; Cho, Y.-M.; Yoon, C.-H.; Shin, J.-H.; Kwak, H.W.; Choi, I.-G. Enhancement of elongation at break and UV-protective properties of poly(lactic acid) film with cationic ring opening polymerized (CROP)-lignin. Int. J. Biol. Macromol. 2023, 253, 127293. [Google Scholar] [CrossRef] [PubMed]
- Claire L Bourmaud, Stefania Bertella, Anna Bosch Rico, Steven D Karlen, John Ralph, Jeremy S Luterbacher. Quantification of Native Lignin Structural Features with Gel-Phase 2D-HSQC0 Reveals Lignin Structural Changes During Extraction. Angew Chem Int Ed Eng. 2024, 63, e202404442.
- Liu, B.; Liu, L.; Deng, B.; Huang, C.; Zhu, J.; Liang, L.; He, X.; Wei, Y.; Qin, C.; Liang, C.; et al. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef]
- Ding, W.; Sun, H.; Li, X.; Li, Y.; Jia, H.; Luo, Y.; She, D.; Geng, Z. Environmental applications of lignin-based hydrogels for Cu remediation in water and soil: Adsorption mechanisms and passivation effects. Environ. Res. 2024, 250, 118442. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, N. Biochemical and in silico molecular study of caffeic acid-O-methyltransferase enzyme associated with lignin deposition in tall fescue. Amino Acids 2022, 55, 1293–1304. [Google Scholar] [CrossRef]
- Ovejero-Pérez, A.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Acidic depolymerization vs ionic liquid solubilization in lignin extraction from eucalyptus wood using the protic ionic liquid 1-methylimidazolium chloride. Int. J. Biol. Macromol. 2020, 157, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Yuqing Zhang, Xiao Jiang 2 Shanqi Wan, Wenjuan Wu, Shufang Wu, Yongcan Jin. Adsorption behavior of two glucanases on three lignins and the effect by adding sulfonated lignin.J Biotechnol. 2020, 10, 1–8.
- Cheng, X.-C.; Guo, X.-R.; Qin, Z.; Wang, X.-D.; Liu, H.-M.; Liu, Y.-L. Structural features and antioxidant activities of Chinese quince (Chaenomeles sinensis) fruits lignin during auto-catalyzed ethanol organosolv pretreatment. Int. J. Biol. Macromol. 2020, 164, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yuan, J.-Y.; Zhang, Y.-T.; Yang, Q.-L.; Zhang, C.-X.; Guo, Q.; Qin, Z.; Liu, H.-M.; Wang, X.-D.; Mei, H.-X.; et al. Effects of different precursors on the structure of lignin-based biochar and its ability to adsorb benzopyrene from sesame oil. Int. J. Biol. Macromol. 2024, 269, 132216. [Google Scholar] [CrossRef]
- Izaguirre, N.; Erdocia, X.; Labidi, J. Exploring chemical reactions to enhance thermal and dispersion stability of kraft and organosolv lignin. Int. J. Biol. Macromol. 2024, 264, 130518. [Google Scholar] [CrossRef]
- Dias, A.H.S.; Cao, Y.; Skaf, M.S.; de Visser, S.P. Machine learning-aided engineering of a cytochrome P450 for optimal bioconversion of lignin fragments. Phys. Chem. Chem. Phys. 2024, 26, 17577–17587. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, U.; Li, X.; Wang, B.; Geng, F.; Rehman, M.S.U.; Zhang, K.; Xu, J. Structural characterizations of lignins extracted under same severity using different acids. Int. J. Biol. Macromol. 2021, 194, 204–212. [Google Scholar] [CrossRef]
- Fan, Y.; Lei, M.; Han, Y.; Zhang, Z.; Kong, X.; Xu, W.; Zhang, H.; Xiao, R.; Liu, C. Elucidating radical-mediated pyrolysis behaviors of preoxidized lignins. Bioresour. Technol. 2022, 350, 126908. [Google Scholar] [CrossRef]
- Tkachenko, O.; Diment, D.; Rigo, D.; Stro̷mme, M.; Budnyak, T.M. Unveiling the Nature of lignin’s Interaction with Molecules: A Mechanistic Understanding of Adsorption of Methylene Blue Dye. Biomacromolecules 2024, 25, 4292–4304. [Google Scholar] [CrossRef]
- Han, X.; Song, K.; Yu, H.; Zhou, X.; Guo, J. Extraction and characterisation of kudzu root residue lignin based on deep eutectic solvents. Phytochem. Anal. 2024, 35, 786–798. [Google Scholar] [CrossRef]
- Ding, W.; Sun, H.; Li, X.; Li, Y.; Jia, H.; Luo, Y.; She, D.; Geng, Z. Environmental applications of lignin-based hydrogels for Cu remediation in water and soil: Adsorption mechanisms and passivation effects. Environ. Res. 2024, 250, 118442. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, B.K.; Siahkamari, M.; Singh, S.K.; Nejad, M.; Hodge, D.B. Effect of Dilute Acid Pretreatment and Lignin Extraction Conditions on Lignin Properties and Suitability as a Phenol Replacement in Phenol-Formaldehyde Wood Adhesives. J. Agric. Food Chem. 2022, 71, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, S.; Fatima, B.; Hussain, D.; Imran, M.; Jawad, S.E.Z.; Saeed, A.; Majeed, S.; Najam-Ul-Haq, M. Doxorubicin encapsulated blend of sitagliptin-lignin polymeric drug delivery system for effective combination therapy against cancer. Int. J. Biol. Macromol. 2024, 269, 132146. [Google Scholar] [CrossRef] [PubMed]
