Submitted:
29 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
- T (Tumor): The size and extent of the primary tumor.
- N (Nodes): The involvement of regional lymph nodes.
- M (Metastasis): The presence of distant metastasis.
- Stage I-III: This group included patients with resectable or borderline resectable disease. These patients were eligible for curative surgical resection, either through pancreaticoduodenectomy (for tumors located in the head of the pancreas) or distal pancreatectomy with splenectomy (for tumors located in the body or tail of the pancreas).
- Stage IV: This group comprised patients with unresectable, locally advanced, or metastatic disease. These patients either underwent palliative surgery or biopsy depending on the extent of the disease at the time of surgery.
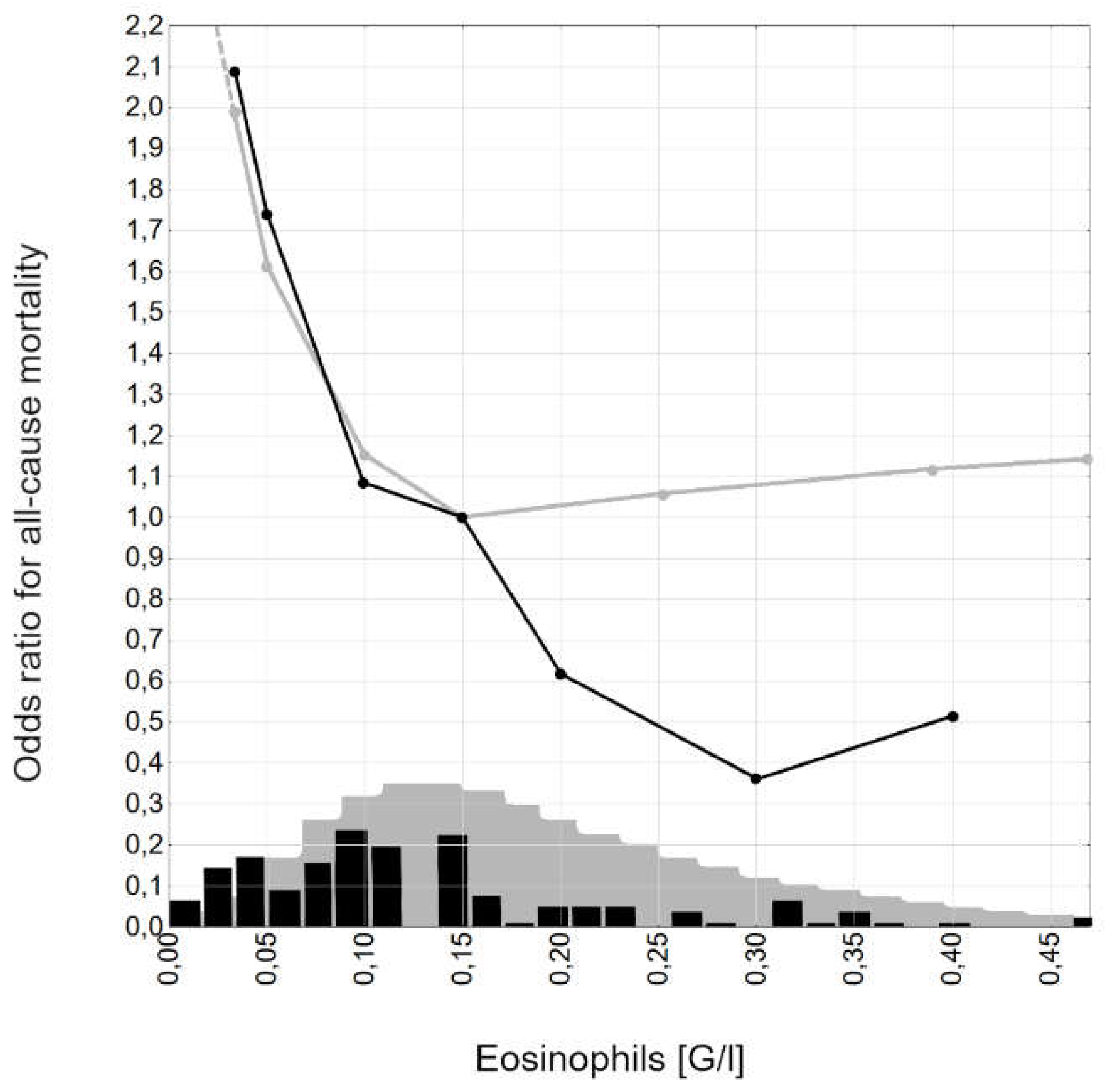
3. Results
4. Discussion
4.1. Eosinophilia and Malignancies
4.2. Eosinophilia in PDAC Patients
- Patient Population: Our cohort included patients with both resectable (Stage I-III) and unresectable (Stage IV) PDAC, while Ohkuma et al. focused solely on resectable stage II patients. The inclusion of patients with advanced-stage disease in our study likely contributed to the overall shorter survival outcomes.
- Follow-up and treatment protocols: The duration of follow-up and the therapeutic approaches, such as adjuvant chemotherapy and radiation, varied between studies. In our cohort, patients with stage IV disease were more likely to have received palliative care, which could explain the shorter survival times observed. The specific treatment protocols followed by patients in the Ohkuma study were also not explicitly comparable to ours, potentially contributing to the differences in outcomes.
