Submitted:
27 September 2024
Posted:
29 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Synthesis of carbon quantum dots (CQDs)
2.2. Synthesis of TiO2/CQDs composite
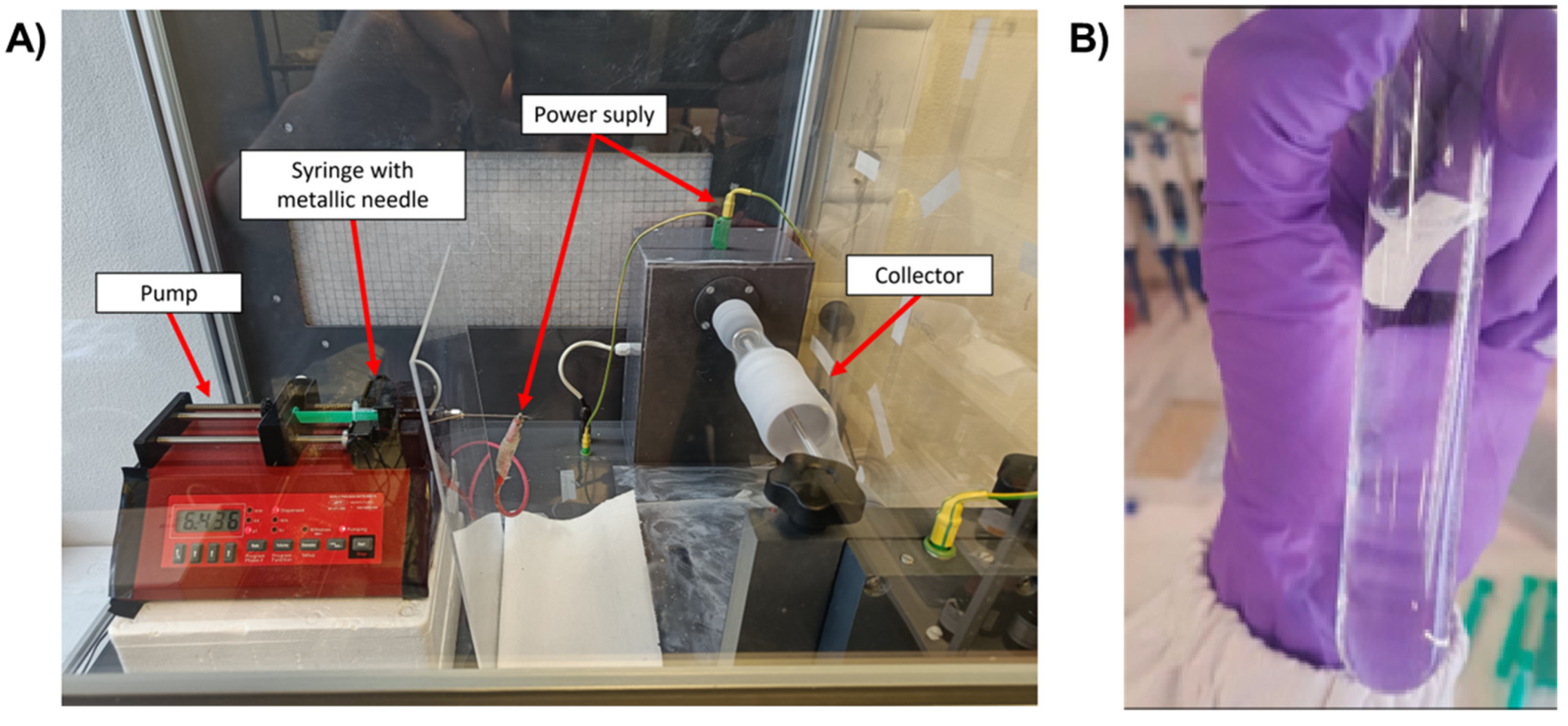
2.3. Preparation of PA66 nanofibers containing TiO2/CQDs by electrospinning
2.4. Nanofibers characterization
2.5. Water matrices characterization
2.6. Photocataytic experiments
2.7. Chromatographic analysis
3. Results and Discussion
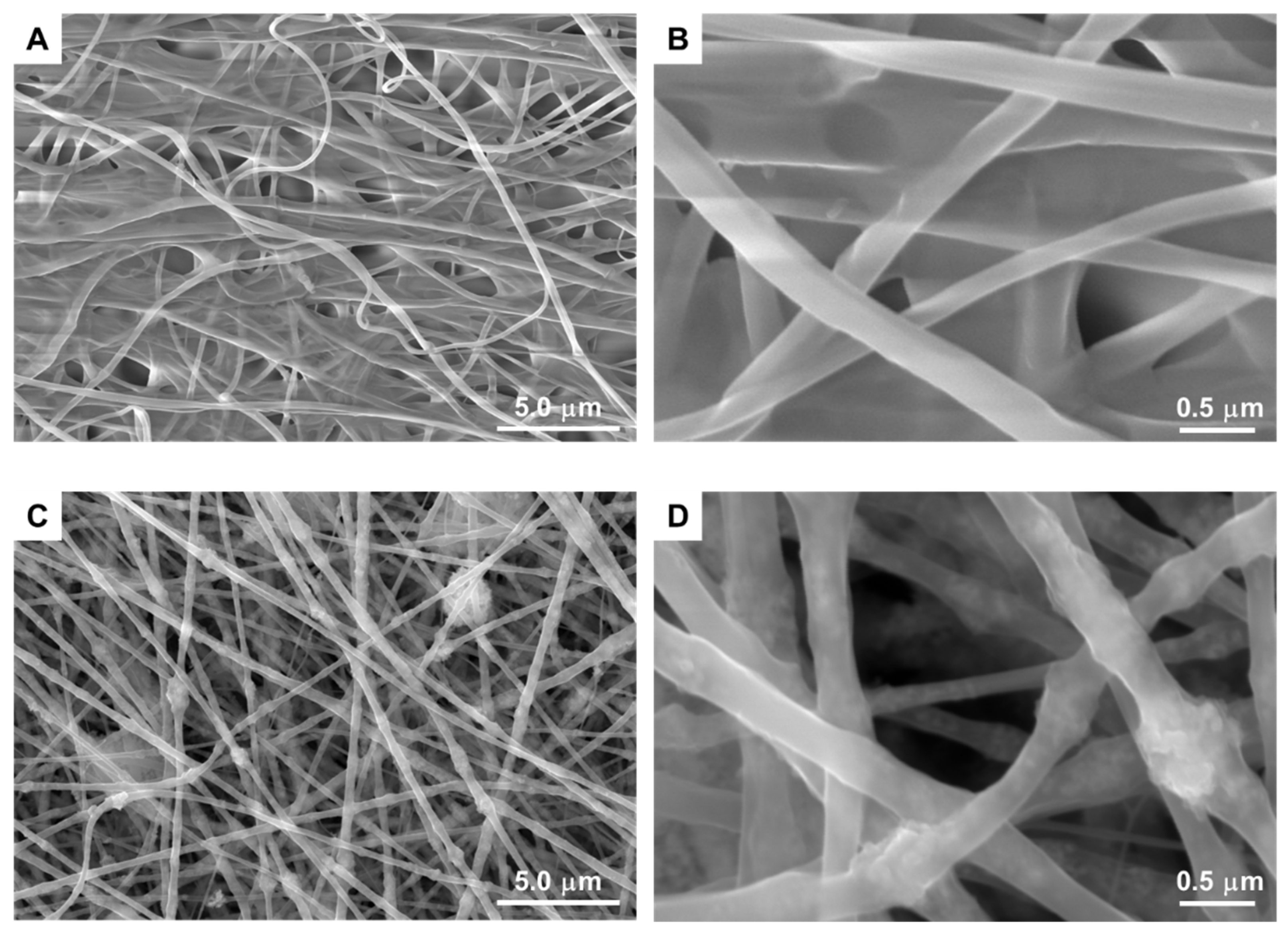
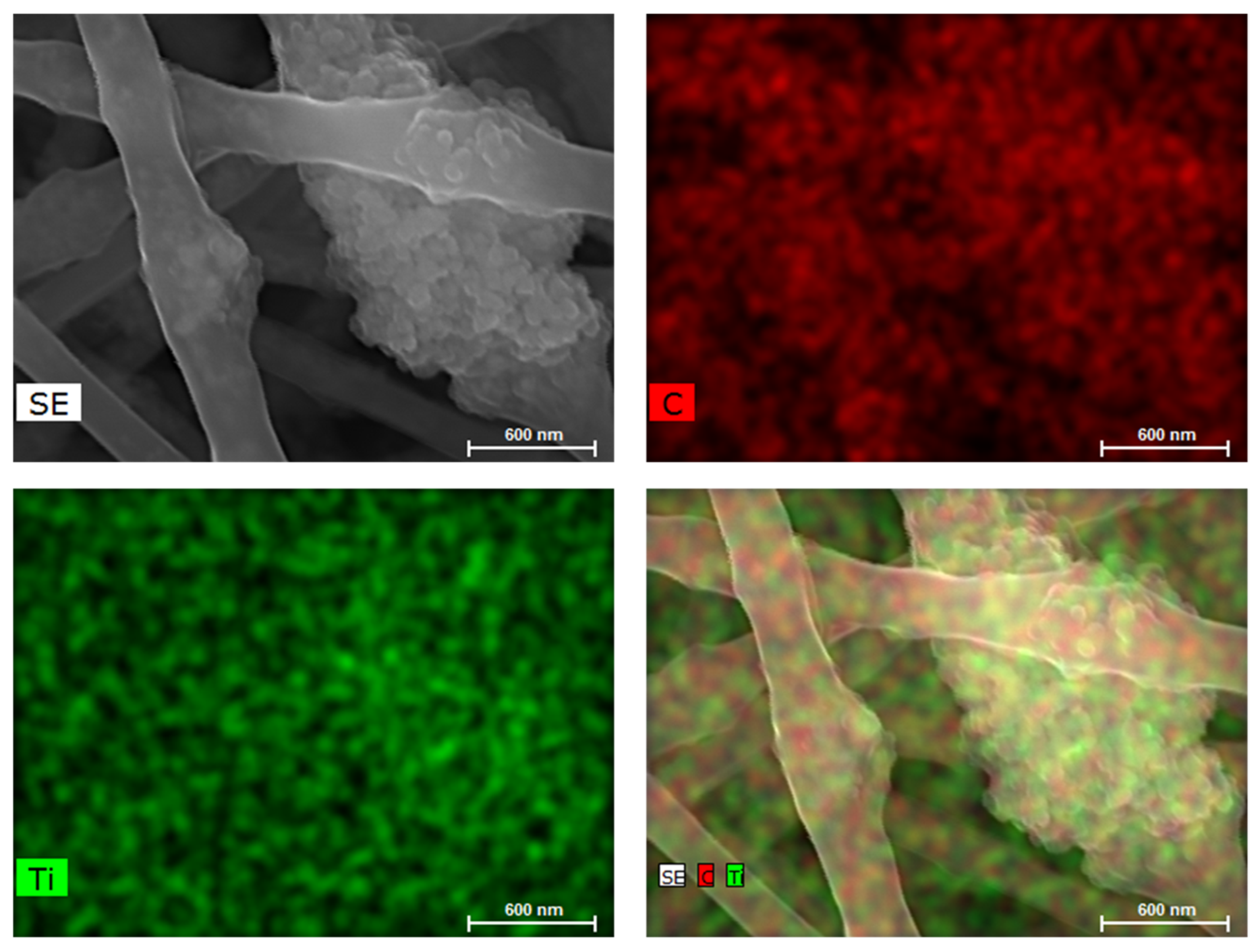
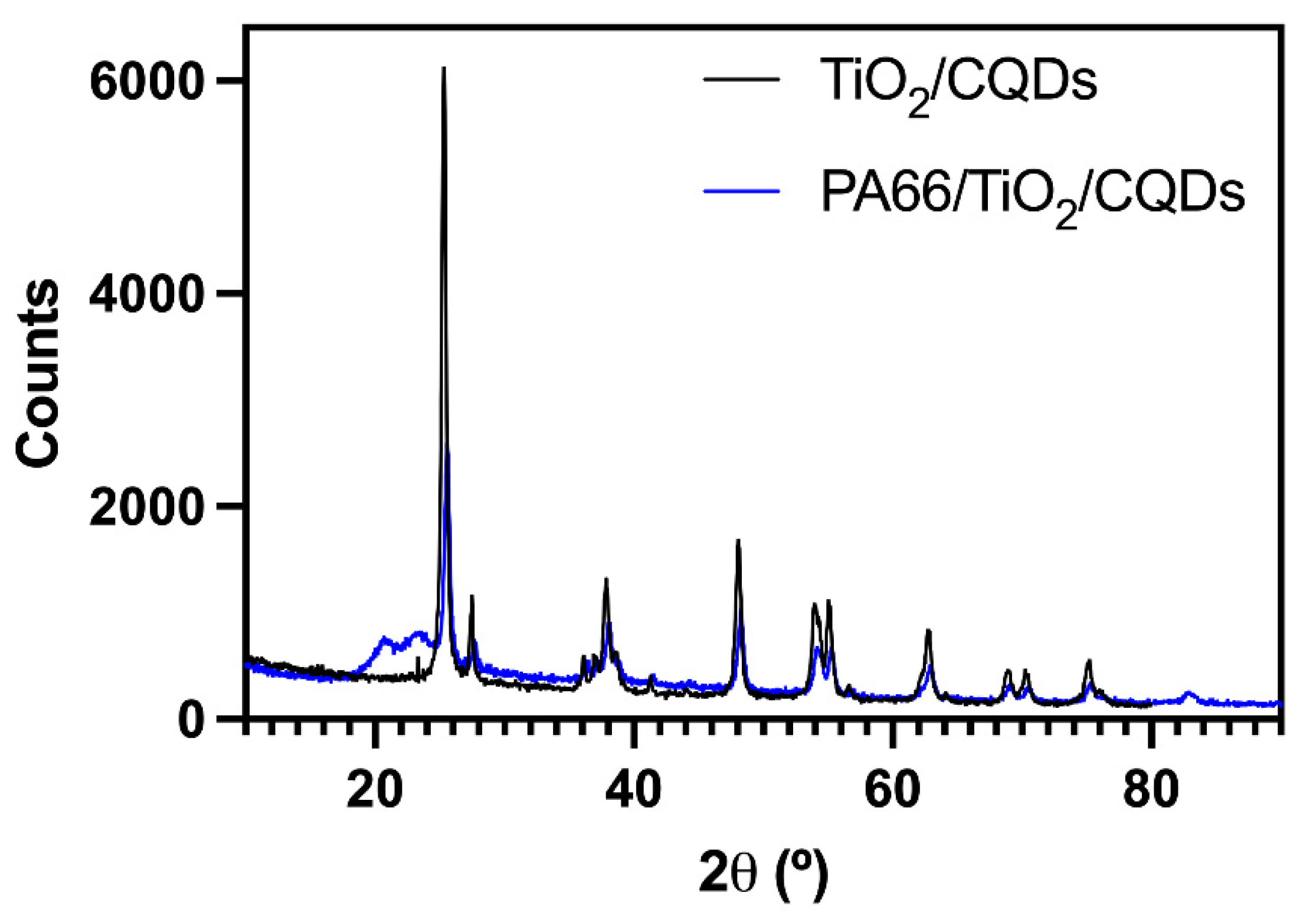
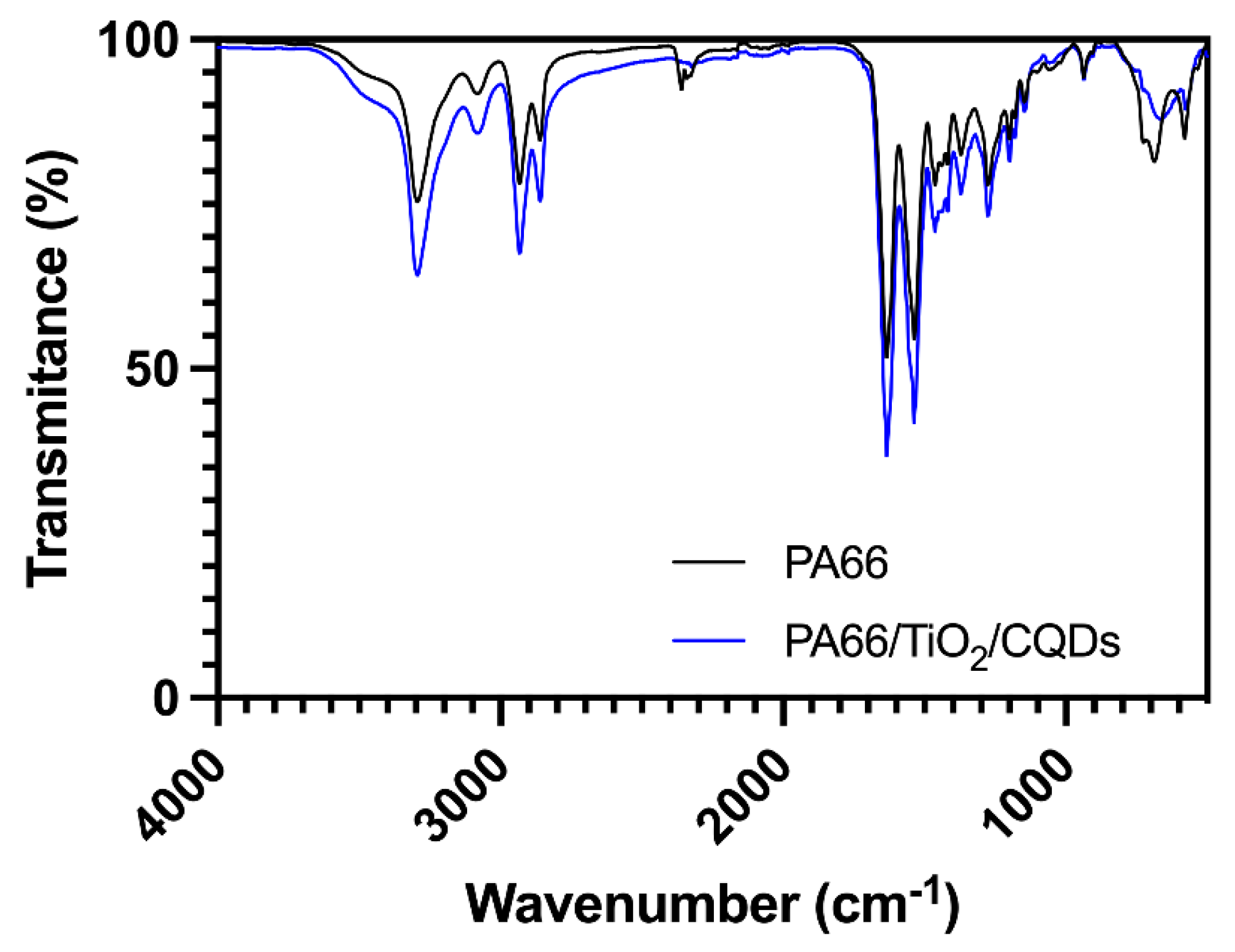
3.1. Preparation of PA66 nanofibers containing TiO2/CQDs by electrospinning
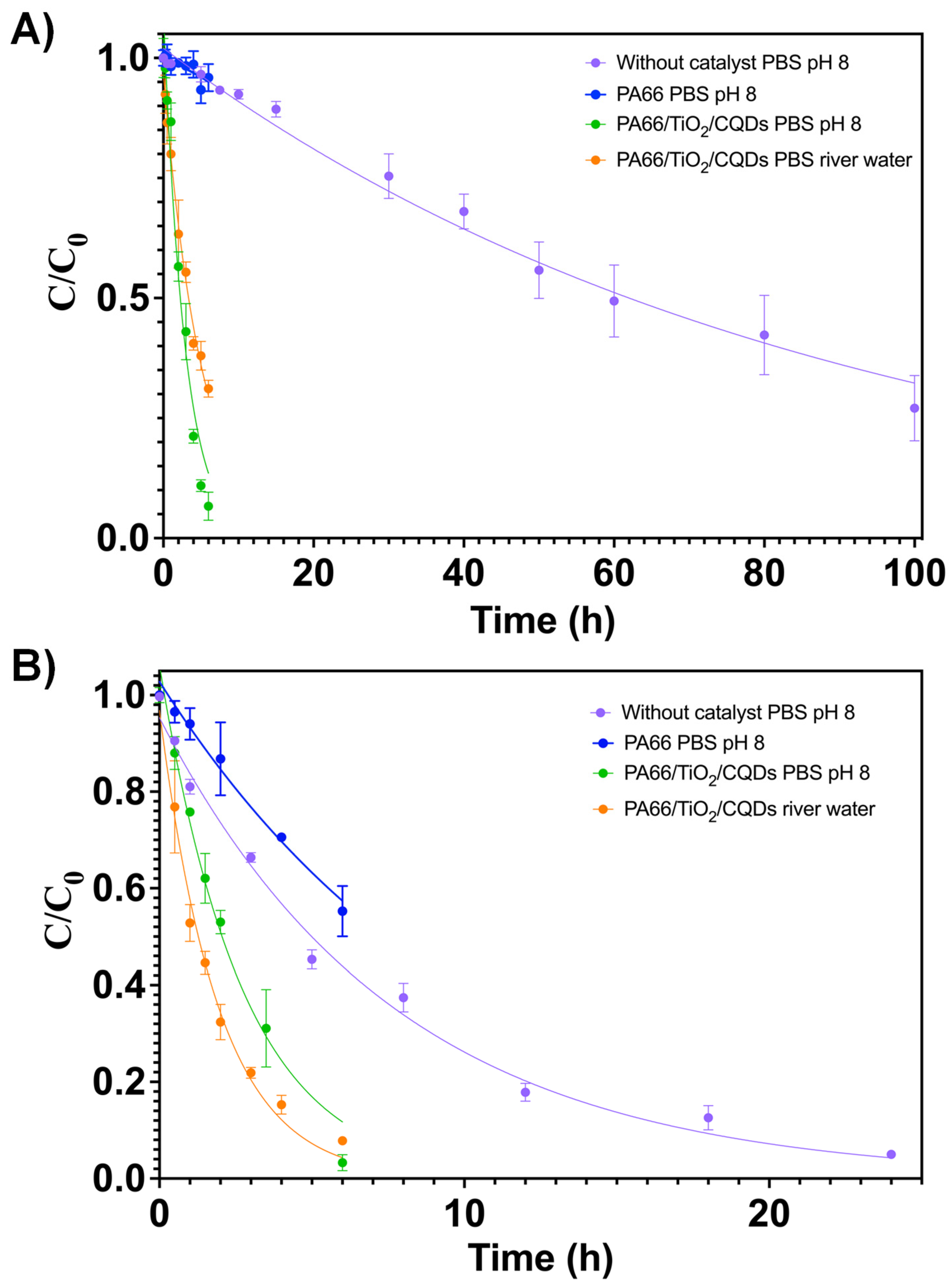
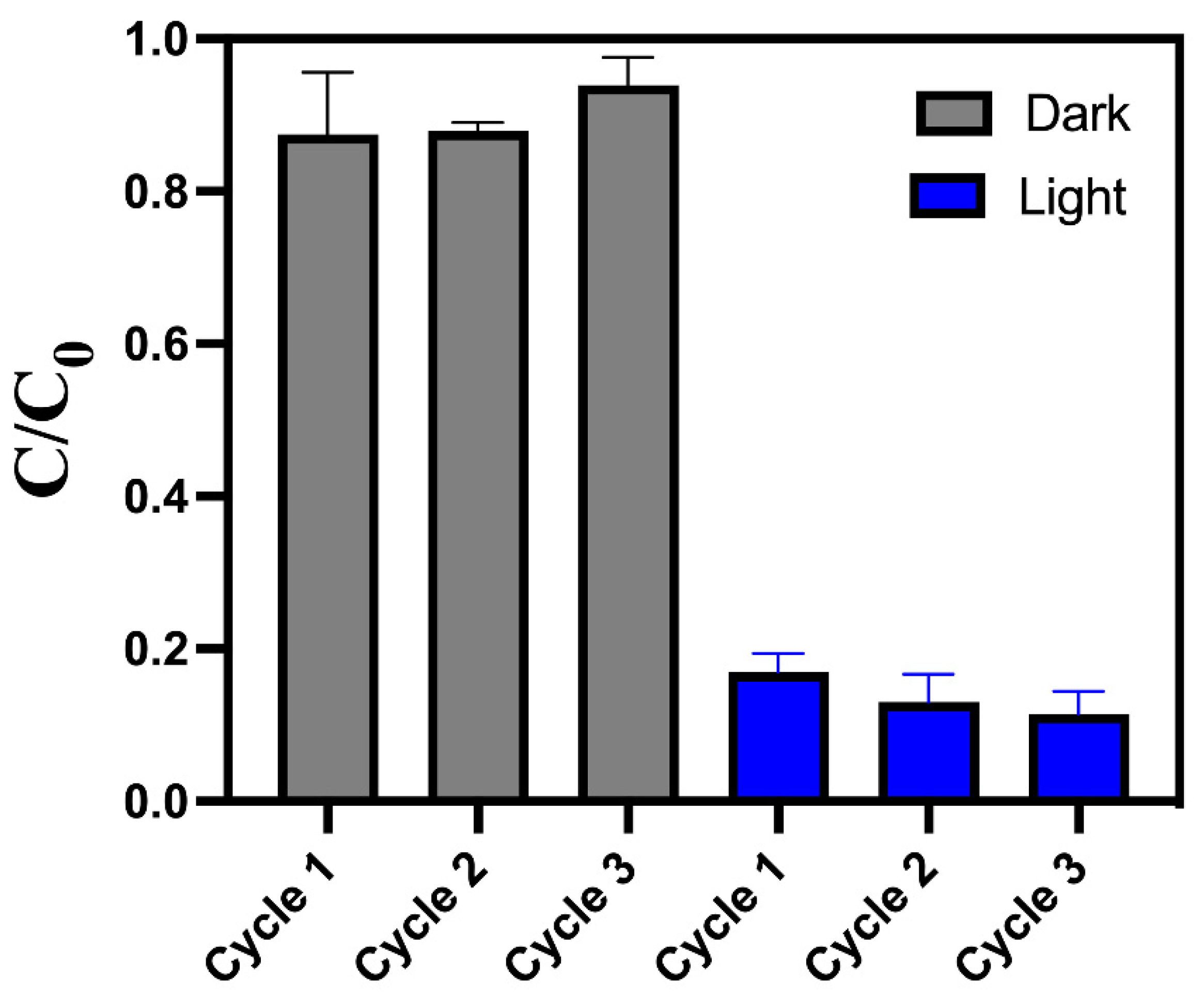
3.2. Photocataytic experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgements
Conflicts of Interest
References
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Reviews in Aquaculture 2020, 12, 640–663. [Google Scholar]
- Harrower, J.; McNaughtan, M.; Hunter, C.; Hough, R.; Zhang, Z.; Helwig, K. Chemical Fate and Partitioning Behavior of Antibiotics in the Aquatic Environment—A Review. Environmental Toxicology and Chemistry 2021, 40, 3275–3298. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, Q.; Li, S.; Gao, M.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Zheng, D.; Xu, Q. Impact of sulfadiazine on performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater. Bioresource Technol 2017, 235, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Feng, Y.; Hu, C.; Xiao, X.; Yu, D.; Zou, X. Mechanistic