- da Mata, A.K.A.; Felipe, V.T.d.A.; Mazzetto, S.E.; Lomonaco, D.; Avelino, F. Development of an eco-friendly acetosolv protocol for tuning the acetylation of coconut shell lignin: Structural, antioxidant, solubility and UV-blocking properties. Int. J. Biol. Macromol. 2022, 211, 271–280. [Google Scholar] [CrossRef]
- ukasz Klapiszewski, Beata Podkościelna, Marta Goliszek, Adam Kubiak, Karolina Młynarczyk, Teofil Jesionowski. . 2021, 178, 344–353.
- Pan, W.; Lin, J. Efficient centrifugal spinning of soda lignin for the production of activated carbon nanofibers with highly porous structure. Int. J. Biol. Macromol. 2022, 222, 1433–1442. [Google Scholar] [CrossRef]
- Maria Vigh. A trashed treasure: Lignin could become a large and renewable source of organic compounds for the chemical industry to replace fossil fuel-based chemicals: Lignin could become a large and renewable source of organic compounds for the chemical industry to replace fossil fuel-based chemicals.EMBO Rep. 2023, 24, e57103.
- Xu, S.; Yuan, J.-Y.; Zhang, Y.-T.; Yang, Q.-L.; Zhang, C.-X.; Guo, Q.; Qin, Z.; Liu, H.-M.; Wang, X.-D.; Mei, H.-X.; et al. Effects of different precursors on the structure of lignin-based biochar and its ability to adsorb benzopyrene from sesame oil. Int. J. Biol. Macromol. 2024, 269, 132216. [Google Scholar] [CrossRef]
- Yang, G.; Gong, Z.; Luo, X.; Chen, L.; Shuai, L. Bonding wood with uncondensed lignins as adhesives. Nature 2023, 621, 511–515. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K.; Ghosh, S.; Verma, A.K. Biogas from lignocellulosic feedstock: Current status and challenges. Environ. Sci. Pollut. Res. 2023, 1–26. [Google Scholar] [CrossRef]
- de Almeida, G.H.G.; Siqueira-Soares, R.d.C.; Mota, T.R.; de Oliveira, D.M.; Abrahão, J.; Foletto-Felipe, M.d.P.; dos Santos, W.D.; Ferrarese-Filho, O.; Marchiosi, R. Aluminum oxide nanoparticles affect the cell wall structure and lignin composition slightly altering the soybean growth. Plant Physiol. Biochem. 2021, 159, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.; Vasil’eva, I.; Shumakovich, G.; Khlupova, M.; Chertkov, V.; Shestakova, A.; Yaropolov, A. Green Extraction of Reed Lignin: The Effect of the Deep Eutectic Solvent Composition on the UV-Shielding and Antioxidant Properties of Lignin. Int. J. Mol. Sci. 2024, 25, 8277. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, K.; Jiang, W.; Liu, Y.; Xie, L.; Liu, F.; Yang, K.; Jiang, Y.; Jia, H. Novel Chemical Routes for Carbon Dioxide and Methane Production from Lignin Photodegradation: The Role of Environmental Free Radicals. Environ. Sci. Technol. 2024, 58, 16055–16065. [Google Scholar] [CrossRef] [PubMed]
- Bing, R.G.; Sulis, D.B.; Wang, J.P.; Adams, M.W.W.; Kelly, R.M. Thermophilic microbial deconstruction and conversion of natural and transgenic lignocellulose. Environ. Microbiol. Rep. 2021, 13, 272–293. [Google Scholar] [CrossRef]
- Song, X.; Zhu, Z.; Chi, X.; Tang, S.; Han, G.; Cheng, W. Efficient downstream valorization of lignocellulose after organosolv fractionation: Synergistic enhancement of waterborne coatings by co-assembled lignin@cellulose nanocrystals. Int. J. Biol. Macromol. 2022, 227, 1325–1335. [Google Scholar] [CrossRef]
- Pongchaiphol, S.; Suriyachai, N.; Hararak, B.; Raita, M.; Laosiripojana, N.; Champreda, V. Physicochemical characteristics of organosolv lignins from different lignocellulosic agricultural wastes. Int. J. Biol. Macromol. 2022, 216, 710–727. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, S.; Wang, D.; Qi, D.; Chen, F.; Qiu, X. Curcumin-loaded high internal phase emulsions stabilized with lysine modified lignin: A biological agent with high photothermal protection and antibacterial properties. Food Funct. 2021, 12, 7469–7479. [Google Scholar] [CrossRef]
- Dimitris S Argyropoulos, Nicolò Pajer, Claudia Crestini. Quantitative 31P NMR Analysis of Lignins and Tannins. J Vis Exp. 2021, 174.