- Methodology: Differences in the statistical methodologies used may also explain the variation in survival data. While both studies used Cox proportional hazard models, the cutoff points for eosinophil levels and the variables included in the multivariate analyses differed. Ohkuma et al. used a cutoff of EOS < 0.126 G/l, while we used EOS < 0.1 G/l. Additionally, our study did not focus specifically on the eosinophil-to-lymphocyte ratio, which may further explain the differences in our results.
4.3. Anti-Eosinophil Treatment and the Risk of Developing Malignancy
4.4. Therapeutic Potential
4.5. Curative versus Palliative Treatment
4.6. Study Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Weyhe, D.; Obonyo, D.; Uslar, V.N.; Stricker, I.; Tannapfel, A. Predictive factors for long-term survival after surgery for pancreatic ductal adenocarcinoma: Making a case for standardized reporting of the resection margin using certified cancer center data. PLOS ONE 2021, 16, e0248633. [Google Scholar] [CrossRef]
- Yang, J.-J.; Hu, Z.-G.; Shi, W.-X.; Deng, T.; He, S.-Q.; Yuan, S.-G. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 2807–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, S.; Fathy, A.H.; Qian, H.; Zhao, Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies. OncoTargets Ther. 2018, 11, 1899–1908. [Google Scholar] [CrossRef]
- Andersen, C.L.; Siersma, V.D.; Hasselbalch, H.C.; Vestergaard, H.; Mesa, R.; Felding, P.; Olivarius, N.D.; Bjerrum, O.W. Association of the blood eosinophil count with hematological malignancies and mortality. Am. J. Hematol. 2014, 90, 225–229. [Google Scholar] [CrossRef]
- Majos, A.; Sewerynek, E.; Grząsiak, O.; Ciesielski, W.; Hogendorf, P.; Hołyński, J.; Strzelczyk, J.; Durczyński, A. FT3 to FT4 Conversion Ratio May Be an Independent Prognostic Factor in Pancreatic Cancer Patients. Biomedicines 2022, 11, 77. [Google Scholar] [CrossRef]
- Szmiel, A.; Majos, A.; Ciesielski, W.; Kumor, A.; Strzelczyk, J.; Szwedziak, K.; Hogendorf, P.; Durczyński, A. Gender-Specific Coagulation Profiles of Peripheral and Portal Blood May Help to Differentiate Malignant from Benign Pancreatic Tumour—Pilot Study. J. Clin. Med. 2022, 11, 1573. [Google Scholar] [CrossRef]
- Upparahalli Venkateshaiah, S.; Manohar, M.; Kandikattu, H.K.; Mishra, A. Experimental Modeling of Eosinophil-Associated Diseases. Methods Mol Biol. 2021; 2241, 275–291. [Google Scholar]
- Fettrelet, T.; Gigon, L.; Karaulov, A.; Yousefi, S.; Simon, H.U. The Enigma of Eosinophil Degranulation. International journal of molecular sciences 2021, 22, 7091. [Google Scholar] [CrossRef]
- Gurtner, A.; Gonzalez-Perez, I.; Arnold, I.C. Intestinal eosinophils, homeostasis and response to bacterial intrusion. Semin. Immunopathol. 2021, 43, 295–306. [Google Scholar] [CrossRef]
- Rodrigo-Muñoz, J.M.; Gil-Martínez, M.; Sastre, B.; del Pozo, V. Emerging Evidence for Pleiotropism of Eosinophils. Int. J. Mol. Sci. 2021, 22, 7075. [Google Scholar] [CrossRef]
- Jacobsen, E.A.; Jackson, D.J.; Heffler, E.; Mathur, S.K.; Bredenoord, A.J.; Pavord, I.D.; Akuthota, P.; Roufosse, F.; Rothenberg, M.E. Eosinophil Knockout Humans: Uncovering the Role of Eosinophils Through Eosinophil-Directed Biological Therapies. Annu. Rev. Immunol. 2021, 39, 719–757. [Google Scholar] [CrossRef] [PubMed]
- Simson, L.; Ellyard, J.I.; Dent, L.A.; Matthaei, K.I.; Rothenberg, M.E.; Foster, P.S.; Smyth, M.J.; Parish, C.R. Regulation of Carcinogenesis by IL-5 and CCL11: A Potential Role for Eosinophils in Tumor Immune Surveillance. J. Immunol. 2007, 178, 4222–4229. [Google Scholar] [CrossRef] [PubMed]
- Holub, K.; Conill, C. Unveiling the mechanisms of immune evasion in pancreatic cancer: may it be a systemic inflammation responsible for dismal survival? Clin Transl Oncol. 2020, 22, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Erdmann, M.; Uslu, U.; Vass, V.; Schuler, G.; Schuler-Thurner, B. Blood Eosinophilia Is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA. Pharmaceutics 2020, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Dias, M.; Campainha, S.; Barroso, A. Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J. Thorac. Dis. 2021, 13, 2716–2727. [Google Scholar] [CrossRef]
- Ghebeh, H.; A Elshenawy, M.; AlSayed, A.D.; Al-Tweigeri, T. Peripheral Blood Eosinophil Count is Associated with Response to Chemoimmunotherapy in Metastatic Triple-Negative Breast Cancer. Immunotherapy 2022, 14, 189–199. [Google Scholar] [CrossRef]
- Andersen, C.L.; Siersma, V.D.; Hasselbalch, H.C.; Lindegaard, H.; Vestergaard, H.; Felding, P.; Olivarius, N.d.F.; Bjerrum, O.W. Eosinophilia in routine blood samples as a biomarker for solid tumor development – A study based on The Copenhagen Primary Care Differential Count (CopDiff) Database. Acta Oncol. 2014, 53, 1245–1250. [Google Scholar] [CrossRef]