model for interpreting the toxic effects of sulfonamides on nitrification. J Hazard Mater 2016, 305, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.K.; Conceição, A.C.S.; Kohatsu, M.Y.; Okamoto, A.B.; Coelho, L.H.; Subtil, E.L.; de Freitas Bueno, R. Effect of Amoxicillin on Nitrogen Oxidation Bacteria Present in Activated Sludge: Respirometry Investigation. Curr Microbiol 2021, 78, 167–178. [Google Scholar] [CrossRef]
- Rocha, H.F.; Silva, V.; Lima, D.L.D.; Calisto, V. Evaluation of the impact of photodegradation processes on the environmental persistence of amoxicillin. Case Studies in Chemical and Environmental Engineering 2024, 9, 100724. [Google Scholar] [CrossRef]
- Dong, F.-X.; Yan, L.; Huang, S.-T.; Liang, J.-Y.; Zhang, W.-X.; Yao, X.-W.; Chen, X.; Qian, W.; Guo, P.-R.; Kong, L.-J. , et al. Removal of antibiotics sulfadiazine by a biochar based material activated persulfate oxidation system: Performance, products and mechanism. Process Safety and Environmental Protection 2022, 157, 411–419. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics traces in the aquatic environment: persistence and adverse environmental impact. Current Opinion in Environmental Science & Health 2020, 13, 68–74. [Google Scholar]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nature Reviews Microbiology 2022, 20, 257–269. [Google Scholar]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environmental Chemistry Letters 2014, 12, 27–47. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem Rev 2014, 114, 9919–9986. [Google Scholar] [PubMed]
