Submitted:
28 September 2024
Posted:
29 September 2024
You are already at the latest version
Abstract
Keywords:
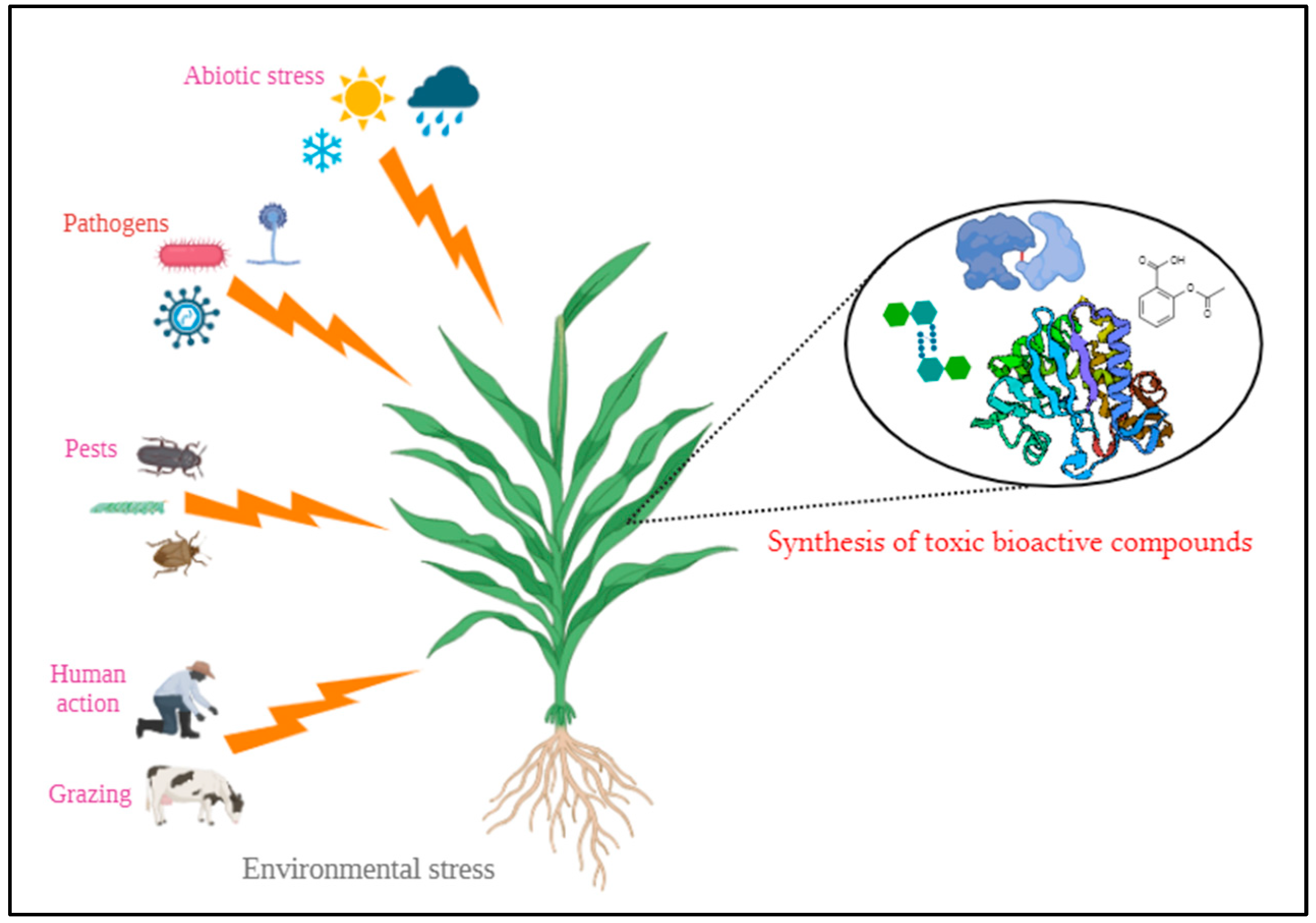
1. Introduction
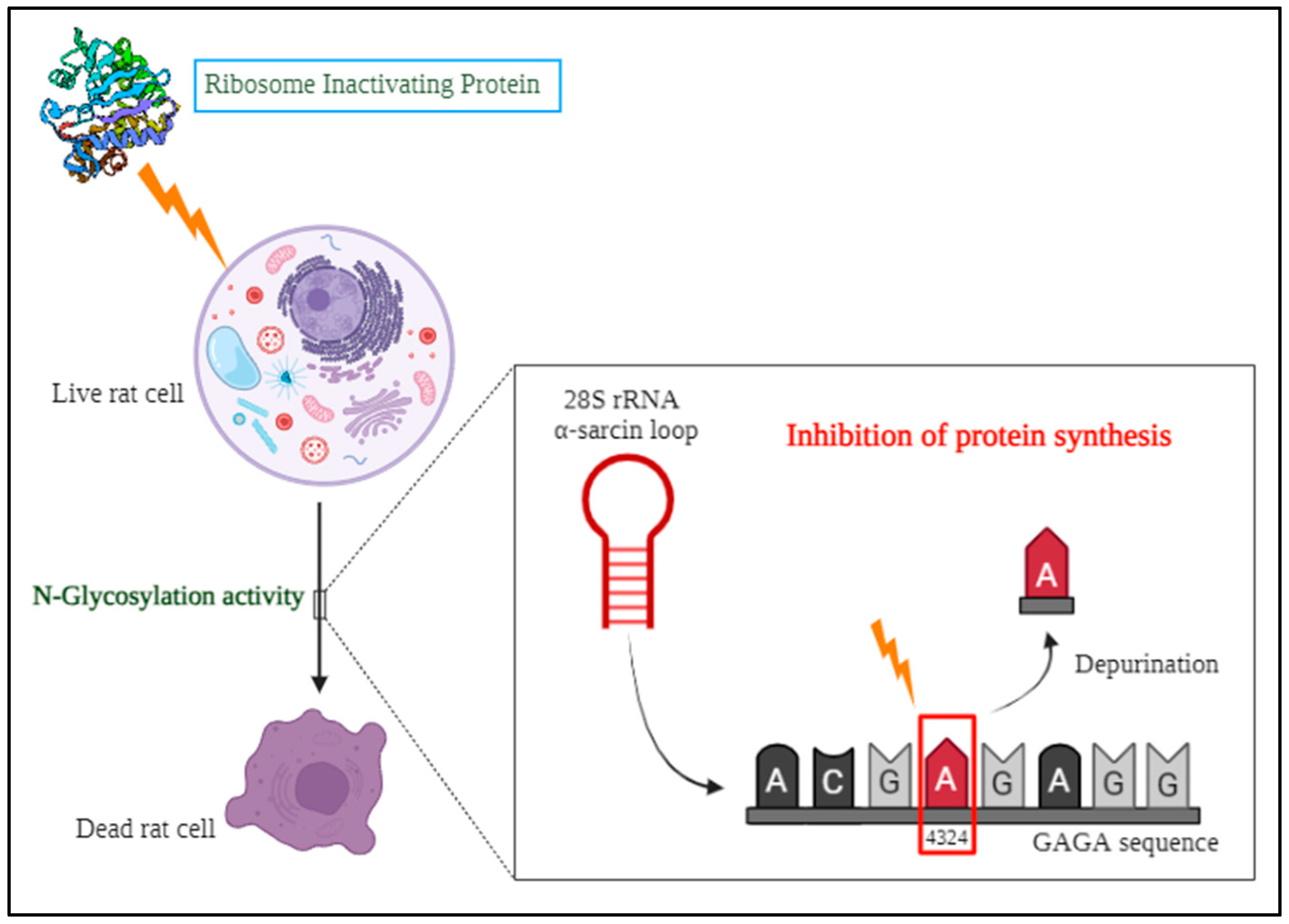
2. An overview of Ribosome Inactivating Proteins (RIPs)
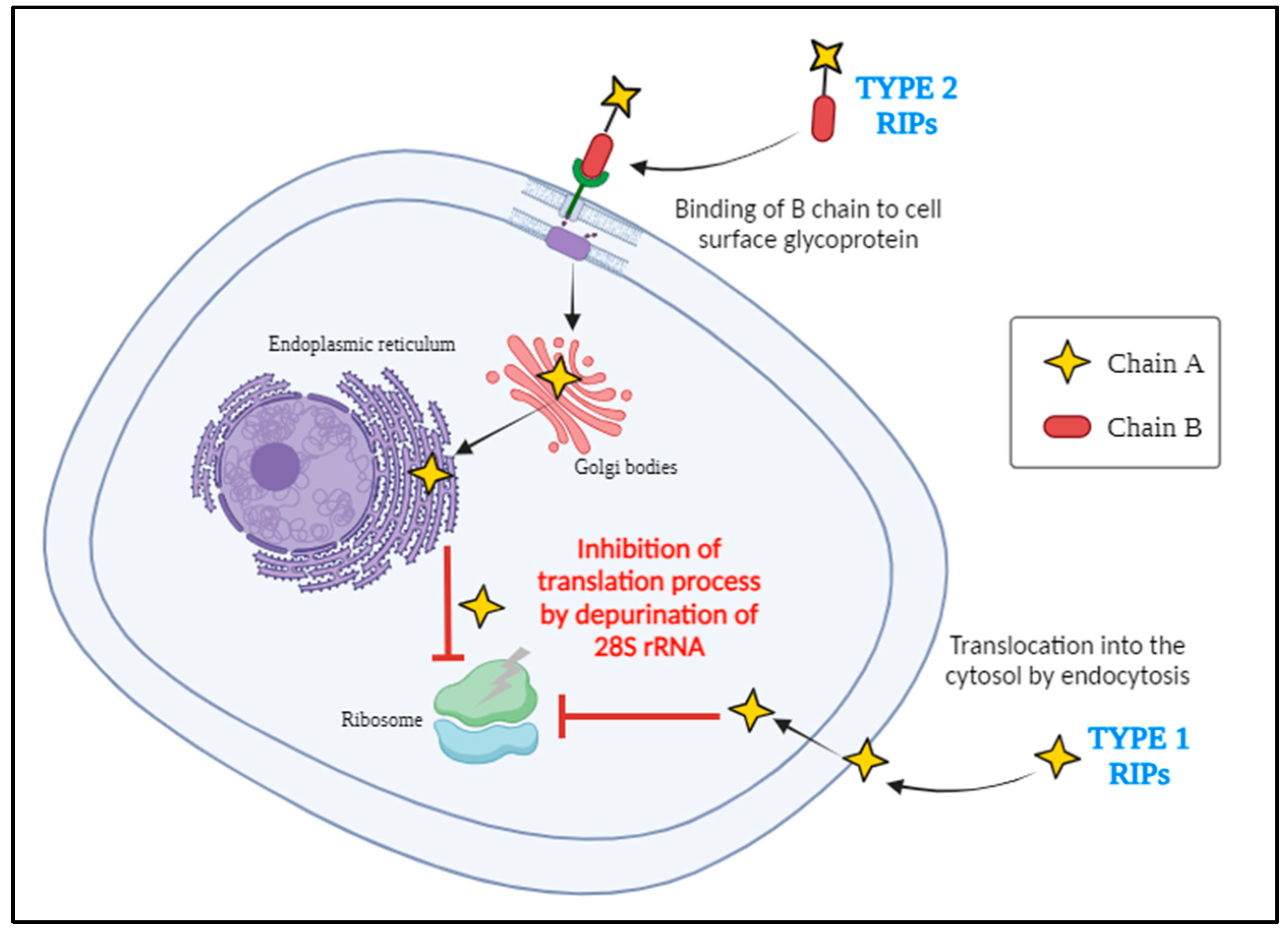
2.1. Origin and Types of RIPs
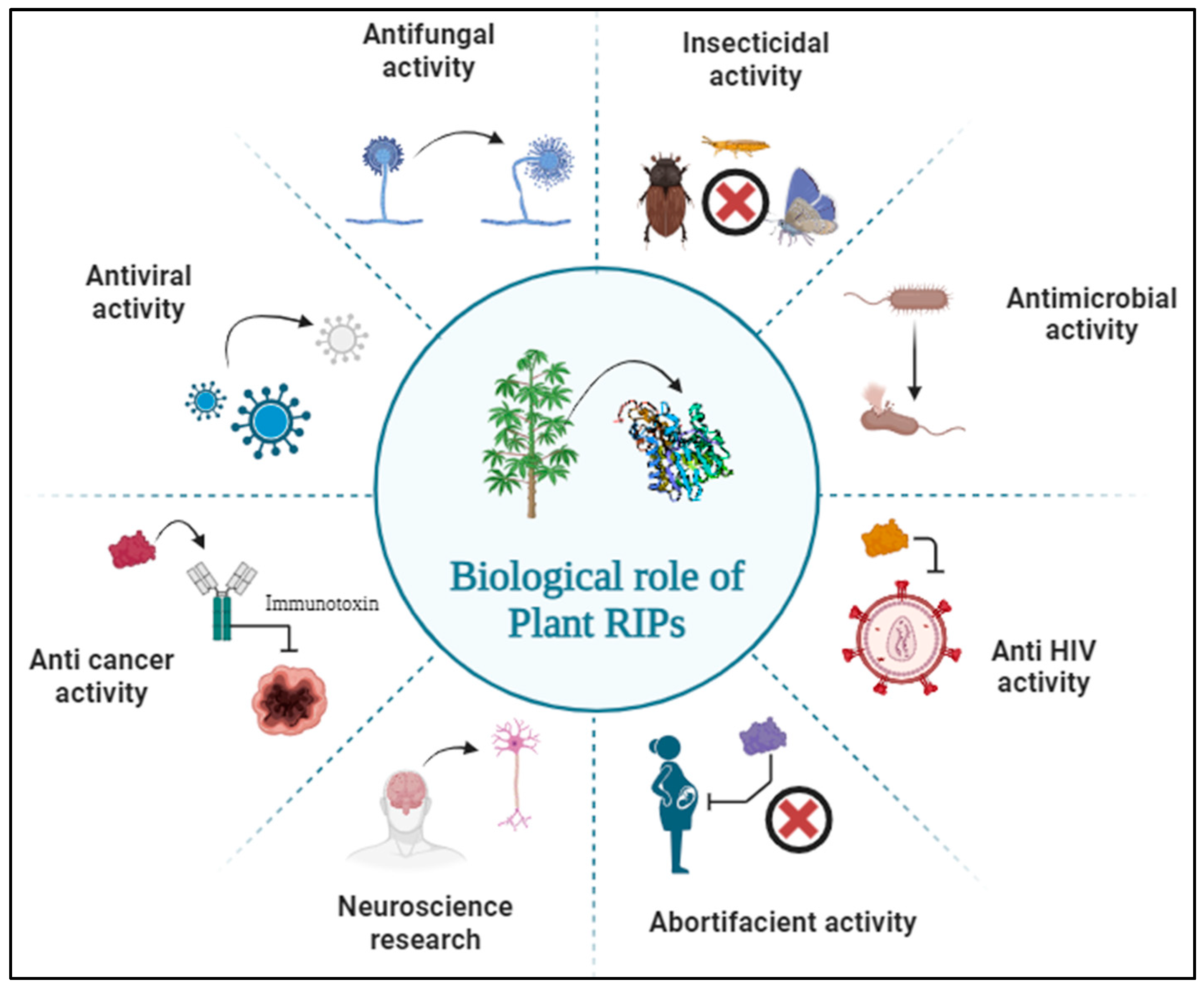
2.2. Biological Role of RIPs
3. Severity of Snakebite and Need for Effective Antivenom
3.1. Composition of Snake Venom
- Neurotoxin—Flaccid paralysis;
- Myotoxin—Systemic skeletal muscle damage;
- Haematotoxin—Interfere in haemostasis, causing either bleeding or thrombosis;
- Necrotoxin—Death of tissues at the bite site;
- Cardiotoxin—Direct damage to cardiovascular tissues;
- Nephrotoxin—Direct damage to renal tissues.