- Curran, C.S.; Bertics, P.J. Eosinophils in glioblastoma biology. J. Neuroinflammation 2012, 9, 11. [Google Scholar] [CrossRef]
- Krishnan, T.; Tomita, Y.; Roberts-Thomson, R. A retrospective analysis of eosinophilia as a predictive marker of response and toxicity to cancer immunotherapy. Future Sci. OA, 2020; 6, FSO608. [Google Scholar]
- Hu, G.; Wang, S.; Zhong, K.; Xu, F.; Huang, L.; Chen, W.; Cheng, P. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: a meta-analysis. BMC Cancer 2020, 20, 454. [Google Scholar] [CrossRef]
- Peurala, E.; Tuominen, M.; Löyttyniemi, E.; Syrjänen, S.; Rautava, J. Eosinophilia is a favorable prognostic marker for oral cavity and lip squamous cell carcinoma. APMIS 2018, 126, 201–207. [Google Scholar] [CrossRef]
- Dorta, R.G.; Landman, G.; Kowalski, L.P.; Lauris, J.R.P.; O Latorre, M.R.D.; Oliveira, D.T. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology 2002, 41, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Bradley, P.J.; Griffin, N.R. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am. J. Surg. 1994, 168, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Leighton, S.E.J.; Teo, J.G.C.; Leung, S.F.; Cheung, A.Y.K.; Lee, J.C.K.; van Hasselt, C.A. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer 1996, 77, 436–440. [Google Scholar] [CrossRef]
- Harbaum, L.; Pollheimer, M.J.; Kornprat, P.; A Lindtner, R.; Bokemeyer, C.; Langner, C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod. Pathol. 2015, 28, 403–413. [Google Scholar] [CrossRef]
- Fernández-Aceñero, M.J.; Galindo-Gallego, M.; Sanz, J.; Aljama, A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 2000, 88, 1544–1548. [Google Scholar] [CrossRef]
- Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner, N.; Moesgaard, F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999, 189, 487–495. [CrossRef]
- Prizment, A.E.; Vierkant, R.A.; Smyrk, T.C.; Tillmans, L.S.; Lee, J.J.; Sriramarao, P.; Nelson, H.H.; Lynch, C.F.; Thibodeau, S.N.; Church, T.R.; et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod. Pathol. 2016, 29, 516–527. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Wang, L.; Ning, Z.; Zhuang, Y.; Gan, J.; Chen, S.; Zhou, D.; Zhu, H.; Tan, D.; et al. Clinical Impact of Tumor-Infiltrating Inflammatory Cells in Primary Small Cell Esophageal Carcinoma. Int. J. Mol. Sci. 2014, 15, 9718–9734. [Google Scholar] [CrossRef]
- Ishibashi, S.; Ohashi, Y.; Suzuki, T.; Miyazaki, S.; Moriya, T.; Satomi, S.; Sasano, H. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006, 26, 1419–24. [Google Scholar] [PubMed]
- Hollander, P.; Rostgaard, K.; Smedby, K.E.; Molin, D.; Loskog, A.; de Nully Brown, P.; Enblad, G.; Amini, R.-M.; Hjalgrim, H.; Glimelius, I. An anergic immune signature in the tumor microenvironment of classical Hodgkin lymphoma is associated with inferior outcome. Eur. J. Haematol. 2018, 100, 88–97. [Google Scholar] [CrossRef]
- Iwasaki, K.; Torisu, M.; Fujimura, T. Malignant tumor and eosinophils: I. Prognostic significance in gastric cancer. Cancer 1986, 58, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Ozawa, M.; Tamura, Y.; Suzuki, T.; Suzuki, K.; Kurokawa, K.; Fukabori, Y.; Yamanaka, H. Tumor-associated tissue eosinophilia of penile cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2002, 9, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Bethwaite, P.B.; Holloway, L.J.; Yeong, M.L.; Thornton, A. Effect of tumour associated tissue eosinophilia on survival of women with stage IB carcinoma of the uterine cervix. . J. Clin. Pathol. 1993, 46, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Tostes Oliveira D, Tjioe KC, Assao A, Sita Faustino SE, Lopes Carvalho A, Landman G, Kowalski LP. Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol. 2009, 17, 244–249. [Google Scholar] [CrossRef]
- Flamm, J. Tumor-associated tissue inflammatory reaction and eosinophilia in primary superficial bladder cancer. Urology 1992, 40, 180–185. [Google Scholar] [CrossRef]
- Paz D, Chang KP, Kao HK, Lao WWK, Huang YC, Chang YL, Huang, Y. Clinical implications of tumor-associated tissue eosinophilia in tongue squamous cell carcinoma. Laryngoscope. 2018, 129, 1123–1129. [Google Scholar]
- Alrawi SJ, Tan D, Stoler DL, Dayton M, Anderson GR, Mojica P, Douglas W, Hicks W, Jr, Rigual, N, Loree, T. Tissue eosinophilic infiltration: a useful marker for assessing stromal invasion, survival and locoregional recurrence in head and neck squamous neoplasia. Cancer J. 2005, 11, 217–225.
- Ercan I, Cakir B, Basak T, Ozdemir, T, Sayin, I, Turgut, S. Prognostic significance of stromal eosinophilic infiltration in cancer of the larynx. Prognostic significance of stromal eosinophilic infiltration in cancer of the larynx. Otolaryngol Head Neck Surg. 2005, 132, 869–873.