- Sakar, M.; Mithun Prakash, R.; Do, T.-O. Insights into the TiO2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Hu, K.; Nie, G.; Yang, Y.; Wang, Y.; Duan, X.; Wang, S. Carbon dots based photocatalysis for environmental applications. Journal of Environmental Chemical Engineering 2022, 10, 107336. [Google Scholar]
- Deng, Y.; Chen, M.; Chen, G.; Zou, W.; Zhao, Y.; Zhang, H.; Zhao, Q. Visible–Ultraviolet Upconversion Carbon Quantum Dots for Enhancement of the Photocatalytic Activity of Titanium Dioxide. ACS Omega 2021, 6, 4247–4254. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Tang, C.; Park, Y.; Du, A.; Ayoko, G.A.; Hwang, Y.; Chae, S. Visible-light-driven photocatalytic degradation of tetracycline using citric acid and lemon juice-derived carbon quantum dots incorporated TiO2 nanocomposites. Separation and Purification Technology 2024, 350, 127836. [Google Scholar]
- Sendão, R.M.S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Photocatalytic removal of pharmaceutical water pollutants by TiO2 – Carbon dots nanocomposites: A review. Chemosphere 2022, 301, 134731. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhu, L.; Li, Y.; Yan, Z.; Shen, Z.; Cao, X. Facile microwave assisted synthesis of N-rich carbon quantum dots/dual-phase TiO2 heterostructured nanocomposites with high activity in CO2 photoreduction. Applied Catalysis B: Environmental 2018, 231, 269–276. [Google Scholar] [CrossRef]
- Silva, V.; Fernandes, J.F.A.; Tomás, M.C.; Silva, C.P.; Calisto, V.; Otero, M.; Lima, D.L.D. Enhanced solar driven photocatalytic removal of antibiotics from aquaculture effluents by TiO2/carbon quantum dot composites. Catalysis Today 2023, 419, 114150. [Google Scholar]