3.1.1. Phospholipase A2 (PLA2)
3.1.2. Three-Finger Toxins (3FTx)
3.1.3. Snake Venom Metalloproteases (SVMP)
3.1.4. Snake Venom Serine Proteases (SVSP)
3.2. Production of Antivenom for Curing Snakebites
4. Plants with Antivenom Properties in Traditional Medicine
5. In Silico Analysis of Plant Derived RIPs against Snake Venom Proteins
6. Future Prospects
7. Conclusions
Author Contributions
Funding and Acknowledgments
Conflicts of Interest
References
- Mackessy, S.P. Venom production and secretion in reptiles. Journal of Experimental Biology 2022, 225, jeb227348. [Google Scholar] [CrossRef]
- Warrell, D.A. Venomous animals. Medicine 2016, 44, 120–124. [Google Scholar] [CrossRef]
- Maag, D.; Erb, M.; Köllner, T.G.; Gershenzon, J. Defensive weapons and defense signals in plants: some metabolites serve both roles. BioEssays 2015, 37, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: chemical aspects. Annual review of plant biology 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, E.; Kocaadam-Bozkurt, B.; Bozkurt, O.; Ağagündüz, D.; Capasso, R. Plant Toxic Proteins: Their Biological Activities, Mechanism of Action and Removal Strategies. Toxins 2023, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Singh, H.; Bhardwaj, N.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Peng, W. An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Critical Reviews in Environmental Science and Technology 2022, 52, 395–435. [Google Scholar] [CrossRef]
- Stirpe, F.; Gilabert-Oriol, R. Ribosome-inactivating proteins: An overview. Plant Toxins; Carlini, CR, Ligabue-Braun, R., Gopalakrishnakone, P., Eds 2015, 153-182.
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-inactivating proteins from plants: A historical overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Bortolotti, M.; Battelli, M.G.; Polito, L. Hyperuricaemia, xanthine oxidoreductase and ribosome-inactivating proteins from plants: the contributions of Fiorenzo Stirpe to frontline research. Molecules 2017, 22, 206. [Google Scholar] [CrossRef]
- Stirpe, F. Ribosome-inactivating proteins: From toxins to useful proteins. Toxicon 2013, 67, 12–16. [Google Scholar] [CrossRef]
- Barbieri, L.; Valbonesi, P.; Righi, F.; Zuccheri, G.; Monti, F.; Gorini, P.; Samori, B.; Stirpe, F. Polynucleotide: adenosine glycosidase is the sole activity of ribosome-inactivating proteins on DNA. The journal of biochemistry 2000, 128, 883–889. [Google Scholar] [CrossRef]
- Nielsen, K.; Boston, R.S. Ribosome-inactivating proteins: a plant perspective. Annual review of plant biology 2001, 52, 785–816. [Google Scholar] [CrossRef] [PubMed]
- Lapadula, W.J.; Ayub, M.J. Ribosome Inactivating Proteins from an evolutionary perspective. Toxicon 2017, 136, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Van Damme, E.J. Evolution of plant ribosome-inactivating proteins. Toxic plant proteins 2010, 1–26. [Google Scholar]
- Dallal, J.A.; Irvin, J.D. Enzymatic inactivation of eukaryotic ribosomes by the pokeweed antiviral protein. FEBS letters 1978, 89, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhou, Y.-K.; Ji, Z.-L.; Chen, X.-R. The plant ribosome-inactivating proteins play important roles in defense against pathogens and insect pest attacks. Frontiers in Plant Science 2018, 9, 146. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Sharma, N.R.; Paul, K. Expanding role of ribosome-inactivating proteins: From toxins to therapeutics. IUBMB life 2023, 75, 82–96. [Google Scholar] [CrossRef]
- Frigerio, L.; Vitale, A.; Lord, J.M.; Ceriotti, A.; Roberts, L.M. Free ricin A chain, proricin, and native toxin have different cellular fates when expressed in tobacco protoplasts. Journal of Biological Chemistry 1998, 273, 14194–14199. [Google Scholar] [CrossRef]
- Zhu, R.H.; Ng, T.B.; Yeung, H.W.; Shaw, P.C. High level synthesis of biologically active recombinant trichosanthin in Escherichia coli. International Journal of Peptide and Protein Research 1992, 39, 77–81. [Google Scholar] [CrossRef]
- Carzaniga, R.; Sinclair, L.; Fordham-Skelton, A.P.; Harris, N.; Croy, R.R. Cellular and subcellular distribution of saporins, type-1 ribosome-inactivating proteins, in soapwort (Saponaria officinalis L.). Planta 1994, 194, 461–470. [Google Scholar] [CrossRef]
- Das, M.K.; Sharma, R.S.; Mishra, V. Induction of apoptosis by ribosome inactivating proteins: importance of N-glycosidase activity. Applied biochemistry and biotechnology 2012, 166, 1552–1561. [Google Scholar] [CrossRef]
- Moshiri, M.; Hamid, F.; Etemad, L. Ricin toxicity: Clinical and molecular aspects. Reports of biochemistry & molecular biology 2016, 4, 60. [Google Scholar]
- Jang, D.H.; Hoffman, R.S.; Nelson, L.S. Attempted suicide, by mail order: Abrus precatorius. Journal of medical toxicology 2010, 6, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Dickers, K.J.; Bradberry, S.M.; Rice, P.; Griffiths, G.D.; Vale, J.A. Abrin poisoning. Toxicological reviews 2003, 22, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, B.; Lei, N.; Zheng, J.; He, Q.; Li, D.; Zhao, X.; Shen, F. Alpha-momorcharin possessing high immunogenicity, immunotoxicity and hepatotoxicity in SD rats. Journal of ethnopharmacology 2012, 139, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.J.; Dodd, J.E.; Hautbergue, G.M. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef]
- Schep, L.J.; Temple, W.A.; Butt, G.A.; Beasley, M.D. Ricin as a weapon of mass terror—Separating fact from fiction. Environment international 2009, 35, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Patočka, J.; Středa, L. Plant toxic proteins and their current significance for warfare and medicine. J Appl Biomed 2003, 1, 141–147. [Google Scholar] [CrossRef]
- Divakar, M.N.; Rajesh, S.; Renukadevi, P.; Rajagopal, B. Cloning, Expression and in silico Characterization of a Truncated Antiviral Protein Gene from Bougainvillea spectabilis Willd. Int. J. Curr. Microbiol. App. Sci 2019, 8, 2828–2836. [Google Scholar] [CrossRef]
- Biology, C.f.P.M. A cDNA (BAPc DNA L1) from Bougainvillea spectabilis willd leaves encoding a protein with antiviral & ribosome inactivating activity and a method for obtaining the same. 24 8606, 2006. [Google Scholar]
- Akkouh, O.; Ng, T.B.; Cheung, R.C.F.; Wong, J.H.; Pan, W.; Ng, C.C.W.; Sha, O.; Shaw, P.C.; Chan, W.Y. Biological activities of ribosome-inactivating proteins and their possible applications as antimicrobial, anticancer, and anti-pest agents and in neuroscience research. Applied microbiology and biotechnology 2015, 99, 9847–9863. [Google Scholar] [CrossRef]
- Organization, W.H. Snakebite envenoming: a strategy for prevention and control. 2019.