- Sassier, A.M.; Mcclatchey, K.D.; Wolf, G.T.; Fisher, S.G. Eosinophilic infiltration in advanced laryngeal squamous cell carcinoma. Laryngoscope 1995, 105, 413–416. [Google Scholar] [CrossRef]
- Keresztes, K.; Szollosi, Z.; Simon, Z.; Tarkanyi, I.; Nemes, Z.; Illes, A. Retrospective analysis of the prognostic role of tissue eosinophil and mast cells in Hodgkin’s lymphoma. Pathol. Oncol. Res. 2007, 13, 237–242. [Google Scholar] [CrossRef]
- von Wasielewski R, Seth S, Franklin J, Fischer R, Hubner K, Hansmann ML, Diehl, V, Georgii, A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood. 2000, 95, 1207–1213. [CrossRef]
- van Driel, W.J.; Hogendoorn, P.C.; Jansen, F.-W.; Zwinderman, A.H.; Trimbos, J.; Fleuren, G.J. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum. Pathol. 1996, 27, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, U.; Asti, D.; Saqib, A.; Mudduluru, B.M.; Ayaz, S.; Odaimi, M. Eosinophilia as the presenting sign in pancreatic cancer: an extremely rare occurrence. Postgrad. Med. 2017, 129, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.H.; Kapoor, H.; Morris, J. Severe eosinophilia associated with a malignant islet cell tumour. CMAJ 1989, 140, 1061–1063. [Google Scholar]
- Hirata, J.; Koga, T.; Nishimura, J.; Ibayashi, H. Pancreatic carcinoma associated with marked eosinophilia: A case report. Eur. J. Haematol. 1987, 39, 462–466. [Google Scholar] [CrossRef]
- Checinska, Z. [A CASE OF SEVERE EOSINOPHILIA IN A CASE OF CANCER OF THE HEAD OF THE PANCREAS]. Pol Arch Med Wewn. 1964, 34, 1373–1376. [Google Scholar]
- Abu-Shawer, O.; Abu-Shawer, M.; Shurman, A.; Lattouf, A.; Haimour, A.; Hamdan, O.; Mansour, R.; Altamimi, T.; Al-Hussaini, M. The clinical value of peripheral immune cell counts in pancreatic cancer. PLOS ONE 2020, 15, e0232043. [Google Scholar] [CrossRef]