- Han, L.; Hou, L.; Du, X.; Li, Y.; Liu, R.; Nikolai, M.; Sun, Z. Electrospinning nanomaterials: a powerful strategy for wastewater treatment applications. Reviews in Environmental Science and Bio/Technology 2024, 23, 471–502. [Google Scholar]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Sheikh Abdul Kadir, S.H.; Kurniawan, T.A.; Jilani, A. Immobilization techniques of a photocatalyst into and onto a polymer membrane for photocatalytic activity. RSC Advances 2021, 11, 6985–7014. [Google Scholar] [CrossRef]
- Zhou, X.; Shao, C.; Yang, S.; Li, X.; Guo, X.; Wang, X.; Li, X.; Liu, Y. Heterojunction of g-C3N4/BiOI Immobilized on Flexible Electrospun Polyacrylonitrile Nanofibers: Facile Preparation and Enhanced Visible Photocatalytic Activity for Floating Photocatalysis. ACS Sustainable Chemistry & Engineering 2018, 6, 2316–2323. [Google Scholar]
- Zhu, J.; Shao, C.; Li, X.; Han, C.; Yang, S.; Ma, J.; Li, X.; Liu, Y. Immobilization of ZnO/polyaniline heterojunction on electrospun polyacrylonitrile nanofibers and enhanced photocatalytic activity. Materials Chemistry and Physics 2018, 214, 507–515. [Google Scholar] [CrossRef]
- Almeida, N.A.; Martins, P.M.; Teixeira, S.; Lopes da Silva, J.A.; Sencadas, V.; Kühn, K.; Cuniberti, G.; Lanceros-Mendez, S.; Marques, P.A.A.P. TiO2/graphene oxide immobilized in P(VDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. Journal of Materials Science 