- Organization, W.H. Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 01 February 2024).
- Yu, X.; Wu, N.C.; Ge, L.; Li, L.; Zhang, Z.; Lei, J. Artificial shelters provide suitable thermal habitat for a cold-blooded animal. Scientific Reports 2022, 12, 5879. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, W.; Warrell, D.; Whitaker, R.; Menon, G.; Rodrigues, R.; Fu, S.H.; Begum, R.; Sati, P.; Piyasena, K.; Bhatia, M. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. Elife 2020, 9, e54076. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.S.E. Global mortality of snakebite envenoming between 1990 and 2019. Nature Communications 2022, 13, 6160. [Google Scholar]
- Ralph, R.; Faiz, M.A.; Sharma, S.K.; Ribeiro, I.; Chappuis, F. Managing snakebite. BMJ 2022, 376. [Google Scholar] [CrossRef] [PubMed]
- Bolon, I.; Finat, M.; Herrera, M.; Nickerson, A.; Grace, D.; Schütte, S.; Martins, S.B.; de Castañeda, R.R. Snakebite in domestic animals: First global scoping review. Preventive Veterinary Medicine 2019, 170, 104729. [Google Scholar] [CrossRef] [PubMed]
- Wallach, V.; Williams, K.L.; Boundy, J. SnakeS of theWorld. A Catalogue of Living and Extinct Species. Boca Raton: CRC Press-Taylor & Francis Group 2014.
- Tednes, M.; Slesinger, T.L. Evaluation and treatment of snake envenomations. In StatPearls [Internet]; StatPearls Publishing: 2022.
- Goswami, P.K.; Samant, M.; Srivastava, R.S. Snake venom, anti-snake venom & potential of snake venom. International Journal of Pharmacy and Pharmaceutical Sciences 2014, 6, 4–7. [Google Scholar]
- Uetz, P. The Reptile Database. 2023.
- Simpson, I.D.; Norris, R.L. Snakes of medical importance in India: is the concept of the “Big 4” still relevant and useful? Wilderness & environmental medicine 2007, 18, 2–9. [Google Scholar]
- Kerkkamp, H.M.; Casewell, N.R.; Vonk, F.J. Evolution of the snake venom delivery system. Evolution of venomous animals and their toxins 2015, 1–11. [Google Scholar]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proceedings of the Royal Society B: Biological Sciences 2009, 276, 2443–2449. [Google Scholar] [CrossRef]
- Ojeda, P.G.; Ramírez, D.; Alzate-Morales, J.; Caballero, J.; Kaas, Q.; González, W. Computational studies of snake venom toxins. Toxins 2017, 10, 8. [Google Scholar] [CrossRef]
- Santhosh, M.S.; Hemshekhar, M.; Sunitha, K.; Thushara, R.; Jnaneshwari, S.; Kemparaju, K.; Girish, K. Snake venom induced local toxicities: plant secondary metabolites as an auxiliary therapy. Mini Rev Med Chem 2013, 13, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake venom peptides: Tools of biodiscovery. Toxins 2018, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A current perspective on snake venom composition and constituent protein families. Archives of Toxicology 2023, 97, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nature reviews Disease primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Toxinology Department, W.s.C.s.H.W. Clinical Toxinology Resources Website. Available online: http://www.toxinology.com/ (accessed on 07 February 2024).
- Marcussi, S.; Sant'Ana, C.D.; Oliveira, C.Z.; Quintero Rueda, A.; Menaldo, D.L.; Beleboni, R.O.; Stabeli, R.G.; Giglio, J.R.; M Fontes, M.R.; Soares, A.M. Snake venom phospholipase A2 inhibitors: medicinal chemistry and therapeutic potential. Current topics in medicinal chemistry 2007, 7, 743–756. [Google Scholar] [CrossRef]
- Doley, R.; Zhou, X.; Kini, R.M. Snake venom phospholipase A2 enzymes. Handbook of venoms and toxins of reptiles 2010, 1, 173–205. [Google Scholar]
- Saikia, D.; Mukherjee, A.K. Anticoagulant and membrane damaging properties of snake venom phospholipase A 2 enzymes. Snake Venoms 2017, 87–104. [Google Scholar]
- Teixeira, C.d.F.P.; Landucci, E.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 2003, 42, 947–962. [Google Scholar] [CrossRef]
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom–milestones of discovery. Toxicon 2013, 62, 50–55. [Google Scholar] [CrossRef]
- Nirthanan, S. Snake three-finger α-neurotoxins and nicotinic acetylcholine receptors: Molecules, mechanisms and medicine. Biochemical Pharmacology 2020, 181, 114168. [Google Scholar] [CrossRef]
- Das, D. Studies on the crude venom and purified three finger toxin of Naja kaouthia from north east India. 2015.
