Submitted:
29 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
1. Methods
1.1. Study Subjects
1.2. Study Content
1.2.1. HFNC Sequential Therapy Protocol
1.2.2. Data Collection
1.3. Statistical Analysis
2. Results
2.1. General Information of the Included Patients
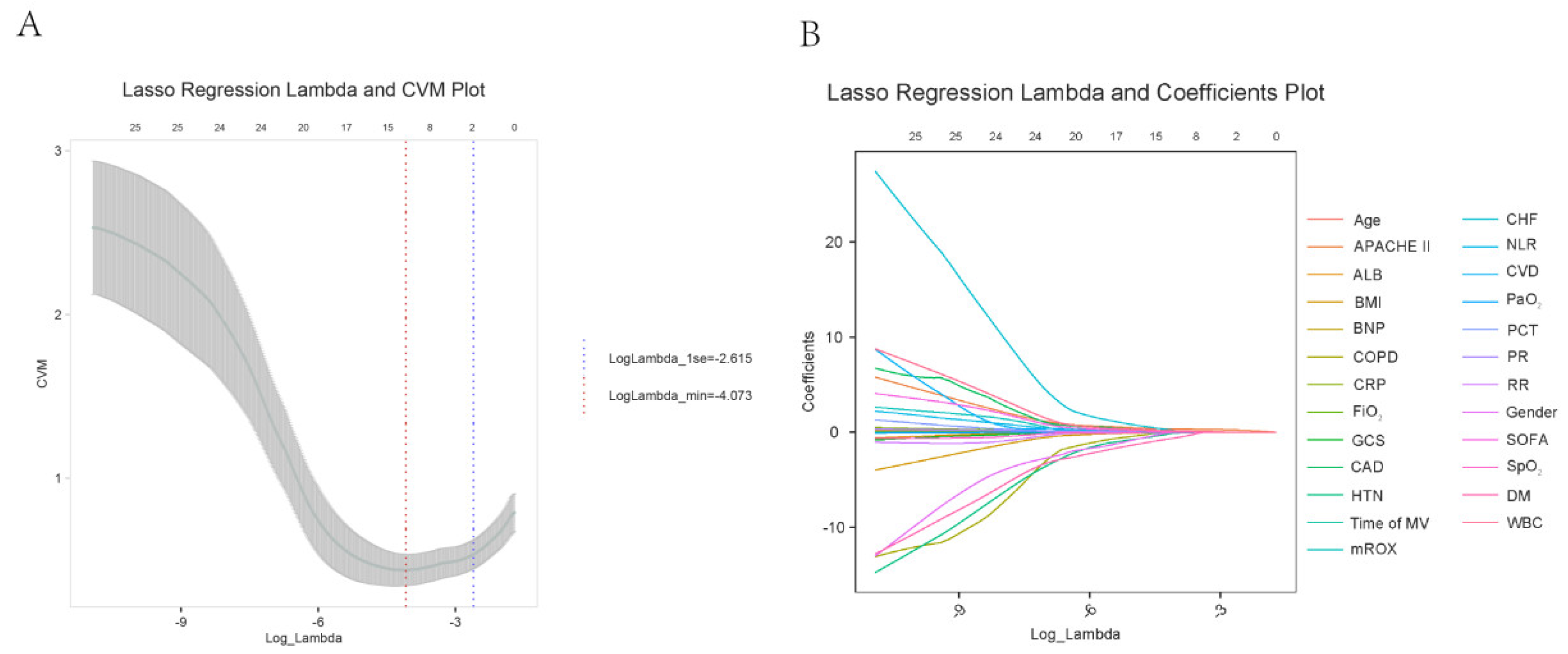
2.2. Lasso Regression Analysis for Feature Selection Related to Outcomes
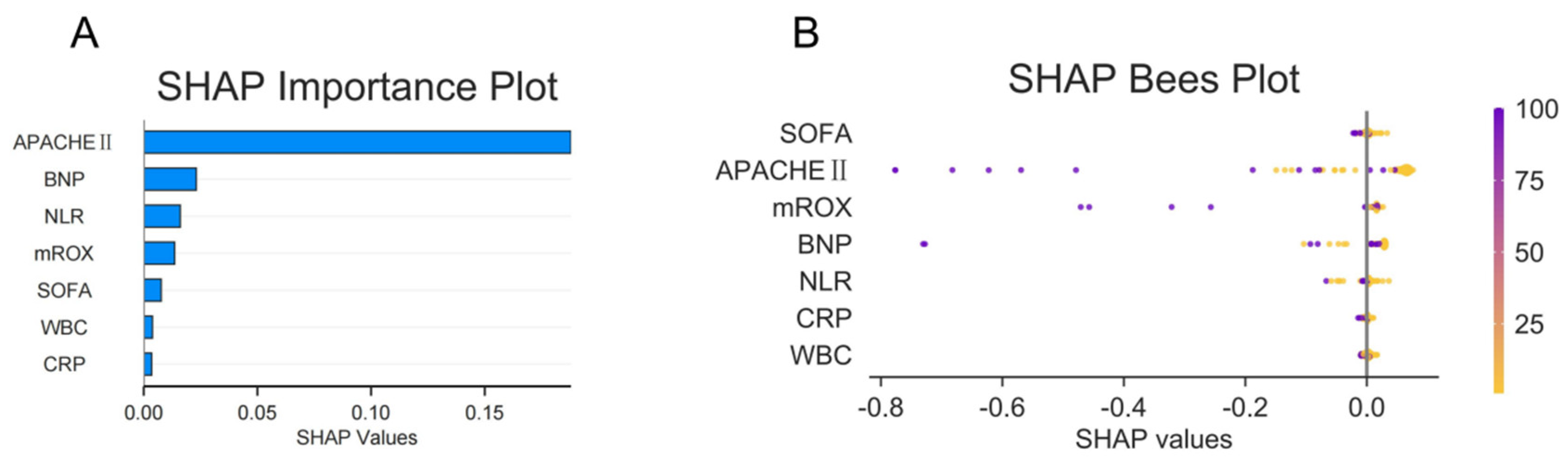
2.3. Random Forest Analysis to Identify Important Features Related to Outcomes
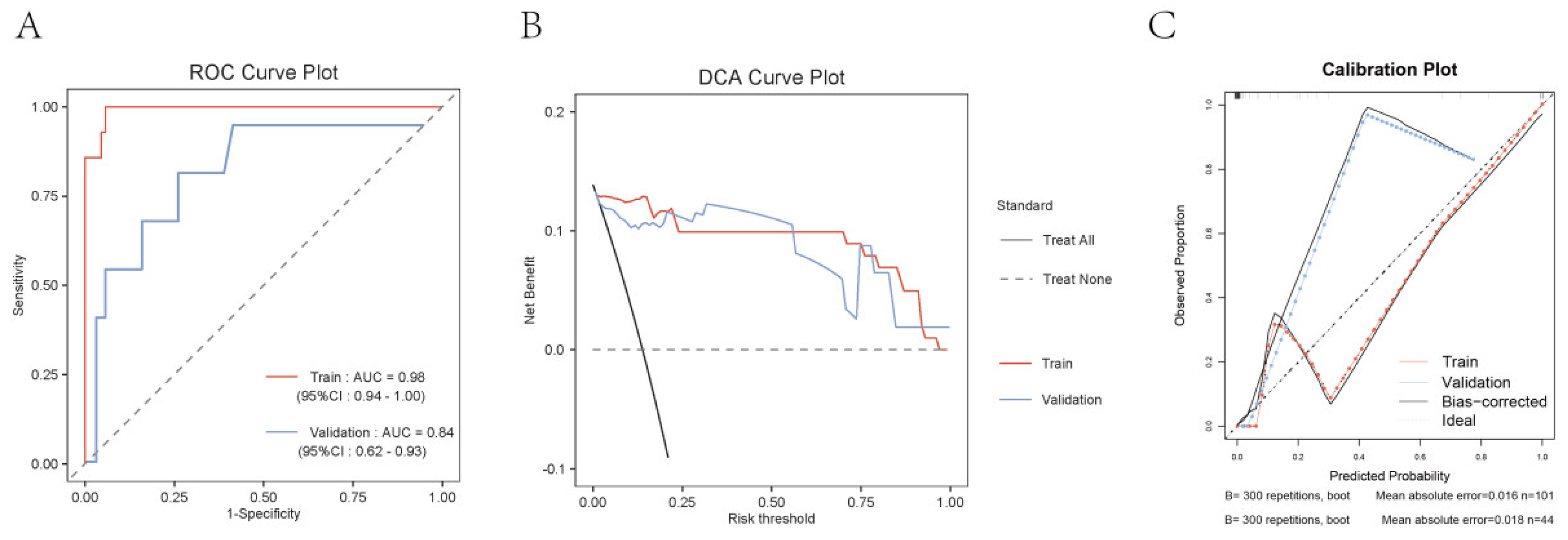
2.4. Construction of an Early Warning Model Nomogram
Discussion
Conclusion
References
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017, 50, 1602426. [Google Scholar] [CrossRef] [PubMed]
- Piraino, T. Noninvasive Respiratory Support in Acute Hypoxemic Respiratory Failure. Respir Care. 2019, 64, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Granton, D.; Wang, D.X.; et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019, 45, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Gu, S.; Lei, W.; et al. High-flow nasal cannula versus conventional oxygen therapy in acute COPD exacerbation with mild hypercapnia: a multicenter randomized controlled trial. Crit Care. 2022, 26, 109 Published 2022 Apr 15. [Google Scholar] [CrossRef]
- Frat, J.P.; Marie, D.; Thille, A.W. Acute respiratory failure: nonintubation assist methods for the acutely deteriorating patient. Curr Opin Crit Care. 2019, 25, 591–596. [Google Scholar] [CrossRef]
- Oczkowski, S.; Ergan, B.; Bos, L.; et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022, 59, 2101574. [Google Scholar] [CrossRef]