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- Lin, X.; Fang, H.; Wang, L.; Sun, D.; Zhao, G.; Xu, J. Photocatalytic Degradation of Sulfamethoxazole and Enrofloxacin in Water Using Electrospun Composite Photocatalytic Membrane. Water 2024, 16, 218. [Google Scholar] [CrossRef]
- Enesca, A.; Cazan, C. Polymer Composite-Based Materials with Photocatalytic Applications in Wastewater Organic Pollutant Removal: A Mini Review. Polymers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Daels, N.; Radoicic, M.; Radetic, M.; Van Hulle, S.W.H.; De Clerck, K. Functionalisation of electrospun polymer nanofibre membranes with TiO2 nanoparticles in view of dissolved organic matter photodegradation. Separation and Purification Technology 2014, 133, 282–290. [Google Scholar] [CrossRef]
- Louros, V.L.; Ferreira, L.M.; Silva, V.G.; Silva, C.P.; Martins, M.A.; Otero, M.; Esteves, V.I.; Lima, D.L.D. Photodegradation of Aquaculture Antibiotics Using Carbon Dots-TiO2 Nanocomposites. Toxics 2021, 9, 330. [Google Scholar] [CrossRef]
- Guan, X.; Zheng, G.; Dai, K.; Liu, C.; Yan, X.; Shen, C.; Guo, Z. Carbon Nanotubes-Adsorbed Electrospun PA66 Nanofiber Bundles with Improved Conductivity and Robust Flexibility. ACS Applied Materials & Interfaces 2016, 8, 14150–14159. [Google Scholar]
- Kahraman, H.T. Fabrication of electrospun PA66 nanofibers loaded with biosynthesized silver nanoparticles: investigation of dye degradation and antibacterial activity. Environmental Science and Pollution Research 2024, 31, 53121–53134. [Google Scholar] [CrossRef]