- Olaoba, O.T.; Dos Santos, P.K.; Selistre-de-Araujo, H.S.; de Souza, D.H.F. Snake venom metalloproteinases (SVMPs): a structure-function update. Toxicon: X 2020, 7, 100052. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, M.; Almeida, J.R.; Rajan, E.; Williams, H.F.; Townsend, F.; Cornish, E.; Mitchell, R.D.; Patel, K.; Vaiyapuri, S. Intramuscular bleeding and formation of microthrombi during skeletal muscle damage caused by a snake venom metalloprotease and a cardiotoxin. Toxins 2023, 15, 530. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.d.F.P.; Fernandes, C.M.; Zuliani, J.P.; Zamuner, S.F. Inflammatory effects of snake venom metalloproteinases. Memórias do Instituto Oswaldo Cruz 2005, 100, 181–184. [Google Scholar] [CrossRef]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake venom proteases affecting hemostasis and thrombosis. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 2000, 1477, 146–156. [Google Scholar] [CrossRef]
- Kini, R.M. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiology of haemostasis and thrombosis 2006, 34, 200–204. [Google Scholar] [CrossRef]
- Latinović, Z.; Leonardi, A.; Koh, C.Y.; Kini, R.M.; Trampuš Bakija, A.; Pungerčar, J.; Križaj, I. The procoagulant snake venom serine protease potentially having a dual, blood coagulation factor V and X-Activating activity. Toxins 2020, 12, 358. [Google Scholar] [CrossRef]
- Dias da Silva, W.; De Andrade, S.A.; Megale Â, A.A.; De Souza, D.A.; Sant'Anna, O.A.; Magnoli, F.C.; Guidolin, F.R.; Godoi, K.S.; Saladini, L.Y.; Spencer, P.J.; et al. Antibodies as Snakebite Antivenoms: Past and Future. Toxins (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K. Polyvalent Snake Antivenoms: Production Strategy and Their Therapeutic Benefits. Toxins 2023, 15, 517. [Google Scholar] [CrossRef]
- Tan, C.H. Snake Venomics: Fundamentals, recent Updates, and a look to the next decade. Toxins 2022, 14, 247. [Google Scholar] [CrossRef]
- Alangode, A.; Rajan, K.; Nair, B.G. Snake antivenom: Challenges and alternate approaches. Biochemical Pharmacology 2020, 181, 114135. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Jackson, T.N.; Laustsen, A.H.; Sunagar, K. Causes and consequences of snake venom variation. Trends in pharmacological sciences 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Modahl, C.M.; Brahma, R.K.; Koh, C.Y.; Shioi, N.; Kini, R.M. Omics technologies for profiling toxin diversity and evolution in snake venom: impacts on the discovery of therapeutic and diagnostic agents. Annual Review of Animal Biosciences 2020, 8, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, R.K.; Munshi, H.; Bawaskar, H.S. National programme for prevention & control of snakebite in India: Key challenges & recommendations. Indian J Med Res 2023, 157, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Vanuopadath, M.; Rajan, K.; Alangode, A.; Nair, S.S.; Nair, B.G. The need for next-generation antivenom for snakebite envenomation in india. Toxins 2023, 15, 510. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Mackessy, S.P. Prevention and improvement of clinical management of snakebite in Southern Asian countries: a proposed road map. Toxicon 2021, 200, 140–152. [Google Scholar] [CrossRef]
- Poddar, S.; Sarkar, T.; Choudhury, S.; Chatterjee, S.; Ghosh, P. Indian traditional medicinal plants: A concise review. International Journal of Botany Studies 2020, 5, 174–190. [Google Scholar]
- MS, V.; More, V.S.; Zameer, F.; Muddapur, U.; More, S.S. Ethnomedicinal plants and isolated compounds against Snake venom activity: A review. 2021.
- Magowska, A. The natural history of the concept of antidote. Toxicology reports 2021, 8, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.M.; Sastry, K.V.; Bhise, S.B. A contemporary exploration of traditional Indian snake envenomation therapies. Tropical Medicine and Infectious Disease 2022, 7, 108. [Google Scholar] [CrossRef]
- Premendran, S.J.; Salwe, K.J.; Pathak, S.; Brahmane, R.; Manimekalai, K. Anti-cobra venom activity of plant Andrographis paniculata and its comparison with polyvalent anti-snake venom. J Nat Sci Biol Med 2011, 2, 198–204. [Google Scholar] [CrossRef]
- Singh, S.; Saxena, N. Traditional healing practices for treatment of animal bites among tribes of India: A systematic review. Indian Journal of Traditional Knowledge (IJTK) 2023, 22, 638–645. [Google Scholar]
- Bala, A.A.; Mohammed, M.; Umar, S.; Ungogo, M.A.; Hassan, M.A.-K.; Abdussalam, U.S.; Ahmad, M.H.; Ishaq, D.U.; Mana, D.; Sha'aban, A. Pre-clinical efficacy of African medicinal plants used in the treatment of snakebite envenoming: A systematic review. Toxicon 2023, 107035. [Google Scholar] [CrossRef] [PubMed]
- Mogha, N.G.; Kalokora, O.J.; Amir, H.M.; Kacholi, D.S. Ethnomedicinal plants used for treatment of snakebites in Tanzania–a systematic review. Pharmaceutical Biology 2022, 60, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, A.; Mallhi, T.H.; Khan, Y.H.; Khokhar, A.; Chaman, S.; Ali, M. Anti-snake venom properties of medicinal plants: a comprehensive systematic review of literature. Brazilian Journal of Pharmaceutical Sciences 2022, 58. [Google Scholar] [CrossRef]
- Upasani, S.V.; Upasani, M.S. Plants from northeast India utilize in snakebite treatment–an ethanobotanical review. 2021.
- Gbolade, A.A. Nigerian medicinal plants with anti-snake venom activity—A review. J Malar Res phytomedicine 2021, 4, 29–44. [Google Scholar]
- Omara, T.; Kagoya, S.; Openy, A.; Omute, T.; Ssebulime, S.; Kiplagat, K.M.; Bongomin, O. Antivenin plants used for treatment of snakebites in Uganda: ethnobotanical reports and pharmacological evidences. Tropical Medicine and Health 2020, 48, 1–16. [Google Scholar] [CrossRef]
- Dey, A.; De, J.N. Traditional use of plants against snakebite in Indian subcontinent: a review of the recent literature. Afr J Tradit Complement Altern Med 2012, 9, 153–174. [Google Scholar] [CrossRef]
- Gómez-Betancur, I.; Gogineni, V.; Salazar-Ospina, A.; León, F. Perspective on the Therapeutics of Anti-Snake Venom. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Singh, P.; Yasir, M.; Hazarika, R.; Sugunan, S.; Shrivastava, R. A Review on Venom Enzymes Neutralizing Ability of Secondary Metabolites from Medicinal Plants. J Pharmacopuncture 2017, 20, 173–178. [Google Scholar] [CrossRef]
- Adrião, A.A.; Dos Santos, A.O.; de Lima, E.J.; Maciel, J.B.; Paz, W.H.; da Silva, F.; Pucca, M.B.; Moura-da-Silva, A.M.; Monteiro, W.M.; Sartim, M.A. Plant-derived toxin inhibitors as potential candidates to complement antivenom treatment in snakebite envenomations. Frontiers in Immunology 2022, 13, 842576. [Google Scholar] [CrossRef]
- Sofyantoro, F.; Yudha, D.S.; Lischer, K.; Nuringtyas, T.R.; Putri, W.A.; Kusuma, W.A.; Purwestri, Y.A.; Swasono, R.T. Bibliometric analysis of literature in Snake venom-related research worldwide (1933–2022). Animals 2022, 12, 2058. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Mukaila, Y.O.; Ajao, A.A.-n.; Sabiu, S. An appraisal of antidotes’ effectiveness: Evidence of the use of phyto-antidotes and biotechnological advancements. Molecules 2020, 25, 1516. [Google Scholar] [CrossRef] [PubMed]
- H. M. Berman, J.W., Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, P.E. Bourne. The Protein Data Bank. Nucleic Acids Research 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Sharma, N.; Naorem, L.D.; Jain, S.; Raghava, G.P.S. ToxinPred2: an improved method for predicting toxicity of proteins. Briefings in Bioinformatics 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Bolognesi, A. Plants Producing Ribosome-Inactivating Proteins in Traditional Medicine. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Schrot, J.; Weng, A.; Melzig, M.F. Ribosome-inactivating and related proteins. Toxins 2015, 7, 1556–1615. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Peshin, S.S. Do herbal medicines have potential for managing snake bite envenomation? Toxicol Int 2012, 19, 89–99. [Google Scholar] [CrossRef]
- Shendge, P.N.; Belemkar, S. Therapeutic Potential of Luffa acutangula: A Review on Its Traditional Uses, Phytochemistry, Pharmacology and Toxicological Aspects. Front Pharmacol 2018, 9, 1177. [Google Scholar] [CrossRef]
- Félix-Silva, J.; Silva-Junior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Medicinal Plants for the Treatment of Local Tissue Damage Induced by Snake Venoms: An Overview from Traditional Use to Pharmacological Evidence. Evid Based Complement Alternat Med 2017, 2017, 5748256. [Google Scholar] [CrossRef]
- Jabbari, M.; Daneshfard, B.; Emtiazy, M.; Khiveh, A.; Hashempur, M.H. Biological Effects and Clinical Applications of Dwarf Elder ( Sambucus ebulus L): A Review. J Evid Based Complementary Altern Med 2017, 22, 996–1001. [Google Scholar] [CrossRef]
- Jenkins, T.P.; Fryer, T.; Dehli, R.I.; Jürgensen, J.A.; Fuglsang-Madsen, A.; Føns, S.; Laustsen, A.H. Toxin neutralization using alternative binding proteins. Toxins 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Ciaramella, V.; Ragucci, S.; Chambery, A.; Ciardiello, F.; Pedone, P.V.; Troiani, T.; Di Maro, A. A Novel EGFR Targeted Immunotoxin Based on Cetuximab and Type 1 RIP Quinoin Overcomes the Cetuximab Resistance in Colorectal Cancer Cells. Toxins 2023, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U. Therapeutic application of genetically engineered ribosome-inactivating toxin proteins for cancer. Journal ISSN 2021, 2766, 2276. [Google Scholar] [CrossRef]
- Flavell, D.J.; Flavell, S.U. Plant-Derived Type I Ribosome Inactivating Protein-Based Targeted Toxins: A Review of the Clinical Experience. Toxins 2022, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Knödler, M.; Buyel, J. Plant-made immunotoxin building blocks: A roadmap for producing therapeutic antibody-toxin fusions. Biotechnology Advances 2021, 47, 107683. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-Q.; Zhu, Z.-N.; Zheng, Y.-T.; Shaw, P.-C. Engineering of ribosome-inactivating proteins for improving pharmacological properties. Toxins 2020, 12, 167. [Google Scholar] [CrossRef]
- Miranda, A.; Ismail, H.; Martien, R.; Ciptasari, U.H.; Kusniasari, A.; Arimurni, D.A.; Wahyudi, S.M.D.P.; Sismindari, S. Double-Coated Nanoparticle of Ribosome Inactivating Protein (RIP) from Mirabilis jalapa L. prepared from Chitosan-Sodium Tripolyphosphate and Alginate-Calcium Chloride: The New Strategy for Protein Drug in Oral Delivery. In Proceedings of the BIO Web of Conferences, 2023; p. 04001.