- Verrillo, M.; Savy, D.; Cangemi, S.; Savarese, C.; Cozzolino, V.; Piccolo, A. Valorization of lignins from energy crops and agro-industrial byproducts as antioxidant and antibacterial materials. J. Sci. Food Agric. 2021, 102, 2885–2892. [Google Scholar] [CrossRef]
- Marangon, C.A.; Otoni, C.G.; Bertuso, P.C.; Rossi, P.F.; dos Santos, D.M.; Lourençon, T.V.; Martins, V.C.; Plepis, A.M.G.; Mattoso, L.H.; Nitschke, M. Side-stream lignins: Potential antioxidant and antimicrobial agents in milk. Food Res. Int. 2024, 180, 114091. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.; Su, W. Review of studies on polysaccharides, lignins and small molecular compounds from three Polygonatum Mill. (Asparagaceae) spp. in crude and processed states. Int. J. Biol. Macromol. 2024, 260, 129511. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Nilvebrant, N.-O. Comment on 'Making the biochemical conversion of lignocellulose more robust'. Trends Biotechnol. 2023, 42, 393–394. [Google Scholar] [CrossRef] [PubMed]
| # | Mechanism | Description | Ref. |
|---|---|---|---|
| 1 | Disruption of Cell Walls and Membranes | Cell Wall Integrity: Lignin and its derivatives can interact with the components of microbial cell walls, particularly in bacteria. This interaction can compromise the integrity of the cell wall, leading to cell lysis and death. Membrane Permeability: Lignin can embed itself into the lipid bilayer of microbial cell membranes, causing increased permeability. This disruption allows the leakage of essential intracellular contents and ultimately results in cell death. |
[21] |
| 2 | Generation of Reactive Oxygen Species (ROS) | Oxidative Stress: Lignin can induce the production of reactive oxygen species (ROS) within microbial cells. ROS are highly reactive molecules that can damage cellular components such as DNA, proteins, and lipids, leading to oxidative stress and cell death. The phenolic groups in Lignin can undergo redox cycling, contributing to ROS generation. This mechanism is particularly effective against a broad range of microorganisms. |
[22] |
| 3 | Enzyme Inhibition | Inhibition of Metabolic Enzymes: Lignin can inhibit key enzymes involved in microbial metabolism. The phenolic compounds in Lignin can bind to the active sites of these enzymes, preventing them from catalyzing essential biochemical reactions. | [23] |
| 4 | Interaction with Nucleic Acids | DNA Binding: Lignin and its derivatives can interact with microbial DNA, causing structural changes or damage. This interaction can inhibit DNA replication and transcription, thereby preventing microbial proliferation. Genetic Damage: The oxidative stress induced by Lignin can lead to mutations and breaks in the microbial DNA, further inhibiting cell viability. |
[24] |
| 5 | Chelation of Metal Ions | Nutrient Deprivation: Lignin can chelate essential metal ions such as iron and magnesium, which are necessary for microbial growth and enzyme function. By sequestering these ions, Lignin deprives microorganisms of critical nutrients, inhibiting their growth. Metabolic Disruption: The chelation of metal ions can also disrupt various metabolic pathways that depend on these cofactors. |
[25] |
| 6 | Chelation of Essential Nutrients | Lignin can chelate metal ions like iron and magnesium, which are essential for microbial growth. By binding these ions, Lignin deprives microorganisms of the nutrients they need for survival and proliferation. | [26] |
| 7 | Disruption of Electron Transport Chain | Lignin can interfere with the electron transport chain in microbial cells, disrupting energy production and leading to cell death. | [27] |
| 8 | Binding to Proteins | Lignin can bind to microbial proteins, causing structural changes or denaturation. This binding can inhibit enzyme activity and interfere with protein function, leading to microbial cell death. | [28] |
| 9 | Interference with Quorum Sensing | Lignin can interfere with quorum sensing, the process by which bacteria communicate and coordinate their behavior. Disrupting quorum sensing can prevent biofilm formation and reduce virulence. | [29] |