- Hernández, G.; Paredes, I.; Moran, F.; et al. Effect of postextubation noninvasive ventilation with active humidification vs high-flow nasal cannula on reintubation in patients at very high risk for extubation failure: a randomized trial [published correction appears in Intensive Care Med. 2023 Mar;49, 385]. Intensive Care Med. 2022, 48, 1751–1759. [Google Scholar]
- Frat, J.P.; Thille, A.W.; Mercat, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Liu, X.; Song, M.; Tao, D.; et al. Random forest construction with robust semisupervised node splitting. IEEE Trans Image Process. 2015, 24, 471–483. [Google Scholar] [CrossRef]
- Wallace, M.L.; Mentch, L.; Wheeler, B.J.; et al. Use and misuse of random forest variable importance metrics in medicine: demonstrations through incident stroke prediction. BMC Med Res Methodol. 2023, 23, 144. [Google Scholar] [CrossRef]
- Middleton, P.M. Practical use of the Glasgow Coma Scale; a comprehensive narrative review of GCS methodology. Australas Emerg Nurs J. 2012, 15, 170–83. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Jin, Z.; Deng, J.; She, Y.; Zhong, Y.; Sun, W.; Ren, Y.; Cao, N.; Chen, C. Development and validation of a deep learning model to predict the survival of patients in ICU. J Am Med Inform Assoc. 2022, 29, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Zhang, Z.; Tan, Z.; Shi, Z.; Li, H.; Zhang, S.; Zhao, X.; Yao, Y.; Li, W.; Gao, Y.; Zhu, M. Modified Respiratory Rate Oxygenation Index: An Early Warning Index for the Need of Intubation in COVID-19 Patients with High-Flow Nasal Cannula Therapy. J Emerg Med. 2023, 65, e93–e100. [Google Scholar] [CrossRef]
- Giri, J.; Al-Lohedan, H.A.; Mohammad, F.; et al. A Comparative Study on Predication of Appropriate Mechanical Ventilation Mode through Machine Learning Approach. Bioengineering 2023, 10, 418. [Google Scholar] [CrossRef]