- Pizzo, E.; Di Maro, A. A new age for biomedical applications of Ribosome Inactivating Proteins (RIPs): from bioconjugate to nanoconstructs. J Biomed Sci 2016, 23, 54. [Google Scholar] [CrossRef]
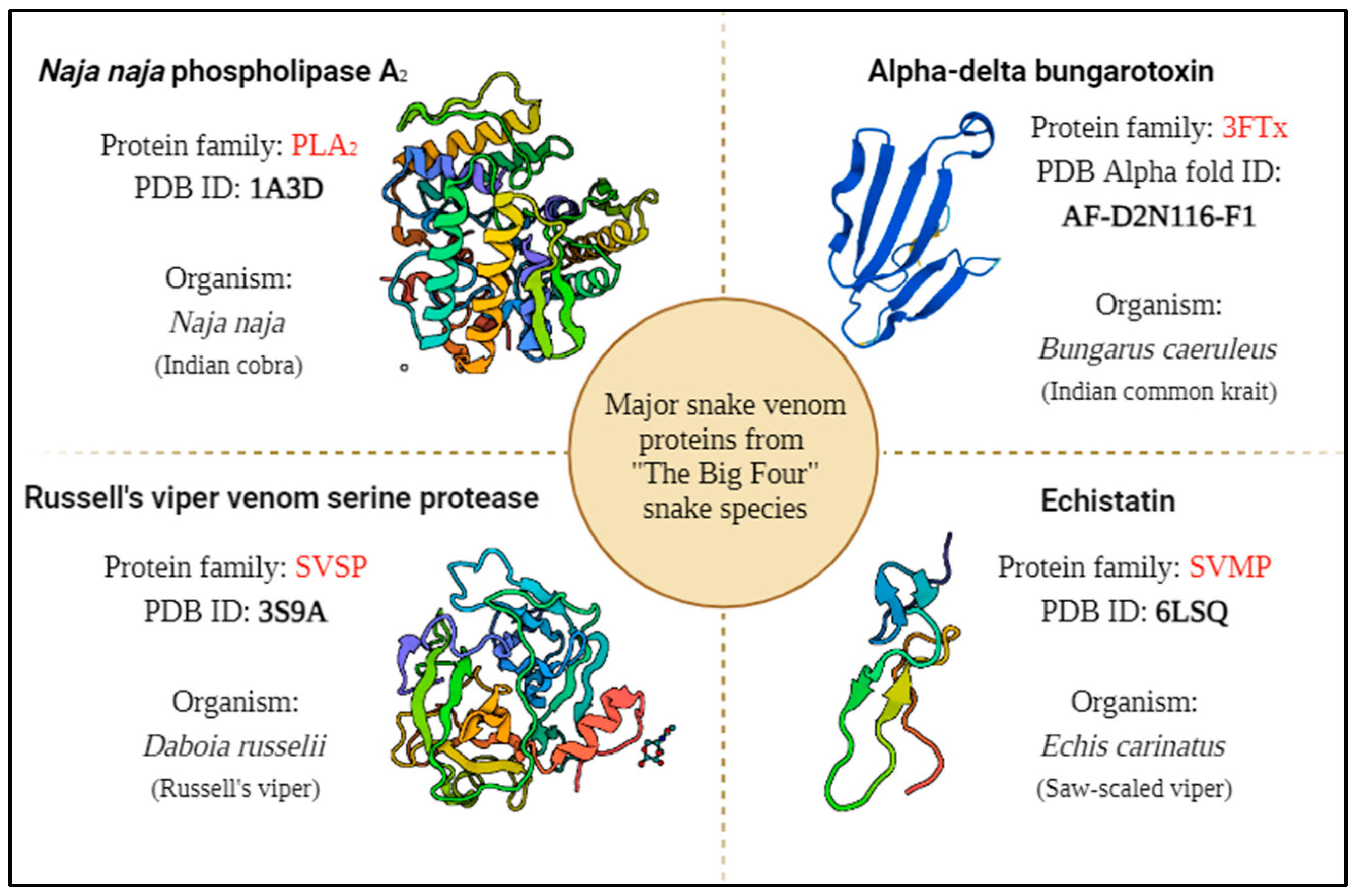
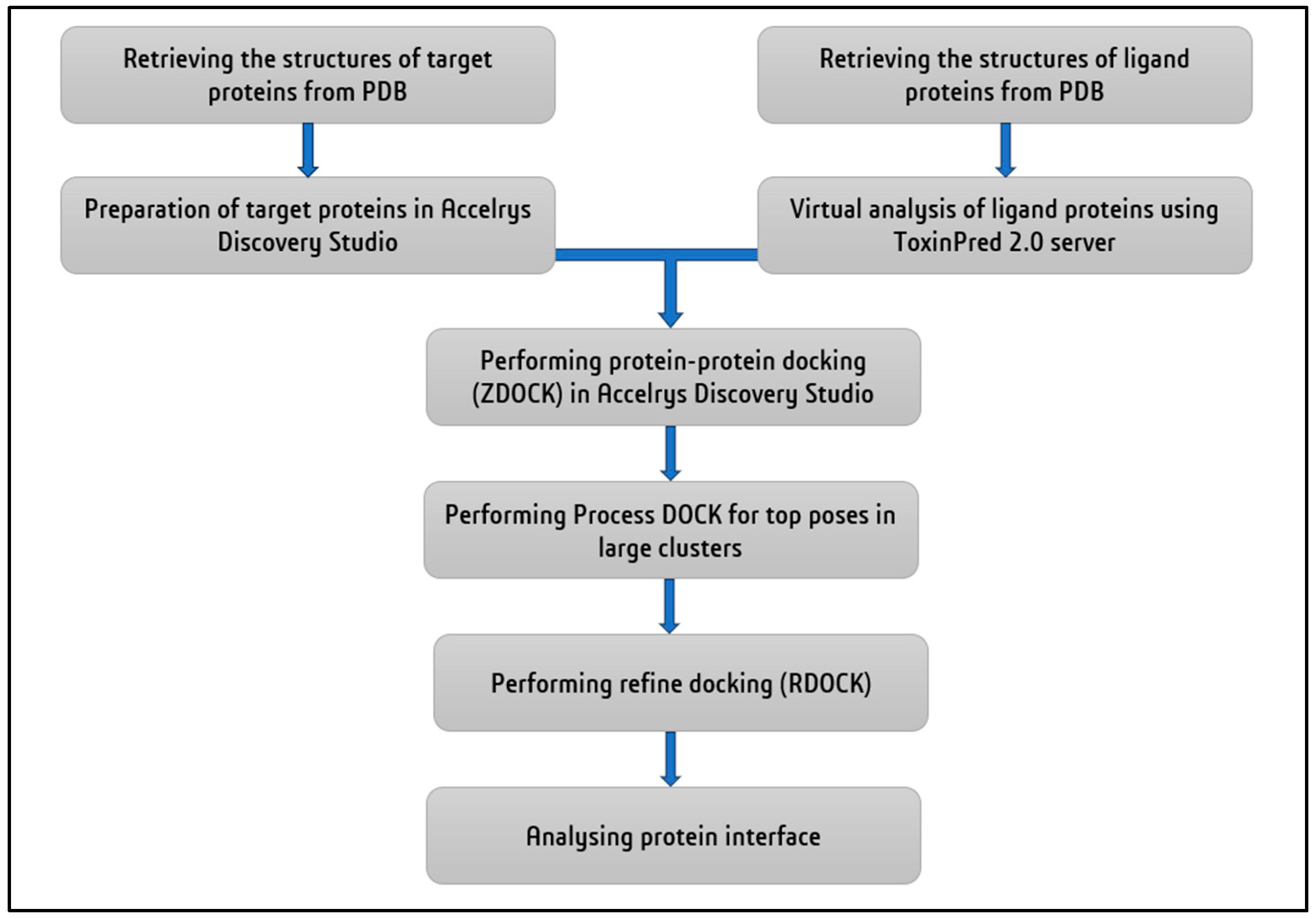
| S.No. | Groups | No. of Families | Family Names | References |
|---|---|---|---|---|
| 1. | Dominant protein family | 4 | Phospholipase A2 (PLA2), Three-finger toxins (3FTx), Snake venom metalloproteases (SVMP) and Snake venom serine proteases (SVSP) |
[49,50] |
| 2. | Secondary protein family | 6 | Kunitz peptides (KUN), L-amino acid oxidases (LAAO), Cysteine-rich secretory proteins (CRiSP), C-type lectins (CTL), Disintegrins (DIS) and Natriuretic peptides (NP) |
|
| 3. | Minor protein family | 9 | Acetylcholinesterase, Hyaluronidase, 5’nucleotidase, Phosphodiesterase, Phospholipase B, Nerve growth factor, Vascular endothelial growth factor, Vespryn/Ohanin and Snake venom metalloprotease inhibitor | |
| 4. | Rare protein family | 36 | Glutaminyl cyclase, Aminopeptidase, Endonuclease, Cobra venom factor, Transferrin, Waprin, Endopeptidase, Glutothione peroxidase, Kazal-type inhibitor, Galactose-binding protein, Trypsinogen, Albumin, Prokineticin, Selectin, Peroxiredoxin, Protein C activator, Cholinesterase, Polyglycine peptides, Glycine-histidine rich peptide, Flavine monoamine oxidase, Lysosomal acid lipase A, Fibrinogenases, Haemoglobins, Neurotrophin, Aspartic protease, Type-B carbyoxylesterase, Cytotoxin, Neuronal membrane glycoprotein, Insulin-like growth factor, Sulfhydryl oxidase, Aminotransferase, Complement decay accelerating factor, Kinesin-like protein, Ribosomal protein, Multiple inositol polyphosphate phosphatase and Phospholipase A2 inhibitor | |
| 5. | Unique protein family | 4 | Defensins, Waglerin, Maticotoxin and Cystatins |
| S.No. | Scientific Name of the Plant | Common Name | Family | Plant Parts Used for Curing Snakebite | Name of the RIPs Isolated | Type | Source of RIPs | PDB/ AlphaFold ID | Molecular Weight (kDa) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Abrus precatorius | Rosary pea | Fabaceae | Roots | Abrin | Type II | Seeds | 1ABR | 60.06 | [7,87,95,96] |