- Ohkuma, R.; Kubota, Y.; Horiike, A.; Ishiguro, T.; Hirasawa, Y.; Ariizumi, H.; Watanabe, M.B.; Onoue, R.B.; Ando, K.; Tsurutani, J.; et al. The Prognostic Impact of Eosinophils and the Eosinophil-to-Lymphocyte Ratio on Survival Outcomes in Stage II Resectable Pancreatic Cancer. Pancreas 2021, 50, 167–175. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Bourdin, A.; Busse, W.W.; Ferguson, G.T.; Brooks, L.; Barker, P.; Martin, U.J. Two-Year Integrated Efficacy And Safety Analysis Of Benralizumab In Severe Asthma. J. Asthma Allergy 2019, ume 12, 401–413. [Google Scholar] [CrossRef]
- Jackson, D.J.; Korn, S.; Mathur, S.K.; Barker, P.; Meka, V.G.; Martin, U.J.; Zangrilli, J.G. Safety of Eosinophil-Depleting Therapy for Severe, Eosinophilic Asthma: Focus on Benralizumab. Drug Saf. 2020, 43, 409–425. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; Fitzgerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Cameron, J.L.; Lillemoe, K.D. i wsp. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann. Surg. 2002, 236, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa T, Wood LD, Itoi T, Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.-Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.-C.; Vlahovic, G.; et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef]
- Aglietta, M.; Barone, C.; Sawyer, M.B.; Moore, M.J.; Miller, W.H., Jr.; Bagalà, C.; Colombi, F.; Cagnazzo, C.; Gioeni, L.; Wang, E.; et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1750–1755. [Google Scholar] [CrossRef]