- Gao, D.; Feng, W.; Qiao, Y.; Jiang, X.; Zhang, Y. Development and validation of a random forest model to predict functional outcome in patients with intracerebral hemorrhage. Neurol Sci. 2023, 44, 3615–3627. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, J.; Qiu, Y.; Fei, N.; Yin, L. Correlations between APACHE-II score and pressure parameters of mechanical ventilation in patients with ARDS and their value in prognostic evaluation. Pak J Med Sci. 2023, 39, 1584–1588. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Wang, Z.; Jia, D.; Zhang, C.; Ma, X.; Han, X.; Zhao, T.; Zhang, Z. The risk factors of reintubation in intensive care unit patients on mechanical ventilation: A systematic review and meta-analysis. Intensive Crit Care Nurs. 2023, 74, 103340. [Google Scholar] [CrossRef]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J Am Coll Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef]
- Cortegiani, A.; Longhini, F.; Carlucci, A.; Scala, R.; Groff, P.; Bruni, A.; Garofalo, E.; Taliani, M.R.; Maccari, U.; Vetrugno, L.; Lupia, E.; Misseri, G.; Comellini, V.; Giarratano, A.; Nava, S.; Navalesi, P.; Gregoretti, C. High-flow nasal therapy versus noninvasive ventilation in COPD patients with mild-to-moderate hypercapnic acute respiratory failure: study protocol for a noninferiority randomized clinical trial. Trials. 2019, 20, 450. [Google Scholar] [CrossRef]
- Capone, M.; Giannarelli, D.; Mallardo, D.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xie, T.; Chen, Y.; Zhou, Y.; Han, X. The Effects of Bivalirudin and Ordinary Heparin on the Incidence of Bleeding Events and the Level of Inflammation after Interventional Therapy for Acute Myocardial Infarction. Altern Ther Health Med. Published online May 24, 2024.