- Silva, V.; Invêncio, I.; Silva, C.P.; Otero, M.; Lima, D.L.D. Photodegradation of oxolinic acid in aquaculture effluents under solar irradiation: is it possible to enhance efficiency by the use of TiO2/carbon quantum dots composites? Chemosphere 2022, 308, 136522. [Google Scholar] [CrossRef]
- Jia, C.; Fu, H.; Wang, Z.; Zhao, C.; Wang, C.-C. Enhanced mineralization capacity for photocatalytic toluene degradation over Ag3PO4/TiO2: the critical role of oxygen vacancy. Journal of Environmental Chemical Engineering 2024, 12, 112747. [Google Scholar] [CrossRef]
- Žerjav, G.; Teržan, J.; Djinović, P.; Barbieriková, Z.; Hajdu, T.; Brezová, V.; Zavašnik, J.; Kovač, J.; Pintar, A. TiO2-β-Bi2O3 junction as a leverage for the visible-light activity of TiO2 based catalyst used for environmental applications. Catalysis Today 2021, 361, 165–175. [Google Scholar] [CrossRef]
- Jose, M.V.; Steinert, B.W.; Thomas, V.; Dean, D.R.; Abdalla, M.A.; Price, G.; Janowski, G.M. Morphology and mechanical properties of Nylon 6/MWNT nanofibers. Polymer 2007, 48, 1096–1104. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.J.; Cadenas Pliego, G.; Andrade-Guel, M. Development of polymeric nanocomposites (Nylon 6/PVA with ZrO2/SiO2) by ultrasound-assisted melt-extrusion for adsorption of lead (II). Mater Lett 2024, 372, 137062. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J.; Xu, S.; Li, J.; Shen, L. Electron beam irradiation influencing the mechanical properties and water absorption of polycaprolactam (PA6) and polyhexamethylene adipamide (PA66). RSC Advances 2020, 10, 21481–21486. [Google Scholar] [CrossRef]