| 10 | Modulation of Gene Expression | : Lignin can affect the expression of genes involved in microbial virulence and survival. This modulation can inhibit microbial growth and pathogenicity. | [30] |
| 11 | Surface Interaction and Biofilm Prevention | Lignin can prevent the attachment of microorganisms to surfaces, thereby inhibiting biofilm formation. Biofilms protect microbes from external threats, so preventing their formation enhances antimicrobial efficacy. | [31] |
| 12 | Disruption of Membrane Potential | Lignin can disrupt the membrane potential of microbial cells, affecting ion gradients and membrane permeability. This disruption can lead to cell death due to the inability to maintain essential cellular processes. | [32] |
| 13 | Interaction with Cell Membrane Proteins | Lignin can bind to membrane proteins, altering their structure and function. This binding can inhibit nutrient transport, signal transduction, and other critical cellular functions. | [33] |
| 14 | Inhibition of ATP Synthesis | Lignin can inhibit ATP synthesis in microbial cells by interfering with the function of ATP synthase or other components of the ATP production pathway, leading to energy depletion and cell death. | [34] |
| 15 | Induction of Apoptosis-like Cell Death | Lignin can induce apoptosis-like cell death in microbial cells, characterized by cell shrinkage, DNA fragmentation, and other apoptotic markers. This programmed cell death can be triggered by oxidative stress and other cellular disruptions caused by Lignin. | [35] |
| 16 | Activation of Antimicrobial Peptides | Lignin can enhance the activity of naturally occurring antimicrobial peptides by interacting with them and increasing their affinity for microbial cell membranes. | [36]. |
| 17 | Synergistic Effects with Other Antimicrobials | Lignin can act synergistically with other antimicrobial agents, enhancing their efficacy. This synergy can occur through various mechanisms, such as disrupting microbial defenses or facilitating the entry of other antimicrobials. | [37] |
| 18 | Altering Microbial Metabolic Pathways | Lignin can alter key metabolic pathways in microbial cells, leading to the accumulation of toxic intermediates or depletion of essential metabolites, which can inhibit growth and survival. | [38] |
| # | Mechanism | Description | Ref |
|---|---|---|---|
| 1 | Antioxidant Properties | Lignin has significant antioxidant properties due to its phenolic structure, which allows it to scavenge free radicals. This activity is beneficial in reducing oxidative stress in biological systems and can be harnessed in the development of health supplements and pharmaceuticals. | [41] |
| 2 | Anti-inflammatory Effects | Some studies suggest that Lignin and its derivatives can reduce inflammation by modulating the activity of inflammatory mediators. This potential makes Lignin a candidate for developing treatments for inflammatory diseases. | [42] |
| 3 | Anticancer Potential | Certain lignin derivatives have been found to exhibit cytotoxic effects on cancer cells, inhibiting their growth and proliferation. This anticancer potential is an area of ongoing research, with the goal of developing lignin-based therapeutic agents. | [43] |
| 4 | Drug Delivery Systems | Due to its biocompatibility and biodegradability, Lignin is being explored as a carrier material for drug delivery systems. Lignin nanoparticles can encapsulate drugs, enhancing their stability and controlled release. | [44] |
| 5 | Prebiotic Activity | Lignin can act as a prebiotic, promoting the growth of beneficial gut bacteria. This property is essential for maintaining a healthy digestive system and could be utilized in the development of functional foods and dietary supplements. | [45] |
| 6 | Environmental Applications | Lignin-degrading microorganisms can be used to break down environmental pollutants, such as pesticides and industrial chemicals, making Lignin a valuable tool in bioremediation efforts. | [46] |
| 7 | Bioremediation | Lignin and its derivatives can enhance plant growth by improving soil structure, increasing nutrient availability, and providing protection against pathogens. This can lead to more sustainable agricultural practices. | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