- VIKAS,GUPTA, VIKRAM,CHAUDHARI, SHAILESH V.,SHRIKHANDE, et al. Does Preoperative Serum Neutrophil to Lymphocyte Ratio (NLR), Platelet to Lymphocyte Ratio (PLR), and Lymphocyte to Monocyte Ratio (LMR) Predict Prognosis Following Radical Surgery for Pancreatic Adenocarcinomas? Results of a Retrospective Study. Journal of gastrointestinal cancer 2022, 53, 641–648.
- Roca, O.; Messika, J.; Caralt, B.; García-de-Acilu, M.; Sztrymf, B.; Ricard, J.D.; Masclans, J.R. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J Crit Care. 2016, 35, 200–5. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Pan, J.; Xu, Z.; Xu, J. The ROX index as a predictor of high-flow nasal cannula outcome in pneumonia patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. BMC Pulm Med. 2022, 22, 121. [Google Scholar] [CrossRef]
- Roca, O.; Messika, J.; Caralt, B.; et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J Crit Care. 2016, 35, 200–205. [Google Scholar] [CrossRef]
- incent, J.L.; de Mendonça, A.; Cantraine, F.; et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
| Succeed(n=126) | Failure(n=19) | t/χ² | P | |
| Age | 56.50±14.94 | 59.32±13.24 | -0.78 | 0.439 |
| BMI(kg/m2) | 24.06±3.25 | 23.63±3.45 | 0.53 | 0.6 |
| Time of MV(h) | 218.47±64.01 | 208.58±63.76 | 0.63 | 0.531 |
| WBC(10^9/L) | 13.89±2.51 | 15.68±1.92 | -3.64 | 0.001 |
| NLR | 7.61±4.42 | 9.37±3.68 | -1.65 | 0.101 |
| CRP(mg/L) | 39.94±22.72 | 58.68±38.93 | -2.05 | 0.054 |
| Alb(g/L) | 33.40±7.53 | 34.16±8.61 | -0.4 | 0.688 |
| PCT(ng/mL) | 7.49±3.92 | 7.63±4.02 | -0.14 | 0.886 |
| BNP(pg/mL) | 148.45±109.65 | 291.26±140.73 | -5.09 | <.001 |
| RR | 21.75±3.55 | 21.05±3.39 | 0.8 | 0.426 |
| PR | 93.44±12.76 | 94.63±11.28 | -0.38 | 0.702 |
| SpO2 (%) | 93.73±2.21 | 94.16±2.46 | -0.78 | 0.439 |
| PaO2(mmHg) | 77.23±20.09 | 72.26±19.42 | 1.01 | 0.315 |
| FiO2 | 0.77±0.20 | 0.72±0.19 | 1.01 | 0.315 |