- Domingo, G.D.; Souza, A.M.C. PA6/PA66/talc composite: Effect of reprocessing on the structure and properties. Journal of Applied Polymer Science 2022, 139, 51869. [Google Scholar] [CrossRef]
- Loureiro dos Louros, V.; Silva, C.P.; Nadais, H.; Otero, M.; Esteves, V.I.; Lima, D.L.D. Photodegradation of sulfadiazine in different aquatic environments – Evaluation of influencing factors. Environmental Research 2020, 188, 109730. [Google Scholar] [CrossRef]
- Gao, B.; Wang, J.; Dou, M.; Xu, C.; Huang, X. Enhanced photocatalytic removal of amoxicillin with Ag/TiO2/mesoporous g-C3N4 under visible light: property and mechanistic studies. Environmental Science and Pollution Research 2020, 27, 7025–7039. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.D.N.; Nguyen, L.H.; Nguyen, X.H.; Phung, H.V.; The Vinh, T.H.; Van Viet, P.; Van Thai, N.; Le, H.N.; Pham, D.T.; Van, H.T. , et al. Enhanced heterogeneous photocatalytic perozone degradation of amoxicillin by ZnO modified TiO2 nanocomposites under visible light irradiation. Materials Science in Semiconductor Processing 2022, 142, 106456. [Google Scholar]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Research 2019, 163, 114841. [Google Scholar]
- Louros, V.L.; Silva, V.; Silva, C.P.; Calisto, V.; Otero, M.; Esteves, V.I.; Freitas, R.; Lima, D.L.D. Sulfadiazine's photodegradation using a novel magnetic and reusable carbon based photocatalyst: Photocatalytic efficiency and toxic impacts to marine bivalves. Journal of Environmental Management 2022, 313, 115030. [Google Scholar] [CrossRef] [PubMed]
| Matrix | pH | Conductivity (µS cm-1) |