| APA-1 (Abrus precatorius Agglutinin 1) |
Type II | Seeds | 2ZR1 | 118.01 | ||||||
| 2. | Amaranthus viridis | Slender amaranth | Amaranthaceae | Leaves, stem | Amaranthin | Type I | Leaves | AF-Q9SAQ5-F1 | 30.4 | [7,95,96,97] |
| 3. | Luffa acutangula | Ridge gourd | Cucurbitaceae | Fruit, seed tendrils | Luffaculin | Type I | Seeds | 2OQA | 53.11 | [7,96,98] |
| 4. | Momordica charantia | Bitter gourd | Cucurbitaceae | Flower | Momordin | Type I | Seeds | 1AHA | 27.53 | [7,95,96,99] |
| β-momorcharin | Type I | Seeds | 1CF5 | 58.3 | ||||||
| 5. | Nicotiana tabacum | Tobacco | Solanaceae | Leaves | Nicotiana tabacum RIP | Type I | Leaves | AF-B0EVM3-F1 | 14.48 | [7,78,86,96] |
| 6. | Sambucus ebulus | Danewort | Adoxaceae | Leaves | Ebulin | Type II | Leaves, green fruits, rhizomes, barks | 1HWM | 58.95 | [7,95,96,100] |
| 7. | Viscum articulatum | Flat mistletoe | Santalaceae | Whole plant | Viscum articulatum RIP | Type II | Whole plant | AF-B3F5I6-F1 | 61.96 | [7,87,96] |
| ZDOCK Scores | ZRANK Scores | |||||||
|---|---|---|---|---|---|---|---|---|
| PLA2 | 3FTx | SVMP | SVSP | PLA2 | 3FTx | SVMP | SVSP | |
| Abrin | 55.99 | 58.22 | 52.88 | 61.72 | −97.538 | −92.226 | −93.556 | −95.095 |
| APA-1 | 54.61 | 48.96 | 49.53 | 54.5 | −93.592 | −90.773 | −96.193 | −102.322 |
| Amaranthin | 60.59 | 48.27 | 51.17 | 55.59 | −109.673 | −81.871 | −87.46 | −89.727 |
| Luffaculin | 55.35 | 52.5 | 45.41 | 51.27 | −118.52 | −99.576 | −85.885 | −114.996 |
| Momordin | 62.84 | 58.19 | 46.26 | 52.51 | −123.173 | −94.896 | −98.891 | −96.195 |
| β-momorcharin | 58.19 | 62.33 | 50.12 | 55.02 | −118.085 | −109.959 | −87.866 | −99.142 |
| Nicotiana tabacum RIP | 56.06 | 62.51 | 46.34 | 54.59 | −125.688 | −113.133 | −93.512 | −127.68 |
| Ebulin | 50.89 | 52.49 | 46.42 | 58.09 | −108.197 | −102.067 | −73.95 | −101.827 |
| Viscum articulatum RIP | 74.06 | 64.39 | 66.08 | 69.31 | −105.068 | −98.496 | −97.277 | −113.711 |