| mROX | 5.16±1.33 | 3.95±1.50 | 3.64 | <.001 |
| APACHEⅡ | 13.07±3.47 | 19.47±3.12 | -7.59 | <.001 |
| GCS | 10.69±3.86 | 11.54±4.59 | 1.9 | 0.16 |
| SOFA | 9.56±3.73 | 12.21±2.76 | -3.7 | <.001 |
| Gender,n(%) | 0 | 0.984 | ||
| Male | 66(52.38) | 10(52.63) | ||
| Female | 60(47.62) | 9(47.37) | ||
| HTN,n(%) | 0.03 | 0.855 | ||
| No | 99(78.57) | 14(73.68) | ||
| Yes | 27(21.43) | 5(26.32) | ||
| DM,n(%) | 0.75 | 0.388 | ||
| No | 101(80.16) | 13(68.42) | ||
| Yes | 25(19.84) | 6(31.58) | ||
| CAD,n(%) | 0.49 | 0.483 | ||
| No | 105(83.33) | 14(73.68) | ||
| Yes | 21(16.67) | 5(26.32) | ||
| CHF,n(%) | 0.37 | 0.543 | ||
| No | 104(82.54) | 14(73.68) | ||
| Yes | 22(17.46) | 5(26.32) | ||
| COPD,n(%) | 0.03 | 0.87 | ||
| No | 82(65.08) | 12(63.16) | ||
| Yes | 44(34.92) | 7(36.84) | ||
| CVD,n(%) | 2.43 | 0.119 | ||
| No | 108(85.71) | 13(68.42) | ||
| Yes | 18(14.29) | 6(31.58) |
| Train set(n=101) | Validation set(n=44) | t/χ² | P | |
| WBC(10^9/L) | 13.97±2.37 | 14.81±2.51 | -1.60 | 0.112 |
| NLR | 7.13±4.04 | 8.42±4.33 | -1.45 | 0.151 |
| CRP(mg/L) | 44.39±28.82 | 46.81±23.05 | -0.41 | 0.681 |
| BNP(pg/mL) | 181.50±124.84 | 170.94±131.88 | 0.39 | 0.701 |
| mROX | 4.93±1.31 | 5.17±1.62 | -0.81 | 0.419 |
| APACHEⅡ | 13.80±4.05 | 13.52±4.46 | 0.32 | 0.754 |
| SOFA | 10.39±3.93 | 10.00±3.45 | 0.47 | 0.638 |
| HTN,n(%) | 0.10 | 0.757 | ||
| No | 78(77.23) | 35(79.55) | ||
| Yes | 23(22.77) | 9(20.45) | ||
| DM,n(%) | 0.03 | 0.858 | ||
| No | 79(78.22) | 35(79.55) | ||
| Yes | 22(21.78) | 9(20.45) | ||
| CHF,n(%) | 0.14 | 0.708 | ||
| No | 83(82.18) | 35(79.55) | ||
| Yes | 18(17.82) | 9(20.45) | ||
| Outcome,n(%) | 1.89 | 0.169 | ||
| Succeed | 63(90.00) | 24(77.42) | ||
| Failure | 7(10.00) | 7(22.58) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