- Renouf, D.J.; Knox, J.J.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; Harb, M.; Aucoin, N.; et al. LBA65 The Canadian Cancer Trials Group PA.7 trial: Results of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab-P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). Ann. Oncol. 2020, 31, S1195. [Google Scholar]
- Renouf, D.J.; Knox, J.J.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; Harb, M.; Aucoin, N.; et al. LBA65 The Canadian Cancer Trials Group PA.7 trial: Results of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab-P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). Ann. Oncol. 2020, 31, S1195. [Google Scholar]
| Parameter - nominal | N, % |
| Grade: 1 2 3 |
26; 19,0% 93; 67,9% 18; 13,1% |
| Tumor localization Head Body/tail |
98; 71,5% 39; 28,5% |
| Stage I-III IV |
65; 47,4% 72; 52,6% |
| Sex Female Male |
79; 57,7% 58; 42,3% |
| Survival 6-months 12-months 24-months |
58; 42,3% 74; 54,0% 5; 3,7% |
| Parameter – linear |
Mean ±SD; median; IQR; minimum; maximum |
| Age, years | 64,2±7,7; 65,0; 59,0-70,0; 46,0; 82,0 |
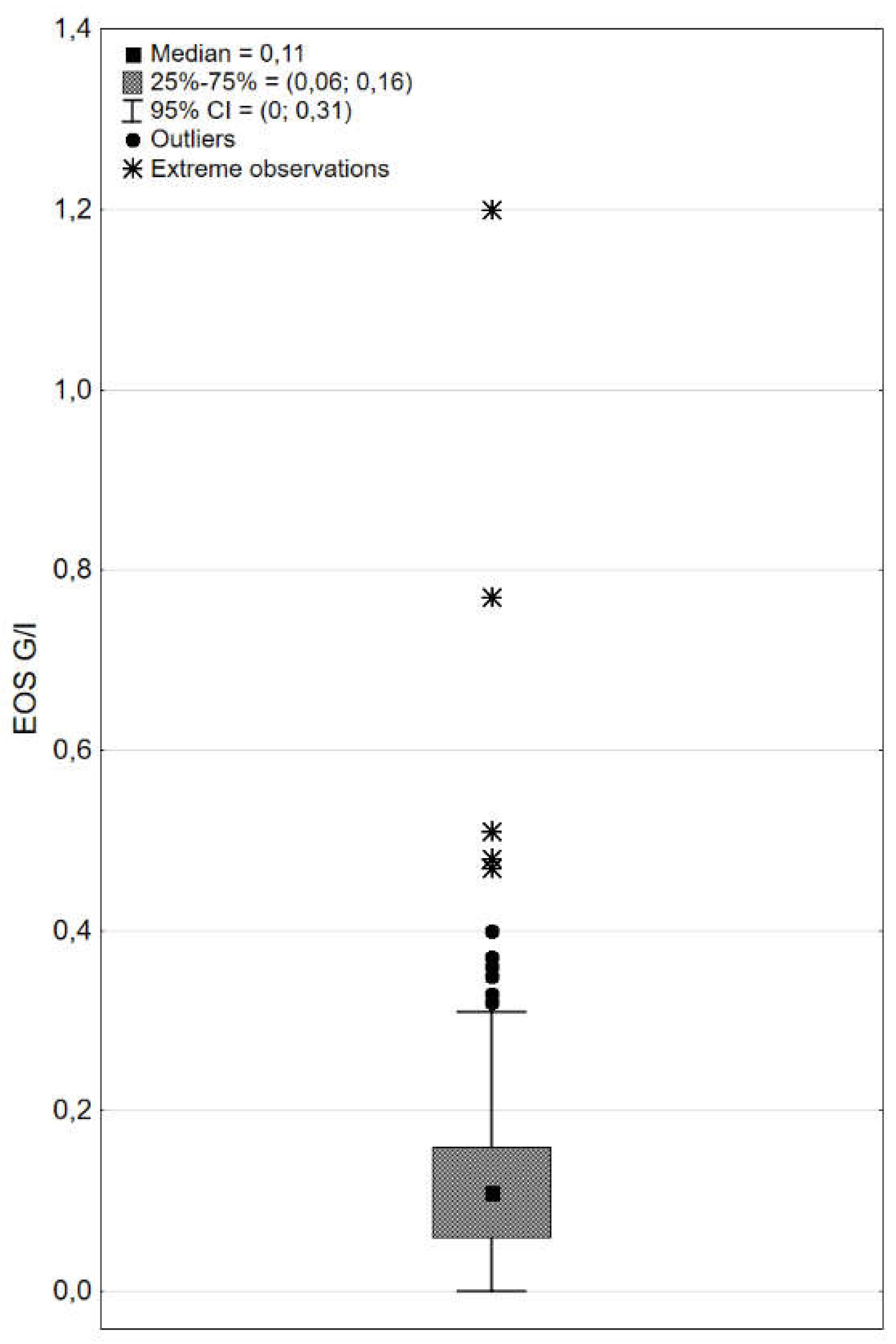
| EOS, G/l | 0,15±0,15; 0,11; 0,06-0,16; 0,00; 1,2 |
| Relative eosinophilia, % | 2,0±1,9; 1,6; 0,8-2,5; 0,0; 13,8 |
| Parameter - nominal |
Stage I-III N, % |
Stage IV N, % |
p |
| Grade: 1 2 3 |
11; 17,46% 45; 71,43% 7; 11,11% |
15; 21,74% 45; 65,22% 9; 13,04% |
0,743 |
| Tumor localization Head Body/tail |
46; 73,02% 17; 26,98% |
47; 68,12% 22; 31,88% |
0,537 |
| Sex Female Male |
29; 46,03% 34; 53,97% |
29; 42,03% 40; 57,97% |
0,644 |
| Survival 6-months 12-months 24-months |
40; 63,49% 31; 49,21% 14; 22,22% |
34; 49,28% 17; 24,64% 1; 1,45% |
0,100 0,003 0,000 |
| Parameter – linear |
Mean ±SD; median; IQR; minimum; maximum |