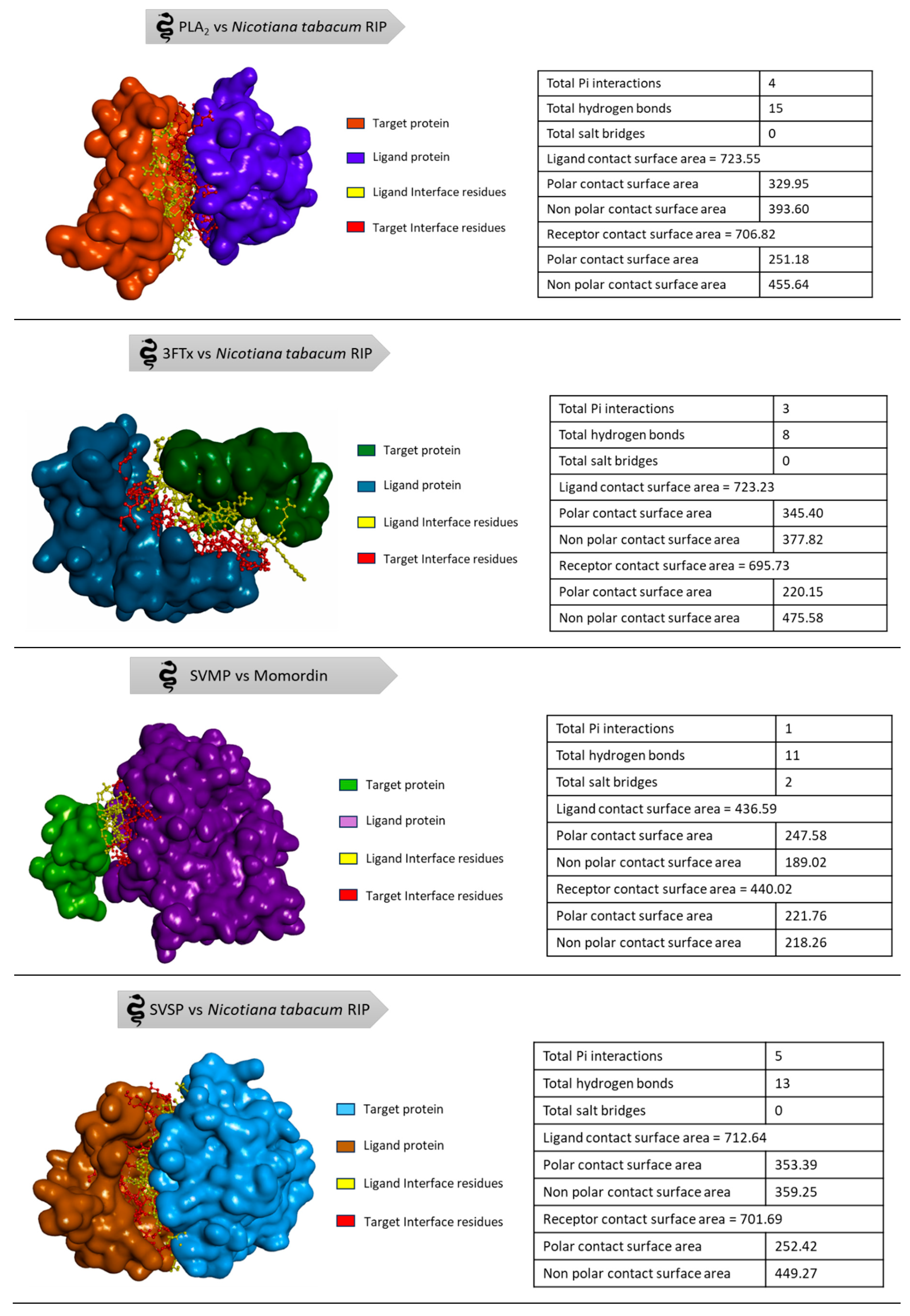
| Best PPI. | ZRANK Scores | Interface Residues of Target Protein Involved in the Interaction | Interface Residues of Ligand Protein Involved in the Interaction | |
|---|---|---|---|---|
| Target Protein | Ligand Protein | |||
| PLA2 | Nicotiana tabacum RIP | −125.688 | ASP39, ARG42, CYS43, GLN45, VAL46, ASN49, CYS50, GLU53, ALA54, GLU70, SER72, GLN73, GLY74, THR75, LEU76, THR77, CYS78, LYS79, GLY80, ASN82, SER88, ASP91, CYS92, ARG94, LEU95, ALA96, ILE98, CYS99, ALA101, GLY102, ALA103, PRO104 | THR1, ASN2, VAL3, VAL5, MET6, GLY7, TYR8, LEU9, VAL10, ASN11, SER12, ALA25, GLN27, TYR28, VAL29, PHE30, LYS31, GLY32, SER33, THR34, PHE62, PHE65, ILE69, PHE73, ILE87, THR90, THR91, ALA92, ALA94, SER95, ILE104 |
| 3FTx | Nicotiana tabacum RIP | −113.133 | TYR1, LEU3, SER20, GLY21, ASN23, LEU24, THR27, MET29, LYS40, ALA48, THR49, CYS50, PRO51, GLN52, PRO53, GLU58, THR60, CYS61, CYS62, SER63, THR64, ASP65, LYS66, CYS67, ASN68, PRO69, PRO71, GLN73, ARG74, PRO75, GLY76 | THR1, ASN2, VAL3, TYR4, VAL5, MET6, GLY7, TYR8, LEU9, VAL10, ASN11, SER12, PHE16, ALA25, TYR28, VAL29, PHE30, LYS31, GLY32, SER33, PHE62, PHE65, ASP66, SER67, ILE69, THR70, LEU72, PHE73, ILE87, THR90, THR91, ALA92, SER95, TRP125 |
| SVMP | Momordin | −98.891 | GLU1, CYS2, GLU3, SER4, GLY5, PRO6, CYS8, ARG9, ASN10, CYS11, LYS12 | ILE116, ALA117, ALA118, GLY119, LYS120, LYS124, ILE125, PRO126, PRO130, ALA131, ASP133, SER134, SER137, THR138, HIS141, ASP143, THR145, ALA146, ALA147, GLY149, VAL153, ASP178 |
| SVSP | Nicotiana tabacum RIP | −127.68 | ALA56, HIS57, ASP59, ARG60, ARG62, LYS88, TYR89, PHE90, CYS91, LEU92, ASN93, THR94, LYS95, PRO96, ASN97, GLY98, LEU99, PRO170, LEU171, TYR172, TRP173, HIS192, SER217, GLU218, LYS224, ARG245 | VAL3, TYR4, VAL5, MET6, GLY7, TYR8, ASN11, TYR28, VAL29, PHE30, LYS31, GLY32, PHE62, PHE65, ASP66, ILE69, THR70, PHE73, ILE87, ILE88, THR90, THR91, ALA94, SER95, GLU101, ILE104, VAL105, ILE108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