Salinity (PSU) | TDS (mg L-1) | DO (%) | TOC (mg L-1) |
|---|---|---|---|---|---|---|
| PBS | 8.06 ± 0.01 | 105 | 0.05 | 53 | 88 | 1.81 ± 0.02 |
| River Water | 7.08 ± 0.01 | 171 | 0.1 | 101 | 120.5 | 4.8 ± 0.1 |
| Antibiotic | Matrix | Catalyst | k ± SD (h-1) | R2 | t1/2 (h) |
|---|---|---|---|---|---|
| AMX | PBS | n.a. | 0.012± 0.002 | 0.9572 | 60 ± 1 |
| PBS | PA66/TiO2/CQDs | 0.35± 0.01 | 0.9625 | 1.98 ± 0.06 | |
| River water | PA66/TiO2/CQDs | 0.197± 0.005 | 0.9702 | 3.52 ± 0.09 | |
| SDZ | PBS | n.a. | 0.129± 0.003 | 0.9887 | 5.4 ± 0.1 |
| PBS | PA66/TiO2/CQDs | 0.37± 0.01 | 0.9649 | 1.87 ± 0.05 | |
| River water | PA66/TiO2/CQDs | 0.52± 0.02 | 0.9641 | 1.33 ± 0.05 |
| Antibiotic | [Antibiotic] (mg L-1) |
Matrix | Photocatalyst | [Catalyst] (g L-1) |
k (h-1) | Ref. |
|---|---|---|---|---|---|---|
| AMX | 5 | Ultra-pure Water | Ag/TiO2(P25)/C3N4 | 1.0 | 3.68 | [38] |
| 50 | Distilated water pH 9.0 | TiO2/ZnO | 0.1 | 0.47 | [39] | |
| 100 | Distilated water pH 6.7 | Chitosan/TiO2(P25) | * | 0.56 ± 0.01 | [40] | |
| 10 | PBS pH 8.06 | TiO2/CQDs/PA66 | 1.5 | 0.35± 0.01 | This work | |
| 10 | River water | TiO2/CQDs/PA66 | 1.5 | 0.197± 0.005 | This work | |
| SDZ | 10 | PBS pH 8.3 | TiO2/CQDs | 0.5 | 0.71 ± 0.02 | [27] |
| 10 | PBS pH 8.6 | TiO2(P25)/CQDs | 0.5 | 4.81 ± 0.06 | [18] | |
| 10 | * | Magnetic biochar/TiO2 | 0.1 | 0.124 ± 0.008 | [41] | |
| 10 | PBS pH 8.06 | TiO2/CQDs/PA66 | 1.5 | 0.37± 0.01 | This work | |
| 10 | River water | TiO2/CQDs/PA66 | 1.5 | 0.52± 0.02 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