p | |
| Age, years | 64,0±7,8; 66,0; 58,0-70,0; 47,0; 82,0 | 54,6±7,4; 65,0; 61,0-71,0; 46,0; 78,0 | 0,149 |
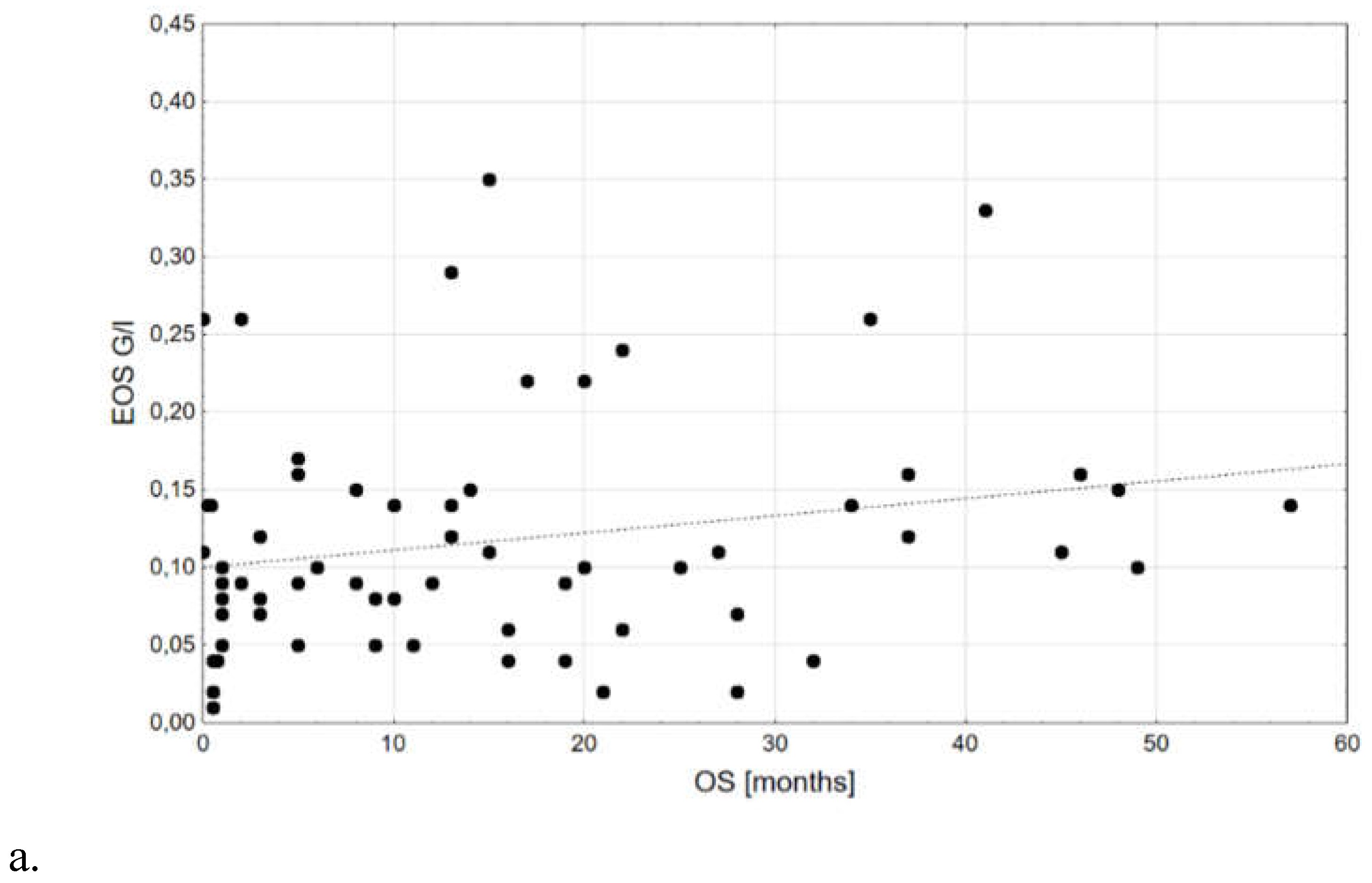
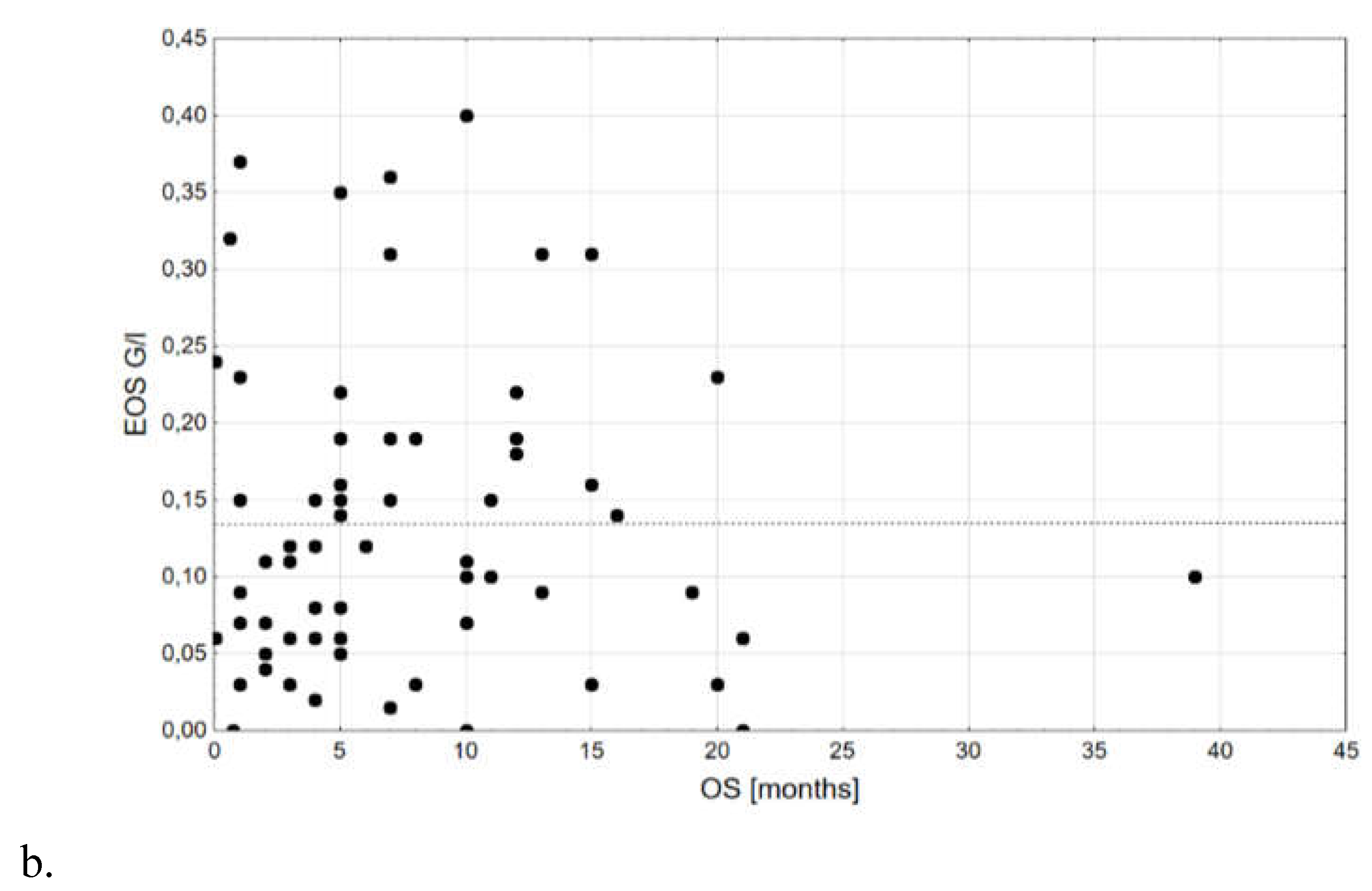
| EOS, G/l | 0,18±0,08; 0,10; 0,06-0,15; 0,01; 0,35 | 0,13±0,10; 0,11; 0,06-0,19; 0,00; 0,40 | 0,773 |
| Relative eosinophilia, % | 1,69±1,14; 1,57; 0,94-2,19; 0,24; 5,77 | 1,89±1,42; 1,62; 0,72-2,53; 0,00; 7,67 | 0,773 |
| Parameter - nominal |
< 0,1 G/l N, % |
≥ 0,1 G/l N, % |
p |
| Grade: 1 2 3 |
9; 15,52% 44; 75,86% 5; 8,62% |
17; 21,52% 49; 62,03% 13; 16,46% |
0,207 |
| Tumor localization Head Body/tail |
40; 68,97% 18; 31,03% |
58; 73,42% 21; 26,58% |
0,568 |
| Stage I-III IV |
29; 50,00% 29; 50,00% |
36; 45,57% 43; 54,45% |
0,607 |
| Sex Female Male |
30; 51,72% 28; 48,28% |
49; 62,03% 30; 37,97% |
0,227 |
| Survival 6-months 12-months 24-months |
27; 46,6% 16; 27,6% 2; 3,45% |
50; 63,29% 35; 44,30% 14; 17,72% |
0,051 0,045 0,011 |
| Parameter – linear | Mean ±SD; median; IQR; minimum; maximum | ||
| Age, years | 63,8±8,6; 63,5; 58,0-70,0; 46,0; 81,0 | 64,5±7,0; 66,0; 59,0-70,0; 49,0; 82,0 | 0,663 |
| EOS, G/l | 0,05±0,03; 0,06 0,03-0,08; 0,0; 0,09 | 0,21±0,16; 0,15; 0,12-0,26; 0,10; 1,20 | 0,000 |
| Relative eosinophilia, % | 0,86±0,53; 0,76; 0,48-1,17; 0,00; 2,28 | 2,92±2,03; 2,35; 1,67-3,28; 0,97; 13,79 | 0,000 |
| Parameter | Effect level -reference level | p | HR | HR 95%CI |
|---|---|---|---|---|
| Grade | 1 - 3 | 0,177 | 0,53 | 0,28-1,00 |
| 2 - 3 | 0,134 | 0,54 | 0,12-0,93 | |
| Localization | Head – body/tail | 0,508 | 0,88 | 0,59-1,30 |
| Stage | IV - I-III | 0,001 | 1,88 | 1,29-2,75 |
| Sex | Female - male | 0,747 | 1,06 | 0,74-1,25 |
| Age>65 years | No - yes | 0,414 | 0,86 | 0,60-1,23 |
| EOS | <0,1 G/l - ≥0,1 G/l | 0,035 | 1,48 | 1,03-2,13 |
| Parameter | Effect level -reference level | p | HR | HR 95%CI |
|---|---|---|---|---|
| Grade | 1 - 3 | 0,422 | 0,62 | 0,33-1,15 |
| 2 - 3 | 0,126 | 0,57 | 0,32-0,99 | |
| Localization | Head – body/tail | 0,427 | 1,18 | 0,79-1,76 |
| Stage | IV - I-III | 0,003 | 1,73 | 1,19-2,50 |
| Sex | Female - male | 0,933 | 1,01 | 0,71-1,45 |
| Age>65 years | No - yes | 0,360 | 0,84 | 0,59-1,21 |
| EOS | <0,1 G/l - ≥0,1 G/l | 0,021 | 1,57 | 1,07-2,31 |
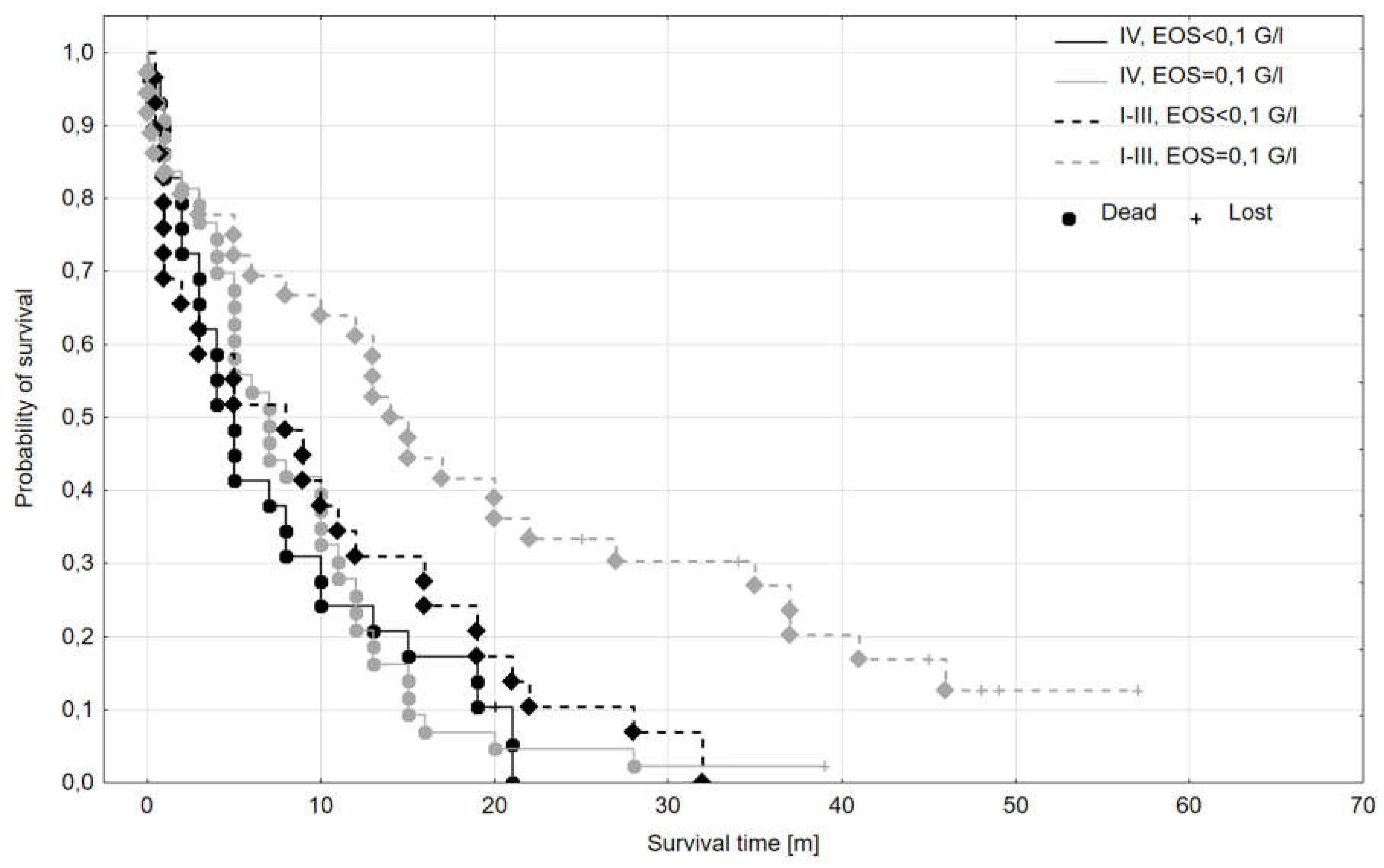
| Subgroup | Median OS [m] | Mean OS [m] | OS SD [m] | No. of complete observations | No. of lost observations | n total |
|---|---|---|---|---|---|---|
| I-III, EOS ≥0,1 G/l | 14,50 | 19,33 | 16,85 | 30 | 6 | 36 |
| I-III, EOS <0,1 G/l | 8,00 | 9,84 | 9,66 | 28 | 1 | 29 |
| IV, EOS ≥0,1 G/l | 7,00 | 8,57 | 7,56 | 42 | 1 | 43 |
| IV, EOS <0,1 G/l | 5,00 | 7,48 | 6,87 | 28 | 1 | 29 |
| Parameter | Holub | Abu-Shawer | Ohkuma | Current study |
|---|---|---|---|---|
| Year | 2020 | 2020 | 2021 | 2023 |
| Protocol | retrospective | retrospective | retrospective | retrospective |
| n | 66 | 355 | 67 | 137 |
| Subjects with distant meta included? | No | Yes | No | No |
| Resection status known | Yes | No | Yes | Yes |
| Chemotherapy | Yes | N/A | N/A | N/A |
| Radiotherapy | Yes | N/A | N/A | N/A |
| EOS: median; mean [G/l] | 0,1; 0,189 | 0,140; 0,190 | N/A | 0,11; 0,15 |
| Cut-off point | 0,5 | 0,143 | 0,126 | 0,1 |
| Result from univariate Cox regression | ≥0,5; HR 1,9 p=0,300 | ≥0,143 HR=0,9 p=0,54 | ≥0,126 HR=0,51 p=0,042 | <0,1: HR 1,48 p=0,035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





